Abstract
Objective
To see if the distribution patterns of phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) intraneuronal inclusions in amyotrophic lateral sclerosis (ALS) permit recognition of neuropathological stages.
Methods
pTDP-43 immunohistochemistry was performed on 70 μm sections from ALS autopsy cases (N=76) classified by clinical phenotype and genetic background.
Results
ALS cases with the lowest burden of pTDP-43 pathology were characterized by lesions in the agranular motor cortex, brainstem motor nuclei of cranial nerves XII-X, VII, V, and spinal cord α-motoneurons (stage 1). Increasing burdens of pathology showed involvement of the prefrontal neocortex (middle frontal gyrus), brainstem reticular formation, precerebellar nuclei, and the red nucleus (stage 2). In stage 3, pTDP-43 pathology involved the prefrontal (gyrus rectus and orbital gyri) and then postcentral neocortex and striatum. Cases with the greatest burden of pTDP-43 lesions showed pTDP-43 inclusions in anteromedial portions of the temporal lobe, including the hippocampus (stage 4). At all stages, these lesions were accompanied by pTDP-43 oligodendroglial aggregates. Ten cases with C9orf72 repeat expansion displayed the same sequential spreading pattern as non-expansion cases but a greater regional burden of lesions, indicating a more fulminant dissemination of pTDP-43 pathology.
Interpretation
pTDP-43 pathology in ALS possibly disseminates in a sequential pattern that permits recognition of four neuropathological stages consistent with the hypothesis that pTDP-43 pathology is propagated along axonal pathways. Moreover, the fact that pTDP-43 pathology develops in the prefrontal cortex as part of an ongoing disease process could account for the development of executive cognitive deficits in ALS.
Introduction
Amyotrophic lateral sclerosis (ALS) is the most frequent adult-onset motor neuron disease characterized by rapidly progressive paresis leading to death with a mean survival of approximately 3 years.1 The phosphorylated 43-kDa TAR DNA-binding protein (pTDP-43) was identified as the major component in ubiquitin-positive neuronal inclusions in sporadic ALS and the largest subset of frontotemporal lobar degeneration (FTLD), now referred to as FTLD-TDP.2 TDP-43 is a highly conserved and widely expressed RNA-binding protein that is a member of the heterogeneous nuclear ribonucleoprotein family of proteins.3 The defining histopathology of ALS and FTLD-TDP is the presence of pTDP-43 aggregates in select neurons and glial cells of the central nervous system (CNS).2 Progressive accumulation of similar protein aggregates is now recognized as a characteristic feature of many neurodegenerative diseases, including accumulation of abnormally phosphorylated tau in Alzheimer’s disease (AD) or α-synuclein in Parkinson’s disease (PD).
Importantly, many neurodegenerative diseases show a characteristic spreading pattern of their underlying pathology across specific brain regions with disease progression. In AD, tau aggregates develop in the locus coeruleus and advance from the transentorhinal cortex and entorhinal region through the hippocampal formation into broad areas of the neocortex 4, 5 whereas in PD α-synuclein aggregation in the CNS begins in the lower medulla and sequentially progresses via the midbrain into cortical regions.6 The cellular mechanisms underlying this process are incompletely understood, but increasing evidence suggests that misfolded protein aggregates could spread by a self-perpetuating process that leads to amplification and spreading of these assemblies.7 Evidence of propagation of Aβ, tau, α-synuclein and polyQ proteins in vitro, in rodents, or in human grafts has been reported.8-13 Whereas the cell-to-cell transmission of pTDP-43 has not been demonstrated conclusively in vivo, a recently discovered C-terminal prion-like domain has been implicated in the aggregation of pTDP-43 in cultured cells. Consequently, observations from other neurodegenerative diseases raise the possibility that pTDP-43 pathology in ALS could sequentially disseminate from a focal site of onset and then propagate in a cell-to-cell manner.14 The detection of disease-specific distribution patterns of pTDP-43 pathology could help to provide a staging system for standardizing the neuropathological description of pTDP-43 pathology in ALS and would also constitute a major step for subsequent elucidation of disease progression mechanisms and neuronal vulnerability in ALS. Here, we present preliminary evidence for the sequential progression of pTDP-43 pathology in four stages in a large cross-sectional cohort of phenotypically well-defined ALS autopsy cases.
Materials and methods
Autopsy cohort
We included 76 patients with a clinical diagnosis of ALS (Table 1) in accordance with modified El Escorial Criteria15 and a confirmed neuropathological diagnosis of ALS, who underwent autopsy in the Center for Neurodegenerative Disease Research (CNDR) at the University of Pennsylvania between 1985 and 2012. Informed written consent was obtained previously from all patients or for autopsy cases from their next of kin. Detailed clinical characteristics (age at onset, age at death, site of onset, disease duration, ALS global disease severity as measured by a functional rating score [ALSFRS-R],16 the Mini Mental Status Examination,17 and gender), were ascertained from an integrated clinical and autopsy database, as described previously,18 and by retrospective chart review of clinical visits within the University of Pennsylvania Health System (Table 1).
Table 1.
ALS autopsy cases (N=76) included in this study; data is shown as median and interquartile range (in parentheses).
| Site of onset | ALS (all) | BLMN | BUMN | CLMN | LLMN | LUMN |
|---|---|---|---|---|---|---|
| N | 76 | 16 | 6 | 25 | 19 | 10 |
| Gender [f/m] | 30/46 | 5/11 | 3/3 | 9/16 | 9/10 | 4/6 |
| Duration [mo] | 33 (24-60) | 30 (23-39) | 24 (23-33) | 30 (22-46) | 34 (30-62) | 58 (28-96)* |
| Age at death [yrs] | 63 (56-70) | 66 (60-71) | 64 (46-72) | 62 (55-71) | 61 (58-66) | 58 (56-74) |
| ALSFRS-R | 18 (14-23) | 24 (14-26) | 24 (19-30) | 18 (15-22) | 17 (14-23) | 21 (13-27) |
| MMSE | 29 (26-30) | 29 (27-30) | 25 (24-28) | 29 (28-30) | 28 (27-29) | 29 (28-30) |
| Brain weight [g] | 1353 (1231- 1427) |
1359 (1333- 1453) |
1352 (11258- 1473) |
1365 (1222- 1456) |
1323 (1252- 1420) |
1233 (1162- 1388) |
Statistical significance across ALS syndromes in Kruskal-Wallis One Way Analysis of Variance on Ranks, p = 0.02. Abbreviations: ALSFRS-R – ALS functional rating scale, revised version, BLMN – bulbar lower motor neuron onset of disease, BUMN – bulbar upper motor neuron onset of disease, CLMN – cervical lower motor neuron onset of disease, duration – duration of disease, f/m – female/male, g – grams, LLMN – lumbar lower motor neuron onset of disease, LUMN – lumbar upper motor neuron onset of disease, mo – months, yrs – years.
The majority of the ALS patients were seen by two neurologists (LE, LM). We excluded all ALS cases in the CNDR Brain Bank (N=35) for which clinical data relating to site of onset or disease duration was incomplete or equivocal. Also excluded were 6 cases, for which ≥ 3/22 CNS regions examined (see below) were unavailable, and 2 cases lacking pTDP-43 pathology, leaving a cohort of N=76 (N=30 females, N=46 males; age range 42-87 years; mean age ± SD: 63.0 ± 10.6 years from a total of 119 autopsy cases (Tables 1-2). For the subjects with missing data, their gender, disease duration, and age of death were compared to the other cases and no differences were found (data not shown). Different ALS syndromes were defined according to clinical onset of disease: cervical lower motor neuron (CLMN) ALS, lumbar lower motor neuron (LLMN) ALS, lumbar upper motor neuron (LUMN) ALS, bulbar lower motor neuron (BLMN) ALS, and bulbar upper motor neuron (BUMN) ALS.19 None of the cases in the cohort had cervical UMN onset of disease (Table 1). Unless otherwise specified, results of clinical testing used in this study were from the visits at initial presentation or disease onset (first occurrence of paresis or bulbar symptoms, e.g., dysarthria, dysphagia) as well as the visit most proximate to death, i.e., occurring within 3 months of death. Of the ALS cases included here, 5 (6.6%) had a clinical history of dementia (ALS-D) (Table 2), and met criteria for FTLD.20-22
Table 2.
ALS patient (N=76) data and distribution of pTDP-43 pathology across four neuropathological stages
| case | f/m | age | dur | onset | SS | SFA | SSA | cog | NFT | NP | ALS | AGN | SC9 | BSM | RF | IO | RN | PFN | STR | DG | CA1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | m | 65 | 30 | CLMN | L | U/L | U/R | 0 | II | 0 | 1 | ++ | +++ | +++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | m | 78 | 16 | CLMN | L | U/L | L/L | 0 | II | 0 | 1 | + | + | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 3 | f | 43 | 156 | LUMN | R | L/R | L/L | ne | 0 | 0 | 1 | + | + | + | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 4 | m | 46 | 34 | LLMN | L | L/L | U/L | 0 | I | 0 | 1 | ++ | ++ | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 5 | m | 59 | 62 | CLMN | R | U/L | U/R | 0 | I | 0 | 1 | + | + | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 6 | f | 82 | 26 | BLMN | R | U/L | np | 0 | I | 0 | 1 | ++ | ++ | ++ | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | m | 61 | 24 | LLMN | L | L/L | U/L | 0 | 0 | A | 2 | +++ | +++ | ++ | + | ++ | + | 0 | 0 | 0 | 0 |
| 8 | f | 48 | 69 | LLMN | R | L/L | L/R | 0 | I | 0 | 2 | ++ | ++ | ++ | + | + | + | 0 | 0 | 0 | 0 |
| 9 | m | 49 | 53 | BLMN | R | np | np | 0 | 0 | 0 | 2 | ++ | ++ | ++ | + | + | + | 0 | 0 | ne | 0 |
| 10 | m | 60 | 40 | CLMN | R | U/R | L/R | 0 | 0 | 0 | 2 | ++ | + | +++ | + | + | + | 0 | 0 | 0 | 0 |
| 11 | m | 42 | 16 | CLMN | L | U/L | U/R | 0 | ne | 0 | 2 | ++ | ++ | +++ | ++ | ++ | 0 | 0 | 0 | 0 | 0 |
| 12 | f | 67 | 31 | LLMN | L | L/L | U/L | 0 | I | 0 | 2 | ++ | ++ | ++ | + | + | 0 | 0 | 0 | ne | ne |
| 13 | m | 66 | 34 | LLMN | R | L/L | U/L | 0 | I | 0 | 2 | ++ | +++ | +++ | + | ++ | + | 0 | 0 | 0 | 0 |
| 14 | m | 58 | 29 | CLMN | L | U/R | U/L | 0 | I | 0 | 2 | ++ | ++ | +++ | + | + | + | 0 | 0 | 0 | 0 |
| 15 | m | 63 | 60 | CLMN | R | U/R | U/L | 0 | 0 | 0 | 2 | + | + | + | + | + | 0 | 0 | 0 | 0 | 0 |
| 16 | m | 48 | 22 | CLMN | L | U/R | L/R | 0 | 0 | 0 | 2 | ++ | ++ | ++ | ++ | + | 0 | 0 | 0 | 0 | 0 |
| 17 | m | 74 | 60 | LUMN | R | L/L | L/R | 0 | I | 0 | 2 | ++ | + | + | 0 | + | 0 | 0 | 0 | 0 | 0 |
| 18 | f | 53 | 46 | CLMN | L | U/R | L/R | 0 | I | 0 | 2 | ++ | ++ | ne | ++ | + | ne | 0 | 0 | 0 | 0 |
| 19 | m | 62 | 40 | CLMN | R | U/R | U/L | 0 | 0 | 0 | 2 | ++ | ++ | + | + | + | 0 | 0 | 0 | 0 | 0 |
| 20 | f | 54 | 120 | LLMN | R | L/L | L/R | ne | 0 | 0 | 2 | ++ | ++ | ++ | + | + | + | 0 | 0 | 0 | 0 |
| 21 | m | 70 | 15 | CLMN | R | U/R | U/L | 0 | 0 | 0 | 2 | ++ | +++ | ++ | + | + | 0 | 0 | 0 | 0 | 0 |
| 22 | f | 74 | 66 | CLMN | L | U/L | U/R | 0 | ne | 0 | 3 | ++ | ++ | ++ | + | + | ++ | + | ++ | 0 | 0 |
| 23 | m | 54 | 21 | BLMN | L | U/L | np | 0 | II | 0 | 3 | +++ | +++ | ++ | ++ | ++ | ++ | + | ++ | 0 | 0 |
| 24 | f | 61 | 24 | LLMN | L | L/R | L/L | 1 | I | 0 | 3 | ++ | ++ | ++ | + | + | + | + | + | 0 | 0 |
| 25 | m | 56 | 19 | CLMN | R | U/L | U/R | 0 | 0 | 0 | 3 | ++ | ++ | +++ | ++ | + | + | + | + | 0 | 0 |
| 26 | m | 64 | 96 | LLMN | R | L/L | L/R | 0 | 0 | 0 | 3 | +++ | ++ | ++ | + | + | + | + | + | 0 | 0 |
| 27 | f | 71 | 32 | BLMN | L | L/R | L/L | 0 | II | B | 3 | +++ | +++ | +++ | ++ | ++ | ++ | + | ++ | 0 | 0 |
| 28 | f | 57 | 39 | LUMN | R | L/L | L/R | 0 | 0 | 0 | 3 | +++ | ++ | +++ | ++ | ++ | + | 0 | + | 0 | 0 |
| 29 | m | 53 | 32 | LLMN | R | L/L | L/R | 0 | 0 | 0 | 3 | ++ | ++ | +++ | + | + | + | + | + | 0 | 0 |
| 30 | m | 67 | 36 | BLMN | R | np | np | 0 | I | 0 | 3 | ++ | ++ | ++ | ++ | + | + | 0 | + | 0 | 0 |
| 31 | f | 55 | 12 | CLMN | L | U/R | L/R | 0 | III | C | 3 | ++ | ++ | ++ | ++ | + | ++ | + | ++ | 0 | 0 |
| 32 | m | 68 | 24 | CLMN | R | U/L | U/R | 0 | I | 0 | 3 | ++ | ++ | ++ | ++ | ++ | +++ | ++ | +++ | 0 | 0 |
| 33 | m | 85 | 96 | LUMN | L | L/ne | L/ne. | 0 | III | C | 3 | ++ | ++ | ne | + | + | + | + | + | 0 | 0 |
| 34 | m | 57 | 60 | LUMN | L | L/L | L/R | 0 | I | 0 | 3 | +++ | +++ | +++ | + | ++ | + | 0 | ++ | 0 | 0 |
| 35 | m | 42 | 9 | BUMN | R | np | np | 0 | II | 0 | 3 | +++ | ++ | +++ | ++ | ++ | + | + | ++ | 0 | 0 |
| 36 | m | 52 | 60 | CLMN | L | U/L | L/L | ne | 0 | 0 | 3 | ++ | ++ | +++ | + | ++ | ne | + | +++ | 0 | 0 |
| 37 | m | 67 | 41 | BLMN | R | np | np | 0 | 0 | 0 | 3 | +++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | 0 | 0 |
| 38 | m | 60 | 11 | LLMN | L | L/R | L/L | 0 | I | 0 | 3 | ++ | ++ | ++ | + | + | + | + | + | 0 | 0 |
| 39 | m | 70 | 24 | BLMN | R | U/R | np | ne | I | 0 | 3 | ++ | ++ | ++ | + | + | + | + | + | 0 | 0 |
| 40 | m | 79 | 84 | LLMN | R | L/R | L/L | 0 | I | A | 3 | ++ | ++ | ++ | + | + | + | 0 | + | 0 | 0 |
| 41 | m | 54 | 48 | LLMN | R | L/L | L/R | 1 | I | 0 | 3 | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | 0 | 0 |
| 42 | m | 74 | 108 | LUMN | L | L/L | L/R | 0 | I | B | 3 | ++ | ++ | ne | + | + | + | + | + | 0 | 0 |
| 43 | f | 59 | 62 | LLMN | L | L/L | L/R | 0 | I | 0 | 3 | +++ | +++ | +++ | ++ | ++ | ne | + | + | 0 | 0 |
| 44 | m | 58 | 37 | BLMN | R | U/L | np | 0 | I | 0 | 3 | +++ | ++ | ++ | + | + | + | + | + | 0 | 0 |
| 45 | f | 54 | 33 | CLMN | L | U/R | U/L | 0 | I | 0 | 3 | ++ | ++ | ++ | ++ | + | + | + | + | 0 | 0 |
| 46 | f | 87 | 55 | CLMN | R | U/R | U/L | 0 | I | B | 3 | ++ | ++ | ++ | + | + | + | 0 | + | 0 | 0 |
| 47 | m | 73 | 16 | LUMN | R | L/R | L/L | 0 | I | 0 | 3 | ++ | ++ | ++ | + | + | + | + | + | 0 | 0 |
| 48 | f | 87 | 55 | CLMN | L | U/L | U/R | 0 | 0 | 0 | 3 | ++ | ++ | ne | + | + | + | + | + | 0 | 0 |
| 49 | m | 61 | 28 | BLMN | R | np | np | 0 | I | 0 | 3/4 | +++ | ++ | ++ | ++ | ++ | 0 | + | + | ne | ne |
| 50 | f | 56 | 12 | LUMN | L | U/L | U/R | 0 | I | 0 | 3/4 | +++ | ++ | +++ | + | + | + | 0 | + | ne | ne |
| 51 | f | 54 | 33 | CLMN | L | U/L | L/L | 0 | 0 | 0 | 3/4 | ++ | ++ | +++ | ++ | + | + | + | + | ne | ne |
| 52 | f | 73 | 24 | BUMN | L | L/L | L/R | ne | 0 | ne | 3/4 | +++ | +++ | ++ | ++ | ++ | + | + | + | ne | ne |
| 53 | m | 46 | 24 | BUMN | L | U/R | U/L | 0 | I | ne | 4 | +++ | ++ | +++ | ++ | ++ | ++ | +++ | ++ | +++ | ++ |
| 54 | f | 64 | 15 | CLMN | R | U/R | U/L | 0 | II | 0 | 4 | ++ | ++ | ++ | ++ | ++ | +++ | ++ | +++ | +++ | ++ |
| 55 | m | 72 | 34 | BUMN | L | U/L | U/R | 0 | I | B | 4 | +++ | ++ | ++ | ++ | ++ | ne | ++ | ++ | + | + |
| 56 | f | 59 | 24 | LLMN | R | L/L | np | 0 | I | 0 | 4 | ++ | ++ | ++ | + | ++ | + | + | ++ | + | 0 |
| 57 | m | 63 | 35 | LLMN | L | L/R | U/R | 0 | I | C | 4 | +++ | ++ | ++ | + | ++ | + | + | ++ | + | 0 |
| 58 | f | 59 | 55 | LUMN | R | U/L | U/R | 1 | I | 0 | 4 | +++ | ++ | +++ | + | ++ | + | ++ | ++ | ++ | ++ |
| 59 | f | 69 | 24 | BUMN | L | U/R | U/L | 0 | II | B | 4 | +++ | ++ | ++ | ++ | ++ | + | ++ | ++ | +++ | +++ |
| 60 | m | 76 | 72 | LLMN | R | L/R | U/R | 0 | I | A | 4 | ++ | ++ | ++ | + | ++ | + | ++ | ++ | +++ | ++ |
| 61 | f | 71 | 30 | CLMN | L | U/L | U/R | 0 | 0 | 0 | 4 | ne | +++ | +++ | ++ | + | ++ | + | ++ | +++ | ++ |
| 62 | m | 66 | 19 | BLMN | L | U/L | U/R | 1 | I | 0 | 4 | +++ | ++ | +++ | + | ++ | ++ | ++ | ++ | +++ | +++ |
| 63 | m | 61 | 30 | CLMN | R | U/R | U/L | 0 | II | A | 4 | ++ | ++ | ++ | ++ | ++ | ne | ++ | ++ | +++ | + |
| 64 | f | 66 | 60 | LLMN | L | L/R | ne | 0 | II | 0 | 4 | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | +++ | ++ |
| 65 | m | 83 | 38 | CLMN | L | U/L | U/R | 0 | III | C | 4 | ++ | ++ | +++ | ++ | + | + | ++ | + | +++ | + |
| 66 | m | 56 | 28 | LUMN | R | L/L | U/L | 0 | 0 | 0 | 4 | +++ | ++ | ne | ++ | ++ | + | ++ | ++ | +++ | +++ |
| 67 | f | 64 | 60 | BLMN | L | U/R | np | 0 | 0 | 0 | 4 | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | ++ | + |
| 68 | f | 81 | 28 | BLMN | R | L/R | U/R | 0 | II | ne | 4 | +++ | ++ | +++ | + | ++ | ++ | ++ | ++ | +++ | + |
| 69 | m | 65 | 18 | BLMN | L | U/L | U/R | 0 | II | 0 | 4 | +++ | ++ | ++ | + | ++ | ++ | ++ | ++ | +++ | ++ |
| 70 | f | 75 | 22 | BLMN | L | U/L | U/R | 0 | I | 0 | 4 | +++ | +++ | ++ | + | + | ne | + | + | + | + |
| 71 | f | 59 | 33 | BUMN | L | U/L | U/R | 1 | I | 0 | 4 | +++ | +++ | +++ | ++ | ++ | ++ | + | +++ | +++ | ++ |
| 72 | m | 64 | 33 | BLMN | L | U/R | L/R | 0 | II | 0 | 4 | ++ | ++ | ++ | + | + | + | + | + | + | + |
| 73 | m | 53 | 108 | BLMN | R | U/L | U/R | 0 | III | 0 | 4 | +++ | ++ | +++ | ++ | ++ | ++ | ++ | ++ | + | 0 |
| 74 | f | 67 | 63 | LLMN | L | L/L | U/L | 0 | I | A | 4 | +++ | +++ | +++ | ++ | ++ | ++ | + | ++ | + | + |
| 75 | m | 57 | 25 | CLMN | L | U/L | L/L | 0 | I | 0 | 4 | +++ | ++ | +++ | +++ | ++ | ne | ++ | +++ | +++ | ++ |
| 76 | f | 59 | 28 | LLMN | R | L/L | L/R | 0 | 0 | 0 | 4 | ++ | ++ | ++ | + | ++ | ++ | ++ | + | + | + |
Abbreviations: case – case number; f/m – female/male; age – age at death in years; dur – disease duration in months; onset – BLMN = bulbar lower motor neuron, BUMN = bulbar upper motor neuron, CLMN = cervical lower motor neuron, LLMN = lumbar lower motor neuron, LUMN = lumbar upper motor neuron; SS – side of brain sampled, L= left, R = right; SFA – side and extremity first affected (L/L =lower left extremity, L/R = lower right extremity, U/L = upper left extremity, U/R = upper right extremity), SSA – side and extremity second affected (L/L =lower left extremity, L/R = lower right extremity, U/L = upper left extremity, U/R = upper right extremity); np – no extremity involvement (paresis); cog – cognitive/executive dysfunction (0 = no, 1 = yes); NFT – Braak neurofibrillary tangle stages 0-VI; NP – neuritic plaques (CERAD 0-3); ALS – stages 1-4 semiquantitatively assessed using phosphorylated TDP-43 (pTDP-43) immunocytochemistry as follows: 0 = none/not detectable or ≤2 aggregates per region, + mild, ++ moderate, +++ severe/numerous; AGN – frontal agranular cortex (Brodmann 4 and 6); SC9 – spinal cord (layer 9, all levels); BSM – bulbar somatomotor (except oculomotor) neurons: hypoglossal motor nucleus, ambiguus nucleus, facial motor nucleus, trigeminal motor nucleus; RF – reticular formation; IO – inferior olive, pontine gray (precerebellar nuclei chiefly with cortical afferents); RN – red nucleus, parvocellular portions; PFN – prefrontal neocortex (gyrus rectus and orbital gyri); STR – caudate nucleus and putamen, medium-sized nerve cells; DG – dentate gyrus; CA1 – Ammon’s horn, sector 1; ne – not evaluated/not available.
Tissue preparation, staining, and immunohistochemistry
Pathology was examined in 22 regions of the CNS: middle frontal gyrus, gyrus rectus and orbital gyri, agranular motor cortex (Brodmann areas 4 and 6), somatosensory cortex, superior or middle temporal gyrus, visual cortex (Brodmann areas 17 and 18), anterior cingulate gyrus, hypothalamus, amygdala, hippocampal formation, striatum and pallidum (the striatum block included anterior portions of the caudate nucleus and putamen plus the accumbens nucleus, where the caudate nucleus and putamen merge), thalamus, midbrain (including substantia nigra and red nucleus), upper pons at the level of the locus coeruleus, lower pons (including the motor nucleus of cranial nerve VII), medulla oblongata at level of the hypoglossal nucleus (XII), cerebellum (including both a portion of the cerebellar cortex and dentate nucleus), cervical spinal cord, thoracic spinal cord, lumbar spinal cord, and sacral spinal cord. The spinal cord (SC) was sectioned from the brainstem at the level of C2 and extracted in a single block. After removing the dural sac, 0.5 cm thick blocks representing the cervical SC, thoracic SC, lumbar SC, and sacral SC were selected. The cervical tissue block was obtained two spinal nerve roots below C2 (corresponding to C4), Additional blocks were obtained at the level equidistant between the cervical and lumbosacral enlargements (corresponding to Th7 or Th8). The lumbar block was taken from the level of the second or third spinal nerve root after the beginning of the lumbosacral enlargement (corresponding to L3 or L4), and the sacral cord was sampled equidistant between the filum terminale and the cranial end of the lumbosacral enlargement (corresponding to S2 or S3).
After fixation, all tissue samples were embedded in paraffin, sectioned at 6-7 μm, and stained with hematoxylin and eosin (HE). Immunohistochemistry was performed with antibodies to hyperphosphorylated tau (monoclonal antibody PHF1; 1:1000, gift from Dr. Peter Davies), α-synuclein (monoclonal antibody Syn303; 1:4000, generated in CNDR),22 pTDP-43 (rat antibody p409/410, 1:1000, gift from Dr Manuela Neumann),24 and amyloid-β (monoclonal antibody NAB228; 1:15 000; generated in CNDR).25 Neurofibrillary tangle (NFT) stages and neuritic plaques (CERAD scores) are shown in Table 2. The extent of pTDP-43 intraneuronal inclusions (dots, wisps, skeins) was rated for each region on a 4-point ordinal scale (0, none/not detectable; 1, mild; 2, moderate; 3, severe/numerous) as previously described (data not shown).26, 27
To study each of the 22 CNS regions in greater neuroanatomical detail in all 76 cases, two additional sets of 70 μm sections were prepared as described previously28 from the same paraffin blocks used above. The first set was stained with aldehyde fuchsin-Darrow red for topographical orientation and evaluation of neuronal loss (lipofuscin pigment deposits, basophilic Nissl material).4, 6 pTDP-43 pathology was analyzed in the second set using a commercially available 409/410 rabbit polyclonal antibody (Cosmo Bio, Carlsbad, CA, USA) at 1:10 000 and assessed according to a semiquantitative rating scale (0, not detectable or ≤2 aggregates per region; +, mild; ++, moderate; 3, +++ severe/numerous in Table 2). In assigning cases to a given stage, the extent (topographical distribution pattern) was assigned more weight than the degree of pTDP-43 pathology.
Genetic analysis
Genomic DNA was extracted from peripheral blood or brain tissue following the manufacturer’s protocols [Flexigene (Qiagen, Valencia, CA, USA) or QuickGene DNA whole blood kit L (Autogen, Holliston, MA, USA) for blood, and QIAsymphony DNA Mini Kit (Qiagen) for brain]. Genotyping for a C9orf72 repeat expansion was performed as previously described in detail.29 The coding regions of ubiquilin 2 (UBQLN2) and exon 6 of TDP-43 (TARDBP) were sequenced as described.30, 31 Sequence data were analyzed using Mutation Surveyor software (SoftGenetics, State College, PA, USA).
Statistical analysis
Data analysis was performed using SPSS (Version 17.0 SPSS Inc., Chicago, IL, USA). The “average” (and “range”) of data on patient characteristics were described using the median (and interquartile range). Wilcoxon Mann-Whitney Test was used to test differences between two clinical subgroups, and Kruskal-Wallis analysis of variance on ranks was applied in case of three or more groups, followed in the event of significance by Dunn’s Method. Trend analysis was conducted using the Mantel-Haenszel Chi-square test. All correlations were studied using Spearman’s rank order correlation coefficient. Bonferroni-correction for multiple testing was applied when contrasts were not driven by a specific hypothesis. For all other tests, p-values < 0.05 were considered significant. All statistical tests were 2-sided.
Results
Aggregated pTDP-43 in neurons and oligodendrocytes
Generally, pTDP-43 pathology presented in the form of intraneuronal dash-like, skein-like, or dot-like inclusions in the cytoplasm. Remarkably, these lesions were accompanied by similar changes in oligodendrocytes (Figs. 1h, 2d, 2e). pTDP-43 pathology did not develop in non-myelinating oligodendrocytes located along the somata of affected cortical pyramidal cells (satellite cells). pTDP-43-immunoreactive oligodendrocytes were seldom encountered in the spinal cord posterior funiculi but were seen in 100% of cases in the anterior and lateral funiculi, particularly within corticospinal projections. By contrast, the medial lemniscus, medial longitudinal fascicle, and the superior cerebellar peduncle did not display altered oligodendrocytes. Pontine sections often showed pTDP-43 pathology in oligodendrocytes in corticobulbar and corticospinal fiber tracts, contrasting with its infrequent appearance in pontocerebellar pathways. Four stages in the progressive dissemination of pTDP-43 pathology could be distinguished in our ALS cohort, as follows:
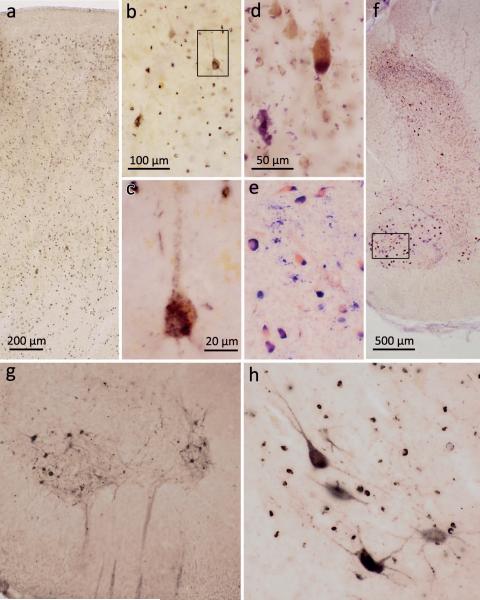
Figure 1. pTDP-43 pathology in the motocortex and spinal cord.
a. ALS-associated pTDP-43 pathology was observed in Betz pyramidal cells of layer Vb and in smaller projection neurons of layers II, III, V, and VI in the primary motor area (Brodmann area 4) (case 75, stage 4). b. Layer Vb of the primary motor cortex with affection of Betz pyramidal cells (case 75, stage 4). c. Detail micrograph (framed area in b) shows single Betz cell in higher resolution with pTDP-43 aggregates detectable in the somatodendritic compartment and in unmyelinated initial portions of the axon. d. Pigment-Nissl stain of layer Vb of the motor cortex depicting a Betz pyramidal cell (upper right) and loss of a Betz cell indicated by lipofuscin pigment remnants in the neuropil (pigment incontinence, lower left) (case 14, stage 2). e. Pigment-Nissl stain of spinal cord ventral horn (framed area in f is rotated 90° to the right in e) depicting α-motoneurons alongside neuronal loss indicated by lipofuscin pigment remnants in the neuropil (case 13, stage 2). f. Pigment-Nissl overview of spinal cord ventral and dorsal horns. g,h. Spinal cord α-motoneurons of layer 9 showing pTDP-43 aggregates in neuronal cell bodies as well as in dendrites and axons (heading towards the ventral root) (case 48, stage 3). Note affected oligodendrocytes in h (case 38, stage 3). Scale in a applies to g; scale bar in b is also valid for e; scale bar in d applies to h.
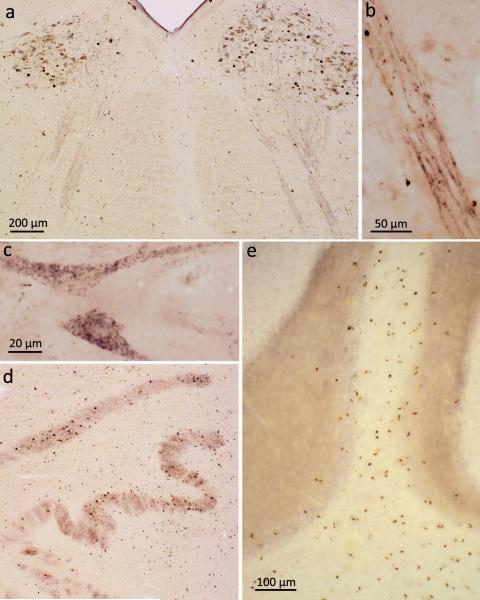
Figure 2. pTDP-43 pathology in bulbar somatomotor nuclei, precerebellar nuclei, and cerebellum.
a. Medulla oblongata section showing extensive ALS-associated pTDP-43 aggregates in neurons of both hypoglossal nuclei (XII) as well as within axons of these cells (case 75, stage 4). b. High resolution micrograph of the intramedullary course of fiber tracts generated from motoneurons of the hypoglossal nucleus. The tract itself contains intraaxonal pTDP-43 aggregates; in addition, immunoreactive oligodendrocytes are located in close proximity to the affected axons (case 75, stage 4). c. Cytoplasmic dash-like pTDP-43 aggregates in motoneurons of the hypoglossal nucleus (case 75, stage 4). d. Section through the medulla oblongata at the level of the hypoglossal nucleus shows involvement of precerebellar nuclei characteristic of stage 2 with extensive pTDP-43 pathology in neurons of the inferior olive. Note the predominant involvement of the superior accessory olivary nucleus and upper lamella of the principal olivary nucleus (case 34, stage 3). e. Section of cerebellar cortex demonstrates multiple pTDP-43-immunopositive oligodendrocytes in cerebellar cortical white matter and deep portions of the cerebellar granular layer (case 10, stage 2). Scale bar in a. is also valid for d. Paraffin-embedded 70 μm sections.
Stage 1: Agranular motor cortex, bulbar and spinal somatomotor neurons
Stage 1 cases (N=6) were characterized by the presence of pTDP-43 immunoreactive inclusions in projection neurons of the agranular motor neocortex, in α-motoneurons of the spinal cord ventral horn, and in bulbar motor neurons of cranial nerves XII-X, VII, and V (Table 2). Within the motor cortex (Brodmann fields 4 and 6), pTDP-43 pathology occurred in Betz pyramidal cells of layer Vb (Fig. 1a-c) and in smaller projection neurons of layers II, III, V, and VI (Fig. 1a). The intraneuronal pathology was accompanied by pTDP-43 immunoreactive inclusions in oligodendrocytes of the cortex and subcortical white matter, as well as among corticobulbar and corticospinal projections.
In the ventral horn of the spinal cord, pTDP-43 pathology predominantly occurred in α-motoneurons of layer 9 (Fig. 1e-h), whereby in some instances the lesions were most extensive in motoneurons localized in lateral portions of the ventral horn (Fig. 1g). Notably, oligodendrocytes of the anterior and lateral portion of the corticospinal tract were also immunoreactive for pTDP-43. In the brainstem, pTDP-43 pathology was present bilaterally in the hypoglossal nucleus (XII) (Fig. 2a-c), in the ambiguus nucleus of the vagal nerve (X), as well as in motor nuclei of the cranial nerves XI, VII, and V.
In contrast, the motor neurons of cranial nerves III, IV, and VI did not contain pTDP-43 aggregates and were virtually uninvolved even in severe ALS cases. Similarly, bulbar visceromotor nuclei, i.e., the dorsal motor nuclei of the vagal and glossopharyngeal nerves, failed to display pTDP-43 inclusions. Additional brainstem nuclei that notably remained free of pTDP-43 pathology included the noradrenergic locus coeruleus and the serotoninergic nuclei of the raphe system.
Stage 2: Reticular formation and precerebellar nuclei
Stage 2 cases (N=15) displayed the pTDP-43 pathology of stage 1, some involvement of the prefrontal neocortex, as assessed in the middle frontal gyrus, and lesions in the reticular formation (both parvocellular and magnocellular portions) and in precerebellar nuclei.31 Representative for the involvement of the large group of precerebellar nuclei was the inferior olivary complex (Table 2). Within this nucleus, pTDP-43 inclusions mainly occurred in circumscribed groups of neurons within the upper lamella of the principal olivary nucleus, within the dorsal accessory olivary nucleus (Fig. 2d), and, somewhat more infrequently, in the medial accessory olive. Similarly to the inferior olivary complex, pTDP-43 aggregates in the pontine gray matter were seen in isolated groups of neurons, creating a patchy or insular pattern. Additionally involved precerebellar nuclei (not essential for neuropathological staging and therefore not shown in Table 2) included the lateral reticular nucleus, conterminal nucleus, interfascicular nucleus, nucleus of Roller, vermiform nucleus, subventricular nucleus, dorsal paramedian reticular nucleus, arcuate nucleus, and the pontobulbar body.32 Because the neurons in parvocellular portions of the red nucleus also contained pTDP-43 pathology and project to the inferior olivary complex, we evaluated them within the context of the precerebellar nuclei. Inasmuch as the available slides did not include the magnocellular portion, we could not assess it here.
pTDP-43-immunopositive oligodendroglial cells were observed along projections from the precerebellar nuclei to the cerebellum. These oligodendrocytes were most dense within the white matter beneath the cerebellar cortex (Fig. 2e). Neuronal types of the cerebellar cortex did not become involved.
We found isolated neuromelanin-containing cells with pTDP-43 inclusions within the pars compacta of the substantia nigra, and, in addition, circumscribed areas of the reticulate portion displayed a punctate pattern similar to that seen in the pallidum (discussed below in greater detail). Finally, in (and beyond) stage 2, the large neurons of the thalamic nuclei that project to layer IV of the cerebral cortex developed pTDP-43 aggregates, but we did not consider them absolutely essential for staging ALS and, thus, these regions do not appear in Table 2. Finally, no pTDP-43 pathology was detected in the subthalamic nucleus.
Stage 3: Prefrontal neocortex and basal ganglia
Stage 3 cases (N=27) were characterized by the presence of additional lesions in the prefrontal granular neocortex, as assessed in the gyrus rectus and orbital gyri, where the pathology was most extensive in cortical layers II, III, and V, VI while conspicuously sparing the internal granular layer (IV). From there, the pathology extended into post-centrally located sensory areas reaching temporal neocortical areas (as evaluated in the superior and middle temporal gyrus). The exclusive involvement of long-axoned cortical pyramidal cells was frequently accompanied by pTDP-43-immunoreactive oligodendrocytes in both the cortex and subcortical white matter. The occipital cortex (Brodmann areas 17 and 18) rarely showed pTDP-43 inclusions.
Within the subcortical gray matter, pTDP-43 aggregates occurred in large, deep layer projection cells of the inferior colliculus, but the main subcortical focus of the pathology in stage 3 cases was the striatum. Within the three subdivisions of the striatum, the accumbens nucleus (ventral striatum) displayed the heaviest burden of pathology. Striatal lesions were seen chiefly in the medium-sized projection neurons33 (Fig. 3a-c) together with occasional involvement of the large cholinergic local circuit neurons (Fig. 3b). Bundles of striato-pallidal axons contained dust-like insoluble pTDP-43-immunoreactive material. The diameters of these axons increased gradually, reaching a maximum within the internal segment of the pallidum. Terminal portions showed numerous globular enlargements. Inclusions were seldom observed in spindle-shaped pallidal projection neurons. The projection neurons of the claustrum also developed pTDP-43 pathology.
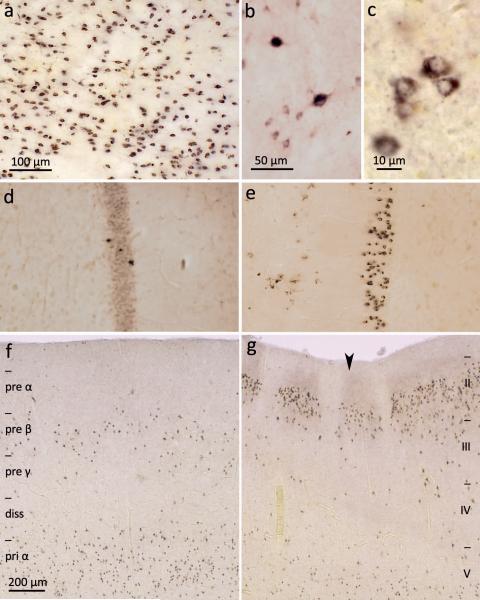
Figure 3. pTDP-43 pathology in the striatum, anteromedial fields of the temporal lobe and the hippocampal formation.
a. Severe pTDP-43 pathology in striatum involving mainly medium-sized projection neurons (case 69, stage 4). b. Micrograph of a group of medium-sized projection neurons and two large cholinergic local circuit neurons of the striatum showing ALS-associated lesions (case 70, stage 4). c. Immunoreactive medium-sized spiny projection cells of the striatum at higher magnification (case 70, stage 4). d. Mild involvement of hippocampal formation with pTDP-43 pathology in few granular cells of the dentate fascia (case 60, stage 4). e. Marked pTDP-43 pathology in granular cells of the dentate fascia (case 69, stage 4). Uninvolved plexiform layer and portion of the fourth sector of the Ammon’s horn is seen in the right halves of d and e. f. pTDP-43 pathology in pyramidal cells of the entorhinal region, mainly affecting layers pre-β and pri-α. Borders of allocortical entorhinal layers are indicated at the left (diss – lamina dissecans, pre – layers of the external main stratum, pri – layers of the internal main stratum) (case 69, stage 4). g. Transition between entorhinal region (left) and transentorhinal region (right) is indicated by large arrowhead. pTDP-43 pathology in pyramidal cells of the transentorhinal region affects neocortical layers: here, borders of neocortical layers II-V (at right) (case 69, stage 4). Scale bar in a also applies to d and e; scale bar in f is also valid for g. Paraffin-embedded 70 μm sections.
Stage 4: Anteromedial areas of the temporal lobe and the hippocampal formation
Stage 4 cases (N=24) displayed the most extensive pTDP-43 distribution pattern not only in regions typical for the preceding stages 1-3 but also in anteromedial portions of the temporal lobe. Affected cortical pyramidal cells were seen in the transentorhinal region (Fig. 3g) and adjoining allocortical entorhinal region, mainly in layers pre-β and pri-α (Fig. 3f).34 Whereas the small-celled islands of the presubiculum were devoid of pathology, the hippocampal formation displayed relatively strong involvement that was helpful for characterizing stage 4 cases. Particularly the granular cells of the dentate fascia showed marked pTDP-43 pathology accompanied by lesions in pyramidal neurons of the Ammon’s horn (CA), initially in sectors CA1-CA2 and then in sectors CA3-CA4. In some ALS cases, the inclusions were confined to isolated dentate granular cells in the absence of pTDP-43 inclusions in the Ammon’s horn (Table 2, cases 53-55; Fig. 3d). By contrast, in others, virtually all granular neurons of the dentate fascia were severely involved (Table 2, cases 64-76; Fig. 3e). Finally and notably, a minority of stage 4 cases (N=4) also displayed mild involvement of the dentate nucleus in the cerebellum (data not shown) while the cerebellar cortical gray matter generally remained unaffected by pTDP-43 pathology.
Recommended regions for staging of pTDP-43 neuronal inclusions in ALS
Although we evaluated pTDP-43 pathology in multiple regions of the CNS, we intentionally limited the regions selected per stage to key sites to make the staging procedure as practicable as possible. The number of regions examined per stage could be increased, but the potential benefits of doing so for the sake of completeness need to be weighed against those of expediency. The ALS staging protocol proposed here requires a minimum of five tissue blocks (Table 3), the sizes of which fit standard embedding cassettes. Paraffin-embedded blocks can be sectioned at 6-10 μm or 70-100 μm28: The first block includes the agranular motor neocortex (Brodmann areas 4 and 6), if possible cut perpendicular to the central sulcus approximately 2 cm away from the longitudinal fissure (stage 1). The second block should be made through the medulla oblongata, perpendicular to the brainstem axis and at the level of the hypoglossal nucleus. This specimen is used to assess the hypoglossal nucleus bilaterally (stage 1 and following stages) and the possible presence of pTDP-43 pathology in the inferior olive (stage 2 and following stages). Importantly, all stage 1 cases examined here consistently displayed pTDP-43 pathology not only in the agranular motor cortex and spinal cord ventral horn but also in somatomotor nuclei of cranial nerves XII-X, VII, and V (Table 2). This means that sections from the spinal cord are not absolutely necessary to assess stage 1 – the rationale for which is that the spinal cord may be unavailable in some instances where staging is, nonetheless, desirable. The third block should include a section through the middle frontal gyrus midway between the agranular areas and the frontal pole; the fourth block includes the striatum to evaluate pTDP-43 pathology in the caudate nucleus and/or putamen (stage 3 and following). The fifth block represents the conventional slice through the hippocampal formation and includes a portion of the dentate gyrus (stage 4).
Table 3.
Tissue blocks and regions for staging of pTDP-43 pathology in ALS
| Stage 1: | block 1: agranular motor neocortex – Brodmann areas 4, 6 block 2: medulla oblongata at the level of N. XII – bulbar somatomotor neurons of N. XII optional: spinal cord layer 9 – ventral horn α-motoneurons |
| Stage 2: | block 1 block 2: inferior olive, medullary reticular formation optional: parvocellular portion of the red nucleus |
| Stage 3: | blocks 1 and 2 block 3: prefrontal neocortex (e.g., gyrus rectus, orbital gyri) block 4: striatum optional: postcentral neocortex |
| Stage 4: | blocks 1-4 block 5: hippocampal formation, entorhinal region, adjoining temporal neocortex |
Staging is based on a minimum of five tissue blocks, additional blocks, e.g., from the spinal cord or midbrain, are optional. When assigning stages, the extent (topographical distribution pattern) is accorded more weight than the degree (severity) of the pTDP-43 pathology in each region.
Relation of pTDP-43 lesions to genotype and other pathology
All cases with DNA available (N=75) were tested for C9orf72, TARDBP, and UBQLN2 mutations. No mutations in the TARDBP or UBQLN2 gene were observed. A C9orf72 hexanucleotide repeat expansion was detected in 11 cases (14.7%). Of C9orf72 expansion cases with family history information available, an expansion was present in 5 of 7 cases (71%) with a positive family history for FTD or ALS (N=4) or another neurodegenerative disorder (N=1), whereas 2 (29%) were identified in apparently sporadic cases. Overall, a family history of FTD and/or ALS was noted in 15.7% (12/76) of the cohort and a family history of a neurodegenerative disease other than FTD or ALS in 15.7% (12/76). Apparent sporadic cases made up 48.7% (37/76) of the cohort, and there was insufficient information to evaluate family history in 19.8% (15/76). The site of onset in the 11 cases with C9orf72 expansion was as follows: extremity onset (N=5), BLMN (N=4), and BUMN (N=2). Cases with the C9orf72 mutation showed a shorter disease duration (median 24 months, interquartile range 14-36 months), as compared with non-expansion cases (median 34 months, interquartile range 25-60 months) (p=0.03). While cases with the C9orf72 hexanucleotide expansion showed the same sequential distribution pattern as ALS cases without the expansion, they displayed a greater regional burden of pathology (Supplementary figure 1).
A correlation existed between pTDP-43 intraneuronal pathology and neuronal loss across several of the regions analyzed here (superior and middle temporal gyrus, cingulate gyrus, cervical spinal cord, amygdala, hippocampal formation, entorhinal cortex, Suppl. Table 1). pTDP-43 pathology also tended to increase with neuronal loss in the substantia nigra, although this failed to reach statistical significance. There was no significant correlation of pTDP-43 with age of onset or disease duration in any of the regions analyzed (Suppl. Table 1). The stages of TDP-43 pathology as defined here did not show a significant difference regarding age at onset (p=0.25), disease duration (p=0.93), or ALSFRS-R (p=0.59). Data on the MMSE was available for 43 (56.6%) of our autopsy cases. ALS cases with stage 4 pathology (data available for N= 16 cases, median 29, interquartile range 28-30) showed a significantly lower score on the MMSE as compared to stage 1 and 2 cases (data available for N=18 cases, median 26.5, interquartile range 24-28) (p=0.02). Finally, stages of pTDP-43 pathology did not show a significant difference with regard to clinical subtypes of ALS (p=0.56).
Discussion
This study presents preliminary evidence for a sequential propagation of pTDP-43 pathology in a large cohort of clinically characterized ALS autopsy cases. To determine potential dissemination of pTDP-43 aggregates, we used an approach based on the assumption that intraneuronal pathological lesions are likely to develop consecutively at different CNS sites and usually increase in severity with disease progression. This rationale is in line with previous staging attempts for other neurodegenerative diseases.4, 35, 36 Furthermore, it is based on the assumption derived from clinical observation that motor neuron degeneration in ALS is a focal process that spreads contiguously through the anatomy of the motor system.37-39 Potential drawbacks to the approach used here are that early (i.e., preclinical) stage cases are often lacking and the fact that such studies are, by definition, cross-sectional. This problem is accentuated in a rapidly progressive disease, such as ALS, which leads to death within a short time after clinical onset. We not only analyzed cases with little overall pTDP-43 pathology to detect possible sites of onset but we also placed greater emphasis on sequential changes in the regional distribution pattern of pTDP-43 inclusions than on grading based on lesion intensities at each stage. An additional drawback is that our analysis of the brainstem pathology is more detailed than that of the lesions in the far more expansive cortex. Thus, the staging protocol proposed here for the cortical lesions in ALS is preliminary, and neuropathological studies of the entire cortex using serial hemisphere sections are underway now, to map the distribution and progression of pTDP-43 in more detail in the cerebral cortex.
Neuronal and oligodendroglial pTDP-43 pathology
The presence of selective degeneration of pTDP-43 inclusion-bearing neurons supports the notion that pTDP-43 aggregates are tightly linked with neurodegeneration.3 Others observed that the extent of pTDP-43 pathology correlates with neuronal loss across many regions of the CNS.38, 39 Although mechanisms of pTDP-43-mediated neurodegeneration remain to be clarified, phosphorylation, aggregation, cleavage, and clearance from the cell nucleus could result in a loss of physiological pTDP-43 function that then entails neuronal dysfunction and death.3 However, it also is plausible that increasing accumulation of pTDP-43 aggregates gradually impairs neuronal function before crossing an indeterminate threshold that leads to neuronal death. The frequently observed presence of dash-like pTDP-43 immunoreactive inclusions may represent an early state of pTDP-43 accumulation,40 and neuronal death may occur only with further aggregation into massive lesions that encompass large portions of the neuronal cell body and its neurites.
The neuronal pTDP-43 pathology assessed here was accompanied by abnormal changes within oligodendrocytes. Only a minority of the total number of oligodendrocytes present in the tissue displayed cytoplasmic pTDP-43-positive aggregates. Involved cells were usually widely spaced apart and accompanied long fiber tracts, indicating that oligodendroglial cells could develop the lesions owing to contact with involved axons of diseased nerve cells. The lesions may result from an uptake of soluble or partially soluble substances (possibly pTDP-43 aggregates) through axonal contact zones into the oligodendrocytes. Our observation that lesions did not develop in satellite cells located along the somata of affected cortical pyramidal cells and that diseased oligodendroglia did not induce additional pathological changes in nearby (local) but previously uninvolved oligodendrocytes supports the notion of transmission of pTDP-43 pathology along axonal pathways. Similar to neurons, severely involved oligodendrocytes are likely to become dysfunctional, which could then contribute to the observed demyelination of affected axons in ALS.41, 42
Spreading pattern of pTDP-43 pathology in ALS cases
Stage 1
The first of the proposed stages was characterized by the presence of pTDP-43 pathology in the agranular motor cortex, spinal cord α-motor neurons, and brainstem motor nuclei of cranial nerves XII-X, VII, and V (Table 2). No differences regarding the pattern of pTDP-43 were observed between different types of onset of disease. Further differentiation of very early pTDP-43 pathology will require autopsy material from pre-clinical or “incidental” cases.
In accordance with previous studies, we observed pTDP-43 inclusions in large Betz pyramidal neurons of layer Vb, which in addition to smaller types of pyramidal cells have projections that make up the corticospinal and corticobulbar tracts.2, 27, 43 Moreover, we could confirm the results reported by other groups 2, 27, 29, 39 with respect to the presence of extensive pTDP-43 pathology in spinal cord ventral horn α-motoneurons. Interestingly, this pathology frequently was accentuated in lateral portions of the ventral horn. The motor nuclei columns of the lateral portion of the ventral horn (i.e., the anterolateral and posterolateral column of the cervical and lumbar spinal cord) innervate muscles of distal parts of the extremities, which are often affected by paresis early in the disease course.1 Finally, although the severe bilateral involvement of brainstem somatomotor nuclei seen here (e.g., hypoglossal nucleus, ambiguus nucleus) is not new,27, 44, 45 it is highly consistent with the available clinical evidence of early bulbar involvement in ALS.1
The brainstem nuclei of cranial nerves VI, IV, and III were virtually uninvolved, and this remained true even in cases with greater regional involvement. This observation corroborates previously published clinical and pathological findings, where involvement of motoneurons controlling the extrinsic eye muscles in ALS is rare and presents mainly in patients with prolonged survival rates.45-48 Modulation of the eye movements essentially takes place via axons that form the medial longitudinal fascicle.49 The nerve cells belonging to these axons do not become involved in ALS, and this may account for why the motoneurons that control the extrinsic eye muscles remain largely intact during the course of the disorder.
Stage 2
This stage was marked by pTDP-43 immunoreactive lesions in precerebellar nuclei of the brainstem. The inferior olivary complex and the pontine gray matter neurons receive indirect and direct afferents from layer V of the agranular neocortex,50 as relay stations of the cortico-pontine-cerebellar or corticorubro-olivary-cerebellar tracts. Clinically, involvement of precerebellar nuclei would be anticipated to produce symptoms of cerebellar dysfunction: axial or limb ataxia in association with intention tremor and an impairment of alternating movements. However, none of the ALS cases here was reported as showing cerebellar deficits upon clinical examination, and prior reports of cerebellar symptoms in ALS are limited to small case series.51 As a possible explanation, we observed only patchy or isolated pTDP-43 pathology in the inferior olivary complex and pontine gray matter at this point. Accordingly, it is likely that larger portions of these nuclei with increasing burdens of pathology would be necessary before neuronal dysfunction and clinically detectable cerebellar deficits gradually evolve in such individuals. Furthermore, clinical deficits, such as limb ataxia, are difficult to detect in ALS, as they may be eclipsed or masked by severe pareses owing to α-motoneuron degeneration. In contrast, the well-preserved function of neurons that control eye movements offers a promising and novel approach to the diagnosis of cerebellar deficits in ALS via analysis of associated eye movement abnormalities.52 In fact, several studies have detected impaired smooth pursuit eye movements in ALS, which are functionally controlled by cerebro-pontocerebellar pathways.53
Stage 3
During this stage pTDP-43 pathology extended into prefrontal and later, presumably via direct connections between the frontal agranular region and postcentral sensory areas, the postcentral neocortex, and it also reached the basal ganglia, including the caudate nucleus, putamen, and especially the ventral striatum (accumbens nucleus). The possible spread of pTDP-43 pathology from agranular motor cortex to prefrontal neocortical areas is consistent with clinical evidence that executive cognitive deficits affecting executive function up to full-scale FTD occur in up to 50% of ALS patients.54, 55 It is also in line with imaging correlates of cognitive decline that reveal frontal lobe atrophy and hypometabolism in the frontotemporal cortex and supports findings by our own group that the extent of pTDP-43 in the prefrontal neocortex correlates with the presence of executive cognitive dysfunction.39, 56, 57 Importantly, our data imply that pTDP-43 lesions obligatorily disseminate to the prefrontal cortex with ongoing disease, thereby lending support to the idea that all ALS patients eventually develop “frontal type” cognitive deficits depending on disease duration and the rapidity of disease propagation. In fact, the ALS cases with dementia included here all displayed stage 3 or 4 pTDP-43 pathology (Table 2). Inasmuch as it is known that the extent of pTDP-43 pathology in prefrontal cortical areas correlates with tests of executive function,38 such test batteries could provide a promising tool to detect stage 3 pTDP-43 pathology in vivo.
The anatomical correlate of this frontal dissemination of pTDP-43 pathology is still unclear. Nevertheless, our data suggest that layers V and VI of the motor cortex are early foci of pTDP-43 pathology. From there, the lesions could reach other cortical layers, particularly layers II and III, which are closely linked to adjacent cortical areas via association fibers, thereby providing a potential route for pTDP-43 dissemination within the neocortex.58-63 Furthermore, the main intracortical projections of the primary motor cortex are chiefly directed towards more frontally localized areas, such as Brodmann area 6 (premotor cortex) and adjoining prefrontal areas.58-63 These projections could serve as a pre-existing pathway to canalize dissemination of pTDP-43 pathology from the agranular motor cortex in a frontal trajectory.58
Although involvement of the sensory neocortex may seem to contradict the perception of ALS as a motor neuron disease, several earlier studies observed clinical or neuropathological evidence of mild sensory system involvement.64-66 Furthermore, a previous study by our own group performed on 6 μm sections detected mild pTDP-43 pathology in the postcentral neocortex.27 We therefore postulate that pTDP-43 could spread to the postcentral association neocortex by way of long association fibers originating from prefrontal neocortical areas.67, 68
Cases assigned to stage 3 were also characterized by the presence of severe ALS-associated pathology in the caudate nucleus, putamen, and ventral striatum, thereby corroborating previous reports that pTDP-43 pathology and/or ubiquitin-immunoreactive inclusions can occur in these regions in ALS.27, 64, 69, 70 The involvement of the striatum and medial pallidum as well as the lack of pTDP-43 inclusions in the subthalamic nucleus favor the interpretation that the pathology in ALS mainly affects the direct pathway through the cortico-basal ganglia-cortical circuit.71 However, clinical signs of basal ganglia involvement (e.g., bradykinesia, rigidity, postural instability) were not reported in our patient cohort and are, in fact, rare in ALS.1, 51, 70 Similar to potential cerebellar deficits, evolving extrapyramidal motor symptoms could be eclipsed by severe pareses of later-stage patients. Furthermore, the occurrence of rigidity may well require the integrity of spinal cord α-motoneurons72 – and these, as reported here, appear to become involved early. Finally, the presence of severe pTDP-43 pathology in the ventral striatum, which processes information coming from the limbic cortical regions,73 could diminish striatal input to the emotional motor system during ALS by hampering an adequate response to external stimuli associated with emotionally weighted motor reactions.
Stage 4
The fourth stage was characterized by the dissemination of pTDP-43 pathology into anteromedial areas of the temporal lobe, including the hippocampal formation. Whereas hippocampal pTDP-43 pathology has been reported by several groups,2, 27, 39, 70 its clinical relevance has been questioned because AD-like memory deficits are considered a rarity in ALS.54, 75 Nonetheless, the extensive and consistent presence of pTDP-43 inclusions observed in the hippocampal formation of stage 4 cases and the worse performance by such cases on the MMSE as compared to stage 1 and 2 cases strongly suggests that ALS patients may inevitably develop amnestic dysfunctions in late phases of the disease. In neuropsychological testing, such deficits may be overshadowed by executive dysfunction already precipitated by pTDP-43 dissemination to prefrontal neocortical areas.
Mechanisms of pTDP-43 propagation
While this study provides neuropathological data in a large cohort that pTDP-43 aggregates disseminate in a sequential manner in ALS, the underlying mechanisms of the pathological process are far from clear. Misfolded protein aggregates could induce a self-perpetuating process that leads to amplification, templating, and propagation of pathological protein assemblies.7, 76 Although such transmission mechanisms originally were thought to be uniquely associated with prions, an increasing number of neurodegenerative diseases were found to spread by “prion-like” mechanisms.8, 9, 11, 19, 77-80 Both SOD1 and pTDP-43 can form aggregates that seed misfolding of the corresponding wild type protein in vitro,81, 82 and a C-terminal prion-like domain has been identified in TDP-43.14 Importantly, a recent study has shown that pTDP-43 is actively transported in axons of somatomotor neurons at a speed consistent with fast axonal transport.83 The sequential spreading pattern of pTDP-43 lesions observed in this study strongly supports the notion that pTDP-43 aggregates disseminate via axonal transport, but further studies in cell culture and animal models are needed to establish this with certainty.
A key characteristic of ALS is the presence of pTDP-43 pathology in regions that are far apart from each other. For instance, the Betz cells of the agranular motor cortex and the α-motoneurons in the spinal cord ventral horn are not only topographically distant from another but also interconnected by the corticospinal tract that could help to propagate pTDP-43 aggregates. That axonal transport may be crucial for pTDP-43 dissemination is further indicated by our observation that in stage 1-2 cases pTDP-43 pathology occurs chiefly in regions of the CNS that are closely connected by major axonal pathways. On the other hand, neurons without major cortical input, such as in nuclei of cranial nerves VI, IV, and III, are apparently inured to pTDP-43 pathology and spreading mechanisms. The notion of axonal propagation of pTDP-43 aggregates is furthermore supported by our observations on oligodendroglial pTDP-43 pathology that affected only cells with potential close axonal contact, whereas satellite cells located along the somata of diseased neurons remain uninvolved.
Finally, our data make it possible to speculate that ALS genotypes, like the C9orf72 hexanucleotide repeat expansion,84, 85 might be capable of modifying the potential neuron-to-neuron propagation of pTDP-43: The high burden and fast progression of pTDP-43 pathology seen in ALS cases with the C9orf72 expansion could have contributed to the fulminant disease course observed in those individuals.85
Summary
The findings reported here imply that pTDP-43 pathology in ALS may disseminate in a sequential pattern that permits recognition of four neuropathological disease stages. However, while such staging protocols can help to further our understanding of disease progression in ALS, they cannot mimic exactly the underlying pathological process that continually evolves. Furthermore, while our data indicate that pTDP-43 may disseminate from one region to the next, the biochemical substrates of this process must be elucidated in future studies.
Supplementary Material
Supplementary Figure 1. Burden of pTDP-43 pathology in ALS with/without C9orf72 repeat expansion.
This bar plot illustrates the burden of pTDP-43 pathology in ALS with(N=11) and without (N=64) theC9orf72 repeat expansion. Bars indicate median and 95% confidence interval, significant difference in Wilcoxon Mann-Whitney Test (p<0.05) is indicated by asterisk. Abbreviations: AM – amygdala, CE – cerebellum, CG – anterior cingulate gyrus, ENT – entorhinal region, GP – pallidum, HIP – hippocampal formation, HTH – hypothalamus, IO – inferior olive, MF– middle frontal gyrus, MO – agranular motor cortex (Brodmann areas 4 and 6), OR– gyrus rectus and orbital gyri, PG – pontine gray, RF – reticular formation, RU – red nucleus, SC – spinal cord anterior horn, SEN – somatosensory cortex, SMT – superior or middle temporal gyrus, ST – striatum, TH – thalamus, VIS – visual cortex (Brodmann areas 17 and 18), XII – hypoglossal nucleus.
Acknowledgements
We thank the many patients who contributed to this study. We gratefully acknowledge Kevin Raible, Terry Schuck, and Phuong Thu Brettschneider for technical support, and David Ewert (University of Ulm) for assistance with the graphics. This study was supported by the NIH (AG033101, AG017586, AG010124, AG032953, AG039510, NS044266), the Wyncote Foundation, the Koller Family Foundation, and the Deutsche Forschungsgemeinschaft (DFG, grant number TR 1000/1-1). VMYL is the John H. Ware, 3rd, Professor of Alzheimer’s Disease Research. JQT is the William Maul Measey-Truman G. Schnabel, Jr. Professor of Geriatric Medicine and Gerontology. JBT is supported by a grant of the Fundación Alfonso Martin Escudero. DJI is supported by T32-AG000255.
References
- 1.Kiernan MC, Vucic S, Cheah BC, et al. Amyotrophic lateral sclerosis. Lancet. 2011 Mar 12;377(9769):942–55. doi: 10.1016/S0140-6736(10)61156-7. [DOI] [PubMed] [Google Scholar]
- 2.Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006 Oct 6;314(5796):130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 3.Lee EB, Lee VM, Trojanowski JQ. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nature reviews Neuroscience. 2012 Jan;13(1):38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braak H, Thal DR, Ghebremedhin E, Del Tredici K. Stages of the pathologic process in Alzheimer disease: age categories from 1 to 100 years. Journal of neuropathology and experimental neurology. 2011 Nov;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. This is the correct reference and not Braak et al. 2006.
- 5.Braak H, Del Tredici K. Where, when, and in what form does sporadic Alzheimer’s disease begin? Current opinion in neurology. 2012 Dec;25(6):708–14. doi: 10.1097/WCO.0b013e32835a3432. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiology of aging. 2003 Mar-Apr;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 7.Polymenidou M, Cleveland DW. Prion-like spread of protein aggregates in neurodegeneration. The Journal of experimental medicine. 2012 May 7;209(5):889–93. doi: 10.1084/jem.20120741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clavaguera F, Bolmont T, Crowther RA, et al. Transmission and spreading of tauopathy in transgenic mouse brain. Nature cell biology. 2009 Jul;11(7):909–13. doi: 10.1038/ncb1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nature medicine. 2008 May;14(5):501–3. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 10.Mougenot AL, Bencsik A, Nicot S, et al. Transmission of prion strains in a transgenic mouse model overexpressing human A53T mutated alpha-synuclein. Journal of neuropathology and experimental neurology. 2011 May;70(5):377–85. doi: 10.1097/NEN.0b013e318217d95f. [DOI] [PubMed] [Google Scholar]
- 11.Volpicelli-Daley LA, Luk KC, Patel TP, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011 Oct 6;72(1):57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological alpha-synuclein initiates a rapidly progressive neurodegenerative alpha-synucleinopathy in mice. The Journal of experimental medicine. 2012 May 7;209(5):975–86. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luk KC, Kehm V, Carroll J, et al. Pathological alpha-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012 Nov 16;338(6109):949–53. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cushman M, Johnson BS, King OD, Gitler AD, Shorter J. Prion-like disorders: blurring the divide between transmissibility and infectivity. Journal of cell science. 2010 Apr 15;123(Pt 8):1191–201. doi: 10.1242/jcs.051672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders: official publication of the World Federation of Neurology. Research Group on Motor Neuron Diseases. 2000 Dec;1(5):293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 16.Cedarbaum JM, Stambler N, Malta E, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III) Journal of the neurological sciences. 1999 Oct 31;169(1-2):13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 17.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of psychiatric research. 1975 Nov;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 18.Xie SX, Baek Y, Grossman M, et al. Building an integrated neurodegenerative disease database at an academic health center. Alzheimer’s & dementia : the journal of the Alzheimer’s Association. 2011 Jul;7(4):e84–93. doi: 10.1016/j.jalz.2010.08.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanouchi T, Ohkubo T, Yokota T. Can regional spreading of amyotrophic lateral sclerosis motor symptoms be explained by prion-like propagation? Journal of neurology, neurosurgery, and psychiatry. 2012 Jul;83(7):739–45. doi: 10.1136/jnnp-2011-301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neary D, Snowden JS, Gustafson L, et al. Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology. 1998 Dec;51(6):1546–54. doi: 10.1212/wnl.51.6.1546. [DOI] [PubMed] [Google Scholar]
- 21.Rascovsky K, Hodges JR, Knopman D, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain : a journal of neurology. 2011 Sep;134(Pt 9):2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011 Mar 15;76(11):1006–14. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Annals of neurology. 2002 Aug;52(2):205–10. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- 24.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta neuropathologica. 2009 Feb;117(2):137–49. doi: 10.1007/s00401-008-0477-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee EB, Leng LZ, Zhang B, et al. Targeting amyloid-beta peptide (Abeta) oligomers by passive immunization with a conformation-selective monoclonal antibody improves learning and memory in Abeta precursor protein (APP) transgenic mice. The Journal of biological chemistry. 2006 Feb 17;281(7):4292–9. doi: 10.1074/jbc.M511018200. [DOI] [PubMed] [Google Scholar]
- 26.Uryu K, Nakashima-Yasuda H, Forman MS, et al. Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. Journal of neuropathology and experimental neurology. 2008 Jun;67(6):555–64. doi: 10.1097/NEN.0b013e31817713b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geser F, Brandmeir NJ, Kwong LK, et al. Evidence of multisystem disorder in whole-brain map of pathological TDP-43 in amyotrophic lateral sclerosis. Arch Neurol. 2008 May;65(5):636–41. doi: 10.1001/archneur.65.5.636. [DOI] [PubMed] [Google Scholar]
- 28.Feldengut S, Del Tredici K, Braak H. Paraffin sections of 70-100 μm: A novel technique and its benefits for studying the nervous system. Journal of neuroscience methods. 2013 Mar 25; doi: 10.1016/j.jneumeth.2013.03.010. doi:10.1016/j.neumeth.2013.03.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 29.Brettschneider J, Van Deerlin VM, Robinson JL, et al. Pattern of ubiquilin pathology in ALS and FTLD indicates presence of C9ORF72 hexanucleotide expansion. Acta neuropathologica. 2012 Jun;123(6):825–39. doi: 10.1007/s00401-012-0970-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011 Sep 8;477(7363):211–5. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van Deerlin VM, Leverenz JB, Bekris LM, et al. TARDBP mutations in amyotrophic lateral sclerosis with TDP-43 neuropathology: a genetic and histopathological analysis. Lancet neurology. 2008 May;7(5):409–16. doi: 10.1016/S1474-4422(08)70071-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braak H, Rüb U, Del Tredici K. Involvement of precerebellar nuclei in multiple system atrophy. Neuropathology and applied neurobiology. 2003 Feb;29(1):60–76. doi: 10.1046/j.1365-2990.2003.00432.x. [DOI] [PubMed] [Google Scholar]
- 33.Braak H, Braak E. Neuronal types in the striatum of man. Cell and tissue research. 1982 Nov;227(2):319–342. doi: 10.1007/BF00210889. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt S, Braak E, Braak H. Parvalbumin-immunoreactive structures of the adult human entorhinal and transentorhinal region. Hippocampus. 1993 Oct;3(4):459–70. doi: 10.1002/hipo.450030407. [DOI] [PubMed] [Google Scholar]
- 35.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta neuropathologica. 1991;82(4):239–59. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 36.Ozawa T, Paviour D, Quinn NP, et al. The spectrum of pathological involvement of the striatonigral and olivopontocerebellar systems in multiple system atrophy: clinicopathological correlations. Brain : a journal of neurology. 2004 Dec;127(Pt 12):2657–71. doi: 10.1093/brain/awh303. [DOI] [PubMed] [Google Scholar]
- 37.Ravits JM, La Spada AR. ALS motor phenotype heterogeneity, focality, and spread: deconstructing motor neuron degeneration. Neurology. 2009 Sep 8;73(10):805–11. doi: 10.1212/WNL.0b013e3181b6bbbd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ravits J, Paul P, Jorg C. Focality of upper and lower motor neuron degeneration at the clinical onset of ALS. Neurology. 2007 May 8;68(19):1571–5. doi: 10.1212/01.wnl.0000260965.20021.47. [DOI] [PubMed] [Google Scholar]
- 39.Ravits J, Laurie P, Fan Y, et al. Implications of ALS focality: rostral-caudal distribution of lower motor neuron loss postmortem. Neurology. 2007 May 8;68(19):1576–82. doi: 10.1212/01.wnl.0000261045.57095.56. [DOI] [PubMed] [Google Scholar]
- 40.Nishihira Y, Tan CF, Hoshi Y, et al. Sporadic amyotrophic lateral sclerosis of long duration is associated with relatively mild TDP-43 pathology. Acta neuropathologica. 2009 Jan;117(1):45–53. doi: 10.1007/s00401-008-0443-6. [DOI] [PubMed] [Google Scholar]
- 41.Brettschneider J, Libon DJ, Toledo JB, et al. Microglial activation and TDP-43 pathology correlate with executive dysfunction in amyotrophic lateral sclerosis. Acta neuropathologica. 2012 Jan 1; doi: 10.1007/s00401-011-0932-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mori F, Tanji K, Zhang HX, et al. Maturation process of TDP-43-positive neuronal cytoplasmic inclusions in amyotrophic lateral sclerosis with and without dementia. Acta neuropathologica. 2008 Aug;116(2):193–203. doi: 10.1007/s00401-008-0396-9. [DOI] [PubMed] [Google Scholar]
- 43.Lee Y, Morrison BM, Li Y, et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature. 2012 Jul 26;487(7408):443–8. doi: 10.1038/nature11314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Philips T, Bento-Abreu A, Nonneman A, et al. Oligodendrocyte dysfunction in the pathogenesis of amyotrophic lateral sclerosis. Brain : a journal of neurology. 2013 Feb;136(Pt 2):471–82. doi: 10.1093/brain/aws339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mackenzie IR, Bigio EH, Ince PG, et al. Pathological TDP-43 distinguishes sporadic amyotrophic lateral sclerosis from amyotrophic lateral sclerosis with SOD1 mutations. Annals of neurology. 2007 May;61(5):427–34. doi: 10.1002/ana.21147. [DOI] [PubMed] [Google Scholar]
- 46.Hirano A. Neuropathology of ALS: an overview. Neurology. 1996 Oct;47(4 Suppl 2):S63–6. doi: 10.1212/wnl.47.4_suppl_2.63s. [DOI] [PubMed] [Google Scholar]
- 47.Al-Chalabi A, Jones A, Troakes C, King A, Al-Sarraj S, van den Berg LH. The genetics and neuropathology of amyotrophic lateral sclerosis. Acta neuropathologica. 2012 Sep;124(3):339–52. doi: 10.1007/s00401-012-1022-4. [DOI] [PubMed] [Google Scholar]
- 48.Okamoto K, Hirai S, Amari M, et al. Oculomotor nuclear pathology in amyotrophic lateral sclerosis. Acta neuropathologica. 1993;85(5):458–62. doi: 10.1007/BF00230482. [DOI] [PubMed] [Google Scholar]
- 49.Lawyer T, Jr., Netsky MG. Amyotrophic lateral sclerosis. AMA archives of neurology and psychiatry. 1953 Feb;69(2):171–92. doi: 10.1001/archneurpsyc.1953.02320260029002. [DOI] [PubMed] [Google Scholar]
- 50.Sharma R, Hicks S, Berna CM, Kennard C, Talbot K, Turner MR. Oculomotor dysfunction in amyotrophic lateral sclerosis: a comprehensive review. Arch Neurol. 2011 Jul;68(7):857–61. doi: 10.1001/archneurol.2011.130. [DOI] [PubMed] [Google Scholar]
- 51.Büttner-Ennever JA. The extraocular motor nuclei: organization and functional neuroanatomy. Progress in brain research. 2006;151:95–125. doi: 10.1016/S0079-6123(05)51004-5. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki L, Coulon P, Sabel-Goedknegt EH, Ruigrok TJ. Organization of cerebral projections to identified cerebellar zones in the posterior cerebellum of the rat. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012 Aug 8;32(32):10854–69. doi: 10.1523/JNEUROSCI.0857-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gilbert RM, Fahn S, Mitsumoto H, Rowland LP. Parkinsonism and motor neuron diseases: twenty-seven patients with diverse overlap syndromes. Movement disorders : official journal of the Movement Disorder Society. 2010 Sep 15;25(12):1868–75. doi: 10.1002/mds.23200. [DOI] [PubMed] [Google Scholar]
- 54.Donaghy C, Thurtell MJ, Pioro EP, Gibson JM, Leigh RJ. Eye movements in amyotrophic lateral sclerosis and its mimics: a review with illustrative cases. Journal of neurology, neurosurgery, and psychiatry. 2011 Jan;82(1):110–6. doi: 10.1136/jnnp.2010.212407. [DOI] [PubMed] [Google Scholar]
- 55.Leveille A, Kiernan J, Goodwin JA, Antel J. Eye movements in amyotrophic lateral sclerosis. Arch Neurol. 1982 Nov;39(11):684–6. doi: 10.1001/archneur.1982.00510230010003. [DOI] [PubMed] [Google Scholar]
- 56.Phukan J, Pender NP, Hardiman O. Cognitive impairment in amyotrophic lateral sclerosis. Lancet neurology. 2007 Nov;6(11):994–1003. doi: 10.1016/S1474-4422(07)70265-X. [DOI] [PubMed] [Google Scholar]
- 57.Strong MJ, Grace GM, Orange JB, Leeper HA, Menon RS, Aere C. A prospective study of cognitive impairment in ALS. Neurology. 1999 Nov 10;53(8):1665–70. doi: 10.1212/wnl.53.8.1665. [DOI] [PubMed] [Google Scholar]
- 58.Jeong Y, Park KC, Cho SS, et al. Pattern of glucose hypometabolism in frontotemporal dementia with motor neuron disease. Neurology. 2005 Feb 22;64(4):734–6. doi: 10.1212/01.WNL.0000152047.58767.9D. [DOI] [PubMed] [Google Scholar]
- 59.Ludolph AC, Langen KJ, Regard M, et al. Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta neurologica Scandinavica. 1992 Feb;85(2):81–9. doi: 10.1111/j.1600-0404.1992.tb04003.x. [DOI] [PubMed] [Google Scholar]
- 60.Fang PC, Stepniewska I, Kaas JH. Ipsilateral cortical connections of motor, premotor, frontal eye, and posterior parietal fields in a prosimian primate, Otolemur garnetti. The Journal of comparative neurology. 2005 Sep 26;490(3):305–33. doi: 10.1002/cne.20665. [DOI] [PubMed] [Google Scholar]
- 61.Wu CW, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. The Journal of comparative neurology. 2000 Jul 17;423(1):140–77. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 62.Matsumura M, Kubota K. Cortical projection to hand-arm motor area from post-arcuate area in macaque monkeys: a histological study of retrograde transport of horseradish peroxidase. Neuroscience letters. 1979 Mar;11(3):241–6. doi: 10.1016/0304-3940(79)90001-6. [DOI] [PubMed] [Google Scholar]
- 63.Muakkassa KF, Strick PL. Frontal lobe inputs to primate motor cortex: evidence for four somatotopically organized ‘premotor’ areas. Brain research. 1979 Nov 9;177(1):176–82. doi: 10.1016/0006-8993(79)90928-4. [DOI] [PubMed] [Google Scholar]
- 64.Tokuno H, Tanji J. Input organization of distal and proximal forelimb areas in the monkey primary motor cortex: a retrograde double labeling study. The Journal of comparative neurology. 1993 Jul 8;333(2):199–209. doi: 10.1002/cne.903330206. [DOI] [PubMed] [Google Scholar]
- 65.Lu MT, Preston JB, Strick PL. Interconnections between the prefrontal cortex and the premotor areas in the frontal lobe. The Journal of comparative neurology. 1994 Mar 15;341(3):375–92. doi: 10.1002/cne.903410308. [DOI] [PubMed] [Google Scholar]
- 66.Piao YS, Wakabayashi K, Kakita A, et al. Neuropathology with clinical correlations of sporadic ayotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol. 2003 Jan;13(1):10–22. doi: 10.1111/j.1750-3639.2003.tb00002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matheson JK, Harrington HJ, Hallett M. Abnormalities of multimodality evoked potentials in amyotrophic lateral sclerosis. Arch Neurol. 1986 Apr;43(4):338–40. doi: 10.1001/archneur.1986.00520040026013. [DOI] [PubMed] [Google Scholar]
- 68.Radtke RA, Erwin A, Erwin CW. Abnormal sensory evoked potentials in amyotrophic lateral sclerosis. Neurology. 1986 Jun;36(6):796–801. doi: 10.1212/wnl.36.6.796. [DOI] [PubMed] [Google Scholar]
- 69.Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex; a journal devoted to the study of the nervous system and behavior. 2012 Jan;48(1):82–96. doi: 10.1016/j.cortex.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 70.Kawamura K, Naito J. Corticocortical projections to the prefrontal cortex in the rhesus monkey investigated with horseradish peroxidase techniques. Neuroscience research. 1984 Apr;1(2):89–103. doi: 10.1016/s0168-0102(84)80007-3. [DOI] [PubMed] [Google Scholar]
- 71.Zhang H, Tan CF, Mori F, et al. TDP-43-immunoreactive neuronal and glial inclusions in the neostriatum in amyotrophic lateral sclerosis with and without dementia. Acta neuropathologica. 2008 Jan;115(1):115–22. doi: 10.1007/s00401-007-0285-7. [DOI] [PubMed] [Google Scholar]
- 72.Miki Y, Mori F, Nunomura J, et al. Sporadic amyotrophic lateral sclerosis with pallido-nigro-luysian degeneration: a TDP-43 immunohistochemical study. Neuropathology : official journal of the Japanese Society of Neuropathology. 2010 Apr;30(2):149–53. doi: 10.1111/j.1440-1789.2009.01046.x. [DOI] [PubMed] [Google Scholar]
- 73.Nambu A. A new dynamic model of the cortico-basal ganglia loop. Progress in brain research. 2004;143:461–6. doi: 10.1016/S0079-6123(03)43043-4. [DOI] [PubMed] [Google Scholar]
- 74.Delwaide PJ. Parkinsonian rigidity. Functional neurology. 2001 Apr-Jun;16(2):147–56. [PubMed] [Google Scholar]
- 75.Saper CB. Role of the cerebral cortex and striatum in emotional motor response. Progress in brain research. 1996;107:537–50. doi: 10.1016/s0079-6123(08)61886-5. [DOI] [PubMed] [Google Scholar]
- 76.Boxer AL, Mackenzie IR, Boeve BF, et al. Clinical, neuroimaging and neuropathological features of a new chromosome 9p-linked FTD-ALS family. Journal of neurology, neurosurgery, and psychiatry. 2011 Feb;82(2):196–203. doi: 10.1136/jnnp.2009.204081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kew JJ, Leigh PN, Playford ED, et al. Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain : a journal of neurology. 1993 Jun;116(( Pt 3):655–80. doi: 10.1093/brain/116.3.655. [DOI] [PubMed] [Google Scholar]
- 78.Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E. Cell-to-cell transmission of non-prion protein aggregates. Nature reviews Neurology. 2010 Dec;6(12):702–6. doi: 10.1038/nrneurol.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baker HF, Ridley RM, Duchen LW, Crow TJ, Bruton CJ. Induction of beta (A4)-amyloid in primates by injection of Alzheimer’s disease brain homogenate. Comparison with transmission of spongiform encephalopathy. Molecular neurobiology. 1994 Feb;8(1):25–39. doi: 10.1007/BF02778005. [DOI] [PubMed] [Google Scholar]
- 80.Kane MD, Lipinski WJ, Callahan MJ, et al. Evidence for seeding of beta -amyloid by intracerebral infusion of Alzheimer brain extracts in beta -amyloid precursor protein-transgenic mice. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000 May 15;20(10):3606–11. doi: 10.1523/JNEUROSCI.20-10-03606.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mougenot AL, Nicot S, Bencsik A, et al. Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiology of aging. 2011 Aug 1; doi: 10.1016/j.neurobiolaging.2011.06.022. [DOI] [PubMed] [Google Scholar]
- 82.Nath S, Agholme L, Kurudenkandy FR, Granseth B, Marcusson J, Hallbeck M. Spreading of neurodegenerative pathology via neuron-to-neuron transmission of beta-amyloid. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012 Jun 27;32(26):8767–77. doi: 10.1523/JNEUROSCI.0615-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chia R, Tattum MH, Jones S, Collinge J, Fisher EM, Jackson GS. Superoxide dismutase 1 and tgSOD1 mouse spinal cord seed fibrils, suggesting a propagative cell death mechanism in amyotrophic lateral sclerosis. PloS one. 2010;5(5):e10627. doi: 10.1371/journal.pone.0010627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Furukawa Y, Kaneko K, Watanabe S, Yamanaka K, Nukina N. A seeding reaction recapitulates intracellular formation of Sarkosyl-insoluble transactivation response element (TAR) DNA-binding protein-43 inclusions. The Journal of biological chemistry. 2011 May 27;286(21):18664–72. doi: 10.1074/jbc.M111.231209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fallini C, Bassell GJ, Rossoll W. The ALS disease protein TDP-43 is actively transported in motor neuron axons and regulates axon outgrowth. Human molecular genetics. 2012 Aug 15;21(16):3703–18. doi: 10.1093/hmg/dds205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dejesus-Hernandez M, Mackenzie IR, Boeve BF, et al. Expanded GGGGCC Hexanucleotide Repeat in Noncoding Region of C9ORF72 Causes Chromosome 9p-Linked FTD and ALS. Neuron. 2011 Oct 20;72(2):245–56. doi: 10.1016/j.neuron.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Renton AE, Majounie E, Waite A, et al. A Hexanucleotide Repeat Expansion in C9ORF72 Is the Cause of Chromosome 9p21-Linked ALS-FTD. Neuron. 2011 Oct 20;72(2):257–68. doi: 10.1016/j.neuron.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Byrne S, Elamin M, Bede P, et al. Cognitive and clinical characteristics of patients with amyotrophic lateral sclerosis carrying a C9orf72 repeat expansion: a population-based cohort study. Lancet neurology. 2012 Mar;11(3):232–40. doi: 10.1016/S1474-4422(12)70014-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Burden of pTDP-43 pathology in ALS with/without C9orf72 repeat expansion.
This bar plot illustrates the burden of pTDP-43 pathology in ALS with(N=11) and without (N=64) theC9orf72 repeat expansion. Bars indicate median and 95% confidence interval, significant difference in Wilcoxon Mann-Whitney Test (p<0.05) is indicated by asterisk. Abbreviations: AM – amygdala, CE – cerebellum, CG – anterior cingulate gyrus, ENT – entorhinal region, GP – pallidum, HIP – hippocampal formation, HTH – hypothalamus, IO – inferior olive, MF– middle frontal gyrus, MO – agranular motor cortex (Brodmann areas 4 and 6), OR– gyrus rectus and orbital gyri, PG – pontine gray, RF – reticular formation, RU – red nucleus, SC – spinal cord anterior horn, SEN – somatosensory cortex, SMT – superior or middle temporal gyrus, ST – striatum, TH – thalamus, VIS – visual cortex (Brodmann areas 17 and 18), XII – hypoglossal nucleus.