CLINICAL VIGNETTE
A 3-year-old boy presents with a history of difficult-to-treat atopic dermatitis (AD). He has had an eczematous rash “since birth.” The rash initially involved his cheeks and the extensor aspects of the extremities, but more recently, it has also involved the antecubitals, wrists, fingers, lower posterior trunk, popliteals, and ankles. He has had increased itching and has not been sleeping well. In the past, the eczema had responded to topical corticosteroids. He has also been treated with a moisturizing lotion and cetirizine once daily. More recently, he was treated with oral antibiotics twice for suspected skin infections. His parents are concerned that food allergy might be the cause of the child’s AD. Recently, his pediatrician measured his serum IgE level, which was 3176 IU/mL, and also sent off sIgE measurements to a panel of common food allergens. The parents state that these were “all positive.” The parents have struggled to eliminate all of the foods reported as positive, but the child’s AD continues to flare.
The patient has wheezed with several viral upper respiratory tract infections and, more recently, when he slept over at a friend’s home, where a cat was present. There are no furred animals in the home, and the child is not in day care. His mother has a history of childhood asthma, and his older brother has hay fever.
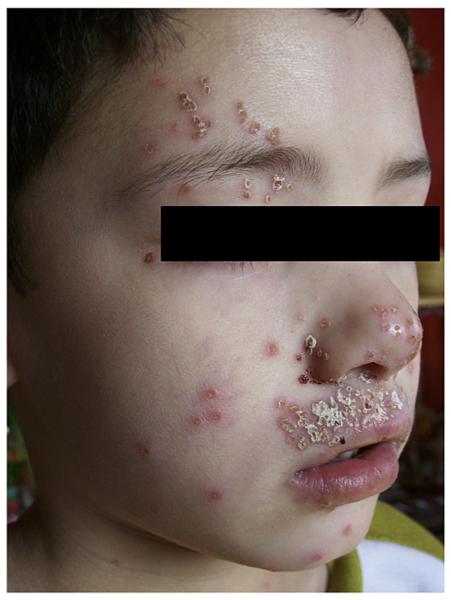
On examination, the patient was uncomfortable and scratched continuously when undressed. He had excoriated, lichenified eczematous lesions involving his lower back, antecubitals, wrists, popliteals, posterior thighs, and ankles. He had scaling of the anterior distal lower extremities and hyperlinear palms. He had no pustular or vesicular lesions, although multiple individual punched-out crusted papules were noted on his face (see Fig E1 in this article’s Online Repository at www.jacionline.org). His hair and nails were normal. He had shotty, nontender cervical and inguinal nodes bilaterally. The remainder of his examination was unremarkable.
FIG E1.
Child with ADEH.
Because of poorly controlled AD not responding to outpatient therapy and complicated by cutaneous infection, this child was admitted to our multidisciplinary eczema day unit (described in Boguniewicz et alE1). Swabs of excoriated lesions were sent for culture and sensitivity, and PCR testing was performed on punched-out lesions. While awaiting results, the patient was started on oral acyclovir. Skin cultures showed a few areas of methicillin-sensitive Staphylococcus aureus, and PCR was positive for herpes simplex virus (HSV). He was started on a nurse-supervised skin care regimen with soaking baths in warm water for 10 minutes twice daily with the assistance of a child life therapist to distract him. A midpotency topical steroid ointment was applied to eczema lesions on the trunk and the extremities twice daily, and moisturizing cream was applied to clear areas. A sedating medication was added at bedtime. Wet wraps were applied to the most severe eczema of the extremities at bedtime for 4 days and then discontinued. Behavioral modification for itch control was instituted by a pediatric psychologist, and parents received hands-on education from the nursing staff. The patient had significant clearing of skin lesions and a dramatic decrease in pruritus and scratching. The parents were provided with a written stepped-care plan for home management.
The full version of this article, including a review of relevant issues to be considered, can be found online at www.jacionline.org. If you wish to receive CME or MOC credit for the article, please see the instructions above.
REVIEW
High prevalence rates of AD have been observed in a number of countries, with data from more than a million children in 97 countries showing that AD is a major problem in developing, as well as developed, countries.E1,E2 Pruritus is a key symptom and, together with sleep disturbance, significantly affects the quality of life of patients and their families.
Skin barrier and immune abnormalities
A number of investigations into the pathogenesis of AD have pointed to both immune dysregulation and epidermal barrier abnormalities.E3 The complexity of the skin barrier was recently reviewed with a physical barrier, including the stratum corneum and tight junctions; a microbiome barrier of colonizing organisms; a chemical barrier of antimicrobial peptides; and an immunologic barrier.E4 Mutations in the gene encoding the epidermal barrier protein filaggrin (FLG) have been shown to be strongly associated with the risk for AD.E5 Not only is filaggrin a major structural component of the stratum corneum, but also amino acids from filaggrin breakdown products contribute to skin hydration and affect skin pH. In turn, the latter can influence the activity of endogenous and exogenous proteases, as well as microbial colonization.E6 Importantly, patients with FLG mutations have been shown to have earlier onset and more severe and persistent AD and are more likely to wheeze and have allergic sensitization. Also, they have been shown to have an increased risk for eczema herpeticum.
Although FLG mutations are the most important risk factor for AD and occur in approximately 50% of patients with severe AD,E6 not all patients with AD have FLG mutations, and even those with mutations can go into remission. Indeed, the majority of patients with AD will have filaggrin deficiency caused by cytokine dysregulation. In fact, filaggrin expression has been shown to be downregulated by TH2 and TH22 cytokines.E6
Susceptibility of patients with AD to microbial infection or colonization
Patients with AD have an increased susceptibility to infection or colonization with a variety of organisms. These include infections with HSV and S aureus. Of note, patients with AD in whom eczema herpeticum develops (ADEH) have more severe TH2-polarized disease with greater allergen sensitization. They are also much more likely to experience cutaneous infections with S aureus. Patients with ADEH have also been shown to have reduced IFN-γ production, and IFNGR1 variants are significantly associated with ADEH.E7
Therapeutic implications of research insights
Key observations with important therapeutic implications are that normal-appearing skin of patients with AD contains infiltrating lymphocytes and that these patients manifest skin barrier abnormalities in nonlesional skin.E8 These observations provide the rationale for maintenance or proactive therapy in patients with AD. Of note, increased binding of S aureus to AD skin is related to underlying skin inflammation, and anti-inflammatory treatment with both topical steroids and calcineurin inhibitors reduces S aureus colonization.E9 In patients with a relapsing course, after a period of stabilization, proactive therapy with either a topical steroid or calcineurin inhibitor improves disease control and reduces the number of flares.E10
More recent insights into the pathophysiology of AD with therapeutic implications include those addressing mechanisms of disease onset and persistence. Gittler et alE11 investigated intra-personal sets of transcriptomes from nonlesional skin and acute and chronic lesions of patients with AD through genomic, molecular, and cellular profiling. Onset of acute lesions was associated with an increase in a subset of terminal differentiation proteins and significant increases in gene expression levels of TH22 and TH2 cytokines. Further significant intensification of TH22 and TH2 cytokines was observed between acute and chronic lesions. In addition, AD lesions tend to recur in the same locations after treatment, suggesting that residual cellular and molecular alterations might predispose to new lesions.E12 These findings will need to be addressed as novel targeted and pharmacogenomics-based therapies are developed for AD.
Fundamentals of therapy in patients with severe AD
In approaching the management of patients with severe AD, clinicians need to review the fundamentals of therapy before considering the patient a treatment failure and prescribing systemic immunosuppressive treatments.E13 This includes assessment of irritants, allergens, infectious agents, and emotional stressors. Negative skin test results with proper controls have a high predictive value for ruling out suspected allergens, whereas positive skin test results have a lower correlation with clinical symptoms in patients with suspected food allergen–induced AD.E14 Specific IgE to food allergens measured by using the ImmunoCAP assay (Phadia, Uppsala, Sweden) can be helpful in determining the probability of a clinical reaction for a limited number of foods, although the levels do not identify the type or severity of reaction. Extensive elimination diets are almost never warranted. In a study of children predominantly with AD evaluated for food allergy, 89% of oral food challenge results were found to be negative.E15 In this cohort milk, egg, peanut, soy, wheat, tree nuts, and shellfish accounted for the majority of clinically relevant food allergies. The 95% predictive decision points for food-specific IgE were useful for milk, egg, and peanut.
Because patients with AD have increased transepidermal water loss, reduced water-binding capacity, and decreased ceramide levels in their skin, hydration through soaking in warm water for approximately 10 minutes combined with an occlusive agent to retain the absorbed water is an essential component of therapy. Bathing also removes allergens and reduces colonization by S aureus.
The practice parameter update reaffirmed the fundamental anti-inflammatory and antipruritic role of topical steroids in patients with AD, with higher-potency steroids reserved for acute exacerbations of AD.E13 Of note, patients or caregivers often delay treatment with these medications during flares of AD. Thus clinicians should use a “what, when, and where” approach to reviewing skin care. Inadequate prescription size is another contributing factor for suboptimally controlled AD.E1 Written instructions using a stepped-care approach are critical to improving outcomes (an example of such a plan is provided in Boguniewicz et alE1). Of note, a limited number of topical steroids are approved for children less than 2 years of age, and even those are labeled for use up to 4 weeks. For the most part, systemic steroids, including oral prednisone, should be avoided in the management of a chronic relapsing disorder, such as AD. Although patients or caregivers often demand immediate relief and find systemic steroids more convenient to use than topical therapy, the dramatic improvement observed with their use is often followed by an equally dramatic flaring of AD after discontinuation, leading to a vicious cycle or erythrodermic rebound.
Topical calcineurin inhibitors (TCIs) as nonsteroidal agents for the treatment of AD have proved effective with a good safety profile, even with long-term administration. Because use of TCIs is not associated with skin atrophy, they are particularly useful for treatment of eczema involving the face, axillae, or groin or on skin with atrophic changes. Ongoing surveillance has not shown a trend for increased frequency of infections or problems with response to childhood vaccinations. Currently, tacrolimus ointment 0.03% is approved for intermittent treatment of children aged 2 years and older with moderate-to-severe AD, tacrolimus ointment 0.1% is approved for intermittent treatment of adults with moderate-to-severe AD, and pimecrolimus cream 1% is approved for intermittent treatment of patients aged 2 years and older with mild-to-moderate AD. Although there is no evidence of a causal link of cancer and the use of TCIs, the US Food and Drug Administration has issued a “boxed” warning for TCIs because of a lack of long-term safety data. The labeling states that these drugs are recommended as second-line treatments and that their use in children less than 2 years of age is currently not recommended. Proactive therapy with tacrolimus ointment in both adults and children with AD, similarly to topical steroids, has been shown to be more effective than a reactive approach.E10 Although this would be considered off-label therapy in the United States, in Europe proactive therapy with tacrolimus ointment has been approved for use in children 2 years or older with AD for up to 12 months.
Addressing the itch-scratch cycle in patients with AD is a key part of successful management because pruritus is typically the least tolerated symptom of AD. The cause of pruritus in patients with AD is complex, with a number of mediators other than histamine, including neuropeptides and cytokines, involved. Systemic antihistamines and anxiolytics remain useful for many patients through their tranquilizing and sedative effects and should be used primarily at bedtime. If nocturnal pruritus remains severe, short-term use of a sedative to allow adequate rest might be appropriate. Behavioral modification and biofeedback therapy have also been useful as adjunctive therapy.
Although patients with AD are typically colonized by S aureus and often secondarily infected, a number of therapies directed at healing the skin barrier, including hydration and use of anti-inflammatory therapies, can reduce bacterial load. Systemic antibiotic therapy might be necessary to treat overt infections. Antibiotic sensitivities from culture should be obtained to direct therapy, especially with the increase in colonization or infection by methicillin-resistant S aureus.E16 Bleach baths with dilute sodium hypochlorite (¼-½ cup of household bleach per full tub of water) can be considered for patients with recurrent skin infections, especially with methicillin-resistant S aureus, but might cause skin irritation, with pruritus and scratching leading to further skin barrier damage.E13 Rinsing off afterward might remove irritating chemical residue from the skin.
HSV infection is often missed in patients with AD, especially if lesions become impetiginized. HSV can be diagnosed either by doing a Tzanck smear of cells scraped from the base of a freshly unroofed vesicle, viral PCR, or culture. Patients with disseminated HSV (ADEH) require treatment with systemic acyclovir or related antiviral agents, and if they appear toxic, they should be hospitalized with intravenous therapy. Periocular lesions should be evaluated by an ophthalmologist. Daily prophylactic oral acyclovir can be considered in patients with AD with recurrent HSV infections.
In patients with severe recalcitrant AD, selective use of wet-wrap therapy in conjunction with skin hydration and topical steroids can break their vicious cycle of itch-scratch-relapse (see Boguniewicz et alE1). Wet-wrap dressings reduce pruritus and inflammation, act as a barrier to trauma associated with scratching, and improve penetration of topical steroids. In addition, wet-wrap therapy has been shown to improve epidermal barrier recovery, which persists even after wrap therapy is discontinued.E17 Wet-wrap therapy should be reserved for difficult to manage AD and should not be overused because it can result in secondary infection. Of note, the package inserts for TCIs recommend that they should not be used under any occlusive dressing.
New directions in therapy
Patients with AD have a relative defect in innate immunity because they express lower levels of antimicrobial peptides, such as human β-defensins 2 and 3 and cathelicidin LL-37, in inflamed skin. The observation that cathelicidin expression in human subjects is induced by 1,25-dihydroxyvitamin D3 points to just one aspect of a complex interaction of this vitamin with a number of immune cells (as reviewed by Muehleisen and GalloE18) and suggests a role for vitamin D3 therapy in patients with AD. The current practice parameter states that patients with AD might benefit from supplementation with vitamin D, particularly if they have a documented low level of vitamin D.E13
Because TH2 cytokines have been shown to inhibit filaggrin expression and antimicrobial peptide responses, cytokine-modulating therapies could ameliorate barrier abnormalities. In this respect both topical steroids and TCIs (as discussed above) have been shown to reverse reduced filaggrin expression in patients with lesional AD. Targeted immunomodulatory therapy, including IL-4 receptor antibody or anti–thymic stromal lymphopoietin, offers potential advances in therapeutics for subsets of patients with AD.E5,E11,E13 In addition, gene expression and immunohisto-chemistry studies of lesional and nonlesional skin in patients with moderate-to-severe chronic AD treated with narrow-band UVB phototherapy showed that epidermal hyperplasia and differentiation normalized with suppression of TH2 and TH22 immune pathways.E19
THE CASE REVISITED
This child with AD complicated by HSV infection illustrates a number of problems encountered by clinicians dealing with this common, chronic, relapsing disease. This child’s pruritic eczematous rash with flexural distribution and a relapsing course, along with a personal and positive family history of atopy and increased serum IgE levels, fulfills both the major and minor criteria of Hanifin and Rajka for the diagnosis of AD. However, as shown in the flowchart of the diagnosis and management of AD in the current practice parameter,E13 clinicians should consider other conditions in patients not responding to the usual therapies. This patient’s history of onset in infancy with a persistent severe course, atopic sensitization, and recurrent respiratory symptoms suggestive of asthma is strongly suggestive of an AD phenotype with associated FLG loss-of-function mutation, and the physical findings of skin scaling and palmar hyperlinearity further support this association.E6 The punched-out appearance of the patient’s lesions, even without blistering, should raise suspicion for HSV. It is important that clinicians consider immunodeficiency with mutations in dedicator of cytokinesis 8 in the differential diagnosis in their evaluation of a patient with severe AD complicated by HSV infections.E20
Although there are currently no specific treatments for correcting filaggrin deficiency, basic skin care measures using an ABC approach of avoidance of triggers, barrier repair and maintenance, and control of inflammation and infection (Table E1) are essential. Education is a key component of a successful treatment plan because patients and caregivers often have a poor understanding of the chronic relapsing nature of the disease and triggers, as well as having concerns about prescribed medications.E1 Although patients and caregivers might provide a history suggesting adherence to a prescribed treatment plan, closer review or direct observation frequently reveals incorrect or inadequate implementation of the recommendations. Under supervision, this child was able to improve his skin hydration regimen significantly, which, together with appropriate use of moisturizers, topical anti-inflammatory therapy, a short course of wet wraps, and antiviral therapy, led to dramatic clinical improvement in a short period of time. With clearing of his eczema and resolution of HSV infection, this patient was able to transition to a twice-weekly regimen with fluticasone 0.05% cream to previously involved areas of recurring eczema. Additionally, clearing of eczema allowed for select allergy skin prick testing after the patient was able to hold oral antihistamine for 4 days. This patient was tested only to the 5 most common food allergens that he ate on a regular basis (milk, egg, peanut, wheat, and soy), with egg eliciting the only positive result. The parents elected to eliminate all egg from the patient’s diet for 3 to 4 weeks while restarting the foods eliciting negative skin test results individually, one per week. In addition, the parents reintroduced foods with positive results on in vitro testing but with poor clinical correlation, without any signs or symptoms noted (eg, orange, tomato, and corn).
TABLE E1.
ABC’s of atopic dermatitis
| A = Avoidance of triggers (eg, irritants and proved allergens) |
| • Educate patients and caregivers about role of irritants |
| • Approach allergy evaluation in a critical manner, informing patient/caregiver before testing about predictive values of positive and negative test results, pros/cons of dietary eliminations, risks vs benefits of food challenges, and environmental control measures |
| B = Barrier repair and maintenance |
| • Emphasis on hydration and moisturizers |
| • Address itch-scratch cycle with medications but also behavioral modification |
| • Consider wet-wrap therapy for limited periods of time to areas of recalcitrant AD |
| C = Control of inflammation and infections |
| • Consider proactive (twice weekly) therapy in patients with relapsing course |
| • Use diagnostics effectively (eg, culture and sensitivity test, viral culture, or PCR) |
| • Use antimicrobial agents appropriately |
| • Use topical corticosteroids and TCIs appropriately (eg, prescribe appropriate potency, vehicle, and quantity) |
INSTRUCTIONS.
Credit can now be obtained, free for a limited time, by reading the review articles in this issue. Please note the instructions listed below:
Review the target audience, learning objectives and author disclosures.
Complete the pre-test online at www.jacionline.org (click on the Online CME heading).
Follow the online instructions to read the full version of the article, including the clinical vignette and review components.
Complete the post-test. At this time, you will have earned 1.00 AMA PRA Category 1 CME Credit™.
Approximately 4 weeks later you will receive an online assessment regarding your application of this article to your practice. Once you have completed this assessment, you will be eligible to receive 2 MOC Part II Self-Assessment credits from the American Board of Allergy and Immunology.
Acknowledgments
Supported by the National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (HHSN272201000020C), the Atopic Dermatitis Research Network, and NIH grant AR41256. The authors wish to acknowledge the Edelstein Family Foundation for their generous support of this work.
Footnotes
Target Audience: Physicians and researchers within the field of allergic disease.
Accreditation/Provider Statements and Credit Designation: The American Academy of Allergy, Asthma & Immunology (AAAAI) is accredited by the Accreditation Council for Continuing Medical Education (ACCME) to provide continuing medical education for physicians. The AAAAI designates this journal-based CME activity for a maximum of 1 AMA PRA Category 1 Credit™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
List of Design Committee Members: Mark Boguniewicz, MD, and Donald Y. M. Leung, MD, PhD (authors), and James T. Li, MD, PhD (series editor)
- To discuss recent insights into skin barrier and immune abnormalities in patients with atopic dermatitis.
- To recognize the reasons for treatment failure in patients with severe atopic dermatitis.
- To educate patients and caregivers regarding the basics of skin care for atopic dermatitis.
Recognition of Commercial Support: This CME activity has not received external commercial support.
Disclosure of Significant Relationships with Relevant Commercial Companies/Organizations: D. Y. M. Leung has received a grant from the National Institutes of Health and is employed by National Jewish Health. J. T. Li has consulted for Abbott. M. Boguniewicz declares that he has no relevant conflicts of interest.
REFERENCES
- E1.Boguniewicz M, Nicol N, Kelsay K, Leung DY. A multidisciplinary approach to evaluation and treatment of atopic dermatitis. Semin Cutan Med Surg. 2008;27:115–27. doi: 10.1016/j.sder.2008.05.001. [DOI] [PubMed] [Google Scholar]
- E2.Odhiambo JA, Williams HC, Clayton TO, Robertson CF, Asher MI. Global variations in prevalence of eczema symptoms in children from ISAAC Phase Three. J Allergy Clin Immunol. 2009;124:1251–8. doi: 10.1016/j.jaci.2009.10.009. [DOI] [PubMed] [Google Scholar]
- E3.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–46. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E4.Kuo I-H, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol. 2013;131:266–78. doi: 10.1016/j.jaci.2012.12.1563. [DOI] [PubMed] [Google Scholar]
- E5.Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. N Engl J Med. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- E6.McAleer MA, Irvine AD. The multifunctional role of filaggrin in allergic skin disease. J Allergy Clin Immunol. 2013;131:280–91. doi: 10.1016/j.jaci.2012.12.668. [DOI] [PubMed] [Google Scholar]
- E7.Leung DY, Gao PS, Grigoryev DN, Rafaels NM, Streib JE, Howell MD, et al. Human atopic dermatitis complicated by eczema herpeticum is associated with abnormalities in IFN-gamma response. J Allergy Clin Immunol. 2011;127:965–73. doi: 10.1016/j.jaci.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E8.Suarez-Farinas M, Tintle SJ, Shemer A, Chiricozzi A, Nograles K, Cardinale I, et al. Nonlesional atopic dermatitis skin is characterized by broad terminal differentiation defects and variable immune abnormalities. J Allergy Clin Immunol. 2011;127:954–64. doi: 10.1016/j.jaci.2010.12.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E9.Hung SH, Lin YT, Chu CY, Lee CC, Liang TC, Yang YH, et al. Staphylococcus colonization in atopic dermatitis treated with fluticasone or tacrolimus with or without antibiotics. Ann Allergy Asthma Immunol. 2007;98:51–6. doi: 10.1016/S1081-1206(10)60859-9. [DOI] [PubMed] [Google Scholar]
- E10.Schmitt J, von Kobyletzki L, Svensson A, Apfelbacher C. Efficacy and tolerability of proactive treatment with topical corticosteroids and calcineurin inhibitors for atopic eczema: systematic review and meta-analysis of randomized controlled trials. Br J Dermatol. 2011;164:415–28. doi: 10.1111/j.1365-2133.2010.10030.x. [DOI] [PubMed] [Google Scholar]
- E11.Gittler JK, Shemer A, Suárez-Fariñas M, Fuentes-Duculan J, Gulewicz KJ, Wang CQ, et al. Progressive activation of T(H)2/T(H)22 cytokines and selective epidermal proteins characterizes acute and chronic atopic dermatitis. J Allergy Clin Immunol. 2012;130:1344–54. doi: 10.1016/j.jaci.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E12.Suárez-Fariñas M, Gittler JK, Shemer A, Cardinale I, Krueger JG, Guttman-Yassky E. Residual genomic signature of atopic dermatitis despite clinical resolution with narrow-band UVB. J Allergy Clin Immunol. 2013;131:577–9. doi: 10.1016/j.jaci.2012.11.010. [DOI] [PubMed] [Google Scholar]
- E13.Schneider L, Tilles S, Lio P, Boguniewicz M, Beck L, Lebovidge J, et al. Atopic dermatitis: a practice parameter update 2012. J Allergy Clin Immunol. 2013;131:295–9. doi: 10.1016/j.jaci.2012.12.672. [DOI] [PubMed] [Google Scholar]
- E14.Bergmann MM, Caubet J-C, Boguniewicz M, Eigenmann PA. Evaluation of food allergy in patients with atopic dermatitis. J Allergy Clin Immunol: In Practice. 2013;1:22–8. doi: 10.1016/j.jaip.2012.11.005. [DOI] [PubMed] [Google Scholar]
- E15.Fleischer DM, Bock SA, Spears GC, Wilson CG, Miyazawa NK, Gleason MC, et al. Oral food challenges in children with a diagnosis of food allergy. J Pediatr. 2011;158:578–83. doi: 10.1016/j.jpeds.2010.09.027. [DOI] [PubMed] [Google Scholar]
- E16.Boguniewicz M. New strategies for dealing with Staphylococcus aureus colonization and the emerging methicillin-resistant Staphylococcus aureus epidemic in atopic dermatitis. Chem Immunol Allergy. 2012;96:113–9. doi: 10.1159/000331910. [DOI] [PubMed] [Google Scholar]
- E17.Lee JH, Lee SJ, Kim D, Bang D. The effect of wet dressing on epidermal barrier in patients with atopic dermatitis. J Eur Acad Dermatol Venereol. 2007;21:1360–8. doi: 10.1111/j.1468-3083.2007.02277.x. [DOI] [PubMed] [Google Scholar]
- E18.Muehleisen B, Gallo RL. Vitamin D in allergic disease: shedding light on a complex problem. J Allergy Clin Immunol. 2013;131:324–9. doi: 10.1016/j.jaci.2012.12.1562. [DOI] [PubMed] [Google Scholar]
- E19.Tintle S, Shemer A, Suarez-Farinas M, Fujita H, Gilleaudeau P, Sullivan-Whalen M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and bio-markers for therapeutic response. J Allergy Clin Immunol. 2011;128:583–93. e1–4. doi: 10.1016/j.jaci.2011.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E20.Englelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. 2009;124:1289–302. doi: 10.1016/j.jaci.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]