Abstract
Oil-polluted water is a worldwide problem due to the increasing industrial oily wastewater and the frequent oil spill accidents. Here, we report a novel kind of superhydrophilic hybrid membranes for effective oil/water separation. They were prepared by depositing CaCO3-based mineral coating on PAA-grafted polypropylene microfiltration membranes. The rigid mineral-coating traps abundant water in aqueous environment and forms a robust hydrated layer on the membrane pore surface, thus endowing the membranes with underwater superoleophobicity. Under the drive of either gravity or external pressure, the hybrid membranes separate a range of oil/water mixtures effectively with high water flux (>2000 L m−2 h−1), perfect oil/water separation efficiency (>99%), high oil breakthrough pressure (>140 kPa) and low oil fouling. The oil/water mixtures include not only free mixtures but also oil-in-water emulsions. Therefore, the mineral-coated membrane enables an efficient and energy-saving separation for various oil/water mixtures, showing attractive potential for practical oil/water separation.
Oil pollution is a worldwide problem due to the increasing oily wastewater produced by petrochemical, textile, and food industries, as well as the frequent oil spill accidents1,2,3. The oil-polluted water usually contains harmful chemicals, which will bring about harm to people's health and even will have damaging impact on the ecosystem. Therefore, advanced materials or techniques are urgently needed to effectively separate various oil/water mixtures. Conventional techniques such as gravity separation, skimming and flotation are useful for the separation of free oil/water mixtures, but are not applicable to oil/water emulsions4,5. Besides, they suffer from the limits of low efficiency and high operation cost. As an alternative way, recent researches have emphasized the effectiveness of using special wettability to design novel materials for oil/water separation6,7. Superwetting materials with different affinities towards water and oil are believed to be promising in realizing high-efficient oil/water separation, since water and many oils are intrinsically immiscible. For example, superhydrophobic-superoleophilic materials, such as PTFE-coated mesh8, silicone-modified polyester textile9, and polysiloxane-based gel10, were fabricated to separate free oil/water mixtures by either membrane filtration or affinity absorption of oil from water11,12,13,14. In fact, the separation of water-in-oil emulsions also becomes available if the pore size of a hydrophobic-oleophilic membrane is rationally designed to be smaller than the emulsified water droplets15,16. However, there are some drawbacks for such “oil-removing” type of materials. The oil adhered on the material surfaces are generally difficult to be removed, which makes a washing process necessary and leads to unavoidable wastes of oils, cleaning fluids and the materials themselves. Besides, water normally has higher density than oil, which tends to settle below oil and then forms a barrier layer above the materials for oil permeation or adsorption. These disadvantages limit the suitability of the hydrophobic-oleophilic membranes for effective separation of free oil/water mixtures.
Recently, considerable attention has been focused on the underwater oil-repellency of fish scales17. Studies reveal that superhydrophilic materials such as fish scales can trap abundant water on their rough surfaces. The trapped water greatly weakens the direct contact between oils and materials, which results in “superoleophobic surfaces” with low oil-adhesion for the superhydrophilic materials18,19,20,21,22,23,24,25,26,27. Inspired by this phenomenon, several underwater superoleophobic materials, such as pH-responsive polymer-grafted textile28, hydrogel-coated29 or zeolite-coated mesh membranes30, were fabricated to separate free oil/water mixtures. These membranes showed high separation efficiency and low oil fouling. Moreover, a recent breakthrough was achieved by Tuteja and co-workers31,32. They reported a fluorodecyl POSS + x-PEGDA-coated hygro-responsive membrane, which can effectively separate both oil/water emulsions and free mixtures. Nevertheless, the pore size is larger than 30 μm for all these superoleophobic membranes. According to the permeation theory33, membrane pores with large size can reduce the flow resistance of water and facilitate water permeation, hence enabling the membranes with gravity-driven filtration and high flux. However, it also declines the flow resistance of oil and the oil breakthrough/intrusion pressure is less than 2 kPa for all these membranes. It means the height of the liquid column that the membranes can support is lower than several centimeters, limiting their practical applications. Therefore, it is imperative to develop novel membranes with high water flux, perfect oil/water separation efficiency, high oil breakthrough pressure and low oil fouling for practical oil/water separation.
Here, we report one novel kind of hybrid membranes for oil/water separation, which were previously found to have perfect water permeation with high water flux and energy-saving filtration under ultralow trans-membrane pressure34. These membrane were optimized by depositing CaCO3-based nano-sized minerals on the commercial microporous polypropylene membranes (MPPM) grafted with ~ 30 wt.% of poly(acrylic acid) (PAA). The as-prepared membranes are superhydrophilic, underwater superoleophobic, and antifouling to oils. It is very promising that, under the drive of either gravity or external pressure, our membranes can effectively separate not only free oil/water mixtures but also oil-in-water emulsions with high water flux (>2000 L m−2 h−1) and perfect oil/water separation efficiency (>99%). One of the most important advantages is the oil breakthrough pressure is high enough (>140 kPa) for the membranes, which is 2–3 orders of magnitude higher than those of reported superoleophobic membranes, showing attractive potentials for oil/water separation in real situations.
Results
Surface morphology of the mineral-coated membrane
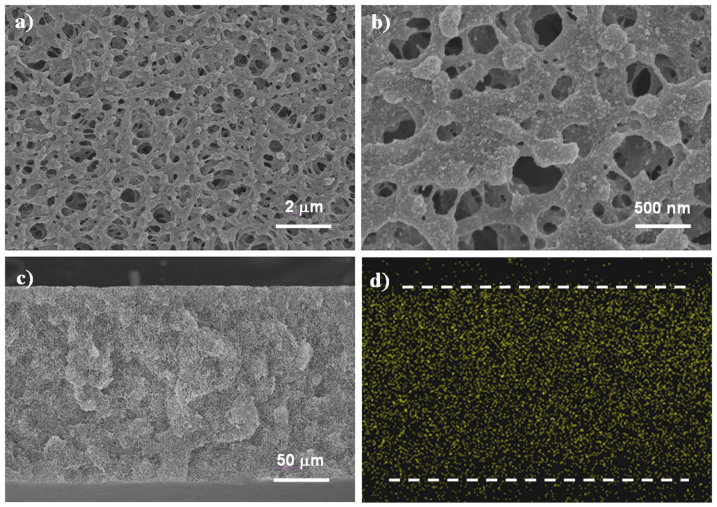
The hydrophilic CaCO3 coating was deposited on the membrane pore surface through photoinitiated grafting of PAA and then followed by an alternate soaking process34. Figure 1 shows typical SEM images of the mineral-coated membranes. It can be seen that a rough coating is formed on the smooth surface of PAA-grafted membrane (Figure S1). The coating distributes conformally and the porous structure is mostly retained for the prepared membrane. The enlarged view of membrane (Figure 1b) indicates that the coating is composed of CaCO3 nanoparticles with size in 38.7 ± 9.0 nm. This size is much smaller than the diameter of membrane pores (200 ~ 750 nm), which ensures the separation performances of the membrane. Additionally, Ca element EDX mapping of the membrane cross-section further confirms that the mineral coating distributes evenly throughout the membrane (Figure 1c,d).
Figure 1. Morphology of the mineral-coated membranes prepared by grafting PAA and then depositing CaCO3 nanoparticles on polypropylene microfiltration membranes.
(a) SEM image of the membrane surface in large-area view. (b) Enlarged view of the membrane surface. (c) SEM image of the membrane cross-section. (d) Distribution of calcium element (points measured by EDX mapping analysis) on the membrane cross-section (The dash lines indicate the edges of the cross-section. The dense spots below the lower dash line originate from the bottom surface of the membrane).
Underwater oil wettability of the mineral-coated membrane
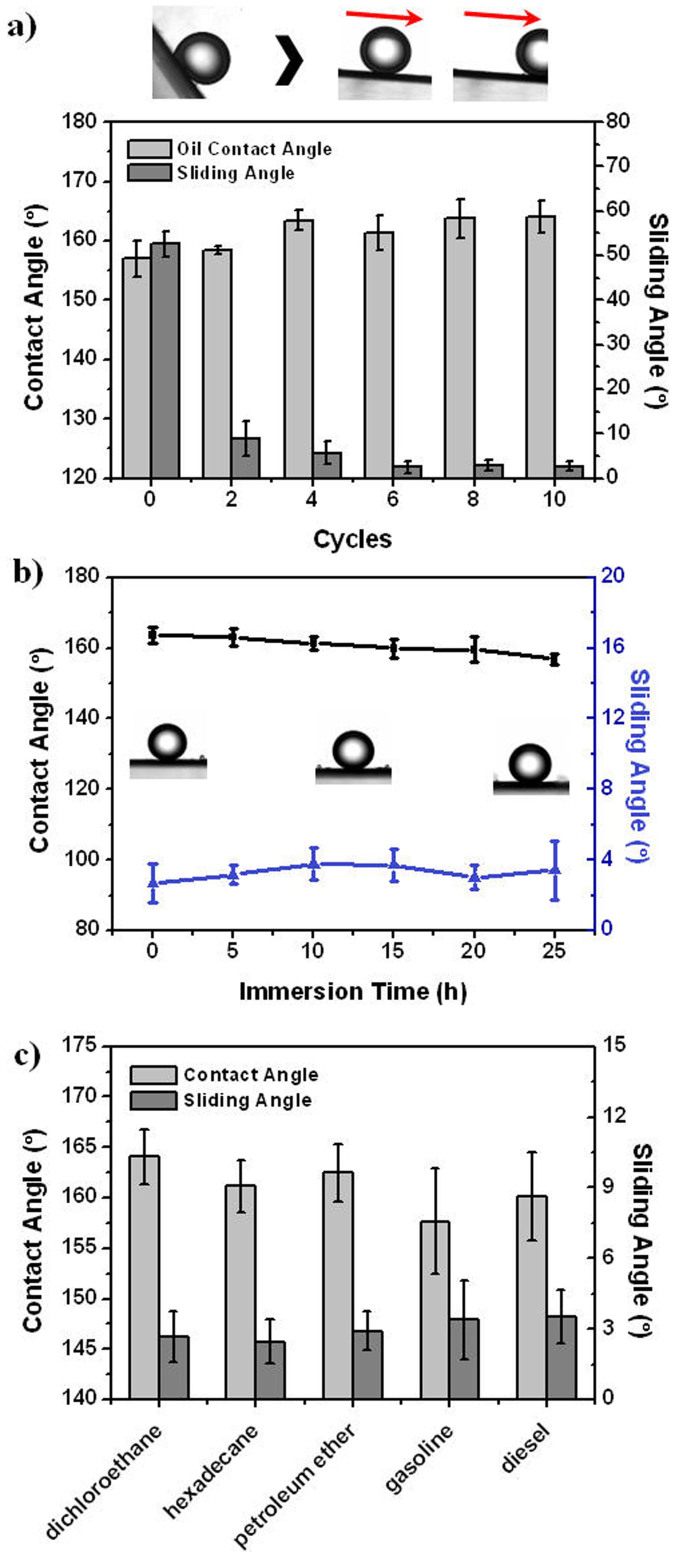
Our previous work has proved that the mineral-coated membranes are superhydrophilic (Figure S3)34. In order to evaluate the performances of the membranes in oil/water separation, we further investigated their underwater oil wettability. Figure 2a shows the wetting behavior of 1,2-dichloroethane (DCE) on the membranes prepared with different mineral-coating cycles. Both PAA-grafted and mineral-coated membranes are underwater superoleophobic with oil contact angles (OCA) above 150°. As is well-known, the wettability of a surface is mainly governed by its chemical composition and roughness6,7,35,36. Particularly, a micro/nano-scale hierarchical structure is recognized to be crucial for constructing super-antiwetting surface, which promotes the “Cassie-Baxter” state of liquid in three-phase system. MPPM possesses porous structure with certain roughness. After being grafted with PAA, a hydrophilic layer is introduced onto the membrane pore surface and helps to trap water into the membrane. The trapped water decreases the contact area between the membrane surface and the oil droplet, leading to a dramatic increase in oleophobicity. However, this PAA-grafted membrane surface still suffers from high oil-adhesion. The sliding angle (SA) is found to be 52.6 ± 2.9°. It is due to the limited roughness of PAA-grafted MPPM and the instability of trapped water in the soft PAA brushes.
Figure 2. Underwater oil wettability of the mineral-coated membranes.
(a) The contact angle and sliding angle of 1,2-dichloroethane (DCE) droplets on the membranes prepared from different mineral-coating cycles. Inset photographs show that the oil-adhesion declines after mineral-coating. (b) The wetting behavior of DCE droplets on the membranes that are immersed in DCE for a certain time. The membranes exhibit stable superoleophobicity. (c) The underwater superoleophobic property of the mineral-coated membranes for a series of oils.
After being mineral-coated, these drawbacks can be totally overcome. Firstly, the coating is composed of CaCO3 nanoparticles, which forms micro/nano-hierarchical structure when combining with the porous morphology of MPPM. The roughness of the membrane surface is greatly increased and the contact area between the membrane surface and the oil droplet is decreased. As a result, oil-adhesion of the membrane declines gradually with increasing the mineral-coating cycles (Figure 2a). The SA is only 2.7 ± 1.1° for those optimal membranes prepared from 10 mineral-coating cycles, indicating low oil-adhesion property. Besides, CaCO3 is more rigid and stable than PAA brushes, which enhances the stability of trapped water. To verify it, the optimal as-prepared membranes were pre-wetted with water and then immersed into DCE for a certain time. The OCA and SA were measured and the results are shown in Figrue 2b. It is found that the membranes are superoleophobic even after being immersed in DCE for 25 h, presenting stable superoleophobicity. In addition to DCE, the membranes are also highly repulsive to other organic solvents and oils, including hexadecane, petroleum ether, gasoline and diesel. Figure 2c shows the underwater wetting behavior of various oils. The OCAs of all oil droplets are larger than 150° and the SAs are smaller than 5°. Therefore, the optimal as-prepared membranes were used in the following studies unless otherwise specified.
Separation of free oil/water mixtures
In view of the contrary wettability of water and oil on the mineral-coated membranes, it is reasonable to expect that these two liquids will have different permeabilities towards the membranes. As a proof of this, we first tested the separation of free oil/water mixture. As shown in Figure S4, a mixture of DCE and water was poured onto the membrane sample which was mounted in a dead-end cell. The sole driving force used for separation is the gravity of the mixture. It can be seen that the wetting phase (water) permeates through the sample with a flux of about 90 L m−2 h−1, while the non-wetting phase (DCE) is rejected above the membrane sample. The oil rejection ratio is higher than 99.9%. No visible oil was observed in the filtrate. Besides, the mineral-coated membranes are easily cleaned by water due to their low oil-adhesion property, exhibiting promising performances in oil/water separation.
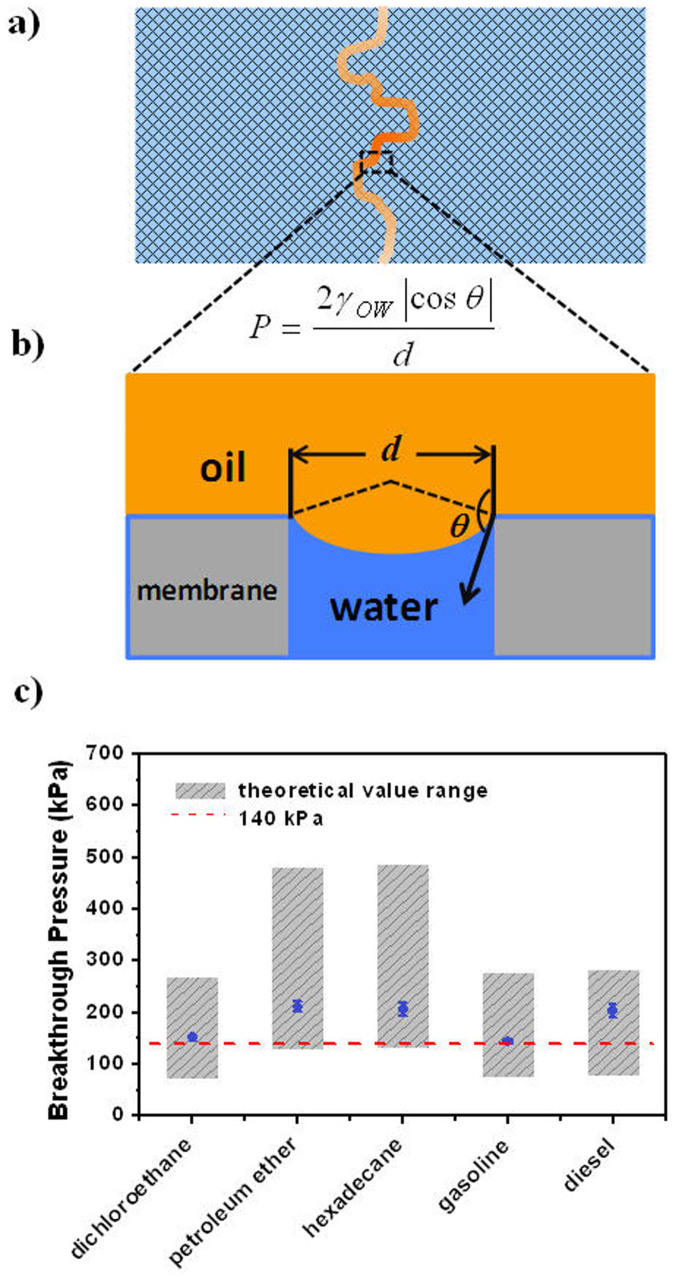
In practical processes, the membranes are usually applied with an external pressure (100 kPa or higher) to improve the water flux and enhance the process efficiency. However, the external trans-membrane pressure also has the risk of exceeding the oil breakthrough pressure. In this case the oil is forced to permeate through the membranes, decreasing the separation efficiency quickly. Therefore, the breakthrough pressure of non-wetting phase is a crucial parameter for oil/water separation membranes. We measured the pressure of various oils for the mineral-coated membranes. The results show that all the pressures are higher than 140 kPa (Figure 3c). To the best of our knowledge, these values are 2–3 orders of magnitude greater than those of reported separation materials with similar superoleophobicity. In order to validate the high Pbreakthrough, theoretical values of different oils were also estimated using equation (1) (Figure 3b, see SI for details)29,32:
here d is the distance between two adjacent peaks (corresponding to the pore size, Figure S5), γOW is the oil/water interfacial tension and θ is the underwater oil contact angle on the flat mineral coating. According to the equation, MPPM with relatively small pore size (200–750 nm) provides a perfect framework for oil/water separation, which enhances the capillary resistance for oil to penetrate. On the other hand, the stable hydrated mineral layer offers a robust oil-repellent interface (high OCA), further increasing the oil-flow resistance. Theoretical Pbreakthrough range was calculated based on this equation (Table S1). The results are consistent with the experimental values (Figure 3c).
Figure 3. Oil breakthrough pressures of the mineral-coated membranes.
(a) Schematic illustration of the oil channel in a membrane when the membrane is intruded by oil. (b) Illustration for the calculation of theoretical breakthrough pressure. (c) Measured and theoretical breakthrough pressures of different oils.
Separation of oil-in-water emulsions
Compared with free oil/water mixtures, it is more difficult to separate oil/water emulsions owing to those surfactants added for emulsion formation. Membrane-based technology is of particular interest among the existing methods for emulsion separation, since it integrates demulsification and separation into a single unit15,16,31,32. In order to improve oil rejection, ultrafiltration membranes are commonly preferred because their pore size is smaller than the emulsified oil droplets37,38,39. However, common ultrafiltration membranes have drawbacks such as low permeate flux and poor resistance to oil fouling, due to their small pore size, low porosity and oleophilic membrane surface. Given that the mineral-coated membranes are superoleophobic and have relatively larger pores (microfiltration membrane), we systematically studied their separation performances for oil-in-water emulsions.
Diesel was utilized as the target oil for preparing oil-in-water emulsions. As mentioned above, the mineral-coated membranes have high breakthrough pressure for diesel. Therefore, the separation experiments were carried out under a series of external pressures in a dead-end filtration mode. The dead-end mode provides a “worst case scenario” for oil accumulation and fouling study40. Figure S7 shows the dependence of permeation flux on trans-membrane pressure for emulsions with different oil contents. The water flux increases linearly with increasing the external pressure, but decreases dramatically with the oil content. This decrease is attributed to the increasing content of emulsified oil droplets and the high rejection of the membranes. Since oil can not penetrate through the membranes below their breakthrough pressure, the rejected emulsified oil reduces the contact area between the membrane surface and the water phase. As a result, the available pores for water permeation are decreased, leading to the decline of water flux.
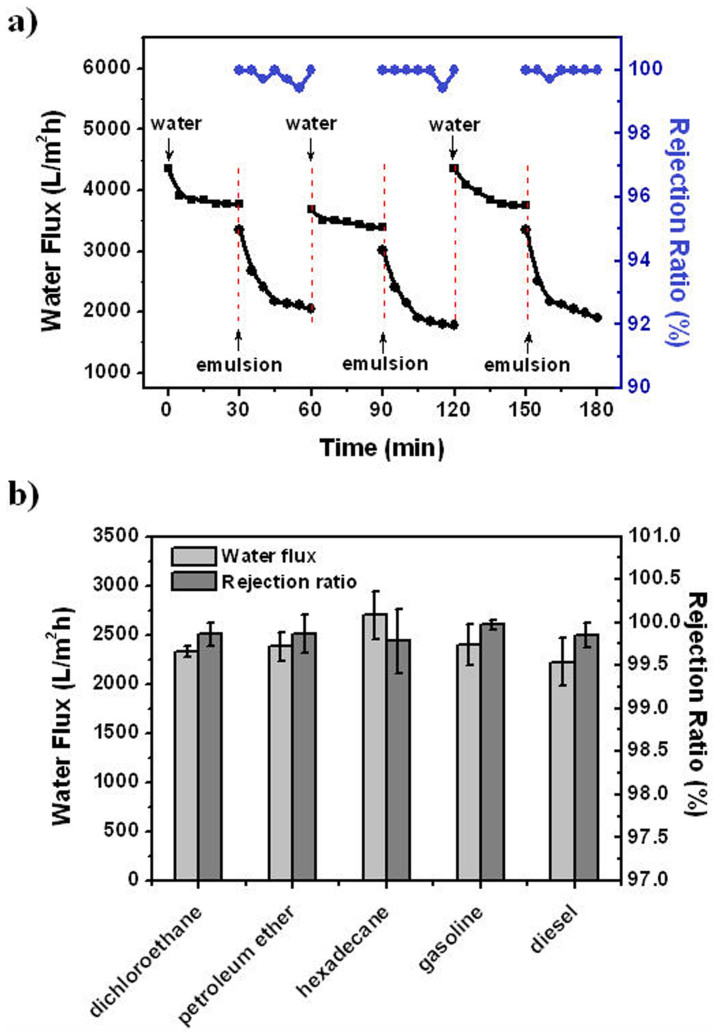
In addition, a repeating separation experiment was performed on the mineral-coated membranes to further study their separation efficiency and recyclability. As shown in Figure 4a, the filtration process includes three cycles. In each cycle, pure water and oil-in-water emulsion were sequentially filtered through the membranes. During the interval of different cycles, the membranes were simply rinsed with pure water. Experimental results show that the permeate flux can reach 2000 L m−2 h−1. This value is much higher than conventional ultrafiltration membranes (<300 L m−2 h−1), while the external pressure used (0.05 MPa) is lower than that of latter (>0.1 MPa). The gradual decline of flux during emulsion separation is ascribed to the oil droplet accumulation above the membrane surface. The oil rejection ratio is measured to be >99%, which is comparable to those reported in literatures15,16,31,32,37,38,39,40. Moreover, the membranes are highly resistant to oil fouling. In the second cycle, the flux of pure water filtration recovers to about 90% of the initial flux and the rejection ratio remains >99%. The slight decrease of flux is due to the mineral loss when a large amount of water flows through the membranes. Although CaCO3 is added to the water during filtration, the concentration of enriched Ca in water is still much lower than the value recommended by World Health Organization for soft water or safe drinking water34. After re-mineral coating, the flux recovery ratio is nearly 100% (Figure 4a, the third cycle). In addition to diesel, the coated membranes can effectively separate emulsions composed by a range of oils such as DCE, gasoline, etc (Figure 4b). The water flux of all experiments is larger than 2000 L m−2 h−1. Meanwhile, the rejection ratio is above 99%. As such, the mineral-coated membranes enable an efficient and energy-saving separation for various oil/water emulsions with both high flux and high rejection ratio.
Figure 4. Separation of oil-in-water emulsions with the mineral-coated membranes.
(a) Time-dependent flux and rejection ratio of the mineral-coated membranes during emulsion separation (diesel-in-water, 1:100 v:v). The filtration contains three cycles and every cycle includes two steps: water filtration and emulsion filtration. Before the third cycle, the membrane sample was re-mineral coated with 3 cycles. (b) Separation performances of the mineral-coated membranes for a range of oil-in-water emulsions (1:100 v:v). The trans-membrane pressure used is 0.05 MPa during emulsion separation.
Discussion
In this paper, we have prepared one novel kind of hybrid membranes for oil/water separation via the combination of microfiltration membrane and superhydrophilic CaCO3-based mineral coating. Under aqueous environment, the rigid mineral-coating traps abundant water in its rough structure and forms a robust hydrated layer on the membrane pore surface, which endows the membranes with underwater superoleophobicity and low oil-adhesion. Accordingly, the hybrid membranes can effectively separate various oil/water mixtures with high separation efficiency (>99%), high flux (>2000 L m−2 h−1), ultrahigh oil breakthrough pressure (>140 kPa) and low oil fouling. These oil/water mixtures include not only free mixtures but also oil-in-water emulsions because of the rational pore size and high oil-repellency of the membranes. Moreover, the hybrid membranes only involve common chemicals yet possess high separation performances. We anticipate that these membranes will have promising applications in many real situations, such as industrial emulsified wastewater treatment, oil spill cleanup and fuel purification.
Methods
Materials
Microporous polypropylene membrane (MPPM, porosity 75%) was commercially obtained from Membrana GmbH (Germany). Acrylic acid (AA, analytical grade) was purchased from Sinopharm Chemical (China) and distilled under reduced pressure before use. Calcium chloride (CaCl2, anhydrous, analytical grade), sodium carbonate (Na2CO3, anhydrous, analytical grade), and other chemicals were purchased from Sinopharm Chemical and used without further purification. Water used in all experiments was deionized and ultrafiltrated to 18.2 MΩ with an ELGA LabWater system (France).
Fabrication of the mineral-coated membranes
MPPM was firstly grafted with PAA using a photoinitiated graft polymerization method as described elsewhere34. Mineral coating was then formed on the membrane surfaces by an alternate soaking process (ASP). In a typical experiment, MPPM grafted with 30 wt.% PAA was pre-wetted with ethanol and washed with water to exchange the ethanol. Then the membranes were sequentially soaked in CaCl2 aqueous solution (200 mM) for 30 s, rinsed with water for 30 s, soaked in Na2CO3 aqueous solution (200 mM) for 30 s, and rinsed again with water for 30 s. The soaking process was repeated for certain cycles. After ASP, the membranes were rinsed with ethanol to remove any weakly bound CaCO3 and dried under reduced pressure. Unless otherwise specified, optimal membranes were prepared with 10 ASP cycles.
Preparation of oil-in-water emulsions
Oil-in-water emulsions were prepared by adding Tween80 as the emulsifier. Typically, 500 mg of Tween80 was added into 500 mL of water, then 5 mL of oil (hexadecane, dichloroethane, petroleum ether, gasoline or diesel) was added. The mixture was stirred for 1 h to obtain a stable and turbid emulsion. The size of oil droplets, which was measured by a Malvern Zetasizer Nano ZS instrument, was in the range of 0.14–5.56 μm (Figure S6).
Oil/water separation
The as-prepared membrane sample was pre-wetted with water and then mounted in a dead-end filtration cell (Millipore, USA), which connected to a solution reservoir and a nitrogen tank for adjusting the trans-membrane pressure. The separation process can be driven either by the gravity of oil/water mixture or by an external trans-membrane pressure. Flux was calculated based on the water quantity permeating through the membranes. Rejection ratios of oil were determined by measuring the oil content in feed and in corresponding filtrate with a UV-Vis spectrophotometer (Shimadzu, UV-1601PC, Japan, the oil was dyed with Sudan Blue II).
Instruments and charaterization
Field emission scanning electron microscope (FE-SEM, Hitachi S4800, Japan) was used to observe the membrane morphologies. Energy dispersive X-ray spectroscope (EDAX) was used to map the calcium distribution. Underwater oil contact angles (OCA) were recorded at room temperature with a DropMeter A-200 contact angle system (MAIST Vision Inspection & Measurement Co. Ltd., China). For the static OCA, 10 μL of oil droplets were carefully dropped onto the membrane surface and the OCAs were measured. In measuring dynamic OCA, the membrane surface was tilted slowly until the 10 μL oil droplet started to slide along the surface. Then the sliding angle was recorded. All OCA tests were repeated for five times per sample and the average values were adopted. Oil/water interfacial tensions were evaluated by pendant drop method using the DropMeter A-200 system.
Author Contributions
P.C.C. and Z.K.X. conceived the project, designed the experiments, analyzed the data and wrote the manuscript. P.C.C. performed the experiments.
Supplementary Material
Supporting Information
Acknowledgments
The authors are grateful to the financial support in Grant number 50933006 by the National Natural Science Foundation of China and in Grant number 2009CB623401 by the National Basic Research Program of China. We also thank Dr. Xiao-Jun Huang and Dr. Ling-Shu Wan for their helpful disscussion during the early stages of this work.
References
- Asatekin A. & Mayes A. M. Oil industry wastewater treatment with fouling resistant membranes containing amphiphilic comb copolymers. Environ. Sci. Technol. 43, 4487–4492 (2009). [DOI] [PubMed] [Google Scholar]
- McNutt M. K. et al. Review of flow rate estimates of the Deepwater Horizon oil spill. Proc. Natl. Acad. Sci. U.S.A 109, 20260–20267 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson C. H. et al. Long-term ecosystem response to the Exxon Valdez oil spill. Science 302, 2082–2086 (2003). [DOI] [PubMed] [Google Scholar]
- Cheryan M. & Rajagopalan N. Membrane processing of oily streams. Wastewater treatment and waste reduction. J. Membr. Sci. 151, 13–28 (1998). [Google Scholar]
- Gaaseidnes K. & Turbeville J. Separation of oil and water in oil spill recovery operations. Pure Appl. Chem. 71, 95–101 (1999). [Google Scholar]
- Liu M. J., Zheng Y. M., Zhai J. & Jiang L. Bioinspired super-antiwetting interfaces with special liquid-solid adhesion. Acc. Chem. Res. 43, 368–377 (2010). [DOI] [PubMed] [Google Scholar]
- Xue Z. X., Liu M. J. & Jiang L. Recent developments in polymeric superoleophobic surfaces. J. Polym. Sci. Part B: Polym. Phys. 50, 1209–1224 (2012). [Google Scholar]
- Feng L. et al. A super-hydrophobic and super-oleophilic coating mesh film for the separation of oil and water. Angew. Chem. Int. Ed. 43, 2012–2014 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang J. P. & Seeger S. Polyester materials with superwetting silicone nanofilaments for oil/water separation and selective oil absorption. Adv. Funct. Mater. 21, 4699–4704 (2011). [Google Scholar]
- Hayase G., Kanamori K., Fukuchi M., Kaji H. & Nakanishi K. Facile Synthesis of marshmallow-like macroporous gels usable under harsh conditions for the separation of oil and water. Angew. Chem. Int. Ed. 52, 1986–1989 (2013). [DOI] [PubMed] [Google Scholar]
- Deng D. et al. Hydrophobic meshes for oil spill recovery devices. ACS Appl. Mater. Interfaces 5, 774–781 (2013). [DOI] [PubMed] [Google Scholar]
- Lu P. et al. Macroporous silicon oxycarbide fibers with luffa-like superhydrophobic shells. J. Am. Chem. Soc. 131, 10346–10347 (2009). [DOI] [PubMed] [Google Scholar]
- Tian D. L., Zhang X. F., Wang X., Zhai J. & Jiang L. Micro/nanoscale hierarchical structured ZnO mesh film for separation of water and oil. Phys. Chem. Chem. Phys. 13, 14606–14610 (2011). [DOI] [PubMed] [Google Scholar]
- Yuan J. K. et al. Superwetting nanowire membranes for selective absorption. Nat. Nanotechnol. 3, 332–336 (2008). [DOI] [PubMed] [Google Scholar]
- Shi Z. et al. Ultrafast separation of emulsified oil/water mixtures by ultrathin free-standing single-walled carbon nanotube network films. Adv. Mater. 25, 2422–2427 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang W. B. et al. Superhydrophobic and superoleophilic PVDF membranes for effective separation of water-in-oil emulsions with high flux. Adv. Mater. 25, 2071–2076 (2013). [DOI] [PubMed] [Google Scholar]
- Liu M. J., Wang S. T., Wei Z. X., Song Y. L. & Jiang L. Bioinspired design of a superoleophobic and low adhesive water/solid interface. Adv. Mater. 21, 665–669 (2009). [Google Scholar]
- Cheng Q. F. et al. Janus interface materials: superhydrophobic air/solid interface and superoleophobic water/solid interface inspired by a lotus leaf. Soft Matter 7, 5948–5951 (2011). [Google Scholar]
- Hejazi V. & Nosonovsky M. Wetting transitions in two-, three-, and four-phase systems. Langmuir 28, 2173–2180 (2012). [DOI] [PubMed] [Google Scholar]
- Hejazi V., Nyong A. E., Rohatgi P. K. & Nosonovsky M. Wetting transitions in underwater oleophobic surface of brass. Adv. Mater. 24, 5963–5966 (2012). [DOI] [PubMed] [Google Scholar]
- Huang Y. et al. Controllable underwater oil-adhesion-interface films assembled from nonspherical particles. Adv. Funct. Mater. 21, 4436–4441 (2011). [Google Scholar]
- Jung Y. C. & Bhushan B. Wetting behavior of water and oil droplets in three-phase interfaces for hydrophobicity/philicity and oleophobicity/philicity. Langmuir 25, 14165–14173 (2009). [DOI] [PubMed] [Google Scholar]
- Kobayashi M. et al. Wettability and antifouling behavior on the surfaces of superhydrophilic polymer brushes. Langmuir 28, 7212–7222 (2012). [DOI] [PubMed] [Google Scholar]
- Lin L. et al. Bio-inspired hierarchical macromolecule-nanoclay hydrogels for robust underwater duperoleophobicity. Adv. Mater. 22, 4826–4830 (2010). [DOI] [PubMed] [Google Scholar]
- Liu X. L. et al. Clam's dhell inspired high-energy inorganic coatings with underwater low adhesive superoleophobicity. Adv. Mater. 24, 3401–3405 (2012). [DOI] [PubMed] [Google Scholar]
- Xu L. P. et al. An ion-induced low-oil-adhesion organic/inorganic hybrid film for stable superoleophobicity in seawater. Adv. Mater. 25, 606–611 (2013). [DOI] [PubMed] [Google Scholar]
- Xue B. L., Gao L. C., Hou Y. P., Liu Z. W. & Jiang L. Temperature controlled water/oil wettability of a surface fabricated by a block copolymer: application as a dual water/oil on-off switch. Adv. Mater. 25, 273–277 (2013). [DOI] [PubMed] [Google Scholar]
- Zhang L. B., Zhang Z. H. & Wang P. Smart surfaces with switchable superoleophilicity and superoleophobicity in aqueous media: toward controllable oil/water separation. NPG Asia Mater. 4, e8 (2012). [Google Scholar]
- Xue Z. X. et al. A novel superhydrophilic and underwater superoleophobic hydrogel-coated mesh for oil/water separation. Adv. Mater. 23, 4270–4273 (2011). [DOI] [PubMed] [Google Scholar]
- Wen Q., Di J. C., Jiang L., Yu J. H. & Xu R. R. Zeolite-coated mesh film for efficient oil-water separation. Chem. Sci. 4, 591–595 (2013). [Google Scholar]
- Kota A. K., Kwon G., Choi W., Mabry J. M. & Tuteja A. Hygro-responsive membranes for effective oil-water separation. Nat. Commun. 3, 1025 (2012). [DOI] [PubMed] [Google Scholar]
- Kwon G. et al. On-demand separation of oil-water mixtures. Adv. Mater. 24, 3666–3671 (2012). [DOI] [PubMed] [Google Scholar]
- Baker R. W. Membrane Technology and Applications (Wiley, Weinheim, 2004). [Google Scholar]
- Chen P. C., Wan L. S. & Xu Z. K. Bio-inspired CaCO3 coating for superhydrophilic hybrid membranes with high water permeability. J. Mater. Chem. 22, 22727–22733 (2012). [Google Scholar]
- Liu K. S., Yao X. & Jiang L. Recent developments in bio-inspired special wettability. Chem. Soc. Rev. 39, 3240–3255 (2010). [DOI] [PubMed] [Google Scholar]
- Sun T. L., Feng L., Gao X. F. & Jiang L. Bioinspired surfaces with special wettability. Acc. Chem. Res. 38, 644–652 (2005). [DOI] [PubMed] [Google Scholar]
- Chen W. J. et al. Separation of oil/water emulsion using Pluronic F127 modified polyethersulfone ultrafiltration membranes. Sep. Purif. Technol. 66, 591–597 (2009). [Google Scholar]
- Chen W. J., Su Y. L., Zheng L. L., Wang L. J. & Jiang Z. Y. The improved oil/water separation performance of cellulose acetate-graft-polyacrylonitrile membranes. J. Membr. Sci. 337, 98–105 (2009). [Google Scholar]
- Ju H. et al. Crosslinked poly(ethylene oxide) fouling resistant coating materials for oil/water separation. J. Membr. Sci. 307, 260–267 (2008). [Google Scholar]
- Chen W. J. et al. Engineering a robust, versatile amphiphilic membrane surface through forced surface segregation for ultralow flux-decline. Adv. Funct. Mater. 21, 191–198 (2011). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information