Abstract
Hypoxia has wide-ranging impact in normal physiology and disease processes. This stimulus evokes changes in gene expression mediated by transcription factors termed hypoxia-inducible factors (HIFs) that affect numerous processes: angiogenesis, cell survival, cellular metabolism, stem cell self- renewal and multipotency, migration, invasiveness and metastatic progression in tumour cells. Over the past decade increasing numbers of reports have emerged documenting differential roles of HIF1α and HIF2α in these processes. In cells of the sympathoadrenal lineage both HIFs differentially mediate influences of hypoxia on catecholamine synthesis and secretion, but HIF2α signalling has particularly prominent functions in regulating developmental processes of growth and differentiation. This article discusses the role of HIF2α and HIF1α in the context of the development, phenotypic features and functions of chromaffin cells. Moreover, current knowledge about tumour formation in cells of the sympathoadrenal lineage, leading to catecholamine producing pheochromocytomas and paragangliomas, is analysed in the light of the HIF2α signalling network.
Keywords: Hypoxia-inducible factor 2, hypoxia, catecholamines, chromaffin cell, sympathoadreanal development, pheochromocytoma, paraganglioma
I. Introduction
Oxygen tension differs widely across tissues and is much lower than under ambient conditions (21% O2). The term mild hypoxia, the lack of sufficient oxygen supply, is generally used for oxygen concentrations of 1–5% ; in contrast, severe hypoxia is defined as below 1% (Koh & Powis, 2012). Oxygen shortage leads to stabilisation of a class of transcription factors, termed hypoxia-inducible factors (HIFs). HIFs are comprised of a stable β subunit and an oxygen-sensitive α subunit. Protein stability of the latter is regulated by several processes, including modification by prolyl hydroxylases (PHDs) with subsequent normoxic proteasomal degradation; the latter is partly mediated by the von Hippel-Lindau (VHL) tumour suppressor. The molecular mechanisms for these processes are well described in several reviews (Kaelin & Ratcliffe, 2008; Koh & Powis, 2012).
There are two main HIFα isoforms with partly overlapping, though mostly complementary functions (Carroll & Ashcroft, 2006; Hu et al., 2003; Rankin et al., 2007). HIF1α is activated during short periods of severe hypoxia, whereas HIF2α (also referred to as EPAS1, endothelial PAS protein 1) is active under mild hypoxia for prolonged periods of time (Holmquist-Mengelbier et al., 2006). This differential effect is mediated by hypoxia-associated factor, which marks HIF1α for degradation, but transactivates HIF2α by binding to a different protein site than in HIF1α (Koh et al., 2008; Koh et al., 2011). This differential regulation leads to distinct cellular functions, reflected in the expression patterns of the two transcription factors. HIF1α is ubiquitously present in most cell types, whereas HIF2α displays a more restricted expression pattern. HIF2α was first identified in endothelial cells, but has since been shown to be expressed in several other cell types, specifically in retina, lungs, heart, glial and neural crest cells.
Both HIF1α and HIF2α employ at least two mechanisms for regulating gene expression. In addition to their well-known interaction with HIFβ, followed by C-terminal transactivation of genes possessing hypoxia responsive elements (HRE), both HIFα subunits also functionally interact with other signal transduction and transcriptional systems. These non-HRE-mediated mechanisms include NOTCH, WNT, and MYC pathway interactions (Kaelin & Ratcliffe, 2008). Some evidence suggests that HIF1α and HIF2α can regulate the interaction of MYC and MAX, resulting in opposing functional effects on MYC-dependent cell proliferation, apoptosis, differentiation and stemness (Dang et al., 2008; Gordan et al., 2007).
The present article focusses on the roles of HIF2α and HIF1α in cells of the sympathoadrenal lineage, and in particular their influences on catecholamine synthesis and secretion, developmental processes and tumourigenesis.
II. Regulation of catecholamine synthesis and secretion by hypoxia
Hypoxia is a well-established potent stimulus for secretion of catecholamines both in vivo and in vitro in isolated cell systems (Cheung, 1989; Donnelly & Doyle, 1994; Kumar et al., 1998). Direct effects of hypoxia on chromaffin cell catecholamine release are vital for maintaining physiological homeostasis of foetuses before sympathetic innervation is fully developed (Phillippe, 1983; Ream et al., 2008). Increased release of catecholamines at birth facilitates appropriate haemodynamic adjustments and stimulation of surfactant production by the lungs (Padbury, 1989; Paulick et al., 1985). Thereafter, responses of catecholamine systems to hypoxic stress, such as associated with high altitude, remain important for maintenance of cardio-respiratory homeostasis (Gamboa et al., 2006; Kanstrup et al., 1999). On the other hand, chronic hypoxic stress-associated catecholamine release can also lead to pathological complications, such as hypertension associated with increased sympathetic activity in patients with sleep apnea (Dimsdale et al., 1995; Donnelly, 2005; Prabhakar & Kumar, 2010).
Intermittent hypoxia (5% O2 in the gas phase) increased the efflux of both norepinephrine and epinephrine from ex vivo adrenal medullae of rats 10 days after beginning of treatment indicating that catecholamine secretion is upregulated under low oxygen tension (Kumar et al., 2006). Further studies demonstrated that hypoxia increases cellular calcium influx, leading to elevated exocytosis (Bournaud et al., 2007; Carpenter et al., 2000; Mojet et al., 1997; Taylor et al., 1999). More recently, the involvement of NADPH oxidase and reactive oxygen species signalling in hypoxia-evoked catecholamine secretion has been established (Souvannakitti et al., 2010).
Besides stimulating catecholamine secretion, hypoxia induces expression of tyrosine hydroxylase (TH), the rate-limiting enzyme of catecholamine synthesis, in numerous catecholamine-producing cells both in vivo and in vitro (Czyzyk-Krzeska et al., 1992; Czyzyk-Krzeska et al., 1994; Schmitt et al., 1992; Schmitt et al., 1993). This induction is explained by the presence of a functional HRE on the TH promoter; both HIF isoforms are able to activate this promoter in a reporter construct assay (Schnell et al., 2003). It has also been shown that levels of both TH and dopamine β hydroxylase (DBH) protein are increased after intermittent and sustained hypoxia (10% O2 in the gas phase) in the rat carotid body and to lesser extents in superior cervical ganglia and adrenal glands; in the carotid bodies this resulted in an increase in contents of dopamine and norepinephrine (Hui et al., 2003). In this study, increased TH activity was shown to result not only from increased levels of TH protein, but also from post-translation activation of the enzyme by phosphorylation at serines 19, 31, and 40. This effect is most likely mediated by AMP-activated kinase (AMPK), since AMPK inhibition by AICAR (5- aminoimidazole-4-carboxamide 1-β-D-ribofuranoside) in PC12 cells prevents TH phosphorylation on relevant serine residues (Fukuda et al., 2007).
Surprisingly, TH mRNA was not downregulated by RNAi knockdown of Hif2α in immortalised rat chromaffin-cell-derived MAH cells; instead HIF2α was shown to directly regulate dopa decarboxylase (DDC) by binding to an HRE within its promoter (Brown et al., 2009). The authors demonstrated that besides DDC also DBH mRNA is decreased by RNAi knockdown of Hif2α. Although no HRE was found in the Dbh promoter region, the authors speculated that HIF2α regulation is due to either the presence of an HRE within the gene or a mediating factor. The same group also showed that HIF2α directly affects adenosine A2A receptor expression in MAH cells (Brown et al., 2011). Receptor activation induces an increase in intracellular calcium in a HIF2α-dependent manner, leading to increased catecholamine release.
The above findings are in tune with an earlier observation that Hif2α−/− mouse embryos at 12.5 days have dramatically reduced norepinephrine levels compared to wildtypes (Tian et al., 1998). These Hif2α−/− embryos die in midgestation similarly to TH- or DBH-deficient mice (Kobayashi et al., 1995; Thomas et al., 1995; Zhou et al., 1995), emphasising the importance of catecholamines during mammalian development. This crucial requirement is reinforced by findings that maternal oxygen (inspired O2 33 or 63%) prevents mid-gestational lethality of TH-deficient embryos indicating that catecholamines mediate fetal survival by maintaining oxygen homeostasis (Ream, et al., 2008).
As reviewed in detail by Wong and coworkers (Wong et al., 2010), HIF1α also appears important in regulating adrenergic responses to stress by activating phenylethanolamine N-methyltransferase (PNMT), the enzyme that converts norepinephrine to epinephrine. This effect appears to be indirectly mediated by HIF1α stimulation of EGR-1 and SP-1 transcription factors and is in agreement with other findings that hypoxia increases expression of PNMT, HIF1α and EGR-1 in mouse pheochromocytoma cells (Evinger et al., 2002).
Taking all above findings together, it appears that PNMT is predominantly responsive to HIF1α, whereas TH is responsive to both HIFs and DDC and DBH are regulated mainly by HIF2α. This might suggest differential effects on expression of catecholamine biosynthetic enzymes dependent on the nature of the hypoxic stimulus. In support of this, rat embryos exposed to long-term hypoxia, a state of predominant HIF2α signalling, develop adrenal medullae with decreased epinephrine and increased norepinephrine content, and a decreased percentage of chromaffin cells expressing PNMT (Mamet et al., 2002). Similar findings of reduced numbers of PNMT positive adrenal medullary cells were observed after long-term hypoxia in fetal sheep (Ducsay et al., 2007). In contrast, but still in line with differential effects on catecholamine biosynthetic machinery, acute short-term hypoxia in fetal sheep increased expression of PNMT, but decreased that of TH (Adams & McMillen, 2000). Other studies have characterised differential effects of long-term and intermittent hypoxia on expression of TH and HIF isoforms in catecholamine-producing cells of the carotid body and brain (Gozal et al., 2005; Lam et al., 2008; Raghuraman et al., 2012). As outlined below, at least some of the differential effects of hypoxia on expression of catecholaminergic biosynthetic enzyme may also partly reflect influences of HIF2α on chromaffin cell growth and differentiation rather than direct actions on expression of biosynthetic enzymes.
III. The role of HIF2α in chromaffin cell development
Initial investigations concerning the role of HIF2α in sympathoadrenal development assessed expression patterns during embryogenesis. A study with chicken embryos demonstrated strong expression in endothelial and vascular smooth muscle cells, liver, kidney, and cellular progenitors of the sympathetic nervous system characterised by TH expression (Favier et al., 1999). HIF2α distribution in the sympathetic lineage was investigated more closely in mouse embryos where HIF2α-positive cells were observed in the sympathetic chain at embryonic day E11.5 (Tian, et al., 1998). This expression was lost soon after, but followed by a strong immunohistochemical staining signal in forming paraganglia; this signal was maintained until E15.5. Lower levels of expression were also found in the adrenal. Death of Hif2α-null embryos coincided with the time of expression in sympathoadrenal cells. Furthermore, HIF2α colocalised with TH protein in paraganglia of a human fetus at week 8.5 corresponding to E15 in mice (Nilsson et al., 2005).
The above findings provide strong evidence that HIF2α is important in the regulation of developmental processes of sympathoadrenal cells. As discussed earlier, HIF2α regulates catecholamine synthesis and secretion; hence Hif2α-null embryos contain less norepinephrine than wildtype mice (Tian, et al., 1998). Since PHD3 was shown to preferentially hydroxylate HIF2α, labelling the latter for degradation (Appelhoff et al., 2004), Phd3−/− mice should contain higher HIF2α levels than their wildtype littermates. However, contrary to what one would expect, these mice display a hypofunctional sympathoadrenal system with reduced tissue innervation, lower plasma levels of epinephrine and norepinephrine, and decreased systolic and diastolic blood pressures (Bishop et al., 2008). The authors also demonstrated that nerve growth factor (NGF)-stimulated neuronal survival was increased in Phd3−/− mice in a HIF2α-dependent manner. Moreover, increased numbers of TH-positive cells were measured in the adrenal medulla, carotid body, and superior cervical ganglion. These results suggest that HIF2α renders cells more responsive to neurite growth- promoting effects, but at the same time has dedifferentiating effects leading to the occurrence of a hypofunctional sympathoadrenal system in Phd3−/− mice.
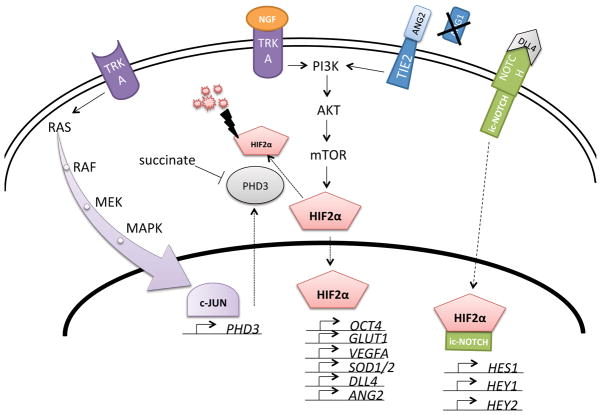
During the development of multicellular organisms the balance between cell proliferation, differentiation and death is constantly changing leading to processes, such as organ morphogenesis. The balance is maintained by the presence of different factors at certain developmental stages. One such factor, with extreme importance for the sympathetic nervous system is NGF (Figure 1). NGF inhibits both basal and hypoxia-induced Hif2α but not Hif1α expression in PC12 cells (Naranjo-Suarez et al., 2003). Depriving sympathetic neurons of NGF results in reduced glucose uptake, elevated levels of reactive oxygen species, and hence increased cell death (Lomb et al., 2009). This process is diminished by PHD inhibitors and, in concordance with this, knockdown of Hif2α by shRNA in mouse neurons decreases survival in the presence of NGF compared to control.
Figure 1. HIF2α signalling network in chromaffin cells.
Nerve growth factor (NGF) binding to its receptor tyrosine kinase TRKA induces PI3K/AKT signalling, which in turn activates the global translational regulator mTOR. One of its targets is hypoxia inducible factor 2α (HIF2α), a transcription factor able to induce a number of different genes, such as the NOTCH ligand delta-like ligand 4 (DLL4). HIF2α and intracellular NOTCH (ic-NOTCH) jointly activate genes, such as stem cell marker HES1. The HIF2α protein is labelled for degradation by prolyl hydroxylase 3 (PHD3) and processed by the proteasome complex, part of which is the Von Hippel- Lindau (VHL) protein. PHD3 is inhibited by high concentrations of succinate, which can be achieved by inactivation of the enzyme succinate dehydrogenase. When TRKA is not activated by NGF the RAS/MAPK pathway is activated instead leading to induction of the transcription factor c-JUN, which stimulates increased transcription of PHD3. An alternative way of PI3K activation is signalling via the TIE2 receptor, which is normally activated by its ligand angiopoietin-1 (ANG1). When angiopoietin-2 (ANG2) is present ANG1 effects can be inhibited, but when ANG1 is absent ANG2 is also able to induce TIE2 signalling.
In keeping with the above concepts, PC12 cells overexpressing Phd3 (also referred to as EGLN3 or SM-20) display increased cytochrome c and caspase-dependent apoptosis (Straub et al., 2003). Similar observations were made in sympathetic neurons, where the authors also demonstrated increased expression of Phd3 after NGF withdrawal (Lipscomb et al., 1999; Lipscomb et al., 2001). It is well established that NGF deprivation causes apoptosis by activating the transcription factor c-JUN (Estus et al., 1994; Ham et al., 1995; Schlingensiepen et al., 1994; Xia et al., 1995). More recently, Lee and coworkers showed that PHD3 but not PHD1 or PHD2 is required for c-JUN dependent apoptosis by a mechanism in which c-JUN directly binds to the PHD3 promoter (Lee et al., 2005).
Besides regulation of cell death, HIF2α is also involved in differentiation processes; thus similar to chromaffin progenitors, Hif2α is expressed in pancreatic progenitor cells, but not in differentiated endocrine or exocrine cells (Chen et al., 2010). In the same study, Hif2α-null embryos were found to have less HES1 (hairy and enhancer of split-1)-positive cells than wildtype, indicating a less differentiated state. This effect appears to be independent of the canonical hypoxia pathway, since Hif1β deletion did not impair normal development. The authors also demonstrated that HIF2α binds to the NOTCH intracellular domain (ic-NOTCH) explaining the activation of the classical NOTCH signalling effector HES1.
Knockdown of HIF2α in neuroblastoma tumour-initiating stem cells resulted in decreased expression of NOTCH target genes and increased expression of neural differentiation markers (Pietras et al., 2009). A similar effect was seen with rapamycin indicating that HIF2α translation is dependent on the mammalian target of rapamycin (mTOR) pathway. In keeping with the above, hypoxia was shown to induce neural crest genes, including NOTCH-1 and HES1, in neuroblastoma cell lines and the embryonic carcinoma cell line P19 (Gustafsson et al., 2005; Jogi et al., 2002; Nilsson, et al., 2005). These effects are however partly dependent on HIF1α, since it was shown that HIF1α is also able to associate with ic-NOTCH (Gustafsson, et al., 2005; Pietras et al., 2011).
There is some evidence that neural crest stem cells cultured under mild hypoxia undergo sympathoadrenal differentiation to cells expressing TH and DBH with measurable release of dopamine and norepinephrine (Morrison et al., 2000). In a similar way PC12 cells treated with a PHD inhibitor or shRNA against Phd1 or Phd2 show increased TH activity and dopamine release (Johansen et al., 2010); however it is not clear if these effects are HIF1α or HIF2α mediated. On the other hand, HIF2α was shown to act directly upstream of OCT4 (octamer-binding transcription factor 4), a transcription factor known to be essential for maintenance of pluripotency (Covello et al., 2006; Koh, et al., 2011).
The above results strongly indicate that HIF2α is responsible for maintaining a balance between stemness and differentiation in the sympathoadrenal lineage. Interestingly, HIF2α was found to be repressed in murine embryonic stem cells (Hu et al., 2006), suggesting this transcription factor is needed in later developmental stages. This is in agreement with the observation that Hif2α expression is only induced at E11.5 in chromaffin cell progenitors (Tian, et al., 1998). On the other hand, HIF1α signalling is fully functional in embryonic stem cells, where it activates the Wnt/β-catenin pathway, which regulates neural stem cell proliferation and differentiation e.g. in the subgranular zone of the hippocampus (Mazumdar, O’Brien, et al., 2010).
In summary, HIF1α and HIF2α have distinct roles in development of cells of the mammalian sympatho-adrenal lineage; wherein HIF2α is a central player in regulating chromaffin cell phenotypic features.
IV. HIF2α signalling in tumourigenesis
HIF2α expression has been linked to malignant progression and poor prognosis in a number of tumours, including astrocytoma, glioma, neuroblastoma, head and neck cancers, melanoma and others (Keith et al., 2012). In most cases, both overexpression of HIF1α and HIF2α have negative effects on outcome; however, in neuroblastoma, HIF1α staining in tissue sections correlated with favourable prognosis, whereas HIF2α staining indicated poor outcome (Noguera et al., 2009).
Similar observations have been reported in renal cell carcinoma (RCC), in which VHL mutations lead to decreased HIFα degradation. It was shown that VHL wildtype cells expressing a stable HIF1α mutant are not able to reproduce the tumourigenic phenotype (Maranchie et al., 2002; Raval et al., 2005); in contrast, HIF2α suppression abrogated tumour formation in mice (Kondo et al., 2003). Raval et al. also demonstrated that in RCC lines proapoptotic genes, such as BNIP3 are predominantly regulated by HIF1α, whereas protumorigenic genes, such as VEGF, transforming growth factor alpha and cyclin D are more dependent on HIF2α.
In a Kras-driven lung tumour mouse model, Hif2α deletion but not Hif1α deletion contributed to tumour growth and progression by direct downregulation of the tumour suppressor secretoglobin 3A1, an inhibitor of AKT signalling (Mazumdar, Hickey, et al., 2010). Interestingly, overexpression of a stable mutant of Hif2α also increased tumour formation in the same Kras mouse model; this was shown to be dependent on increased angiogenesis by induction of Vegf and increased invasiveness, demonstrated by increased markers of epithelial-mesenchymal transition (EMT), such as Snail (Kim et al., 2009). These results indicate that Hif2α expression has to strike a certain balance and that both upregulation and downregulation can promote tumourigenesis.
It is well known that HIF2α is a potent inducer of angiogenesis. In 2000, Peng and coworkers showed that mouse embryos originating from Hif2α-deficient embryonic stem cells display severe vascular defects in the embryo itself and the yolk sac, where vessels are formed but fail to connect and establish the correct network (Peng et al., 2000). Overexpression of Hif2α in rat glioma tumours increases Vegf mRNA and vascular tumour area, whereas the expression of the dominant negative form of Hif2α resulted in the opposite (Acker et al., 2005).
HIF2α expression has also been shown to be associated with increased vascular density in breast tumours (Giatromanolaki et al., 2006), as well as increased VEGF expression and advanced clinical stage in neuroblastoma (Holmquist-Mengelbier, et al., 2006). In VHL-deficient mouse livers Vegf expression and the development of hemangiomas was demonstrated to be dependent on Hif2α and not Hif1α expression (Rankin et al., 2008). Patients with VHL germline mutations are at higher risk to develop hemangioblastomas. These highly vascularised, but nonmalignant tumours, mainly originate from stromal cells in the central nervous system, retina, but can also occur in other organs. Further tumours associated with the VHL syndrome are clear cell RCC and pheochromocytoma.
Another area related to angiogenesis and where HIF2α plays a critical role is hematopoiesis. Mice lacking Hif2α have pancytopenia but intact multilineage maturation processes in the bone marrow, which led the authors to suggest a mechanism related to a disturbed microenvironment (Scortegagna et al., 2003). Later it was established that HIF2α directly regulates erythropoietin expression (Rankin, et al., 2007; Scortegagna et al., 2005; Warnecke et al., 2004), and is a crucial regulator of iron absorption, a process essential for the normal functionality of erythrocytes (Mastrogiannaki et al., 2009).
Other processes important for both normal development of the vasculature and tumour progression are cell migration and matrix vascular remodelling. Epidermal growth factor receptor activation in hypoxic foci in head and neck squamous cell carcinoma leads to enhanced cell migration, but not proliferation, a process shown to be dependent on the expression of HIF2α (Wang & Schneider, 2010). This pathway is proposed to promote a more aggressive phenotype in this type of cancer. A HIF2α target gene possibly mediating this effect is plasminogen activator inhibitor-1 (PAI1) (Sato et al., 2004), a serine protease inhibitor shown to induce cancer invasion and vascularisation by facilitating attachment and migration of cancer cells (Bajou et al., 1998; Chazaud et al., 2002).
Another HIF2α target identified in RCC cells lacking the VHL gene is type-1 matrix metalloproteinase, a protein capable of extracellular matrix degradation (Petrella et al., 2005). In the context of cartilage destruction a number of other matrix metalloproteinases and matrix catabolic factors were identified to be HIF2α targets (S. Yang et al., 2010). In RCC cells deficient for VHL, HIF2α was shown to induce genes that drive metastasis, including chemokine (C-X-C motif) receptor 4 and cytohesin 1 interacting protein; they are involved in chemotactic cell invasion and protection from death cytokine signalling, respectively (Vanharanta et al., 2013). These effects were dependent on the induction of epigenetic changes, such as histone H3 and DNA methylation. HIF2α also activates expression of the adenosine A2A receptor; activation does not only affect catecholamine release (Brown, et al., 2011), but also other processes, including cell proliferation, cell migration, and tube formation in primary cultures of human lung endothelial cells (Ahmad et al., 2009).
Interestingly, in murine endothelial cells HIF2α appears to have an inhibitory effect on tumour cell migration and metastasis mediated by reduction of nitric oxide (NO) synthesis; in contrast, HIF1α has an opposing effect (Branco-Price et al., 2012; Takeda et al., 2010). This is consistent with the finding of Skuli et al., where Hif2α deletion resulted in increased migration and invasion, but dysfunctional arteriogenesis (Skuli et al., 2012), which was associated with decreased expression of delta-like ligand 4 (DLL4, NOTCH ligand), Hes1 and other NOTCH target genes and the proangiogenic factor angiopoietin-2 (ANG2). Hif2α expression in host endothelial cells was also shown to be crucial for tumour neovascularisation in a xenograft model of melanoma, where this process was identified to be dependent on the HIF2α-driven expression of ephrin A1 (Yamashita et al., 2008).
These above examples emphasise that not only tumour cells need to be considered in the process of tumourigenesis but also cells of the stromal microenvironment. High HIF2α levels are found in tumour-associated macrophages (TAMs) (Talks et al., 2000), and their number has been associated with poor clinical outcome in different cancers (Tang et al., 2013). HIF2α is crucial for TAM infiltration into tumour lesions, and through this mechanism promotes tumour progression in mouse models (Imtiyaz et al., 2010). On the other hand, in a melanoma mouse model it was shown that HIF2α stabilisation by a PHD3 inhibitor decreases tumour growth; this was attributed to TAMs secreting an increased amount of soluble VEGFR that inhibits VEGF function (Roda et al., 2012).
TAMs appear to reside close to a HIF2α expressing immature neural crest-like cell population in the perivascular niche of neuroblastomas (Pietras et al., 2008). These cells resemble a population termed neural crest-like neuroblastoma tumour-initiating stem cells, which have been isolated from patient bone marrows (Pietras, et al., 2009). Similarly, tumour associated stem cells with high HIF2α expression have been identified in glioblastomas (Li et al., 2009).
In summary, the role of HIF2α in tumourigenesis appears to be complex and context dependent in that both increased and decreased expression can result in tumour development. Hence targeting hypoxic signalling pathways as cancer therapy requires careful evaluation of tumour type and phenotypic features.
V. Genotype-phenotype relationships of chromaffin cell tumours
Pheochromocytomas (PHEOs) and paragangliomas (PGLs) are catecholamine producing tumours derived respectively from chromaffin cells of the adrenal medulla and extraadrenal paraganglia. Most catecholamine-producing PGLs occur in abdominal and thoracic regions. Other PGLs are found in the head and neck region, but these usually produce negligible or low amounts of catecholamines. Occurrence of PHEOs/PGLs in the general population is rare, but a substantial portion of these tumours have a hereditary basis, where about one third are caused by germline mutations in one of the following genes: neurofibromatosis type 1 (NF1); rearranged during transfection (RET) protooncogene, transmembrane protein 127 (TMEM127); myc-associated factor (MAX); VHL or one of the genes for succinate dehydrogenase subunits (SDHA, B, C, D).
The above diverse genotypic backgrounds of PHEOs/PGLs are associated with distinct differences in clinical presentation (Eisenhofer, Pacak, et al., 2011). This includes tumour location, propensity to malignancy, tumour tissue catecholamine contents, the dominant type of catecholamine (dopamine, norepinephrine, or epinephrine) produced and catecholamine secretory characteristics (Figure 2). Tumours due to mutations of succinate dehydrogenase subunit genes predominantly occur at extraadrenal locations, whereas those due to RET, NF1, TMEM127 and MAX mutations predominantly occur at adrenal locations. Tumours due to VHL mutations can occur at both locations, but predominate at adrenal locations. PGLs due to SDHB mutations are particularly prone to metastasis (Blank et al., 2010; King et al., 2011).
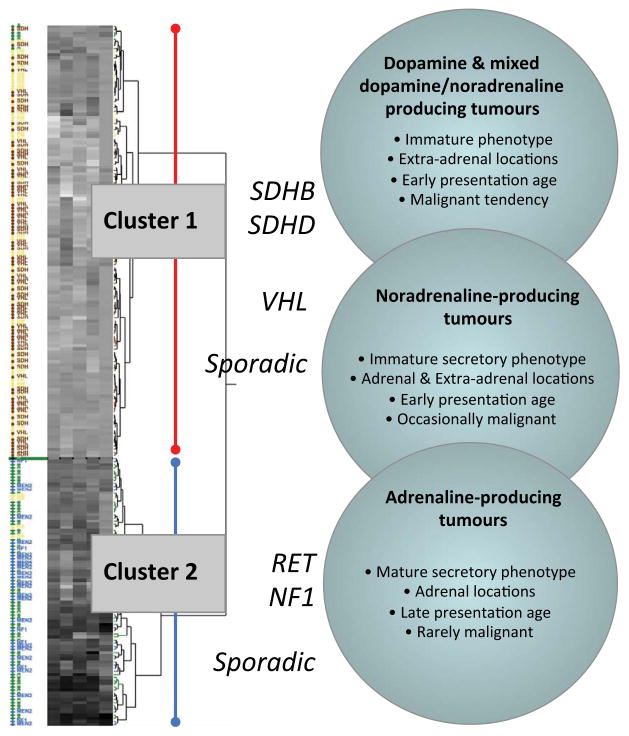
Figure 2. Unsupervised hierarchical clustering based on measured values for epinephrine-related analytes in plasma and urine.
Increasing analyte levels are illustrated in grey scale by progression from lighter to darker heat map areas. Patients with VHL or SDHx mutations shown in red are confined to cluster 1, whereas patients with RET and NF1 mutations are depicted in blue and are confined to cluster 2. Patients without evidence of hereditary syndrome are illustrated in green for those with adrenaline-producing tumours, and in yellow for noradrenergic or dopaminergic tumours. Image modified from (Eisenhofer, Pacak, et al., 2011)
Hereditary tumours associated with RET mutations in multiple endocrine neoplasia type 2 (MEN 2) produce epinephrine, whereas VHL tumours lack expression of PNMT leading to termination of catecholamine synthesis at the level of norepinephrine (Eisenhofer et al., 2001). Similarly, tumours due to NF1 mutations express PNMT and thus produce epinephrine, whereas those due to mutations of SDH subunit genes do not and tend towards immature phenotypic features of low tissue catecholamine contents with significant production of dopamine and its O-methylated metabolite methoxytyramine (Eisenhofer et al., 2012; Eisenhofer, Pacak, et al., 2011).
Interestingly, while total tissue catecholamine contents are highest in epinephrine-producing hereditary and sporadic tumours and lowest in those that do not produce epinephrine, rates of catecholamine secretion and excretion into urine are highest in dopamine- and norepinephrine- producing tumours (Eisenhofer, Pacak, et al., 2011). This difference in catecholamine secretory characteristics has been linked to a more fully developed regulatory secretory pathway in tumours that produce epinephrine than in those that do not (Eisenhofer et al., 2008). Lack of regulatory controls in the more immature norepinephrine- and dopamine-producing tumours leads to more continuous or constitutive catecholamine-secretory activity than in the more fully differentiated tumours that produce epinephrine.
VI. What role does HIF2α play in the development of chromaffin cell tumours?
The differences in genetic backgrounds of PHEOs/PGLs are reflected by distinct differences in gene expression profiles, with consistent differences among hereditary groups observed in several studies (Burnichon et al., 2011; Dahia et al., 2005; Eisenhofer et al., 2004; Lopez-Jimenez et al., 2010). In particular, these various gene expression profiling studies have all described two cluster groups with epinephrine-producing tumours due to RET, NF1 and TMEM127 mutations in one cluster group (cluster 2) and norepinephrine- or dopamine-producing tumours due to VHL and SDHx mutations in the other (cluster 1).
The first of the above studies compared gene expression profiles in VHL and MEN2 tumours in relationship to sporadic epinephrine- and norepinephrine-producing tumours (Eisenhofer, et al., 2004). This study noted distinct differences in gene expression between both hereditary and sporadic norepinephrine- versus epinephrine-producing tumours with over-expression of genes involved in hypoxia-angiogenic pathways in the former tumours. The most important upregulated gene in both hereditary and sporadic norepinephrine-producing tumours was HIF2α. Taking into account the role of HIF2α in maintaining stem cell-like traits in chromaffin cells, these findings were interpreted to suggest a key role of HIF2α in development of PHEOs/PGLs with an immature catecholamine phenotype. Expression of HIF2α in developing chromaffin progenitors was considered to confer susceptibility of these cells to mutations of genes, such as VHL and SDHx, that impact hypoxia pathways. Hypoxia effects of high altitude have also been associated with the occurrence of tumours in the carotid bodies (Astrom et al., 2003; Cerecer-Gil et al., 2010; Rodriguez-Cuevas et al., 1998).
The second gene profiling study also clustered PHEOs/PGLs in two groups, one containing tumours with VHL, SDHB and SDHD mutations and another with RET and NF1 mutations (Dahia, et al., 2005). Notably, almost all extraadrenal PGLs analysed in this cohort were confined to cluster group 1, which was comprised of both adrenal and extra-adrenal tumours in equal proportions. In agreement with the earlier study, cluster 1 displayed a gene signature of activated hypoxia pathways and enhanced angiogenesis and extracellular matrix processes. Furthermore, the cluster 1 gene profile showed a suppressed mitochondrial function, an observation confirmed by decreased SDHB protein in the majority of all cluster 1 tumours, including VHL and sporadic cases. These features, according to gain- of-function and loss-of-function experiments in cell line models, were described as dependent on HIF1α signalling.
Subsequent gene expression profiling studies confirmed the strong HIF2α expression in VHL and SDHx tumours compared to cluster 2 tumours (Burnichon, et al., 2011; Lopez-Jimenez, et al., 2010). This was also further confirmed by immunohistochemical analyses of HIF2α in tumour sections (Favier et al., 2009). HIF1α expression on the other hand was not different between clusters.
In 2005 Lee et al. proposed that the PHEO/PGL gene mutations NF1, c-RET, VHL, and SDHx, all act on the same HIF2α signalling network (Figure 1) resulting in decreased apoptosis during chromaffin cell development, and hence tumour formation (Lee, et al., 2005). NF1 is a negative regulator of RAS signalling induced by stimulation of the NGF receptor TrkA. c-RET is the receptor for GDNF (glial cell line-derived neurotrophic factor) that crosstalks with TrkA (Dechant, 2002; Peterson & Bogenmann, 2004; Tsui-Pierchala et al., 2002) and induces JUNB, an antagonist of c-JUN, leading to decreased apoptosis (Lee, et al., 2005). In the same study, it was demonstrated that loss of VHL, similar to c-RET activation, results in JUNB induction. The authors went on to show that succinate inhibits PHD3. The former accumulates in the cell when SDH is inhibited (Selak et al., 2005; Smith et al., 2007). NF1 mutations as well as the more recently identified TMEM127 mutations were shown to hyperphosphorylate mTOR (Dasgupta et al., 2005; Qin et al., 2010), potentially inducing HIF2α translation. The fundamental differences observed between clusters could potentially be due to a more severe activation of HIF2α in cluster 1 due to the impairment of the degradation machinery.
Given the highly divergent phenotypic features and gene expression profiles observed between different groups of hereditary PHEOs/PGLs it seems counterintuitive that all these tumours might develop through a single signalling network as proposed by Lee et al. (Lee, et al., 2005). Nevertheless, this remains plausible should components of this network be differentially susceptible to the mutations of the various tumour-susceptibility genes in chromaffin progenitors or mature cells at different locations and stages of development. In this way expression of HIF2α in specific chromaffin cell types at specific locations or in sympathoadrenal progenitors at a particular stage in chromaffin cell development might make only these cells vulnerable to mutations impacting hypoxia-pathways. As originally proposed (Eisenhofer, et al., 2004), the expected result is development of tumours with the same immature catecholamine phenotypic features characteristic of the sympatho-adrenal progenitor cells from which the tumours derive.
Support for the above concept has been derived from analysis of a large clinical dataset documenting highly significant differences in age of diagnosis of sporadic and hereditary PHEOs/PGLs according to catecholamine phenotypic features (Eisenhofer, Timmers, et al., 2011). Specifically, patients with epinephrine-producing cluster 2 type tumours are diagnosed on average a decade later than patients with norepinephrine- or dopamine-producing cluster 1 tumours. While hereditary PHEOs/PGLs also present on average 10 to 15 years earlier than sporadic tumours, the differences in ages of presentation of tumours with different catecholamine phenotypes occur independently of this additional influence (Table 1). Thus, patients with dopamine- or norepinephrine-producing hereditary tumours showed the youngest ages of disease presentation. Importantly, among this group, presentation of disease was much earlier in patients with multifocal extraadrenal tumours than in those with solitary PHEOs/PGLs.
Table 1.
Age at diagnosis of hereditary and sporadic PHEO/PGLs from nine different studies
| RET | NF1 | VHL | SDHB | SDHD | Sporadic | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | n | Age | n | Age | n | Age | n | Age | n | Age | n | |
| (Neumann et al., 2002) | 36 | 13 | - | - | 18 | 30 | 26 | 12 | 29 | 11 | 44 | 205 |
| (Amar et al., 2005) | 30 | 16 | 40 | 13 | 24 | 25 | 34 | 21 | 31 | 11 | 46 | 228 |
| (Mannelli et al., 2009) | 37 | 27 | 42 | 5 | 30 | 48 | 29 | 24 | 40 | 47 | 50 | 340 |
| (Cascon et al., 2009) | 39 | 36 | - | - | 28 | 20 | 30 | 25 | 25 | 11 | 46 | 143 |
| (Walther et al., 1999) | - | - | 42 | 148 | - | - | - | - | - | - | - | - |
| (Casanova et al., 1993) | 38 | 100 | - | - | - | - | - | - | - | - | - | - |
| (Pomares et al., 1998) | 38 | 23 | - | - | - | - | - | - | - | - | 47 | 23 |
| (Ricketts et al., 2010) | - | - | - | - | - | - | 27 | 153 | 21 | 18 | - | - |
| (Eisenhofer, Timmers, et al., 2011) | 40 | 38 | 42 | 10 | 31 | 66 | 31 | 48 | 31 | 11 | 47 | 182 |
| Grouped mean & total n | 37.7 | 253 | 41.9 | 176 | 27.4 | 189 | 28.6 | 283 | 32.4 | 109 | 47.0 | 1121 |
The above observations not only support origins of PHEOs/PGLs from different chromaffin progenitor cells with variable developmental susceptibility to disease causing mutations, but also suggest development of multifocal disease from HIF2α-overexpressing tumour stem cells that have suffered a second hit to inactivate gene function before migration to different sites. Later age of presentation of tumours that produce epinephrine than norepinephrine is expected, since in those patients any genetic abnormalities leading to tumourigenesis can only be expected to have impact after chromaffin progenitors have migrated and differentiated into adrenaline-producing chromaffin cells within the adrenals.
VII. HIF2α and metastatic pheochromocytoma/paraganglioma
In addition to phenotypic differences between tumours of the two main gene-expression cluster groups, there are also differences between tumours of the same cluster; SDHB-related tumours are often extraadrenal and have an enhanced tendency to metastatic progression, whereas VHL tumours are mostly adrenal and have a low risk of malignancy (Brouwers, Eisenhofer, et al., 2006; Burnichon, et al., 2011; Gimenez-Roqueplo et al., 2003). Gene expression profiling also clearly distinguishes between those two hereditary groups of tumours (Favier, et al., 2009; Lopez-Jimenez, et al., 2010). VHL tumours have a more HIF1α-driven signature than SDHx-related tumours, which manifests in upregulation of glycolytic and suppression of mitochondrial genes, the so called Warburg effect. These differences were additionally associated with increased expression of apoptotic target genes such as BNIP3.
In line with the above observations, overexpression of HIF2α in a VHL-deficient RCC line increased mitochondrial and decreased glycolytic metabolism compared to controls, suggesting that SDHx- related tumours are more dependent on HIF2α (Biswas et al., 2010). Pollard and colleagues not only confirmed higher levels of BNIP3 in VHL tumours by immunohistochemistry, but also found somewhat increased VEGF, cyclin D1 and HIF2α levels (Pollard et al., 2006). The authors, however, concluded that VHL tumours are more HIF2α-driven than those with SDHx mutations; nevertheless, based on their observations that both target genes of HIF1α (BNIP3) and a HIF2 α (cyclin D1) are increased and that VEGF expression is known to be induced by both transcription factors (Keith, et al., 2012), the question of whether one signalling pathway is more prominent than the other may not have such a simple answer. Interestingly, PHD3 is more highly expressed in VHL tumours, which is however not reflected in the protein level analysed by immunohistochemistry indicating some form of posttranscriptional mechanism (Eisenhofer, et al., 2004; Lopez-Jimenez, et al., 2010).
More than 50% of childhood PHEO/PGL cases have metastatic disease, and about 70 of these carry SDHB mutations (King, et al., 2011). This again is in line with concepts that that phenotypically immature tumors develop earlier in life than more fully differentiated tumors, but also indicates an additional link to aggressiveness of tumours. Nevertheless, SDHB mutations carry a poor prognosis in both children and adults (Blank, et al., 2010; King, et al., 2011). Gene profiling studies attempting to shed light on the distinctive biology between SDHx-mutated and VHL tumours have so far failed to show a definitive lead (Brouwers, Elkahloun, et al., 2006; Waldmann et al., 2010). Both studies however established downregulation of JUNB in malignant PHEOs/PGLs, pointing towards a potential involvement of the HIF2α signalling network (Lee, et al., 2005).
Recently, Favier and colleagues established increased vascularisation coupled with increased VEGF and ANG2 expression in cluster 1 tumours (Favier, Igaz, et al., 2012). Interestingly, malignant tumours of both clusters showed strongly reduced ANG1 expression. Generally ANG2 is a weaker agonist for the TIE2 receptor, and in the presence of ANG1 it actually inhibits its actions; but if ANG1 is absent ANG2 activates the TIE2 receptor and downstream signalling of PI3K and AKT (Yuan et al., 2009)(Figure 1). This evidence points to a possible activation of the mTOR pathway in malignant PHEOs/PGLs; however the sample size of this study was too low for any definite conclusions (Favier, Igaz, et al., 2012). Preliminary investigations concerning mTOR inhibition in the mouse PHEO cell lines indicate a concentration-dependent decrease in cell survival (Nolting & Grossman, 012).
The question remains, why are SDHB-related tumours so much more malignant than VHL tumours? They are more immature, have dramatically decreased total catecholamine contents with high proportions of dopamine, and they are larger at diagnosis compared to other PHEOs/PGLs (Eisenhofer, et al., 2012). Is this solely mediated by HIF2α signalling? Or more likely, are there another factors involved? SDHB mutations alter the balance of energy metabolites in these cells dramatically by strongly elevating succinate concentrations (Selak, et al., 2005; Smith, et al., 2007). Succinate not only broadly inhibits α-ketoglutarate-dependent enzymes, such as PHDs, but also affects the TET family of DNA hydroxylases and inhibits other enzymes, such as histone demethylases leading to increased histone H3 methylation (Cervera et al., 2009; Smith, et al., 2007; Xiao et al., 2012). The same effects have been demonstrated for fumarate accumulation caused by fumarate hydratase knockdown (Xiao, et al., 2012). These studies demonstrate that changes in metabolite levels can lead to profound epigenetic alterations and hence changes in gene expression that will no doubt differ from those observed in VHL mutated tumours.
VIII. Mutations of HIF2α as a cause of chromaffin cell tumours
A central role of HIF2α signalling in PHEO/PGL development has been substantiated by findings of somatic HIF2α gain-of-function mutations in two patients with multiple PGLs and somatostatinomas (Zhuang et al., 2012). Shortly after this initial report, there followed several further publications from different groups describing either somatic or germline HIF2α mutations in patients characterised with mostly multiple PGLs and polycythemia (Comino-Mendez et al., 2013; Favier, Buffet, et al., 2012; Lorenzo et al., 2012; Pacak, 2013; C. Yang et al., 2013). These gain-of-function mutations protect HIF2α from degradation processes mediated by PHDs, but whether they predispose or rather are a direct cause of tumourigenesis is currently unclear.
HIF2α mutated tumours have increased levels of HIF2α mRNA, similar to cases with VHL and SDHx mutations (Comino-Mendez, et al., 2013). However, Favier et al. noted that the activation of hypoxia- inducible genes is rather mild compared to other cluster 1 tumours (Favier, Buffet, et al., 2012). Interestingly, all subjects described in these studies who presented with multiple PGLs and increased red blood cell mass were first diagnosed at the age 35 or younger, with most being younger than 20 years; In contrast, patients without polycythemia presented later with PHEO/PGL and were less often characterised by multifocal disease. This may point to different degrees of HIF2α activation in these patients. Hence it would be of considerable interest to establish whether mice overexpressing Hif2α (e.g. by introduction of a nondegradable gene variant) have a higher tumour incidence. Phd3 knockout mice exhibit mild hyperplasia in the adrenal, carotid body and superior cervical ganglia (Bishop, et al., 2008). However, no PHEOs or PGLs have been found in Sdhd or Sdhd/H19 knockout mice (Bayley et al., 2009).
IX. Conclusion
HIF2α signalling appears to be a central pathway in chromaffin cell development and differentiation, with cells highly expressing HIF2α exhibiting a less differentiated and more stem cell-like phenotype. Recent evidence suggests that HIF2α signalling in PHEO/PGL patients leads to tumours with less mature catecholamine phenotypes that occur earlier in life and are more often multifocal and potentially more aggressive than tumours that do not display features of upregulated HIF2α signalling. This may indicate a mutational second hit during fetal development activating HIF2α (by gene mutation or inhibition of degradation through VHL or SDHx inactivation), which results in maintenance of a more undifferentiated phenotype. Hence HIF2α may be a useful biomarker for more aggressive disease. Moreover, targeting HIF2α by small molecule inhibitors could be a valid therapeutic strategy for PHEOs/PGLs of the cluster 1 type, especially since inhibition of HIF2α in a cell line model of VHL-deficient RCC increased cell death and sensitivity to radiation (Bertout et al., 2009).
Abbreviations
- AMPK
AMP-activated kinase
- ANG
Angiopoietin
- DDC
Dopa decarboxylase
- DBH
Dopamine β hydroxylase
- DLL4
Delta-like ligand 4
- HES1
Hairy and enhancer of split-1
- HIF
Hypoxia-inducible factors
- HRE
Hypoxia responsive element
- MEN2
Multiple endocrine neoplasia type 2
- mTOR
Mammalian target of rapamycin
- NADPH
Nicotinamidadenindinukleotidphosphat
- NGF
Nerve growth factor
- OCT4
Octamer-binding transcription factor 4
- PGL
Paraganglioma
- PHD
Prolyl hydroxylase
- PHEO
Pheochromocytoma
- PI3K
Phosphoinositide 3-kinase
- PNMT
Phenylethanolamine N-methyltransferase
- RCC
Renal cell carcinoma
- SDH
Succinate dehydrogenase
- TAMs
Tumour-associated macrophages
- TH
Tyrosine hydroxylase
- VEGF(R)
Vascular endothelial growth factor (receptor)
- VHL
Von Hippel-Lindau
Footnotes
Conflict of Interest statement: The authors have no conflicts of interest to declare.
References
- Acker T, Diez-Juan A, Aragones J, Tjwa M, Brusselmans K, Moons L, et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell. 2005;8(2):131–141. doi: 10.1016/j.ccr.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Adams MB, McMillen IC. Actions of hypoxia on catecholamine synthetic enzyme mRNA expression before and after development of adrenal innervation in the sheep fetus. J‘Physiol. 2000;529(Pt 3):519–531. doi: 10.1111/j.1469-7793.2000.00519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad A, Ahmad S, Glover L, Miller SM, Shannon JM, Guo X, et al. Adenosine A2A receptor is a unique angiogenic target of HIF-2alpha in pulmonary endothelial cells. Proc Natl Acad Sci USA. 2009;106(26):10684–10689. doi: 10.1073/pnas.0901326106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amar L, Bertherat J, Baudin E, Ajzenberg C, Bressac-de Paillerets B, Chabre O, et al. Genetic testing in pheochromocytoma or functional paraganglioma. Journal of Clinical Oncology. 2005;23(34):8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- Appelhoff RJ, Tian YM, Raval RR, Turley H, Harris AL, Pugh CW, et al. Differential function of the prolyl hydroxylases PHD1, PHD2, and PHD3 in the regulation of hypoxia- inducible factor. J Biol Chem. 2004;279(37):38458–38465. doi: 10.1074/jbc.M406026200. [DOI] [PubMed] [Google Scholar]
- Astrom K, Cohen JE, Willett-Brozick JE, Aston CE, Baysal BE. Altitude is a phenotypic modifier in hereditary paraganglioma type 1: evidence for an oxygen-sensing defect. Hum Genet. 2003;113(3):228–237. doi: 10.1007/s00439-003-0969-6. [DOI] [PubMed] [Google Scholar]
- Bajou K, Noel A, Gerard RD, Masson V, Brunner N, Holst-Hansen C, et al. Absence of host plasminogen activator inhibitor 1 prevents cancer invasion and vascularization. Nat Med. 1998;4(8):923–928. doi: 10.1038/nm0898-923. [DOI] [PubMed] [Google Scholar]
- Bayley JP, van Minderhout I, Hogendoorn PC, Cornelisse CJ, van der Wal A, Prins FA, et al. Sdhd and SDHD/H19 knockout mice do not develop paraganglioma or pheochromocytoma. PLoS One. 2009;4(11):e7987. doi: 10.1371/journal.pone.0007987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertout JA, Majmundar AJ, Gordan JD, Lam JC, Ditsworth D, Keith B, et al. HIF2alpha inhibition promotes p53 pathway activity, tumor cell death, and radiation responses. Proc Natl Acad Sci USA. 2009;106(34):14391–14396. doi: 10.1073/pnas.0907357106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop T, Gallagher D, Pascual A, Lygate CA, de Bono JP, Nicholls LG, et al. Abnormal sympathoadrenal development and systemic hypotension in PHD3−/− mice. Mol Cell Biol. 2008;28(10):3386–3400. doi: 10.1128/MCB.02041-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Troy H, Leek R, Chung YL, Li JL, Raval RR, et al. Effects of HIF-1alpha and HIF2alpha on Growth and Metabolism of Clear-Cell Renal Cell Carcinoma 786–0 Xenografts. J Oncol. 2010;2010:757908. doi: 10.1155/2010/757908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank A, Schmitt AM, Korpershoek E, van Nederveen F, Rudolph T, Weber N, et al. SDHB loss predicts malignancy in pheochromocytomas/sympathethic paragangliomas, but not through hypoxia signalling. Endocr Relat Cancer. 2010;17(4):919–928. doi: 10.1677/ERC-09-0316. [DOI] [PubMed] [Google Scholar]
- Bournaud R, Hidalgo J, Yu H, Girard E, Shimahara T. Catecholamine secretion from rat foetal adrenal chromaffin cells and hypoxia sensitivity. Pflugers Arch. 2007;454(1):83–92. doi: 10.1007/s00424-006-0185-z. [DOI] [PubMed] [Google Scholar]
- Branco-Price C, Zhang N, Schnelle M, Evans C, Katschinski DM, Liao D, et al. Endothelial cell HIF-1alpha and HIF-2alpha differentially regulate metastatic success. Cancer Cell. 2012;21(1):52–65. doi: 10.1016/j.ccr.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwers FM, Eisenhofer G, Tao JJ, Kant JA, Adams KT, Linehan WM, et al. High frequency of SDHB germline mutations in patients with malignant catecholamine-producing paragangliomas: implications for genetic testing. J Clin Endocrinol Metab. 2006;91(11):4505–4509. doi: 10.1210/jc.2006-0423. [DOI] [PubMed] [Google Scholar]
- Brouwers FM, Elkahloun AG, Munson PJ, Eisenhofer G, Barb J, Linehan WM, et al. Gene expression profiling of benign and malignant pheochromocytoma. Ann N Y Acad Sci. 2006;1073:541–556. doi: 10.1196/annals.1353.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown ST, Kelly KF, Daniel JM, Nurse CA. Hypoxia inducible factor (HIF)-2 alpha is required for the development of the catecholaminergic phenotype of sympathoadrenal cells. J Neurochem. 2009;110(2):622–630. doi: 10.1111/j.1471-4159.2009.06153.x. [DOI] [PubMed] [Google Scholar]
- Brown ST, Reyes EP, Nurse CA. Chronic hypoxia upregulates adenosine 2a receptor expression in chromaffin cells via hypoxia inducible factor-2alpha: role in modulating secretion. Biochem Biophys Res Commun. 2011;412(3):466–472. doi: 10.1016/j.bbrc.2011.07.122. [DOI] [PubMed] [Google Scholar]
- Burnichon N, Vescovo L, Amar L, Libe R, de Reynies A, Venisse A, et al. Integrative genomic analysis reveals somatic mutations in pheochromocytoma and paraganglioma. Hum Mol Genet. 2011;20(20):3974–3985. doi: 10.1093/hmg/ddr324. [DOI] [PubMed] [Google Scholar]
- Carpenter E, Hatton CJ, Peers C. Effects of hypoxia and dithionite on catecholamine release from isolated type I cells of the rat carotid body. J Physiol. 2000;523(Pt 3):719–729. doi: 10.1111/j.1469-7793.2000.00719.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll VA, Ashcroft M. Role of hypoxia-inducible factor (HIF)-1alpha versus HIF-2alpha in the regulation of HIF target genes in response to hypoxia, insulin-like growth factor-I, or loss of von Hippel-Lindau function: implications for targeting the HIF pathway. Cancer Res. 2006;66(12):6264–6270. doi: 10.1158/0008-5472.CAN-05-2519. [DOI] [PubMed] [Google Scholar]
- Casanova S, Rosenberg-Bourgin M, Farkas D, Calmettes C, Feingold N, Heshmati HM, et al. Phaeochromocytoma in multiple endocrine neoplasia type 2 A: survey of 100 cases. Clin Endocrinol (Oxf) 1993;38(5):531–537. doi: 10.1111/j.1365-2265.1993.tb00350.x. [DOI] [PubMed] [Google Scholar]
- Cascon A, Pita G, Burnichon N, Landa I, Lopez-Jimenez E, Montero-Conde C, et al. Genetics of pheochromocytoma and paraganglioma in Spanish patients. J Clin Endocrinol Metab. 2009;94(5):1701–1705. doi: 10.1210/jc.2008-2756. [DOI] [PubMed] [Google Scholar]
- Cerecer-Gil NY, Figuera LE, Llamas FJ, Lara M, Escamilla JG, Ramos R, et al. Mutation of SDHB is a cause of hypoxia-related high-altitude paraganglioma. Clin Cancer Res. 2010;16(16):4148–4154. doi: 10.1158/1078-0432.CCR-10-0637. [DOI] [PubMed] [Google Scholar]
- Cervera AM, Bayley JP, Devilee P, McCreath KJ. Inhibition of succinate dehydrogenase dysregulates histone modification in mammalian cells. Mol Cancer. 2009;8:89. doi: 10.1186/1476-4598-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chazaud B, Ricoux R, Christov C, Plonquet A, Gherardi RK, Barlovatz-Meimon G. Promigratory effect of plasminogen activator inhibitor-1 on invasive breast cancer cell populations. Am J Pathol. 2002;160(1):237–246. doi: 10.1016/S0002-9440(10)64367-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Houshmand G, Mishra S, Fong GH, Gittes GK, Esni F. Impaired pancreatic development in Hif2-alpha deficient mice. Biochem Biophys Res Commun. 2010;399(3):440–445. doi: 10.1016/j.bbrc.2010.07.111. [DOI] [PubMed] [Google Scholar]
- Cheung CY. Direct adrenal medullary catecholamine response to hypoxia in fetal sheep. J Neurochem. 1989;52(1):148–153. doi: 10.1111/j.1471-4159.1989.tb10909.x. [DOI] [PubMed] [Google Scholar]
- Comino-Mendez I, de Cubas AA, Bernal C, Alvarez-Escola C, Sanchez-Malo C, Ramirez-Tortosa CL, et al. Tumoral EPAS1 (HIF2A) mutations explain sporadic pheochromocytoma and paraganglioma in the absence of erythrocytosis. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt069. [DOI] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20(5):557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Bayliss DA, Lawson EE, Millhorn DE. Regulation of tyrosine hydroxylase gene expression in the rat carotid body by hypoxia. J Neurochem. 1992;58(4):1538– 1546. doi: 10.1111/j.1471-4159.1992.tb11376.x. [DOI] [PubMed] [Google Scholar]
- Czyzyk-Krzeska MF, Furnari BA, Lawson EE, Millhorn DE. Hypoxia increases rate of transcription and stability of tyrosine hydroxylase mRNA in pheochromocytoma (PC12) cells. J Biol Chem. 1994;269(1):760–764. [PubMed] [Google Scholar]
- Dahia PL, Ross KN, Wright ME, Hayashida CY, Santagata S, Barontini M, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1(1):72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang CV, Kim JW, Gao P, Yustein J. The interplay between MYC and HIF in cancer. Nat Rev Cancer. 2008;8(1):51–56. doi: 10.1038/nrc2274. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Yi Y, Chen DY, Weber JD, Gutmann DH. Proteomic analysis reveals hyperactivation of the mammalian target of rapamycin pathway in neurofibromatosis 1- associated human and mouse brain tumors. Cancer Res. 2005;65(7):2755–2760. doi: 10.1158/0008-5472.CAN-04-4058. [DOI] [PubMed] [Google Scholar]
- Dechant G. Chat in the trophic web: NGF activates Ret by inter-RTK signaling. Neuron. 2002;33(2):156–158. doi: 10.1016/s0896-6273(02)00564-0. [DOI] [PubMed] [Google Scholar]
- Dimsdale JE, Coy T, Ziegler MG, Ancoli-Israel S, Clausen J. The effect of sleep apnea on plasma and urinary catecholamines. Sleep. 1995;18(5):377–381. [PubMed] [Google Scholar]
- Donnelly DF. Development of carotid body/petrosal ganglion response to hypoxia. Respir Physiol Neurobiol. 2005;149(1–3):191–199. doi: 10.1016/j.resp.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Donnelly DF, Doyle TP. Developmental changes in hypoxia-induced catecholamine release from rat carotid body, in vitro. J Physiol. 1994;475(2):267–275. doi: 10.1113/jphysiol.1994.sp020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducsay CA, Hyatt K, Mlynarczyk M, Root BK, Kaushal KM, Myers DA. Long-term hypoxia modulates expression of key genes regulating adrenomedullary function in the late gestation ovine fetus. Am J Physiol Regul Integr Comp Physiol. 2007;293(5):R1997–2005. doi: 10.1152/ajpregu.00313.2007. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Huynh TT, Elkahloun A, Morris JC, Bratslavsky G, Linehan WM, et al. Differential expression of the regulated catecholamine secretory pathway in different hereditary forms of pheochromocytoma. Am J Physiol Endocrinol Metab. 2008;295(5):E1223–1233. doi: 10.1152/ajpendo.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Huynh TT, Pacak K, Brouwers FM, Walther MM, Linehan WM, et al. Distinct gene expression profiles in norepinephrine- and epinephrine-producing hereditary and sporadic pheochromocytomas: activation of hypoxia-driven angiogenic pathways in von Hippel-Lindau syndrome. Endocr Relat Cancer. 2004;11(4):897–911. doi: 10.1677/erc.1.00838. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lenders JW, Siegert G, Bornstein SR, Friberg P, Milosevic D, et al. Plasma methoxytyramine: a novel biomarker of metastatic pheochromocytoma and paraganglioma in relation to established risk factors of tumour size, location and SDHB mutation status. Eur J Cancer. 2012;48(11):1739–1749. doi: 10.1016/j.ejca.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Pacak K, Huynh TT, Qin N, Bratslavsky G, Linehan WM, et al. Catecholamine metabolomic and secretory phenotypes in phaeochromocytoma. Endocr Relat Cancer. 2011;18(1):97–111. doi: 10.1677/ERC-10-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Timmers HJ, Lenders JW, Bornstein SR, Tiebel O, Mannelli M, et al. Age at diagnosis of pheochromocytoma differs according to catecholamine phenotype and tumor location. J Clin Endocrinol Metab. 2011;96(2):375–384. doi: 10.1210/jc.2010-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhofer G, Walther MM, Huynh TT, Li ST, Bornstein SR, Vortmeyer A, et al. Pheochromocytomas in von Hippel-Lindau syndrome and multiple endocrine neoplasia type 2 display distinct biochemical and clinical phenotypes. J Clin Endocrinol Metab. 2001;86(5):1999–2008. doi: 10.1210/jcem.86.5.7496. [DOI] [PubMed] [Google Scholar]
- Estus S, Zaks WJ, Freeman RS, Gruda M, Bravo R, Johnson EM., Jr Altered gene expression in neurons during programmed cell death: identification of c-jun as necessary for neuronal apoptosis. J Cell Biol. 1994;127(6 Pt 1):1717–1727. doi: 10.1083/jcb.127.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evinger MJ, Cikos S, Nwafor-Anene V, Powers JF, Tischler AS. Hypoxia activates multiple transcriptional pathways in mouse pheochromocytoma cells. Ann N Y Acad Sci. 2002;971:61–65. doi: 10.1111/j.1749-6632.2002.tb04434.x. [DOI] [PubMed] [Google Scholar]
- Favier J, Briere JJ, Burnichon N, Riviere J, Vescovo L, Benit P, et al. The Warburg effect is genetically determined in inherited pheochromocytomas. PLoS One. 2009;4(9):e7094. doi: 10.1371/journal.pone.0007094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier J, Buffet A, Gimenez-Roqueplo AP. HIF2A mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(22):2161. doi: 10.1056/NEJMc1211953. author reply 2161–2162. [DOI] [PubMed] [Google Scholar]
- Favier J, Igaz P, Burnichon N, Amar L, Libe R, Badoual C, et al. Rationale for anti- angiogenic therapy in pheochromocytoma and paraganglioma. Endocr Pathol. 2012;23(1):34–42. doi: 10.1007/s12022-011-9189-0. [DOI] [PubMed] [Google Scholar]
- Favier J, Kempf H, Corvol P, Gasc JM. Cloning and expression pattern of EPAS1 in the chicken embryo. Colocalization with tyrosine hydroxylase. FEBS Lett. 1999;462(1–2):19–24. doi: 10.1016/s0014-5793(99)01476-3. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Ishii K, Nanmoku T, Isobe K, Kawakami Y, Takekoshi K. 5-Aminoimidazole- 4-carboxamide-1-beta-4-ribofuranoside stimulates tyrosine hydroxylase activity and catecholamine secretion by activation of AMP-activated protein kinase in PC12 cells. J Neuroendocrinol. 2007;19(8):621–631. doi: 10.1111/j.1365-2826.2007.01570.x. [DOI] [PubMed] [Google Scholar]
- Gamboa A, Gamboa JL, Holmes C, Sharabi Y, Leon-Velarde F, Fischman GJ, et al. Plasma catecholamines and blood volume in native Andeans during hypoxia and normoxia. Clin Auton Res. 2006;16(1):40–45. doi: 10.1007/s10286-006-0305-z. [DOI] [PubMed] [Google Scholar]
- Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI. Hypoxia-inducible factor-2 alpha (HIF-2 alpha) induces angiogenesis in breast carcinomas. Appl Immunohistochem Mol Morphol. 2006;14(1):78–82. doi: 10.1097/01.pai.0000145182.98577.10. [DOI] [PubMed] [Google Scholar]
- Gimenez-Roqueplo AP, Favier J, Rustin P, Rieubland C, Crespin M, Nau V, et al. Mutations in the SDHB gene are associated with extra-adrenal and/or malignant phaeochromocytomas. Cancer Res. 2003;63(17):5615–5621. [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11(4):335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyzyk-Krzeska MF, et al. Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol. 2005;99(2):642–649. doi: 10.1152/japplphysiol.00880.2004. [DOI] [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9(5):617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Ham J, Babij C, Whitfield J, Pfarr CM, Lallemand D, Yaniv M, et al. A c-Jun dominant negative mutant protects sympathetic neurons against programmed cell death. Neuron. 1995;14(5):927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- Holmquist-Mengelbier L, Fredlund E, Lofstedt T, Noguera R, Navarro S, Nilsson H, et al. Recruitment of HIF-1alpha and HIF-2alpha to common target genes is differentially regulated in neuroblastoma: HIF-2alpha promotes an aggressive phenotype. Cancer Cell. 2006;10(5):413– 423. doi: 10.1016/j.ccr.2006.08.026. [DOI] [PubMed] [Google Scholar]
- Hu CJ, Iyer S, Sataur A, Covello KL, Chodosh LA, Simon MC. Differential regulation of the transcriptional activities of hypoxia-inducible factor 1 alpha (HIF-1alpha) and HIF-2alpha in stem cells. Mol Cell Biol. 2006;26(9):3514–3526. doi: 10.1128/MCB.26.9.3514-3526.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia- inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23(24):9361–9374. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui AS, Striet JB, Gudelsky G, Soukhova GK, Gozal E, Beitner-Johnson D, et al. Regulation of catecholamines by sustained and intermittent hypoxia in neuroendocrine cells and sympathetic neurons. Hypertension. 2003;42(6):1130–1136. doi: 10.1161/01.HYP.0000101691.12358.26. [DOI] [PubMed] [Google Scholar]
- Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, et al. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120(8):2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jogi A, Ora I, Nilsson H, Lindeheim A, Makino Y, Poellinger L, et al. Hypoxia alters gene expression in human neuroblastoma cells toward an immature and neural crest-like phenotype. Proc Natl Acad Sci U S A. 2002;99(10):7021–7026. doi: 10.1073/pnas.102660199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JL, Sager TN, Lotharius J, Witten L, Mork A, Egebjerg J, et al. HIF prolyl hydroxylase inhibition increases cell viability and potentiates dopamine release in dopaminergic cells. J Neurochem. 2010;115(1):209–219. doi: 10.1111/j.1471-4159.2010.06917.x. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Jr, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30(4):393–402. doi: 10.1016/j.molcel.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Kanstrup IL, Poulsen TD, Hansen JM, Andersen LJ, Bestle MH, Christensen NJ, et al. Blood pressure and plasma catecholamines in acute and prolonged hypoxia: effects of local hypothermia. J Appl Physiol. 1999;87(6):2053–2058. doi: 10.1152/jappl.1999.87.6.2053. [DOI] [PubMed] [Google Scholar]
- Keith B, Johnson RS, Simon MC. HIF1alpha and HIF2alpha: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12(1):9–22. doi: 10.1038/nrc3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, et al. HIF2alpha cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest. 2009;119(8):2160–2170. doi: 10.1172/JCI38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KS, Prodanov T, Kantorovich V, Fojo T, Hewitt JK, Zacharin M, et al. Metastatic pheochromocytoma/paraganglioma related to primary tumor development in childhood or adolescence: significant link to SDHB mutations. J Clin Oncol. 2011;29(31):4137–4142. doi: 10.1200/JCO.2011.34.6353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Morita S, Sawada H, Mizuguchi T, Yamada K, Nagatsu I, et al. Targeted disruption of the tyrosine hydroxylase locus results in severe catecholamine depletion and perinatal lethality in mice. J Biol Chem. 1995;270(45):27235–27243. doi: 10.1074/jbc.270.45.27235. [DOI] [PubMed] [Google Scholar]
- Koh MY, Darnay BG, Powis G. Hypoxia-associated factor, a novel E3-ubiquitin ligase, binds and ubiquitinates hypoxia-inducible factor 1alpha, leading to its oxygen-independent degradation. Mol Cell Biol. 2008;28(23):7081–7095. doi: 10.1128/MCB.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MY, Lemos R, Jr, Liu X, Powis G. The hypoxia-associated factor switches cells from HIF-1alpha- to HIF-2alpha-dependent signaling promoting stem cell characteristics, aggressive tumor growth and invasion. Cancer Res. 2011;71(11):4015–4027. doi: 10.1158/0008-5472.CAN-10-4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MY, Powis G. Passing the baton: the HIF switch. Trends Biochem Sci. 2012;37(9):364–372. doi: 10.1016/j.tibs.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Kim WY, Lechpammer M, Kaelin WG., Jr Inhibition of HIF2alpha is sufficient to suppress pVHL-defective tumor growth. PLoS Biol. 2003;1(3):E83. doi: 10.1371/journal.pbio.0000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar GK, Overholt JL, Bright GR, Hui KY, Lu H, Gratzl M, et al. Release of dopamine and norepinephrine by hypoxia from PC-12 cells. Am J Physiol. 1998;274(6 Pt 1):C1592– 1600. doi: 10.1152/ajpcell.1998.274.6.C1592. [DOI] [PubMed] [Google Scholar]
- Kumar GK, Rai V, Sharma SD, Ramakrishnan DP, Peng YJ, Souvannakitti D, et al. Chronic intermittent hypoxia induces hypoxia-evoked catecholamine efflux in adult rat adrenal medulla via oxidative stress. J Physiol. 2006;575(Pt 1):229–239. doi: 10.1113/jphysiol.2006.112524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SY, Tipoe GL, Liong EC, Fung ML. Differential expressions and roles of hypoxia- inducible factor-1alpha, -2alpha and -3alpha in the rat carotid body during chronic and intermittent hypoxia. Histol Histopathol. 2008;23(3):271–280. doi: 10.14670/HH-23.271. [DOI] [PubMed] [Google Scholar]
- Lee S, Nakamura E, Yang H, Wei W, Linggi MS, Sajan MP, et al. Neuronal apoptosis linked to EglN3 prolyl hydroxylase and familial pheochromocytoma genes: developmental culling and cancer. Cancer Cell. 2005;8(2):155–167. doi: 10.1016/j.ccr.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15(6):501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb EA, Sarmiere PD, Crowder RJ, Freeman RS. Expression of the SM-20 gene promotes death in nerve growth factor-dependent sympathetic neurons. J Neurochem. 1999;73(1):429–432. doi: 10.1046/j.1471-4159.1999.0730429.x. [DOI] [PubMed] [Google Scholar]
- Lipscomb EA, Sarmiere PD, Freeman RS. SM-20 is a novel mitochondrial protein that causes caspase-dependent cell death in nerve growth factor-dependent neurons. J Biol Chem. 2001;276(7):5085–5092. doi: 10.1074/jbc.M008407200. [DOI] [PubMed] [Google Scholar]
- Lomb DJ, Desouza LA, Franklin JL, Freeman RS. Prolyl hydroxylase inhibitors depend on extracellular glucose and hypoxia-inducible factor (HIF)-2alpha to inhibit cell death caused by nerve growth factor (NGF) deprivation: evidence that HIF-2alpha has a role in NGF-promoted survival of sympathetic neurons. Mol Pharmacol. 2009;75(5):1198–1209. doi: 10.1124/mol.108.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Jimenez E, Gomez-Lopez G, Leandro-Garcia LJ, Munoz I, Schiavi F, Montero-Conde C, et al. Research resource: Transcriptional profiling reveals different pseudohypoxic signatures in SDHB and VHL-related pheochromocytomas. Mol Endocrinol. 2010;24(12):2382– 2391. doi: 10.1210/me.2010-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo FR, Yang C, Tang Ng, Fui M, Vankayalapati H, Zhuang Z, Huynh T, et al. A novel EPAS1/HIF2A germline mutation in a congenital polycythemia with paraganglioma. J Mol Med (Berl) 2012 doi: 10.1007/s00109-012-0967-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamet J, Peyronnet J, Roux JC, Perrin D, Cottet-Emard JM, Pequignot JM, et al. Long-term prenatal hypoxia alters maturation of adrenal medulla in rat. Pediatr Res. 2002;51(2):207–214. doi: 10.1203/00006450-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Mannelli M, Castellano M, Schiavi F, Filetti S, Giacche M, Mori L, et al. Clinically guided genetic screening in a large cohort of italian patients with pheochromocytomas and/or functional or nonfunctional paragangliomas. J Clin Endocrinol Metab. 2009;94(5):1541–1547. doi: 10.1210/jc.2008-2419. [DOI] [PubMed] [Google Scholar]
- Maranchie JK, Vasselli JR, Riss J, Bonifacino JS, Linehan WM, Klausner RD. The contribution of VHL substrate binding and HIF1-alpha to the phenotype of VHL loss in renal cell carcinoma. Cancer Cell. 2002;1(3):247–255. doi: 10.1016/s1535-6108(02)00044-2. [DOI] [PubMed] [Google Scholar]
- Mastrogiannaki M, Matak P, Keith B, Simon MC, Vaulont S, Peyssonnaux C. HIF- 2alpha, but not HIF-1alpha, promotes iron absorption in mice. J Clin Invest. 2009;119(5):1159– 1166. doi: 10.1172/JCI38499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, Hickey MM, Pant DK, Durham AC, Sweet-Cordero A, Vachani A, et al. HIF-2alpha deletion promotes Kras-driven lung tumor development. Proc Natl Acad Sci U S A. 2010;107(32):14182–14187. doi: 10.1073/pnas.1001296107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazumdar J, O’Brien WT, Johnson RS, LaManna JC, Chavez JC, Klein PS, et al. O2 regulates stem cells through Wnt/beta-catenin signalling. Nat Cell Biol. 2010;12(10):1007–1013. doi: 10.1038/ncb2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojet MH, Mills E, Duchen MR. Hypoxia-induced catecholamine secretion in isolated newborn rat adrenal chromaffin cells is mimicked by inhibition of mitochondrial respiration. J Physiol. 1997;504(Pt 1):175–189. doi: 10.1111/j.1469-7793.1997.175bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Csete M, Groves AK, Melega W, Wold B, Anderson DJ. Culture in reduced levels of oxygen promotes clonogenic sympathoadrenal differentiation by isolated neural crest stem cells. J Neurosci. 2000;20(19):7370–7376. doi: 10.1523/JNEUROSCI.20-19-07370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naranjo-Suarez S, Castellanos MC, Alvarez-Tejado M, Vara A, Landazuri MO, del Peso L. Down-regulation of hypoxia-inducible factor-2 in PC12 cells by nerve growth factor stimulation. J Biol Chem. 2003;278(34):31895–31901. doi: 10.1074/jbc.M304079200. [DOI] [PubMed] [Google Scholar]
- Neumann HP, Bausch B, McWhinney SR, Bender BU, Gimm O, Franke G, et al. Germ-line mutations in nonsyndromic pheochromocytoma. N Engl J Med. 2002;346(19):1459– 1466. doi: 10.1056/NEJMoa020152. [DOI] [PubMed] [Google Scholar]
- Nilsson H, Jogi A, Beckman S, Harris AL, Poellinger L, Pahlman S. HIF-2alpha expression in human fetal paraganglia and neuroblastoma: relation to sympathetic differentiation, glucose deficiency, and hypoxia. Exp Cell Res. 2005;303(2):447–456. doi: 10.1016/j.yexcr.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Noguera R, Fredlund E, Piqueras M, Pietras A, Beckman S, Navarro S, et al. HIF-1alpha and HIF-2alpha are differentially regulated in vivo in neuroblastoma: high HIF-1alpha correlates negatively to advanced clinical stage and tumor vascularization. Clin Cancer Res. 2009;15(23):7130–7136. doi: 10.1158/1078-0432.CCR-09-0223. [DOI] [PubMed] [Google Scholar]
- Nolting S, Grossman AB. Signaling pathways in pheochromocytomas and paragangliomas: prospects for future therapies. Endocr Pathol. 2012;23(1):21–33. doi: 10.1007/s12022-012-9199-6. [DOI] [PubMed] [Google Scholar]
- Pacak KJI, Prodanov T, Yang C, Merino M, Fojo T, Prchal JT, Tischler AS, Lechan RM, Zhuang Z. A new syndrome of paraganglioma and somatostatinoma associated with polycythemia. Journal of clinical oncology. 2013 doi: 10.1200/JCO.2012.47.1912. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padbury JF. Functional maturation of the adrenal medulla and peripheral sympathetic nervous system. Baillieres Clin Endocrinol Metab. 1989;3(3):689–705. doi: 10.1016/s0950-351x(89)80049-7. [DOI] [PubMed] [Google Scholar]
- Paulick R, Kastendieck E, Wernze H. Catecholamines in arterial and venous umbilical blood: placental extraction, correlation with fetal hypoxia, and transcutaneous partial oxygen tension. J Perinat Med. 1985;13(1):31–42. doi: 10.1515/jpme.1985.13.1.31. [DOI] [PubMed] [Google Scholar]
- Peng J, Zhang L, Drysdale L, Fong GH. The transcription factor EPAS-1/hypoxia- inducible factor 2alpha plays an important role in vascular remodeling. Proc Natl Acad Sci U S A. 2000;97(15):8386–8391. doi: 10.1073/pnas.140087397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S, Bogenmann E. The RET and TRKA pathways collaborate to regulate neuroblastoma differentiation. Oncogene. 2004;23(1):213–225. doi: 10.1038/sj.onc.1206980. [DOI] [PubMed] [Google Scholar]
- Petrella BL, Lohi J, Brinckerhoff CE. Identification of membrane type-1 matrix metalloproteinase as a target of hypoxia-inducible factor-2 alpha in von Hippel-Lindau renal cell carcinoma. Oncogene. 2005;24(6):1043–1052. doi: 10.1038/sj.onc.1208305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillippe M. Fetal catecholamines. Am J Obstet Gynecol. 1983;146(7):840–855. doi: 10.1016/0002-9378(83)91088-8. [DOI] [PubMed] [Google Scholar]
- Pietras A, Gisselsson D, Ora I, Noguera R, Beckman S, Navarro S, et al. High levels of HIF-2alpha highlight an immature neural crest-like neuroblastoma cell cohort located in a perivascular niche. J Pathol. 2008;214(4):482–488. doi: 10.1002/path.2304. [DOI] [PubMed] [Google Scholar]
- Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, et al. HIF- 2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor- initiating cells. Proc Natl Acad Sci U S A. 2009;106(39):16805–16810. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras A, von Stedingk K, Lindgren D, Pahlman S, Axelson H. JAG2 induction in hypoxic tumor cells alters Notch signaling and enhances endothelial cell tube formation. Mol Cancer Res. 2011;9(5):626–636. doi: 10.1158/1541-7786.MCR-10-0508. [DOI] [PubMed] [Google Scholar]
- Pollard PJ, El-Bahrawy M, Poulsom R, Elia G, Killick P, Kelly G, et al. Expression of HIF- 1alpha, HIF-2alpha (EPAS1), and their target genes in paraganglioma and pheochromocytoma with VHL and SDH mutations. J Clin Endocrinol Metab. 2006;91(11):4593–4598. doi: 10.1210/jc.2006-0920. [DOI] [PubMed] [Google Scholar]
- Pomares FJ, Canas R, Rodriguez JM, Hernandez AM, Parrilla P, Tebar FJ. Differences between sporadic and multiple endocrine neoplasia type 2A phaeochromocytoma. Clin Endocrinol (Oxf) 1998;48(2):195–200. doi: 10.1046/j.1365-2265.1998.3751208.x. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR, Kumar GK. Mechanisms of sympathetic activation and blood pressure elevation by intermittent hypoxia. Respir Physiol Neurobiol. 2010;174(1–2):156–161. doi: 10.1016/j.resp.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Yao L, King EE, Buddavarapu K, Lenci RE, Chocron ES, et al. Germline mutations in TMEM127 confer susceptibility to pheochromocytoma. Nat Genet. 2010;42(3):229– 233. doi: 10.1038/ng.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghuraman G, Prabhakar NR, Kumar GK. Differential regulation of tyrosine hydroxylase by continuous and intermittent hypoxia. Adv Exp Med Biol. 2012;758:381–385. doi: 10.1007/978-94-007-4584-1_51. [DOI] [PubMed] [Google Scholar]
- Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, et al. Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest. 2007;117(4):1068–1077. doi: 10.1172/JCI30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rankin EB, Rha J, Unger TL, Wu CH, Shutt HP, Johnson RS, et al. Hypoxia- inducible factor-2 regulates vascular tumorigenesis in mice. Oncogene. 2008;27(40):5354–5358. doi: 10.1038/onc.2008.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raval RR, Lau KW, Tran MG, Sowter HM, Mandriota SJ, Li JL, et al. Contrasting properties of hypoxia-inducible factor 1 (HIF-1) and HIF-2 in von Hippel-Lindau-associated renal cell carcinoma. Mol Cell Biol. 2005;25(13):5675–5686. doi: 10.1128/MCB.25.13.5675-5686.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ream MA, Chandra R, Peavey M, Ray AM, Roffler-Tarlov S, Kim HG, et al. High oxygen prevents fetal lethality due to lack of catecholamines. Am J Physiol Regul Integr Comp Physiol. 2008;295(3):R942–953. doi: 10.1152/ajpregu.00860.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricketts CJ, Forman JR, Rattenberry E, Bradshaw N, Lalloo F, Izatt L, et al. Tumor risks and genotype-phenotype-proteotype analysis in 358 patients with germline mutations in SDHB and SDHD. Hum Mutat. 2010;31(1):41–51. doi: 10.1002/humu.21136. [DOI] [PubMed] [Google Scholar]
- Roda JM, Wang Y, Sumner LA, Phillips GS, Marsh CB, Eubank TD. Stabilization of HIF-2alpha induces sVEGFR-1 production from tumor-associated macrophages and decreases tumor growth in a murine melanoma model. J Immunol. 2012;189(6):3168–3177. doi: 10.4049/jimmunol.1103817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cuevas S, Lopez-Garza J, Labastida-Almendaro S. Carotid body tumors in inhabitants of altitudes higher than 2000 meters above sea level. Head Neck. 1998;20(5):374–378. doi: 10.1002/(sici)1097-0347(199808)20:5<374::aid-hed3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Sato M, Tanaka T, Maemura K, Uchiyama T, Sato H, Maeno T, et al. The PAI-1 gene as a direct target of endothelial PAS domain protein-1 in adenocarcinoma A549 cells. Am J Respir Cell Mol Biol. 2004;31(2):209–215. doi: 10.1165/rcmb.2003-0296OC. [DOI] [PubMed] [Google Scholar]
- Schlingensiepen KH, Wollnik F, Kunst M, Schlingensiepen R, Herdegen T, Brysch W. The role of Jun transcription factor expression and phosphorylation in neuronal differentiation, neuronal cell death, and plastic adaptations in vivo. Cell Mol Neurobiol. 1994;14(5):487–505. doi: 10.1007/BF02088833. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Garcia C, Soulier V, Pujol JF, Pequignot JM. Influence of long-term hypoxia on tyrosine hydroxylase in the rat carotid body and adrenal gland. J Auton Nerv Syst. 1992;40(1):13–19. doi: 10.1016/0165-1838(92)90221-2. [DOI] [PubMed] [Google Scholar]
- Schmitt P, Pequignot J, Hanchin F, Pujol JF, Pequignot JM. Regional specificity of long-term regulation of tyrosine hydroxylase in some catecholaminergic rat brainstem areas. II. Effect of a chronic dihydralazine treatment. Brain Res. 1993;611(1):61–66. doi: 10.1016/0006-8993(93)91777-p. [DOI] [PubMed] [Google Scholar]
- Schnell PO, Ignacak ML, Bauer AL, Striet JB, Paulding WR, Czyzyk-Krzeska MF. Regulation of tyrosine hydroxylase promoter activity by the von Hippel-Lindau tumor suppressor protein and hypoxia-inducible transcription factors. J Neurochem. 2003;85(2):483–491. doi: 10.1046/j.1471-4159.2003.01696.x. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Ding K, Zhang Q, Oktay Y, Bennett MJ, Bennett M, et al. HIF-2alpha regulates murine hematopoietic development in an erythropoietin-dependent manner. Blood. 2005;105(8):3133–3140. doi: 10.1182/blood-2004-05-1695. [DOI] [PubMed] [Google Scholar]
- Scortegagna M, Morris MA, Oktay Y, Bennett M, Garcia JA. The HIF family member EPAS1/HIF-2alpha is required for normal hematopoiesis in mice. Blood. 2003;102(5):1634–1640. doi: 10.1182/blood-2003-02-0448. [DOI] [PubMed] [Google Scholar]
- Selak MA, Armour SM, MacKenzie ED, Boulahbel H, Watson DG, Mansfield KD, et al. Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-alpha prolyl hydroxylase. Cancer Cell. 2005;7(1):77–85. doi: 10.1016/j.ccr.2004.11.022. [DOI] [PubMed] [Google Scholar]
- Skuli N, Majmundar AJ, Krock BL, Mesquita RC, Mathew LK, Quinn ZL, et al. Endothelial HIF-2alpha regulates murine pathological angiogenesis and revascularization processes. J Clin Invest. 2012;122(4):1427–1443. doi: 10.1172/JCI57322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EH, Janknecht R, Maher LJ., 3rd Succinate inhibition of alpha-ketoglutarate- dependent enzymes in a yeast model of paraganglioma. Hum Mol Genet. 2007;16(24):3136–3148. doi: 10.1093/hmg/ddm275. [DOI] [PubMed] [Google Scholar]
- Souvannakitti D, Nanduri J, Yuan G, Kumar GK, Fox AP, Prabhakar NR. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci. 2010;30(32):10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub JA, Lipscomb EA, Yoshida ES, Freeman RS. Induction of SM-20 in PC12 cells leads to increased cytochrome c levels, accumulation of cytochrome c in the cytosol, and caspase-dependent cell death. J Neurochem. 2003;85(2):318–328. doi: 10.1046/j.1471-4159.2003.01688.x. [DOI] [PubMed] [Google Scholar]
- Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, et al. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24(5):491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157(2):411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Mo C, Wang Y, Wei D, Xiao H. Anti-tumour strategies aiming to target tumour- associated macrophages. Immunology. 2013;138(2):93–104. doi: 10.1111/imm.12023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Batten TF, Peers C. Hypoxic enhancement of quantal catecholamine secretion. Evidence for the involvement of amyloid beta-peptides. J Biol Chem. 1999;274(44):31217–31222. doi: 10.1074/jbc.274.44.31217. [DOI] [PubMed] [Google Scholar]
- Thomas SA, Matsumoto AM, Palmiter RD. Noradrenaline is essential for mouse fetal development. Nature. 1995;374(6523):643–646. doi: 10.1038/374643a0. [DOI] [PubMed] [Google Scholar]
- Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia- responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12(21):3320– 3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui-Pierchala BA, Milbrandt J, Johnson EM., Jr NGF utilizes c-Ret via a novel GFL- independent, inter-RTK signaling mechanism to maintain the trophic status of mature sympathetic neurons. Neuron. 2002;33(2):261–273. doi: 10.1016/s0896-6273(01)00585-2. [DOI] [PubMed] [Google Scholar]
- Vanharanta S, Shu W, Brenet F, Hakimi AA, Heguy A, Viale A, et al. Epigenetic expansion of VHL-HIF signal output drives multiorgan metastasis in renal cancer. Nat Med. 2013;19(1):50–56. doi: 10.1038/nm.3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldmann J, Fendrich V, Holler J, Buchholz M, Heinmoller E, Langer P, et al. Microarray analysis reveals differential expression of benign and malignant pheochromocytoma. Endocr Relat Cancer. 2010;17(3):743–756. doi: 10.1677/ERC-09-0118. [DOI] [PubMed] [Google Scholar]
- Walther MM, Herring J, Enquist E, Keiser HR, Linehan WM. von Recklinghausen’s disease and pheochromocytomas. J Urol. 1999;162(5):1582–1586. [PubMed] [Google Scholar]
- Wang X, Schneider A. HIF-2alpha-mediated activation of the epidermal growth factor receptor potentiates head and neck cancer cell migration in response to hypoxia. Carcinogenesis. 2010;31(7):1202–1210. doi: 10.1093/carcin/bgq078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnecke C, Zaborowska Z, Kurreck J, Erdmann VA, Frei U, Wiesener M, et al. Differentiating the functional role of hypoxia-inducible factor (HIF)-1alpha and HIF-2alpha (EPAS-1) by the use of RNA interference: erythropoietin is a HIF-2alpha target gene in Hep3B and Kelly cells. FASEB J. 2004;18(12):1462–1464. doi: 10.1096/fj.04-1640fje. [DOI] [PubMed] [Google Scholar]
- Wong DL, Tai TC, Wong-Faull DC, Claycomb R, Siddall BJ, Bell RA, et al. Stress and adrenergic function: HIF1alpha, a potential regulatory switch. Cell Mol Neurobiol. 2010;30(8):1451–1457. doi: 10.1007/s10571-010-9567-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270(5240):1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xiao M, Yang H, Xu W, Ma S, Lin H, Zhu H, et al. Inhibition of alpha-KG-dependent histone and DNA demethylases by fumarate and succinate that are accumulated in mutations of FH and SDH tumor suppressors. Genes Dev. 2012;26(12):1326–1338. doi: 10.1101/gad.191056.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Ohneda K, Nagano M, Miyoshi C, Kaneko N, Miwa Y, et al. Hypoxia- inducible transcription factor-2alpha in endothelial cells regulates tumor neovascularization through activation of ephrin A1. J Biol Chem. 2008;283(27):18926–18936. doi: 10.1074/jbc.M709133200. [DOI] [PubMed] [Google Scholar]
- Yang C, Sun MG, Matro J, Huynh TT, Rahimpour S, Prchal JT, et al. Novel HIF2A mutations disrupt oxygen sensing leading to polycythemia, paragangliomas and somatostatinomas. Blood. 2013 doi: 10.1182/blood-2012-10-460972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Kim J, Ryu JH, Oh H, Chun CH, Kim BJ, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16(6):687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- Yuan HT, Khankin EV, Karumanchi SA, Parikh SM. Angiopoietin 2 is a partial agonist/antagonist of Tie2 signaling in the endothelium. Mol Cell Biol. 2009;29(8):2011–2022. doi: 10.1128/MCB.01472-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou QY, Quaife CJ, Palmiter RD. Targeted disruption of the tyrosine hydroxylase gene reveals that catecholamines are required for mouse fetal development. Nature. 1995;374(6523):640–643. doi: 10.1038/374640a0. [DOI] [PubMed] [Google Scholar]
- Zhuang Z, Yang C, Lorenzo F, Merino M, Fojo T, Kebebew E, et al. Somatic HIF2A gain- of-function mutations in paraganglioma with polycythemia. N Engl J Med. 2012;367(10):922–930. doi: 10.1056/NEJMoa1205119. [DOI] [PMC free article] [PubMed] [Google Scholar]