Abstract
Rheumatoid arthritis (RA) is a complex disease, with contributions from systemic autoimmunity and local inflammation. Persistent synovial joint inflammation and invasive synovial pannus tissue lead to joint destruction. RA is characterized by the production of inflammatory mediators, many of which are regulated by the Rel/NF-κB transcription factors. Although an attractive target for therapeutic intervention in inflammatory diseases, Rel/NF-κB is involved in normal physiology, thus global inhibition could be harmful. An alternate approach is to identify and target the Rel/NF-κB subunits critical for components of disease. To assess this, mice with null mutations in c-rel or nfkb1 were used to examine directly the roles of c-Rel and p50 in models of acute and chronic inflammatory arthritis. We found c-Rel–deficient mice were resistant to collagen-induced arthritis but had a normal response in an acute, destructive arthritis model (methylated BSA/IL-1 induced arthritis) suggesting c-Rel is required for systemic but not local joint disease. In contrast, p50-deficient mice were refractory to induction of both the chronic and acute arthritis models, showing this subunit is essential for local joint inflammation and destruction. Our data suggest Rel/NF-κB subunits play distinct roles in the pathogenesis of inflammatory arthritis and may provide a rationale for more specific therapeutic blockade of Rel/NF-κB in RA.
Introduction
Rheumatoid arthritis (RA) is a complex autoimmune disease that results in chronic inflammation of synovial joints. A common paradigm for the pathogenesis of RA is that major histocompatibility-restricted T-cell activation in response to an unknown joint-derived antigen results in production of autoantibodies and systemic disease induction. Rheumatoid synovitis then occurs, characterized by the infiltration of inflammatory cells into the synovial compartment and the production of inflammatory mediators, many of which are thought to be regulated by the Rel/nuclear factor-κB (NF-κB) transcription factor family (1, 2). Collagen-induced arthritis (CIA) is a widely used murine model of RA (3). Recent studies on CIA in RAG-1–deficient mice, which lack mature T and B cells and yet still develop CIA, support the concept of two components of disease development (4). One is the systemic immune component outlined above, whereas a second involves local synovial cell activation that may be less dependent on a systemic immune response. This concept is further supported by the observation that synovial cells from patients with RA are intrinsically invasive when engrafted into SCID mice (5). The formation of synovial pannus tissue is a characteristic feature of RA and leads to destruction of underlying articular cartilage and bone.
The Rel/NF-κB family of transcription factors are homo- and heterodimeric proteins comprising subunits encoded by a multigene family related to the c-rel proto-oncogene. Five mammalian Rel/NF-κB proteins have been described to date: NF-κB1 (p50, p105), NF-κB2 (p52, p100), RelA (p65), RelB, and c-Rel subunits, encoded by the nfkb1, nfkb2, rela, relb, and c-rel genes, respectively (see refs. 6 and 7 for reviews). In unstimulated cells, the majority of Rel/NF-κB dimers are retained in the cytoplasm as an inactive complex bound to inhibitor proteins (IκBs). In response to a wide variety of stimuli, IκB proteins are phosphorylated by the IKK complex (8), targeting the IκBs for intracellular degradation (9, 10). Rel/NF-κB dimers then translocate to the nucleus and bind to decameric DNA sequences (κB elements) required for the transcription of many genes, including cytokines, chemokines, and adhesion molecules (1). Functional effects of the different Rel/NF-κB dimers may be dependent on binding preferences for particular κB sequences, as well as varying expression profiles of the individual subunits in tissues (11, 12).
Much of our understanding of the function of the Rel/NF-κB family members developed from in vitro experiments, but more recent studies with gene-knockout mice have illuminated the in vivo functions of individual subunits. Lack of RelA causes embryonic lethality due to apoptosis of fetal hepatocytes (13), whereas c-Rel (14), p50 (15), and RelB (16, 17) are required for normal hemopoietic and immune cell function. Although c-Rel–deficient (c-rel–/–) and p50-deficient (nfkb1–/–) mice develop normally, their mature B and T cells exhibit selective activation defects in response to certain mitogenic stimuli (14, 15).
Rel/NF-κB has been considered a potential target for therapeutic intervention in inflammatory disease. Indeed, it has now been realized that at least part of the anti-inflammatory effects of widely used drugs, such as dexamethasone and aspirin, is through inhibition of Rel/NF-κB (18–20). However, because Rel/NF-κB plays such a critical role in immune regulation, nonspecific inhibition might compromise normal host defenses. An alternative approach is to identify and target the Rel/NF-κB subunits critical for disease development.
In this study, we employed mice homozygous for null mutations in genes encoding the Rel/NF-κB subunits, c-Rel and p50, to directly examine the role of these transcription factors in inflammatory arthritis. Two models were examined: CIA, a model of chronic systemic autoimmune arthritis, and methylated BSA/IL-1–induced arthritis, an acute monoarticular arthritis. Each of these models has features of human RA. We report that Rel/NF-κB is an essential mediator of both chronic autoimmune and acute inflammatory arthritis. Both c-rel–/– and nfkb1–/– mice had impaired cellular and humoral immunity to type II collagen (CII) and largely failed to develop CIA. However, c-Rel–deficient mice had a normal response in the acute arthritis model, suggesting c-Rel is not required for the destructive phase of joint disease. In contrast, p50-deficient mice were refractory to the induction of acute arthritis, showing that this Rel/NF-κB subunit is essential for local joint inflammation and destruction. Our data suggest that Rel/NF-κB subunits play distinct roles in the pathogenesis of inflammatory arthritis and may provide the basis for more specific therapeutic intervention in RA.
Methods
Mice.
Mice deficient in the c-rel gene (c-rel–/–), were derived as described previously (14) and backcrossed onto the C57BL/6 strain for 10 generations before use. The p50-deficient mice (nfkb1–/–) (15), were further backcrossed onto C57BL/6 mice for eight generations before use. C57BL/6 mice, obtained from The Walter and Eliza Hall Institute animal services (Kew, Victoria, Australia), were used as wild-type (WT) controls in all experiments. All mice were aged 8 to 13 weeks.
Collagen-induced arthritis.
Chick CII (Sigma Chemical Co., St. Louis, Missouri, USA), dissolved in 10 mM acetic acid at a concentration of 2 mg/mL, was emulsified in an equal volume of CFA containing 5 mg/mL heat-killed Mycobacterium tuberculosis (strain H37Ra; Difco Laboratories, Detroit, Michigan, USA), as described previously (21, 22). Arthritis was induced by injecting mice intradermally at several sites into the base of the tail with 100 μL emulsion at day 0 and 21. Individual experiments contained at least nine mice per group, and all experiments were performed two or more times.
Animals were assessed for swelling of limbs, and a clinical score was allocated to each mouse two to three times per week, for up to 60 days. The scoring system was as described previously (23), where 0 indicates normal, 1 indicates slight swelling, 2 indicates extensive swelling, and 3 indicates joint distortion and/or rigidity. The maximum score per mouse was 12. Mice were considered to have arthritis when two consecutive positive evaluations were obtained. Where swelling was restricted to digits, the maximum score for the affected limb was 1, regardless of severity. At sacrifice, paws were removed, fixed, decalcified, and processed for paraffin embedding. Hematoxylin and eosin–stained sections (5 μm) were evaluated for arthritis as described previously (21), where mild indicated minimal synovitis with cartilage and bone erosions confined to discrete foci; moderate indicated synovitis and erosions with intact joint architecture; and severe indicated extensive erosions and disrupted joint architecture. Clinical and histological evaluations were assessed by two independent investigators blinded to the experimental groups.
Acute monoarticular arthritis.
Acute monoarticular arthritis was induced as described (24, 25). On day 0, mice were anesthetized and the skin over the knees incised to expose the stifle joints. Two hundred micrograms of methylated BSA (mBSA; Sigma Chemical Co.) was injected into the left knee joint, and the contralateral joint received 10 μL saline. The incisions were then closed with wound clips. Human recombinant IL-1β (250 ng) in 0.5% (vol/vol) normal mouse serum (in saline) was injected subcutaneously into the left footpad on days 0, 1, and 2. Mice were sacrificed at day 7 and their rear limbs removed and processed, as above, for histological assessment of hematoxylin and eosin–stained frontal sections of the stifle joints. Coded joint sections were assessed histologically for five features of inflammatory arthritis, each on a scale of 0 (normal) to 5 (severe), as described (24). These were exudate (presence of inflammatory cells or fibrinlike material in the joint space), synovitis (thickening of the synovium, infrapatella fatpad, and periosteum, but excluding pannus), pannus (synovial hyperplasia immediately adjacent to cartilage or bone), cartilage degradation, and bone degradation. Sections were assessed by two independent observers without knowledge of the treatment groups. There were six to ten mice per experimental group.
ELISA for detection of Abs to CII.
ELISAs were performed to detect Abs to CII as previously described (21). Horseradish peroxidase–conjugated goat anti-mouse IgG (Sigma Chemical Co.) or IgM (Southern Biotechnology Associates, Birmingham, Alabama, USA) antisera were used as detection Abs. Standard curves for anti-CII IgG and IgM were constructed from sera of CII-hyperimmunized mice using arbitrary units.
T-cell proliferation assay.
A single-cell suspension of inguinal lymph node cells (2 × 106 cells/mL in RPMI containing 50 μM 2-mercaptoethanol and 5% FBS), prepared from mice (n ≥ 3) at least 10 days after CII immunization, was incubated at 37°C (5% CO2) for 72 hours with 0–50 μg/mL of denatured CII (boiled for 10 minutes). Cultures were performed in 96-well round-bottomed plates (Becton Dickinson Labware, Franklin Lakes, New Jersey, USA) with 0.2 mL per well (4–6 wells per group). For the final 6-12 hours, 5 μCi/mL [3H]thymidine (Amersham International, Amersham, United Kingdom) was added and cells harvested with an Inotech cell harvester (Rockville, Maryland, USA). A microplate scintillation counter (Canberra Packard, Victoria, Australia) was used to determine radioactive incorporation as a measure of T-cell proliferation.
Electrophoretic mobility shift analysis.
Synovial tissue was dissected from acutely arthritic joints (n = 20), 7 days after injection of mBSA, and a single-cell suspension was prepared by digestion for 1 hour at 37°C in RPMI containing 2.4 mg/mL dispase II (Boehringer-Mannheim GmbH, Mannheim, Germany), 1 mg/mL type II Clostridial collagenase (Sigma Chemical Co.), and 0.1 mg/mL bovine pancreatic DNAse I (Boehringer-Mannheim GmbH). After overnight incubation in RPMI plus 10% FBS, nuclear extracts were prepared from the washed, adherent synovial cells for electrophoretic mobility shift analysis (EMSA) as described (26). The Igκ probe (5′-GTACGAGGGGACTTTCCGA-3′) was used in these experiments. For supershift analyses, extracts were incubated for 1 hour on ice with 0.2 mg/mL rabbit Ab to mouse p65, c-Rel, and RelB (Santa Cruz Biotechnology, Santa Cruz, California, USA), and p50 (gift from N. Rice, National Cancer Institute, Frederick, Maryland, USA) before addition of radioactively labeled probe. Bands were visualized using a PhosphoImager with Image Quant v5.0 software (Molecular Dynamics, Sunnyvale, California, USA).
Statistics.
For CIA clinical scores and acute monoarticular arthritis histological scores the Mann-Whitney two-sample rank test was used to determine the level of significance between means of groups. For data pertaining to serum Ab and the T-cell proliferation assay, Student’s t test for the difference of two means was employed. The incidence of CIA between different groups and the CIA histological assessments were evaluated by the χ2 test. For each test P less than 0.05 was considered statistically significant.
Results
c-rel–/– and nfkb1–/– mice are resistant to CIA.
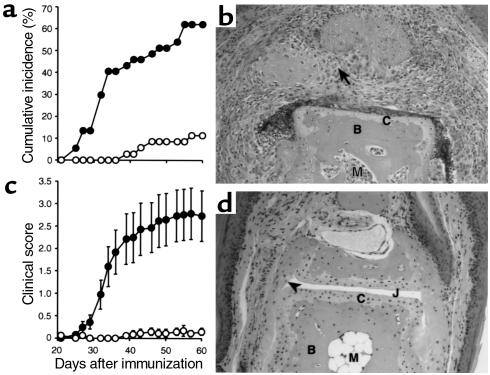
To examine the role of Rel/NF-κB in a model of autoimmune arthritis, WT mice and mice homozygous for null mutations either in the c-rel or nfkb1 genes were compared for incidence and severity of CIA induced by intradermal immunization with chick CII in CFA, followed by a boost injection 21 days later. We showed previously that this procedure successfully elicits arthritis in C57BL/6 mice (21) and is dependent on T and B cells (22). Paws were serially assessed and a clinical score given for each mouse according to the procedure outlined in Methods. Figure 1 shows the kinetics of the response in c-rel–/– and WT mice both in terms of incidence (Figure 1a) and clinical severity (Figure 1c). Only four of 35 (11%) c-Rel–deficient mice showed clinical signs of arthritis compared with 23 of 37 (62%) WT mice (P < 0.001) by day 60 (Table 1). The clinical scores of the c-rel–/– mice ranged from 1 to 4 compared with 1 to 12 in WT mice and were significantly lower (P < 0.0001; 0.3 ± 0.1 vs. 3.1 ± 0.6, mean ± SEM for c-rel–/– and WT mice, respectively). Therefore, c-Rel–deficient mice had a lower incidence and reduced severity of CIA compared with WT controls. Compared with 15 of 25 WT mice, p50-deficient mice were completely resistant to CIA, with no clinical evidence of disease in a total of 24 mice tested (Table 1).
Figure 1.
Clinical and histological assessment of CIA in c-rel–/– versus WT mice. The incidence of arthritis (shown as cumulative percentage) (a) and mean clinical scores (± SEM) (c) of c-rel–/– (open circles, n = 35) and WT (filled circles, n = 37) mice are shown with time after primary immunization with CII. Data are pooled from three experiments. For statistical analysis see Table 1. At 60 days after primary immunization with CII, mice were sacrificed, their hind limbs removed, and the paws processed for histology (see Methods). Frontal sections of the interphalangeal joints of WT (b) and c-rel–/– (d) mice are shown. WT mouse joints frequently showed severe pathology with pannus invading into the subchondral bone (arrow). The majority of c-rel–/– mouse joints examined appeared normal, with intact articular cartilage (C) and no inflammatory cells in the joint space (J) or synovium (arrowhead). B, bone; M, bone marrow. Hematoxylin and eosin stained. ×200.
Table 1.
Reduced incidence and clinical severity of CIA in c-rel–/– and nfkb1–/– miceA
Histological analysis of CIA.
To confirm the clinical analyses, hematoxylin and eosin–stained sections of joints from c-rel–/–, nfkb1–/–, and WT mice were assessed by histology. There was a strong concordance between the clinical and histological assessments. Joints of WT mice frequently (62% of joints examined) showed severe inflammation and joint tissue destruction with invasive pannus tissue associated with bone and cartilage degradation (Figure 1b and Table 2). In contrast, the majority (83%; see Table 2) of joints from the c-rel–/– mice appeared normal (Figure 1d). However, in the few c-rel–/– arthritic joints, the histological features were similar to that of the WT arthritic mice (not shown), with 6% of the joints examined showing severe pathology (Table 2). Therefore, c-Rel plays an important but not indispensable role in the full expression of CIA. In accordance with clinical assessments, none of the nfkb1–/– mouse joints showed any signs of arthritis.
Table 2.
Histopathological assessment of joints from c-Rel–deficient and WT miceA
Altered humoral and reduced cellular immune responses to CII in c-rel–/– and nfkb1–/– mice.
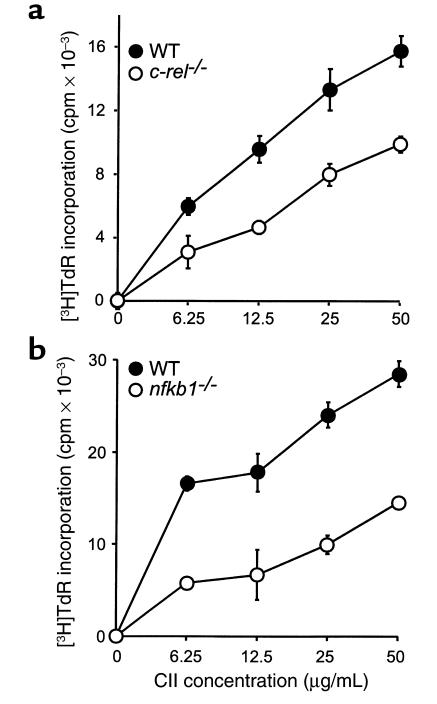
Studies were undertaken to determine why c-rel–/– and nfkb1–/– mice are resistant to CIA. Since CIA is dependent on both humoral and cellular immune responses to CII (27), these were examined. The c-rel–/– mice had a dramatically lower IgG response compared with WT mice at all time points (Figure 2a, upper panel). However, the c-rel–/– IgM response, although lower at day 12, was comparable to WT mice at day 28 and 60 (Figure 2a, lower panel). In contrast, the nfkb1–/– mice had no detectable IgG response and a significantly reduced IgM response to CII at all time points (Figure 2b). In vitro T-cell responses were also examined. Both c-rel–/– and nfkb1–/– T lymphocytes proliferated to CII in a dose-dependent manner (Figure 3, a and b, respectively), however the responses were significantly reduced (by 40–50% at the highest CII concentration, 50 μg/mL) in the gene-knockout mice, compared with WT controls.
Figure 2.
Humoral response to CII in c-rel–/– (a) and nfkb1–/– (b) mice. Serum levels of CII-specific Abs (IgG and IgM) were determined by ELISA in c-rel–/–, nfkb1–/–, and WT mice (n = 9–10) at different times after primary immunization with CII. Results show the mean + SEM values for IgG and IgM in arbitrary units per milliliter for individual experiments. AP < 0.001, BP < 0.01, CP < 0.05 compared with WT control mice.
Figure 3.
Cellular response to CII in c-rel–/– (a) and nfkb1–/– (b) mice. Single-cell suspensions of inguinal lymph node cells were prepared from c-rel–/–, nfkb1–/–, and WT mice immunized with CII (see Methods). The cells were stimulated for 72 hours with different doses of denatured CII and labeled with [3H]thymidine (TdR) over the last 6 (a) or 12 hours (b) of culture. The CII-specific T-cell proliferative response was measured as the increase in [3H]TdR incorporation over that in the absence of CII, in counts per minute. Cells of the c-rel–/– and nfkb1–/– mice showed significantly less [3H]TdR incorporation than those of WT mice at all CII concentrations (P < 0.05).
Differential requirement for Rel/NF-κB subunits in acute inflammatory arthritis.
The majority of c-rel–/– mice failed to develop CIA due, at least partly, to a diminished systemic immune response to CII. However, in a small minority of c-rel–/– mice CIA did develop, and within the affected joints it progressed to destructive joint disease. An acute inflammatory arthritis model was therefore employed to further assess the requirement for c-Rel in the destructive phase of arthritis. This model develops over 1 week, occurs in all mBSA-injected joints, and is dependent on IL-1 (24) and CD4+ T cells but not B cells (K. Lawlor et al., manuscript submitted for publication). Sections of mBSA-injected joints from c-rel–/– and WT mice were indistinguishable in appearance (compare Figure 4, c and b, respectively) and this was reflected in the histological scores (Figure 4e), with each genotype showing a similar degree of inflammatory cell infiltration, pannus formation, and loss of articular cartilage and bone. Saline-injected contralateral control joints appeared normal in all instances (Figure 4a).
Figure 4.
Acute inflammatory arthritis in Rel/NF-κB–deficient and WT mice. Monoarticular arthritis was induced as described in Methods. Frontal sections of control (a) and mBSA-injected patello-femoral joints of WT (b), c-rel–/– (c), and nfkb1–/– (d) mice are shown (hematoxylin and eosin–stained). ×200. E, inflammatory exudate; F, femur; I, inflammatory cell infiltrate; P, pannus; Pt, patella. Arrow indicates synovial lining. Coded joint sections were assessed histologically for five features of inflammatory arthritis, each on a scale of 0 (normal) to 5 (severe). Results (e and f) compare the mean + SEM values for each of the histological features of c-rel–/– (n = 6) (e) and nfkb1–/– (n = 10) (f) mice (open bars) to those of WT mice (filled bars). Control (saline) joints scored 0 for all categories (not shown). AP < 0.005.
To determine whether the pathological mechanisms in the acute arthritis model were independent of Rel/NF-κB involvement, we also evaluated the requirement for p50. In contrast to c-Rel–deficient mice, p50-deficient mice had markedly reduced arthritis compared with WT mice (compare Figure 4, d and b, respectively). The most notable differences were the absence of inflammatory cells in the joint space and soft connective tissues (i.e., subsynovium, infrapatella fatpad, periosteum) and a reduction in pannus formation in the mBSA-injected joints of nfkb1–/– mice (see Figure 4f for histological assessment). The reduced response of the nfkb1–/– mice was observed in all joints examined (n = 10).
Nuclear translocation of p50 but not c-Rel in acute arthritis synovial cells.
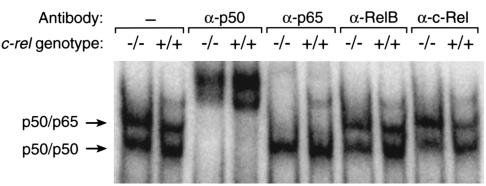
To further investigate the role of Rel/NF-κB subunits in acute inflammatory arthritis, EMSA and supershift analyses were performed on nuclear extracts of primary adherent synovial cells obtained from mBSA-injected joints of WT and c-rel–/– mice. Figure 5 shows two major bands were detected for each genotype; a slower migrating band supershifted by Abs to p50 and p65 (top arrow) and a faster migrating band supershifted by Abs to p50 only (bottom arrow). Abs to c-Rel and RelB had no effect on the electrophoretic mobilities of either band. These findings are consistent with the activation in WT and c-rel–/– arthritic joint synovial cells of p50/p65 heterodimers and p50 homodimers, but not of c-Rel– or RelB–containing complexes.
Figure 5.
Subunit composition of Rel/NF-κB complexes in synovial cells from acute arthritic joints. Nuclear extracts of primary synovial cells obtained by enzymatic digestion of synovial tissue from mBSA-injected joints (day 7) of WT (+/+) and c-rel–/– (–/–) mice were examined by EMSA in the presence and absence of specific Abs to Rel/NF-κB subunits.
Discussion
The Rel/NF-κB family of transcription factors regulate a wide variety of inflammatory mediators, most of which are found in synovial joints of patients with RA (1, 2). Several immunohistochemical studies have reported Rel/NF-κB activation in synovial tissue from RA patients (28, 29) and in animal models of inflammatory arthritis (30, 31), thereby implicating Rel/NF-κB in disease pathogenesis. In this study we used mice homozygous for null mutations in genes encoding the Rel/NF-κB subunits, c-Rel or p50, to obtain direct evidence for Rel/NF-κB involvement in acute, monoarticular, and chronic polyarticular inflammatory arthritis models. Our results show that Rel/NF-κB is essential for the development of inflammatory arthritis and, to our knowledge, provide the first evidence that selective blockade of Rel/NF-κB subunits may prevent it. We also show that the absence of different Rel/NF-κB subunits can have differential effects suggesting distinct roles, rather than redundancy, for the various Rel/NF-κB subunits in different stages of disease.
An interesting result from this study was the almost complete absence of CIA in c-Rel–deficient mice, but a typical response of these mice in the acute arthritis model. CIA is a widely used model of RA thought to be dependent on the establishment of both cellular and humoral immunity to CII (27), although recent evidence suggests that innate immune responses to CII are also important (4). Both a reduced incidence and clinical severity of CIA were exhibited by c-rel–/– mice. In the few c-rel–/– mice that did develop arthritis (4 of 35) it was limited to isolated digits. In contrast, at the histological level, the pannus formation and bone and cartilage erosion in affected joints was comparable to that seen in arthritic WT mice. Therefore, c-Rel is needed for the polyarticular expression of CIA, but its absence is not absolutely protective against CIA. In contrast, p50-deficient mice were completely protected from CIA when assessed both clinically and histologically.
It was reported previously that c-rel–/– and nfkb1–/– mice had impaired Ab responses (14, 15). Production of anti-CII IgM was essentially normal in c-rel–/– mice, but deficient in nfkb1–/– mice. IgG Abs to CII were markedly suppressed in both knockout mice compared with WT mice. Thus c-Rel is required for the switch from an IgM to an IgG response to CII in vivo, which confirms previous in vitro studies on c-rel–/– B cells (32). The absence of high levels of IgG anti-CII could account for the lack of CIA response in both knockout mice, because IgG anti-CII and fixation of complement C5 are considered essential for CIA (33, 34). However, because some c-rel–/– mice did develop limited CIA, it is also possible that high local IgM anti-CII may have substituted for IgG in affected joints. The complete absence of CIA in nfkb1–/– mice, which lacked an IgM response to CII, would be consistent with this hypothesis. Alternatively, the limited CIA observed in c-rel–/– mice may have resulted from innate immune responses to CII (4) that are dependent on p50 but not c-Rel.
Deficiencies in other immune or inflammatory mechanisms may also limit CIA in c-rel–/– and nfkb1–/– mice. Both knockout mice had reduced T-cell proliferative responses to CII (Figure 3), consistent with previous findings that showed a diminished response to mitogens and/or altered production of cytokines by T cells (14, 15, 35–37). However, antigen-presenting cell defects could also be involved. Whereas c-rel–/– and nfkb1–/– mice exhibited both impaired humoral and cellular immune responses to CII that together could account for the dramatic reduction of CIA, other contributing influences could be the cytokine profile and activation state of macrophage populations (15, 38). Macrophages are important both as antigen-presenting cells (39) and as a source of inflammatory mediators in CIA (40, 41).
In contrast to the response of c-rel–/– mice in CIA, these mice were indistinguishable from WT in the acute inflammatory arthritis model, which is dependent on IL-1 and CD4+ T cells, but not B cells. The knee sections of each showed infiltration of neutrophils and mononuclear cells, pannus formation, soft tissue inflammation, and cartilage and bone erosion. This is consistent with the histological features observed in the few CIA-affected joints of the c-rel–/– mice. Taken together, these results suggest that c-Rel is required for establishment of the immune response in CIA but not for the destructive (effector) phase of inflammatory arthritis. In contrast to c-rel–/– mice, nfkb1–/– mice had a markedly diminished response in the acute arthritis model. There were few inflammatory cells in the joint space and connective tissues and a striking reduction in synovial hyperplasia. Since Rel/NF-κB regulates the transcription of adhesion molecules, prostaglandin-producing enzymes, and chemokines (1), this may explain the reduced cellular trafficking into the mBSA-injected joints of p50-deficient mice.
The effect of p50 deletion on T-cell function is unclear. There are conflicting reports on nfkb1–/– T-cell proliferation and cytokine production (15, 36, 37). The p50-deficient mice were protected in a T cell–dependent model of allergic airway inflammation (37); antigen-specific T-cell responses were maintained in these mice, but IL-5 production was reduced. It is possible that different T-cell cytokine profiles in the c-rel–/– and nfkb1–/– mice may account for responses in the acute arthritis model. Both c-rel–/– and nfkb1–/– mice had antigen-specific T-cell proliferative responses, but these were reduced — to a similar degree — compared with WT mice (Figure 3). In spite of this, T cells from c-rel–/– mice were able to elicit a normal response in the T cell–dependent acute arthritis model, but nfkb1–/– mice showed almost no disease.
A recent study showed Rel/NF-κB inhibition enhanced synovial apoptosis in a rat model of arthritis (42), consistent with the potential involvement of Rel/NF-κB transcription factors in synovial hyperplasia. Once formed, pannus is thought to mediate cartilage and bone destruction in RA through matrix metalloproteinase (MMP) production (43). The transcription of MMPs appears to be regulated in part by Rel/NF-κB (44). Inhibition of Rel/NF-κB in human rheumatoid synovial cell cultures reduced proinflammatory cytokine production, together with MMP-1 and MMP-3 (45). In IL-1β–stimulated rabbit synovial fibroblasts, p50 bound to a regulatory element in the distal promoter of MMP-1 (46). These findings, together with the results presented here, suggest p50 is involved in pannus formation and perhaps in regulating joint tissue destruction through effects on MMP gene transcription.
The importance of p50 in synovial cell gene transcription was confirmed by EMSAs, which showed p50/p65 heterodimers and p50 homodimers in nuclear extracts of cells isolated from the acutely inflamed joints of WT and c-rel–/– mice (Figure 5). In contrast, c-Rel was not involved in the activated synovial cell response. Because primary cells were used, the Rel/NF-κB complexes could have been derived from macrophage-like (type A) or fibroblast-like (type B) synovial cells, or both. Using immunohistochemistry, p50 and p65 have been found in type A cells of rheumatoid synovium (28, 29) and in passaged rheumatoid synovial fibroblasts by EMSA (47, 48). We have now also identified p50 homodimers in synovial cell nuclei. Whereas regulation of gene transcription by p50-containing dimers is complex — indeed it has been suggested that p50 homodimers can function as transcriptional repressors (11) — our data clearly demonstrate that the net effect of p50 deletion is a marked reduction of inflammatory joint disease.
In summary, our results show that Rel/NF-κB is essential for the development of inflammatory arthritis and provide strong evidence that selective blockade of Rel/NF-κB subunits may prevent major components of the disease. We also show that the absence of different Rel/NF-κB subunits can have differential effects in acute inflammatory arthritis suggesting distinct roles, rather than redundancy, for the various Rel/NF-κB subunits. Several strategies have been used to block global Rel/NF-κB activity in various disease models (31, 42, 49–51). However, targeting a specific Rel/NF-κB subunit, especially within a given anatomical compartment, may cause fewer side effects than those that inhibit all Rel/NF-κB functions. Such a specific approach with p65 antisense phosphorothioate oligonucleotides was used to prevent experimental colitis (52). Our results support several previous studies that indicate total Rel/NF-κB blockade would prevent inflammatory arthritis (31, 42, 49). However, we have demonstrated that specific Rel/NF-κB subunits may play distinct roles at different stages of disease. Consequently, local inhibition of p50 may prevent joint inflammation and the synovial tissue responses that lead to joint destruction. We conclude that p50 and genes regulated by p50 are targets for rational drug design in inflammatory arthritis.
Acknowledgments
This work was supported by the Reid Charitable Trusts, the Arthritis Foundation of Australia, the National Health and Medical Research Council of Australia, the Anti-Cancer Council of Victoria, and a Commonwealth AIDS Research Grant (SG; 971274). We thank D. Baltimore (California Institute of Technology, Pasadena, California, USA) and W. Sha (University of California, Berkeley, California, USA) for the provision of nfkb1–/– mice. We are grateful to J. Rikmanspoel for technical assistance, A. Milligan for animal care, S. Mihajlovic for histology, and S. Olding for help with figures.
References
- 1.Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- 2.Feldmann M, Brennan FM, Maini RN. Role of cytokines in rheumatoid arthritis. Annu Rev Immunol. 1996;14:397–440. doi: 10.1146/annurev.immunol.14.1.397. [DOI] [PubMed] [Google Scholar]
- 3.Myers LK, Rosloniec EF, Cremer MA, Kang AH. Collagen-induced arthritis, an animal model of autoimmunity. Life Sci. 1997;61:1861–1878. doi: 10.1016/s0024-3205(97)00480-3. [DOI] [PubMed] [Google Scholar]
- 4.Plows D, Kontogeorgos G, Kollias G. Mice lacking mature T and B lymphocytes develop arthritic lesions after immunization with type II collagen. J Immunol. 1999;162:1018–1023. [PubMed] [Google Scholar]
- 5.Müller-Ladner U, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am J Pathol. 1997;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen F, Castranova V, Shi X, Demers LM. New insights into the role of nuclear factor-κB, a ubiquitous transcription factor in the initiation of diseases. Clin Chem. 1999;45:7–17. [PubMed] [Google Scholar]
- 7.Baldwin AS., Jr The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–681. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 8.Stancovski I, Baltimore D. NF-κB activation. The IκB kinase revealed? Cell. 1997;91:299–302. doi: 10.1016/s0092-8674(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 9.DiDonato J, et al. Mapping of the inducible IκB phosphorylation sites that signal its ubiquitination and degradation. Mol Cell Biol. 1996;16:1295–1304. doi: 10.1128/mcb.16.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IκBα by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84:853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 11.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 12.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 13.Beg AA, Sha WC, Bronson RT, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 14.Köntgen F, et al. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 15.Sha WC, Liou H-C, Tuomanen EI, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 16.Burkly L, et al. Expression of relB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 17.Weih F, et al. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 18.Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: inhibition of NF-κB activity through induction of IκB synthesis. Science. 1995;270:286–290. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 19.Scheinman RI, Cogswell PC, Lofquist AK, Baldwin A., Jr Role of transcriptional activation of IκBα in mediation of immunosuppression by glucocorticoids. Science. 1995;270:283–286. doi: 10.1126/science.270.5234.283. [DOI] [PubMed] [Google Scholar]
- 20.Kopp E, Ghosh S. Inhibition of NF-κB by sodium salicylate and aspirin. Science. 1994;265:956–959. doi: 10.1126/science.8052854. [DOI] [PubMed] [Google Scholar]
- 21.Campbell IK, et al. Protection from collagen-induced arthritis in granulocyte-macrophage colony-stimulating factor-deficient mice. J Immunol. 1998;161:3639–3644. [PubMed] [Google Scholar]
- 22.Campbell, I.K., Hamilton, J.A., and Wicks, I.P. 2000. Collagen-induced arthritis in C57BL/6 (H-2b) mice: new insights into an important disease model of rheumatoid arthritis. Eur. J. Immunol. In press. [DOI] [PubMed]
- 23.Campbell IK, Bendele A, Smith DA, Hamilton JA. Granulocyte-macrophage colony stimulating factor exacerbates collagen induced arthritis in mice. Ann Rheum Dis. 1997;56:364–368. doi: 10.1136/ard.56.6.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Staite ND, et al. Induction of an acute erosive monoarticular arthritis in mice by interleukin-1 and methylated bovine serum albumin. Arthritis Rheum. 1990;33:253–260. doi: 10.1002/art.1780330215. [DOI] [PubMed] [Google Scholar]
- 25.Bischof RJ, Zafiropoulos D, Hamilton JA, Campbell IK. Exacerbation of acute inflammatory arthritis by the colony-stimulating factors CSF-1 and GM-CSF: evidence of macrophage infiltration and local proliferation. Clin Exp Immunol. 2000;119:361–367. doi: 10.1046/j.1365-2249.2000.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens LA, et al. Tumour necrosis factor-—activated cell death pathways in NIT-1 insulinoma cells and primary pancreatic β cells. Endocrinology. 1999;140:3219–3227. doi: 10.1210/endo.140.7.6873. [DOI] [PubMed] [Google Scholar]
- 27.Seki N, et al. Type II collagen-induced murine arthritis. I. Induction and perpetuation of arthritis require synergy between humoral and cell-mediated immunity. J Immunol. 1988;140:1477–1484. [PubMed] [Google Scholar]
- 28.Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-κB in rheumatoid arthritis. Localization of p50 and p65. Arthritis Rheum. 1995;38:1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- 29.Marok R, et al. Activation of the transcription factor nuclear factor-κB in human inflamed synovial tissue. Arthritis Rheum. 1996;39:583–591. doi: 10.1002/art.1780390407. [DOI] [PubMed] [Google Scholar]
- 30.Han Z, Boyle DL, Manning AM, Firestein GS. AP-1 and NF-κB regulation in rheumatoid arthritis and murine collagen-induced arthritis. Autoimmunity. 1998;28:197–208. doi: 10.3109/08916939808995367. [DOI] [PubMed] [Google Scholar]
- 31.Tsao PW, et al. The effect of dexamethasone on the expression of activated NF-κB in adjuvant arthritis. Clin Immunol Immunopathol. 1997;83:173–178. doi: 10.1006/clin.1997.4333. [DOI] [PubMed] [Google Scholar]
- 32.Zelazowski P, et al. B cells genetically deficient in the c-rel transactivation domain have selective defects in germline CH transcription and Ig class switching. J Immunol. 1997;159:3133–3139. [PubMed] [Google Scholar]
- 33.Watson WC, Townes A. Genetic susceptibility to murine collagen II autoimmune arthritis. Proposed relationship to the IgG2 autoantibody subclass response, complement C5, major histocompatibility complex (MHC) and non-MHC loci. J Exp Med. 1985;162:1878–1891. doi: 10.1084/jem.162.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Rollins SA, Madri JA, Matis LA. Anti-C5 monoclonal antibody therapy prevents collagen-induced arthritis and ameliorates established disease. Proc Natl Acad Sci USA. 1995;92:8955–8959. doi: 10.1073/pnas.92.19.8955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gerondakis S, et al. Rel-deficient T cells exhibit defects in production of interleukin 3 and granulocyte-macrophage colony-stimulating factor. Proc Natl Acad Sci USA. 1996;93:3405–3409. doi: 10.1073/pnas.93.8.3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hilliard B, Samoilova EB, Liu T-ST, Rostami A, Chen Y. Experimental autoimmune encephalomyelitis in NF-κB-deficient mice: roles of NF-κB in the activation and differentiation of autoreactive T cells. J Immunol. 1999;163:2937–2943. [PubMed] [Google Scholar]
- 37.Yang L, et al. Essential role of nuclear factor κB in the induction of eosinophilia in allergic airway inflammation. J Exp Med. 1998;188:1739–1750. doi: 10.1084/jem.188.9.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigoriadis G, et al. The Rel subunit of NF-κB-like transcription factors is a positive and negative regulator of macrophage gene expression: distinct roles for Rel in different macrophage populations. EMBO J. 1996;15:7099–7107. [PMC free article] [PubMed] [Google Scholar]
- 39.Michaëlsson E, et al. Macrophages, but not dendritic cells, present collagen to T cells. Eur J Immunol. 1995;25:2234–2241. doi: 10.1002/eji.1830250818. [DOI] [PubMed] [Google Scholar]
- 40.Marinova-Mutafchieva L, et al. Dynamics of proinflammatory cytokine expression in the joints of mice with collagen-induced arthritis (CIA) Clin Exp Immunol. 1997;107:507–512. doi: 10.1046/j.1365-2249.1997.2901181.x. [DOI] [PubMed] [Google Scholar]
- 41.van Lent PLEM, et al. Phagocytic lining cells determine local expression of inflammation in type II collagen-induced arthritis. Arthritis Rheum. 1996;39:1545–1555. doi: 10.1002/art.1780390915. [DOI] [PubMed] [Google Scholar]
- 42.Miagkov AV, et al. NF-κB activation provides the potential link between inflammation and hyperplasia in the arthritic joint. Proc Natl Acad Sci USA. 1998;95:13859–13864. doi: 10.1073/pnas.95.23.13859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagase, H., and Okada, Y. 1997. Proteinases and matrix degradation. In Textbook of rheumatology. W. Kelley, E. Harris, Jr., S. Ruddy, and C. Sledge, editors. W.B. Saunders Co. Philadelphia, Pennsylvania, USA. 323–341.
- 44.Benbow U, Brinckerhoff CE. The AP-1 site and MMP gene regulation: what is all the fuss about? Matrix Biol. 1997;15:519–526. doi: 10.1016/s0945-053x(97)90026-3. [DOI] [PubMed] [Google Scholar]
- 45.Bondeson J, Foxwell B, Brennan F, Feldmann M. Defining therapeutic targets by using adenovirus: blocking NF-κB inhibits both inflammatory and destructive mechanisms in rheumatoid synovium but spares anti-inflammatory mediators. Proc Natl Acad Sci USA. 1999;96:5668–5673. doi: 10.1073/pnas.96.10.5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vincenti MP, Coon CI, Brinckerhoff CE. Nuclear factor κB/p50 activates an element in the distal matrix metalloproteinase 1 promoter in interleukin-1β-stimulated synovial fibroblasts. Arthritis Rheum. 1998;41:1987–1994. doi: 10.1002/1529-0131(199811)41:11<1987::AID-ART14>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 47.Fujisawa K, et al. Activation of transcription factor NF-κB in human synovial cells in response to tumor necrosis factor α. Arthritis Rheum. 1996;39:197–203. doi: 10.1002/art.1780390205. [DOI] [PubMed] [Google Scholar]
- 48.Roshak AK, et al. Manipulation of distinct NFκB proteins alters interleukin-1β-induced human rheumatoid synovial fibroblast prostaglandin E2 formation. J Biol Chem. 1996;271:31496–31501. doi: 10.1074/jbc.271.49.31496. [DOI] [PubMed] [Google Scholar]
- 49.Palombella, et al. Role of the proteasome and NF-κB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci USA. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu SF, Ye XB, Malik AB. In vivo inhibition of nuclear factor-κB activation prevents inducible nitric oxide synthase expression and systemic hypotension in a rat model of septic shock. J Immunol. 1997;159:3976–3983. [PubMed] [Google Scholar]
- 51.Essani NA, Fisher MA, Jaeschke H. Inhibition of NF-κB activation by dimethyl sulfoxide correlates with suppression of TNF-α formation, reduced ICAM-1 gene transcription, and protection against endotoxin-induced liver injury. Shock. 1997;7:90–96. doi: 10.1097/00024382-199702000-00003. [DOI] [PubMed] [Google Scholar]
- 52.Neurath MF, Pettersson S, Meyer zum Büschenfelde K-H, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-κB abrogates established experimental colitis in mice. Nat Med. 1996;2:998–1004. doi: 10.1038/nm0996-998. [DOI] [PubMed] [Google Scholar]