Abstract
Recurrence after urinary tract infection (rUTI) is common in adult women. The majority of recurrences are believed to be reinfection from extraurinary sources such as the rectum or vagina. However, uropathogenic Escherichia coli are now known to invade urothelial cells and form quiescent intracellular bacterial reservoirs. Management of women with frequent symptomatic rUTI can be particularly vexing for both patients and their treating physicians. This review addresses available and promising management strategies for rUTI in healthy adult women.
Key words: Recurrent urinary tract infection, Uropathogenic Escherichia coli, Prophylaxis
Recurrence after urinary tract infection (rUTI) is common in adult women. One study showed that, with healthy college age women who were followed for 6 months after an index UTI, 20.9% had at least one symptomatic recurrence.1 In another study of 179 Finnish women who were followed for 1 year after an index Escherichia coli UTI, 44% had a least one rUTI and 5% had more than three rUTIs.2 Natural history studies suggest that, after an index infection, rUTIs tend to cluster in the first 3 to 4 months. The most likely time for recurrence is 30 to 60 days, and the frequency of rUTI declines with increasing duration.3,4
The majority of rUTIs are believed to be reinfection from extraurinary sources such as the rectum or vagina. However, uropathogenic E coli (UPEC) are now known to invade urothelial cells and form quiescent intracellular bacterial reservoirs (QIRS). It is thought QIRS may provide a source for bacterial persistence and UTI recurrence.5–7
Management of women with frequent symptomatic rUTI can be particularly vexing for both patients and their treating physicians. For the patient, each UTI recurrence is associated with days of lower urinary tract symptoms, general malaise, and restrictions on everyday activities.8 For physicians, an etiology is often never elucidated, making patient counseling difficult. Additionally, current prophylactic measures are limited, often ineffective, and may be associated with untoward side effects.
Urologic Evaluation
The first step in the management of rUTI is to obtain a detailed history and perform a thorough physical examination. A proper history must include information pertaining to previously documented UTI episodes including number, frequency, and temporal associations. Other important historical elements include menopausal status, recent antibiotic use, and sexual history, including number of partners, new partners, spermicide use, and use of barrier contraceptives. A physical examination must include a complete pelvic examination in which the quality of the vaginal epithelium and presence or absence of pelvic organ prolapse is assessed. The urethra should be carefully palpated for any evidence of diverticulum or Skene gland cyst or infection.
Additional urologic investigations are generally unnecessary in patients with a history of uncomplicated lower rUTI. A cohort study of 100 young, healthy women with lower rUTI found that abdominal radiograph, cystoscopy, intravenous pyelogram, and abdominal ultrasound were all low yield.9 If history or physical examination are suggestive of complicating factors, then further evaluation with postvoid residual urine volume, urinary tract ultrasound, and cystoscopy may be justified.
Interventions
Behavioral Modifications
Case-control studies confirm that women with rUTI differ in a number of sexual activity variables. Any lifetime sexual activity, sexual activity in the past 12 months, and recent 1-month intercourse frequency are all strong independent risk factors for rUTI.4,10 Additionally, spermicide use, barrier contraceptives, new sex partners, multiple sexual partners, or history of sexually transmitted infections are established independent risk factors for rUTI.10 Sexually active women may consider changing their mode of contraception if using barrier contraceptives or spermicidal products. Pre- and postcoital voiding, frequency of urination, fluid intake, personal hygiene practices (including wiping back to front after a bowel movement), hot tub use, douching, or tampon use have not been shown to be important risk factors for rUTI.10,11 Patients may be reassured that the available evidence is insufficient to recommend increased fluid intake, changes in hygiene, or pericoital voiding for rUTI prevention.
A recent cross-sectional study utilized claims data to show an association between obesity and UTI. At all stratifications, obese women were more likely to be diagnosed with a UTI compared with nonobese women. However, this was only statistically significant for those with a body mass index of 30 to 34.9 (odds ratio [OR] 1.22; 95% confidence interval [CI], 1.15–1.28).12 Prospective weight loss studies will be necessary to further establish obesity as an important modifiable risk factor for rUTI.
Estrogen Replacement
A Cochrane systematic review was conducted to examine the efficacy and safety of estrogens in decreasing the rate of rUTI in postmenopausal women with signs of vaginal atrophy. This review found that the application of intravaginal estrogens was effective and safe whereas oral estrogens were not. Nine randomized, controlled trials (RCTs), including 3345 postmenopausal women, found that oral estrogens did not reduce the occurrence of rUTI and were associated with more adverse events such as vaginal bleeding and breast tenderness when compared with placebo.13 Two RCTs included in the review showed a reduction in the number of rUTIs when estrogen was administered locally within the vagina. At 8 months, Raz and Stamm14 found that topical vaginal estrogen cream significantly reduced the incidence of rUTI when compared with placebo (0.5 vs 5.9 episodes per patient year; P < .001). Postmenopausal women were randomized to either an estrogen-eluting silicone vaginal ring or placebo and, at 36 weeks, the risk ratio was 0.64 (95% CI, 0.47–0.86) in favor of the vaginal ring.15
Antibiotics
Self-start Therapy
Self-start therapy involves providing patients with instructions and materials that allow them to both diagnose and treat their UTI at the onset of symptoms. This is typically accomplished with urine dipstick or conventional culture and a refillable prescription for a short course of antimicrobial therapy. It has been shown that motivated and adherent rUTI patients are able to accurately self-diagnose (88%–92%) and effectively treat rUTI using this strategy.16–18 Self-start therapy is associated with a higher infection rate when compared with continuous prophylaxis (2.2 episodes per patient-year vs 0.2 episodes per patient year).16 However, patient satisfaction with self-start therapy is high, clinical and microbiologic resolution is prompt, and there are few adverse outcomes.16–18 Using this strategy it makes sense to treat each rUTI as an acute uncomplicated cystitis. Consideration should be given to patient allergy, availability, and local community resistance prevalence when choosing the appropriate antimicrobial. The Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases recommends nitrofurantoin monohydrate/macrocrystals, 100 mg, twice daily for 5 days; trimethoprim/sulfamethoxazole, 160/800 mg (one double-strength tablet), twice daily for 3 days; fosfomycin trometamol, 3 g, single-dose; or pivmecillinam, 400 mg, twice daily for 5 days. If the patient cannot take any of the aforementioned antimicrobials, then either a fluoroquinolone or β-lactam may be used.19
Postcoital Prophylaxis
In sexually active women, a single postcoital dose of antibiotic can be an effective and efficient way to prevent rUTI. A small RCT randomized healthy premenopausal women with a history of rUTI to receive a single postcoital dose of trimethoprim-sulfamethoxazole or placebo; 9 of 11 patients who took the placebo developed UTIs (3.6 UTI per patient-year), compared with 2 of 16 patients who received trimethoprim-sulfamethoxazole (0.3 UTI per patient-year). Of note, postcoital trimethoprim-sulfamethoxazole was effective regardless of intercourse frequency.20 Postcoital prophylaxis appears to have similar efficacy when compared with continuous prophylaxis; 152 women with a history of rUTI were randomized to either daily or postcoital ciprofloxacin (125 mg). The incidence of rUTI decreased in both groups (3.66 to 0.031 episodes per year for daily prophylaxis vs 3.62 to 0.043 episodes per year for postcoital prophylaxis), which was not statistically different (P = .7).21 See Table 1 for postcoital antimicrobial prophylaxis regimens.
Table 1.
Postcoital Antimicrobial Prophylaxis for Recurrent Urinary Tract Infection
| Antimicrobial | Dose (once daily) |
|---|---|
| Trimethoprim-sulfamethoxazole | 40 mg/200 mg |
| 80 mg/400 mg | |
| Nitrofurantoin | 50 mg or 100 mg |
| Cephalexin | 250 mg |
| Ciprofloxacin | 125 mg |
| Norfloxacin | 200 mg |
| Ofloxacin | 100 mg |
Continuous Prophylaxis
A Cochrane review of 19 RCTs, including 1120 healthy women, found that continuous antimicrobial prophylaxis, when compared with placebo, reduced the rate of UTI during prophylaxis (0 to 0.9 vs 0.8 to 3.6 for microbiologic recurrences per patient-year; P < .01 and 0 to .27 vs 1.12 to 3.6 for clinical recurrences per patient-year; P < .01). In order to prevent one symptomatic rUTI, the number needed to treat was 1.85 (95% CI, 1.60–2.2) by microbiological criteria and 2.2 (95% CI, 1.80–2.80) by clinical criteria. Notably, upon discontinuation of antimicrobial prophylaxis, rUTI incidence did not differ between groups (relative risk [RR] 0.82; 95% CI, 0.44–1.53). Side effects were more common in patients taking prophylactic antimicrobials (RR 1.58; 95% CI, 0.47–5.28) and the number needed to harm for any side effect was 13.5.22 Comparisons of efficacy and safety between different antimicrobials were not possible in this Cochrane review due to significant heterogeneity among studies.22
The ideal schedule for continuous prophylaxis is unclear. There are no studies comparing daily with every-other-day or weekly prophylaxis, but weekly prophylaxis does appear to be better than monthly prophylaxis. Guibert and colleagues23 compared weekly versus monthly prophylaxis with 400 mg of pefloxacin in women with rUTI; 17 of the 185 patients (9.1%) in the weekly group experienced at least one reinfection during the 48 weeks of prophylaxis compared with 52 of 176 patients (29.5%) in the monthly prophylaxis group (P < .0001).
Table 2 outlines the typical antimicrobial agents used for continuous prophylaxis. For those patients requiring > 6 months of nitrofurantoin prophylaxis, it is recommended that liver function tests (LFTs) and a chest radiograph be obtained. Our practice is to perform these tests every 12 months in patients taking daily low-dose nitrofurantoin. Others have suggested repeated LFTs and chest radiographs every 6 months to monitor for hepatotoxicity and pneumonitis.24
Table 2.
Continuous Antimicrobial Prophylaxis for Recurrent Urinary Tract Infection
| Antimicrobial | Dose | Frequency |
|---|---|---|
| Trimethoprim-sulfamethoxazole | 40 mg/200 mg | Daily or 3 × wk |
| Trimethoprim | 100 mg | Daily |
| Nitrofurantoin | 50–100 mg | Daily |
| Cephalexin | 125–250 mg | Daily |
| Ciprofloxacin | 125 mg | Daily |
| Norfloxacin | 200 mg | Daily |
| Ofloxacin | 100 mg | Daily |
| Fosfomycin | 3 g every 10 d | 3 g every 10 d |
Nonantibiotic Prophylaxis
Cranberry
In vitro and ex vivo research has confirmed that proanthocyanidin, a chemical found in high concentration in cranberry, has a dose-dependent effect on E coli adherence to and displacement from urothelial cells.25–27 However, the results of large well-designed clinical trials are conflicting.
Stothers28 randomized 150 healthy women (aged 21–72 y) with rUTI to placebo only, placebo juice, and cranberry tablets, or cranberry juice and placebo tablets. Both cranberry juice and cranberry tablets were found to significantly reduce the proportion of women experiencing at least one symptomatic UTI over 12 months when compared with placebo (20% cranberry juice, 18% cranberry extract vs 32% for placebo; P < .05).28
A recent double-blind RCT found that cranberry juice failed to prevent rUTI; 319 college-aged women were randomized to receive either 8 oz of 27% low-calorie cranberry juice twice daily or 8 oz of placebo juice. Patients were followed for 6 months or until the first documented recurrent UTI. Intent-to-treat analysis revealed that the distribution of recurrences was similar between groups (19.3% vs 14.6%; P = .21). However, the overall recurrence rate in the placebo group was lower than predicted, leading the authors of this study to question their choice of placebo.29
Cranberry may be useful for rUTI prevention but further standardized study is necessary. For those patients interested in taking cranberry prophylaxis, cranberry tablets have been shown to be twice as cost effective as cranberry juice.28
Ascorbic Acid
Ascorbic acid (vitamin C) is often recommended as a supplement that can prevent rUTI by acidification of the urine. Strong clinical evidence to support this claim in healthy adult women is lacking.
In young women, Foxman and Chi11 found a weak association between dietary vitamin C and decreased UTI risk (no prior UTI: OR 0.59; 95% CI, 0.35–0.98; one or more prior UTIs: OR 0.85; 95% CI, 0.58–1.25].11 A total of 110 pregnant women were randomized to receive ferrous sulfate, 200 mg/d, folic acid, 5 mg/d, and ascorbic acid, 100 mg/d, or daily ferrous sulfate and folic acid only. At 3 months, the presence of urinary infections in the ascorbic acid-treated group was significantly lower than in the ferrous sulfate and folic acid only group (OR 0.35; CI 95%, 0.13–0.91).30
In vitro data now suggest that vitamin C can have a bacteriostatic effect in the urine. This effect was shown to be mediated by the reduction of urinary nitrites to reactive nitrogen oxides rather then by lowering urinary pH.31,32
Methenamine Salts
Methenamine salts are hydrolyzed in the urine to form ammonia and formaldehyde. Formaldehyde is widely bacteriostatic and lacks bacterial resistance. These features, along with a limited side-effect profile, make methenamine salts attractive agents for rUTI prophylaxis. However, there is a paucity of good clinical evidence to support their use.
A number of small studies have compared methenamine hippurate with placebo in healthy pre- and postmenopausal women. The evidence is weak but does suggest that methenamine hippurate may be more effective at reducing rUTI at 12 months compared with placebo.33–35 Two small RCTs have compared continuous methenamine hippurate and continuous antimicrobial prophylaxis in healthy adult women. Brumfitt and colleagues36 compared nitrofurantoin, 50 mg, twice daily, to methenamine hippurate, 1 g/d, and found that nitrofurantoin more effectively reduced rUTI over 12 months. A second study by the same group compared trimethoprim, 100 mg/d, methenamine hippurate, 1 g twice daily, and twice daily perineum cleansing with povidone-iodine over 12 months. This study found no significant difference between trimethoprim and methenamine hippurate with regard to microbiologic rUTI rate (40% trimethoprim vs 40% methenamine hippurate; RR 1.00; 95% CI, 0.49–2.05).37 A Cochrane review concluded that methenamine hippurate may be safe and effective for short-term prevention of rUTI in patients without neurogenic bladder or urinary tract abnormalities.38
d-Mannose
Studies utilizing rodents and human urothelial cell lines have demonstrated that d-mannose-based inhibitors of FimH can block UPEC adhesion and invasion of uroepithelial cells.39,40 This basic science evidence has formed the basis for the promotion of d-mannose in human rUTI prevention. However, it is important to note that there are virtually no clinical studies in which d-mannose has been evaluated for rUTI prevention. In fact, there is in vitro evidence that mannose can inhibit macrophage activity,41 which could theoretically retard bacterial clearance from the urinary tract. Additionally, d- mannose may not be effective against certain strains of UPEC or other uropathogenic bacteria that do not express type 1 pili and FimH.42
Probiotics
The efficacy and safety of intravaginal Lactobacillus crispatus for rUTI prevention was recently examined in a phase 2 RCT. After successful treatment of a documented UTI, 100 premenopausal women (aged 18–40 years) with a history of at least one prior UTI within the past 12 months were randomized to receive either 108 CFU/mL L crispatus CTV-05 or placebo. Both were given intravaginally once daily for 5 days followed by once weekly administration for 10 weeks. At the end of 10 weeks, women in the L crispatus CTV-05 arm were found to have a significant reduction in the incidence of rUTI when compared with the placebo-treated group (RR 0.5; 95% CI, 0.2–1.2). Adverse effects were similar between groups (56% for L crispatus CTV-05 and 50% for placebo) and included vaginal discharge, vaginal itching, and abdominal discomfort.43 A prospective RCT is now enrolling postmenopausal women to examine the efficacy of intravaginal lactobacilli in combination with low-dose estriol for rUTI prevention.
Hyaluronic Acid and Chondroitin Sulphate
With the aim of restoring the integrity of the bladder glycosaminoglycan layer, Damiano and associates44 evaluated the efficacy and safety of intravesical hyaluronic acid and chondroitin sulfate (IALURIL); 57 women with a history of frequent rUTI were randomized to receive either a 50-mL solution containing 1.6% hyaluronic acid and 2.0% chondroitin sulfate or 50 mL of intravesical placebo. Serial bladder instillations with IALURIL, given over the course of 12 months, significantly reduced UTI rates (−86.6% ± 47.6 vs −9.6% ± 24.6%) and improved urinary symptoms and quality of life. The instillations were well tolerated and there were no severe side effects.44 This study demonstrates promise for this form of prophylaxis. Further study is needed to assess generalizability, long-term outcomes, and economic feasibility.
Vaccination
A number of different vaccine strategies have been developed in an attempt to prevent rUTI. To date, clinical success has been limited and therefore no licensed vaccine is available for rUTI prevention.
Naber and associates45 systematically reviewed the use of bacterial lysates as immunoactive prophylaxis for rUTI. Only two products were found to have published data, Uro-Vaxom® (OM Pharma, Meyrin, Switzerland) and Solco Urovac® (Solco Basel, Basel, Switzerland). Uro-Vaxom is a daily oral capsule containing 18 E coli strains. Meta-analysis of five studies (1000 adult patients) revealed that Uro-Vaxom significantly reduced rUTI over a 6- to 12-month period compared with placebo (41.7% vs 62.4%; OR 0.43; 95% CI, 0.34–0.55; P < .001). Four studies (220 adult patients) examined the effect of Solco Urovac, a vaginal suppository containing 10 uropathogenic bacterial strains, on UTI recurrence. The result of these studies together suggest moderate efficacy but only with a booster cycle.45 These products are widely used in parts of Europe but remain unavailable in North America at the present time.
A phase 2 trial randomized 54 women with rUTI (aged 18–74 years) to receive either placebo, primary immunization with a vaginal vaccine, or primary immunization plus booster immunizations at 1, 2, 6, 10, and 14 weeks. Vaginal suppositories were prepared using Urovac, a whole-cell vaccine containing heat-killed bacteria from 10 human uropathogenic strains including 6 E coli strains, Klebsiella pneumoniae, Proteus mirabilis, P morganii, and Enterococcus faecalis. Time to first recurrence, number of UTIs, and adverse reactions were documented over 6 months of follow-up. Time until first recurrence was significantly longer for women in the booster immunization group but not in the primary immunization or placebo group (P = .02). There were no reinfections during the 6-month trial period in 55.6% of the booster immunization group, 22.2% of the primary immunization group, and 22.2% of the placebo group (P = .06). The average number of infections per patient in the 6-month period was 1.1, 1.7, and 1.5 in the booster immunization, primary immunization, and placebo groups, respectively (P = .6). Adverse events were low and did not differ between vaccine or placebo groups.46 The promising results of this trial have yet to be followed-up with a large phase 3 trial.
In mice, a live-attenuated E coli vaccine was recently found to have a significant and long-lasting protective effect against a broad range of clinical UPEC isolates.47 This vaccine remains in the preclinical stages of investigation.
Recent technologic advances have allowed researchers to better characterize E coli gene expression in human UTI. It is hoped that this will allow the development of more specific and durable vaccines for rUTI prevention. Recent work has identified several attractive UPEC vaccine candidates, including the outer membrane proteins involved in bacterial iron acquisition.48 Continued research on vaccine candidates and larger phase 3 trials will be important in establishing a broader-based and widely reliable vaccine strategy.
Conclusions
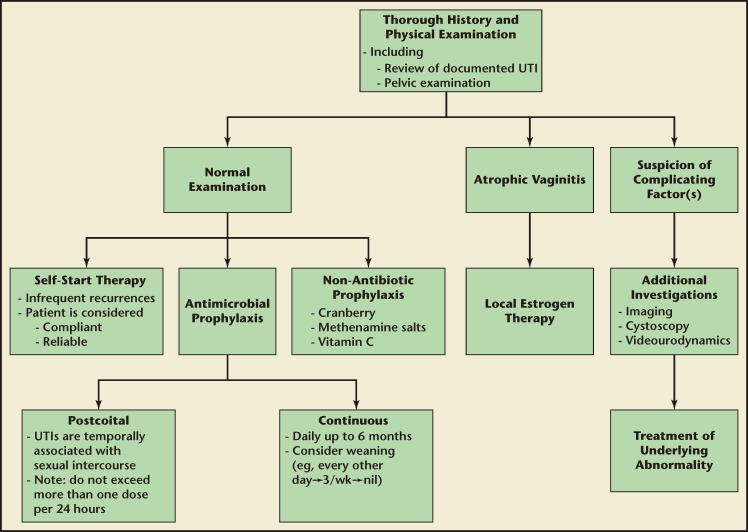
Recurrent UTIs are common in healthy adult women. It is important to evaluate all patients with a thorough history and physical examination as this will help to uncover underlying abnormalities or modifiable risk factors. Antimicrobials continue to be the most effective form of prophylaxis but are associated with untoward side effects. The type of antimicrobial agent and its schedule should be tailored to region and to the individual patient. The evidence backing other forms of rUTI prophylaxis is poor. However, for those who wish to try nonantimicrobial prophylaxis, cranberry, vitamin C, and methenamine salts are reasonable alternatives. See Figure 1 for a suggested rUTI treatment algorithm. There are a number of promising agents for rUTI prevention on the horizon. Further basic science research and large-scale RCTs are necessary before these agents can be added to the rUTI prevention armamentarium.
Figure 1.
Suggested treatment algorithm for recurrent urinary tract infection in adult women. UTI, urinary tract infection.
Main Points.
Recurrence after urinary tract infection (rUTI) is common in adult women. The majority of recurrences are believed to be reinfection from extraurinary sources such as the rectum or vagina. However, uropathogenic Escherichia coli are now known to invade urothelial cells and form quiescent intracellular bacterial reservoirs.
Management of women with frequent symptomatic rUTI can be particularly vexing for both patients and their treating physicians. However, several promising strategies exist for management of rUTI.
Spermicide use, barrier contraceptives, new sex partners, multiple sexual partners, and history of sexually transmitted infections are established independent risk factors for rUTI.
Both postcoital and continuous prophylaxis with antimicrobial agents has been proven to reduce the incidence of rUTI.
Sources of nonantibiotic prophylaxis include cranberry and ascorbic acid. There are number of promising agents for rUTI prevention on the horizon, but further study is warranted.
References
- 1.Foxman B, Gillespie B, Koopman J, et al. Risk factors for second urinary tract infection among college women. Am J Epidemiol. 2000;151:1194–1205. doi: 10.1093/oxfordjournals.aje.a010170. [DOI] [PubMed] [Google Scholar]
- 2.Ikähelmo R, Siitonen A, Heiskanen T, et al. Recurrence of urinary tract infection in a primary care setting: analysis of a 1-year follow-up of 179 women. Clin Infect Dis. 1996;22:91–99. doi: 10.1093/clinids/22.1.91. [DOI] [PubMed] [Google Scholar]
- 3.Kraft KJ, Stamey TA. The natural history of symptomatic recurrent bacteriuria in women. Medicine (Baltimore) 1977;56:55–60. [PubMed] [Google Scholar]
- 4.Stamm WE, McKevitt M, Roberts PL, White NJ. Natural history of recurrent urinary tract infections in women. Rev Infect Dis. 1991;13:77–84. doi: 10.1093/clinids/13.1.77. [DOI] [PubMed] [Google Scholar]
- 5.Mulvey MA, Schilling JD, Hultgren SJ. Establishment of a persistent Escherichia coli reservoir during the acute phase of a bladder infection. Infect Immun. 2001;69:4572–4579. doi: 10.1128/IAI.69.7.4572-4579.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosen DA, Hooton TM, Stamm WE, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berry RE, Klumpp DJ, Schaeffer AJ. Urothelial cultures support intracellular bacterial community formation by uropathogenic Escherichia coli. Infect Immun. 2009;77:2762–2772. doi: 10.1128/IAI.00323-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Little P, Merriman R, Turner S, et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ. 2010;340:b5633. doi: 10.1136/bmj.b5633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Haarst EP, van Andel G, Heldeweg EA, et al. Evaluation of the diagnostic workup in young women referred for recurrent lower urinary tract infections. Urology. 2001;57:1068–1072. doi: 10.1016/s0090-4295(01)00971-2. [DOI] [PubMed] [Google Scholar]
- 10.Scholes D, Hooton TM, Roberts PL, et al. Risk factors for recurrent urinary tract infection in young women. J Infect Dis. 2000;182:1177–1182. doi: 10.1086/315827. [DOI] [PubMed] [Google Scholar]
- 11.Foxman B, Chi JW. Health behavior and urinary tract infection in college-aged women. J Clin Epidemiol. 1990;43:329–337. doi: 10.1016/0895-4356(90)90119-a. [DOI] [PubMed] [Google Scholar]
- 12.Semins MJ, Shore AD, Makary MA, et al. The impact of obesity on urinary tract infection risk. Urology. 2012;79:266–269. doi: 10.1016/j.urology.2011.09.040. [DOI] [PubMed] [Google Scholar]
- 13.Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Obstet Gynecol. 2008;112:689–690. doi: 10.1097/AOG.0b013e318185f7a5. [DOI] [PubMed] [Google Scholar]
- 14.Raz R, Stamm WE. A controlled trial of intravaginal estriol in postmenopausal women with recurrent urinary tract infections. N Engl J Med. 1993;329:753–756. doi: 10.1056/NEJM199309093291102. [DOI] [PubMed] [Google Scholar]
- 15.Eriksen B. A randomized, open, parallel-group study on the preventive effect of an estradiol-releasing vaginal ring (Estring) on recurrent urinary tract infections in postmenopausal women. Am J Obstet Gynecol. 1999;180:1072–1079. doi: 10.1016/s0002-9378(99)70597-1. [DOI] [PubMed] [Google Scholar]
- 16.Wong ES, McKevitt M, Running K, et al. Management of recurrent urinary tract infections with patient-administered single-dose therapy. Ann Intern Med. 1985;102:302–307. doi: 10.7326/0003-4819-102-3-302. [DOI] [PubMed] [Google Scholar]
- 17.Schaeffer AJ, Stuppy BA. Efficacy and safety of self-start therapy in women with recurrent urinary tract infections. J Urol. 1999;161:207–211. [PubMed] [Google Scholar]
- 18.Gupta K, Hooton TM, Roberts PL, Stamm WE. Patient-initiated treatment of uncomplicated recurrent urinary tract infections in young women. Ann Intern Med. 2001;135:9–16. doi: 10.7326/0003-4819-135-1-200107030-00004. [DOI] [PubMed] [Google Scholar]
- 19.Gupta K, Hooton TM, Naber KG, et al. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis. 2011;52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 20.Stapleton A, Latham RH, Johnson C, Stamm WE. Postcoital antimicrobial prophylaxis for recurrent urinary tract infection. A randomized, double-blind, placebo-controlled trial. JAMA. 1990;264:703–706. [PubMed] [Google Scholar]
- 21.Melekos MD, Asbach HW, Gerharz E, et al. Post-intercourse versus daily ciprofloxacin prophylaxis for recurrent urinary tract infections in premenopausal women. J Urol. 1997;157:935–939. [PubMed] [Google Scholar]
- 22.Albert X, Huertas I, Pereiró II, et al. Antibiotics for preventing recurrent urinary tract infection in non-pregnant women. Cochrane Database Syst Rev. 2004;(3) doi: 10.1002/14651858.CD001209.pub2. CD001209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guibert J, Humbert G, Meyrier A, et al. Antibioprevention of recurrent cystitis. A randomized doubleblind comparative trial of 2 dosages of pefloxacin [in French] Presse Med. 1995;24:213–216. [PubMed] [Google Scholar]
- 24.Cetti RJ, Venn S, Woodhouse CR. The risks of long-term nitrofurantoin prophylaxis in patients with recurrent urinary tract infection: a recent medico-legal case. BJU Int. 2009;103:567–569. doi: 10.1111/j.1464-410X.2008.08008.x. [DOI] [PubMed] [Google Scholar]
- 25.Schmidt DR, Sobota AE. An examination of the anti-adherence activity of cranberry juice on urinary and nonurinary bacterial isolates. Microbios. 1988;55:173–181. [PubMed] [Google Scholar]
- 26.Lavigne JP, Bourg G, Combescure C, et al. In-vitro and in-vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect. 2008;14:350–355. doi: 10.1111/j.1469-0691.2007.01917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howell AB, Botto H, Combescure C, et al. Dosage effect on uropathogenic Escherichia coli anti-adhesion activity in urine following consumption of cranberry powder standardized for proanthocyanidin content: a multicentric randomized double blind study. BMC Infect Dis. 2010;10:94. doi: 10.1186/1471-2334-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stothers L. A randomized trial to evaluate effectiveness and cost effectiveness of naturopathic cranberry products as prophylaxis against urinary tract infection in women. Can J Urol. 2002;9:1558–1562. [PubMed] [Google Scholar]
- 29.Barbosa-Cesnik C, Brown MB, Buxton M, et al. Cranberry juice fails to prevent recurrent urinary tract infection: results from a randomized placebo-controlled trial. Clin Infect Dis. 2011;52:23–30. doi: 10.1093/cid/ciq073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ochoa-Brust GJ, Fernández AR, Villanueva-Ruiz GJ, et al. Daily intake of 100 mg ascorbic acid as urinary tract infection prophylactic agent during pregnancy. Acta Obstet Gynecol Scand. 2007;86:783–787. doi: 10.1080/00016340701273189. [DOI] [PubMed] [Google Scholar]
- 31.Carlsson S, Wiklund NP, Engstrand L, et al. Effects of pH, nitrite, and ascorbic acid on nonenzymatic nitric oxide generation and bacterial growth in urine. Nitric Oxide. 2001;5:580–586. doi: 10.1006/niox.2001.0371. [DOI] [PubMed] [Google Scholar]
- 32.Carlsson S, Govoni M, Wiklund NP, et al. In vitro evaluation of a new treatment for urinary tract infections caused by nitrate-reducing bacteria. Antimicrob Agents Chemother. 2003;47:3713–3718. doi: 10.1128/AAC.47.12.3713-3718.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cronberg S, Welin CO, Henriksson L, et al. Prevention of recurrent acute cystitis by methenamine hippurate: double blind controlled crossover long term study. Br Med J (Clin Res Ed) 1987;294:1507–1508. doi: 10.1136/bmj.294.6586.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gundersen R, Høivik HO, Osmundsen K. Frequent cystitis in elderly women. A double-blind comparison of Hiprex and placebo in general practice [in Norwegian] Tidsskr Nor Laegeforen. 1986;106:2048–2049. [PubMed] [Google Scholar]
- 35.Høivik HO, Gundersen R, Osmundsen K, et al. Prevention of recurrent cystitis in fertile women. A double-blind comparison of Hiprex and placebo in general practice [in Norwegian] Tidsskr Nor Laegeforen. 1984;104:1150–1152. [PubMed] [Google Scholar]
- 36.Brumfitt W, Cooper J, Hamilton-Miller JM. Prevention of recurrent urinary infections in women: a comparative trial between nitrofurantoin and methenamine hippurate. J Urol. 1981;126:71–74. doi: 10.1016/s0022-5347(17)54386-4. [DOI] [PubMed] [Google Scholar]
- 37.Brumfitt W, Hamilton-Miller JM, Gargan RA, et al. Long-term prophylaxis of urinary infections in women: comparative trial of trimethoprim, methenamine hippurate and topical povidone-iodine. J Urol. 1983;130:1110–1114. doi: 10.1016/s0022-5347(17)51709-7. [DOI] [PubMed] [Google Scholar]
- 38.Lee BB, Simpson JM, Craig JC, Bhuta T. Methenamine hippurate for preventing urinary tract infections. Cochrane Database Syst Rev. 2007;(4) doi: 10.1002/14651858.CD003265.pub2. CD003265. [DOI] [PubMed] [Google Scholar]
- 39.Michaels EK, Chmiel JS, Plotkin BJ, Schaeffer AJ. Effect of D-mannose and D-glucose on Escherichia coli bacteriuria in rats. Urol Res. 1983;11:97–102. doi: 10.1007/BF00256954. [DOI] [PubMed] [Google Scholar]
- 40.Wellens A, Garofalo C, Nguyen H, et al. Intervening with urinary tract infections using anti-adhesives based on the crystal structure of the FimH-oligomannose-3 complex. PLoS One. 2008;3:e2040. doi: 10.1371/journal.pone.0002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felipe I, Bochio EE, Martins NB, Pacheco C. Inhibition of macrophage phagocytosis of Escherichia coli by mannose and mannan. Braz J Med Biol Res. 1991;24:919–924. [PubMed] [Google Scholar]
- 42.van der Bosch JF, Verboom-Sohmer U, Postma P, et al. Mannose-sensitive and mannose-resistant adherence to human uroepithelial cells and urinary virulence of Escherichia coli. Infect Immun. 1980;29:226–233. doi: 10.1128/iai.29.1.226-233.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stapleton AE, Au-Yeung M, Hooton TM, et al. Randomized, placebo-controlled phase 2 trial of a Lactobacillus crispatus probiotic given intravaginally for prevention of recurrent urinary tract infection. Clin Infect Dis. 2011;52:1212–1217. doi: 10.1093/cid/cir183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Damiano R, Quarto G, Bava I, et al. Prevention of recurrent urinary tract infections by intravesical administration of hyaluronic acid and chondroitin sulphate: a placebo-controlled randomised trial. Eur Urol. 2011;59:645–651. doi: 10.1016/j.eururo.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 45.Naber KG, Cho YH, Matsumoto T, Schaeffer AJ. Immunoactive prophylaxis of recurrent urinary tract infections: a meta-analysis. Int J Antimicrob Agents. 2009;33:111–119. doi: 10.1016/j.ijantimicag.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 46.Uehling DT, Hopkins WJ, Elkahwaji JE, et al. Phase 2 clinical trial of a vaginal mucosal vaccine for urinary tract infections. J Urol. 2003;170:867–869. doi: 10.1097/01.ju.0000075094.54767.6e. [DOI] [PubMed] [Google Scholar]
- 47.Billips BK, Yaggie RE, Cashy JP, et al. A live-attenuated vaccine for the treatment of urinary tract infection by uropathogenic Escherichia coli. J Infect Dis. 2009;200:263–272. doi: 10.1086/599839. [DOI] [PubMed] [Google Scholar]
- 48.Hagan EC, Lloyd AL, Rasko DA, et al. Escherichia coli global gene expression in urine from women with urinary tract infection. PLoS Pathog. 2010;6:e1001187. doi: 10.1371/journal.ppat.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfau A, Sacks TG. Effective postcoital quinolone prophylaxis of recurrent urinary tract infections in women. J Urol. 1994;152:136–138. doi: 10.1016/s0022-5347(17)32837-9. [DOI] [PubMed] [Google Scholar]
- 50.Pfau A, Sacks T, Engelstein D. Recurrent urinary tract infections in premenopausal women: prophylaxis based on an understanding of the pathogenesis. J Urol. 1983;129:1153–1157. doi: 10.1016/s0022-5347(17)52617-8. [DOI] [PubMed] [Google Scholar]
- 51.Lichtenberger P, Hooton TM. Antimicrobial prophylaxis in women with recurrent urinary tract infections. Int J Antimicrob Agents. 2011;38(suppl):36–41. doi: 10.1016/j.ijantimicag.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 52.Rudenko N, Dorofeyev A. Prevention of recurrent lower urinary tract infections by long-term administration of fosfomycin trometamol. Double blind, randomized, parallel group, placebo controlled study. Arzneimittelforschung. 2005;55:420–427. doi: 10.1055/s-0031-1296881. [DOI] [PubMed] [Google Scholar]
- 53.Nicolle LE, Harding GK, Thompson M, et al. Prospective, randomized, placebo-controlled trial of norfloxacin for the prophylaxis of recurrent urinary tract infection in women. Antimicrob Agents Chemother. 1989;33:1032–1035. doi: 10.1128/aac.33.7.1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gower PE. The use of small doses of cephalexin (125 mg) in the management of recurrent urinary tract infection in women. J Antimicrob Chemother. 1975;1(3 suppl):93–98. doi: 10.1093/jac/1.suppl_3.93. [DOI] [PubMed] [Google Scholar]
- 55.Bailey RR, Roberts AP, Gower PE, De Wardener HE. Prevention of urinary-tract infection with low-dose nitrofurantoin. Lancet. 1971;2:1112–1114. doi: 10.1016/s0140-6736(71)91270-0. [DOI] [PubMed] [Google Scholar]
- 56.Stamm WE, Counts GW, Wagner KF, et al. Antimicrobial prophylaxis of recurrent urinary tract infections: a double-blind, placebo-controlled trial. Ann Intern Med. 1980;92:770–775. doi: 10.7326/0003-4819-92-6-770. [DOI] [PubMed] [Google Scholar]
- 57.Brumfitt W, Smith GW, Hamilton-Miller JM, Gargan RA. A clinical comparison between Macrodantin and trimethoprim for prophylaxis in women with recurrent urinary infections. J Antimicrob Chemother. 1985;16:111–120. doi: 10.1093/jac/16.1.111. [DOI] [PubMed] [Google Scholar]