Abstract
We used wild-type (WT) mice and mice engineered to express either apoB-100 only (B100 mice) or apoB-48 only (B48 mice) to examine the effects of streptozotocin-induced diabetes (DM) on apoB-100– and apoB-48–containing lipoproteins. Plasma lipids increased with DM in WT mice, and fat tolerance was markedly impaired. Lipoprotein profiles showed increased levels and cholesterol enrichment of VLDL in diabetic B48 mice but not in B100 mice. C apolipoproteins, in particular apoC-I in VLDL, were increased. To investigate the basis of the increase in apoB-48 lipoproteins in streptozotocin-treated animals, we characterized several parameters of lipoprotein metabolism. Triglyceride and apoB production rates were normal, as were plasma lipase activity, VLDL glycosaminoglycan binding, and VLDL lipolysis. However, β-VLDL clearance decreased due to decreased trapping by the liver. Whereas LRP activity was normal, livers from treated mice incorporated significantly less sulfate into heparan sulfate proteoglycans (HSPG) than did controls. Hepatoma (HepG2) cells and endothelial cells cultured in high glucose also showed decreased sulfate and glucosamine incorporation into HSPG. Western blots of livers from diabetic mice showed a decrease in the HSPG core protein, perlecan. Delayed clearance of postprandial apoB-48–containing lipoproteins in DM appears to be due to decreased hepatic perlecan HSPG.
Introduction
Insulin-deficient diabetes mellitus (Type I DM) is associated with a striking increase in the risk of atherosclerotic disease that is of uncertain origin (1, 2). Modest abnormalities of fasting lipid levels, most commonly hypertriglyceridemia, have been observed in Type I DM (3) but are absent in patients with good glycemic control (4). However, potentially atherogenic lipoprotein compositional abnormalities are observed persistently, consisting of increased numbers and cholesterol enrichment of the smaller VLDL particles (5–8). Patients with Type I DM also show delayed clearance of postprandial lipoproteins (9). Delayed clearance of postprandial lipoproteins and the accumulation of cholesterol-enriched “remnant” particles have been associated with the development of coronary heart disease in nondiabetics (10–13). Such abnormalities may play a particularly important role in the pathogenesis of the atherosclerotic complications of DM.
Chylomicron remnant clearance recently has been reviewed comprehensively (14). The mechanism of delayed chylomicron remnant clearance in DM remains unclear. Lipoprotein lipase (LPL), the rate-limiting enzyme for triglyceride (TG) hydrolysis in plasma, is an insulin-regulated enzyme, and decreased LPL activity has been observed under some circumstances in Type I DM (15, 16). Moreover, the infusion of insulin and glucose into normal subjects leads to a rapid increase in LPL activity (17). DM-induced hypertriglyceridemia has been prevented in rats and in transgenic mice by increased LPL expression (18, 19). ApoC-III is an insulin-regulated VLDL constituent that acts to decrease lipolysis, in part through decreasing VLDL interaction with the glycosaminoglycan (GAG) matrix, where cell-surface lipolytic enzymes reside (20–23). ApoC-III also decreases the interaction of lipoproteins with lipoprotein receptors (24–26). Increased expression of apoC-III due to insulin deficiency or resistance might play an important role in the hypertriglyceridemia of DM (27, 28).
A defect in the LDL receptor–related protein (LRP) pathway may also be a cause for the chylomicron remnant-clearance defect in DM. In humans, dietary (intestinal) lipoproteins contain the truncated form of apoB, apoB-48, whereas nondietary (hepatic) lipoproteins contain the full-length form of apoB, apoB-100. The apoB-containing lipoproteins have two well-defined catabolic pathways. The LDL receptor (LDL-R) interacts with apoB-100, leading to the removal of LDL, and with apoE on VLDL and chylomicron remnants, leading to their clearance (25). Genetic deficiency of the LDL-R (familial hypercholesterolemia) leads to marked LDL elevation, confirming the essential role for this receptor in LDL metabolism. However, the clearance of remnant lipoproteins is not severely affected in this disorder (29). The LRP has been shown to provide an alternative pathway for removal of these lipoproteins (30–32). LRP does not interact directly with apoB and uses apoE as its principal apolipoprotein ligand (33).
We hypothesized that the decreased clearance of postprandial lipoproteins in DM is due primarily to a specific defect in catabolic pathways required for apoB-48 TG-rich lipoproteins. LRP expression is positively regulated by insulin (34), and elevated apoB-48, along with delayed postprandial lipid clearance, has been reported in human Type II DM (35). Though much of the postprandial increase in lipids in humans is observed in apoB-100–containing particles (36), the extent of this increase may be driven by the rate of clearance of intestinally derived apoB-48 particles.
We tested this hypothesis in wild-type mice and in gene-targeted mice that express exclusively either apoB-48 or apoB-100, which were made diabetic by the injection of the pancreatic islet–cell toxin streptozotocin (37). We observed a selective decrease in the catabolism of apoB-48–containing lipoproteins in streptozotocin-treated mice, and we demonstrate an associated decrease, not in LRP, but in heparan sulfate proteoglycans (HSPG) that is its likely explanation.
Methods
Animals.
Mice were caged in an approved animal care facility with a period of light from 0700 to 1900. Except as indicated, animals were fed a standard mouse chow diet containing 4.5% fat (10% of calories) and 0.02% cholesterol. Access to food was ad libitum, except as indicated. Fasting blood was drawn at 1700, 8 hours after the removal of food. Nonfasted blood was drawn at 0900. Animals were anesthetized with methoxyflurane for retro-orbital plexus phlebotomy and femoral vein intravenous injections. One hundred microliters of plasma was required for analyses, and animals were allowed to recover for 10 days between phlebotomies.
C57BL/6 mice were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). Mice secreting only apoB-100 (available from the Jackson Laboratory as B6,129S-Apobtm2Sgy) and mice secreting only apoB-48 (available from the Jackson Laboratory as B6,129S-Apobtm1Sgy), in an equally mixed genetic background of 129/SV and C57BL/6, were the gift of Stephen G. Young (Gladstone Institute, University of California at San Francisco, San Francisco, California, USA). The mice were produced by gene targeting in embryonic stem cells (38). The gene-targeted animals were outbred and exhibited greater variability, leading to lesser statistical power to detect differences in lipid parameters than in the C57BL/6 mice.
DM was induced in male mice by intraperitoneal injection of streptozotocin, 50 mg/kg, on 5 successive days as described by Kunjathoor et al. (39). Studies were performed beginning 4 weeks after streptozotocin injection. Mice were of similar age and genetic background in all experiments.
Lipoprotein analysis.
TG and cholesterol concentrations were measured using Boehringer Mannheim commercial kits (Roche Molecular Biochemicals, Indianapolis, Indiana, USA) on a Hitachi autoanalyzer model 705 (Hitachi Instruments Inc., San Jose, California, USA). Lipoprotein fractions were isolated from three pools, each containing plasma from three animals, for each genotype/phenotype. Mean values are shown. VLDL (d < 1.006 g/mL), IDL plus LDL (d = 1.006–1.063 g/mL), and HDL (d = 1.063–1.21 g/mL) were separated by sequential density ultracentrifugation of pooled mouse plasma obtained at 16 weeks of age (40). Protein concentrations in lipoprotein fractions were determined by the BCA protein assay (Pierce Chemical Co., Rockford, Illinois, USA). Gel filtration chromatography was performed, as described, on 500 μL of pooled plasma, obtained in the fasted state from five mice that were 6 to 7 months old (23). FPLC fractions are dilute; the FPLC data are qualitative because of the threshold of sensitivity of the assays applied to the fractions.
Fatty acid levels.
Blood samples were obtained in the fasted state and kept on ice. The plasma was separated promptly, snap-frozen in liquid nitrogen, and stored at –70°C until the time of assay. Fatty acid levels were determined using the Wako NEFA-C kit according to the manufacturer’s instructions (Wako Chemicals USA Inc., Richmond, Virginia, USA).
Fat tolerance testing.
Animals were gavaged with 0.4 mL of peanut oil. Plasma TG was determined at the baseline and then hourly for 7 hours. The baseline TG level was subtracted from the postprandial levels at each time point. TG excursions were calculated and were then normalized to body weight by multiplying by individual body weight (grams) and then dividing by a typical body weight of 30 g.
Apolipoprotein analysis.
Whole plasma, VLDL and HDL samples (as constant amounts of protein) were analyzed for mouse apolipoprotein levels by SDS-PAGE, as described (41), and by Western blot analysis. Antibodies to mouse apoE (K23100R) and mouse apoC (K23200R, primarily detects apoC-III) were obtained from BIODESIGN International (Kennebunk, Maine, USA). Anti-mouse apoC-I (42) was the gift of Karl H. Weisgraber (Gladstone Institute). Antibody to mouse apoB (43) was the gift of Stephen G. Young (Gladstone Institute). Blots were developed with enhanced chemiluminescence (ECL) reagent (Amersham Pharmacia Biotech, Piscataway, New Jersey, USA), exposed to film, and scanned using a Molecular Dynamics 300A laser densitometer (Molecular Dynamics, Sunnyvale, California, USA) and Image QuaNT software. The identity of the bands was confirmed by molecular weight and by immunodetection with each of the antibodies used separately.
RNase protection assay.
RNase protection assay was performed as described (44). The mouse apoC-I cDNA was the gift of Marten H. Hofker (University of Leiden, Leiden, the Netherlands) and was subcloned into pGEM1. The mouse apoC-III cRNA probe vector (45) was the gift of Todd Leff (Parke-Davis Pharmaceuticals, Ann Arbor, Michigan, USA). Mouse apoE mRNA was detected using a rat apoE cRNA probe vector, which was the gift of Elaine M. Quinet (Wyeth-Ayerst Research, Radnor, Pennsylvania, USA). RNase protection assays were performed on a constant amount of hepatic total RNA, obtained in the morning (postabsorptive state).
VLDL GAG binding and lipolysis studies.
VLDL TG was 3H-labeled in vivo as described (46). Three VLDL preparations for each genotype and state (diabetic/nondiabetic) were isolated by ultracentrifugation from distinct plasma pools. The binding of VLDL to heparin-Sepharose was assayed as described (41). The lipolysis of VLDL was quantitated as free fatty acids (FFA) released upon incubation of VLDL with LPL (Sigma L-2254) that had been prebound to heparan sulfate (Sigma H-4777) as described (47). Constant amounts of TG from unlabeled VLDL pools were used as substrates.
Plasma lipase activities.
Hepatic lipase and LPL activities were determined in postheparin plasma on 20 animals (five each of each genotype/phenotype) as described (23, 48).
TG and apoB production rates.
TG and apoB production rates were determined by inhibiting the plasma catabolism of apoB and TG with the injection of Triton and simultaneously radiolabeling apoB with 35S methionine, as described (41). Only apoB-48 was detected in B48 mice and only apoB-100 was detected in the B100 mice, confirming the identity of the counted bands as apoB.
Remnant clearance studies.
Six male B48 mice were fed the high-fat, high-cholesterol, 0.5% cholic acid diet (C13002, Research Diets) described by Paigen et al., for 2 weeks (49). The animals were sacrificed and lipoproteins (d < 1.006) were isolated from fasting plasma. It was radioiodinated, as described (50), and 1 million counts per minute (cpm) of the dialyzed preparation was injected into six B48 and six B48-diabetic mice fed chow. Diabetic and control animals were of identical weight. Tracer apoB in plasma was determined at 2, 10, 20, 40, 80, and 120 minutes by SDS-PAGE of whole plasma followed by autoradiography and γ-counting of the excised apoB-48 band. The rate of clearance of postlipolysis lipoprotein remnant particles was determined by the disappearance of tracer apoB from this preparation, as described (51).
Hepatic remnant uptake.
Trapping of the remnant lipoprotein preparation in the liver was assayed in a separate experiment: Plasma cpm were determined 30 seconds after the injection and terminally. Animals were sacrificed 5 minutes after the injection and were perfused with PBS. Livers were rapidly removed, as described below, and cpm in homogenized whole liver were determined in a scintillation counter. Parallel experiments were performed in which the animals were injected with 10 U (∼400 U/kg) of bovine heparin before injection of the radioactive tracer.
LRP levels.
LRP activity was estimated by the rate of clearance of radioiodinated α2-macroglobulin, as described (52). One million cpm of labeled preparation was injected into each of six control (body wt 27.3 ± 0.58 g) and six diabetic (body wt 29.3 ± 1.5 g) male, age-matched C57BL/6 mice. Plasma was obtained for γ-counting at 0.5, 5, 10, 20, 40, 80, and 120 minutes. Essentially all the change in plasma cpm was observed over the first four time points. The clearance of the tracer was calculated as the slope of the natural logarithm of the cpm values at these four time points.
Proteoglycan sulfation in vivo.
Production of total proteoglycans and HSPG in tissues was determined using radiolabeling with [35S]SO4 and selective digestion of chondroitin (CS) and dermatan (DS) sulfates using chondroitinase (53). One hundred microcuries of [35S]SO4 (Amersham) was injected through the tail vein. Liver, heart, and epididymal fat pads (both sides) were homogenized in ice-cold homogenization buffer (20 mM Tris, pH 7.4, 3 M urea, 0.5 % CHAPS, 0.1 M NaCl, 1 mM PMSF, 1 mM benzamidine), and proteoglycans were precipitated with 3 vol 100% ethanol. One aliquot of the proteoglycans was incubated overnight with 0.5 U of chondroitinase ABC (Seikagaku Corp., Atlanta, Georgia, USA) at 37°C. CS/DS was calculated as the difference in cpm between the chondroitinase-treated aliquot (heparan sulfate proteoglycans) and the untreated aliquot (total proteoglycans).
Cell culture.
Human hepatoma (HepG2) cells were maintained in MEM supplemented with 10% FBS, 1% human nonessential amino acids, 1% sodium pyruvate, 2 mM L-glutamine. Bovine aortic endothelial cells were isolated and cultured as described (54). The cells (5–15 passages) were grown in MEM containing 10% FBS (Life Technologies, Rockville, Maryland, USA). Cells were seeded at a density of 100,000 cells/well on collagen-coated six-well plates. Media were renewed every 12 hours to minimize glucose depletion. In both cases, the medium was supplemented to a final concentration of either 25 mM D-glucose (high glucose) or 5.5 mM D-glucose, 19.9 mM mannitol (osmotic control). In pilot experiments, culture in 5.5 mM glucose in the absence of mannitol gave identical results to the osmotic control. At 5 days, when the cells were uniformly confluent, labeling was performed for 12 hours with [35S]SO4 (see below).
Proteoglycan sulfation: cultured cells.
Labeling with [35S]SO4 was performed for 12 hours, essentially as described (55, 56). At 5 days, when the cells were uniformly confluent, labeling was performed for 12 hours with [35S]SO4. Cells were collected with a rubber policeman and washed three times with cold PBS. An aliquot of the resuspended cells was removed for protein assay (Bio-Rad Laboratories Inc., Hercules, California, USA). Proteoglycans were isolated from the remainder as follows. Cells were lysed with 4 M guanidine hydrochloride and then dialyzed against 0.03 mol/L Na2SO4 and 0.02 NaCl at 4°C to remove free isotope and guanidine. Total proteoglycan radioactivity was measured after precipitation with 3 vol absolute ethanol containing sodium acetate (0.8 g/L) for 18 hours at –20°C. Samples were spun at 500 g for 1 hour, the supernatant was removed, and the pellet was solubilized in 0.5 N NaOH. The radioactivity in an aliquot of the aqueous phase was determined in scintillation fluid (Hydrofluor; National Diagnostic, Atlanta, Georgia, USA) in a liquid scintillation counter (model 1800; Beckman Instruments Inc., Fullerton, California, USA) and normalized to cell protein content. Assessment of HSPG and DS/CS proteoglycans (DS/CSPG) (non-HSPG) was as follows. Aliquots of dialyzed proteoglycans were treated with 0.05 U of chondroitinase ABC in enzyme buffer containing 0.01 M N-ethylmalemide, 0.07 mM pepstatin, 0.001 M PMSF (protease inhibitors), 1 mg/mL BSA, and then incubated for 20 hours at 37°C. HSPG radioactivity was measured as activity that would precipitate in ethanol. DS/CSPG (non-HSPG) was determined by subtracting HSPG from the total proteoglycan. Counts were adjusted for each plate by total protein. Nine plates were examined for each condition (12 in the case of bovine aortic endothelial cells).
Proteoglycan sulfation: cultured cells SDS-PAGE.
HepG2 cells were labeled for 12 hours with [35S]SO4 as described above after culturing in high glucose and osmotic control conditions. Constant amounts of cell protein were fractionated by 3–15% gradient SDS-PAGE. Dried gels were visualized and quantitated in a PhosphorImager. Three pools of cells were examined for each condition.
Proteoglycan [3H]glucosamine incorporation: cultured cells.
Confluent HepG2 cells were labeled with 100 kBq/mL [3H]glucosamine for 12 hours as a measure of GAG carbohydrate synthesis. To eliminate glucosamine incorporated via non-GAG pathways, GAGs were purified according to the method described by Wagner et al. (57). Samples were digested for at least 5 hours with 1 μg of papain per milligram of tissue in a buffer containing 0.1 M sodium acetate, 0.05 M cysteine HCl, and 0.01 M EDTA, pH 6. After digestion, GAG were precipitated with 1% 1-hexadecyl pyridinium chloride. GAG precipitates were dissolved in 1 mL of a solution of 2 M NaCl and absolute ethanol (in a ratio of 100:215) followed by precipitation by the addition of a further 2 mL of absolute ethanol. Precipitates were washed three times with ethanol and then dissolved in water. HSPG and non-HSPG GAG were quantitated and normalized to total cell protein as described above.
Perlecan immunoblot.
Liver samples from three control and three diabetic male six-month-old C57BL/6 mice were homogenized in a buffer containing 20 mM of Tris, pH 7.4, 3 M urea, 0.5% CHAPS, 0.1 M NaCl, 1 mM PMSF, and 1 mM benzamidine. The homogenate was centrifuged 15 minutes at 16,000 g at 4°C. The supernatant was analyzed for protein content and 50 μg of protein was loaded onto a 3–15% SDS-PAGE gel. The gels were electroblotted onto nitrocellulose, and the blots were stained with a rat mAb that specifically recognizes perlecan (05-209; Upstate Biotechnology, Lake Placid, New York, USA), followed by goat anti-rat IgG linked to horseradish peroxidase. Blots were developed with ECL reagent (Amersham) and exposed to film. Densitometry was performed as described above.
Statistics.
The logarithms of the TG values were used as the basis for statistical comparison. Other values were used as measured. All bar graphs display means plus or minus SD. All comparisons were by t test. Two-tailed P values of 0.05 or less were considered statistically significant.
Results
Plasma lipoproteins and apolipoproteins: WT mice.
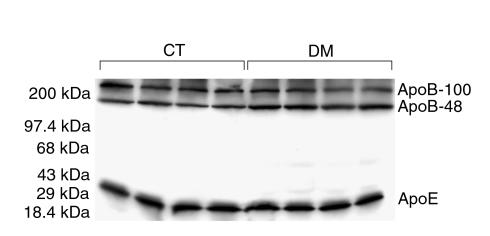
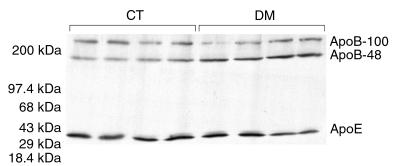
Diabetic C57BL/6 (WT) inbred mice showed moderate, but highly statistically significant (P < 0.00001), elevations of total cholesterol (+24%) and total TG (+59%) without significant change in HDL cholesterol (HDL-C), as shown in Table 1. Apolipoprotein levels in whole plasma were determined by SDS-PAGE and Western blot analysis (Figure 1). ApoB-100 and apoE levels did not change with DM, whereas apoB-48 levels increased by 37% (P = 0.002). The apoB-48/apoB-100 ratio increased by 46% (P = 0.0008) and apoB-48/apoE increased by 49% (P = 0.006).
Table 1.
Male C57BL/6 morning (nonfasting) plasma lipids (mg/dL ± SD)
Figure 1.
Western blot analysis of whole plasma from control (CT) and diabetic (DM) C57BL/6 mice for apoB and apoE. Constant volumes of plasma were loaded. ApoB-48 is increased in the diabetic animals, whereas apoB-100 and apoE are unchanged.
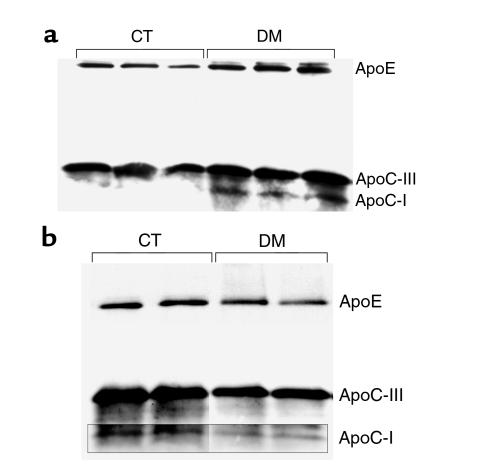
The cholesterol, TG, and phospholipid composition of lipoprotein fractions isolated by sequential density ultracentrifugation was unchanged (data not shown). Aliquots of the isolated lipoprotein preparations were blotted for apoE, apoC-III, and apoC-I. VLDL from diabetic animals (Figure 2a) showed increased apoC-III and apoE and a marked increase in apoC-I (undetectable in WT animals). HDL from diabetic mice showed modest decreases of apoE, apoC-III, and apoC-I, suggesting the transfer of these apolipoproteins from HDL to VLDL and the accumulation of remnants (Figure 2b). RNase protection assays of apoE, apoC-III, and apoC-I mRNA levels in liver showed no change in the diabetic animals (data not shown).
Figure 2.
(a) Western blot analysis of isolated VLDL from CT and DM C57BL/6 mice for apoE, apoC-III, and apoC-I. Constant amounts of protein were loaded. ApoE, apoC-III, and apoC-I are increased in DM, apoC-I most prominently. (b) Composite of blot of isolated HDL separately developed for these antibodies. In contrast, apoE, apoC-III, and apoC-I are modestly decreased in DM.
Fat tolerance testing.
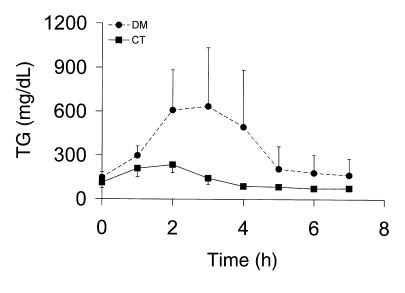
The observation that plasma apoB-48 was increased in diabetic mice suggested that there was a defect in the removal of intestinally derived lipoproteins. Because apoB-48 is also made in the liver in mice, we evaluated the metabolism of gut-derived particles by assessing the extent of postprandial lipemia. Diabetic animals administered an oral fat load showed a large increase in postprandial TG from 1–4 hours after the fat load (Figure 3a). The integrated 7-hour TG area above the baseline was 9,916 ± 7,741 mg/dL/h vs. 944 ± 2,414 in controls (n = 6 of each, P = 0.02). These results suggested that prolonged postprandial hyperlipidemia was likely a major contributor to the fasting dyslipidemia and elevation in apoB-48 that we had observed in diabetic animals.
Figure 3.
Fat tolerance testing in CT (squares) and DM (circles) C57BL/6 mice. Plasma TG after the oral administration of 0.4 mL of peanut oil are shown. A marked increase in the plasma TG response to the fat load is noted in the diabetic animals.
Plasma lipoprotein profiles: gene-targeted mice.
We then tested whether the defect in the metabolism of apoB-48 particles was specific to apoB-48 per se or specific to intestinal lipoproteins. To distinguish these possibilities, we studied gene-targeted mice that produce either apoB-48 only (B48 mice) in both liver and intestine, or apoB-100 only (B100 mice), also in both sites. Any differential effects of DM in these two strains would reflect inherent differences between apoB-48 and apoB-100 lipoproteins and not simply their sites of synthesis. Plasma glucose and lipid levels in these animals are shown in Table 2. Cholesterol levels were higher at the baseline in the B48 animals, as has been documented previously (38), whereas TG and glucose levels were the same. Plasma levels 4 weeks after the induction of streptozotocin DM are also shown. Glucose levels increased similarly in both groups. Plasma FFA levels in the fasted state increased with DM in both B100 and B48 animals. In contrast, cholesterol and TG remained unchanged with DM in the B100 animals but increased in the B48 animals, supporting a specific effect of DM on apoB-48. The gene-targeted animals were outbred and hence more variable than the C57BL/6 mice. The changes in plasma lipids in the B48 animals achieved statistical significance for TG (+111%; P = 0.02) only in the fasted state and for cholesterol (+23%; P = 0.03) in the nonfasted state.
Table 2.
B48-only and B100-only mice: plasma lipids, glucose, and FFA before and after streptozotocin
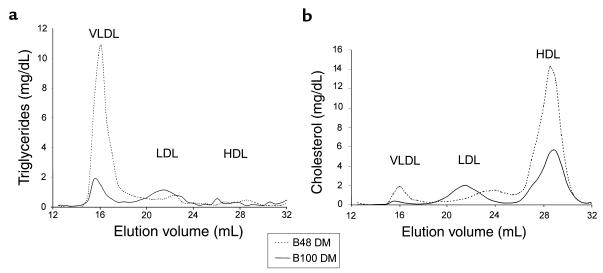
Fasting lipoprotein profiles were also determined by gel-filtration chromatography on plasma from B48 and B100 animals before and after the induction of DM. In the basal state the B48 animals had lower levels of IDL/LDL cholesterol and TG, as has been described (38), and higher levels of HDL. VLDL levels were very low in both B48 and B100 mice (data not shown). With DM, the B48 mice, but not the B100, showed an increase in VLDL TG and cholesterol and a further increase in HDL cholesterol (Figure 4, a and b).
Figure 4.
Gel filtration chromatography of whole plasma from diabetic B48 (dashed line) and B100 (solid line) animals. (a) TG. (b) Cholesterol. VLDL TG and cholesterol levels are markedly higher in diabetic B48 plasma, as are HDL cholesterol levels.
When VLDL were isolated by ultracentrifugation from the gene-targeted mice, a compositional difference was observed. Cholesterol/TG was 0.11 in B48 mice, 0.20 in diabetic B48 mice, 0.064 in B100 mice, and 0.067 in diabetic B100 mice. VLDL from the diabetic B48 animals, but not the diabetic B100 animals, were cholesterol enriched.
We then produced mice that were heterozygous for both gene-targeted alleles to allow us to compare DM-mediated effects on apoB-48 and apoB-100 in the same animal. One allele expressed only apoB-48 in both liver and intestine, and the other allele expressed apoB-100 in both sites. This model permitted us to evaluate simultaneously the clearance of apoB-100 and apoB-48 particles, apart from any possible differences in apoB mRNA editing, in the production or clearance of intestinal versus hepatic lipoproteins and apart from any possible genetic or environmental variation. These animals were made diabetic, and levels of apoB-48, apoB-100, and apoE were determined in whole plasma by SDS-PAGE/Western blot analysis (Figure 5). ApoB-48 levels more than doubled (P = 0.001), whereas apoB-100 and apoE levels remained unchanged.
Figure 5.
Western blot analysis of whole plasma from mice heterozygous for both the B48 and B100 gene-targeted alleles of apoB, developed for apoB and apoE. There is a marked increase in apoB-48 relative to apoB-100 and apoE in the DM samples.
VLDL production.
To establish the mechanism for the specific increase in apoB-48 particles, we evaluated the gene-targeted animals for a DM-related increase in VLDL production. Lipolysis and clearance were blocked with Triton, allowing the estimation of both TG production (change in plasma levels) and apoB production (after the administration of 35S-methionine). TG production rates were unchanged with DM, in both B48 and B100 mice (B48, 168 ± 69 mg/dL/h vs. B48 DM, 159 ± 72; P = not significant [NS]; B100, 219 ± 24 vs. B100 DM, 141 ± 93; P = NS, n = 6 for each genotype/state). Simultaneous apo-B production rates (cpm) tended to be lower with DM but were variable and not significantly different (B48, 320 ± 271 vs. B48 DM, 186 ± 79; P = NS; B100, 550 ± 542 vs. B100 DM, 279 ± 170; P = NS).
Plasma lipase activities and VLDL lipolysis.
We next examined possible causes of a VLDL clearance defect. To exclude hypertriglyceridemia related to a DM-related decrease in plasma lipase activity, we determined LPL and hepatic lipase (HL) activity in postheparin plasma. Other than a slight decrease in HL in B100 DM, there was no change in the activity of either enzyme with DM, neither in B100 nor in B48 mice. (LPL: B48, 44.6 ± 7.7 μMol FFA/mL/h vs. B48 DM, 54.2 ± 9.9, P = NS; B100, 38.2 ± 5.0 vs. B100 DM, 27.7 ± 7.3, P = NS. HL: B48, 11.1 ± 0.36 μMol FFA/mL/h vs. B48 DM, 10.9 ± 1.7, P = NS; B100, 11.3 ± 1.35 vs. B100 DM, 9.1 ± 0.75, P = 0.03.)
We then tested VLDL preparations for differences in their susceptibility to lipolysis, presumably related to the observed apolipoprotein compositional changes. First, we examined the binding of VLDL to heparin-Sepharose, a model of the cell-surface GAG matrix where lipolytic enzymes reside. B48 VLDL showed no change in heparin-releasable binding with the induction of DM (control, 8.3 ± 1.2% binding vs. DM, 7.47 ± 1.7 % binding; P = NS). B100 VLDL showed an increase in binding with DM (control, 6.2 ± 0.9 % binding vs. DM, 9.7 ± 1.7% binding; P = 0.001) the opposite of the change one would expect from any increase in VLDL apoC-III (23). We then examined the actual hydrolysis of VLDL TG by a model of the endothelial surface: LPL bound to heparan sulfate. VLDL from both diabetic B48 mice and diabetic B100 animals showed paradoxically increased lipolysis (FFA release) with DM (B48, 0.28 ± 0.08 mEq/L vs. B48, DM 2.00 ± 1.15, P < 0.02; B100, 0.49 ± 0.08 vs. 1.27 ± 0.36, P < 0.02).
Remnant clearance.
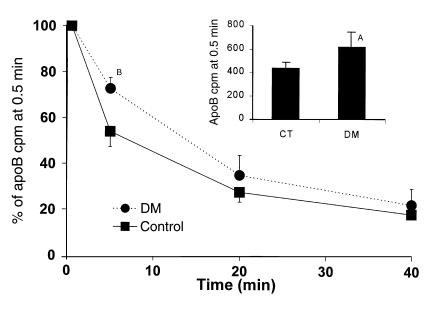
The catabolic rate of radiolabeled VLDL isolated from cholesterol-fed B48 animals (B48 β-VLDL) was determined. The isolated lipoprotein preparation had a cholesterol concentration of 61 mg/dL and a TG concentration of 16 mg/dL. We used these TG-depleted particles, which are unlikely to require significant lipolysis, as a means to directly assess receptor-mediated lipoprotein uptake. ApoB-48 clearance from β-VLDL was significantly slower in the diabetic C57BL/6 animals (fractional catabolic rate ± SD, 3.2 ± 1.3 pools/h vs. 8.0 ± 4.1 pools/h in controls; n = 6 of each, P = 0.02). The difference was greatest at the first two time points (0.5 and 5 minutes) implicating early events in lipoprotein particle uptake (Figure 6). The inset bar graph shows cpm in plasma at 0.5 minutes. This was already significantly higher in the diabetic animals. The line graph normalizes the 0.5-minute values and reveals the significant decrease in relative clearance at 5 minutes. This prominent effect at very early time points suggested that DM produced a defect in lipoprotein trapping rather than in receptor-mediated uptake.
Figure 6.
Clearance of radiolabeled apoB in highly cholesterol-enriched VLDL (remnant clearance). ApoB cpm are significantly higher in DM at the initial 0.5-minute time point (bar graph inset, AP = 0.01). The decrease relative to these initial points is also significantly less at 5 minutes in the DM mice (BP = 0.0002), as is the calculated fractional catabolic rate (see text).
Liver uptake of remnants.
To address the mechanism of this decrease in rapid clearance of lipoproteins from the plasma, we examined the trapping of apoB-48 remnant lipoproteins by the liver. The same amount of radioiodinated lipoprotein preparation was injected into seven control and five diabetic C57BL/6 mice. The control animals were heavier (25 ± 3 g, n = 7, vs. 21 ± 3 g, n = 5, in mice with DM). Perhaps due to this difference, cpm in an aliquot of plasma obtained 30 seconds after injection were somewhat lower, though not significantly so, in control animals versus DM animals (7,468 ± 1,627 vs. 8,672 ± 1,814). By the time of sacrifice at 5 minutes, plasma cpm were significantly lower in the control animals, consistent with the delayed clearance in DM that we had observed previously (3,521 ± 337 vs. 5,898 ± 2,098 in DM; P = 0.01). In contrast, cpm in liver at 5 minutes were significantly higher in controls (200,186 ± 42,652 vs. 111,589 ± 72,794 in mice with DM; P = 0.02). These results indicated that the decrease in remnant clearance in DM mice was due to decreased uptake by the liver. Because rapid accumulation of remnants in the liver is due to binding to proteoglycans, we repeated the experiment in heparin-injected mice. This eliminated binding to HSPG. In the heparinized animals, plasma cpm at 30 seconds (controls, 6,723 ± 550; DM, 6,758 ± 1,356) and at 5 minutes (controls, 5,739 ± 464; DM:, 5,877 ± 1,138) declined more slowly and were identical in control (n = 6) and diabetic (n = 5) animals. Liver cpm at 5 minutes were also the same in controls and diabetics (controls, 44,409 ± 1,431; DM, 39,880 ± 11,866).
LRP levels.
Based on these results, we hypothesized that the difference in hepatic uptake was based primarily on differences in binding to HSPG rather than in levels of the LRP itself. We tested this by measuring the clearance of radiolabeled activated α2-macroglobulin. The α2-macroglobulin is a ligand for the LRP whose clearance is not dependent on prior binding to HSPG (58). Clearance rates for α2-macroglobulin (in ln(cpm)/min ± SD) were identical in diabetic and control C57BL/6 mice (–0.1257 ± 0.011 vs. –0.1257 ± 0.004; P = NS, n = 6 of each).
HSPG production in vivo.
To establish whether the diabetic mice did have an abnormality in HSPG, newly synthesized proteoglycans were labeled with [35S]SO4. We then digested the DS/CSPG and selectively precipitated HSPG. 35S-incorporation into HSPG was reduced by 52% in the livers of diabetic mice (DM, 2709 ± 1121 cpm/mg of liver protein vs. 5612 ± 1942 in controls; n = 5 of each, P < 0.0004). 35S incorporation into dermatan sulfate plus chondroitin sulfate did not change (DM, 2903 ± 1038 vs. 2995 ± 1403 in controls; P = NS). No significant change in 35S-incorporation was noted in heart and adipose tissue. Thus, a reduction in liver HSPG was associated with the defect in the trapping of apoB-48 lipoproteins.
HSPG production in vitro.
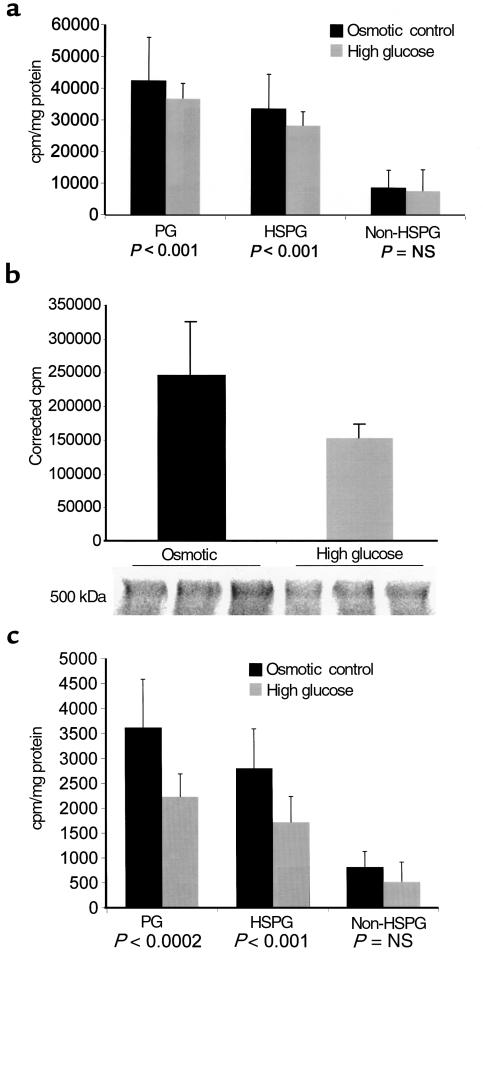
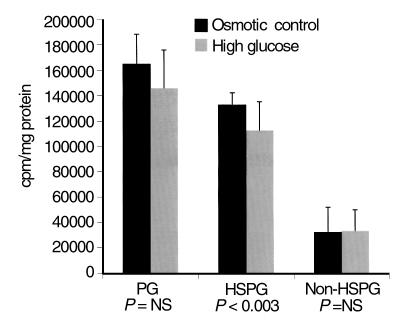
To examine the mechanism of this decrease in HSPG, we studied the effects of elevated glucose in the medium on proteoglycan production by cultured hepatoma (HepG2) cells, a simplified system where variation in insulin and other effects of DM are eliminated and the cell type producing the proteoglycan is certain. Elevated glucose in the medium produced a 13% decrease in proteoglycan sulfation that was predominantly due to a 15.4% decrease in HSPG sulfation (P < 0.001, n = 9). No significant difference was noted in non-HSPG (Figure 7a). SDS-PAGE of [35S]SO4-labeled HepG2 cells followed by autoradiography revealed a decrease in a high-molecular-weight (> 500 kDa) region consistent with the HSPG perlecan (Figure 7b). The incorporation of radiolabeled glucosamine was then assessed in order to discriminate between a primary decrease in sulfation and decreased GAG synthesis (Figure 7c). A 39% decrease in glucosamine incorporation into HSPG was noted (P < 0.001). Because of the possibility that the HSPG in Disse’s space may be of endothelial origin, the sulfation assay was also performed with cultured bovine aortic endothelial cells (BAEC) (Figure 8). Once again, a statistically significant (15%; P = 0.003, n = 12) decrease in HSPG was noted, without change in non-HSPG.
Figure 7.
(a) [35S]SO4 incorporation (mean ± SD) into total proteoglycans, HSPG, and non-HSPG (calculated) in HepG2 cells cultured in either 25 mM D-glucose (high glucose) or 5.5 mM D-glucose, 19.9 mM mannitol (osmotic control). (b) SDS-PAGE autoradiogram of [35S]SO4-labeled HepG2 cells cultured under these conditions. The cpm per milligram of protein in the indicated diffuse proteoglycan bands (molecular weight consistent with perlecan HSPG) are shown. (c) [3H]glucosamine incorporation into isolated proteoglycan from HepG2 cells cultured under these conditions.
Figure 8.
[35S]SO4 incorporation into total proteoglycans, HSPG, and non-HSPG (calculated), in BAEC cultured in either 25 mM D-glucose (high glucose) or 5.5 mM D-glucose, 19.9 mM mannitol (osmotic control).
Perlecan levels in vivo.
We then returned to the diabetic mice and determined the levels of perlecan in the liver, the core protein of the predominant HSPG species in Disse’s space, the site of lipoprotein sequestration before clearance (59). An immunoblot of whole-liver homogenates probed with a specific antiperlecan antibody is shown in Figure 9. Decreased perlecan is evident in the livers of the diabetic animals. Densitometry of this band yielded values (OD units ± SD) of 109 ± 40 in controls versus 49 ± 23 in the diabetic livers (P < 0.05).
Figure 9.
Immunoblot of whole-liver homogenates (50 μg of protein from supernatant) from control (CT) and diabetic (DM) mice stained with an antiperlecan antibody. Decreased perlecan immunoreactivity is evident in the livers from the diabetic animals.
Discussion
To understand the basis for the lipoprotein abnormalities in Type I DM when under inadequate glycemic control, we examined the effects of DM in several mouse models. We examined inbred WT mice, gene-targeted mice producing only apoB-100, the apolipoprotein of LDL, or only apoB-48, the form of apoB in chylomicrons and their remnants, and compound heterozygotes with both gene-targeted alleles. In all mice that produced apoB-48, i.e., WT, B48, and B100/B48 mice, streptozotocin-induced DM increased plasma levels of apoB-48–containing lipoproteins. No DM-related changes in apoB-100 were found in WT or gene-targeted mice. Plasma TG also increased and TG-rich lipoproteins were cholesterol-enriched, as is found in human DM. Furthermore, the tolerance of a dietary fat load was markedly impaired in the DM mice, out of proportion to the elevation in fasting TG.
The etiology of this selective increase in apoB-48 lipoproteins was explored. Plasma FFA levels increased with DM, as would be anticipated. However, no consequent DM-related increase in VLDL TG or apoB production was found, as has been reported recently (60). A lipoprotein catabolic defect remained as the only possibility. However, there was no decrease in plasma lipoprotein lipase activity, consistent with the literature both on streptozotocin-diabetic rats and mice (60, 61) and on moderately hyperglycemic humans (62, 63). Hepatic lipase also plays a role in chylomicron remnant clearance (64), but no change in activity was observed in the B48 mice, and only a modest change was seen in the B100 mice. Whereas VLDL apoC-III increased, there was no evidence of any functional impairment in VLDL lipolysis attributable to this. As opposed to findings in human apoC-III–transgenic mice, VLDL GAG binding was not reduced, and the lipolysis of diabetic VLDL was actually improved, perhaps due to increased VLDL particle size or increased apoC-II content, the concentration of which may be limiting (65). Our data do not support a prominent role for the overexpression of apoC-III in diabetic dyslipidemia. Increased expression of apoA-II may also have an inhibitory effect on lipolysis (66). However, we did not observe any change in apoA-II protein or mRNA levels with DM (data not shown).
Lipid and apolipoprotein compositional analysis of the VLDL fraction from diabetic mice was most consistent with remnant accumulation. Clearance studies performed with model remnant apoB-48 lipoprotein particles confirmed the presence of a defect in the removal of apoB-48 lipoproteins from the plasma. We considered that this might be due to the altered apolipoprotein composition of the particles. The increase in VLDL apoC-I was greater than that of apoC-III, and the diabetic phenotype was very reminiscent of the phenotype of the apoC-I–overexpressing transgenic mouse. Human apoC-I transgenic mice exhibit increased levels and cholesterol-enrichment of VLDL and decreased lipoprotein clearance through the LRP (41, 67, 68). Since apoC-I was a proportionately greater constituent of apoB-48–containing lipoproteins than of apoB-100 lipoproteins, increased apoC-I on TG-rich lipoproteins remains a possible explanation for a selective decrease in apoB-48 clearance, though not the one we favor.
The decrease in remnant clearance was marked by significantly decreased early trapping of the remnant preparation by the liver that was glycosaminoglycan-dependent, as indicated by the abolition of differential trapping and clearance by intravenous heparin. However, the activity of the two principal receptors for lipoprotein remnants was unchanged: LDL-R activity was essentially normal, based on the unchanged apoB-100 levels in the context of essentially unchanged hepatic apoB secretion, and clearance by the LRP of a nonlipoprotein ligand, α2-macroglobulin, was unaffected. Moreover, the unchanged clearance of α2-macroglobulin in the diabetic mice (along with the equal lipoprotein trapping in the heparinized mice) also indicated that reduced remnant clearance was unlikely to have resulted from such nonspecific processes as dehydration or reduced cardiac output. Lipoprotein clearance through the LRP is a two-step process, with an initial association of the lipoproteins with HSPG followed by receptor uptake. The proteoglycan-mediated step is thought to concentrate the lipoproteins in proximity to the receptor and allow further enrichment with apoE, thereby greatly enhancing their interaction with the receptor (69). Such a process is not needed for LDL-R uptake of lipoproteins or for the clearance by the LRP of nonlipoprotein ligands (58, 70). Inhibition of the LRP pathway by overexpression of the receptor antagonist RAP (71) or by liver-specific LRP knockout (72) leads to an increase in plasma levels of chylomicron remnants in mice that also lack the LDL-R. In otherwise normal mice, decreased LRP activity produces LDL-R upregulation and increased plasma apoB-48 but no significant alterations in plasma TG and cholesterol have been reported (72). It has been uncertain whether modulation of non-LDL-R pathways can have an important role in lipoprotein metabolism under physiological conditions and when the LDL-R is present (38, 73).
Because of the importance of HSPG in facilitating lipoprotein clearance through the LRP and, possibly, in directly mediating remnant uptake (74, 75), we assessed liver proteoglycan production in diabetic and control mice. A marked and specific decrease in HSPG production, as assessed by sulfation, was noted with DM. Decreased HSPG sulfation in liver in DM has been reported (76–79). We propose that this decrease in HSPG produced decreased trapping, which led to the delay in clearance of apoB-48–containing lipoproteins. A similar effect may explain why apoE-null mice, for whom HSPG may constitute the principal residual apoB-48–clearance pathway, develop particularly marked increases (doubling) in cholesterol after the induction of DM (80). The principal proteoglycan in Disse’s space, the site of hepatic lipoprotein trapping, appears to be perlecan (59, 81). Whereas proliferating hepatocytes actively secrete perlecan, endothelial cells may be principally responsible for its synthesis in normal liver (81). Our in vitro findings, both in cultured hepatoma cells and in endothelial cells, support a conclusion that a decrease in secreted HSPG is likely in DM and that this decrease is due to the effects of elevated glucose alone. The decrease was evident both at the level of sulfation and of carbohydrate incorporation. A decrease in HSPG of the molecular weight of perlecan was apparent on SDS-PAGE/autoradiography of sulfate-labeled HepG2 cells. Decreased perlecan core protein was evident on specific immunoblot of livers from the diabetic mice.
Decreased HSPG in DM has been studied predominantly in the context of diabetic nephropathy, although it has been observed in a variety of tissues (82–84). Decreased expression of perlecan, specifically, has been described in a variety of renal models (85, 86). However, any connection of the HSPG changes in DM with atherosclerotic disease has focused on effects in the vessel wall (87). Decreased sulfation of liver (but, as we found, not heart HSPG) in DM has been reported but no association with diabetic dyslipidemia was proposed (88, 89). Decreased glucosaminyl N-deacetylase activity, the first step in GAG sulfation, has been proposed as a mechanism for this observation (90). This would not be supported by our observation of decreased glucosamine incorporation into HSPG, which indicates decreased GAG carbohydrate synthesis. Our observation of decreased perlecan levels in the livers of the diabetic mice suggests that a defect in perlecan biosynthesis may be primary, as has been proposed in the kidney (86).
In summary, our data reveal a primary defect in the clearance of apoB-48 remnants in a mouse model of diabetic dyslipidemia. This led to fasting and severe postprandial dyslipidemia in animals with no genetic defect in the LDL-R or LRP and no evidence for LDL-R or LRP downregulation. These data are the first to demonstrate that selective modulation of a non–LDL-receptor remnant-removal pathway can occur with pathophysiologically meaningful consequences in WT animals. The normal clearance of apoB-48–containing lipoproteins is likely to be substantially mediated by this pathway, which appears to involve binding to HSPG. In addition, our findings illustrate a likely explanation for the increased levels of atherogenic chylomicron remnants found in diabetic humans (91). Further work defining the precise biochemical mechanism by which hyperglycemia produces its effects on HSPG production may lead to new approaches to preventing the end-organ pathologies of DM.
Acknowledgments
We thank Henry N. Ginsberg, Sivaram Pillarisetti, Joseph C. Obunike, and George Steiner for advice; Kristine Uffelman, Theresa Vanni-Reyes, and Colleen Ngai for technical advice and assistance; and Stephen G. Young, Karl H. Weisgraber, and Karen Reue for the gift of scarce reagents. This research was supported by National Heart, Lung, and Blood Institute/National Institutes of Health (NHLBI/NIH) grant R01 HL56232 and Project 1 of NHLBI/NIH P01 HL57217 (in association with the Juvenile Diabetes Foundation) to Neil S. Shachter.
Footnotes
Karin Conde and Tetsu Ebara contributed equally to this work.
References
- 1.Królewski AS, et al. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 2.Jensen T, Borch-Johnsen K, Kofoed-Enevoldsen A, Deckert T. Coronary heart disease in young Type I (insulin-dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia. 1987;30:144–148. doi: 10.1007/BF00274218. [DOI] [PubMed] [Google Scholar]
- 3.Winocour PH, Durrington PN, Ishola M, Hillier VF, Anderson DC. The prevalence of hyperlipidemia and related clinical features in insulin-dependent diabetes mellitus. Q J Med. 1989;70:265–276. [PubMed] [Google Scholar]
- 4.Lipid and lipoprotein levels in patients with IDDM diabetes control and complication. Trial experience. The DCCT Research Group. Diabetes Care. 1992;15:886–894. doi: 10.2337/diacare.15.7.886. [DOI] [PubMed] [Google Scholar]
- 5.Rivellese A, et al. Presence of very low density lipoprotein compositional abnormalities in Type I (insulin-dependent) diabetic patients; effects of blood glucose optimisation. Diabetologia. 1988;31:884–888. doi: 10.1007/BF00265371. [DOI] [PubMed] [Google Scholar]
- 6.Patti L, et al. Abnormal distribution of VLDL subfractions in Type I (insulin-dependent) diabetic patients: could plasma lipase activities play a role? Diabetologia. 1993;36:155–160. doi: 10.1007/BF00400698. [DOI] [PubMed] [Google Scholar]
- 7.Perez A, et al. Lipoprotein compositional abnormalities in Type I diabetes: effect of improved glycaemic control. Diabetes Res Clin Pract. 1997;36:83–90. doi: 10.1016/s0168-8227(97)00033-8. [DOI] [PubMed] [Google Scholar]
- 8.Dunn FL. Plasma lipid and lipoprotein disorders in IDDM. Diabetes. 1992;41(Suppl. 2):102–106. doi: 10.2337/diab.41.2.s102. [DOI] [PubMed] [Google Scholar]
- 9.Georgopoulos A, Phair RD. Abnormal clearance of postprandial Sf 100-400 plasma lipoproteins in insulin-dependent diabetes mellitus. J Lipid Res. 1991;32:1133–1141. [PubMed] [Google Scholar]
- 10.Phillips NR, Waters D, Havel RJ. Plasma lipoproteins and progression of coronary artery disease evaluated by angiography and clinical events. Circulation. 1993;88:2762–2770. doi: 10.1161/01.cir.88.6.2762. [DOI] [PubMed] [Google Scholar]
- 11.Groot PHE, et al. Postprandial lipoprotein metabolism in normolipidemic men with and without coronary artery disease. Arterioscler Thromb. 1991;11:653–662. doi: 10.1161/01.atv.11.3.653. [DOI] [PubMed] [Google Scholar]
- 12.Ginsberg HN, et al. Association of postprandial triglyceride and retinyl palmitate responses with newly diagnosed exercise-induced myocardial ischemia in middle-aged men and women. Arterioscler Thromb Vasc Biol. 1995;15:1829–1838. doi: 10.1161/01.atv.15.11.1829. [DOI] [PubMed] [Google Scholar]
- 13.Sharrett AR, Chambless LE, Heiss G, Paton CC, Patsch W. Association of postprandial triglyceride and retinyl palmitate responses with asymptomatic carotid artery atherosclerosis in middle-aged men and women. The Atherosclerosis Risk in Communities (ARIC) Study. Arterioscler Thromb Vasc Biol. 1995;15:2122–2129. doi: 10.1161/01.atv.15.12.2122. [DOI] [PubMed] [Google Scholar]
- 14.Cooper AD. Hepatic uptake of chylomicron remnants. J Lipid Res. 1997;38:2173–2192. [PubMed] [Google Scholar]
- 15.Fried SK, Russell CD, Grauso NL, Brolin RE. Lipoprotein lipase regulation by insulin and glucocorticoid in subcutaneous and omental adipose tissues of obese women and men. J Clin Invest. 1993;92:2191–2198. doi: 10.1172/JCI116821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ginsberg HN. Lipoprotein physiology in nondiabetic and diabetic states: relationship to atherogenesis. Diabetes Care. 1991;14:839–855. doi: 10.2337/diacare.14.9.839. [DOI] [PubMed] [Google Scholar]
- 17.Eckel RH, Goldberg IJ, Steiner L, Yost TJ, Paterniti JR. Plasma lipolytic activity. Relationship to postheparin lipolytic activity and evidence for metabolic regulation. Diabetes. 1988;37:610–615. doi: 10.2337/diab.37.5.610. [DOI] [PubMed] [Google Scholar]
- 18.Tsutsumi K, Inoue Y, Shima A, Murase T. Correction of hypertriglyceridemia with low high-density lipoprotein cholesterol by the novel compound NO-1886, a lipoprotein lipase-promoting agent, in STZ-induced diabetic rats. Diabetes. 1995;44:414–417. doi: 10.2337/diab.44.4.414. [DOI] [PubMed] [Google Scholar]
- 19.Shimada M, et al. Overexpression of human lipoprotein lipase protects diabetic transgenic mice from diabetic hypertriglyceridemia and hypercholesterolemia. Arterioscler Thromb Vasc Biol. 1995;15:1688–1694. doi: 10.1161/01.atv.15.10.1688. [DOI] [PubMed] [Google Scholar]
- 20.Li WW, et al. Common genetic variation in the promoter of the human apoC-III gene abolishes regulation by insulin and may contribute to hypertriglyceridemia. J Clin Invest. 1995;96:2601–2605. doi: 10.1172/JCI118324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown WV, Baginsky ML. Inhibition of lipoprotein lipase by an apoprotein of human very low density lipoprotein. Biochem Biophys Res Commun. 1972;46:375–382. doi: 10.1016/s0006-291x(72)80149-9. [DOI] [PubMed] [Google Scholar]
- 22.Havel RJ, et al. Cofactor activity of protein components of human very low density lipoproteins in the hydrolysis of triglycerides by lipoprotein lipase from different sources. Biochemistry. 1973;12:1828–1833. doi: 10.1021/bi00733a026. [DOI] [PubMed] [Google Scholar]
- 23.Ebara T, Ramakrishnan R, Steiner G, Shachter NS. Chylomicronemia due to apolipoprotein C-III overexpression in apolipoprotein E-null mice. Apolipoprotein CIII-induced hypertriglyceridemia is not mediated by effects on apolipoprotein E. J Clin Invest. 1997;99:2672–2681. doi: 10.1172/JCI119456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowal RC, et al. Opposing effects of apolipoprotein E and C on lipoprotein binding to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265:10771–10779. [PubMed] [Google Scholar]
- 25.Windler EE, et al. The estradiol-stimulated lipoprotein receptor of rat liver. A binding site that membrane mediates the uptake of rat lipoproteins containing apoproteins B and E. J Biol Chem. 1980;255:10464–10471. [PubMed] [Google Scholar]
- 26.Sehayek E, Eisenberg S. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Biol Chem. 1991;266:18259–18267. [PubMed] [Google Scholar]
- 27.Al Muhtaseb N, Al Yousuf AR, Bajaj JS. Apolipoprotein A-I, A-II, B, C-II, and C-III in children with insulin-dependent diabetes mellitus. Pediatrics. 1992;89:936–941. [PubMed] [Google Scholar]
- 28.Al-Muhtaseb N, Al-Yusef AR, Bajaj JS. Lipoprotein lipids and apolipoproteins (AI, AII, B, CII, CIII) in Type 1 and Type 2 diabetes mellitus in young Kuwaiti women. Diabet Med. 1991;8:732–737. doi: 10.1111/j.1464-5491.1991.tb01692.x. [DOI] [PubMed] [Google Scholar]
- 29.Rubinsztein DC, et al. Chylomicron remnant clearance from the plasma is normal in familial hypercholesterolemic homozygotes with defined receptor defects. J Clin Invest. 1990;86:1306–1312. doi: 10.1172/JCI114839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hui DY, Innerarity TL, Mahley RW. Lipoprotein binding to canine hepatic membranes. Metabolically distinct apoE and apoB,E receptors. J Biol Chem. 1981;256:5646–5655. [PubMed] [Google Scholar]
- 31.Kita T, et al. Hepatic uptake of chylomicron remnants in WHHL rabbits: a mechanism genetically distinct from the low density lipoprotein receptor. Proc Natl Acad Sci USA. 1982;9:3623–3627. doi: 10.1073/pnas.79.11.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herz J, et al. Surface location and high affinity for calcium of a 500-kd liver membrane protein closely related to the LDL-receptor suggest a physiological role as lipoprotein receptor. EMBO J. 1988;7:4119–4127. doi: 10.1002/j.1460-2075.1988.tb03306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kowal RC, Herz J, Goldstein JL, Esser V, Brown MS. Low density lipoprotein receptor-related protein mediates uptake of cholesteryl esters derived from apoprotein E-enriched lipoproteins. Proc Natl Acad Sci USA. 1989;86:5810–5814. doi: 10.1073/pnas.86.15.5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Misra UK, Gawdi G, Gonzalez-Gronow M, Pizzo SV. Coordinate regulation of the α2-macroglobulin signaling receptor and the low density lipoprotein receptor-related protein/α2-macroglobulin receptor by insulin. J Biol Chem. 1999;274:25785–25791. doi: 10.1074/jbc.274.36.25785. [DOI] [PubMed] [Google Scholar]
- 35.Curtin A, et al. Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetol. 1996;33:205–210. doi: 10.1007/BF02048544. [DOI] [PubMed] [Google Scholar]
- 36.Karpe F, Hellenius ML, Hamsten A. Differences in postprandial concentrations of very-low-density lipoprotein and chylomicron remnants between normotriglyceridemic and hypertriglyceridemic men with and without coronary heart disease. Metabolism. 1999;48:301–307. doi: 10.1016/s0026-0495(99)90076-8. [DOI] [PubMed] [Google Scholar]
- 37.Farese RV, Jr, et al. Phenotypic analysis of mice expressing exclusively apolipoprotein B48 or apolipoprotein B100. Proc Natl Acad Sci USA. 1996;93:6393–6398. doi: 10.1073/pnas.93.13.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Véniant MM, et al. Lipoprotein clearance mechanisms in LDL receptor-deficient “apoB-48-only” and “apoB-100-only” mice. J Clin Invest. 1998;102:1559–1568. doi: 10.1172/JCI4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kunjathoor VV, Wilson DL, LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest. 1996;97:1767–1773. doi: 10.1172/JCI118604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Havel RJ, Eder HA, Bragdon JH. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shachter NS, et al. Combined hyperlipidemia in transgenic mice overexpressing human apolipoprotein C-I. J Clin Invest. 1996;98:846–855. doi: 10.1172/JCI118857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Silva HV, et al. Overexpression of human apolipoprotein C-III in transgenic mice results in an accumulation of apolipoprotein B48 remnants that is corrected by excess apolipoprotein E. J Biol Chem. 1994;269:2324–2335. [PubMed] [Google Scholar]
- 43.McCormick SP, et al. Transgenic mice that overexpress mouse apolipoprotein B. Evidence that the DNA sequences controlling intestinal expression of the apolipoprotein B gene are distant from the structural gene. J Biol Chem. 1996;271:11963–11970. doi: 10.1074/jbc.271.20.11963. [DOI] [PubMed] [Google Scholar]
- 44.Smith JD, Plump AS, Hayek T, Walsh A, Breslow JL. Accumulation of human apolipoprotein E in the plasma of transgenic mice. J Biol Chem. 1990;265:14709–14712. [PubMed] [Google Scholar]
- 45.Chen M, Breslow JL, Li W, Leff T. Mechanisms of inhibition by apolipoprotein C of apolipoprotein E-dependent cellular metabolism of human triglyceride-rich lipoproteins through the low density lipoprotein receptor pathway. J Lipid Res. 1994;35:1918–1924. [Google Scholar]
- 46.Aalto-Setälä K, et al. Mechanism of hypertriglyceridemia in human apo CIII transgenic mice: diminished VLDL fractional catabolic rate associated with increased apo CIII and reduced apo E on the particles. J Clin Invest. 1992;90:1889–1900. doi: 10.1172/JCI116066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jong MC, et al. Both lipolysis and hepatic uptake of VLDL are impaired in transgenic mice coexpressing human apolipoprotein E*3Leiden and human apolipoprotein CI. Arterioscler Thromb Vasc Biol. 1996;16:934–940. doi: 10.1161/01.atv.16.8.934. [DOI] [PubMed] [Google Scholar]
- 48.Baginsky ML, Brown WV. Differential characteristics of purified hepatic triglyceride lipase and lipoprotein lipase from human postheparin plasma. J Lipid Res. 1977;18:423–437. [PubMed] [Google Scholar]
- 49.Paigen B, Morrow A, Brandon C, Mitchell D, Holmes P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis. 1985;57:65–73. doi: 10.1016/0021-9150(85)90138-8. [DOI] [PubMed] [Google Scholar]
- 50.Bilheimer DW, Eisenberg J, Levy RI. The metabolism of very low density lipoproteins. I. Preliminary in vivo and in vitro observations. Biochim Biophys Acta. 1972;26:212–221. doi: 10.1016/0005-2760(72)90034-3. [DOI] [PubMed] [Google Scholar]
- 51.Ramakrishnan R, Arad Y, Wong S, Ginsberg HN. Nonuniform radiolabeling of VLDL apolipoprotein B: implications for the analysis of studies of the kinetics of the metabolism of lipoproteins containing apolipoprotein B. J Lipid Res. 1990;31:1031–1042. [PMC free article] [PubMed] [Google Scholar]
- 52.Wu SM, Boyer CM, Pizzo SV. The binding of receptor-recognized α2-macroglobulin to the low density lipoprotein receptor-related protein and the α2M signaling receptor is decoupled by oxidation. J Biol Chem. 1997;272:20627–20635. doi: 10.1074/jbc.272.33.20627. [DOI] [PubMed] [Google Scholar]
- 53.Paka L, Kako Y, Obunike JC, Pillarisetti S. Apolipoprotein E containing high density lipoprotein stimulates endothelial production of heparan sulfate rich in biologically active heparin-like domains. A potential mechanism for the anti-atherogenic actions of vascular apolipoprotein E. J Biol Chem. 1999;274:4816–4823. doi: 10.1074/jbc.274.8.4816. [DOI] [PubMed] [Google Scholar]
- 54.Pillarisetti S, Klein MG, Goldberg IJ. Identification of a heparin-releasable lipoprotein lipase binding protein from endothelial cells. J Biol Chem. 1992;267:16517–16522. [PubMed] [Google Scholar]
- 55.Obunike JC, Pillarisetti S, Paka L, Low MG, Goldberg IJ. Lipoprotein lipase degradation by adipocytes: receptor-associated protein (RAP)-sensitive and proteoglycan-mediated pathways. J Lipid Res. 1996;37:2439–2449. [PubMed] [Google Scholar]
- 56.Obunike JC, et al. The heparin-binding proteins apolipoprotein E and lipoprotein lipase enhance cellular proteoglycan production. Arterioscler Thromb Vasc Biol. 2000;20:111–118. doi: 10.1161/01.atv.20.1.111. [DOI] [PubMed] [Google Scholar]
- 57.Wagner WD, Nohlgren SR. Aortic glycosaminoglycans in genetically selected WC-2 pigeons with increased atherosclerosis susceptibility. Arteriosclerosis. 1981;1:192–201. doi: 10.1161/01.atv.1.3.192. [DOI] [PubMed] [Google Scholar]
- 58.Choi SY, Cooper AD. A comparison of the roles of the low density lipoprotein (LDL) receptor and the LDL receptor-related protein/2-macroglobulin receptor in chylomicron remnant removal in the mouse in vivo. J Biol Chem. 1993;268:15804–15811. [PubMed] [Google Scholar]
- 59.Roskams T, et al. Heparan sulfate proteoglycan expression in normal human liver. Hepatology. 1995;21:950–958. [PubMed] [Google Scholar]
- 60.Kako Y, et al. Streptozotocin-induced diabetes in human apolipoprotein B transgenic mice. Effects on lipoproteins and atherosclerosis. J Lipid Res. 1999;40:2185–2194. [PubMed] [Google Scholar]
- 61.Bar-on H, Chen Y, Reaven G. Evidence for a new cause of defective plasma removal of very low density lipoproteins in insulin-deficient rats. Diabetes. 1981;30:496–499. doi: 10.2337/diab.30.6.496. [DOI] [PubMed] [Google Scholar]
- 62.Laakso M, et al. Relationship between postheparin plasma lipases and high-density lipoprotein cholesterol in different types of diabetes. Diabetologia. 1987;30:703–706. doi: 10.1007/BF00296992. [DOI] [PubMed] [Google Scholar]
- 63.Hansen PM, et al. Skeletal muscle lipoprotein-lipase activity in insulin-dependent diabetic patients with and without albuminuria. J Diabetes Complications. 1997;11:230–235. doi: 10.1016/s1056-8727(96)00048-7. [DOI] [PubMed] [Google Scholar]
- 64.Donner C, et al. Accelerated lipoprotein uptake by transplantable hepatomas that express hepatic lipase. J Lipid Res. 1998;39:1805–1815. [PubMed] [Google Scholar]
- 65.Hegele RA, et al. Elevated LDL triglyceride concentrations in subjects heterozygous for the hepatic lipase S267F variant. Arterioscler Thromb Vasc Biol. 1998;18:1212–1216. doi: 10.1161/01.atv.18.8.1212. [DOI] [PubMed] [Google Scholar]
- 66.Boisfer E, et al. Overexpression of human apolipoprotein A-II in mice induces hypertriglyceridemia due to defective very low density lipoprotein hydrolysis. J Biol Chem. 1999;274:11564–11572. doi: 10.1074/jbc.274.17.11564. [DOI] [PubMed] [Google Scholar]
- 67.Weisgraber KH, et al. Apolipoprotein C-I modulates the interaction of apolipoprotein E with β-migrating very low density lipoproteins (β-VLDL) and inhibits binding of β-VLDL to low density lipoprotein receptor-related protein. J Biol Chem. 1990;265:22453–22459. [PubMed] [Google Scholar]
- 68.Jong MC, et al. In the absence of the low density lipoprotein receptor, human apolipoprotein C-I overexpression in transgenic mice inhibits the hepatic uptake of very low density lipoproteins via a receptor-associated protein-sensitive pathway. J Clin Invest. 1996;98:2259–2267. doi: 10.1172/JCI119036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mahley RW, Ji Z-S. Remnant lipoprotein metabolism: key pathways involving cell-surface heparan sulfate proteoglycans and apolipoprotein E. J Lipid Res. 1999;40:1–16. [PubMed] [Google Scholar]
- 70.Ji ZS, Sanan DA, Mahley RW. Intravenous heparinase inhibits remnant lipoprotein clearance from the plasma and uptake by the liver: in vivo role of heparan sulfate proteoglycans. J Lipid Res. 1995;36:583–592. [PubMed] [Google Scholar]
- 71.Willnow TE, Sheng Z, Ishibashi S, Herz J. Inhibition of hepatic chylomicron remnant uptake by gene transfer of a receptor antagonist. Science. 1994;264:1471–1474. doi: 10.1126/science.7515194. [DOI] [PubMed] [Google Scholar]
- 72.Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci USA. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ji Z-S, et al. Role of heparan sulfate proteoglycans in the binding and uptake of apolipoprotein E-enriched remnant lipoproteins by cultured cells. J Biol Chem. 1993;268:10160–10167. [PubMed] [Google Scholar]
- 75.Al-Haideri M, et al. Heparan sulfate proteoglycan-mediated uptake of apolipoprotein E-triglyceride-rich lipoprotein particles: a major pathway at physiological particle concentrations. Biochemistry. 1997;36:12766–12772. doi: 10.1021/bi9631024. [DOI] [PubMed] [Google Scholar]
- 76.Kjellen L, Bielefeld D, Hook M. Reduced sulfation of liver heparan sulfate in experimentally diabetic rats. Diabetes. 1983;32:337–342. doi: 10.2337/diab.32.4.337. [DOI] [PubMed] [Google Scholar]
- 77.Spiro MJ. Sulfate metabolism in the alloxan-diabetic rat: relationship of altered sulfate pools to proteoglycan sulfation in heart and other tissues. Diabetologia. 1987;30:259–267. doi: 10.1007/BF00270425. [DOI] [PubMed] [Google Scholar]
- 78.Unger E, Pettersson I, Eriksson UJ, Lindahl U, Kjellen L. Decreased activity of the heparan sulfate-modifying enzyme glucosaminyl N-deacetylase in hepatocytes from streptozotocin-diabetic rats. J Biol Chem. 1991;266:8671–8674. [PubMed] [Google Scholar]
- 79.Kofoed-Enevoldsen A, Noonan D, Deckert T. Diabetes mellitus induced inhibition of glucosaminyl N-deacetylase: effect of short-term blood glucose control in diabetic rats. Diabetologia. 1993;36:310–315. doi: 10.1007/BF00400233. [DOI] [PubMed] [Google Scholar]
- 80.Park L, et al. Suppression of accelerated diabetic atherosclerosis by the soluble receptor for advanced glycation end products. Nat Med. 1998;4:1025–1031. doi: 10.1038/2012. [DOI] [PubMed] [Google Scholar]
- 81.Rescan PY, et al. Distribution and origin of the basement membrane component perlecan in rat liver and primary hepatocyte culture. Am J Pathol. 1993;142:199–208. [PMC free article] [PubMed] [Google Scholar]
- 82.van der Woude FJ, van Det NF. Heparan sulphate proteoglycans and diabetic nephropathy. Exp Nephrol. 1997;5:180–188. [PubMed] [Google Scholar]
- 83.Bollineni JS, Alluru I, Reddi AS. Heparan sulfate proteoglycan synthesis and its expression are decreased in the retina of diabetic rats. Curr Eye Res. 1997;16:127–130. doi: 10.1076/ceyr.16.2.127.5089. [DOI] [PubMed] [Google Scholar]
- 84.Yokoyama H, et al. Immunohistochemical quantification of heparan sulfate proteoglycan and collagen IV in skeletal muscle capillary basement membranes of patients with diabetic nephropathy. Diabetes. 1997;46:1875–1880. doi: 10.2337/diab.46.11.1875. [DOI] [PubMed] [Google Scholar]
- 85.Kanwar YS, et al. D-glucose-induced dysmorphogenesis of embryonic kidney. J Clin Invest. 1996;98:2478–2488. doi: 10.1172/JCI119066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Templeton DM, Fan MY. Posttranscriptional effects of glucose on proteoglycan expression in mesangial cells. Metabolism. 1996;45:1136–1146. doi: 10.1016/s0026-0495(96)90014-1. [DOI] [PubMed] [Google Scholar]
- 87.Jensen T. Pathogenesis of diabetic vascular disease: evidence for the role of reduced heparan sulfate proteoglycan. Diabetes. 1997;46(Suppl. 2):S98–S100. doi: 10.2337/diab.46.2.s98. [DOI] [PubMed] [Google Scholar]
- 88.Kjellen L, Bielefeld D, Hook M. Reduced sulfation of heparin sulfate in experimentally diabetic rats. Diabetes. 1983;32:337–342. doi: 10.2337/diab.32.4.337. [DOI] [PubMed] [Google Scholar]
- 89.Spiro MJ. Sulfate metabolism in the alloxan-diabetic rat: relationship of altered sulfate pools to proteoglycan sulfation in heart and other tissues. Diabetologia. 1987;30:259–267. doi: 10.1007/BF00270425. [DOI] [PubMed] [Google Scholar]
- 90.Unger E, Pettersson I, Eriksson UJ, Lindahl U, Kjellen L. Decreased activity of the heparan sulfate-modifying enzyme glucosaminyl N-deacetylase in hepatocytes from streptozotocin-diabetic rats. J Biol Chem. 1991;266:8671–8674. [PubMed] [Google Scholar]
- 91.Curtin A, et al. Elevated triglyceride-rich lipoproteins in diabetes. A study of apolipoprotein B-48. Acta Diabetol. 1996;33:205–210. doi: 10.1007/BF02048544. [DOI] [PubMed] [Google Scholar]