Abstract
Objective
To assess the efficacy of brief fatigue self-management for medically unexplained chronic fatigue (UCF) and chronic fatigue syndrome (CFS) in primary care.
Methods
A randomized controlled design was used wherein 111 patients with UCF or CFS were randomly assigned to: two sessions of fatigue self-management (FSM), two sessions of symptom monitoring support (attention control; AC), or a usual care control condition (UC). Participants were assessed at baseline and at 3- and 12-months post-treatment. The primary outcome, the Fatigue Severity Scale, measured fatigue impact on functioning. Analysis was by intention-to-treat (multiple imputation) and also by per protocol.
Results
A group by time interaction across the 15 month trial showed significantly greater reductions in fatigue impact in the FSM group in comparison to the AC group (p< .023) and the UC group (p< .013). Medium effect sizes for reduced fatigue impact in the FSM group were found in comparison with the AC group (d=.46) and the UC group (d=.40). The per protocol analysis revealed large effect sizes for the same comparisons. Clinically significant decreases in fatigue impact were found for 53% of participants in the FSM condition, 14% in the AC condition, and 17% in the UC condition. Dropout rates at the 12 month follow-up were high (42%–53%) perhaps attributable to the burden of monthly phone calls to assess health care utilization.
Conclusion
A brief self-management intervention for patients with UCF or CFS appeared to be clinically effective for reducing the impact of fatigue on functioning.
Keywords: Fatigue self-management, unexplained chronic fatigue, chronic fatigue syndrome, cognitive-behavior therapy, primary care, nurse-delivered intervention
Introduction
Given the high prevalence and poor outcomes for medically unexplained chronic fatigue (UCF) and chronic fatigue syndrome (CFS) in medical care (1–4), efficacious self-management interventions may offer hope and help to these underserved patients. A randomized booklet-based self-management study of patients with UCF or CFS in primary care (5) found significant reductions in fatigue and psychiatric symptoms at a three month follow-up. Two later randomized trials of UCF and CFS patients in primary care tested nurse-conducted cognitive-behavioral therapy (CBT) in comparison to graded exercise therapy (GET; 6) or non-directive counseling (7). At an eight month follow-up in both studies, the findings indicated substantial improvements (at least 50% reductions) in fatigue in all treatment conditions with no significant differences between the conditions within each study. However, the high dropout rates (range: 29%–35%) in these six-session primary care studies as compared to the simple booklet-based trial (5) suggest perhaps that the number of face-to-face visits may have played a role in study retention and overall efficacy.
Although the favorable outcomes for CBT and GET in primary care (6, 7) were expected, the success of the (attention control) non-directive counseling condition (7) was not. Thus, it is not clear how effective these behavioral interventions would have been in comparison to a usual care/no treatment condition that controls for passage of time, a critical question in determining efficacy. By comparison, the booklet-based self-management trial (5) did include a no treatment control, but not an attention control condition to assess nonspecific effects.
In order to address the methodological limitations of the above intervention trials in primary care, the design of the current cognitive-behavioral self-management study in UCF and CFS included (1) both a no treatment (usual care) control condition and an attention control condition; and (2) a brief (two-session) behavioral intervention that may plausibly show reduced attrition in comparison to the more intensive six-session interventions in the above studies. Our treatment model (8) was based on ameliorating the detrimental influences of affective distress, absence of pleasant affect and experiences, and maladaptive activity patterns. No assumptions were made about physical deconditioning and fear-based avoidance (9), the principles that guided the above intervention studies.
Our hypotheses were as follows: cognitive-behavioral self-management for UCF/CFS in primary care is more effective in reducing fatigue impact on functioning than (a) usual medical care alone and (b) an attention control condition of symptom monitoring support. The long-range objective was to develop an easy access cost effective behavioral self-management intervention in primary care to help these medically underserved patients.
Method
Sample
Participant Recruitment
Recruitment was obtained from the Stony Brook family medicine/primary care practice which included 14 attending physicians and 21 family practice residents who provided referrals of chronic fatigue patients to this study. In addition, study invitation letters were sent from the medical director to primary care patients with diagnoses suggestive of UCF and CFS. This study complied with the research ethics protocols established by the Stony Brook University Institutional Review board (IRB). All participants signed IRB-approved consent forms in a face-to-face meeting with the research staff during their initial appointment. Candidates were offered up to $420 as compensation for full participation in the study. Data collection began in February, 2009 and ended in December, 2011.
Initial Screening
Primary care patients eligible for the study met these criteria: (1) age between18–65, not pregnant, and ability to fully participate in the study; (2) at least six months of persistent fatigue with participant-reported impairment in physical, social, and/or role domains; (3) no medical or psychiatric exclusions, as determined by the patient's primary care physician and a psychiatric nurse. An initial phone interview conducted by F.F. screened for CFS diagnostic criteria (10) using a validated assessment protocol (11). Medical exclusions consisted of cases of fatigue clearly attributable to identifiable medical conditions (e.g., autoimmune disease) or to medications (e.g., beta blockers) taken by prospective participants (10, 12).
Psychiatric exclusions were as follows: any psychosis or dementia, alcohol or substance abuse in the two years prior to illness onset and at any time afterward, current or past depression with melancholic or psychotic features within five years of illness onset or within five years of evaluation, and present or past anorexia nervosa or bulimia nervosa. To verify the absence of psychiatric exclusions, Axis I psychiatric diagnoses were formally identified with a nurse-conducted Structured Clinical Interview for DSM-IV (SCID; 13).
Randomization and Partial Blinding
Following an individual's documented consent to participate and completion of baseline assessments, each participant was assigned to one of the three study conditions via a computer-generated randomization schedule. The randomization schedule was completed prior to enrollment of the first participant. We addressed possible seasonal effects over the recruitment period by running participants in small, variable size blocks (block sizes of 6 or 9). Randomly varying the block size avoided potential selection bias by project personnel because it was difficult to determine where blocks start and stop and thus determine the next assignment. After completion of all screening assessments for a participant, a graduate student sent an email to one of the nurse interventionists with the condition assignment for that participant. In order to minimize demand characteristics associated with completion of the outcome measures, the staff collecting outcome data (not the interventionists) were blinded to the participant's treatment condition.
Treatment Procedure
The treatment phase began in April 2009 and ended in December 2011. Participants in the behavioral self-management and symptom monitoring support conditions were seen individually for two face-to-face sessions of up to 60 minutes by one of the two nurse interventionists.
Active Treatment: Fatigue Self-Management (FSM)
This two-session nurse-conducted individual self-management protocol was based on a modified version of an efficacious 12-session cognitive-behavioral treatment program for CFS (14) and a self-help book for CFS and fibromyalgia (15). A 61-page self-management booklet provided to these participants contained material discussed and assigned in therapy sessions for the three month self-management period.
Session 1
This session educated the participant about (1) diagnosis and possible causal factors in UCF and CFS and (2) stress factors and behaviors that play a role in disturbed sleep patterns, post-exertional symptoms, and push-crash activity cycles. Persistent fatigue was explained as a symptom associated with doing too much or too little. Optimal self-management was intended to achieve a healthy balance between mental and physical exertion and periods of rest (8). Assignments included the self-management booklet and a daily web diary to identify baseline activities, symptoms, and stress levels.
Session 2
Scheduled two weeks after session 1, this session identified unhelpful behaviors and beliefs about the illness followed by development of more useful cognitive and behavioral coping strategies. With information gathered from the week 1 web diary, the scheduling of home-based activities, rest/sleep assignments, and cognitive coping skills was individualized for each participant. Walking, if included, was intended as a voluntary leisure activity, rather than a fitness regimen. For instance, a relatively low functioning individual might be assigned a regular sleep /wake schedule and gradual low effort walking to increase tolerance of physical activity. A higher functioning participant might respond more favorably to pacing of activity and low effort pleasant activities. The final topic was post-intervention planning for maintenance of new self-management skills which included recognizing and managing early symptoms of setbacks before they affected functioning.
Attention Control: Symptom Monitoring Support (AC)
To control for therapist attention, homework assignments, and other non-specific effects, a two-session AC control condition was incorporated into this study (16). This condition included (1) in-session emotional support and (2) home-based self-monitoring of symptoms, affect and stress as recorded in web diaries. The two face-to-face sessions in this condition were separated by two weeks.
This protocol was based on the attention control condition used in a 12-week CBT trial in lupus (17). This condition was found to have participant-rated credibility (18) comparable to their active CBT intervention. In addition, their attention control condition was associated with small to medium effect sizes for fatigue, affect, and pain. These improvements were determined not to be statistically significant, nor were they of a magnitude that suggested clinical significance. Based on these findings, our AC condition was intended to serve as a credible attention control condition without the skills-training and practice components of the active intervention.
Session 1
A motivational description was given for the in-session disclosure and support element of the protocol (17) including its potential behavioral and physiological benefits, such as less physical and affective dysfunction (19), potential symptom reduction (e.g., 20), and improvements in immune functioning (21). In addition, the ability of symptom monitoring at home to reduce symptoms was explained as an evidence-based technique (e.g., 22).
During this session, patients were asked to report their current symptoms of UCF/CFS and a psychosocial history was taken. Topics discussed included a history of symptoms and diagnosis, interactions with health care professionals, the associations between fatigue, pain, and stress, and the impact of UCF/CFS on family and other interpersonal relationships. The nurse therapist listened empathically but avoided making suggestions for change or teaching stress or symptom management skills to the patient. Participants were also given a two-page information sheet (modified from 17) that explained the potential therapeutic value of emotional support and symptom monitoring. Symptom monitoring was scheduled as homework using a web diary at the end of the day to record participant ratings on an 11-point numerical rating scale (0–10) for physical fatigue, mental fatigue, fatigue interference, pain, stress, and negative and positive feelings.
Session 2
Symptom monitoring homework was reviewed including any comments or reactions from participants. The nurse again listened empathically to the list of illness issues compiled by the therapist on the basis of session 1 (stress and symptoms, family impact, etc.) and incorporated any specific issue that the participant wished to discuss. A continuation of the diary-based symptom monitoring homework was assigned for a 3-month period to match the duration of the active treatment arm. No behavioral recommendations, explicit or implicit, were made.
Usual Medical Care/No Treatment Control (UC)
This “no (additional) treatment” control condition consisted of the patient's usual medical care plus all three study assessments (baseline, 3- and 12-month follow-ups).
Assessment of Treatment Fidelity
Two nurse interventionists successfully completed 15 hours of face-to-face training as taught by a clinical psychologist (F.F) who is an experienced CBT interventionist for patients with chronic fatigue. During the intervention period, biweekly clinical supervision of the providers was conducted by the psychologist. In order to independently verify the distinctiveness of the FSM intervention, a validated fidelity rating scale developed in a previous CBT trial in CFS (14) and modified for this study was used. A selected random sample of 14% (n=11) of the audio recorded sessions was rated for therapist behaviors and techniques that distinguished the active FSM intervention from the AC condition. Fidelity ratings assessed the consistency of implementation of the two nurse-conducted sessions, as specified in the FSM and AC treatment manuals, to ensure that these two study conditions were in fact distinct. Two fidelity raters (graduate students) were trained by a licensed psychologist (F.F.).
Assessments and Outcome Measures
Primary outcome measure
Fatigue Severity Scale (FSS). This measure of the effect of fatigue on functioning is comprised of nine items rated on a Likert-type rating scale (1–7), where one indicates no impairment and seven indicates severe impairment (score range: 1.00–7.00). In the initial validation study (23), internal consistency for the FSS was excellent (α =.80) and the scale clearly distinguished between patients and controls. The scale has been recommended for use in CFS (24) and has shown sensitivity to treatment change (14).
Secondary outcome measures
Short Form-36 Physical Function subscale (SF-36PF). Physical functioning was measured with the SF-36PF. Limitations of ill health are measured on a scale of 0 (limited in all activities, including basic self-care) to 100 (no limitations, able to carry out vigorous activities). Test construction studies for the SF-36 (25, 26) have shown high internal consistency for the physical function subscale (α =.91–.94) and substantial differences in scores between patient and non-patient populations.
Beck Depression Inventory—second edition (BDI-II). Depressive symptomatology was measured with the BDI-II (27, 28), a 21-item self-report instrument with well-established psychometric properties (27, 28).
Beck Anxiety Inventory (BAI). Anxiety symptoms were measured with the BAI, a 21-item self-report measure with high internal consistency (a = .92) and established and replicated construct validity (29, 30).
Web diary
All study conditions
A time-stamped web diary (Science Trax; Macon, Georgia) recorded patient-reported activities, and symptom and stress ratings (e.g., “Stress Now” on a 0–10 numerical rating scale) at the end of the day for each one week assessment (baseline, 3- and 12-month follow-up). For ratings of fatigue, fatigue interference, and stress, response-activated screens were displayed, each with a numerical rating scale (0–10). The end point anchors on the numerical scales were None (0) and Highest (10).
AC and FSM conditions only
Daily symptom and stress ratings were scheduled for the entire 3-month intervention period.
FSM condition only
Questions were included to assess the following activities: daily duration of booklet reading and self-management activities, e.g., pacing, activity/exercise, pleasant activities, coping statements practice. In addition, three multiple choice questions assessed knowledge of each chapter in the booklet, such as facts about chronic fatigue illness, stress and lifestyle factors, and self-management techniques.
Power Calculation
The sample size for this study was chosen to ensure that there would be adequate power to detect differential treatment effects for fatigue impact, assuming that the actual effects are comparable to those reported in Powell et al. (31). In an intention-to-treat analysis of CFS patients who either completed a self-management behavioral intervention or received usual care (31), the differential improvement at the 3- month follow-up between the minimum intervention (two-session) group and the control group in fatigue on the Fatigue Scale was a full 5 times the baseline standard deviations. Even assuming zero autocorrelation between baseline and follow-up (extremely conservative), the proposed study design with 29 subjects in each group had a greater than 99% power to detect differences between the active treatment group and each control group in a two-tailed, α =.05, RM-ANOVA test of the group*time interaction.
Procedure
All baseline and intervention visits took place at the primary care/family medicine practice at Stony Brook University. Formal assessments (questionnaires, web diaries) were scheduled at baseline and at the three-month and 12-month follow-ups. The study sequence involved (a) an initial phone screening conducted by F.F. and documentation of a UCF or CFS diagnosis from the patient's doctor; (b) a face-to-face baseline assessment visit that included a nurse-conducted SCID, a brief program overview, and web diary instruction; (c) two intervention visits with a primary care nurse (FSM and AC conditions only); (d) home-based implementation of the FSM and AC conditions with daily web diary verification; (e) monthly phone calls to assess health care utilization (to be reported separately); and (f) face-to-face follow-up assessments at 3- and 12-months post-treatment.
Data Analysis
To test the primary hypotheses concerning treatment efficacy, both intention to treat (ITT) and per protocol analyses were done on the primary outcome measure of fatigue impact and the secondary measures of physical functioning, depression and anxiety. To minimize possible selective attrition bias or bias due to selective non-adherence, all outcome measures were analyzed (SPSS v20 software package) with an ITT approach of multiple imputation (MI; 32) to handle missing data. Missing data were assumed to be missing at random (33). The per protocol analysis used a one-way ANOVA.
The primary hypotheses concerned the simple effects of time (i.e., pre- vs. post-treatment change) within the experimental group and, more often, interaction effects of time-by-group (i.e., differential change) for the primary and secondary outcome measures. In addition, the data were analyzed to determine if the effect of study condition was moderated by diagnostic (CFS vs. UCF) group. To assess clinically significant change (34, 35) for fatigue impact, a patient was considered clinically improved if his/her 12 month post-treatment score was more than two standard deviations below the pre-treatment sample mean on the FSS (M = 5.57; SD = .94). Finally, compliance with home assignments and their relationship with fatigue impact outcomes were evaluated with web diary data.
Results
Participant Characteristics
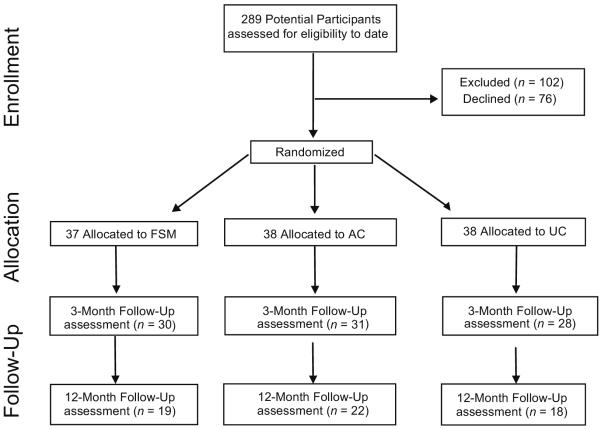
Figure 1 shows the flow of participants through the study. Over a period of 18 months, 289 individuals contacted by letter who had a physician diagnosis of CFS or UCF, were identified as potential participants. Of the initial 289 people, 102 were excluded because they did meet entry criteria and 76 declined to participate. After a psychiatric interview (SCID) with a psychiatric nurse to verify the absence of psychiatric exclusions, 111 patients were randomized (a response rate of 111 of 187 or 59.3%). The majority of participants (Table 1) were women (77%) and white (76%), and the mean age was lower to mid-40s. Thirty-nine percent of the sample met symptom criteria for CFS and the remaining 61% who did not meet full CFS criteria were classified as UCF. At baseline, the primary outcome measure of fatigue impact (FSS) was significantly higher in those with a CFS diagnosis as compared to those with a UCF diagnosis (t (109) = −2.55, p = .012). However, large majorities of both groups (UCF: 77%; CFS: 84%) exhibited FSS scores that exceeded 5.0, considered to be “high fatigue” impact, i.e., 2 SDs above healthy controls (36, 37).
Figure 1.
Study Flow-Chart. FSM = fatigue self-management; AC = symptom monitoring Support; UC = usual care condition.
TABLE 1.
Baseline Demographic Characteristics of UCF/CFS Participants in the Fatigue Self-Management (FSM), Attention Control (AC), and Usual Care Conditions (UC)
| FSM Group (n = 37) | AC Group (n = 38) | UC Group (n = 36) | |
|---|---|---|---|
| Age, mean (SD) | 42.47 (15.45) | 42.49 (12.10) | 43.64 (13.34) |
| Length of illness in years mean (SD) | 11.67 (9.11) | 8.93 (7.45) | 9.81 (8.37) |
| Sex, women, n (%) | 29 (78.4%) | 29 (76.3%) | 28 (77.8%) |
| SF36, mean (SD) | 70.29 (24.31) | 61.17 (24.53) | 60.05 (24.05) |
| Marital status n % | |||
| Single/divorced/separated | 17 (45.9%) | 10 (26.3%) | 12 (33.4%) |
| Living with partner | 14 (37.8%) | 25 (65.8%) | 19 (52.8%) |
| Ethnicity n % | |||
| European | 30 (81.1%) | 28 (73.7%) | 26 (72.2%) |
| Latino | 1 (2.7%) | 2 (5.3%) | 5 (13.9%) |
| Black | 1 (2.7%) | 2 (5.3%) | 1 (2.8%) |
| Employment | |||
| Working full time n % | 16 (43.2%) | 21 (55.3%) | 20 (55.6%) |
| Unemployed n % | 7 (18.9%) | 1 (2.6%) | 4 (11.1%) |
No significant differences were found between the three study conditions on any demographic variable.
Thirty seven participants were randomly assigned to the FSM condition, 38 to the AC condition, and 36 to the UC condition. No significant differences were found across study conditions at baseline on any demographic or symptom variable (Table 1). At baseline, 53.7% of participants at baseline had one or more non-exclusionary SCID diagnosis. The rates of SCID diagnoses did not significantly differ among the three study conditions (χ2 (2) = 1.40, p = .40).
For the FSM group, 30 participants (81%) completed the two-session intervention and three month follow-up, and 19 (51%) completed the 12 month follow-up. For the AC group, 31 participants (82%) completed the two session intervention and three month follow-up, and 22 (58%) completed the 12 month follow-up. For the UC group, 28 participants (78%) completed the two session intervention and three month follow-up and 18 (47%) the 12 month follow-up. In total, 53 patients dropped out of the study after randomization. The most frequently given reasons for dropping out were: too much work to do in study, didn't feel that the type of treatment was appropriate, no longer interested, and “unknown” for those who did not return phone calls (the largest category). Between study completers and dropouts, no significant differences were found on any baseline demographic or symptom variable. In addition, no significant differences were found in dropout rates between those diagnosed at baseline with UCF as compared to CFS (Z = .94, p = .17).
Treatment Fidelity
Inter-rater reliabilities on the fidelity rating scale for 11 (14%) audio recorded treatment sessions revealed a median inter-rater percent agreement for item reliability of .77, considered to be an acceptable level (14). The two nurses did not significantly differ on their ratings for delivery of skills in the FSM (p = .40) and AC (p = .80) conditions or with respect to their ratings for general interpersonal skills (p = .60). The scores for the 11 rated sessions indicated that the FSM (t (9) = 2.29, p = .048) and AC (t (9) = 2.38, p = .041) treatment conditions were significantly differentiated by the questions tapping their corresponding categories. Overall, the analysis of treatment fidelity suggested that the therapists implemented the two distinct treatment arms as specified.
Enactment of behavioral skills in the FSM condition was assessed in part with web diary answers to multiple choice questions regarding self-management knowledge for CFS. A significant association was found between the proportion of correct answers (range: .07–.97) and the degree of improvement in FSS scores at the three month follow-up (r = .56, p < .001). Also, 12-month follow-up scores on the FSS were significantly associated (r =.38, p < .001) with more days of completed web diary (range: 0–89 days) homework during the 3-month intervention period.
Primary Outcome Measure
For the analyses of the MI data, the relative efficiencies for the pooled estimates of the regression parameters were all greater than 90%. Changes in FSS scores from baseline to 12-month were unrelated to diagnostic group (CFS vs. UCF; p = .38) or to the interaction of diagnostic group by treatment condition (p = .45). The change in FSS scores in the active FSM group was significantly greater than that found in the UC (B = .86, t(105) = 2.53, p = .013) and the AC (B = −722, t(105) = −2.30, p = .023) control groups (Figure 2). These differences in FSS scores reflect moderate effect sizes for the FSM vs. UC comparison (d=.46) and the FSM vs. AC comparison (d=.40). The mean change in FSS scores did not significantly differ (p = .67) between the two control groups.
Figure 2.

Profile Plot of FSS Means by Time and Study Condition. Study Condition: FSM=Fatigue Self-Management; AC= Symptom Monitoring Support Attention Control; UC=Usual Care Control; FSS=Fatigue Severity Scale.
Within group findings (Table 3) for the mean change in FSS scores from baseline to the 12-month follow-up for the FSM group indicated significant (t (35) = −4.75; p < .001) improvement and a large effect size (d=.78). The changes in FSS scores for the AC (t(36)= −1.95, p = .059) and UC (t (34) = −1.35, p = .19) group were both non-significant.
Secondary Outcome Measures
The MI analyses for the secondary measures (SF-36PF, BAI and BDI; Table 3) revealed no significant differences in changes scores by time, treatment group, or diagnostic group and no significant interaction of these factors (all p-values > .05).
Per Protocol Analysis for the Primary Outcome Measure
In comparison to the ITT analysis, differences found between treatments were similar but of greater magnitude in the per-protocol analysis. The main effect for Diagnostic Group was significant (F(1, 53) = 11.4, p < .001, η2p = .18), indicating that the mean FSS score for the UCF group was reliably lower (less fatigue impact) than the mean FSS score for the CFS group. The interactions effects for Diagnostic Group × Time (p = .67), Diagnostic Group × Treatment (p = .54), and Diagnostic Group × Treatment × Time (p = .66) were all non-significant.
The simple effect of Condition at the 12-month follow-up was statistically significant (F(2,53) = 5.27, p < .01, η2p = .17). Post hoc means comparison tests indicate that the FSM group had significantly (p < .01) lower FSS scores than both the AC (d=.87) and the UC (d=.89) control groups, reflecting large effect sizes. The AC and UC groups had nearly identical and statistically equivalent FSS means (p= .81). The simple effect of Time for the FSM group was statistically significant (F (2,52) = 14.9, p < .001, η2p = .37). The simple effects of Time for the AC and the UC control groups were both non-significant (p=.100) indicating that the FSS means for these groups did not change significantly over the 15 months of the study.
Per Protocol Analysis for the Secondary Outcome Measures
For the secondary outcome measures of physical functioning (PF), anxiety (BAI), and depression (BDI), the main effects for condition and diagnostic group as well as the interactions of these factors with time (baseline, 3-month, 12-month) were all non-significant (all p-values > .07).
Clinical Significance
Using our criterion for clinical significance of the primary outcome measure (FSS mean <= 3.9) in the per protocol data, the number of clinically improved participants at the 12-month follow-up was as follows: 10/19 (53%) in the FSM condition, 3/22 (14%) in the AC condition, and 3/18 (17%) in the UC condition. In healthy adults, the FSS mean is 2.3 (SD = .70) (23). FSS baseline scores were significantly associated (r = −.49; p <.001) with clinically significant changes at 12 month follow-up indicating that lower fatigue impact scores at baseline were less likely to show clinically significant change.
Discussion
This study found that a brief behavioral self-management intervention for patients with UCF or CFS in a primary care setting was successful in reducing fatigue impact on functioning in comparison to two control conditions, an attention control group and a usual care control group. The intervention did not have a significant effect on physical function, depression or anxiety symptoms. Clinically significant improvements in fatigue impact were found for 53% of the intervention group, 14% of the attention control group and 17% of the usual care control group. Moderate attrition rates (18%–22%) were evidenced at the 3-month follow-up, but contrary to expectation, high dropout rates (42%–53%) were observed at the 12-month follow-up. Attrition was not related to any demographic or symptom variable.
The theory of illness used in the current study was based on a clinical model of CFS (8) which posits maladaptive activity levels (too high or too low or an alternating combination) in association with emotional stress and/or a lack of pleasant experiences. Although substantial improvements are considered possible in this model, interventions are not assumed to lead to recovery or cure. The physical deconditioning and avoidance model cited in previous intervention trials (9) is based on different underlying assumptions; however, the intervention techniques used are similar.
Comparisons with Prior Behavioral Intervention Trials
This study replicated the Powell et al. (31) trial which found strong effect sizes at a one-year follow-up for their two-session behaviorally focused educational intervention for patients with CFS. In the current study, the percentage of participants showing clinically significant improvement in comparison to the control conditions exceeded that reported in a review of CBT randomized trials in CFS (38) and in the recent large sample PACE trial (39). Given that about 60% of our sample were diagnosed with UCF rather than full-blown CFS, the potential for self-directed improvement may have been greater in this more functional, less ill sample, consistent with more favorable outcomes for patients with less fatigue in prospective studies (40, 41).
The inclusion of both attention control and no treatment usual care conditions in this study revealed that the significant reduction found in fatigue impact could not be accounted for by nonspecific factors in the active intervention or to simple passage of time. At the 12-month follow-up, no significant differences were found between the two control conditions, suggesting that any nonspecific effects largely if not entirely dissipated over the long follow-up interval. Yet it appeared that the attention control condition had credibility given that the number of web diary days completed was significantly greater (p<.05) in the attention control condition as compared to the active treatment condition.
Perhaps our brief two-session protocol provided less opportunity for nonspecific effects in the attention control condition as compared to the effective six-session attention control condition in the Ridsdale et al. (7) primary care study. In addition, their attention control condition of non-directive counseling may have delivered a more active intervention because it was originally devised as a treatment for patients with depression and anxiety in primary care.
Attrition
The attrition rate at the 12-month follow-up was high (42–53%) in comparison to other UCF and CFS behavioral intervention studies in primary care (5–7). Attrition appeared to be random given that dropouts in comparison to study completers did not show significant differences on any demographic or symptom variable including diagnosis (CFS or UCF). One notable difference in our follow-up protocol in comparison to other UCF/CFS behavioral intervention studies in primary care (5–7) was the scheduling of 15 min. monthly phone calls to assess health care utilization and resource use for a future economic analysis. Perhaps the additional burden of the monthly phone calls played a role in the high rate of attrition during the 12 month follow-up interval. Often, when participants dropped out during this period, no reason could be identified because they did not answer or return pre-scheduled phone calls. This high dropout rate suggests that a lower subject burden may be important for patients in primary care. Similar to a previous booklet-based UCF/CFS self-management trial in primary care (5), we are finding a much lower dropout rate (<5%) in an ongoing fatigue self-management study in primary care patients that is entirely home-based, i.e., no face-to-face visits.
Study Limitations
The main limitations of this study are its high rate of attrition and the relatively high functioning participants (usually diagnosed with UCF) which may have elevated the level of improvement in comparison to samples consisting only of CFS cases. Also, only self-report measures were used, given that data collection for our more objective assessments (six-minute walk test and actigraphy) was not adequate for analysis. Finally, the lack of improvement in physical functioning is a potential limitation, although this finding is consistent with the absence of significant improvements in physical function reported in a review of randomized CBT trials in CFS (42).
Conclusions and Implications
This study found that the impact of fatigue on functioning in UCF and CFS can be effectively treated for a notable proportion of these patients in a primary care setting. The intervention consisted of a brief self-management program conducted by trained primary care nurses and augmented with a self-management booklet and daily web diary assignments. The findings of reduced fatigue impact at the 12 month follow-up reflected medium to large effect sizes in comparison to attention control and no treatment control conditions. However, a high rate of attrition at the 12 month follow-up was a limitation that may in part be explained by the additional burden of monthly phone calls to collect health care utilization data. The importance of the dropout issue in this study indicates the need to look further into the determinants of retention and dropout in these primary care patients. In sum, our findings suggest that unexplained, often intractable fatigue in primary care patients can be effectively addressed for many (but not all) of these patients by teaching behavioral self-management skills. A cost effectiveness analysis of this intervention will be published separately.
Table 2.
Primary and Secondary Outcome Measures for Active Intervention and Control Groups at Baseline and Follow-up Assessments
| Measure | (Δ) from | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | M | SE | 95% CI | N | Baseline | SE Δ | t for Δ | p for Δ | d | ||
| FSS | |||||||||||
| FSM | Baseline | 5.40 | .16 | 5.09 | 5.71 | 37 | |||||
| 3 Month | 4.94 | .19 | 4.56 | 5.31 | |||||||
| 12 Month | 4.19 | .26 | 3.66 | 4.71 | −1.21* | .25 | −4.75 | <.001 | .78 | ||
| AC | Baseline | 5.71 | .16 | 5.40 | 6.01 | 38 | |||||
| 3 Month | 5.39 | .19 | 5.00 | 5.77 | |||||||
| 12 Month | 5.16 | .29 | 4.57 | 5.75 | −.55 | .28 | −1.95 | .059 | .32 | ||
| UC | Baseline | 5.72 | .16 | 5.42 | 6.03 | 36 | |||||
| 3 Month | 5.45 | .19 | 5.07 | 5.83 | |||||||
| 12 Month | 5.31 | .32 | 4.63 | 5.98 | −.42 | .31 | −1.34 | .19 | .22 | ||
| SF-36PF | |||||||||||
| FSM | Baseline | 68.49 | 4.06 | 60.53 | 76.45 | 37 | |||||
| 3 Month | 74.82 | 4.01 | 66.90 | 82.74 | |||||||
| 12 Month | 66.30 | 4.89 | 56.31 | 76.29 | −2.19 | 4.64 | −.47 | .64 | .08 | ||
| AC | Baseline | 62.53 | 4.03 | 54.63 | 70.43 | 38 | |||||
| 3 Month | 67.07 | 3.72 | 59.77 | 74.37 | |||||||
| 12 Month | 65.96 | 4.24 | 57.58 | 74.34 | 3.43 | 3.95 | .87 | .39 | .14 | ||
| UC | Baseline | 59.77 | 4.02 | 51.90 | 67.65 | 36 | |||||
| 3 Month | 64.92 | 3.67 | 57.73 | 72.11 | |||||||
| 12 Month | 56.44 | 4.21 | 48.12 | 64.75 | −3.34 | 3.92 | −.85 | .40 | .14 | ||
| BAI | |||||||||||
| FSM | Baseline | 11.97 | 1.47 | 9.08 | 14.86 | 37 | |||||
| 3 Month | 10.98 | 1.60 | 7.84 | 14.13 | |||||||
| 12 Month | 12.02 | 1.89 | 8.20 | 15.85 | .05 | 1.91 | .03 | .98 | .00 | ||
| AC | Baseline | 14.91 | 1.46 | 12.05 | 17.78 | 38 | |||||
| 3 Month | 14.49 | 1.63 | 11.27 | 17.71 | |||||||
| 12 Month | 13.88 | 1.86 | 10.13 | 17.64 | −1.03 | 1.88 | −.55 | .59 | .09 | ||
| UC | Baseline | 14.99 | 1.46 | 12.13 | 17.85 | 36 | |||||
| 3 Month | 14.50 | 1.65 | 11.25 | 17.75 | |||||||
| 12 Month | 14.39 | 2.23 | 9.65 | 19.13 | −.60 | 2.24 | −.27 | .79 | .04 | ||
| BDI | |||||||||||
| FSM | Baseline | 17.10 | 1.45 | 14.26 | 19.95 | 37 | |||||
| 3 Month | 13.52 | 1.64 | 10.28 | 16.76 | |||||||
| 12 Month | 14.89 | 1.86 | 11.15 | 18.63 | −2.22 | 1.87 | −1.18 | .25 | .19 | ||
| AC | Baseline | 15.29 | 1.44 | 12.47 | 18.12 | 38 | |||||
| 3 Month | 16.41 | 1.55 | 13.37 | 19.45 | |||||||
| 12 Month | 14.56 | 1.90 | 10.70 | 18.42 | −.73 | .73 | −1.00 | .32 | .16 | ||
| UC | Baseline | 18.31 | 1.44 | 15.50 | 21.13 | 36 | |||||
| 3 Month | 16.17 | 1.83 | 12.46 | 19.88 | |||||||
| 12 Month | 14.03 | 1.86 | 10.27 | 17.79 | −4.28 | 1.88 | −2.28 | .029 | .38 | ||
Significant using a Bonferroni adjusted to alpha (αadj = .004)
Measure: FSS=Fatigue Severity Scale; SF-36PF= Short Form-36 Physical Function subscale; BAI=Beck Anxiety Inventory; BDI=Beck Depression Inventory
Group: FSM= Fatigue Self-management; AC= Symptom Monitoring Support Attention Control; UC= Usual Care Control
Acknowledgments
The project described was supported by NIH grants 5R01NR010229 (National Institute of Nursing Research; PI: F. Friedberg) and MO1RR10710 (General Clinical Research Center at Stony Brook University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Nursing Research or the National Institutes of Health.
Acronyms
- UCF
unexplained chronic fatigue
- CFS
chronic fatigue syndrome
- FSM
fatigue self-management
- CBT
cognitive-behavior therapy
- AC
symptom monitoring attention control group
- UC
usual care control group
- FSS
fatigue severity scale
- SF-36PF
short-form 36 physical function subscale
- BDI
Beck Depression inventory
- BAI
Beck Anxiety Inventory
Footnotes
All authors declared no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–236. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 2.Joyce J, Hotopf M, Wessely S. The prognosis of chronic fatigue syndrome: a systematic review. QJM. 1997;90:223–233. doi: 10.1093/qjmed/90.3.223. [DOI] [PubMed] [Google Scholar]
- 3.Kroenke K, Wood DR, Mangelsdorff D, Meier NJ, Powell JB. Chronic fatigue in primary care: prevalence, patient characteristics, and outcome. JAMA. 1988;260:929–934. [PubMed] [Google Scholar]
- 4.Ridsdale L, Evans A, Jerrett W, Mandalia S, Osler K, Vora H. Patients with fatigue in general practice: a prospective study. BMJ. 1993;307:103–106. doi: 10.1136/bmj.307.6896.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chalder T, Wallace P, Wessely S. Self-management treatment of chronic fatigue in the community: A randomized controlled trial. Brit J Health Psych. 1997;2:189–197. [Google Scholar]
- 6.Ridsdale L, Darbishire L, Seed PT. Is graded exercise better than cognitive behaviour therapy for fatigue? A UK randomized trial in primary care. Psychol Med. 2004;34:37–49. doi: 10.1017/s0033291703001247. [DOI] [PubMed] [Google Scholar]
- 7.Ridsdale L, Godfrey E, Chalder T, Seed P, King M, Wallace P, Wessely S. Chronic fatigue in general practice: is counselling as good as cognitive behaviour therapy? Brit J Gen Pract. 2001;51:19–24. [PMC free article] [PubMed] [Google Scholar]
- 8.Friedberg F. Chronic fatigue syndrome, fibromyalgia, and related illnesses: A clinical model of assessment and intervention. J Clin Psychol. 2010;6:641–665. doi: 10.1002/jclp.20676. [DOI] [PubMed] [Google Scholar]
- 9.Chalder T, Deale A, Wessely S. The treatment of chronic fatigue syndrome. In: Rader M, Naber D, editors. Difficult clinical problems in psychiatry. Martin Dunitz; London: 1999. pp. 135–153. [Google Scholar]
- 10.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The Chronic Fatigue Syndrome: A comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–959. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Friedberg F, Sohl S. Longitudinal change in chronic fatigue syndrome: What home-based assessments reveal. J Behav Med. 2009;32:209–218. doi: 10.1007/s10865-008-9189-9. [DOI] [PubMed] [Google Scholar]
- 12.Reeves WC, Lloyd A, Vernon SD, Klimas N, Jason LA, Bleijenberg G, Evengard B, White PD, Nisenbaum R, Unger ER. International Chronic Fatigue Syndrome Study Group. Identification of ambiguities in the 1994 chronic fatigue syndrome research case definition and recommendations for resolution. BMC Health Services Research. 2003;3(1):25. doi: 10.1186/1472-6963-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) New York State Psychiatric Institute; New York, NY: 2001. [Google Scholar]
- 14.Jason LA, Torres-Harding S, Friedberg F, Corradi K, Njoku MG, Donalek J, Reynolds N, Brown M, Weitner BB, Rademaker A, Papernik M. Non-pharmacologic Interventions for CFS: A randomized trial. J Clin Psycholo Med S. 2007;14:275–296. [Google Scholar]
- 15.Friedberg F. Fibromyalgia and chronic fatigue syndrome: Seven proven steps to less pain and more energy. New Harbinger; Oakland, CA: 2006. [Google Scholar]
- 16.Paterson C, Dieppe P. Characteristic and incidental (placebo) effects in complex interventions such as acupuncture. BMJ. 2005;330:1202–1205. doi: 10.1136/bmj.330.7501.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greco CM, Rudy TE, Manzi S. Effects of a stress-reduction program on psychological function, pain, and physical function of systemic lupus erythematosus patients: a randomized controlled trial. Arthritis Rheum. 2004;51:625–34. doi: 10.1002/art.20533. [DOI] [PubMed] [Google Scholar]
- 18.Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psy. 1972;3:257–260. [Google Scholar]
- 19.Kelley JE, Lumley MA, Leisen JCC. Health effects of emotional disclosure in rheumatoid arthritis patients. Health Psychol. 1997;16:331–340. doi: 10.1037//0278-6133.16.4.331. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg MA, Wortman CB, Stone AA. Emotional expression and physical health: Revising traumatic memories or fostering self-regulation? J Pers Soc Psychol. 1996;71:588–602. doi: 10.1037//0022-3514.71.3.588. [DOI] [PubMed] [Google Scholar]
- 21.Pennebaker JW, Kiecolt-Glaser JK, Glaser R. Disclosure of traumas and immune function: Health implications for psychotherapy. J Consult Clin Psych. 1988;56:239–245. doi: 10.1037//0022-006x.56.2.239. [DOI] [PubMed] [Google Scholar]
- 22.Hoekstra J, de Vos R, van Duijn NP, Schade E, Bindels PJ. Using the symptom monitor in a randomized controlled trial: the effect on symptom prevalence and severity. J Pain Symptom Manag. 2006;31(1):22–30. doi: 10.1016/j.jpainsymman.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol-Chicago. 1989;46:1121–23. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 24.Taylor RR, Jason LA, Torres A. Fatigue rating scales: An empirical comparison. Psychol Med. 2000;30:849–856. doi: 10.1017/s0033291799002500. [DOI] [PubMed] [Google Scholar]
- 25.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 26.McHorney CA, Ware JE, Lu AW, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36): III. Tests of data quality, scaling assumptions, and reliability across diverse patient groups. Med Care. 1994;32:40–66. doi: 10.1097/00005650-199401000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996. [Google Scholar]
- 28.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67:588–97. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 29.Hewitt PL, Norton GR. The Beck Anxiety Inventory: A psychometric analysis. Psychol Assessment. 1993;5:408–412. [Google Scholar]
- 30.Steer RA, Clark DA, Beck AT, Ranieri WF. Common and specific dimensions of self-reported anxiety and depression: a replication. J Abnorm Psychol. 1995;104:542–545. doi: 10.1037//0021-843x.104.3.542. [DOI] [PubMed] [Google Scholar]
- 31.Powell P, Bentall RP, Nye FJ, Edwards RH. Randomised controlled trial of patient education to encourage graded exercise in chronic fatigue syndrome. BMJ. 2001;322:387–390. doi: 10.1136/bmj.322.7283.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubin DB. Inference and missing data. Biometrika. 1976;63:581–590. [Google Scholar]
- 33.Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst's perspective. Multivar Behav Res. 1998;33(4):545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- 34.Ogles BM, Lunnen KM, Bonesteel K. Clinical significance: History, application and current practice. Clin Psychol Rev. 2001;21:421–446. doi: 10.1016/s0272-7358(99)00058-6. [DOI] [PubMed] [Google Scholar]
- 35.Speer DC. Clinically significant change: Jacobson and Truax (1991) revisited. J Consult Clin Psych. 1992;60(3):402–408. doi: 10.1037//0022-006x.60.3.402. doi:10.1037/0022-006X.60.3.402. [DOI] [PubMed] [Google Scholar]
- 36.Roelcke U, Kappos L, Lechner-Scott J, Brunnschweiler H, Huber S, Ammann W, Plohmann A, Dellas S, Maquire J, Radu EW, Steck A, Leenders KL. Reduced glucose metabolism in the frontal cortex and basal ganglia of multiple sclerosis patients with fatigue: A 18F-fluorodeoxyglucose positron emission tomography study. Neurology. 1997;48:1566–1571. doi: 10.1212/wnl.48.6.1566. [DOI] [PubMed] [Google Scholar]
- 37.Bakshi R, Miletich RS, Henschel K, Shaikh ZA, Janardhan V, Wasay M, Stengel LM, Ekes R, Kinkel MT, Kinkel PR. Fatigue in multiple sclerosis: Cross-sectional correlation with brain MRI findings in 71 patients. Neurology. 1999;53:1151–1153. doi: 10.1212/wnl.53.5.1151. [DOI] [PubMed] [Google Scholar]
- 38.Price JR, Mitchell E, Tidy E, Hunot V. Cognitive behaviour therapy for chronic fatigue syndrome in adults. Cochrane Database of Systematic Reviews. 2008;(3):CD001027. doi: 10.1002/14651858.CD001027.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White PD, Goldsmith KA, Johnson AL, Potts L, Walwyn R, DeCesare JC, Baber HL, Burgess M, Clark LV, Cox DL, Bavinton J, Angus BJ, Murphy G, Murphy M, O'Dowd H, Wilks D, McCrone P, Chalder T, Shapre M. Comparison of adaptive pacing therapy, cognitive behaviour therapy, graded exercise, and specialist medical care for chronic fatigue syndrome (PACE): a randomized trail. Lancet. 2011;377(9768):823–836. doi: 10.1016/S0140-6736(11)60096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nijrolder I, van der Horst J, van der Windt D. Prognosis of fatigue: A systematic review. J Psychosom Res. 2008;64(4):335–349. doi: 10.1016/j.jpsychores.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 41.Cairns R, Hotopf M. A systematic review describing the prognosis of chronic fatigue syndrome. Occu Med-Oxford. 2005;55(1):20–31. doi: 10.1093/occmed/kqi013. [DOI] [PubMed] [Google Scholar]
- 42.Price JR, Cooper J. Cognitive behaviour therapy for adults with chronic fatigue syndrome. Cochrane Database of Systematic Reviews. 2008;(2):CD001027. doi: 10.1002/14651858.CD001027. [DOI] [PubMed] [Google Scholar]