Abstract
Pancreatic islet transplantation represents a potential treatment for insulin-dependent diabetes mellitus. However, the precise cellular and molecular mechanisms of the immune reactions against allogeneic and xenogeneic transplanted islets remain unclear. Here, we demonstrate that CD4+ Vα14 natural killer T (NKT) cells, a recently identified lymphoid cell lineage, are required for the acceptance of intrahepatic rat islet xenografts. An anti-CD4 mAb, administrated after transplantation, allowed islet xenografts to be accepted by C57BL/6 mice, with no need for immunosuppressive drugs. The dose of anti-CD4 mAb was critical, and the beneficial effect appeared to be associated with the reappearance of CD4+ NKT cells at around 14 days after transplantation. Interestingly, rat islet xenografts were rejected, despite the anti-CD4 mAb treatment, in Vα14 NKT cell–deficient mice, which exhibit the normal complement of conventional lymphoid cells; adoptive transfer of Vα14 NKT cells into Vα14 NKT cell–deficient mice restored the acceptance of rat islet xenografts. In addition, rat islet xenografts were accepted by Vα14 NKT mice having only Vα14 NKT cells and no other lymphoid cells. These results indicate that Vα14 NKT cells play a crucial role in the acceptance of rat islet xenografts in mice treated with anti-CD4 antibody, probably by serving as immunosuppressive regulatory cells.
Introduction
It is well documented that CD4+ helper T cells can be classified into two distinct subpopulations on the basis of their cytokine production patterns and are designated as type 1 T helper (Th1) and type 2 T helper (Th2) cells (1). Th1 cells produce IL-2, IFN-γ, and TNF-β, whereas Th2 cells produce IL-4, IL-5, and IL-10. In allograft immunity, the development of Th1 cells is reported to be critical and required for the induction of cytotoxic effector T cells responsible for graft rejection (2). Thus, the inhibition of CD4 T-cell function may result in the prevention of rejection of transplanted organs or tissues. In fact, it has been reported that the administration of anti-CD4 mAb prevents rejection in various experimental transplantation systems, including those with allogeneic and those with xenogeneic grafts (3–8).
Recently, natural killer T (NKT) cells have been identified as a novel lymphoid lineage distinct from conventional T cells or NK cells. NKT cells express both invariant Vα14 NKT specific antigen receptor as well as an NK marker (NK1.1) (9–13). Vα14 NKT cells specifically recognize CD1d molecules (14–17). The function of Vα14 NKT cells appears to involve important roles in the development of autoimmune diseases, including lpr mouse model (18), diabetes mellitus (19, 20), systemic lupus erythematosus and systemic sclerosis (21), and the prevention of experimental tumor metastasis (22, 23). Thus, it appears evident that Vα14 NKT cells are involved in the regulation of the development of various diseases. In addition, NKT cells have been suggested to play an important role in bone marrow transplantation (24–26). However, the roles of NKT cells in other organ grafts, such as allogeneic and xenogeneic pancreatic islet grafts, have not been elucidated.
Here, we investigate the role of Vα14 NKT cells in islet xenotransplantation by using Vα14 NKT–deficient mice in which only Vα14 NKT cells are lacking, whereas other conventional immune systems, such as T cells, B cells, and NK cells, remain intact. The results clearly show the requirement of Vα14 NKT cells for the acceptance of rat islet xenografts.
Methods
Animals.
Lewis rats (7–10 weeks old) and C57BL/6 (B6) mice (7–10 weeks old) were purchased from Charles River Japan (Kanagawa, Japan) and used as donors and recipients, respectively. Jα281-deficient (Vα14 NKT cell–deficient [KO]) mice with a B6 background (22) were also used as recipients. In Vα14 NKT-KO mice, other lymphoid populations, such as T, B, and NK cells, remain intact (22). Vα14 NKT mice (RAG1–/–, Vα14 Tg, and Vβ8.2 Tg) with a B6 background were also used (22). This mouse line expresses only transgenic Vα14 and Vβ8.2, resulting in the preferential development of Vα14 NKT cells with no conventional T cells, B cells, and NK cells detectable. IL-4–deficient mice were originally generated by M. Kopf et al. (27). IFN-γ-KO mice (28) were provided by Y. Iwakura (Institute of Medical Science, University of Tokyo, Tokyo, Japan).
Induction of diabetes in mice.
Diabetes was induced in the recipient mice by an intravenous injection of streptozotocin (STZ; 200 mg/kg; Sigma Chemical Co., St. Louis, Missouri, USA). Plasma glucose levels were measured by a Beckman glucose analyzer (Beckman Instruments Japan, Tokyo, Japan). The plasma glucose levels exceeded 400 mg/dL by 2 or 3 days after the STZ injection, and the mice remained hyperglycemic at the time of islet transplantation.
Islet isolation and transplantation.
Islets were isolated using collagenase (29) and separated by Ficoll-Conray gradients (30). Five hundred islets were transplanted into the liver of a diabetic mouse via the portal vein at 5–8 days after STZ injection as described elsewhere (31). Nonfasting plasma glucose levels and body weight were monitored three times per week before and after transplantation. Rejection was considered to have occurred when two consecutive plasma glucose levels exceeded 200 mg/dL after transplantation.
mAb treatment.
Anti-CD4 mAb (GK1.5; rat IgG2b) (32) was administered intraperitoneally three times after transplantation on days 0, 2, and 4.
Morphological study.
For light microscopic examinations, the liver and pancreata were fixed with Bouin’s solution, processed, and embedded in paraffin. The sections were stained with hematoxylin and eosin, or aldehyde and fuchsin.
Cell preparation.
Hepatic mononuclear cells were prepared as described previously (33). The liver was excised, cut into small pieces with scissors, pressed through a stainless steel mesh, and suspended in Eagle’s minimal essential medium (MEM; Nissui Pharmaceutical Co., Tokyo, Japan) containing 5 mM HEPES (Nissui Pharmaceutical Co.) and 2% heat-inactivated bovine calf serum. Hepatic mononuclear cells were separated by Percoll gradient centrifugation. Splenocytes were obtained by forcing the spleen through a 200-gauge stainless steel mesh.
Flow cytometry analysis.
The following FITC-, biotin-, or phycoerythrin- conjugated mAb’s were purchased from PharMingen Co. (San Diego, California, USA); anti-CD3 (145-2C11), anti–IL-2 receptor β chain (TMβ1), anti-CD4 (GK1.5), anti-CD8α (53-6.7), anti-CD8β (53-5.8), anti-TCRβ (H57-597), and anti-NK1.1(PK136). The stained cells were analyzed by FACScan (Becton Dickinson Co., Mountain View, California, USA). Ten thousand live cells were collected and analyzed.
Statistical analysis.
The statistical significance of graft survival and the flow cytometry data were determined by Fisher’s exact test and Student’s t test, respectively.
Results
Prevention of rat xenograft rejection in C57BL/6 mice with anti-CD4 treatment.
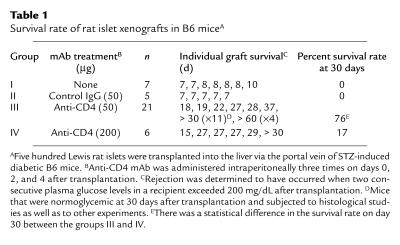
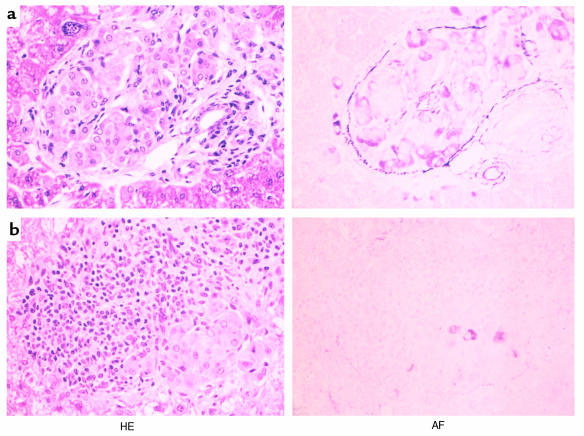
The goal of this study is to identify the role of Vα14 NKT cells in rejection and maintenance of intrahepatic rat islet xenografts. As shown in Table 1, intrahepatic islet xenografts were rejected with mean survival time of 7.6 ± 1.1 days (n = 10) without mAb treatment. Histological study at the time of rejection revealed multiple foci containing mononuclear cells and no transplanted islet grafts in the liver (data not shown). However, when transplanted mice were treated with anti-CD4 mAb (50 μg on days 0, 2, and 4), graft survival was significantly prolonged, and about 76% (16/21) of the mice were normoglycemic 30 days after transplantation (Table 1, group III). Histologically, intact islet grafts were seen in the liver of the normoglycemic mice (Figure 1a). Small foci of mononuclear cells were scattered adjacent to the islet grafts, whereas no infiltration was observed. Interestingly, however, when the dose of anti-CD4 mAb was increased to 200 μg, the prevention of rejection was less impressive, and five of six mice became hyperglycemic within 30 days after transplantation, suggesting that the islet xenografts were rejected (Table 1, group IV). As expected, a massive infiltration of mononuclear cells was seen around the islet grafts in the liver of mice in group IV. Representative results are shown in Figure 1b.
Table 1.
Survival rate of rat islet xenografts in B6 miceA
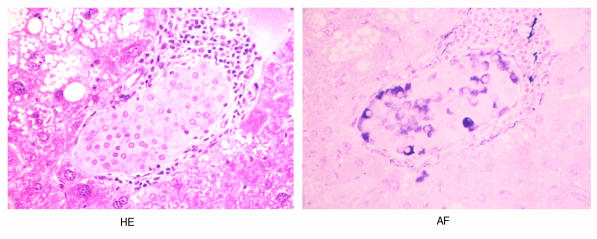
Figure 1.
Light photomicrographs of transplanted islet grafts in the liver. (a) An intact islet with well-granulated β cells observed in the liver of a mouse treated with 50 μg of anti-CD4 mAb. The sample was prepared 60 days after transplantation. (b) A representative islet xenograft in the liver of a mouse treated with 200 μg of anti-CD4 mAb. HE, hematoxylin and eosin staining; AF, aldehyde and fuchsin staining. ×160.
Appearance of CD4+ NKT cells in the liver of mice receiving anti-CD4 mAb.
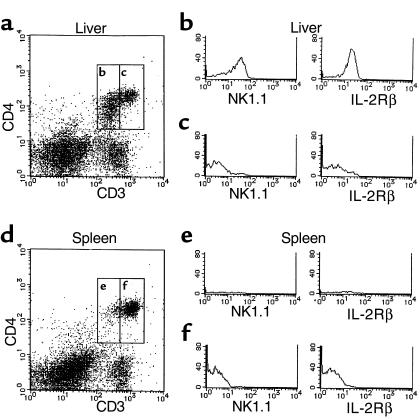
To elucidate the dosage effect of anti-CD4 mAb treatment on xenograft survival, hepatic mononuclear cells were examined by flow cytometry analysis periodically after transplantation, with particular attention paid to cells expressing CD4. Without mAb treatment, the percentage of CD3+CD4+ cells in the liver was 25.7% ± 5.9% (n = 3; data not shown). The CD3+CD4+ cells can be divided into two subpopulations with respect to the expression level of TCR/CD3, i.e., TCR/CD3-intermediate cells and TCR/CD3-bright cells. The former expresses NK1.1 and the IL-2Rβ that is characteristic of NKT cells (Figure 2). The percentages of CD4+NK1.1+ and CD4+NK1.1– were 9.7 ± 0.4% (n = 3) and 16.0 ± 3.0% (n = 3), respectively (data not shown).
Figure 2.
CD3+CD4+ cells in the liver. CD4/CD3 profiles of hepatic mononuclear cells and splenocytes of B6 mice (a and d). NK1.1 and IL-2Rβ expression on CD3intCD4+ cells (b and e) and CD3brightCD4+ (c and f).
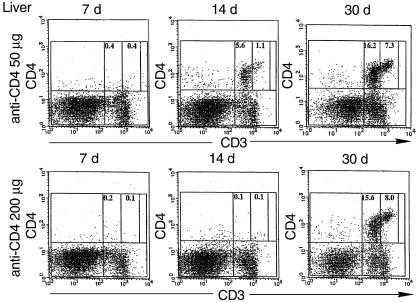
As shown in the left panels of Figure 3, the percentages of both CD4+ NKT cells and conventional CD4+ T cells decreased dramatically at around 7 days after transplantation. This suggests that treatment with anti-CD4 mAb (50 or 200 μg) depleted CD4+ cells from the liver almost completely regardless of their CD3 expression. Interestingly, however, on day 14 after transplantation, significant numbers of CD3-intermediate CD4+ cells (CD4+ NKT cells) reappeared in the liver of mice treated with 50 μg, but not with 200 μg, anti-CD4 (Figure 3, middle panels). After 30 days, both CD4+CD3int NKT cells and conventional CD4+CD3+ T cells were recovered at similar levels in both groups (Figure 3, right panels). Similar patterns of recovery were observed in the spleen (data not shown).
Figure 3.
CD4/CD3 profiles of hepatic mononuclear cells after anti-CD4 mAb treatment. Hepatic mononuclear cells were isolated from mice receiving islet isografts and anti-CD4 mAb. The percentages of CD3intCD4+ cells and CD3highCD4+ cells are shown.
Requirement of Vα14 NKT cells for the acceptance of rat islet xenografts in mice treated with anti-CD4 mAb.
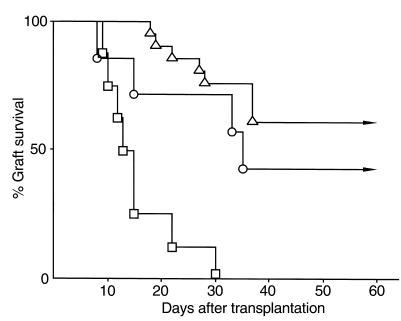
To address the role of Vα14 NKT cells in the acceptance of rat islet xenografts, Vα14 NKT-KO mice were used as recipients. As shown in Figure 4, all Vα14 NKT cell–deficient mice (n = 8) rejected the xenografts within 30 days after transplantation, even at a dose of the 50 μg anti-CD4 mAb. Under the same conditions, 16 of 21 wild-type mice accepted the grafts (triangles in Figure 4).
Figure 4.
The survival of rat islet xenografts in Vα14 NKT-KO mice treated with anti-CD4 mAb. Rat islet xenografts were transplanted into the livers of STZ-induced diabetic Vα14 NKT-KO mice (squares; n = 8) or wild-type littermate mice (triangles; n = 21), and the transplanted mice were treated with 50 μg of anti-CD4 mAb on days 0, 2, and 4. The difference in graft survival between these two experimental groups was statistically significant (P < 0.01). Vα14 NKT-KO mice received 2 × 107 splenocytes from Vα14 NKT mice via the portal vein at the time of islet transplantation, and then the mice were treated with 50 μg of anti-CD4 mAb (circles; n = 7).
Rescue of the acceptance of islet xenografts by the adoptive transfer of Vα14 NKT cells.
The role of Vα14 NKT cells in the acceptance of rat islet xenografts was further investigated by cell transfer experiments. Splenocytes from Vα14 NKT mice were transferred into STZ-induced diabetic Vα14 NKT-KO mice via the portal vein at the time of islet transplantation. The mice were treated with 50 μg of anti-CD4 mAb (on days 0, 2, and 4). The survival rate of islet xenografts in mice receiving 2 × 107 splenocytes from Vα14 NKT mice was significantly increased and was 71% (5/7) 30 days after transplantation (Figure 4). Three of seven mice receiving 2 × 107 splenocytes were normoglycemic for more than 60 days after islet transplantation (Figure 4). Similar to wild-type mice, well-granulated β cells and accumulations of mononuclear cells adjacent to the grafts were observed in the liver of normoglycemic recipients (Figure 5). These results suggest that Vα14 NKT cells are essential for the acceptance of rat islet xenografts in mice treated with anti-CD4 mAb.
Figure 5.
Histological analysis of rat islet xenografts in the livers of Vα14 NKT-KO mice receiving transferred Vα14 NKT cells. Vα14 NKT-KO mice receiving Vα14 NKT cell transfers as described for Figure 4 were normoglycemic for more than 60 days. A representative islet graft in the liver is shown. ×160.
Regulatory role of Va14 NKT cells in islet xenograft transplantation.
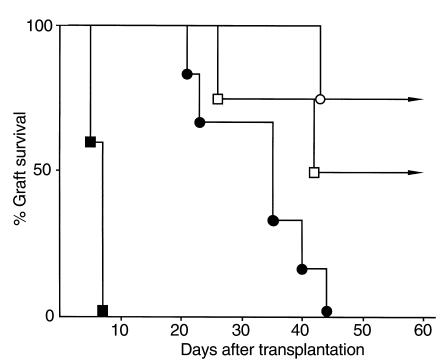
Vα14 NKT cells are known to produce both IL-4 and IFN-γ after TCR cross-linking (34–37), and therefore IL-4-KO and IFN-γ-KO mice were used as recipients to address whether these cytokines are involved in the process leading to the acceptance of islet xenografts. As shown in Figure 6, IL-4-KO mice rejected islet xenografts within 10 days after transplantation if anti-CD4 mAb was not administrated (closed squares; mean survival time is 6.2 ± 1.1 days; n = 5). The survival rate was significantly increased by treatment with anti-CD4 mAb (open squares) to 75% (3/4) after 30 days, a result similar to that seen in wild-type mice (see Table 1). This result suggests that IL-4 is not involved in the process of Vα14 NKT cell–mediated acceptance of islet xenografts. In contrast, a significant prolongation of islet xenograft survival was seen in IFN-γ–deficient mice even without anti-CD4 treatment (Figure 6, closed circles). The mean survival time was 33.0 ± 9.2 days (n = 6). However, it was also noted that all the grafts in IFN-γ-KO mice were rejected by 44 days. When IFN-γ-KO mice were treated with anti-CD4 mAb, the survival rate was 100% on day 30 and 75% on day 60 after transplantation (Figure 6, open circles). This result suggests that IFN-γ is involved in the process of xenograft rejection, but not in the process of Vα14 NKT cell–mediated suppression of xenograft rejection.
Figure 6.
Survival of rat islet xenografts in IL-4-KO and IFN-γ-KO mice treated with anti-CD4 mAb. IL-4-KO mice treated with (open squares; n = 4) or without (filled squares; n = 5) anti-CD4 mAb (50 μg), and IFN-γ-KO mice treated with (open circles; n = 4) or without (filled circles; n = 6) anti-CD4 (50 μg) were used as recipients.
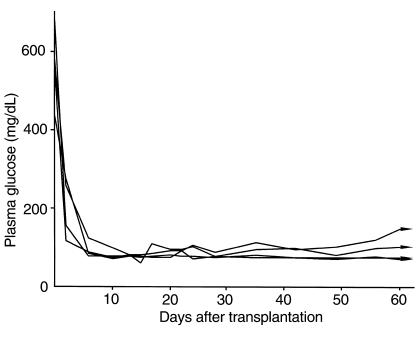
Finally, we used Vα14 NKT mice as recipients to determine whether Vα14 NKT cells serve as effector cells for the rejection of islet xenografts. As reported previously, only Vα14 NKT cells develop in Vα14 NKT mice in which essentially no conventional T cells were detected (22). Figure 7 shows the plasma glucose levels of Vα14 NKT mice with islet xenografts; all islet xenografts (n = 4) were accepted even without anti-CD4 treatment, and the mice were normoglycemic more than 60 days after transplantation. These results suggest that Vα14 NKT cells do not serve as effector cells in the rejection of islet xenografts.
Figure 7.
Plasma glucose levels of STZ-induced diabetic Vα14 NKT mice receiving rat islet xenografts without anti-CD4 mAb treatment. The individual lines represent the nonfasting plasma glucose levels of each animal after transplantation (n = 4).
Discussion
It has been reported that the rejection of islet allografts is dependent on both CD4+ and CD8+ T cells, as evidenced by the fact that a single treatment with either anti-CD4 (38) or anti-CD8 (39) mAb is sufficient to prevent graft rejection. In the allotransplantation experimental system, CD4 T cells are thought to activate and induce CD8 effector T cells, and treatment with mAb specific for either CD4 or CD8 may interfere with the processes of activation and/or the function of CD8 cytotoxic T cells. In contrast, the immune responses leading to islet xenograft rejection appear to be CD8 T cell–independent based on the findings that anti-CD8 treatment fails to prevent islet xenograft rejection (40) and that the rejection of islet xenografts is observed in CD8 T cell–deficient mice (41). Several studies have demonstrated that the administration of anti-CD4 mAb induces various levels of prolongation of xenograft survival in the case of rat islets transplanted into mouse (39, 42, 43) and that of pig islets transplanted into mouse (44), suggesting that the rejection of xenografts is CD4 T-cell dependent.
In the present study, we confirmed that rat islet xenografts were accepted in mice receiving anti-CD4 mAb. Interestingly, it was also noted that the dose of anti-CD4 mAb appeared to be critical. Treatment with low doses of anti-CD4 mAb (50 μg) showed potent inhibition of xenograft rejection, whereas a high anti-CD4 dose (200 μg) had a significantly diminished inhibitory effect (Table 1). To understand this paradox, careful flow cytometry analyses of hepatic mononuclear cells from mice receiving anti-CD4 treatment were performed. The appearance of CD3int CD4+ NKT cells was found to be correlated with the beneficial effects (Figure 3). The behavior of conventional CD3+CD4+ T cells, another anti-CD4–sensitive cell type in the liver, remained unchanged with either of these two treatments. From these results, we hypothesized that CD4+ NKT cells play an important regulatory role in immune responses leading to xenograft rejection, and a set of experiments with Vα14 NKT-KO mice was performed. We found that Vα14 NKT cells are crucial for the acceptance of islet xenografts in the liver of mice treated with anti-CD4 mAb. Vα14 NKT cells are likely to serve as regulatory cells but not effector cells, because Vα14 NKT mice, which have only Vα14 NKT cells without conventional lymphocytes, did not reject islet xenografts.
Although it is not clear why CD4+ NKT cells reappeared more quickly than conventional CD4+ T cells in the liver (Figure 3), one possible explanation is that NKT cells are more resistant to apoptosis. Several investigators reported that NKT cells are more resistant to radiation- and glucocorticoid-induced apoptotic cell death (45, 46), perhaps because of high expression levels of Bcl-2 (45). Although this unique feature of NKT cells could explain the differences in susceptibility to anti–CD4-mAb–mediated cell death, this seems unlikely because almost all CD4+ NKT cells and conventional CD4+ T cells were depleted in the liver 7 days after transplantation (Figure 3). Another possibility is that NKT cells regenerate more quickly than CD4+ T cells. This possibility is consistent with our observation (Figure 3).
It has been well documented that NKT cells produce large amounts of both IL-4 and IFN-γ upon activation (34–37). Given that IL-4 and IFN-γ have opposite effects on the development of Th1 and Th2 cells, extensive analyses with various experimental systems have been performed, and conflicting results have been reported (47–52). More recently, using α-galactosylceramide, a ligand for the Vα14 NKT cell receptor (53), the regulatory role of Vα14 NKT cells in Th1/Th2 cell development has been addressed. Again, evidence suggesting both the IFN-γ–mediated inhibition of Th2 responses and IL-4–induced Th2-cell induction have been reported (54–56). In addition, an IL-4–induced augmentation of NKT cell–mediated cytotoxic function has been noted (57). In any event, it is conceivable that either IL-4 or IFN-γ is involved in the process of the Vα14 NKT cell–mediated inhibition of xenograft rejection. However, the results from IL-4-KO mice and IFN-γ-KO mice suggest little involvement of these two cytokines (Figure 6). Our preliminary results suggest that activated Vα14 NKT cells with α-galactosylceramide produce other inhibitory cytokines such as TNF-α, TGF-β, IL-6, lymphotoxin, and IL-10 (data not shown). However, the effector molecules of the Vα14 NKT cell–mediated inhibition of xenograft rejection have not yet identified. Cytokine production profiles of Vα14 NKT cells in the liver of mice undergoing xenotransplantation and anti-CD4 treatment are worth determining.
In IFN-γ-KO mice, a prolongation of graft survival was observed even without anti-CD4 treatment (Figure 6). This finding suggests that IFN-γ may play a certain role at the effector phase, probably on other cell populations such as cytotoxic T cells rather than the regulatory Vα14 NKT cells. The IFN-γ required for the rejection of xenografts may be produced by activated CD4+ T cells or NKT cells. However, a set of cell transfer experiments is required to address this issue.
Recently, Eberl et al. revealed that NKT cells can be divided into the two subpopulations depending on the reactivity of NKT cells toward the monomorphic MHC class I–like molecule CD1d (58). It has also been suggested that the percentages of these two NKT cells differ among organs. In the present study, we addressed only Vα14 NKT cells, which belong to the cell type with a CD1d restriction. It is conceivable that the molecular requirement for activation, subsequent production of cytokines, and resulting regulatory effects are distinct between these two NKT cell subpopulations. Thus, an analysis focusing on the function of each NKT cell subpopulation would be of interest and helpful for understanding the precise molecular mechanisms operating in the immune responses against xenografts.
In summary, the present study provides evidence suggesting that CD4+ Vα14 NKT cells are essential for the acceptance of islet xenografts in mice treated with anti-CD4 mAb. These results may provide new insights for understanding transplantation immunobiology and are helpful for the establishment of a novel treatment for diabetes involving islet transplantation in humans.
Acknowledgments
This work was supported by a Grant-in-Aid for Highly Advanced Medical Technology and a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and by funds from the Central Research Institute of Fukuoka University.
References
- 1.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 2.Chandler C, Passaro E., Jr Transplant rejection. Mechanisms and treatment. Arch Surg. 1993;128:279–283. doi: 10.1001/archsurg.1993.01420150033006. [DOI] [PubMed] [Google Scholar]
- 3.Sablinski T, Hancock WW, Tilney NL, Kupiec-Weglinski JW. CD4 monoclonal antibodies in organ transplantation: a review of progress. Transplantation. 1991;52:579–589. doi: 10.1097/00007890-199110000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Cobbold SP, Adams E, Marshall SE, Davies JD, Waldmann H. Mechanisms of peripheral tolerance and suppression induced by monoclonal antibodies to CD4 and CD8. Immunol Rev. 1996;149:5–33. doi: 10.1111/j.1600-065x.1996.tb00897.x. [DOI] [PubMed] [Google Scholar]
- 5.Benjamin RJ, Waldmann H. Induction of tolerance by monoclonal antibody therapy. Nature. 1986;320:449–451. doi: 10.1038/320449a0. [DOI] [PubMed] [Google Scholar]
- 6.Carteron NL, Wofsy D, Seaman WE. Induction of immune tolerance during administration of monoclonal antibody to L3T4 does not depend on depletion of L3T4+ cells. J Immunol. 1988;140:713–716. [PubMed] [Google Scholar]
- 7.Darby CR, Morris PJ, Wood KJ. Evidence that long-term cardiac allograft survival induced by anti-CD4 monoclonal antibody does not require depletion of CD4+ T cells. Transplantation. 1992;54:483–490. doi: 10.1097/00007890-199209000-00019. [DOI] [PubMed] [Google Scholar]
- 8.Darby CR, Bushell A, Morris PJ, Wood KJ. Nondepleting anti-CD4 antibodies in transplantation. Evidence that modulation is far less effective than prolonged CD4 blockade. Transplantation. 1994;57:1419–1426. [PubMed] [Google Scholar]
- 9.Imai K, et al. Sequence and expression of transcripts of the T-cell antigen receptor α-chain gene in a functional, antigen-specific suppressor-T-cell hybridoma. Proc Natl Acad Sci USA. 1986;83:8708–8712. doi: 10.1073/pnas.83.22.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makino Y, Kanno R, Ito T, Higashino K, Taniguchi M. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int Immunol. 1995;7:1157–1161. doi: 10.1093/intimm/7.7.1157. [DOI] [PubMed] [Google Scholar]
- 11.MacDonald HR. NK1.1+ T cell receptor-α/β+ cells: new clues to their origin, specificity, and function. J Exp Med. 1995;182:633–638. doi: 10.1084/jem.182.3.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taniguchi M, et al. Vα14+ NK T cells: a novel lymphoid cell lineage with regulatory function. J Allergy Clin Immunol. 1996;98(Suppl. 2):S263–S269. doi: 10.1016/s0091-6749(96)70074-x. [DOI] [PubMed] [Google Scholar]
- 13.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 14.Bendelac A, et al. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–865. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 15.Brossay L, et al. CD1d-mediated recognition of an α-galactosylceramide by natural killer T cells is highly conserved through mammalian evolution. J Exp Med. 1998;188:1521–1528. doi: 10.1084/jem.188.8.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spada FM, Koezuka Y, Porcelli SA. CD1d-restricted recognition of synthetic glycolipid antigens by human natural killer T cells. J Exp Med. 1998;188:1529–1534. doi: 10.1084/jem.188.8.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawano T, et al. A novel recognition motif of human NKT antigen receptor for a glycolipid ligand. Int Immunol. 1999;11:881–887. doi: 10.1093/intimm/11.6.881. [DOI] [PubMed] [Google Scholar]
- 18.Mieza MA, et al. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J Immunol. 1996;156:4035–4040. [PubMed] [Google Scholar]
- 19.Baxter AG, Kinder SJ, Hammond KJ, Scollay R, Godfrey DI. Association between αβTCR+CD4-CD8– T-cell deficiency and IDDM in NOD/Lt mice. Diabetes. 1997;46:572–582. doi: 10.2337/diab.46.4.572. [DOI] [PubMed] [Google Scholar]
- 20.Wilson SB, et al. Extreme Th1 bias of invariant Vα24JαQ T cells in type 1 diabetes [erratum 1999, 399:84] Nature. 1998;391:177–181. doi: 10.1038/34419. [DOI] [PubMed] [Google Scholar]
- 21.Sumida T, et al. Selective reduction of T cells bearing invariant Vα24JαQ antigen receptor in patients with systemic sclerosis. J Exp Med. 1995;182:1163–1168. doi: 10.1084/jem.182.4.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cui J, et al. Requirement for Vα14 NKT cells in IL-12–mediated rejection of tumors. Science. 1997;278:1623–1626. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 23.Kawano T, et al. Natural killer–like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci USA. 1998;95:5690–5693. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yankelevich B, Knobloch C, Nowicki M, Dennert G. A novel cell type responsible for marrow graft rejection in mice. T cells with NK phenotype cause acute rejection of marrow grafts. J Immunol. 1989;142:3423–3430. [PubMed] [Google Scholar]
- 25.Dennert G, Knobloch C, Sugawara S, Yankelevich B. Evidence for differentiation of NK1+ cells into cytotoxic T cells during acute rejection of allogeneic bone marrow grafts. Immunogenetics. 1990;31:161–168. doi: 10.1007/BF00211551. [DOI] [PubMed] [Google Scholar]
- 26.Kikly K, Dennert G. Evidence for extrathymic development of TNK cells. NK1+ CD3+ cells responsible for acute marrow graft rejection are present in thymus-deficient mice. J Immunol. 1992;149:403–412. [PubMed] [Google Scholar]
- 27.Kopf M, et al. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature. 1993;362:245–248. doi: 10.1038/362245a0. [DOI] [PubMed] [Google Scholar]
- 28.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ–/– mice, but not in TNF-α–/– mice: role for IFN-γ in activating apoptosis of hepatocytes. J Immunol. 1997;159:1418–1428. [PubMed] [Google Scholar]
- 29.Sutton R, Peters M, McShane P, Gray DW, Morris PJ. Isolation of rat pancreatic islets by ductal injection of collagenase [erratum 1987, 43:608] Transplantation. 1986;42:689–691. doi: 10.1097/00007890-198612000-00022. [DOI] [PubMed] [Google Scholar]
- 30.Okeda T, Ono J, Takaki R, Todo S. Simple method for the collection of pancreatic islets by the use of Ficoll-Conray gradient. Endocrinol Jpn. 1979;26:495–499. doi: 10.1507/endocrj1954.26.495. [DOI] [PubMed] [Google Scholar]
- 31.Kemp CB, Knight MJ, Scharp DW, Lacy PE, Ballinger WF. Transplantation of isolated pancreatic islets into the portal vein of diabetic rats. Nature. 1973;244:447. doi: 10.1038/244447a0. [DOI] [PubMed] [Google Scholar]
- 32.Dialynas DP, et al. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe H, et al. Details of an isolation method for hepatic lymphocytes in mice. J Immunol Methods. 1992;146:145–154. doi: 10.1016/0022-1759(92)90223-g. [DOI] [PubMed] [Google Scholar]
- 34.Zlotnik A, Godfrey DI, Fischer M, Suda T. Cytokine production by mature and immature CD4-CD8- T cells. αβ-T cell receptor+ CD4-CD8– T cells produce IL-4. J Immunol. 1992;149:1211–1215. [PubMed] [Google Scholar]
- 35.Bendelac A, Schwartz RH. CD4+ and CD8+ T cells acquire specific lymphokine secretion potentials during thymic maturation. Nature. 1991;353:68–71. doi: 10.1038/353068a0. [DOI] [PubMed] [Google Scholar]
- 36.Yoshimoto T, Paul WE. CD4pos, NK1.1pos T cells promptly produce interleukin 4 in response to in vivo challenge with anti-CD3. J Exp Med. 1994;179:1285–1295. doi: 10.1084/jem.179.4.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arase H, Arase N, Nakagawa K, Good RA, Onoe K. NK1.1+ CD4+ CD8- thymocytes with specific lymphokine secretion. Eur J Immunol. 1993;23:307–310. doi: 10.1002/eji.1830230151. [DOI] [PubMed] [Google Scholar]
- 38.Shizuru JA, Gregory AK, Chao CT, Fathman CG. Islet allograft survival after a single course of treatment of recipient with antibody to L3T4. Science. 1987;237:278–280. doi: 10.1126/science.2955518. [DOI] [PubMed] [Google Scholar]
- 39.Wolf LA, Coulombe M, Gill RG. Donor antigen-presenting cell-independent rejection of islet xenografts. Transplantation. 1995;60:1164–1170. doi: 10.1097/00007890-199511270-00018. [DOI] [PubMed] [Google Scholar]
- 40.Gill RG, Coulombe M. Rejection of pancreatic islet xenografts does not require CD8+ T-lymphocytes. Transplant Proc. 1992;24:2877–2878. [PubMed] [Google Scholar]
- 41.Desai NM, et al. Islet allograft, islet xenograft, and skin allograft survival in CD8+ T lymphocyte-deficient mice. Transplantation. 1993;55:718–722. doi: 10.1097/00007890-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman DS, Kong CS, Shizuru JA, Gregory AK, Fathman CG. Use of anti-L3T4 and anti-Ia treatments for prolongation of xenogeneic islet transplants. Transplantation. 1988;46:210–215. doi: 10.1097/00007890-198808000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Lacy PE, Ricordi C, Finke EH. Effect of transplantation site and aL3T4 treatment on survival of rat, hamster, and rabbit islet xenografts in mice. Transplantation. 1989;47:761–766. doi: 10.1097/00007890-198905000-00002. [DOI] [PubMed] [Google Scholar]
- 44.Simeonovic CJ, Ceredig R, Wilson JD. Effect of GK1.5 monoclonal antibody dosage on survival of pig proislet xenografts in CD4+ T cell–depleted mice. Transplantation. 1990;49:849–856. doi: 10.1097/00007890-199005000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Tamada K, Harada M, Abe K, Li T, Nomoto K. IL-4-producing NK1.1+ T cells are resistant to glucocorticoid-induced apoptosis: implications for the Th1/Th2 balance. J Immunol. 1998;161:1239–1247. [PubMed] [Google Scholar]
- 46.Kimura M, et al. Radioresistance of intermediate TCR cells and their localization in the body of mice revealed by irradiation. Microbiol Immunol. 1993;37:641–652. doi: 10.1111/j.1348-0421.1993.tb01687.x. [DOI] [PubMed] [Google Scholar]
- 47.Guery JC, Galbiati F, Smiroldo S, Adorini L. Selective development of T helper (Th) 2 cells induced by continuous administration of low dose soluble proteins to normal and β(2)-microglobulin-deficient BALB/c mice. J Exp Med. 1996;183:485–497. doi: 10.1084/jem.183.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yoshimoto T, Bendelac A, Watson C, Hu-Li J, Paul WE. Role of NK1.1+ T cells in a TH2 response and in immunoglobulin E production. Science. 1995;270:1845–1847. doi: 10.1126/science.270.5243.1845. [DOI] [PubMed] [Google Scholar]
- 49.Yoshimoto T, Bendelac A, Hu-Li J, Paul WE. Defective IgE production by SJL mice is linked to the absence of CD4+, NK1.1+ T cells that promptly produce interleukin 4. Proc Natl Acad Sci USA. 1995;92:11931–11934. doi: 10.1073/pnas.92.25.11931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4–secreting CD1-dependent cells. Science. 1997;275:977–979. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 51.Brown DR, et al. β2-microglobulin–dependent NK1.1+ T cells are not essential for T helper cell 2 immune responses. J Exp Med. 1996;184:1295–1304. doi: 10.1084/jem.184.4.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang BW, et al. T cell responses in calcineurin A α–deficient mice. J Exp Med. 1996;183:413–420. doi: 10.1084/jem.183.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawano T, et al. CD1d-restricted and TCR-mediated activation of Va14 NKT cells by glycosylceramides. Science. 1997;278:1626–1629. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 54.Cui J, et al. Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Vα14 natural killer T cells. J Exp Med. 1999;190:783–792. doi: 10.1084/jem.190.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burdin N, Brossay L, Kronenberg M. Immunization with α-galactosylceramide polarizes CD1-reactive NK T cells towards Th2 cytokine synthesis. Eur J Immunol. 1999;29:2014–2025. doi: 10.1002/(SICI)1521-4141(199906)29:06<2014::AID-IMMU2014>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 56.Singh N, et al. Cutting edge: activation of NK T cells by CD1d and α- galactosylceramide directs conventional T cells to the acquisition of a Th2 phenotype. J Immunol. 1999;163:2373–2377. [PubMed] [Google Scholar]
- 57.Kaneko Y, et al. Augmentation of Vα14 NKT cell–mediated cytotoxicity by interleukin 4 in an autocrine mechanism resulting in the development of concanavalin A–induced hepatitis. J Exp Med. 2000;191:105–114. doi: 10.1084/jem.191.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Eberl G, et al. Tissue-specific segregation of CD1d-dependent and CD1d-independent NK T cells. J Immunol. 1999;162:6410–6419. [PubMed] [Google Scholar]