Abstract
Background
The AJCC/UICC staging system for gastric cancer incorporates the absolute number of metastatic lymph nodes (N status) and is optimally used when ≥15 nodes are examined. The ratio of metastatic to examined nodes (N ratio) is an effective prognostic tool, but has not been examined in Western patients undergoing primarily D1 lymphadenectomy.
Methods
257 patients with gastric adenocarcinoma who underwent gastric resection between 1995 and 2005 at our institution were examined. Novel N ratio intervals were determined using the best cutoff approach (Nr0: N ratio=0 and ≥15 nodes examined; Nr1: 0 ≤ N ratio ≤ 0.3, Nr2: 0.3 < N ratio ≤ 0.7, and Nr3: N ratio > 0.7). Overall survival was examined according to N status and N ratio.
Results
83% of patients underwent D1 lymphadenectomy with a median of 14 lymph nodes examined. Overall survival stratified by N status was significantly different in patients with <15 nodes examined compared to those with ≥15 nodes examined. When we stratified N ratio intervals, there was no significant difference in overall survival in patients with <15 vs. ≥15 nodes examined. On multivariate analysis, N ratio but not N status was retained as an independent prognostic factor.
Conclusions
The use of N status for staging patients undergoing primarily D1 lymphadenectomy results in significant stage migration due to varying numbers of nodes examined. Use of N ratio reduces stage migration and may be a more reliable method of staging such patients.
INTRODUCTION
Gastric adenocarcinoma is a relatively uncommon solid malignancy in the United States with an estimated 21,130 newly diagnosed cases and 10,620 deaths in 2009.1 However, gastric adenocarcinoma is the second leading cause of cancer death worldwide, and in countries such as Japan and Korea, the age-adjusted incidences are up to ten times that of the United States.2;3 In addition to the worldwide variations in incidence, there are also significant differences in the extent of lymphadenectomy performed for gastric adenocarcinoma. To broadly generalize, more extensive lymphadenectomies (D2 or greater) are often performed in Japan and Korea and less extensive lymphadenectomies (D1 or less) are often performed in the United States and some other Western countries. Thus, the average number of lymph nodes examined following gastrectomy and lymphadenectomy for gastric adenocarcinoma is significantly lower in the United States than in Japan and Korea. In an analysis of United States Surveillance, Epidemiology, and End Results (SEER) data from 1973–1999, the median number of lymph nodes examined was 8 and less than 25% of patients had ≥15 nodes examined.4 The regional differences in lymph node examination may also be due to variations in pathological analysis.
Currently, the most commonly used staging system for gastric adenocarcinoma is the 2002 American Joint Committee on Cancer/Union Internationale Contre le Cancer (AJCC/UICC) system, which stages patients based on characteristics of the primary tumor (T status), regional lymph nodes (N status), and distant metastases (M status).5 The N status is based on the absolute number of positive nodes. Patients with no regional lymph node metastases are designated as N0, 1–6 regional lymph node metastases as N1, 7–15 as N2, and ≥15 as N3. The AJCC/UICC staging system further states that “a regional lymphadenectomy specimen will ordinarily contain at least 15 lymph nodes”. Stage migration refers to instances in which evaluation of an inadequate number of lymph nodes leads to understaging and subsequent underestimation of disease severity.6 This phenomenon clearly occurs more commonly in regions where less extensive lymphadenectomies are performed.7
Lymph node ratio (N ratio) is defined as the number of positive lymph nodes divided by the number of examined lymph nodes.8 Several studies have analyzed the prognostic role of N ratio in the staging of gastric cancer. These studies have found that metastatic lymph node ratio can be an independent prognostic factor 9–15 and even better stratify patient survival than AJCC/UICC N stage.9;10;16;17 However, most of these studies have either excluded patients with <15 lymph nodes examined,10 focused on patients with D2 lymphadenectomy,15;18 been based in Asian institutions10;15;19, or been multi-institution series.11;20 We hypothesized that N ratio may attenuate stage migration based on N status resulting from varying numbers of examined lymph nodes, especially in patients undergoing less than D2 lymphadenectomy, and analyzed this hypothesis in 257 patients undergoing primarily D1 lymphadenectomy at a single Western institution.
PATIENTS AND METHODS
In this IRB-approved retrospective study, data was collected from the medical records of 802 patients treated for gastric cancer at our institution from January 1995 to December 2005. Of these 802 patients, the following patients were excluded: patients without histologically confirmed gastric adenocarcinoma (n=155), patients who did not undergo gastric resection (n=293), patients who had Siewert type I lesions (n=93),21 and patients who had non-invasive (<T1) disease (n=4). A total of 545 patients were excluded, leaving 257 patients for analysis.
All 257 patients underwent gastric resection. Patient demographic and clinical data, details of the surgical procedure, pathology, and follow-up information were collected from patient records and the MGH Cancer Registry. Distant metastasis was defined to include nodal metastases beyond N2 lymph node stations as defined by the Japanese Research Society for Gastric Cancer,22 peritoneal metastases, malignant ascites, and metastases to other organs. Grossly positive margin was defined as gross tumor present at the resection specimen margin. Microscopically positive margin was defined as microscopic tumor present at the resection specimen margin.
D1 lymphadenectomy technique was based on surgeon preference and training. For upper tumors, lymph node stations 1, 2, 3, 4sa, and 4sb were resected; for middle tumors, stations 1, 3, 4sb, 5, and 6 were resected, and for lower tumors, stations 3, 4d, 5, and 6 were resected 23.
The median follow-up for all patients was 19 months. Six patients were lost to follow-up. In the survivors, the median follow-up was 56.9 months. In non-survivors, the median follow-up was 14.2 months. Follow-up time was calculated from the date of definitive gastric resection.
Node ratio (N ratio) was calculated by dividing the number of metastatic lymph nodes by the total number of lymph nodes examined.8 Novel N ratio intervals were determined using the best cutoff approach after evaluating the Martingale residuals to ensure entry of the proper functional form of the variable into the model.24 The N ratio intervals identified were the following: Nr1: 0 ≤ node ratio ≤ 0.3, Nr2: 0.3 < node ratio ≤ 0.7, and Nr3: node ratio > 0.7. A unique category, Nr0, was designated for patients with no regional lymph node metastases who also had ≥15 lymph nodes examined. Patients with no regional lymph nodes metastases who had <15 nodes examined were categorized as Nr1.
The statistical analysis was performed using the SAS/JMP software (SAS Institute Inc., Cary, NC). The distribution of tumor and treatment specific factors between Groups 1 and 2 was evaluated using Fisher’s exact test or Pearson χ2 test for categorical variables and Student t-test for continuous variables. Overall survival rates were determined using the Kaplan Meier method25 and the log-rank test26 was used to evaluate differences between groups. Univariate analysis of overall survival by clinicopathological factors was performed using the Cox proportional hazards method.27 The following pathologic variables were used to assess for prognostic factors: (1) age as a continuous variable, (2) male gender, (3) white race, (4) Asian race, (5) history of smoking, (6) tumor size, (7) tumor level of invasion, (8) number of nodes examined (<15 or ≥ 15), (9) 2002 AJCC/UICC N status (N0, N1, N2, N3), (10) metastatic lymph node ratio, (11) presence of distant metastasis at time of surgery, (12) 2002 AJCC overall stage (I, II, III, IV), (13) tumor location, (14) Borrmann classification, (15) lymphovascular (small vessel) invasion, (16) venous (large vessel) invasion, (17) perineural invasion, (18) margin of resection (negative, positive), (19) poorly differentiated tumor (poorly/undifferentiated tumor vs. well/moderately differentiated tumor), (20) chemotherapy, and (21) radiation therapy.
Multivariate survival analysis was performed with the Cox proportional hazards method using variables found to have a p value <0.05 on the univariate analysis. Small vessel invasion, large vessel invasion and perineural invasion were excluded from the multivariate model due to a high percentage of missing data. AJCC/UICC overall stage was excluded as it would be collinear with T, N and M status, which were included in the model.
RESULTS
This study examined 257 consecutive patients with gastric adenocarcinoma who underwent surgical resection at our institution between January 1995 and December 2005. The age range was between 23 to 93 years old, with a median age of 71. The group was predominantly Caucasian (84%) (Table 1). The most common symptoms at presentation were gastrointestinal bleeding or anemia, abdominal pain/discomfort, nausea, vomiting, and weight loss.
Table 1.
Patient Characteristics
| Characteristic | n | % |
|---|---|---|
|
| ||
| Male | 153 | 59.5 |
|
| ||
| Race | ||
| White | 216 | 84.0 |
| Hispanic | 10 | 3.9 |
| Black | 8 | 3.1 |
| Asian | 16 | 6.2 |
| Unknown | 7 | 2.7 |
|
| ||
| Symptoms at presentation | 240 | 93.4 |
| Gastrointestinal bleeding or anemia | 100 | 38.9 |
| Abdominal pain or discomfort | 97 | 37.7 |
| Nausea, anorexia, and/or weight loss | 83 | 32.2 |
| Dyspepsia or heartburn | 20 | 7.8 |
| Dysphagia | 18 | 7.0 |
|
| ||
| Prior gastric resection | 19 | 7.4 |
|
| ||
| History of chemotherapy or radiation | 18 | 7.0 |
|
| ||
| History of smoking | 121 | 47.0 |
The surgical treatment of patients is summarized in Table 2. About one-half of resections were distal or subtotal gastrectomies, and the remainder were proximal gastrectomies or esophagogastrectomies (21.4%) and total gastrectomies (28.8%). Reconstruction following gastrectomy consisted primarily of Billroth II gastrojejunostomy after subtotal gastrectomy, Roux-en-Y esophagojejunostomy after total gastrectomy, and esophagogastric anastomosis after proximal gastrectomy or esophagogastrectomy. A few patients underwent distal pancreatectomy (5.4%), splenectomy (12.1%), or resection of other adjacent organs (10.5%). Following gastric resection, 16.7% of patients had a positive microscopic margins and 1.2% of patients had a grossly positive margin. Median length of stay was 9 days, and 10 patients (3.9%) died during the first 30 days following surgery. Forty-one percent of patients received adjuvant chemotherapy and 31% received adjuvant radiation therapy. Of these patients, 5 patients received neoadjvuant chemotherapy and 14 patients underwent neoadjuvant chemoradiation. The median number of lymph nodes examined was 14, and there were no significant differences in terms of analyzed treatment variables in patients who had <15 nodes examined compared to patients who had ≥15 lymph nodes examined.
Table 2.
Surgical Treatment
| All patients (n=257) | Patients with <15 nodes examined (n=129) | Patients with ≥ 15 nodesexamined (n=128) | p – value <15 vs. ≥15 nodes examined | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n or median | % or range | n or median | % or range | n or median | % or range | ||
|
| |||||||
| Resection | 0.9929 | ||||||
| Distal/subtotal gastrectomy | 128 | 49.8 | 64 | 49.6 | 64 | 50.0 | |
| Proximal /esophagogastrectomy | 55 | 21.4 | 28 | 21.7 | 27 | 21.1 | |
| Total gastrectomy | 74 | 28.8 | 37 | 28.7 | 37 | 28.9 | |
|
| |||||||
| Reconstruction | 1.0000 | ||||||
| Billroth I | 3 | 1.2 | 2 | 1.6 | 1 | 0.8 | |
| Billroth II | 113 | 44.0 | 57 | 44.2 | 56 | 43.8 | |
| Esophagogastrostomy | 50 | 19.5 | 25 | 19.4 | 25 | 19.5 | |
| Roux-en-Y | 91 | 35.4 | 45 | 34.9 | 46 | 35.9 | |
|
| |||||||
| Distal pancreatectomy | 14 | 5.4 | 6 | 4.7 | 8 | 6.3 | 0.5962 |
|
| |||||||
| Splenectomy | 31 | 12.1 | 14 | 10.9 | 17 | 13.3 | 0.5715 |
|
| |||||||
| Other adjacent organs resection | 27 | 10.5 | 16 | 12.4 | 11 | 8.6 | 0.4164 |
|
| |||||||
| Resection margin | 0.5896 | ||||||
| No gross or microscopic disease | 211 | 82.1 | 108 | 83.7 | 103 | 80.5 | |
| Microscopic disease | 43 | 16.7 | 19 | 14.7 | 24 | 18.8 | |
| Gross disease | 3 | 1.2 | 2 | 1.6 | 1 | 0.8 | |
|
| |||||||
| 30 day mortality | 10 | 3.9 | 6 | 4.7 | 4 | 3.1 | 0.5270 |
|
| |||||||
| Length of stay (days) | 9 | 3–156 | 9d | 3–156 | 9d | 4–109 | 0.6143 |
|
| |||||||
| Chemotherapy | 104 | 40.5 | 45 | 34.9 | 59 | 46.1 | 0.0758 |
|
| |||||||
| Radiation therapy | 81 | 31.5 | 37 | 28.7 | 44 | 34.4 | 0.3493 |
The pathological analysis of surgical specimens and pathological staging of patients are summarized in Tables 3 and 4. Tumors were roughly equally distributed between the true gastroesophageal (GE) junction/cardia, body/fundus, and antrum. Nearly two-thirds of tumors were poorly or undifferentiated, and over half of tumor were penetrating the serosa or invaded adjacent organs. Overall, 1,405 metastatic lymph nodes were identified after the examination of 3,939 lymph nodes. As noted earlier, the median number of nodes examined was 14 with a range of 0 to 50. About two-thirds of patients had positive lymph nodes. In comparing the tumor characteristics of patients with <15 nodes examined and patients with ≥15 nodes examined, there were statistically significant differences in tumor size, lymphovascular (small vessel) invasion, venous (large vessel) invasion (Table 3), distribution of T stage, distribution of N stage, and distribution of TNM stage (Table 4). Based on the factors which were significantly different between patients, with <15 and ≥15 nodes examined, the patients with ≥15 nodes were generally a poorer prognosis group.
Table 3.
Pathological Characteristics
| All patients (n=257) | Patients with < 15 nodes examined (n=129) | Patients with ≥ 15 nodes examined (n=128) | p – value <15 vs. ≥15 nodes examined | ||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n or median | % or range | n or median | % or range | n or median | % or range | ||
|
| |||||||
| Tumor size | 4.5cm | <0.5cm–20.0cm | 3.5cm | <0.5cm–20.0cm | 5.5cm | <0.5cm–16.0cm | 0.0006 |
|
| |||||||
| Tumor location | 0.8898 | ||||||
| GE junction/cardia | 69 | 26.8 | 33 | 25.6 | 36 | 28.1 | |
| Body/fundus | 83 | 32.3 | 44 | 34.1 | 39 | 30.5 | |
| Antrum | 100 | 38.9 | 50 | 38.8 | 50 | 39.1 | |
| Diffuse/multifocal | 5 | 1.9 | 2 | 1.6 | 3 | 2.3 | |
|
| |||||||
| Tumor grade | 0.173 | ||||||
| Well differentiated | 7 | 2.7 | 4 | 3.1 | 3 | 2.3 | |
| Moderately differentiated | 77 | 30 | 44 | 34.1 | 33 | 25.8 | |
| Poorly differentiated | 147 | 57.2 | 70 | 54.3 | 77 | 60.2 | |
| Undifferentiated | 17 | 6.6 | 5 | 3.9 | 12 | 9.4 | |
| Unknown | 9 | 3.5 | 6 | 4.7 | 3 | 2.3 | |
|
| |||||||
| Lauren classification | 1 | ||||||
| Intestinal | 55 | 21.4 | 27 | 20.9 | 28 | 21.9 | |
| Diffuse | 33 | 12.8 | 16 | 12.4 | 17 | 13.3 | |
| Mixed | 3 | 1.2 | 1 | 0.8 | 2 | 1.6 | |
| Unknown | 167 | 64.6 | 85 | 65.9 | 81 | 63.3 | |
|
| |||||||
| Borrmann classification | 0.6061 | ||||||
| Polypoid | 36 | 14 | 19 | 14.7 | 17 | 13.3 | |
| Fungating | 37 | 14.4 | 15 | 11.6 | 22 | 17.2 | |
| Ulcerated | 135 | 52.3 | 70 | 54.3 | 65 | 50.8 | |
| Infiltrative | 35 | 13.6 | 19 | 14.7 | 16 | 12.5 | |
| Unknown | 14 | 5.4 | 6 | 4.7 | 8 | 6.3 | |
|
| |||||||
| Lymphovascular (small vessel) invasion | 140/201 | 69.7 | 56/98 | 57.1 | 84/103 | 81.6 | 0.0002 |
|
| |||||||
| Venous (large vessel) invasion | 71/160 | 44.4 | 23/77 | 29.9 | 48/83 | 57.8 | 0.0005 |
|
| |||||||
| Perineural invasion | 104/164 | 63.4 | 46/74 | 62.2 | 58/90 | 64.4 | 0.8707 |
Table 4.
Pathological Staging
| All patients (n=257) | Group 1 Patients with nodes < 15 (n=129) | Group 2 Patients with nodes ≥ 15 (n=128) | p – value <15 vs. >15 nodes examined |
||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n or median | % or range | n or median | % or range | n or median | % or range | ||
|
| |||||||
| AJCC/UICC T status | 0.0148 | ||||||
| T1 | 40 | 15.6 | 29 | 22.5 | 11 | 8.6 | |
| T2 | 71 | 27.6 | 30 | 23.3 | 41 | 32 | |
| T3 | 119 | 46.3 | 58 | 45 | 61 | 47.7 | |
| T4 | 26 | 10.1 | 11 | 8.5 | 15 | 11.7 | |
| Unavailable | 1 | 0.4 | 1 | 0.8 | 0 | 0 | |
|
| |||||||
| AJCC/UICC N status | <0.0001 | ||||||
| N0 | 85 | 33.1 | 58 | 45.0 | 27 | 21.1 | |
| N1 | 90 | 35.0 | 56 | 43.4 | 34 | 26.6 | |
| N2 | 58 | 22.6 | 15 | 11.6 | 43 | 33.6 | |
| N3 | 24 | 9.3 | 0 | 0 | 24 | 18.8 | |
|
| |||||||
| N ratio (mean) | 0.21 | 0–1.0 | 0.08 | 0–1.0 | 0.35 | 0–1.0 | 0.0036 |
|
| |||||||
| Node ratio intervals | <0.0001 | ||||||
| Nr 0 | 28 | 10.9 | N/A | N/A | 28 | 21.9 | |
| Nr 1 | 111 | 43.2 | 80 | 62 | 31 | 24.2 | |
| Nr 2 | 62 | 24.1 | 24 | 18.6 | 38 | 29.7 | |
| Nr 3 | 49 | 19.1 | 18 | 14 | 31 | 24.2 | |
| Unavailable | 7 | 2.7 | 7 | 5.4 | 0 | 0 | |
|
| |||||||
| AJCC/UICC M status | 0.532 | ||||||
| MO | 207 | 80.5 | 106 | 82.2 | 101 | 78.9 | |
| M1 | 50 | 19.5 | 23 | 17.8 | 27 | 21.1 | |
|
| |||||||
| AJCC TNM Stage | 0.0234 | ||||||
| I | 67 | 26.1 | 42 | 32.6 | 25 | 19.5 | |
| II | 44 | 17.1 | 27 | 20.9 | 17 | 13.3 | |
| III | 75 | 29.2 | 32 | 24.8 | 43 | 33.6 | |
| IV | 70 | 27.2 | 27 | 20.9 | 43 | 33.6 | |
| Unavailable | 1 | 0.4 | 1 | 0.8 | 0 | 0 | |
As described in the Methods, node ratio (N ratio) was calculated by dividing the number of metastatic lymph nodes by the total number of nodes examined, and the following N ratio intervals were used for stratification: Nr1: 0 ≤ node ratio ≤ 0.3, Nr2: 0.3 < node ratio ≤ 0.7, and Nr3: node ratio > 0.7. Patients with no regional lymph node metastases and ≥15 lymph nodes examined were given a designation of Nr0. There was a significant difference in median N ratio between patients with <15 nodes examined and patients with ≥15 nodes examined (p=0.0036) (Table 4).
The median overall survival was 24.2 months, and the estimated 5-year overall survival rate was 29.3% (95% CI of 23.8% to 36.0%). A univariate analysis of clinicopathological factors was performed (Table 5). A history of smoking, tumor size, AJCC/UICC T status, N status, M status, overall stage, N ratio, lymphovascular invasion, venous invasion, perineural invasion, positive margin, and poorly or undifferentiated tumor grade were found to be significant prognostic factors. Tumors located in the cardia/GE junction and diffusely throughout the stomach and tumors classified as infiltrative based on Borrmann classification also had significantly worse survival. On multivariate analysis, M status, positive margin, and tumors located in the cardia or diffusely throughout the stomach remained significant factors (Table 6). Also on multivariate analysis, AJCC/UICC N status was not significant while N ratio was highly significant.
Table 5.
Univariate Analysis of Prognostic Factors
| Variable | p – value | Hazard ratio (95% CI) |
|---|---|---|
|
| ||
| Age as a continuous variable | 0.0847 | 1.011 (0.998–1.024) |
|
| ||
| Male | 0.5731 | 0.916 (0.677–1.241) |
|
| ||
| White | 0.0687 | 1.574 (0.966–2.567) |
|
| ||
| Asian | 0.1657 | 0.605 (0.298–1.231) |
|
| ||
| History of smoking | 0.0120 | 0.683 (0.507–0.920) |
|
| ||
| Tumor size (cm) | 0.0002 | 1.088 (1.040–1.138) |
|
| ||
| AJCC/UICC T status | <.0001 | 1.811 (1.486–2.207) |
|
| ||
| AJCC/UICC N status | <.0001 | 1.979 (1.683–2.327) |
|
| ||
| AJCC/UICC M status | <.0001 | 3.319 (2.335–4.719) |
|
| ||
| Overall AJCC/UICC stage | <.0001 | 1.952 (1.672–2.278) |
|
| ||
| N ratio | <.0001 | 10.061 (6.286–16.103) |
|
| ||
| Tumor location compared to Antrum | ||
| GE junction/Cardia | 0.0052 | 1.684 (1.168–2.427) |
| Fundus/body | 0.1855 | 1.276 (0.889–1.832) |
| Diffuse | 0.0022 | 4.998 (1.782–14.016) |
|
| ||
| Borrmann classification compared to Polypoid | ||
| Fungating | 0.9864 | 1.005 (0.556–1.817) |
| Ulcerated | 0.2678 | 1.307 (0.814–2.099) |
| Infiltrative | 0.0307 | 1.878 (1.060–3.327) |
|
| ||
| Lymphovascular invasion | <.0001 | 2.666 (1.710–4.156) |
|
| ||
| Venous invasion | <.0001 | 2.730 (1.807–4.122) |
|
| ||
| Perineural invasion | 0.0002 | 2.296 (1.482–3.558) |
|
| ||
| Positive margin | <.0001 | 3.211 (2.234–4.615) |
|
| ||
| 15 or greater nodes examined | 0.4941 | 1.109 (0.824–1.492) |
|
| ||
| Poorly differentiated or undifferentiated tumor | 0.0125 | 1.515 (1.094–2.100) |
|
| ||
| Chemotherapy | 0.9894 | 0.998 (0.740–1.346) |
|
| ||
| Radiation therapy | 0.0655 | 0.741 (0.539–1.019) |
Table 6.
Multivariate Analysis of Prognostic Factors
| Variable | p-value | Hazard ratio (95% CI) |
|---|---|---|
| Poorly differentiated or undifferentiated | 0.7806 | 1.052 (0.737–1.501) |
| History of smoking | 0.4596 | 0.876 (0.618–1.243) |
| AJCC/UICC N status | 0.3861 | 1.146 (0.842–1.560) |
| Tumor size (cm) | 0.1111 | 0.952 (0.897–1.011) |
| AJCC/UICC T status | 0.0563 | 1.246 (0.994–1.562) |
| AJCC/UICC M status | 0.0093 | 1.831 (1.161–2.890) |
| Positive margin | 0.0080 | 1.862 (1.176–2.946) |
| N ratio | 0.0061 | 3.705 (1.452–9.454) |
| Tumor located in GE junction/cardia or diffuse | 0.0035 | 1.757 (1.203–2.564) |
There was no statistically significant difference in overall survival between patients with <15 nodes examined and patients with ≥15 nodes examined (p = 0.4947). Overall survival by AJCC/UICC N status is shown in Figure 1A. In comparing overall survival of patients with <15 nodes examined and ≥15 nodes examined, AJCC/UICC N status yielded significantly different survival curves in these two subgroups (Figure 1B). N0 patients with <15 nodes examined had a median survival of 56.8 months with a 5-year survival of 45.8%, and N0 patients with ≥15 nodes examined did not reach median survival and had a 5-year survival of 74.2%. This 28% absolute difference in 5-year survival was statistically significant (p = 0.0309). N1 patients with <15 nodes examined had a median survival of 17.7 months with a 5-year survival of 19.2%, and N1 patients with ≥15 nodes examined had a median survival of 33.3 months with a 5-year survival of 35.5%. This 18.4% absolute difference in 5-year survival was also statistically significant (p = 0.0347). There was no significant difference in overall survival in N2 patients with <15 nodes compared to the N2 patients with ≥15 nodes examined. By definition, there were no N3 patients in Group 1 since these patients had <15 nodes examined. The median survival of N3 patients in with ≥15 nodes examined was 6 months and the 5-year overall survival was 0%.
Figure 1.
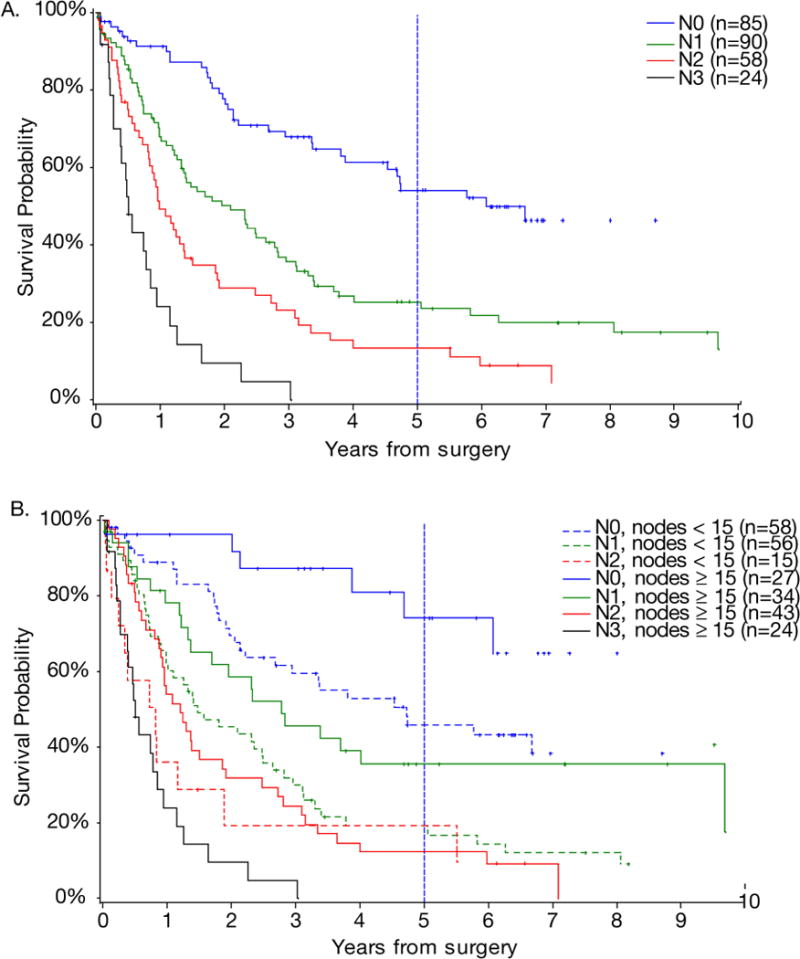
(A) Overall survival of patients based on 2002 AJCC/UICC N status. (B) Overall survival of patients with <15 nodes examined and ≥15 nodes examined based on 2002 AJCC/UICC N status.
Overall survival of all patients by N ratio intervals is shown in Figure 2A. In contrast to the above analysis using AJCC/UICC N status, overall survival stratified by N ratio intervals resulted in similar survival curves regardless of the number of examined nodes (Figures 2B). The Nr0 group had a 75.5% 5-year survival. The Nr1 patients with <15 nodes examined had a median survival of 40.8 months and 5-year survival of 42.2% compared to the Nr1 patients with ≥15 nodes examined who had a median survival of 37.9 months and 35.7% 5-year survival (p= 0.6621). The Nr2 patients with <15 nodes examined had a median survival of 13.9 months and 5-year survival of 4.8%, and Nr2 patients with ≥15 nodes examined had a median survival of 14.5 months and 13.5% 5-year survival (p=0.4706). Finally, the Nr3 patients with <15 nodes and ≥15 nodes examined had a median survivals of 8.6 months and 9.2 months, respectively (p=0.6932).
Figure 2.
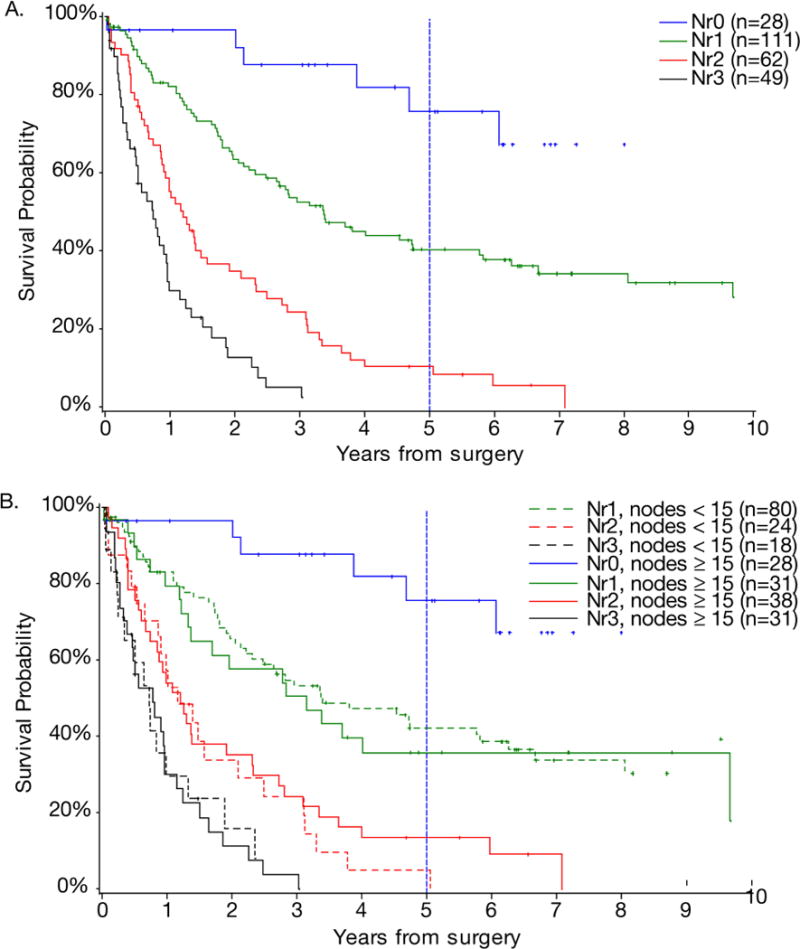
(A) Overall survival of patients based on N ratio intervals. (B) Overall survival of patients with <15 nodes examined and ≥15 nodes examined based on N ratio intervals.
DISCUSSION
In this study, we examined 257 patients who underwent surgical resection involving primarily D1 lymphadenectomy for gastric adenocarcinoma at a single institution and investigated the impact of the number of examined lymph nodes in influencing the accuracy of various nodal staging systems. There was no significant difference in overall survival between patients with <15 lymph nodes examined and patients with ≥15 lymph nodes examined. However, examination of the survival curves for these two groups based on AJCC/UICC N status revealed significant differences in overall survival based on N status, with overall survival significantly lower in N0 and N1 patients with <15 nodes examined compared to patients with ≥15 nodes examined. N ratio is the number of positive lymph nodes divided by the number of examined lymph nodes. Based on analysis using the best cutoff approach, we developed unique N ratio intervals (Nr1: 0 ≤ node ratio ≤ 0.3, Nr2: 0.3 < node ratio ≤ 0.7, and Nr3: node ratio > 0.7) and defined a unique category, Nr0, for patients with no regional lymph node metastases who also had ≥15 lymph nodes examined. This N ratio classification produced similar survival curves for patients regardless of the number of examined lymph nodes. Thus we conclude that use of N ratio using these novel intervals rather than AJCCC/UICC N status ameliorates the understaging that occurs in patients with <15 lymph nodes examined.
Staging is a critical component of cancer care. The accurate staging of cancer patients conveys the extent of disease, allows more specific means of determining risk of recurrence and survival, impacts significantly on therapy decisions, and allows comparison of patient cohorts across different institutions and countries. The most commonly used staging system for gastric adenocarcinoma is the AJCC/UICC staging system, which states that “a regional lymphadenectomy specimen will ordinarily contain at least 15 lymph nodes” and bases N status on the number of positive nodes.5 The AJCC/UICC staging system works quite well when analyzing patients who undergo extensive lymphadenectomies (D2 or greater).6 In contrast, the AJCC/UICC staging less accurately predicts overall survival when examining patients undergoing less extensive lymphadenectomies or when comparing patients undergoing varying levels of lymphadenectomy. For example, overall survival of patients in the SEER database, with an average of 8 examined lymph nodes is significantly inferior, stage for stage, than in a series of patients from Memorial Sloan-Kettering Cancer Center where about 81% of patients underwent D2 lymphadenectomy and a median of 22 lymph nodes were examined.4;28 However, factors other than extent of lymphadenectomy could explain the stage for stage differences in survival of patients treated at a single center compared to those treated at multiple institutions.
In 1990, Okusa et al. noted that N ratio as well as number of positive lymph nodes were both prognostic factors for overall survival in gastric cancer.8 Siewert et al confirmed this finding in Western patients treated by the German Gastric Carcinoma Study Group.20 In the largest series of over 2000 patients in China who underwent D2 or D3 lymphadenectomy, a median of 20 lymph nodes were examined and N ratio was found to better stratify survival that AJC/UICC N status or JGCA N status.29 Similar to prior studies, this current study confirms that N ratio is an independent prognostic factor for survival and may better stratify patient survival than AJCC/UICC N status. Furthermore, this study has several unique findings. This is the first study to examine N ratio in a Western cohort of patients undergoing primarily D1 lymphadenectomy. More importantly, a unique N ratio classification was developed in this study that essentially eliminated the stage migration seen in patients with <15 lymph nodes examined.
N ratio is likely better than AJCC/UICC N status in staging patients with <15 examined lymph nodes, but patients with <15 examined lymph nodes are still not as well staged as patients with ≥15 nodes examined. This concept is exemplified in the subgroup of lymph node negative patients in this study. The survival of lymph node negative patients with ≥15 nodes examined was significantly better than lymph node negative patients with <15 nodes examined. Analysis of more lymph nodes in the group of patients with <15 nodes examined, either via increased lymph node dissection or increased pathological analysis, would likely have identified some patients who were in fact lymph node positive. Thus, we are not implying that staging via less extensive lymphadenectomies can be completely corrected by alterations in N classification. These data merely support the use of N ratio rather than N status in the staging of patients undergoing D1 lymphadenectomy.
A D1 lymphadenectomy is the minimum lymphadenectomy recommended by most gastric cancer specialists except for low risk, T1 tumors, and many specialists advocate for D2 lymphadenectomy or greater 23. In the United Kingdom Medical Research Council (MRC) and Dutch randomized trials, the median number of lymph nodes examined in the D1 groups was 13 and 17, respectively.30;31 In the United States, many gastric cancer patients undergo less than a D1 lymphadenectomy,32 and the median number of examined lymph nodes based on SEER data is 8.4 Patients undergoing less than a D1 lymphadenectomy will likely be understaged no matter what N classification is used and quite possibly may have decreased overall survival due to increased frequency of involved lymph nodes that are left undissected.33;34The patients examined in this study underwent surgery at our institution between 1995 and 2005. During this period, the standard lymphadenectomy performed for gastric adenocarcinoma was a D1 lymphadenectomy. Since 2005, one author (S.S.Y.) has performed primarily D2 lymphadenectomies, and we have recently reviewed the current literature on the extent of lymphadenectomy 23. There is clearly a learning curve for D2 lymphadenectomy 35. The Dutch and United Kingdom trials of D1 versus D2 lymphadenectomy demonstrated that when D2 lymphadenectomy is performed with excess morbidity and mortality, there is no survival benefit compared to D1 lymphadenectomy 36;37. Subsequent studies have demonstrated that distal pancreatectomy is not required to perform an adequate D2 lymphadenectomy and likely should only be performed when there is direct invasion of tumor 38;39. Splenectomy is not required for distal tumors, but the role of splenectomy for mid or proximal tumors is currently be investigated in a prospective, randomized trial in Japan 40. Deguili and colleagues in Italy have demonstrated that D2 lymphadenectomy can be taught to Western surgeons and result in operations with low morbidity and almost no mortality 41. When a more extensive lymphadenectomy is performed, there is clearly a benefit in terms of staging and possibly in terms of reduced loco-regional recurrence 23. If D2 lymphadenectomy is performed with low morbidity and mortality, there also may be a benefit in overall survival 42, but this potential benefit remains to be demonstrated by prospective, randomized trials in Western patients.
There are several limitations to this study. First, this is a retrospective review with the usual attendant sources of bias. Focusing on the discrete outcome of overall survival minimized this problem. The results of this study will need to be confirmed in other cohorts of patients undergoing D1 lymphadenectomy. Second, one may question whether there was any therapeutic effect attributable to resection of more lymph nodes. Patients in this study with ≥15 nodes examined had, in general, worse prognosis disease compared to patients with <15 nodes examined yet the overall survival of these two groups was equivalent. Third, the pathological assessment of lymph nodes involved simple hematoxylin and eosin staining and no enhanced techniques such as immunohistochemistry or reverse-transcription polymerase chain reaction (RT-PCR) were systematically used.43
In conclusion for patients undergoing primarily a D1 lymphadenectomy, N ratio is likely a better means of staging patients undergoing primarily D1 lymphadenectomy than absolute number of positive nodes (N status) because it reduces the stage migration that occurs when fewer lymph nodes are examined. The new 2010 AJCC/UICC staging system will stratify N status such that no regional lymph node metastases is N0, 1–2 regional lymph node metastases is N1, 3–6 regional lymph node metastases is N2, 7–15 regional lymph node metastases is N3a and >15 regional lymph node metastases is N3b. This new N staging was based on large databases from institutions performing primarily D2 lymphadenectomies. A comparison of this new N staging system to the N ratio intervals described in this study would be useful in patients undergoing primarily D1 lymphadenectomy, and perhaps the N staging system to determine overall TNM stage in future staging systems should vary according to extent of lymphadenectomy.
Acknowledgments
The authors would like to thank Carol Venuti in the Massachusetts General Hospital Cancer Data Registry.
Dr. Maduekwe was supported by the Scholars in Clinical Science Program at Harvard Medical School under National Institutes of Health Grant No. 1 KL2 RR025757-0 1, Harvard Clinical and Translational Science Center (KL1).
Footnotes
Disclosures: None
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Lee J, Demissie K, Lu SE, Rhoads GG. Cancer incidence among Korean-American immigrants in the United States and native Koreans in South Korea. Cancer Control. 2007;14:78–85. doi: 10.1177/107327480701400111. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Fact Sheet No 297, Cancer. 2009 Jan 2; http://www.who.int/mediacentre/factsheets/fs297/en/index.html.
- 4.Smith DD, Schwarz RR, Schwarz RE. Impact of total lymph node count on staging and survival after gastrectomy for gastric cancer: data from a large US-population database. J Clin Oncol. 2005;23:7114–7124. doi: 10.1200/JCO.2005.14.621. [DOI] [PubMed] [Google Scholar]
- 5.Greene FL, Page DL, Fleming ID, et al. AJCC Cancer Staging Manuel. 6. 2002. [Google Scholar]
- 6.Lee HK, Yang HK, Kim WH, Lee KU, Choe KJ, Kim JP. Influence of the number of lymph nodes examined on staging of gastric cancer. Br J Surg. 2001;88:1408–1412. doi: 10.1046/j.0007-1323.2001.01875.x. [DOI] [PubMed] [Google Scholar]
- 7.Bunt AM, Hermans J, Smit VT, van de Velde CJ, Fleuren GJ, Bruijn JA. Surgical/pathologic-stage migration confounds comparisons of gastric cancer survival rates between Japan and Western countries. J Clin Oncol. 1995;13:19–25. doi: 10.1200/JCO.1995.13.1.19. [DOI] [PubMed] [Google Scholar]
- 8.Okusa T, Nakane Y, Boku T, et al. Quantitative analysis of nodal involvement with respect to survival rate after curative gastrectomy for carcinoma. Surg Gynecol Obstet. 1990;170:488–494. [PubMed] [Google Scholar]
- 9.Celen O, Yildirim E, Berberoglu U. Prognostic impact of positive lymph node ratio in gastric carcinoma. J Surg Oncol. 2007;96:95–101. doi: 10.1002/jso.20797. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Nakane Y, Iiyama H, et al. The superiority of ratio-based lymph node staging in gastric carcinoma. Ann Surg Oncol. 2002;9:27–34. doi: 10.1245/aso.2002.9.1.27. [DOI] [PubMed] [Google Scholar]
- 11.Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–552. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nitti D, Marchet A, Olivieri M, et al. Ratio between metastatic and examined lymph nodes is an independent prognostic factor after D2 resection for gastric cancer: analysis of a large European monoinstitutional experience. Ann Surg Oncol. 2003;10:1077–1085. doi: 10.1245/aso.2003.03.520. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez Santiago JM, Munoz E, Marti M, Quintana S, Veloso E, Marco C. Metastatic lymph node ratio as a prognostic factor in gastric cancer. Eur J Surg Oncol. 2005;31:59–66. doi: 10.1016/j.ejso.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 14.Saito H, Fukumoto Y, Osaki T, et al. Prognostic significance of the ratio between metastatic and dissected lymph nodes (n ratio) in patients with advanced gastric cancer. J Surg Oncol. 2008;97:132–135. doi: 10.1002/jso.20929. [DOI] [PubMed] [Google Scholar]
- 15.Xu DZ, Geng QR, Long ZJ, et al. Positive lymph node ratio is an independent prognostic factor in gastric cancer after d2 resection regardless of the examined number of lymph nodes. Ann Surg Oncol. 2009;16:319–326. doi: 10.1245/s10434-008-0240-4. [DOI] [PubMed] [Google Scholar]
- 16.Marchet A, Mocellin S, Ambrosi A, et al. The prognostic value of N-ratio in patients with gastric cancer: validation in a large, multicenter series. Eur J Surg Oncol. 2008;34:159–165. doi: 10.1016/j.ejso.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 17.Persiani R, Rausei S, Antonacci V, et al. Metastatic Lymph Node Ratio: A New Staging System for Gastric Cancer. World J Surg. 2009 doi: 10.1007/s00268-009-0157-5. [DOI] [PubMed] [Google Scholar]
- 18.Bando E, Yonemura Y, Taniguchi K, Fushida S, Fujimura T, Miwa K. Outcome of ratio of lymph node metastasis in gastric carcinoma. Ann Surg Oncol. 2002;9:775–784. doi: 10.1007/BF02574500. [DOI] [PubMed] [Google Scholar]
- 19.Yu J, Yang D, Wei F, et al. The staging system of metastatic lymph node ratio in gastric cancer. Hepatogastroenterology. 2008;55:2287–2290. [PubMed] [Google Scholar]
- 20.Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228:449–461. doi: 10.1097/00000658-199810000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siewert JR, Feith M, Werner M, Stein HJ. Adenocarcinoma of the esophagogastric junction: results of surgical therapy based on anatomical/topographic classification in 1,002 consecutive patients. Ann Surg. 2000;232:353–361. doi: 10.1097/00000658-200009000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Japanese Research Society for Gastric Cancer: the general rules for the gastric cancer study in surgery. Jpn J Surg. 1973;3:61. doi: 10.1007/BF02469463. [DOI] [PubMed] [Google Scholar]
- 23.Yoon SS, Yang HK. Lymphadenectomy for gastric adenocarcinoma: should west meet east? Oncologist. 2009;14:871–882. doi: 10.1634/theoncologist.2009-0070. [DOI] [PubMed] [Google Scholar]
- 24.Therneau TM, Grambsch PM, Fleming TR. Martingale-based residuals for survival models. Biometrika. 1990 [Google Scholar]
- 25.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Amer Stat Assoc. 1958:457–481. [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cox D. Regression models and life-tables. J Royal Stat Soc. 1972;34:187–220. [Google Scholar]
- 28.D’Angelica M, Gonen M, Brennan MF, Turnbull AD, Bains M, Karpeh MS. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg. 2004;240:808–816. doi: 10.1097/01.sla.0000143245.28656.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Z, Zhu GL, Lu C, et al. The impact of N-ratio in minimizing stage migration phenomenon in gastric cancer patients with insufficient number or level of lymph node retrieved: results from a Chinese mono-institutional study in 2159 patients. Ann Oncol. 2009;20:897–905. doi: 10.1093/annonc/mdn707. [DOI] [PubMed] [Google Scholar]
- 30.Bonenkamp JJ, Hermans J, Sasako M, et al. Extended lymph-node dissection for gastric cancer. N Engl J Med. 1999;340:908–914. doi: 10.1056/NEJM199903253401202. [DOI] [PubMed] [Google Scholar]
- 31.Cuschieri A, Weeden S, Fielding J, et al. Patient survival after D1 and D2 resections for gastric cancer: long-term results of the MRC randomized surgical trial. Surgical Co-operative Group. Br J Cancer. 1999;79:1522–1530. doi: 10.1038/sj.bjc.6690243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Macdonald JS, Smalley SR, Benedetti J, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. doi: 10.1056/NEJMoa010187. [DOI] [PubMed] [Google Scholar]
- 33.Hundahl SA, Macdonald JS, Benedetti J, Fitzsimmons T. Surgical treatment variation in a prospective, randomized trial of chemoradiotherapy in gastric cancer: the effect of undertreatment. Ann Surg Oncol. 2002;9:278–286. doi: 10.1007/BF02573066. [DOI] [PubMed] [Google Scholar]
- 34.Peeters KC, Hundahl SA, Kranenbarg EK, Hartgrink H, van d V. Low Maruyama index surgery for gastric cancer: blinded reanalysis of the Dutch D1–D2 trial. World J Surg. 2005;29:1576–1584. doi: 10.1007/s00268-005-7907-9. [DOI] [PubMed] [Google Scholar]
- 35.Lee JH, Ryu KW, Lee JH, et al. Learning curve for total gastrectomy with D2 lymph node dissection: cumulative sum analysis for qualified surgery. Ann Surg Oncol. 2006;13:1175–1181. doi: 10.1245/s10434-006-9050-8. [DOI] [PubMed] [Google Scholar]
- 36.Cuschieri A, Fayers P, Fielding J, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–999. doi: 10.1016/s0140-6736(96)90144-0. [DOI] [PubMed] [Google Scholar]
- 37.Bonenkamp JJ, Songun I, Hermans J, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–748. doi: 10.1016/s0140-6736(95)90637-1. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura K, Nishida S, Ichikawa D, et al. No survival benefit from combined pancreaticosplenectomy and total gastrectomy for gastric cancer. Br J Surg. 1999;86:119–122. doi: 10.1046/j.1365-2168.1999.00967.x. [DOI] [PubMed] [Google Scholar]
- 39.Kodera Y, Yamamura Y, Shimizu Y, et al. Lack of benefit of combined pancreaticosplenectomy in D2 resection for proximal-third gastric carcinoma. World J Surg. 1997;21:622–627. doi: 10.1007/s002689900283. [DOI] [PubMed] [Google Scholar]
- 40.Sano T, Yamamoto S, Sasako M. Randomized controlled trial to evaluate splenectomy in total gastrectomy for proximal gastric carcinoma: Japan clinical oncology group study JCOG 0110-MF. Jpn J Clin Oncol. 2002;32:363–364. doi: 10.1093/jjco/hyf085. [DOI] [PubMed] [Google Scholar]
- 41.Degiuli M, Sasako M, Ponti A, Soldati T, Danese F, Calvo F. Morbidity and mortality after D2 gastrectomy for gastric cancer: results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol. 1998;16:1490–1493. doi: 10.1200/JCO.1998.16.4.1490. [DOI] [PubMed] [Google Scholar]
- 42.Wu CW, Hsiung CA, Lo SS, et al. Nodal dissection for patients with gastric cancer: a randomised controlled trial. Lancet Oncol. 2006;7:309–315. doi: 10.1016/S1470-2045(06)70623-4. [DOI] [PubMed] [Google Scholar]
- 43.Debruyne PR, Waldman SA, Schulz S. Pathological staging and therapy of oesophageal and gastric cancer. Expert Opin Pharmacother. 2003;4:1083–1096. doi: 10.1517/14656566.4.7.1083. [DOI] [PubMed] [Google Scholar]


