Abstract
We designed this study to explore to what extent the excess risk of cardiovascular events in diabetic individuals is attributable to hypertension. We retrospectively analyzed prospectively collected data from the Framingham Original and Offspring cohorts. Of the 1145 Framingham subjects newly diagnosed with diabetes who did not have a prior history of cardiovascular events, 663 (58%) had hypertension at the time diabetes was diagnosed. During 4154 person-years of follow-up, 125 died and 204 suffered a cardiovascular event. Framingham participants with hypertension at the time of diabetes diagnosis exhibited higher rates of all cause mortality (32 versus 20 per 1000 person years, p<0.001) and cardiovascular events (52 versus 31 per 1000 person years, p<0.001) compared with normotensive subjects with diabetes. After adjustment for demographic and clinical covariates, hypertension was associated with a 72% increase in the risk of all cause death and a 57% increase in the risk of any cardiovascular event in individuals with diabetes. The population attributable risk from hypertension in individuals with diabetes was 30% for all-cause death and 25% for any cardiovascular event (increasing to 44% and 41% respectively if the 110 normotensive subjects who developed hypertension during follow-up were excluded from the analysis). In comparison, after adjustment for concurrent hypertension, the population attributable risk from diabetes in Framingham subjects was 7% for all cause mortality and 9% for any CVD event. While diabetes is associated with increased risks of death and cardiovascular events in Framingham subjects, much of this excess risk is attributable to coexistent hypertension.
Keywords: diabetes, hypertension, Framingham, population attributable risk
Hypertension and diabetes are increasing in prevalence, commonly coexist, and patients with both conditions are particularly vulnerable to cardiovascular disease and death.1-4 Hypertension is more common in individuals with diabetes than the general population, with estimates of the prevalence of hypertension in diabetic populations ranging from 40% to 80%. 5-11 Although previous studies have demonstrated that diabetes is associated with increased cardiovascular (CV) events and death,5-9, 11 with population attributable risks varying from 6% to 12%,9 it is not clear whether this risk is due to diabetes per se or due to concomitant hypertension. Certainly, trials of glucose lowering in individuals with diabetes have reported disappointingly small benefits on myocardial infarction, stroke, or death.11 Thus, we designed this study to determine how much of the cardiovascular risk in individuals with diabetes is attributable to hypertension.
Methods
Study population
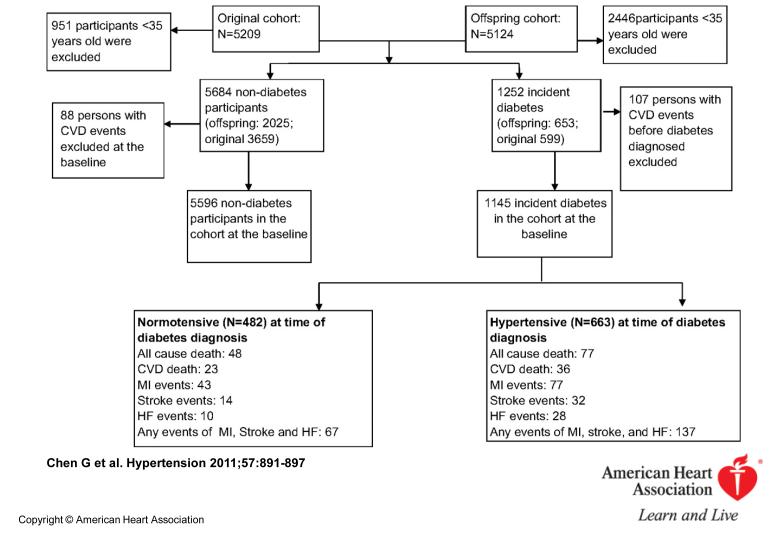
We derived the cohort for this study from both the Original and Offspring subjects of the Framingham Heart Study. The design and inclusion criteria of the Framingham heart study have been described elsewhere12. Although the Framingham study is a prospective cohort, our secondary analysis of the data represents a retrospective cohort study. Of the 10,333 men and women in the Framingham Original (n=5209) and Offspring (n=5124) cohorts, we selected those older than 35 years who had not had a cardiovascular event (defined as myocardial infarction, stroke, or heart failure ) prior to cohort entry (Figure 1): our analytic cohort thus consisted of 1145 individuals with diabetes and 5596 individuals without diabetes.
Figure 1.
Flowchart illustrating derivation of the incident diabetes cohort and cardiovascular outcomes during follow-up.
Similar to previous publications combining data from both the original and offspring Framingham cohorts9, 13-14, we selected subjects for our cohort from 11 cycles of the original cohort examinations, taken 4 years apart and occurring from 1968 to 1996, and from all 7 cycles of the offspring examinations, taken roughly 4 years apart and occurring from1971 to 2001. Although individuals in the Framingham cohort are repeatedly observed and contribute to more than one cycle, we focused our analysis on the first four year risk period for each individual after entry into our analytic cohort15..
Study Outcomes
We examined outcomes in the first 4 years of follow-up within Framingham after diagnosis of diabetes for the diabetic cohort and after Framingham entry for all subjects who did not develop diabetes. Our primary outcomes were all cause mortality and cardiovascular disease (CVD) related mortality. Information on cause of death was obtained from death certificates, medical records, and/or family members. CVD related death was identified as the cause of death if myocardial infarction (MI), heart failure (HF), or stroke were responsible. Our secondary outcomes included nonfatal CVD events such as MI, HF, and stroke. All deaths and CVD events were adjudicated by a panel of 3 physicians using previously described criteria.12
Diabetes and hypertension status assessment
Subjects were considered to have diabetes if they had a random plasma glucose level ≥ 200 mg/dl (11.1mmol/L), fasting plasma glucose ≥ 126mg/dl (7.0 mmol/L), or were taking insulin or an oral hypoglycemic agent. The glycated hemoglobin was not collected in any of the Framingham cycles, random glucose was collected at all Framingham visits except cycles 5 and 7 for the original cohort, and fasting glucose was routinely collected after cycle 3 for the Offspring Cohort.
At each Framingham visit, seated systolic and diastolic blood pressures were measured twice by the examining physician using a mercury sphygmomanometer. Hypertension at the time of entry into our study cohort (ie. time of diabetes diagnosis for those with diabetes and the time of baseline Framingham assessment for those without diabetes) was defined as a systolic blood pressure of at least 130mm Hg or diastolic blood pressure of at least 80 mm Hg among diabetes patients, a systolic blood pressure of 140mm Hg or diastolic blood pressure of 90 mm Hg or more among non-diabetes participants, or current use of antihypertensive therapy in either group. Although our primary analysis defined subjects on the basis of their hypertension status at the time of study cohort entry, in a sensitivity analysis of our diabetic subjects we excluded subjects initially defined as “normotensive” if they developed hypertension during follow-up. We conducted other sensitivity analyses with different definitions for hypertension (> 140/90 mm Hg in both those with and without diabetes) and examined the association between various elements of BP and outcomes imputing BP as a continuous variable into multiple linear regression models.
Covariate assessment
At each Framingham visit, subjects underwent a physical examination and laboratory assessment of cardiovascular risk factors. Height and weight were measured, and obesity was defined as body mass index (BMI) of 30 or greater. Current smoking was defined as at least 1 cigarette per day on average in the year before the examination. The medication history for cholesterol-lowering and antihypertensive agents was defined based on patient self-report and medical records. Laboratory tests, including total cholesterol, high-density lipoprotein (HDL), and random/fasting glucoses were measured as previously described.16-17 We did not have access to data on albuminuria, renal function, or left ventricular hypertrophy.
Statistical analysis
Age- and sex-standardized all cause mortality, CVD mortality and the incidence rates (per 1000 person-years) for CVD events, and their 95% confidence intervals (CI), were calculated stratified for presence/absence of hypertension at baseline in subjects with diabetes. The hazard ratio (HR) and 95% CI for hypertension among non-diabetic participants were also estimated in proportional hazards regression models using the initial 4 years of follow-up.15 Three models were fitted adjusting for sequentially more covariates including age, sex, BMI, current smoking, obesity (BMI≥30), hypercholesterolemia (total cholesterol ≥ 5.2 mmol/L or taking medication for cholesterol-lowering), and low HDL (<1.03 mmol/L for males or <1.28 mmol/L for females).
The population attributable risk (PAR) of hypertension as a risk factor for mortality and CVD events was calculated among Framingham participants with and without diabetes using the following formula: P×(HR-1)/(P×(HR-1)+1). P is prevalence of hypertension among the population of interest, calculated using the person-years of observation with hypertension divided by the total person-years observation, and HR is the hazard ratio for each event associated with hypertension in each population.
Cumulative incidence rates for all cause mortality, CVD mortality, and CVD events as a function of hypertension status at baseline were estimated in proportional hazard regression models after adjustment for age and sex during 4 years of follow-up.
Descriptive data are presented as percentage or mean ± standardized deviation (SD). The baseline characteristics of subjects with diabetes and with/without hypertension were compared using chi-square for categorical variables and t-test for numerical variables. All statistical analyses were conducted with SAS software (SAS Enterprise Guide 9.1, SAS Institute Inc, Cary, North Carolina), and the curves of cumulative incidence rates for mortality and CVD events were drawn using SPSS (version 16.0).
Approval for this study was obtained from the University of Calgary Research Ethics Board.
Results
Of the 1145 eligible subjects with new onset diabetes, 663 (58%) had hypertension at baseline if measured blood pressure (BP) > 130/80 mmHg or current use of antihypertensive therapy was used as the definition; if measured BP > 140/90 mmHg or current use of antihypertensive therapy was used to define hypertension, 642 subjects with diabetes (56%) had hypertension at baseline. Of the 5596 eligible subjects without diabetes, 1309 (23%) had hypertension at baseline (using the definition of 140/90 mmHg). All eligible subjects were followed for a maximum of 4 years (4154 person-years of follow-up in the diabetic cohort [median 3.6 years] and 20157 person-years of follow-up in the non-diabetic cohort [median 3.7 years]). Diastolic and systolic blood pressures and BMI were the only baseline characteristics which were significantly different between Framingham participants with incident diabetes who did versus did not have hypertension at the time their diabetes was diagnosed (Table 1). Subjects taking antihypertensive drugs had lower blood pressures than those with hypertension who were not taking drug therapy at baseline: 145/80 mmHg vs. 149/85 mmHg, p<0.001.
Table 1.
Baseline characteristics of individuals at the time of cohort entry
| Diabetes |
No Diabetes |
|||||
|---|---|---|---|---|---|---|
| Variables at baseline |
Without hypertension (N=482) |
With hypertension (N=663) |
P value |
Without hypertension (N=4287) |
With hypertension (N=1309) |
P value |
| Age, Yrs | 61.1±11.6 | 61.8±11.2 | 0.32 | 43.6±4.1 | 45.9±6.4 | 0.003 |
| Female sex | 45.2 | 44.6 | 0.87 | 46.2 | 45 | 0.004 |
| Systolic Blood pressure (mmHg) |
118.3±9.1 | 146.9±19.0 | <0.001 | 119.5±12.0 | 146.3±19.0 | <0.001 |
| Diastolic Blood pressure (mmHg) |
71.2±6.3 | 83.5±11.1 | <0.001 | 77.4±7.9 | 84.6±9.8 | <0.001 |
| BMI (kg/m2) | 29.5±5.8 | 30.5±6.0 | 0.04 | 25.5±3.8 | 28.1±4.7 | 0.006 |
| Random Plasma Glucose(mmol/L) |
10.6±0.6 | 11.1±0.5 | 0.73 | 5.6±0.5 | 5.9±0.6 | 0.004 |
| Fasting Plasma Glucose (mmol/L) |
8.5±0.5 | 8.3±0.5 | 0.19 | 5.2±0.5 | 5.5±0.6 | 0.004 |
| Cholesterol (mmol/L) |
5.54±1.11 | 5.58±1.12 | 0.32 | 5.32±0.98 | 5.60±1.04 | 0.003 |
| HDL Cholesterol (mmol/L ) |
1.09±0.35 | 1.11±0.37 | 0.55 | 1.35±0.41 | 1.25±0.40 | 0.002 |
| Obesity (BMI 30 or greater) |
33.4 | 40.7 | 0.04 | 25.9 | 32.5 | 0.003 |
| Current Smoker | 25.8 | 28.7 | 0.38 | 30.2 | 24.6 | 0.002 |
| Total cholesterol ≥5.2mmol/L |
60.3 | 62.7 | 0.46 | 57.7 | 67.8 | 0.003 |
| Low HDL cholesterol |
61.2 | 61.3 | 0.90 | 35.1 | 43.8 | 0.002 |
| Treatment for hypertension |
0 | 65.7 | <0.001 | 0 | 63.2 | <0.001 |
| Cholesterol lowering medication use |
5 | 5.9 | 0.6 | 0.8 | 1.6 | 0.004 |
All frequencies reported as percentages and all continuous variables reported as Mean±SD
Low HDL: high-density lipoprotein <1.03mmol/L for male or <1.28mmol/L for female
BMI: Body mass index=mass(kg)/(height(m))2
Age- and sex-standardized rates of all cause mortality and CVD related mortality among Framingham participants with diabetes were 28.7 and 20.1 per 1000 person years, respectively. Those with hypertension at the time of diabetes diagnosis were significantly more likely to suffer these outcomes than those without hypertension at the time of diabetes diagnosis (Table 2). MI, Stroke, HF, and the composite of any CVD event were also more common among subjects with diabetes who had hypertension compared to those without hypertension (all p values < 0.01, Table 3). Event rates were relatively constant over time (please see http://hyper.ahajournals.org for Figure S-1).
Table 2.
All cause mortality and Cardiovascular mortality (per 1000 person years) after diagnosis of diabetes in individuals over age 35, stratified by hypertension status at time of diabetes diagnosis
| All cause death |
Cardiovascular death |
|||
|---|---|---|---|---|
| Subgroups | Number | Rate (95% CI) | Number | Rate (95% CI) |
| Total | ||||
| Normotensive(N=482) | 48 | 19.8(16.4-24.0) | 23 | 12.6(9.4-15.0) |
| Hypertensive(N=663) | 77 | 32.4(28.4-37.0) | 36 | 26.7(22.1-32.2) |
| Age <65 years old | ||||
| Normotensive(N=328) | 16 | 10.2(7.4-13.8) | 7 | 6.1(3.8-7.8) |
| Hypertensive(N=337) | 20 | 14.2(11.0-17.5) | 8 | 9.8(8.6-11.5) |
| Age ≥65 years old | ||||
| Normotensive(N=154) | 32 | 46.1(42.3-48.5) | 16 | 38.6(33.5-44.2) |
| Hypertensive(N=326) | 57 | 57.9(49.6-57 .5) | 28 | 55.6(52.6-59.0) |
| Female | ||||
| Normotensive(N=203) | 18 | 20.1(15.0-25.0) | 10 | 14.8(9.5-20.9) |
| Hypertensive(N=309) | 37 | 31.1(27.5-34.9) | 21 | 29.9(23.1-36.7) |
| Male | ||||
| Normotensive (N=279) | 30 | 19.6(15.3-25.2) | 13 | 11.3(7.5-15.8) |
| Hypertensive(N=354) | 40 | 33.5(28.0-40.1) | 15 | 23.7(18.0-31.3) |
CVD: Cardiovascular disease
Rates adjusted for sex and for age
Table 3.
Cardiovascular events (and rates per 1000 person years) after diagnosis of diabetes in individuals over age 35, stratified by hypertension status at time of diabetes diagnosis
| MI |
Stroke |
HF |
Any MI, stroke or HF event |
|||||
|---|---|---|---|---|---|---|---|---|
| Subgroups | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) | N | Rate (95% CI) |
| Total | ||||||||
| Normotensive(N=482) | 43 | 22.3(17.7-28.0) | 14 | 10.5(7.4-15.0) | 10 | 7.3(4.7-11.4) | 67 | 30.8(25.8-36.7) |
| Hypertensive(N=663) | 77 | 34.5(29.0-41.1) | 32 | 20.8(16.4-24.5) | 28 | 18.6(14.3-24.3) | 137 | 51.7(45.7-58.6) |
| Age <65 years old | ||||||||
| Normotensive(N=328) | 23 | 17.4(13.0-20.3) | 7 | 5.1(3.6-6.6) | 3 | 3.3(1.6-5.0) | 33 | 21.3(16.7-26.1) |
| Hypertensive(N=337) | 39 | 26.1(23.4-33.4) | 6 | 6.5(3.8-10.2) | 11 | 9.1(7.7-14.6) | 56 | 32.1(27.2-39.4) |
| Age ≥65 years old | ||||||||
| Normotensive(N=154) | 20 | 40.4(32.9-47.5) | 7 | 26.5(20.5-32.7) | 7 | 20.9(12.2-26.0) | 34 | 62.6(48.4-70.9) |
| Hypertensive(N=326) | 38 | 51.5(48.1-56.0) | 26 | 44.5(34.1-54.1) | 17 | 35.1(28.6-48.3) | 81 | 81.9(80.0-95.9) |
| Female | ||||||||
| Normotensive(N=203) | 14 | 16.4(10.8-24.9) | 5 | 12.4(10.7-13.9) | 5 | 7.5(3.9-13.4) | 24 | 27.6(20.8-36.6) |
| Hypertensive(N=309) | 35 | 33.5(25.8-41.4) | 13 | 19.4(15.5-23.7) | 10 | 20.3(18.9-29.6) | 58 | 51.3(42.7-61.7) |
| Male | ||||||||
| Normotensive (N=279) | 29 | 26.4(20.0-31.7) | 9 | 8.9(5.3-15.1) | 5 | 7.2(4.0-11.0) | 43 | 33.2(26.5-41.6) |
| Hypertensive(354) | 42 | 35.4(32.9-37.9) | 19 | 22.2(16.1-30.6) | 18 | 17.3(13.9-25.0) | 79 | 52.1(43.9-61.7) |
CVD: cardiovascular disease; MI: myocardial infarction; HF: heart failure
Rates adjusted for sex and for age
On multivariate analysis, hypertension was significantly and strongly associated with increased risks for all cause mortality and CVD related events in both those individuals with and without diabetes (Table 4). After adjustment for other baseline characteristics including age, gender, smoking, obesity, and cholesterol levels, hypertension at baseline was associated with a 72% increase in the risk of all cause death and a 57% increase in the risk of any cardiovascular event in individuals with diabetes; in those individuals without diabetes, hypertension was associated with an 81% increase in the risk of all-cause mortality and a 98% increase in the risk of any CVD event (Table 4). Of note, the population attributable risk from hypertension for the various outcomes ranged from 27% to 44% in individuals without diabetes and from 24% to 34% in individuals with diabetes (Table 4). Results were very similar in a sensitivity analysis in which we defined hypertension in the diabetes cohort as measured BP > 140/90 or current use of antihypertensive therapy (please see http://hyper.ahajournals.org for Table S-1). Baseline SBP, DBP, pulse pressure, and mean arterial pressure (examined as continuous variables in 4 separate multivariate models) were all independently associated with increased risk of CVD death and/or CVD events in our diabetes cohort (please see http://hyper.ahajournals.org for Table S-2A and S-2B).
Table 4.
The adjusted hazard ratio (HR) for hypertension as a risk factor for death or cardiovascular events and population attributable risk (PAR) in Framingham subjects over age 35 with/without diabetes
| Participants with Diabetes (N=1145) |
Participants without Diabetes( N=5596) |
|||||
|---|---|---|---|---|---|---|
| Outcomes | HR (95%CI, Hypertension vs. Normotension) |
Prevalence of Hypertension |
PAR (%)* |
HR (95%CI, Hypertension vs. Normotension) |
Prevalence of Hypertension |
PAR (%)* |
| All cause death | 57.9 | 45.3 | ||||
| Model 1† | 1.34(1.10-1.65) | 16.4 | 1.37(1.26-1.48) | 14.4 | ||
| Model 2‡ | 1.42(l.10-1.85) | 19.6 | 1.72(1.53-1.94 | 24.6 | ||
| Model 3§ | 1.72(1.16-2.56) | 29.5 | 1.81(1.43-2.28) | 26.8 | ||
| CVD death | 56.2 | 39.2 | ||||
| Model 1† | 1.55(1.12-2.17) | 23.6 | 2.45(2.11-2.84) | 36.3 | ||
| Model 2X | 1.62(1.11-2.35) | 25.8 | 2.83(2.28-3.50) | 41.7 | ||
| Model 3§ | 1.90(1.26-3.49) | 33.6 | 3.00(1.82-4.95) | 44.0 | ||
| MI | 55.3 | 41.7 | ||||
| Model 1† | 1.83(1.42-2.35) | 31.5 | 2.11(1.92-2.33) | 36.9 | ||
| Model 2X | 1.64(1.24-2.17) | 26.1 | 2.16(1.90-2.45) | 37.8 | ||
| Model 3§ | 1.89(1.28-2.70) | 33.0 | 2.07(1.69-2.54) | 37.0 | ||
| Stroke | 55.1 | 41.0 | ||||
| Model 1† | 1.94(1.61-2.34) | 34.1 | 2.31(1.99-2.67) | 39.1 | ||
| Model 2‡ | 1.64(1.30-2.07) | 26.1 | 2.40(1.96-2.94) | 42.2 | ||
| Model 3§ | 1.57(1.19-2.24) | 23.9 | 2.93(1.98-4.35) | 40.7 | ||
| HF | 55.1 | 41.0 | ||||
| Model 1† | 1.69(1.46-1.96) | 27.5 | 2.16(1.78-2.61) | 39.6 | ||
| Model 2X | 1.55(1.10-2.11) | 23.2 | 1.92(1.46-2.51) | 38.0 | ||
| Model 3§ | 1.76(1.19-1.97) | 29.5 | 2.34(1.76-3.19) | 39.6 | ||
| Any one of MI, Stroke, or HF event |
57.9 | 45.3 | ||||
| Model 1† | 1.72(1.41-2.08) | 29.5 | 2.00(1.85-2.15) | 31.1 | ||
| Model 2‡ | 1.56(l.25-1.94) | 24.5 | 2.02(1.83-2.24) | 31.7 | ||
| Model 3§ | 1.57(1.22-2.05) | 24.8 | 1.98(1.55-2.04) | 30.7 | ||
PAR = (100*Prevalence*(hazard ratio-1)/(Prevalence*(hazard ratio-1)+1)
Model 1: adjusted for sex, and age)
Model 2 adjusted for sex, age, current smoker at baseline (yes, no), obesity (BMI≥30) at baseline, and hypercholesterolemia at baseline (>5.2mmol/L total cholesterol)
Model 3 adjusted for sex, age, current smoker at baseline (yes, no), obesity (BMI≥30) at baseline, hypercholesterolemia at baseline (≥5.2mmol/L total cholesterol), low HDL at baseline (<1.03mmol/L for male, <1.28mmol/L for female)
While a multivariate model confirmed that both hypertension and diabetes were independent risk factors with similar hazard ratios for all-cause mortality and CVD events (please see http://hyper.ahajournals.org for Table S-3), diabetes was associated with much smaller population attributable risks (for example, 8% for all-cause mortality and 12% for any CVD event) due to the much lower prevalence of diabetes than hypertension in these Framingham participants. Indeed, adjustment for hypertension at baseline in the multivariate models reduced the population attributable risk from diabetes to 7% for all cause death and 9% for any CVD event.
Of note, our estimates of the relative risks and population attributable risks for hypertension are likely underestimates since 23% of the individuals with diabetes who were normotensive at baseline developed hypertension during follow-up. After excluding these 110 normotensive individuals who developed hypertension during follow-up, our sensitivity analyses confirmed this in that the population attributable risk for hypertension increased to 44% for all cause death and 41% for any CVD event (please see http://hyper.ahajournals.org for Table S-4).
Discussion
Our key finding is that the presence of hypertension is the strongest driver of cardiovascular outcomes in individuals with diabetes. More than half of those with diabetes within the Framingham cohort had hypertension and the population attributable risk from hypertension exceeds 25% for any CVD event and 30% for mortality (increasing to 41% and 44% if individuals initially classified as normotensive who developed hypertension during followup were excluded from the analyses). These results suggest that the most important intervention for preventing cardiovascular events and death in individuals with diabetes is control of blood pressure.
Our study is consistent with other studies which have demonstrated an approximately two fold increased risk for cardiovascular events and deaths in diabetic individuals with hypertension compared to those with normal blood pressures.4, 18 However, we have extended this evidence base by exploring the population attributable risk associated with hypertension versus diabetes in a well categorized cohort of individuals who have all been routinely screened for diabetes with complete follow-up and rigorous endpoint ascertainment, and by demonstrating that although diabetes is also associated with excess risk for cardiovascular outcomes, much of this risk is due to concomitant hypertension.
Our data is consistent with other epidemiologic data demonstrating that lower blood pressures are associated with lower cardiovascular event rates,11 and is also consistent with clinical trial data demonstrating that lowering blood pressure in individuals with diabetes confers substantial clinical benefits.6, 19-20 While the recent ACCORD trial raises the possibility that lowering systolic blood pressure below 120 mm Hg in individuals with diabetes may not confer any additional benefits over systolic pressures of 134 mm Hg,21 it is worth noting that the baseline blood pressures in those with diabetes and hypertension in Framingham were substantially higher at 147/84 mm Hg.
However, there are some limitations to our study. First, we were unable to distinguish duration of hypertension and thus those we identified as having hypertension at the time of incident diabetes diagnosis included a mixture of incident hypertensives and individuals with potentially longstanding hypertension but newly recognized diabetes. However, this would not invalidate our results since the inclusion of incident hypertensives would bias our results towards the null. Similarly, we did not have data on follow-up blood pressures (although an analysis of serial changes in blood pressure within Framingham reported relatively modest changes in systolic blood pressure over time)22 and thus could not examine whether those with blood pressures which were subsequently controlled or regressed to the mean exhibited lower event rates than those with persistently elevated blood pressures (although clinical trial data would certainly suggest this should be the case). As some of those we defined as “hypertensive” likely had blood pressures in the normotensive range during follow-up (due to within-person variability in measurement and regression dilution bias), our estimates of event rates in the hypertensive subgroup were likely an underestimate, again biasing our results towards the null. In the same vein, inclusion of individuals who had normal blood pressures at baseline but went on to develop hypertension during follow-up in our “normotensive” comparator group would also have biased our results towards the null – a hypothesis confirmed by our sensitivity analysis. It is noteworthy that all of the limitations of our dataset and analysis discussed thus far would have underestimated the impact of hypertension on CVD outcomes in individuals with diabetes. However, we acknowledge that we did not have any data on albuminuria, renal function, or left ventricular hypertrophy in these subjects, and thus could not adjust for these CV risk factors in our multivariate models. In addition, although we provided data on the association between SBP, DBP, pulse pressure, and mean arterial pressure and outcomes in Tables S-2A and S-2B at http://hyper.ahajournals.org, we acknowledge that these analyses were underpowered to definitively answer the question as to which BP measurement most strongly correlates with individual cardiovascular outcomes in individuals with diabetes.
Perspectives
While diabetes is associated with increased risks for death and cardiovascular events in the Framingham cohort, we have established that much of this excess risk is attributable to coexistent hypertension in diabetic individuals. Increased attention to the role of blood pressure control in preventing cardiovascular events in individuals with diabetes is essential, particularly in light of evidence that hypertension is the most poorly controlled of the cardiac risk factors in patients with diabetes,23,24 yet may be the most cost-effective of the various therapeutic options. 25
Supplementary Material
Acknowledgments
Sources of Funding: Data was obtained from the Framingham Heart Study of the National Heart Lung and Blood Institute of the National Institutes of Health and Boston University School of Medicine. This work was supported by the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195). This manuscript was prepared using a limited access dataset obtained from the NHLBI and does not necessarily reflect the opinions or views of the NHLBI or the Framingham Heart Study Investigators. Guanmin Chen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr McAlister is supported by a Senior Health Scholar salary award from the Alberta Heritage Foundation for Medical Research (AHFMR). Dr Campbell is supported by the Canadian Institutes of Health Research (CIHR) Chair in Hypertension Prevention and control. Dr Hemmelgarn is supported by salary awards from the AHFMR and CIHR. None of the funding agencies had any input into this analysis or manuscript.
Footnotes
Disclosures: None of the authors have any conflicts of interest relevant to this manuscript.
REFERENCES
- 1.Holman RR, Paul SK, Bethel MA, Neil HA, Matthews DR. Long-term follow-up after tight control of blood pressure in type 2 diabetes. N Engl J Med. 2008;359:1565–1576. doi: 10.1056/NEJMoa0806359. [DOI] [PubMed] [Google Scholar]
- 2.Du X, Ninomiya T, de Galan B, Abadir E, Chalmers J, Pillai A, Woodward M, Cooper M, Harrap S, Hamet P, Poulter N, Lip GY, Patel A. Risks of cardiovascular events and effects of routine blood pressure lowering among patients with type 2 diabetes and atrial fibrillation: Results of the advance study. Eur Heart J. 2009;30:1128–1135. doi: 10.1093/eurheartj/ehp055. [DOI] [PubMed] [Google Scholar]
- 3.Alderman MH, Cohen H, Madhavan S. Diabetes and cardiovascular events in hypertensive patients. Hypertension. 1999;33:1130–1134. doi: 10.1161/01.hyp.33.5.1130. [DOI] [PubMed] [Google Scholar]
- 4.Hypertension in diabetes study (hds): Ii. Increased risk of cardiovascular complications in hypertensive type 2 diabetic patients. J Hypertens. 1993;11:319–325. doi: 10.1097/00004872-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 5.Currie CJ, Peters JR, Tynan A, Evans M, Heine RJ, Bracco OL, Zagar T, Poole CD. Survival as a function of hba(1c) in people with type 2 diabetes: A retrospective cohort study. Lancet. 2010;375:481–489. doi: 10.1016/S0140-6736(09)61969-3. [DOI] [PubMed] [Google Scholar]
- 6.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, Woodward M, MacMahon S. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: Results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–1419. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]
- 7.Giorda CB, Avogaro A, Maggini M, Lombardo F, Mannucci E, Turco S, Alegiani SS, Raschetti R, Velussi M, Ferrannini E. Recurrence of cardiovascular events in patients with type 2 diabetes: Epidemiology and risk factors. Diabetes Care. 2008;31:2154–2159. doi: 10.2337/dc08-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almgren T, Wilhelmsen L, Samuelsson O, Himmelmann A, Rosengren A, Andersson OK. Diabetes in treated hypertension is common and carries a high cardiovascular risk: Results from a 28-year follow-up. J Hypertens. 2007;25:1311–1317. doi: 10.1097/HJH.0b013e328122dd58. [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Coady S, Sorlie PD, D’Agostino RB, Sr., Pencina MJ, Vasan RS, Meigs JB, Levy D, Savage PJ. Increasing cardiovascular disease burden due to diabetes mellitus: The framingham heart study. Circulation. 2007;115:1544–1550. doi: 10.1161/CIRCULATIONAHA.106.658948. [DOI] [PubMed] [Google Scholar]
- 10.Hypertension in diabetes study (hds): I. Prevalence of hypertension in newly presenting type 2 diabetic patients and the association with risk factors for cardiovascular and diabetic complications. J Hypertens. 1993;11:309–317. doi: 10.1097/00004872-199303000-00012. [DOI] [PubMed] [Google Scholar]
- 11.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 12.Cupples L, D’Agostino R. Some risk factors related to the annula incidence of cardiovascular disease and death using pooled repeated biennial measurement framingham heart stduy, 30 years follow-up. In: Kannel W, Wolf P, Garrison R, editors. The framingham study: An epidemiological investigation of cardiovascualr disease, section 34. National Institute of Health; Bethesda, MD: 1987. pp. 9–13. [Google Scholar]
- 13.Haider AW, Chen L, Larson MG, Evans JC, Chen MH, Levy D. Antecedent hypertension confers increased risk for adverse outcomes after initial myocardial infarction. Hypertension. 1997;30:1020–1024. doi: 10.1161/01.hyp.30.5.1020. [DOI] [PubMed] [Google Scholar]
- 14.Preis SR, Hwang SJ, Coady S, Pencina MJ, D’Agostino RB, Sr., Savage PJ, Levy D, Fox CS. Trends in all-cause and cardiovascular disease mortality among women and men with and without diabetes mellitus in the framingham heart study, 1950 to 2005. Circulation. 2009;119:1728–1735. doi: 10.1161/CIRCULATIONAHA.108.829176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cupples LA, D’Agostino RB, Anderson K, Kannel WB. Comparison of baseline and repeated measure covariate techniques in the framingham heart study. Stat Med. 1988;7:205–222. doi: 10.1002/sim.4780070122. [DOI] [PubMed] [Google Scholar]
- 16.Rutter MK, Meigs JB, Sullivan LM, D’Agostino RB, Sr., Wilson PW. C-reactive protein, the metabolic syndrome, and prediction of cardiovascular events in the framingham offspring study. Circulation. 2004;110:380–385. doi: 10.1161/01.CIR.0000136581.59584.0E. [DOI] [PubMed] [Google Scholar]
- 17.Murabito JM, Nam BH, D’Agostino RB, Sr., Lloyd-Jones DM, O’Donnell CJ, Wilson PW. Accuracy of offspring reports of parental cardiovascular disease history: The framingham offspring study. Ann Intern Med. 2004;140:434–440. doi: 10.7326/0003-4819-140-6-200403160-00010. [DOI] [PubMed] [Google Scholar]
- 18.The Emerging Risk Factors C. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.UK Prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317:703–713. [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A. Effects of a fixed combination of perindopril and indapamide on macrovascular and microvascular outcomes in patients with type 2 diabetes mellitus (the advance trial): A randomised controlled trial. Lancet. 2007;370:829–840. doi: 10.1016/S0140-6736(07)61303-8. [DOI] [PubMed] [Google Scholar]
- 21.The ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke R, Shipley M, Lewington S, Youngman L, Collins R, Marmot M, Peto R. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]
- 23.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291:335–342. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 24.Brown LC, Johnson JA, Majumdar SR, Tsuyuki RT, McAlister FA. Evidence of suboptimal management of cardiovascular risk in patients with type 2 diabetes mellitus and symptomatic atherosclerosis. CMAJ. 2004;171:1189–1192. doi: 10.1503/cmaj.1031965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The CDC Diabetes Cost-effectiveness Group Cost-effectiveness of intensive glycemic control, intensified hypertension control, and serum cholesterol level reduction for type 2 diabetes. JAMA. 2002;287:2542–2551. doi: 10.1001/jama.287.19.2542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.