Abstract
Background
Colorectal cancer (CRC) incidence and mortality rates are higher in African–Americans as compared with other racial/ethnic groups. The women’s health initiative (WHI) study sample was used to determine whether differences in CRC risk factors explain racial/ethnic differences in incidence and mortality.
Methods
The WHI is a longitudinal study of postmenopausal women recruited from 40 centers. Baseline questionnaires were used to collect sociodemographic and health status information. All CRC diagnoses were centrally adjudicated. Cox regression models were used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for invasive CRC by race/ethnicity.
Results
The study sample included 131,481 (83.7%) White, 14,323 (9.1%) African–American, 6,362 (4.1%) Hispanic, 694 (0.4%) Native American and 4,148 (2.6%) Asian/Pacific Islanders. After a mean follow-up of 10.8 years (SD 2.9), CRC incidence was the highest in African–Americans (annualized rate = 0.14%), followed by Whites and Native Americans (0.12% each), Asian/Pacific Islanders (0.10%), and Hispanics (0.08%). After adjustment for age and trial assignment, Hispanics had a lower risk compared with Whites, HR 0.73 (95% CI: 0.54–0.97) (P = 0.03), and African–Americans had a marginally greater risk, HR 1.16 (95% CI: 0.99–1.34), P = 0.06. Multivariable adjustment attenuated the difference in incidence between African–Americans and Whites (HR 0.99, 95% CI: 0.82–1.20), while strengthening the lower HR for Hispanics (HR 0.68, 95% CI: 0.48–0.97).
Conclusions
African–American/White differences in CRC risk are likely due to sociodemographic/cultural factors other than race.
Impact
A number of modifiable exposures could be a focus for reducing CRC risk in African–Americans.
Introduction
Colorectal cancer (CRC) is the third leading cause of new cancer and cancer death in the United States accounting for 70,480 incident cases and 24,790 deaths among women in 2010 despite overall trends toward decreasing rates in the United States (1). Several studies have reported differences in CRC incidence rates by race and ethnicity, and have consistently shown higher incidence among African–Americans (2–5) and lower incidence among Hispanics (2–4; 6;7). In fact, CRC incidence and mortality rates are the highest in African–American women compared with other racial and ethnic groups. Less information is available on CRC incidence among Asian/Pacific Islanders or Native Americans (5;7;8). In order to optimize cancer control efforts, it is important to develop a better understanding of the factors associated with racial and ethnic variation in CRC incidence and mortality.
In addition to race/ethnicity, patient related factors have been associated with an increased risk of CRC, among these are a history of inflammatory bowel disease (IBD) (9), type II diabetes (10), obesity (11;12), lack of physical activity (13), low fiber diet (14), cigarette smoking (15) and alcohol consumption (16). Use of nonsteroidal antiinflammatory drugs (17), oral contraceptives (18), estrogen, and progesterone therapy (19); calcium (20); vitamins B12, C, E, and selenium (21) have been associated with lower CRC risk. Further, health system-level factors such as regular screening by fecal occult blood testing (FOBT), sigmoidoscopy or colonoscopy also have a protective impact on CRC incidence and mortality (22), although significant racial and ethnic disparities exist in CRC screening (23–26). Data using the North Carolina Cancer Case Control Study database (NCCCS) have shown African–American:White differences in the distribution of known CRC risk factors (27–31); however to our knowledge, no study has evaluated the effect of differences in risk factor distribution on racial and ethnic differences in CRC incidence and mortality.
The hypothesis behind this analysis is that racial and ethnic differences in CRC incidence and mortality could potentially be attenuated by adjustment for differences in the distribution of both patient-level factors such as age, education, health insurance status, and health system-level factors such as medical care utilization and screening. The women’s health initiative (WHI) provides a robust database to explore racial and ethnic differences in cancer rates in a large multicenter population of postmenopausal women for which adjudicated cancer outcomes and robust risk data is available to evaluate these associations more comprehensively. The large sample size allows for more complete comparisons across racial and ethnic groups beyond most studies that limit the focus to a comparison of African Americans and Whites alone.
Materials and Methods
Study population
The WHI is a multi-center longitudinal study consisting of an observational study (OS) and randomized clinical trial (CT) components. The WHI design and recruitment methodologies have been previously described (32–35). The major goals of the WHI were to evaluate the health associated effects, including CRC incidence, of hormonal therapy (HT) [estrogen (E) plus progestin(P) or estrogen(E) alone], dietary modification (DM), and calcium plus vitamin D supplementation in postmenopausal women. Of note, none of these interventions were associated with a significant reduction in CRC risk (36–38). The WHI recruited women who were between the age 50—and 79 years at screening from 40 clinical centers across the United States and were eligible for participation if they were in general good health, with a life expectancy of greater than 3 years and provided written informed consent. Women were offered enrollment in the OS if they were not interested in being randomly assigned, if they were ineligible for a CT, or were directly recruited. The WHI was approved by the human subjects’ committee of each participating institution prior to consenting of participants. Women in the WHI were actively recruited to the trial from 1993 through 1998 and then after informed consent were followed for an additional 5 years in an extension study.
There were 161,808 participants enrolled in either the WHI OS (N = 93,676) or CT (N = 68,132) components of the WHI between October 1, 1993, and December 31, 1998. Of these, 2,262 (1.4%) had incomplete information on race or ethnicity. An additional 947 (0.6%) reported a past history of CRC and 1,591 (1.0%) had an unknown history of CRC leaving 157,008 women in the analytic cohort.
Baseline data collection
Women identified their race or ethnicity by selecting from among six categories listed on the U.S. Census at the time of the initiation of the study including: White, Black/African American, Hispanic, American Indian/Alaskan Native (Native American), Asian/Pacific Islander, or other. Baseline self-administered questionnaires were used to collect additional information on demographics and medical history including a personal history of colon polyps and polyp removal, family history of CRC in a 1st or 2nd degree relative, history of comorbid medical conditions (hypertension, stroke, and coronary heart disease), medical care utilization (current health care provider, last medical visit within one year), screening history (hemoccult stool tests, rectal exams, and sigmoidoscopy or colonoscopy), personal habits including smoking and alcohol use, and physical activity. Dietary intake was assessed by a validated, self-administered semiquantitative food frequency questionnaire (FFQ). The WHI FFQ resulted in estimates of nutrient intake similar to those obtained from short-term and more precise measurements including 24-hour dietary recall and four-day food records (39). Anthropometric measurements were used to assess body mass index (BMI) calculated as weight/ height (kg/m2), and waist circumference (cm). Information about use of postmenopausal hormone therapy (E plus P and E alone), oral contraceptives, medications, and dietary supplements was collected during in-person interviews. Cancer-screening information was updated annually.
Follow-up and colorectal cancer ascertainment
Cancer diagnoses were elicited annually in the OS and semiannually in the CT by mailed or telephone questionnaires. Participant self-reports or next-of-kin (proxy) reports of CRC cancer events were verified by centrally trained physician adjudicators at the WHI Clinical Centers after review of medical records and pathology reports using the Surveillance, Epidemiology, and End Results (SEER) coding system. The follow-up period for these analyses was through August 14, 2009 with an average follow-up time of 10.7 years (S.D. 2.9 years, range up to 15.6 years). Average follow-up time for whites was 10.9 (2.8), African–Americans 9.9 (3.2), Hispanics 9.4 (3.3), Native Americans 9.7 (3.3), and Asian/Pacific Islanders 9.8 (3.0) years. We excluded cancers with the following histologies: adenocarcinoma occurring in the setting of polyposis coli (1); malignant carcinoid tumor (15), neuroendocrine carcinoma (9); infiltrating ductal carcinoma, not otherwise specified (NOS) (2), medullary carcinoma, NOS (1) and malignant melanoma, NOS (2). Cancer site was classified as proximal (cecum, ascending colon, hepatic flexure, and transverse colon), distal (splenic flexure, descending colon, and sigmoid colon), and rectal (rectosigmoid junction and rectum).
Statistical analysis
Association of each variable with race/ethnicity was calculated using χ2 tests for categorical variables or two-sample t-tests for continuous variables. Cox proportional hazards regression was used to compute hazard ratios (HRs) and 95% confidence intervals (CIs) for the incidence of invasive CRC by race or ethnicity as well as the mortality from CRC by race. A number of models were developed for the main analyses. The first (model 1) was adjusted for age both as a categorical (50–59, 60–69, and 79–79) and as a continuous variable, and stratified on WHI trial randomization and extension study participation (yes vs. no). WHI trial randomization refers to whether the participant was randomized to E-alone active; E-alone placebo; E+P active; E+P placebo; DM control–no HT trial; DM intervention–no HT trial; or OS.
Subsequent models were adjusted for patient level and health system-level factors that affect CRC risk including model 1 plus the following groupings of variables: education [less than HS diploma/general education degree (GED); HS diploma/GED]; diabetes (yes; no); lifestyle factors [BMI in kg/m2 (< 25; 25- < 30; ≥ 30), physical activity in metabolic equivalents (METs)/week (0 to < 3.0; 3.0–11.75; > 11.75), smoking (never, past, and current) as well as pack years of smoking (never; < 5; 5 to < 20; ≥ 20) and current alcohol use (none; < 1 drink/week; 1–7 drinks/wk; ≥ 7 drinks/week)]; nonsteroidal anti-inflammatory drug (NSAID) use and duration (none; < 2 years; ≥ 2 years); dietary factors [total dietary energy (kcal), fiber (grams), red meat and fruits and vegetables (median number of servings/day); total calcium intake mg/day (< 400; 400 to < 800; 800 to < 1200; ≥1200)]; CRC risk factors and screening [history of colon polyp removal (yes; no), family history of CRC (yes; no), the occurrence of colonoscopy, sigmoidoscopy, or flexible sigmoidoscopy ever (yes; no); and duration of prior menopausal E alone or E + P use in years (none; < 5; 5 to < 10; ≥10). The effect of race/ethnicity was then assessed with Wald χ2 statistics after incorporation of each of these groupings. Age-adjusted and fully-adjusted time-dependent Cox models were used to examine the effect of colon screening during the study, where any report of colon screening (rectal exam, hemoccult guaiac, colonoscopy, sigmoidoscopy, flexible sigmoidoscopy, or barium enema x-ray), was incorporated on a yearly basis.
Due to small numbers, similar analyses looking at proximal, distal and rectal cancer, death from CRC and death from any cause after invasive CRC diagnosis were restricted to Whites and African–Americans. Hazard ratios for death from any cause after invasive CRC were further adjusted for invasive CRC tumor characteristics at the time of diagnosis. Cox analyses assessing evidence for interaction of race or ethnicity with age at diagnosis, type of insurance, and CRC screening included all adjustments from the fully-adjusted model, as well as the main effects of these variables and their interaction term with race/ethnicity. Wald χ2 tests were used to assess the statistical significance of these terms.
Invasive CRC tumor characteristics were examined among all race and ethnic groups. Due to small numbers, differences by race and ethnicity were compared only for White and African–American women with χ2 or Fisher Exact tests. Associations of each characteristic with race or ethnicity were tested, as well as whether information for each characteristic was missing. All analyses were carried out using Statistical Analysis Systems (SAS) for Windows, version 9.2. A significance level of 0.05 was used to determine the significance of all P-values.
Results
The analytic cohort was comprised of 131,481 (83.7%) Whites, 14,323 (9.1%) African-Americans, 6,362 (4.1%) Hispanics, 694 (0.4%) Native Americans, and 4,148 (2.6%) Asian/Pacific Islanders. Table 1 shows demographic, health, and lifestyle characteristics of women participating in the WHI CT or OS by race and ethnic group. Due to the large sample size, all tests of association between risk factors and race or ethnicity were statistically significant with a P-value < 0.001. Compared with Whites and Asian/Pacific Islanders, African–American, Hispanic, and Native American women were younger, had less education, less private insurance, higher prevalence of diabetes, and were more likely to be obese (Table 1). Whites and Asian/Pacific Islanders were more likely to have higher levels of physical activity and whites had higher consumption of alcohol than any other group. Asian/Pacific Islander and Whites were more likely to have used E alone or E plus P hormone therapies and for longer durations of time. Whites and Asian/Pacific Islanders were also most likely to have undergone colon-screening studies with about 1/3 having colonoscopy or sigmoidoscopy and over ½ having FOBT within the past 5 years. In contrast, Hispanics were least likely to have been screened with almost 2/3 never having a colonoscopy or sigmoidoscopy, and nearly 44% never having FOBT despite a mean age of 60 years at the time of study enrollment.
Table 1.
Demographic, health, and lifestyle characteristics of women participating in the WHI CT or OS by racial and ethnic groups
| Characteristics | White (N = 131,481) |
African–American (N = 14,323) |
Hispanic (N = 6,362) |
Native American (N = 694) |
Asian/Pacific Islander (N = 4,148) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) or % |
N | Mean (SD) or % |
N | Mean (SD) or % |
N | Mean (SD) or % |
N | Mean (SD) or % |
|
| WHI study population | ||||||||||
| Observational study | 76612 | 58.3 | 7445 | 52.0 | 3513 | 55.2 | 406 | 58.5 | 2647 | 63.8 |
| Clinical trial | 54869 | 41.7 | 6878 | 48.0 | 2849 | 44.8 | 288 | 41.5 | 1501 | 36.2 |
| Age at screening, y, mean (SD) |
131481 | 63.5 (7.2) | 14323 | 61.6 (7.1) | 6362 | 60.2 (6.8) | 694 | 61.6 (7.4) | 4148 | 63.0 (7.5) |
| 50–59 | 41325 | 31.4 | 5967 | 41.7 | 3218 | 50.6 | 296 | 42.7 | 1468 | 35.4 |
| 60–69 | 59889 | 45.5 | 6133 | 42.8 | 2479 | 39.0 | 280 | 40.3 | 1738 | 41.9 |
| 70–79 | 30267 | 23.0 | 2223 | 15.5 | 665 | 10.5 | 118 | 17.0 | 942 | 22.7 |
| Education | ||||||||||
| 0–8 years | 843 | 0.6 | 430 | 3.0 | 1137 | 18.2 | 51 | 7.4 | 78 | 1.9 |
| Some high school | 3668 | 2.8 | 1271 | 9.0 | 585 | 9.4 | 66 | 9.6 | 136 | 3.3 |
| High school diploma/GED | 23021 | 17.6 | 1969 | 13.9 | 1024 | 16.4 | 112 | 16.3 | 657 | 16.0 |
| School after high school | 49586 | 38.0 | 5506 | 38.9 | 2193 | 35.1 | 308 | 44.9 | 1427 | 34.7 |
| College degree or higher | 53529 | 41.0 | 4970 | 35.1 | 1311 | 21.0 | 149 | 21.7 | 1820 | 44.2 |
| Type of insurance | ||||||||||
| Private and/or military/VA | 117826 | 90.2 | 11287 | 80.7 | 4266 | 69.5 | 547 | 80.8 | 3808 | 92.6 |
| Medicaid and/or medicare | 9170 | 7.0 | 1715 | 12.3 | 687 | 11.2 | 74 | 10.9 | 222 | 5.4 |
| None | 3588 | 2.7 | 979 | 7.0 | 1185 | 19.3 | 56 | 8.3 | 83 | 2.0 |
| Diabetes ever | 6240 | 4.7 | 1989 | 13.9 | 594 | 9.3 | 113 | 16.4 | 340 | 8.2 |
| BMI, kg/m2, mean (SD) | 130336 | 27.7 (5.8) | 14193 | 31.2 (6.7) | 6295 | 29.1 (5.8) | 680 | 30.0 (6.4) | 4126 | 24.8 (4.6) |
| <25 | 48323 | 37.1 | 2283 | 16.1 | 1566 | 24.9 | 166 | 24.4 | 2415 | 58.5 |
| 25 to <30 | 45506 | 34.9 | 4623 | 32.6 | 2396 | 38.1 | 204 | 30.0 | 1261 | 30.6 |
| ≥30 | 36507 | 28.0 | 7287 | 51.3 | 2333 | 37.1 | 310 | 45.6 | 450 | 10.9 |
| Physical activity, METs/wk | ||||||||||
| 0 to <3 | 32786 | 26.1 | 5397 | 39.0 | 2243 | 37.3 | 233 | 34.5 | 1104 | 26.9 |
| 3 to <11.75 | 40548 | 32.3 | 4515 | 32.6 | 1917 | 31.9 | 221 | 32.7 | 1278 | 31.2 |
| ≥11.75 | 52141 | 41.6 | 3936 | 28.4 | 1855 | 30.8 | 221 | 32.7 | 1718 | 41.9 |
| Current alcohol use | ||||||||||
| None | 33545 | 25.7 | 7190 | 50.9 | 2727 | 43.6 | 301 | 43.8 | 2407 | 58.3 |
| <1 drink/wk | 43434 | 33.2 | 4408 | 31.2 | 2128 | 34.0 | 204 | 29.7 | 1216 | 29.4 |
| 1–6drinks/wk | 36558 | 28.0 | 1935 | 13.7 | 1112 | 17.8 | 132 | 19.2 | 385 | 9.3 |
| ≥7 drinks/wk | 17213 | 13.2 | 604 | 4.3 | 291 | 4.7 | 51 | 7.4 | 123 | 3.0 |
| Smoking status | ||||||||||
| Never | 64894 | 49.9 | 6958 | 49.5 | 3944 | 63.1 | 335 | 49.4 | 2973 | 72.1 |
| Past | 56623 | 43.5 | 5474 | 39.0 | 1858 | 29.7 | 273 | 40.3 | 987 | 23.9 |
| Current | 8512 | 6.5 | 1618 | 11.5 | 453 | 7.2 | 70 | 10.3 | 165 | 4.0 |
| NSAID use | 20936 | 15.9 | 1996 | 13.9 | 903 | 14.2 | 102 | 14.7 | 207 | 5.0 |
| Ibuprofen use | 14749 | 11.2 | 1123 | 7.8 | 620 | 9.7 | 67 | 9.7 | 144 | 3.5 |
| Prescription NSAID use | 6800 | 5.2 | 943 | 6.6 | 319 | 5.0 | 35 | 5.0 | 69 | 1.7 |
| Daily dietary intake | ||||||||||
| Energy, kcal | 128597 | 1649.9 | 13253 | 1616.3 | 5884 | 1656.7 | 647 | 1638.6 | 3946 | 1493.4 |
| (619.3) | (769.9) | (789.6) | (724.7) | (642.9) | ||||||
| Dietary fiber, g | 128597 | 16.4 (6.7) | 13253 | 14.2 (7.1) | 5884 | 15.4 (7.8) | 647 | 15.2 (7.0) | 3946 | 14.8 (6.6) |
| Red meat, med serv/day | 128597 | 0.7 (0.5) | 13253 | 0.8 (0.7) | 5884 | 0.8 (0.7) | 647 | 0.8 (0.6) | 3946 | 0.6 (0.6) |
| Fruits and vegetables, med serv/day |
128592 | 4.2 (2.1) | 13253 | 3.7 (2.2) | 5884 | 3.3 (2.1) | 647 | 3.5 (2.0) | 3946 | 3.9 (2.2) |
| Total calcium (diet+supp +med), mg/d |
||||||||||
| <400 | 7034 | 5.5 | 3115 | 23.5 | 727 | 12.4 | 88 | 13.6 | 600 | 15.2 |
| 400 to <800 | 31270 | 24.3 | 5041 | 38.0 | 1850 | 31.4 | 188 | 29.1 | 1103 | 28.0 |
| 800 to <200 | 32583 | 25.3 | 2662 | 20.1 | 1435 | 24.4 | 163 | 25.2 | 939 | 23.8 |
| ≥1200 | 57709 | 44.9 | 2434 | 18.4 | 1872 | 31.8 | 208 | 32.1 | 1304 | 33.0 |
| Prior HTa use | ||||||||||
| Never | 54869 | 41.8 | 8556 | 59.8 | 3287 | 51.7 | 342 | 49.3 | 1592 | 38.4 |
| Past | 21334 | 16.2 | 2162 | 15.1 | 879 | 13.8 | 117 | 16.9 | 602 | 14.5 |
| Current | 55181 | 42.0 | 3582 | 25.0 | 2186 | 34.4 | 235 | 33.9 | 1952 | 47.1 |
| Duration of prior HT use, y | ||||||||||
| None | 54869 | 41.7 | 8556 | 59.7 | 3287 | 51.7 | 342 | 49.3 | 1592 | 38.4 |
| <5 | 28572 | 21.7 | 2905 | 20.3 | 1470 | 23.1 | 127 | 18.3 | 988 | 23.8 |
| 5 to <10 | 17411 | 13.2 | 1145 | 8.0 | 624 | 9.8 | 79 | 11.4 | 644 | 15.5 |
| ≥10 | 30625 | 23.3 | 1715 | 12.0 | 981 | 15.4 | 146 | 21.0 | 924 | 22.3 |
| First degree relatives with colorectal cancer |
||||||||||
| None | 100868 | 84.4 | 10223 | 85.0 | 4989 | 89.1 | 498 | 85.1 | 3192 | 83.9 |
| 1 | 16662 | 13.9 | 1545 | 12.9 | 545 | 9.7 | 78 | 13.3 | 513 | 13.5 |
| 2 or more | 1961 | 1.6 | 253 | 2.1 | 64 | 1.1 | 9 | 1.5 | 98 | 2.6 |
| Colonoscopy, sigmoidoscopy, or flexible sigmoidoscopy |
66251 | 52.9 | 6355 | 46.1 | 2189 | 36.6 | 288 | 42.7 | 2050 | 50.0 |
| Never | 58983 | 47.2 | 7430 | 54.1 | 3787 | 63.6 | 386 | 57.5 | 2047 | 50.0 |
| Less than 5 years ago | 41609 | 33.3 | 3829 | 27.9 | 1305 | 21.9 | 174 | 25.9 | 1366 | 33.4 |
| 5 or more years ago | 24436 | 19.5 | 2483 | 18.1 | 866 | 14.5 | 111 | 16.5 | 679 | 16.6 |
| FOBT | 95523 | 76.3 | 9908 | 71.9 | 3407 | 57.0 | 457 | 68.2 | 3052 | 74.5 |
| Never | 29592 | 23.7 | 3879 | 28.3 | 2572 | 43.2 | 213 | 31.8 | 1042 | 25.5 |
| Less than 5 years ago | 72450 | 58.0 | 6351 | 46.4 | 2230 | 37.4 | 316 | 47.2 | 2326 | 56.9 |
| 5 or more years ago | 22823 | 18.3 | 3470 | 25.3 | 1155 | 19.4 | 140 | 20.9 | 717 | 17.6 |
| History of colon polyp removal | 11288 | 9.2 | 1067 | 7.9 | 354 | 6.0 | 57 | 8.6 | 402 | 9.9 |
NOTE: All P-values <0.001 for tests of association between descriptive characteristics and race/ethnicity.
Estrogen alone or estrogen plus progesterone.
Abbreviations: VA, Veterans Administration.
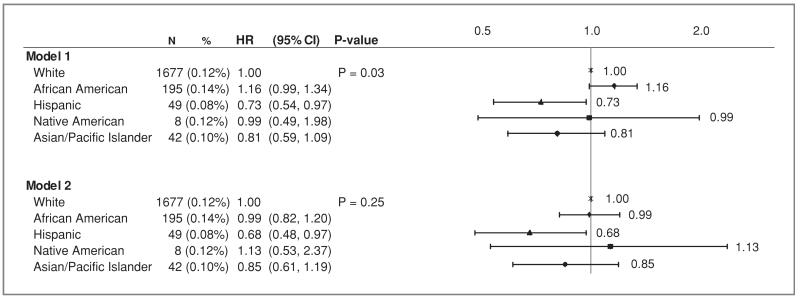
During a mean follow-up through August 14, 2009, of 10.8 years (SD 2.9), there were 1,971 new cases of invasive CRC. The racial/ethnic distribution of CRC cases consisted of: 85% White, 9.9% African–American, 2.5% Hispanic, 0.4% Native American, and 2.1% Asian/Pacific Islander (Fig. 1). CRC incidence rates were highest among African–Americans (annualized percent 0.14%), followed by Whites and Native Americans (both at 0.12%), Asian/Pacific Islanders (0.10%), and Hispanics (0.08%). After adjusting for age and cohort membership, invasive CRC hazard ratios varied by race and ethnicity, P = 0.03 (model 1). Hispanics had a significantly lower risk of CRC than Whites, (HR = 0.73, 95% CI: 0.54–0.97, P = 0.03), and African–Americans had a marginally increased risk, (HR, 1.16, 95% CI: 0.99–1.34, P = 0.06) (Fig. 1). After multivariable adjustment that included known patient-level and health system-level factors, (model 2), no overall difference in invasive CRC incidence between racial/ethnic groups was identified, P = 0.25. The HR for African Americans was nearly the same as that for Whites (HR = 0.99 95% CI: 0.82–1.20), and the difference in incidence between Hispanics and Whites increased, HR = 0.68 (95% CI: 0.48–0.97), P = 0.03. There was no significant difference in CRC risk for Asian Pacific Islanders or Native Americans versus Whites. Model 2 has a lower Akiaike Information Criteria (AIC) (28,994.379) than the AIC for model 1 (37,860.387) which indicate a better fit with model 2.
Figure 1.
Hazard ratios for invasive colorectal cancer (annualized%) by race and ethnicity (Model 1 is adjusted for age and stratified on WHI trial and extension study participation. Model 2 is adjusted for age, education, diabetes, body mass index, physical activity, smoking, alcohol use, NSAID use, total dietary energy, dietary fiber, red meat, fruits and vegetables, total calcium intake, prior colon screening, history of colon polyp removal, family history of colorectal cancer, and prior menopausal hormone use and stratified on WHI trial and extension study participation; Model 2 has a lower Akiaike Information Criteria (AIC) (28994.379) than the AIC for model 1 (37860.387) which indicate a better fit with model 2.)
Table 2 shows details from models showing the addition of groupings of potential confounding variables. In these models, lifestyle factors (BMI, physical activity, smoking and alcohol consumption) seem to have the largest impact on racial/ethnic differences in CRC risk with the greatest reduction in risk for African–Americans. Other variables that had a significant impact on racial/ethnic differences in CRC risk included dietary factors, total calcium intake, and use of hormone therapy.
Table 2.
Invasive colorectal cancer outcome: addition of groupings of potential confounding variables
| White | African–American HR (95% CI) |
Hispanic HR (95% CI) |
Native American HR (95% CI) |
Asian/Pacific Islander HR (95% CI) |
Wald χ | P-valuea | |
|---|---|---|---|---|---|---|---|
| Model 1b | 1.0 (ref) | 1.16 (0.99, 1.34) | 0.73 (0.54, 0.97) | 0.99 (0.49, 1.98) | 0.81 (0.59, 1.09) | 11.07 | 0.03 |
| Model 1 + education (≥HS vs. <HS) | 1.0 (ref) | 1.17 (1.01, 1.36) | 0.76 (0.57, 1.02) | 1.01 (0.50, 2.03) | 0.79 (0.58, 1.08) | 10.64 | 0.03 |
| Model 1 + history of diabetes | 1.0 (ref) | 1.12 (0.96, 1.30) | 0.71 (0.54, 0.95) | 0.95 (0.47, 1.90) | 0.80 (0.59, 1.08) | 10.13 | 0.04 |
| Model 1 + lifestyle factors (BMI, physical activity, smoking, alcohol) |
1.0 (ref) | 1.09 (0.92, 1.28) | 0.75 (0.55, 1.01) | 0.93 (0.44, 1.95) | 0.92 (0.67, 1.25) | 5.26 | 0.26 |
| Model 1 + NSAID use/duration | 1.0 (ref) | 1.15 (0.99, 1.34) | 0.72 (0.54, 0.96) | 0.99 (0.49, 1.99) | 0.84 (0.61, 1.15) | 11.14 | 0.03 |
| Model 1 + dietary factors (total energy, fiber, red meat, fruits and vegetables) |
1.0 (ref) | 1.11 (0.94, 1.29) | 0.73 (0.54, 0.98) | 1.03 (0.51, 2.06) | 0.79 (0.57, 1.09) | 8.49 | 0.08 |
| Model 1 + total calcium intake | 1.0 (ref) | 1.08 (0.92, 1.26) | 0.71 (0.53, 0.96) | 1.02 (0.51, 2.05) | 0.77 (0.56, 1.06) | 8.93 | 0.06 |
| Model 1 + screening, polyp removal and family history | 1.0 (ref) | 1.19 (1.01, 1.40) | 0.70 (0.51, 0.96) | 1.05 (0.50, 2.20) | 0.83 (0.61, 1.14) | 11.32 | 0.02 |
| Model 1 + hormone therapy use | 1.0 (ref) | 1.09 (0.93, 1.26) | 0.71 (0.53, 0.94) | 0.98 (0.49, 1.96) | 0.81 (0.60, 1.11) | 8.97 | 0.06 |
From a Wald χ2 4 degrees of freedom test of the main effect of race/ethnicity
Adjusted for age, and stratified on WHI trial and extension study participation.
Because of the small sample size among the ethnic groups, table 3 lists the distribution of CRC incidence rates by tumor location as well as the distribution of death due to CRC for White and African–American race only. Proximal CRC was slightly more common among African–Americans compared with Whites. After adjustment for age and cohort membership, the HR for proximal tumors was statistically significantly greater than 1.00 for African Americans, HR = 1.25 (95% CI: 1.02–1.54), P = 0.03 (Model 1), although after multivariable adjustment (Model 2), whereas the risk of CRC for African–Americans remained elevated, it was no longer statistically significant, P = 0.21. The overall rates of distal and rectal cancer were lower than for proximal cancer with no significant racial differences. There were also no significant racial differences in death due to CRC. We examined the possibility of an interaction between CRC risk by race with insurance status (private vs. Medicaid, Medicare, and no insurance), screening history (colonoscopy or sigmoidoscopy in the past 5 years versus none) and age group (50–59, 60–69, and 70–79) for Whites and African–Americans and no significant differences were detected. An additional time-dependent analysis incorporating additional colon-screening data evaluated on a yearly basis, over the course of the study provided a consistent overall reduced risk in Hispanics and elevated nonsignificant risk in African Americans (data not shown).
Table 3.
Invasive colorectal cancer outcomes (annualized%) for White and African American women
| Model 1a |
Model 2b |
|||||
|---|---|---|---|---|---|---|
| N (Annualized%) | HR (95% CI) | HR (95% CI) | ||||
| Proximal invasive colorectal cancer | ||||||
| White | 882 | (0.06%) | 1.00 | 1.00 | ||
| African–American | 103 | (0.07%) | 1.25 | (1.02, 1.54) | 1.18 | (0.91, 1.51) |
| P-value c | 0.03 | 0.21 | ||||
| Distal invasive colorectal cancer | ||||||
| White | 403 | (0.03%) | 1.00 | 1.00 | ||
| African–American | 46 | (0.03%) | 1.07 | (0.79, 1.46) | 0.96 | (0.64, 1.42) |
| P-value c | 0.66 | 0.83 | ||||
| Invasive rectal canced | ||||||
| White | 318 | (0.02%) | 1.00 | 1.00 | ||
| African–American | 34 | (0.02%) | 0.99 | (0.69, 1.41) | 0.70 | (0.43, 1.12) |
| P-value c | 0.95 | 0.13 | ||||
| Death from colorectal cancer | ||||||
| White | 411 | (0.03%) | 1.00 | 1.00 | ||
| African–American | 51 | (0.04%) | 0.92 | (0.69, 1.24) | 0.78 | (0.53, 1.13) |
| P-value c | 0.59 | 0.18 | ||||
Adjusted for age and stratified on WHI trial and extension study participation.
Adjusted for age, education, diabetes, body mass index, physical activity, smoking, alcohol use, NSAID use, total dietary energy, dietary fiber, red meat, fruits and vegetables, total calcium intake, prior colon screening, history of colon polyp removal, family history of colorectal cancer, and prior menopausal hormone use and stratified on WHI trial and extension study participation.
P-value is from a Wald χ2 test for the main effect of race/ethnicity.
Includes rectum and rectosigmoid junction sites.
Table 4 shows CRC tumor characteristics by race and ethnicity for all women in the WHI. Significance testing was only done for Whites and African Americans because of low numbers in other race and ethnic groups. The majority of the tumors were staged as localized or regional and there were no statistically significant differences in the distribution of tumor characteristics between White and African American women. Although not statistically significant, Whites had a greater percentage of poorly differentiated or anaplastic tumors. There were no significant differences in either crude or covariate-adjusted mortality rates between African–Americans and Whites among those diagnosed with CRC (P-value ranged from 0.06 to 0.65 in three separate models; data not shown).
Table 4.
Invasive colorectal cancer tumor characteristics by race and ethnicity
| White |
African– American |
Hispanic |
Native American |
Asian/Pacific Islander |
P-valuea | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | N | % | ||
| Tumor size, mm | 0.44 | ||||||||||
| <30 | 339 | 27.1 | 32 | 22.1 | 10 | 29.4 | 1 | 14.3 | 7 | 21.9 | |
| 30–49 | 484 | 38.7 | 60 | 41.4 | 10 | 29.4 | 2 | 28.6 | 13 | 40.6 | |
| ≥50 | 429 | 34.3 | 53 | 36.6 | 14 | 41.2 | 4 | 57.1 | 12 | 37.5 | |
| Missing | 425 | 25.3 | 50 | 25.6 | 15 | 30.6 | 1 | 12.5 | 10 | 23.8 | 0.93 |
| SEER stage | 0.76 | ||||||||||
| Localized | 712 | 44.7 | 76 | 42.0 | 15 | 36.6 | 5 | 62.5 | 14 | 34.1 | |
| Regional | 679 | 42.6 | 82 | 45.3 | 19 | 46.3 | 1 | 12.5 | 18 | 43.9 | |
| Distant | 202 | 12.7 | 23 | 12.7 | 7 | 17.1 | 2 | 25.0 | 9 | 22.0 | |
| Missing | 84 | 5.0 | 14 | 7.2 | 8 | 16.3 | 0 | 0.0 | 1 | 2.4 | 0.20 |
| Tumor grade | 0.08 | ||||||||||
| Well differentiated | 129 | 8.6 | 14 | 8.6 | 5 | 11.6 | 0 | 0.0 | 3 | 7.7 | |
| Moderately differentiated | 1002 | 66.7 | 121 | 74.2 | 24 | 55.8 | 4 | 50.0 | 29 | 74.4 | |
| Poorly differentiated/anaplastic | 372 | 24.8 | 28 | 17.2 | 14 | 32.6 | 4 | 50.0 | 7 | 17.9 | |
| Missing | 174 | 10.4 | 32 | 16.4 | 6 | 12.2 | 0 | 0.0 | 3 | 7.1 | 0.01 |
| Histology | 0.86 | ||||||||||
| Adenocarcinoma, NOS | 1019 | 63.0 | 109 | 58.6 | 28 | 59.6 | 7 | 87.5 | 31 | 75.6 | |
| Adenocarcinoma in adenomatous polyp | 119 | 7.4 | 17 | 9.1 | 4 | 8.5 | 1 | 12.5 | 1 | 2.4 | |
| Adenocarcinoma in villous adenoma | 60 | 3.7 | 9 | 4.8 | 2 | 4.3 | 0 | 0.0 | 1 | 2.4 | |
| Adenocarcinoma in tubulovillous adenoma | 155 | 9.6 | 18 | 9.7 | 5 | 10.6 | 0 | 0.0 | 6 | 14.6 | |
| Mucinous adenocarcinoma | 135 | 8.3 | 17 | 9.1 | 3 | 6.4 | 0 | 0.0 | 0 | 0.0 | |
| Mucin-producing adenocarcinoma | 77 | 4.8 | 11 | 5.9 | 3 | 6.4 | 0 | 0.0 | 1 | 2.4 | |
| Other | 53 | 3.3 | 5 | 2.7 | 2 | 4.3 | 0 | 0.0 | 1 | 2.4 | |
| Missing | 59 | 3.5 | 9 | 4.6 | 2 | 4.1 | 0 | 0.0 | 1 | 2.4 | 0.44 |
| Number of lymph nodes examined | 0.96 | ||||||||||
| None | 170 | 10.8 | 21 | 11.9 | 6 | 14.3 | 1 | 12.5 | 7 | 17.5 | |
| 1–9 | 496 | 31.6 | 56 | 31.6 | 13 | 31.0 | 0 | 0.0 | 8 | 20.0 | |
| 10–15 | 435 | 27.7 | 50 | 28.2 | 9 | 21.4 | 1 | 12.5 | 12 | 30.0 | |
| ≥15 | 470 | 29.9 | 50 | 28.2 | 14 | 33.3 | 6 | 75.0 | 13 | 32.5 | |
| Missing | 106 | 6.3 | 18 | 9.2 | 7 | 14.3 | 0 | 0.0 | 2 | 4.8 | 0.12 |
| Number of positive lymph nodesb | 0.81 | ||||||||||
| None | 916 | 65.5 | 101 | 64.7 | 23 | 63.9 | 4 | 57.1 | 16 | 48.5 | |
| 1 | 145 | 10.4 | 15 | 9.6 | 4 | 11.1 | 0 | 0.0 | 5 | 15.2 | |
| 2–3 | 170 | 12.2 | 23 | 14.7 | 3 | 8.3 | 1 | 14.3 | 2 | 6.1 | |
| ≥4 | 167 | 11.9 | 17 | 10.9 | 6 | 16.7 | 2 | 28.6 | 10 | 30.3 | |
| Missing | 3 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1.00 |
P-values are from χ2 or Fisher Exact tests examining the differences between white and African American race/ethnicities.
For women with lymph nodes examined.
Discussion
As reported by the American Cancer Society in 2010, CRC incidence rates were highest among African–Americans in the United States (52.6 per 100,000), compared with Whites (43.2), American Indians/Alaskan Natives (41.2), Asian–Americans/Pacific Islanders (35.4) and Hispanics/Latinos (32.8). Similarly, mortality rates due to CRC were highest among African–Americans (22.4 per 100,000) compared with Whites (15.3), American Indians/Alaska Natives (14.2), Hispanics/Latinos (10.8), and Asian–Americans/Pacific Islanders (10.2) (1). Our results, using data specific to the WHI cohort corroborate those of SEER. We found that the crude CRC incidence was highest among African–American women compared with women from other racial/ethnic groups. We also showed higher rates of proximal CRC among African–Americans. In contrast, Hispanic women had the lowest incidence of CRC. After adjustment for age and trial participation, the risk of CRC was marginally although not significantly greater for African–Americans than Whites. This relationship was further attenuated after multivariable adjustment suggesting that in the WHI, White: African–American differences in risk thought to be associated with race, can be explained at least in part by patient characteristics especially including lifestyle factors such as BMI, physical activity, smoking, and alcohol as well as other health system related factors. Among Hispanic women in fact, the multivariable adjustment increased the difference in risk, suggesting that there are other un-measured factors, which influence risk in Hispanic women. We found no significant differences in risk for women of Native American or Asian/Pacific Islander origin a result likely explained by the smaller sample in these subgroups.
Our results are consistent with other population based studies that have shown higher CRC incidence rates among African Americans (2–6) compared with other groups (40). It is possible that the difference in CRC risk between African–Americans and Whites in the WHI is not as striking as seen in other studies because of the restricted characteristics of women who self-selected for participation in a longitudinal clinical trial and observational study as well as perhaps by differences in health status of women who choose to enroll. This may also explain the lack of racial/ethnic differences in tumor characteristics such as stage and tumor grade. In fact, compared with other samples, a relatively large percentage of WHI women report advanced education and had regular access to healthcare including cancer screening. The lack of racial/ethnic cancer mortality differences in the WHI may be explained by similar stage of disease at diagnosis, a factor influenced by screening.
Likewise the lower CRC risk seen among Hispanics in the WHI is consistent with findings from other studies (2–4;6;7). Among Hispanics, CRC risk seems to vary by country of origin. Compared with non-Hispanic Whites, higher incidence rates are seen for Cuban Americans, whereas lower incidence rates are seen among Mexicans and New Latina (defined as Central or South American origin) (4). Ecologic trends are also reported for Hispanic immigrants to Florida, with higher rates reported for each immigrant group of Hispanics compared with rates seen in their countries of origin (4), suggesting an environmental or cultural effect related to immigration. Lower rates of CRC diagnoses among Hispanic women may be explained by lack of acceptance of CRC screening in that community, which is supported by lower rates of screening sigmoidoscopy, colonoscopy, and FOBT among Hispanic women in the WHI. However, adjustment for screening as well as other patient related and health systems related variables in our analysis did not attenuate the difference in risk between Hispanics and Whites. It is possible that lower CRC rates seen among Hispanics in the WHI may be a result of other sociocultural (diet, hormone use, parity, etc) or genetic differences inherent in the multifaceted Latino community which we were either not able to assess, or were minimally assessed in the WHI.
Less information is available on CRC incidence among other racial or ethnic groups (5;7;8). In a study of CRC in Hispanics, Native Americans and non-Hispanic Whites using the New Mexico Cancer Registry, CRC incidence decreased between 1969 and 1994 among non-Hispanic Whites, however incidence rates increased among minority women with the greatest increase seen for rectal cancer among Native American women during the same time period (7). In a report from the North American Association of Central Cancer Registries (NAACR), CRC incidence rates were significantly lower for Asian Pacific Islanders compared with Whites and African–Americans across all anatomic subtypes, except for rectal cancer where incidence rates were higher among Asian/Pacific Islanders (5). CRC incidence among Hmong immigrants to California was also lower compared with non-Hispanic whites and Asian/Pacific Islanders (8). The current report adds information to the literature on CRC rates among Native Americans and Asian Pacific Islanders; however, the contribution of these ethnic groups to the study population was relatively low limiting our ability to make definitive statements on risk in those groups particularly in relation to rectal cancer specifically.
While regular screening by FOBT, sigmoidoscopy or colonoscopy have been shown to have a significant protective impact on CRC incidence and mortality (41), significant racial differences exist in the use of these potentially life-saving measures (23; 24; 26). Use of FOBT (23) and screening colonoscopy (24) are less prevalent among African–Americans compared with Whites. In the annual report on the status of cancer in the United States, Whites were more likely than Asian/Pacific Islanders to have used FOBT within the past year (22% vs. 17%) and more likely to have undergone endoscopy in the past 5 years (41% vs.36%). In a study of temporal trends within 19 cancer registries, individuals residing in poorer communities with decreased access to medical care did not experience the same reduction in CRC incidence that has been seen in more affluent communities (42). In addition, whereas endoscopy usage in these communities increased over time for Whites, lower screening rates were noted for Hispanics and African–Americans which was associated with residence in counties with higher poverty rates, lower levels of health insurance, and fewer primary care providers. In the WHI, minority women were less likely to have had screening endoscopies and FOBT, and Hispanics had especially low rates of both procedures. On one hand, screening is likely to have a multiphase influence on CRC incidence with less screening leading to less CRC detection in the short-term, on another hand differences in CRC incidence and mortality in the WHI was not influenced by differences in screening and less screening had no apparent impact on the lower incidence of CRC seen among Hispanics.
National statistics suggest racial and ethnic differences in CRC mortality (6), though we were only able to analyze cancer specific and overall survival differences for African–American and White women in the WHI because of a low number of cases seen in other racial and ethnic groups. Within our cohort, we did not find significant differences in cause specific or overall survival. The lack of differences in CRC mortality in the WHI cohort may be a result of a selected population of women who have relatively equal access to medical care as participants in a large clinical trial, combined with the comparatively uniform tumor characteristics when these variables were compared between races.
The strengths of this study include the large cohort size and well-developed, robust, and validated database of potential covariates that allowed us to study women from a number of different racial and ethnic groups. Limitations include the fact that the majority of study participants were relatively well educated limiting the generalizability of the findings, the lack of additional information on women of Hispanic origin such as country of origin, and the fact that the contribution of some of the racial/ethnic subgroups was small. In addition, a majority of the covariates assessed were self-reported.
In conclusion, African–American/White differences in postmenopausal CRC risk are likely due to sociodemographic or sociocultural factors although no clear determination was made about why CRC risks were lower among Hispanic women. Our data suggests that there are a number of modifiable exposures that could be a focus for reducing the risk of CRC especially in African–Americans.
Acknowledgments
Authors thank the following: Program Office: (National Heart, Lung, and Blood Institute, Bethesda, Maryland) Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Ross Prentice, Garnet Anderson, Andrea LaCroix, Charles L. Kooperberg; (Medical Research Labs, Highland Heights, KY) Evan Stein; and (University of California at San Francisco, San Francisco, CA) Steven Cummings.
Clinical Centers: (Albert Einstein College of Medicine, Bronx, NY) Sylvia Wassertheil-Smoller; (Baylor College of Medicine, Houston, TX) Haleh Sangi-Haghpeykar; (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (Brown University, Providence, RI) Charles B. Eaton; (Emory University, Atlanta, GA) Lawrence S.Phillips; (Fred Hutchinson Cancer Research Center, Seattle, WA) Shirley Beresford; (George Washington University Medical Center, Washington, DC) Lisa Martin; (Los Angeles Biomedical Research Institute at Harbor-UCLA Medical Center, Torrance, CA) Rowan Chlebowski; (Kaiser Permanente Center for Health Research, Portland, OR) Erin LeBlanc; (Kaiser Permanente Division of Research, Oakland, CA) Bette Caan; (Medical College of Wisconsin, Milwaukee, WI) Jane Morley Kotchen; (MedStar Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Northwestern University, Chicago/Evanston, IL) Linda Van Horn; (Rush Medical Center, Chicago, IL) Henry Black; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (State University of New York at Stony Brook, Stony Brook, NY) Dorothy Lane; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Alabama at Birmingham, Birmingham, AL) Cora E. Lewis; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of California at Davis, Sacramento, CA) John Robbins; (University of California at Irvine, CA) F. Allan Hubbell; (University of California at Los Angeles, Los Angeles, CA) Lauren Nathan; (University of California at San Diego, LaJolla/Chula Vista, CA) Robert D. Langer; (University of Cincinnati, Cincinnati, OH) Margery Gass; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Hawaii, Honolulu, HI) J. David Curb; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Massachusetts/Fallon Clinic, Worcester, MA) Judith Ockene; (University of Medicine and Dentistry of New Jersey, Newark, NJ) Norman Lasser; (University of Miami, Miami, FL) Mary Jo O’Sullivan; (University of Minnesota, Minneapolis, MN) Karen Margolis; (University of Nevada, Reno, NV) Robert Brunner; (University of North Carolina, Chapel Hill, NC) Gerardo Heiss; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (University of Tennessee Health Science Center, Memphis, TN) Karen C. Johnson; (University of Texas Health Science Center, San Antonio, TX) Robert Brzyski; (University of Wisconsin, Madison, WI) Gloria E. Sarto; (Wake Forest University School of Medicine, Winston-Salem, NC) Mara Vitolins; and (Wayne State University School of Medicine/Hutzel Hospital, Detroit, MI) Michael S. Simon. Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Grant Support
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100–2, 32105–6, 32108–9, 32111–13, 32115, 32118–32119, 32122, 42107–26, 42129–32, and 44221″, and the Cancer Center Support Grant NIH:NCI P30CA022453.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.American Cancer Society . American cancer society cancer facts & figures 2010. American Cancer Society; Atlanta, GA: 2010. [Google Scholar]
- 2.Soto-Salgado M, Suarez E, Calo W, Cruz-Correa M, Figueroa-Valles NR, Ortiz AP. Incidence and mortality rates for colorectal cancer in Puerto Rico and among Hispanics, non-Hispanic whites, and non-Hispanic blacks in the United States, 1998-2002. Cancer. 2009;115:3016–23. doi: 10.1002/cncr.24340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carozza SE, Howe HL. Patterns of cancer incidence among US Hispanics/Latinos, 1995-2000. Cancer Causes Control. 2006;17:1067–75. doi: 10.1007/s10552-006-0045-3. [DOI] [PubMed] [Google Scholar]
- 4.Pinheiro PS, Sherman RL, Trapido EJ, Fleming LE, Huang Y, Gomez-Marin O, et al. Cancer incidence in first generation U.S. Hispanics: Cubans, Mexicans, Puerto Ricans, and new Latinos. Cancer Epidemiol Biomarkers Prev. 2009;18:2162–69. doi: 10.1158/1055-9965.EPI-09-0329. [DOI] [PubMed] [Google Scholar]
- 5.Wu X, Chen VW, Martin J, Roffers S, Groves FD, Correa CN, et al. Subsite-specific colorectal cancer incidence rates and stage distributions among Asians and Pacific Islanders in the United States, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004;13:1215–22. [PubMed] [Google Scholar]
- 6.American Cancer Society . Cancer facts & figures 2009. American Cancer Society; Atlanta: 2009. [Google Scholar]
- 7.Chao A, Gilliland FD, Hunt WC, Bulterys M, Becker TM, Key CR. Increasing incidence of colon and rectal cancer among Hispanics and American Indians in New Mexico (United States), 1969-94. Cancer Causes Control. 1998;9:137–44. doi: 10.1023/a:1008874025626. [DOI] [PubMed] [Google Scholar]
- 8.Mills PK, Yang RC, Riordan D. Cancer incidence in the Hmong in California, 1988-2000. Cancer. 2005;104:2969–74. doi: 10.1002/cncr.21525. [DOI] [PubMed] [Google Scholar]
- 9.Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut. 1994;35:1590–92. doi: 10.1136/gut.35.11.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berster JM, Goke B. Type 2 diabetes mellitus as risk factor for colorectal cancer. Arch Physiol Biochem. 2008;114:84–98. doi: 10.1080/13813450802008455. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES. Body mass index and colon cancer in a national sample of adult US men and women. Am J Epidemiol. 1999;150:390–98. doi: 10.1093/oxfordjournals.aje.a010018. [DOI] [PubMed] [Google Scholar]
- 12.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, et al. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–31. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 13.Wolin KY, Yan Y, Colditz GA, Lee IM. Physical activity and colon cancer prevention: a meta-analysis. Br J Cancer. 2009;100:611–16. doi: 10.1038/sj.bjc.6604917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Millen AE, Subar AF, Graubard BI, Peters U, Hayes RB, Weissfeld JL, et al. Fruit and vegetable intake and prevalence of colorectal adenoma in a cancer screening trial. Am J Clin Nutr. 2007;86:1754–64. doi: 10.1093/ajcn/86.5.1754. [DOI] [PubMed] [Google Scholar]
- 15.Paskett ED, Reeves KW, Rohan TE, Allison MA, Williams CD, Messina CR, et al. Association between cigarette smoking and colorectal cancer in the Women’s Health Initiative. J Natl Cancer Inst. 2007;99:1729–35. doi: 10.1093/jnci/djm176. [DOI] [PubMed] [Google Scholar]
- 16.Bongaerts BW, van den Brandt PA, Goldbohm RA, de Goeij AF, Weijenberg MP. Alcohol consumption, type of alcoholic beverage and risk of colorectal cancer at specific subsites. Int J Cancer. 2008;123:2411–17. doi: 10.1002/ijc.23774. [DOI] [PubMed] [Google Scholar]
- 17.Sandler RS, Halabi S, Baron JA, Budinger S, Paskett E, Keresztes R, et al. A randomized trial of aspirin to prevent colorectal adenomas in patients with previous colorectal cancer. N Engl J Med. 2003;348:883–90. doi: 10.1056/NEJMoa021633. [DOI] [PubMed] [Google Scholar]
- 18.Lin J, Zhang SM, Cook NR, Manson JE, Buring JE, Lee IM. Oral contraceptives, reproductive factors, and risk of colorectal cancer among women in a prospective cohort study. Am J Epidemiol. 2007;165:794–801. doi: 10.1093/aje/kwk068. [DOI] [PubMed] [Google Scholar]
- 19.Chlebowski RT, Wactawski-Wende J, Ritenbaugh C, Hubbell FA, Ascensao J, Rodabough RJ, et al. Estrogen plus progestin and colorectal cancer in postmenopausal women. N Engl J Med. 2004;350:991–1004. doi: 10.1056/NEJMoa032071. [DOI] [PubMed] [Google Scholar]
- 20.Baron JA, Beach M, Mandel JS, van Stolk RU, Haile RW, Sandler RS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 21.Kune G, Watson L. Colorectal cancer protective effects and the dietary micronutrients folate, methionine, vitamins B6, B12, C, E, selenium, and lycopene. Nutr Cancer. 2006;56:11–21. doi: 10.1207/s15327914nc5601_3. [DOI] [PubMed] [Google Scholar]
- 22.Wilkins T, Reynolds PL. Colorectal cancer: a summary of the evidence for screening and prevention. Am Fam Physician. 2008;78:1385–92. [PubMed] [Google Scholar]
- 23.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States: results from the 2000 National Health Interview Survey. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 24.McAlearney AS, Reeves KW, Dickinson SL, Kelly KM, Tatum C, Katz ML, et al. Racial differences in colorectal cancer screening practices and knowledge within a low-income population. Cancer. 2008;112:391–98. doi: 10.1002/cncr.23156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99:723–28. [PMC free article] [PubMed] [Google Scholar]
- 26.Espey DK, Wu XC, Swan J, Wiggins C, Jim MA, Ward E, et al. Annual report to the nation on the status of cancer, 1975-2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–52. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 27.Vinikoor LC, Long MD, Keku TO, Martin CF, Galanko JA, Sandler RS. The association between diabetes, insulin use, and colorectal cancer among Whites and African Americans. Cancer Epidemiol Biomarkers Prev. 2009;18:1239–42. doi: 10.1158/1055-9965.EPI-08-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Satia-Abouta J, Galanko JA, Martin CF, Ammerman A, Sandler RS. Food groups and colon cancer risk in African-Americans and Caucasians. Int J Cancer. 2004;109:728–36. doi: 10.1002/ijc.20044. [DOI] [PubMed] [Google Scholar]
- 29.Kim S, Martin C, Galanko J, Woosley JT, Schroeder JC, Keku TO, et al. Use of nonsteroidal antiinflammatory drugs and distal large bowel cancer in whites and African Americans. Am J Epidemiol. 2008;168:1292–1300. doi: 10.1093/aje/kwn255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Satia-Abouta J, Galanko JA, Martin CF, Potter JD, Ammerman A, Sandler RS. Associations of micronutrients with colon cancer risk in African Americans and whites: results from the North Carolina Colon Cancer Study. Cancer Epidemiol Biomarkers Prev. 2003;12:747–54. [PubMed] [Google Scholar]
- 31.Satia-Abouta J, Galanko JA, Potter JD, Ammerman A, Martin CF, Sandler RS. Associations of total energy and macronutrients with colon cancer risk in African Americans and Whites: results from the North Carolina colon cancer study. Am J Epidemiol. 2003;158:951–62. doi: 10.1093/aje/kwg248. [DOI] [PubMed] [Google Scholar]
- 32.The Women’s Health Initiative Study Group Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 33.Langer RD, White E, Lewis CE, Kotchen JM, Hendrix SL, Trevisan M. The Women’s Health Initiative Observational Study: baseline characteristics of participants and reliability of baseline measures. Ann Epidemiol. 2003;13:S107–S121. doi: 10.1016/s1047-2797(03)00047-4. [DOI] [PubMed] [Google Scholar]
- 34.Anderson GL, Manson J, Wallace R, Lund B, Hall D, Davis S, et al. Implementation of the Women’s Health Initiative study design. Ann Epidemiol. 2003;13:S5–17. doi: 10.1016/s1047-2797(03)00043-7. [DOI] [PubMed] [Google Scholar]
- 35.Hays J, Hunt JR, Hubbell FA, Anderson GL, Limacher M, Allen C, et al. The Women’s Health Initiative recruitment methods and results. Ann Epidemiol. 2003;13:S18–S77. doi: 10.1016/s1047-2797(03)00042-5. [DOI] [PubMed] [Google Scholar]
- 36.Prentice RL, Pettinger M, Beresford SA, Wactawski-Wende J, Hubbell FA, Stefanick ML, et al. Colorectal cancer in relation to postmenopausal estrogen and estrogen plus progestin in the Women’s Health Initiative clinical trial and observational study. Cancer Epidemiol Biomarkers Prev. 2009;18:1531–37. doi: 10.1158/1055-9965.EPI-08-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, et al. Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA. 2006;295:643–54. doi: 10.1001/jama.295.6.643. [DOI] [PubMed] [Google Scholar]
- 38.Wactawski-Wende J, Kotchen JM, Anderson GL, Assaf AR, Brunner RL, O’Sullivan MJ, et al. Calcium plus vitamin D supplementation and the risk of colorectal cancer. N Engl J Med. 2006;354:684–96. doi: 10.1056/NEJMoa055222. [DOI] [PubMed] [Google Scholar]
- 39.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, gurs-Collins T. Measurement characteristics of the Women’s Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–87. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 40.Thornton JG, Morris AM, Thornton JD, Flowers CR, McCashland TM. Racial variation in colorectal polyp and tumor location. J Natl Med Assoc. 2007;99:723–28. [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkins T, Reynolds PL. Colorectal cancer: a summary of the evidence for screening and prevention. Am Fam Physician. 2008;78:1385–92. [PubMed] [Google Scholar]
- 42.Hao Y, Jemal A, Zhang X, Ward EM. Trends in colorectal cancer incidence rates by age, race/ethnicity, and indices of access to medical care, 1995-2004 (United States) Cancer Causes Control. 2009 doi: 10.1007/s10552-009-9379-y. [DOI] [PubMed] [Google Scholar]