Abstract
Background
Brain serotonin-1A receptors (5-HT1A) are implicated in anxiety. We compared regional brain 5-HT1A binding in medication-free participants with posttraumatic stress disorder (PTSD) and healthy volunteers using fully quantitative positron emission tomography (PET) methods.
Methods
Twenty patients with DSM-IV PTSD (13 with comorbid major depressive disorder, [MDD]) and 49 healthy volunteers underwent PET imaging with 5-HT1A antagonist radioligand [C-11]WAY100635. Arterial blood sampling provided a metabolite-corrected input function and the concentration of free ligand in plasma (fP) for estimation of regional binding potential, BPF ( = Bavailable /KD). Linear mixed modeling compared BPF between groups across regions of interest (ROIs).
Results
The PTSD group had higher 5-HT1A BPF across brain ROIs (P = .0006). Post hoc comparisons showed higher 5-HT1A BPF in PTSD in all cortical ROIs (26–33%), amygdala (34%), and brainstem raphe nuclei (43%), but not hippocampus. The subgroup of seven PTSD patients without comorbid MDD had higher 5-HT1A BPF compared with healthy volunteers (P = .03).
Conclusions
This is the first report of higher brainstem and forebrain 5-HT1A binding in vivo in PTSD. The finding is independent of MDD. PTSD and MDD have in common an upregulation of 5-HT1A binding including midbrain autoreceptors that would favor less firing and serotonin release. This abnormality may represent a common biomarker of these stress-associated brain disorders.
Keywords: posttraumatic stress disorder (PTSD), serotonin-1A (5-HT1A), positron emission tomography, WAY100635, major depressive disorder
INTRODUCTION
Posttraumatic stress disorder (PTSD) is a disabling[1] anxiety disorder that results from severe traumatic experiences with symptoms that persist months to years. These include reexperiencing phenomena (e.g. nightmares, intrusive images), pathological avoidance, emotional numbing, and hyperarousal. Some are more vulnerable to development of PTSD than others exposed to similar trauma; but degree of stress also contributes to risk, as in combat-related PTSD in which risk increases with each tour of duty.[2] Both genetic factors and early life adversity moderate future vulnerability to PTSD.[3,4]
The serotonin (5-HT) neurotransmitter system[5] has cell bodies in brainstem median and dorsal raphe nuclei that project widely in the brain, including to key fear circuitry loci such as amygdala, hippocampus, and ventromedial prefrontal cortex (PFC), mainly targeting GABAergic inhibitory neurons. The serotonin-1A receptor (5-HT1A) in particular is related to anxiety expression in both rodents[6,7] and humans.[8–10] The 5-HT1A receptor is both a somatodentritic autoreceptor on raphe nuclei serotonin neurons, inhibiting neuronal firing; and a terminal field postsynaptic receptor on nonserotonergic neurons in forebrain projection sites, where disruption of 5-HT1A expression during development may lead to a lifelong anxious phenotype.[11]
Despite demonstration of, albeit modest, treatment efficacy for the serotonin selective reuptake inhibitors (SSRIs) in PTSD,[12] little is known about the role of the serotonin system in this disorder. Rodent data suggest 5-HT1A alterations may account for bias toward threatening cues,[6] something observed clinically in PTSD. Moreover, attenuation of contextual fear appears to involve 5-HT1A in the extended amygdala,[13] SSRIs administered concomitantly with extinction training in mice induce an enduring loss of conditioned fear memory; and in PTSD when administered with an extinction-based therapy, SSRIs produce greater improvement in PTSD symptoms and remission rates.[14] Such data suggest regional brain mapping of 5-HT1A in individuals with PTSD is warranted.
Positron emission tomography (PET) employing the radioligand [carbonyl-C-11]WAY-100635 has allowed estimation of regional brain binding to the 5-HT1A receptor in vivo, and regional brain differences in binding measures have been reported in major depressive disorder (MDD),[15–17] social anxiety disorder,[8] and panic disorder.[9,10] There has been one prior report regarding 5-HT1A binding in PTSD by PET using the radioligand [F-18]FC-WAY, reporting no difference in the regional distribution volume (VT) from healthy volunteers.[18] However, limitations of this radiotracer[19] as well as quantitative modeling issues[20] may account for this negative finding. Therefore, we compared regional 5-HT1A binding potential (BPF = Bavailable / KD) using a metabolite-corrected arterial input function and free fraction in a sample of 20 medication-free PTSD participants and 49 healthy volunteers.
MATERIALS AND METHODS
PARTICIPANTS
Twenty participants met DSM-IV criteria for current PTSD. These patients were recruited concurrently with a healthy volunteer group (N = 49) previously reported in refs.16, 21 Diagnoses were determined by experienced masters and PhD-level psychologists using the Structured Clinical Interview for DSM-IV (SCID);[22] and a team of experienced clinical research psychologists and psychiatrists generated best-estimate diagnoses. Inclusion criteria were assessed through psychiatric, chart review, SCID, review of systems, physical exam, routine blood tests, and urine toxicology. Eligibility criteria for PTSD patients included age 18–65 years old; current PTSD; absence of psychotropic medications for at least 2 weeks prior to screening with exception for sedative/hypnotics (one PTSD participant had clonazepam >7 days before scan, and one PTSD participant had zolpidem >7 days before scan); no substance abuse within 2 months nor dependence within 6 months of screening; no lifetime exposure to 3,4-methylenedioxymethamphetamine; no history of psychotic disorder; no significant medical condition; and not pregnant. Criteria for healthy volunteer participants were similar except for a required absence of DSM-IV Axis I psychiatric disorders, and absence of mood or psychotic disorders in any first-degree relative. Beck Depression Inventory,[23] Hamilton Depression Rating Scale,[24] and Global Assessment Scale[25] assessed subjective and objective depression severity and functional impairment, respectively. Brown–Goodwin Aggression Inventory[26] measured lifetime aggression.
Index traumas in the PTSD group meeting DSM-IV-TR PTSD criterion A1 included 11 childhood physical and/or sexual abuse; one domestic abuse and childhood abuse; one domestic abuse; two sexual assault as adults; one physical assault as adult and childhood physical abuse; four with other severe traumatic events that occurred as adults. Of the healthy volunteers, three reported physical and/or sexual abuse, occurring before the age of 15 in each. Thirteen of the 20 PTSD patients also met DSM-IV criteria for a current major depressive episode (MDE) as part of MDD. Other Axis I disorders in the PTSD group included current (n = 5) or lifetime (n = 1) panic disorder, social anxiety disorder (n = 3), simple phobia (n = 1), and binge eating disorder (n = 1). Five PTSD participants had past histories of alcohol and/or substance abuse (one past alcohol dependence; one past alcohol, cannabis, stimulant, and cocaine abuse; one past alcohol abuse and cannabis dependence; one past alcohol abuse, and cannabis, and stimulant dependence; and one hypnotic/anxiolytic and cannabis abuse).
The protocol was approved by the Institutional Review Board of the New York State Psychiatric Institute, and participants gave written informed consent after explanation of the study.
RADIOCHEMISTRY AND INPUT FUNCTION MEASUREMENT
Preparation of [C-11]WAY100635 and measurement of arterial input function, metabolites, and plasma free fraction (fP) has been described.[20,27] The mean ± SD injected dose of [C-11]WAY100635 was comparable between healthy volunteer (8.0 ±3.5 mCi) and PTSD (6.9 ± 2.5 mCi) groups (t = 1.3, df = 67, P = .19). Injected mass was higher (2.8 ± 1.8 versus 1.5 ± 0.8 µg; t = 4.2, df = 67, P < .001) and decay-corrected specific activity (1.6 ± 0.7 versus 2.3 ± 0.8 mCi/nmole; t = −3.5, df = 67, P = .001) was lower in the healthy volunteer group compared with the PTSD group. Later studies differed after our human dosimetry study[28] determined the injected dose (and consequently injected mass) needed to be lowered. No correlation within-groups between injected mass and BPF was found in any region (data not shown). Main analyses were also performed covarying for injected mass and injected dose.
IMAGE ACQUISITION AND ANALYSIS
PET imaging was performed on an ECAT EXACT HR+ (Siemens/CTI, Knoxville, TN). After bolus infusion of [C-11]WAY100635 over 30 s, a 110-min emission scan was acquired in 3D mode as 20 successive frames of increasing duration (3 × 20 s, 3 × 1 min, 3×2 min, 2×5 min, 9×10 min). Automated arterial sampling was conducted every 5 s for first 2 min and then manually at longer intervals thereafter. Image analysis was performed using MATLAB 2006b (The Mathworks, Natick, MA) with extensions to the following: Functional Magnetic Imaging of the Brain’s Linear Image Registration Tool (FLIRT) v 5.2.,[29] Brain Extraction Tool v1.2,[30] Statistical Parametric Mapping (SPM5) normalization,[31] and segmentation routines.[32] To correct for subject motion during PET scan, denoising filter techniques were applied to all PET images starting at frame 5. All frames were aligned to the eighth frame using rigid body FLIRT. A mean of motion-corrected frames 8–18 was registered to the magnetic resonance imaging (MRI) using FLIRT.
Acquisition of T1-weighted MRI for co-registration of PET images and identification of regions of interest (ROIs) were performed for each participant as previously described using a 1.5T Signa Advantage or a 3T Signa HDx system (General Electric, Milwaukee, MI).[27] Twelve ROIs were hand drawn on left and right sides of brain on each subject’s MRI by experienced technicians trained to reliably demarcate these regions using brain atlases[33, 34] and published reports.[35, 36] Test–retest variability between raters was less than 3% for each of the 12 ROIs. These ROIs included ventral, medial, dorsolateral PFC; anterior and posterior cingulate; insular, parietal, temporal, and occipital cortex; amygdala, hippocampus, and parahippocampal gyrus. As a more conservative approach, the ROIs for dorsolateral prefrontal, temporal, parietal, and occipital cortex were included in the main group comparisons despite no direct expected effects in PTSD, noting that our prior work on anxiety symptoms in MDD indicated associations with 5-HT1A binding in these ROIs.[10] An ellipsoid (2 cm3) was manually placed on the raphe nuclei of each individual’s mean PET image, completely encompassing the high [C-11]WAY100635 binding region containing median and dorsal raphe nuclei. A cylindrical reference region was drawn in cerebellar white matter (CWM), a region virtually devoid of 5-HT1A.[20] ROI contours of cortical ROIs were refined using the segmented MRI to reflect the gyral pattern and differences between PET and MRI fields of view.[21] Specifically, a gray matter probability mask was generated in SPM5 using each subject’s segmented MRI. By multiplying all PET voxels within each cortical ROI by the voxel’s corresponding gray matter probability mask value (range between 0 and 1), the cortical ROIs were modified to include only gray matter voxels.
DERIVATION OF REGIONAL OUTCOME MEASURE
Regional distribution volumes (VT) of [C-11]WAY100635 were derived from kinetic analysis using the arterial input function and a two-tissue compartment (2T) model.[27] VT is the sum of the nondisplaceable and specific compartments distribution volumes, noted as VND and VS, respectively.[37] Time-activity curves were fit with a 2T model, with VND fixed to the VT previously estimated in the CWM reference region using a one-tissue compartment model.[20] The main outcome measure of binding potential was BPF ( = Bavailable / KD), the ratio at equilibrium of the concentration of specifically bound radioligand in tissue to the concentration of free radioligand in the tissue (with Bavailable equal to concentration of unoccupied receptor, and KD representing the dissociation constant); BPF was calculated as (VT(ROI) –VND) / fP.[37]
GENOTYPING THE C(-1019)G POLYMORPHISM
Participants were genotyped for the C(-1019)G polymorphism of HTR1A, the 5-HT1A receptor gene, to explore if biallelic genotype status has an effect on regional brain binding of 5-HT1A in PTSD. Genotyping was performed as previously described in ref.38 using allele-specific polymerase chain reaction (PCR) amplification.
STATISTICAL ANALYSIS
To borrow strength across all ROIs and to properly account for correlation among ROIs in the same participant, linear mixed-effects models[39] were fit to the ROI-level BPF estimates with region and diagnostic group as fixed effects and participant as the random effect. This modeling strategy provides a way to partition the expected variability into two sources: the biological variability among subjects within the diagnostic groups and the variability in estimating BPF for each region within each subject, which will include noise in PET scanning, as well as in measures derived from blood samples. This also allows for a single hypothesis to test for a difference between groups that is manifested in each ROI, thus avoiding the multiple comparisons considerations that would arise if the regions were tested separately. To stabilize variance across regions, adjust for slight skewness in distribution of binding measures, and allow testing for a proportional change in binding across regions, we fit the model to log-transformed binding potentials as we[10, 21, 38, 40, 41] and others[15] have reported for PET data analyses. Demonstrating a difference in log(BPF) is equivalent to demonstrating a difference in the same direction of raw BPF, as the natural log is a monotone transformation. Estimated standard errors were computed using a bootstrap algorithm that takes into account errors in metabolite, plasma, and brain data,[42] with observations weighted accordingly. Models were fit using the “nlme” package in the R software environment (www.r-project.org). Post hoc pair-wise comparisons between groups for each individual ROI were also performed, and results are presented with no adjustment for multiple comparisons. Additional statistical analyses include Student’s t-tests, χ2 test, and Fisher’s exact test performed in SPSS 18.0 (SPSS Statistics, 2010) or R.
RESULTS
Table 1 lists demographic and clinical characteristics of the sample. The healthy volunteer and PTSD groups had comparable age and sex distribution. The healthy volunteer group had about 2 years more of education than the PTSD group. Years of education were not related to 5-HT1A BPF (data not shown).
TABLE 1.
Demographic and clinical data of the study groups
| Diagnostic groups |
Group comparisons | ||||||
|---|---|---|---|---|---|---|---|
| HVs (n = 49) |
PTSD (n = 20) |
t-test |
|||||
| Mean | SD | Mean | SD | t-value | df | P-value | |
| Age | 37.3 | 14.8 | 40.7 | 12.2 | −0.91 | 67 | NS (.37) |
| Years of education | 16.7 | 2.9 | 14.9 | 2.9 | 2.36 | 67 | .024 |
| HDRS-24 | 0.7 | 0.9 | 22.3 | 9.3 | −10.41 | 19.16 | <.001 |
| Beck Depression Inventorya | 1.6 | 2.7 | 22.0 | 13.1 | −6.56 | 17.48 | <.001 |
| Lifetime aggression | 15.0 | 3.7 | 20.2 | 5.2 | −4.73 | 67 | <.001 |
| Global Assessment Scalea | 90.2 | 4.5 | 57.0 | 14.8 | 9.35 | 18.15 | <.001 |
| χ2 or FET | |||||||
| No. (%) | No. (%) | Value | df | P-value | |||
| Sex (female) | 28 (57.1) | 14 (70.0) | 0.99 | 1 | NS (.32) | ||
| Comorbid current MDD | 0 (0) | 13 (65.0) | |||||
| Abuse history—Lifetime | 3 (6.1) | 13 (65.0) | <.001 | ||||
| Abuse history —at <15yo | 3 (6.1) | 10 (50.0) | <.001 | ||||
| Prior antidepressant exposure | 0 (0) | 11 (55.0) | |||||
| FET | |||||||
| 5-HT1APR genotypeb | No. (%) | No. (%) | P-value | ||||
| CC | 16 (33.3) | 5 (25) | .004 | ||||
| CG | 29 (60.4) | 7 (35) | |||||
| GG | 3 (6.3) | 8 (40) | |||||
Abbreviations: FET, Fisher’s Exact Test (two-sided); HDRS-24, Hamilton Depression Rating Scale 24-item version; HVs, healthy volunteers; MDD, major depressive disorder; PTSD, posttraumatic stress disorder.
BDI and GAS scores missing for two participants.
Genotype missing for 1 participant.
The free fraction of [C-11]WAY100635 in plasma (fP) was higher in healthy volunteers (8.1 ± 2.4%) compared with PTSD (6.1 ± 1.4%) group (t = 6.9, df = 58.8, P < .0001). The distribution volume of the reference region, VND, did not differ between healthy volunteers (0.29 ± 0.11) and PTSD group (0.27 ± 0.08%; t = 0.69, df = 67, P = .49).
A linear mixed-effects model of regional 5-HT1A BPF, controlling for sex, age, and aggression,[43] demonstrated the PTSD group had higher 5-HT1A BPF compared with healthy volunteers (F1,64 = 12.9, P = .0006). There was also higher binding in females compared with males (F1,64 = 10.8, P =.0016). Neither age (F1,64 = 0.15, P = .69) nor lifetime aggression severity score (F1,64 = 0.35, P = .56) were related to 5-HT1A BPF in group comparison across all regions (although in the healthy volunteer group aggression severity was related to 5-HT1A BPF) Years of education was also not related to 5-HT1A BPF (F1,63 = 1.0, P = .32).
When controlling for between group differences in injected mass, the finding of higher 5-HT1A BPF in the PTSD group remained significant (F1,63 = 12.2, P = .0009); and injected mass was not related to 5-HT1A BPF (F1,63 = 0.60, P = .44). The main finding also remained significant (F1,63 = 12.7, P = .0007) when the model controlled for injected radioactivity of dose, which itself was also not related to 5-HT1A BPF (F1,63 = 0.054, P = .82).
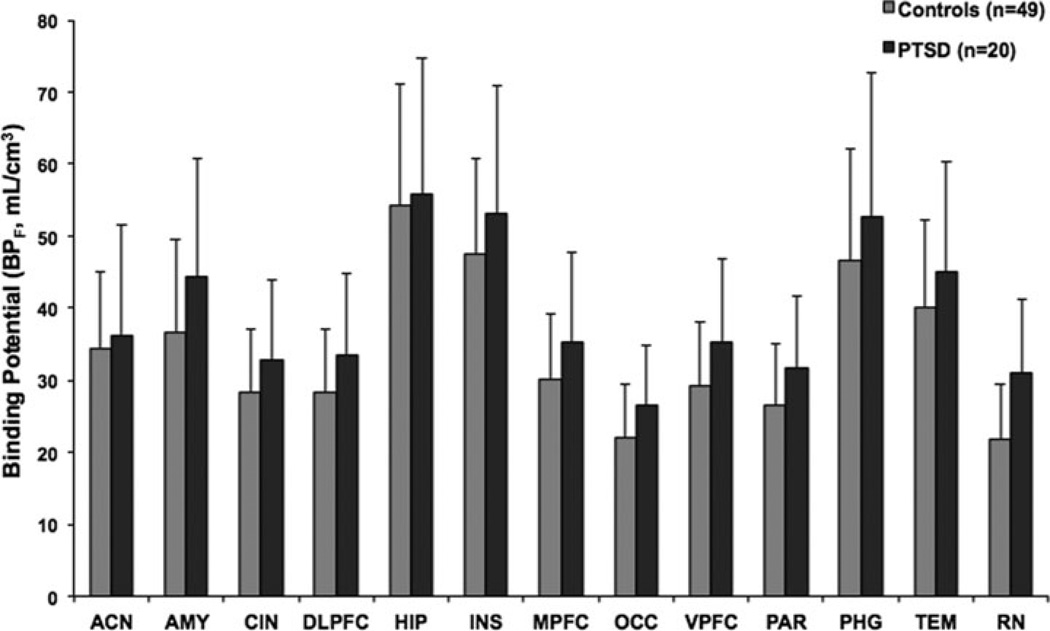
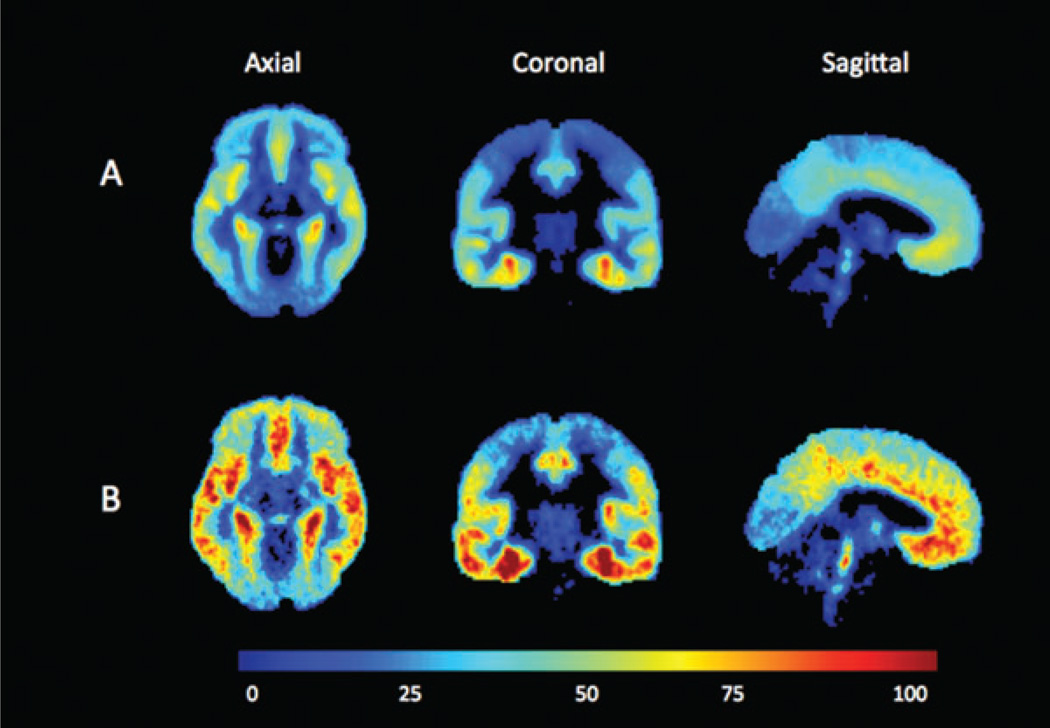
A significant diagnosis × region interaction (F13,803 = 1.9, P = .03) indicated that 5-HT1A BPF differences between diagnostic groups varied between brain region. Post hoc differences in each ROI indicated significantly higher binding in the PTSD group in all ROIs except hippocampus (Table 2; ordered from largest to smallest difference). The greatest difference was seen in raphe nuclei (43% higher in PTSD) and the least in hippocampus (19% higher in PTSD). Figure 1 shows BPF estimates for the PTSD and healthy volunteer groups in the 13 ROIs, and Figure 2 presents voxel maps of mean BPF levels for each of the two groups.
TABLE 2.
Post hoc comparisons of 5-HT1A BPF in PTSD (n = 20) v. HVs (n = 49) in ROIs
|
t-test |
||||
|---|---|---|---|---|
| Region of interest |
Percent higher 5- HT1ABPF in PTSD |
t-value | df | P-value |
| Raphe nuclei | 43% | 2.99 | 803 | .0028 |
| Amygdala | 34% | 2.87 | 803 | .0042 |
| Medial prefrontal cortex |
34% | 3.10 | 803 | .0020 |
| Dorsolateral prefrontal cortex |
33% | 3.02 | 803 | .0026 |
| Ventral prefrontal cortex |
32% | 2.99 | 803 | .0029 |
| Parietal cortex | 30% | 2.81 | 803 | .0050 |
| Anterior cingulate cortex |
30% | 2.78 | 803 | .0055 |
| Posterior cingulate cortex |
30% | 2.78 | 803 | .0056 |
| Occipital cortex | 28% | 2.55 | 803 | .0110 |
| Temporal cortex | 28% | 2.63 | 803 | .0088 |
| Parahippocampal gyrus |
27% | 2.49 | 803 | .0130 |
| Insular cortex | 27% | 2.56 | 803 | .0110 |
| Hippocampus | 19% | 1.75 | 803 | NS (.080) |
Abbreviations: BPF, binding potential; df, degrees of freedom; HVs, healthy volunteers; NS, not significant; PTSD, posttraumatic stress disorder; ROIs, regions of interest; 5-HT1A, serotonin-1A receptor.
Figure 1.
Radiolabeled [Carbonyl-C-11]WAY-100635 binding potential (BPF) estimates for the 5-HT1A receptor in PTSD patients and healthy volunteer participants in 13 ROIs. Comparison of 5-HT1A BPF between PTSD group and healthy volunteer group demonstrates higher 5-HT1A BPF in PTSD patients (P = .0006). Post hoc comparisons demonstrate higher 5-HT1A BPF in the PTSD group in every ROI examined except hippocampus. The height of each vertical bar represents the weighed mean of 5-HT1A BPF for the region, and the error bar represents the standard error of the weighted estimate. ACN, anterior cingulate cortex; AMY, amygdala; BPF, binding potential; [Carbonyl-C-11]WAY-100635, N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclohexanecarboxamide; CIN, posterior cingulate; DLPFC, dorsolateral prefrontal cortex; HIP, hippocampus; 5-HT1A, serotonin-1A receptor; INS, insula; MPFC, medial prefrontal cortex; OCC, occipital cortex; PAR, parietal cortex; PHG, parahippocampal gyrus; TEM, temporal cortex; VPFC, ventral prefrontal cortex; RN, raphe nuclei.
Figure 2.
Voxel-based mean binding potential (BPF) maps were produced as previously described [16] for A) the healthy volunteer group (n = 49) and B) the posttraumatic stress disorder group (n = 20). Each voxel intensity is the mean of the group’s single voxel 5-HT1A BPF measurement. The positron-emission tomography data were registered using each individual’s magnetic resonance image to the Montreal Neurological Institute (MNI) space. The color bar represents 5-HT1A BPF level in milliliters per gram.
Previously published PET studies in PTSD reported 5-HT1A radioligand binding with an alternate outcome measure, BPND (= (VT–VND) / VND; the ratio at equilibrium of specifically bound radioligand to that of nondisplaceable radioligand in tissue). Therefore, we also compared diagnostic groups using this outcome measure and found the PTSD group did not differ from healthy volunteers on 5-HT1A BPND (F1,64 = 1.9, P = .18), nor was there an effect of sex (F1,64 = 0.10, P = .75).
PTSD-ALONE VERSUS PTSD + MDD
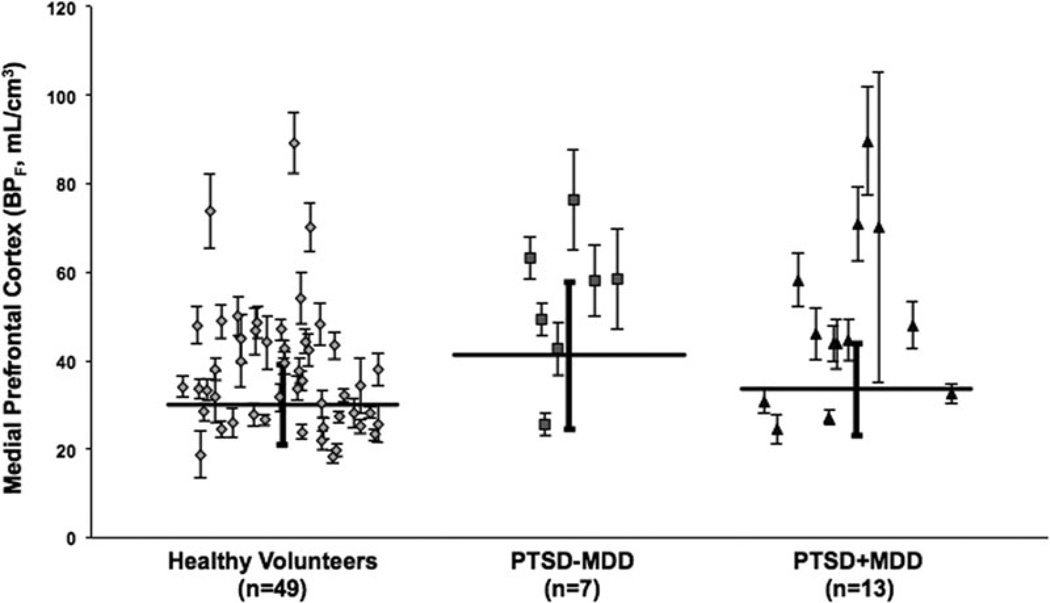
In the PTSD group, 13 of 20 had a current comorbid MDE (PTSD + MDD). To rule out an effect of depression comorbidity on binding, we compared binding in the seven PTSD patients without current MDD (PTSD –MDD) with healthy volunteers,finding higher 5-HT1A BPF in the PTSD-MDD group (F1,66 = 4.9, P = .03). The two PTSD subgroups (PTSD + MDD; PTSD – MDD) did not differ in 5-HT1A BPF from each other (F1,66 = 0.25, P = .62). See Figure 3.
Figure 3.
Radiolabeled [Carbonyl-C-11]WAY-100635 binding potential (BPF) estimates for the 5-HT1A receptor in medial prefrontal cortex for healthy volunteer, PTSD without current MDD (PTSD – MDD), and comorbid PTSD + MDD participants. Diamonds, squares, and triangles represent single measurements of BPF in healthy volunteer participants, the PTSD – MDD participants, and the PTSD + MDD participants, respectively. Thin capped vertical error bars represent standard errors computed using a bootstrap algorithm that takes into account errors in metabolite, plasma, and brain data.[42] Weighted group mean and standard error of the weighted mean of BPF are represented by thick horizontal lines and thick-capped vertical lines, respectively. BPF, binding potential; [Carbonyl-C-11]WAY-100635, N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl)cyclohexanecarboxamide; 5-HT1A, serotonin-1A.
NOT RECENTLY MEDICATED GROUP
The 13 patients (six PTSD – MDD and seven PTSD + MDD) without recent (within 4 months of PET scan) antidepressant exposure (hereafter designated “not recently medicated,” NRM) also had higher 5-HT1A BPF compared with healthy volunteers (F1,65 = 8.07, P = .0060). Moreover, NRM and more recently medicated PTSD subgroup (median time off antidepressants of 47 days) did not differ from each other (F1,65 = 1.38, P = .24).
5-HT1A PROMOTOR POLYMORPHISM
The C(−1019)G genotype showed an association with PTSD (Table 1) whereby the GG genotype was more common in PTSD (40%) compared with healthy volunteers (6.3%: Fisher’s Exact Test, P = .004). Testing for an effect of PTSD diagnosis on raphe nuclei 5-HT1A BPF by the linear mixed effects model, with sex, age, aggression history, and 5-HT1APR genotype as factors, effects of PTSD diagnosis (F1,66.6 = 17.0, P = .00011) and sex (F1,71.8 = 18.4, P = .00007) remained. The effect of genotype was not statistically significant, although there was a strong trend (F2,24.0 = 3.1, P = .053).
DISCUSSION
We found higher regional brain 5-HT1A binding (BPF) in PTSD compared to healthy volunteer participants using the 5-HT1A PET radioligand [11C]WAY100635 and a metabolite-corrected, arterial input function. Higher binding (by 26–43%) was present in PTSD in every ROI examined except hippocampus, and it was highest in raphe nuclei. As we have reported previously for healthy volunteers and those with depression, women had higher 5-HT1A BPF than men. Neither age nor lifetime aggression severity score were related to 5-HT1A BPF in the linear mixed effects model.
Our previous reports,[16, 38] based on two independent samples of MDD patients imaged during a MDE, showed higher 5-HT1A binding in NRM MDD. We have additionally reported that this effect is present in MDD during remission, indicating a trait abnormality.[40] This raises the question as to whether comorbidity of a current MDE in the PTSD group (13/20 PTSD patients) explains the present findings. Since the PTSD subgroup without current MDD (PTSD-MDD group) also had higher binding compared with healthy volunteers, and there was no difference in 5-HT1A binding between the PTSD + MDD and the PTSD – MDD subgroups, we find that higher 5-HT1A BPF in PTSD is not explained by MDD comorbidity.
This similar biological abnormality involving greater 5-HT1A binding in both MDD and PTSD is of potential importance, as it may help explain why comorbidity between PTSD and MDD is common; 48% of the general population who report lifetime PTSD also report lifetime MDD diagnosis.[44] Both PTSD and MDD can be reactions to traumatic exposures, as seen after the attack on the World Trade Center on 11 September 2001.[45] There is also substantial symptom overlap between the disorders. Therefore, we can now add higher 5-HT1A binding to the clinical evidence suggesting that PTSD and MDD to some degree are alternative manifestations of the same underlying diathesis. Greater 5-HT1A binding may be a biological manifestation of this common diathesis or vulnerability to stressful experiences that can result in either MDD or PTSD or both. Moreover, hypothalamic–pituitary–adrenal (HPA) axis abnormalities in comorbid PTSD + MDD may also represent a common central abnormality in glucocorticoid regulation[46, 47] that may be related to regional brain 5-HT1A density. However human and nonhuman primate studies have yet to detect cortisol effects on radioligand binding to 5-HT1A.[48, 49]
Our cross-sectional study needs extension to determine whether higher 5-HT1A binding antedates the development of PTSD (or is still present after remission) indicating that it may be a predisposing biological trait. Reported childhood adversity such as exposure to physical and/or sexual abuse during development, which has been associated with HPA axis dysregulation in adulthood,[50] may contribute to diathesis, consonant with animal models showing lasting changes in 5-HT1A binding resulting from developmental adversity;[51,52] but also see refs.53.
To date, we know of one study of in vivo 5-HT1A binding in PTSD using PET imaging, which used an alternate 5-HT1A ligand [F-18]FC-WAY.[18] The main outcome measure, regional distribution volume (VT), did not differ between 12 PTSD participants and 11 healthy volunteers, nor did the regional nondisplaceable binding (BPND). That no difference was detected may be attributable to (1) challenges for quantitative modeling of 5-HT1A brain binding using this ligand due to a radiometabolite ([F-18]fluorocyclohexanecarboxylic acid) that crosses the blood brain barrier, as well as defluorination with F-18 uptake by skull[19] and (2) use of total cerebellum as reference region, which has measurable 5-HT1A binding,[20] for calculation of BPND, as this runs counter to the assumption for simplified reference tissue modeling (SRTM) of no specific binding.[37] Therefore, methodological limitations may have prevented this prior study from detecting the binding differences we report. We also identified significant differences in free fraction of the tracer (fP) between PTSD patients and healthy volunteers (P < .0001), but our outcome measure, BPF, takes into account any differences in fP between diagnostic groups (note: at equilibrium fND = fP / VND).
The functional C-1019G promoter polymorphism 5-HT1APR GG genotype in vitro is associated with higher 5-HT1A transcript expression in raphe but not hippocampal neurons.[54] Because genotyping for this polymorphism was available for participants in this study, we explored whether there was a role for this genotype in partially explaining elevated raphe nuclei 5-HT1A BPF in the PTSD group; yet such results must be considered very preliminary as the study was grossly underpowered for exploring any relationships with genotype. We have previously reported the G-allele is associated with higher brainstem raphe nuclei 5-HT1A binding in both MDD and bipolar depression.[21, 38] In this sample, the GG genotype is overrepresented in the PTSD group; and when presence of PTSD is considered in the linear mixed effects model, the effect of diagnosis on binding is not all explained by genotype as only a strong trend for genotype effect (P = .053) was identified. Given the lack of statistical power for exploring genotype effects on raphe nuclei 5-HT1A binding, this trend is of interest and raises the possibility that a larger sample may confirm the finding.
A recent course of antidepressants did not explain our findings, because the PTSD subgroup with no antidepressant exposure within 120 days prior to scanning also had higher 5-HT1A BPF compared with healthy volunteers and did not differ from the more recently medicated PTSD subgroup.
Brain regions with the largest differences in binding between PTSD and healthy volunteers, namely the autoreceptors on raphe neurons in midbrain and terminal field receptors in amygdala and several prefrontal cortical regions, can be related to the neural circuits implicated in PTSD. In PTSD, amygdala responses subserving acquisition and recall of fearful responding are putatively exaggerated and prefrontal cortical–hippocampal interactions mediating contextual extinction are deficient, resulting in “failure of recovery” from trauma via impaired extinction memory.[55] The 5-HT1A receptor is implicated in extinction learning.[6, 56] More autoreceptors and less serotonin firing and release may result in deficient serotonergic neurotransmission through forebrain 5-HT1A, thereby impairing extinction memory recall[57] as posited in PTSD.
Recent PET imaging studies have reported regional brain binding differences in other key proteins in the serotonin system, namely the serotonin transporter[58] and the serotonin-1B receptor (5-HT1B),[59] in PTSD (versus healthy volunteers) and in a severe trauma exposed group (versus nonexposed), respectively. The groups compared in the serotonin transporter PET study were similar to those compared in the present study, but transporter-binding differences in the PTSD group appeared localized to amygdala, contrasting with the higher 5-HT1A binding identified throughout several forebrain and raphe region ROIs in our report. The 5-HT1B study compared severe trauma exposure (with and without PTSD) to healthy volunteers without trauma exposure, finding less binding in amygdala, anterior cingulate, and caudate. Thus, the 5-HT1B differences appear to be an effect of severe trauma exposure per se, rather than specific to, or a risk factor for, development of PTSD. Our present study and the transporter study both have the limitation of not including a third matched trauma-exposed healthy volunteer group. Thus, identified differences in 5-HT1A and 5-HT1B in the respective studies may represent effects of trauma exposure per se rather than being PTSD specific.
Another limitation in the present study was heterogeneity of the trauma reported by the PTSD group, both in terms of the developmental stage at which traumas occurred and single versus repeated or chronic trauma. Future work should strive for greater homogeneity in trauma typology and include a trauma-exposed “resilient” control group matched for trauma typology, time elapsed since index trauma, and developmental stage of trauma occurrence, as well as other factors relating to 5-HT1A binding such as sex. Using 5-HT1A BPF as outcome measure, future studies would do well to examine the role of 5-HT1A receptors in other anxiety disorders and the impact of successful treatments.
Acknowledgements
This work was supported by grants from the American Foundation for Suicide Prevention (AFSP), the National Alliance for Research on Schizophrenia and Depression (NARSAD), and United States Public Health Service Grants MH62185 (Conte Center) and K08-MH67015 (GMS). We thank the staff of the Brain Imaging Division and Clinical Evaluation Core of the Conte Translational Center for the Neurobiology of Suicidal Behavior and the Columbia Kreitchman Positron Emission Tomography (PET) Center for expert help. We also thank Brendan J. Carroll, MFA for his indispensable support as research assistant for this project.
REFERENCES
- 1.Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry. 2000;61(Suppl 5):4–12. discussion 13–4. [PubMed] [Google Scholar]
- 2.Phillips CJ, Leardmann CA, Gumbs GR, Smith B. Risk factors for posttraumatic stress disorder among deployed US male marines. BMC Psychiatry. 2010;10:52. doi: 10.1186/1471-244X-10-52. Available at: http://www.biomedcentral.com/1471-244X/10/52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adamec R, Holmes A, Blundell J. Vulnerability to lasting anxiogenic effects of brief exposure to predator stimuli: sex, serotonin and other factors-relevance to PTSD. Neurosci Biobehav Rev. 2008;32(7):1287–1292. doi: 10.1016/j.neubiorev.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holmes A. Genetic variation in cortico-amygdala serotonin function and risk for stress-related disease. Neurosci Biobehav Rev. 2008;32(7):1293–1314. doi: 10.1016/j.neubiorev.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lanfumey L, Mongeau R, Cohen-Salmon C, Hamon M. Corticosteroid-serotonin interactions in the neurobiological mechanisms of stress-related disorders. Neurosci Biobehav Rev. 2008;32(6):1174–1184. doi: 10.1016/j.neubiorev.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Klemenhagen KC, Gordon JA, David DJ, et al. Increased fear response to contextual cues in mice lacking the 5-HT1A receptor. Neuropsychopharmacology. 2006;31(1):101–111. doi: 10.1038/sj.npp.1300774. [DOI] [PubMed] [Google Scholar]
- 7.Menard J, Treit D. The septum and the hippocampus differentially mediate anxiolytic effects of R(+)-8-OH-DPAT. Behav Pharmacol. 1998;9(2):93–101. [PubMed] [Google Scholar]
- 8.Lanzenberger RR, Mitterhauser M, Spindelegger C, et al. Reduced serotonin-1A receptor binding in social anxiety disorder. Biol Psychiatry. 2007;61(9):1081–1089. doi: 10.1016/j.biopsych.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Nash JR, Sargent PA, Rabiner EA, et al. Serotonin 5-HT1A receptor binding in people with panic disorder: positron emission tomography study. Br J Psychiatry. 2008;193(3):229–234. doi: 10.1192/bjp.bp.107.041186. [DOI] [PubMed] [Google Scholar]
- 10.Sullivan GM, Oquendo MA, Simpson N, et al. Brain serotonin1A receptor binding in major depression is related to psychic and somatic anxiety. Biol Psychiatry. 2005;58(12):947–954. doi: 10.1016/j.biopsych.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 11.Gross C, Zhuang X, Stark K, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416(6879):396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 12.Sullivan GM, Neria Y. Pharmacotherapy in post-traumatic stress disorder: evidence from randomized controlled trials. Curr Opin Investig Drugs. 2009;10(1):35–45. [PMC free article] [PubMed] [Google Scholar]
- 13.Gomes FV, Reis DG, Alves FH, et al. Cannabidiol injected into the bed nucleus of the stria terminalis reduces the expression of contextual fear conditioning via 5-HT1A receptors. J Psychopharmacol. 2012;26(1):104–113. doi: 10.1177/0269881110389095. [DOI] [PubMed] [Google Scholar]
- 14.Schneier FR, Neria Y, Pavlicova M, et al. Combined prolonged exposure therapy and paroxetine for PTSD related to the World Trade Center attack: a randomized controlled trial. Am J Psychiatry. 2012;169(1):80–88. doi: 10.1176/appi.ajp.2011.11020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirvonen J, Karlsson H, Kajander J, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-11C]WAY-100635. Int J Neuropsychopharmacol. 2008;11(4):465–476. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 16.Parsey RV, Ogden RT, Miller JM, et al. Higher serotonin 1A binding in a second major depression cohort: modeling and reference region considerations. Biol Psychiatry. 2010;68(2):170–178. doi: 10.1016/j.biopsych.2010.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shrestha S, Hirvonen J, Hines CS, et al. Serotonin-1A receptors in major depression quantified using PET: controversies, confounds, and recommendations. Neuroimage. 2012;59(4):3243–3251. doi: 10.1016/j.neuroimage.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonne O, Bain E, Neumeister A, et al. No change in serotonin type 1A receptor binding in patients with posttraumatic stress disorder. Am J Psychiatry. 2005;162(2):383–385. doi: 10.1176/appi.ajp.162.2.383. [DOI] [PubMed] [Google Scholar]
- 19.Carson RE, Wu Y, Lang L, et al. Brain uptake of the acid metabolites of F-18-labeled WAY 100635 analogs. J Cereb Blood Flow Metab. 2003;23(2):249–260. doi: 10.1097/01.WCB.0000046145.31247.7A. [DOI] [PubMed] [Google Scholar]
- 20.Parsey RV, Arango V, Olvet DM, et al. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25(7):785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 21.Sullivan GM, Ogden RT, Oquendo MA, et al. Positron emission tomography quantification of serotonin-1A receptor binding in medication-free bipolar depression. Biol Psychiatry. 2009;66(3):223–230. doi: 10.1016/j.biopsych.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders, Patient Edition. SCID-I/P, Version 2.0. New York: Biometrics Research, New York State Psychiatric Institute; 1994. [Google Scholar]
- 23.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 24.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Endicott J, Spitzer RL, Fleiss JL, Cohen J. The global assessment scale.A procedure for measuring overall severity of psychiatric disturbance. Arch Gen Psychiatry. 1976;33(6):766–771. doi: 10.1001/archpsyc.1976.01770060086012. [DOI] [PubMed] [Google Scholar]
- 26.Brown GL, Goodwin FK, Ballenger JC, et al. Aggression in humans correlates with cerebrospinal fluid amine metabolites. Psychiatry Res. 1979;1(2):131–139. doi: 10.1016/0165-1781(79)90053-2. [DOI] [PubMed] [Google Scholar]
- 27.Parsey RV, Slifstein M, Hwang DR, et al. Validation and reproducibility of measurement of 5-HT1A receptor parameters with [carbonyl-11C]WAY-100635 in humans: comparison of arterial and reference tissue input functions. J Cereb Blood Flow Metab. 2000;20(7):1111–1133. doi: 10.1097/00004647-200007000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Parsey RV, Belanger MJ, Sullivan GM, et al. Biodistribution and radiation dosimetry of 11C-WAY100,635 in humans. J Nucl Med. 2005;46(4):614–619. [PubMed] [Google Scholar]
- 29.Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- 30.Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Hum Brain Mapp. 1999;7(4):254–266. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 33.Duvernoy HM. The Human Brain: Surface, Three-Dimensional Sectional Anatomy and MRI. Wien: Springer-Verlag; 1991. [Google Scholar]
- 34.Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain : 3-Dimensional Proportional System : An Approach to Cerebral Imaging. Stuttgart: Thieme; 1988. [Google Scholar]
- 35.Kates WR, Abrams MT, Kaufmann WE, et al. Reliability and validity of MRI measurement of the amygdala and hippocampus in children with fragile X syndrome. Psychiatry Res. 1997;75(1):31– 48. doi: 10.1016/s0925-4927(97)00019-x. [DOI] [PubMed] [Google Scholar]
- 36.Killany RJ, Moss MB, Nicholson T, et al. An interactive procedure for extracting features of the brain from magnetic resonance images: the lobes. Hum Brain Mapp. 1997;5:355–363. doi: 10.1002/(SICI)1097-0193(1997)5:5<355::AID-HBM4>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 37.Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27(9):1533–1539. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- 38.Parsey RV, Oquendo MA, Ogden RT, et al. Altered serotonin 1A binding in major depression: a [carbonyl-C-11]WAY100635 positron emission tomography study. Biol Psychiatry. 2006;59(2):106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Pinheiro JC, Bates DM ebrary Inc. Statistics and Computing. New York: Springer; 2000. Mixed-Effects Models in S and S-PLUS. [Google Scholar]
- 40.Miller JM, Brennan KG, Ogden TR, et al. Elevated serotonin 1A binding in remitted major depressive disorder: evidence for a trait biological abnormality. Neuropsychopharmacology. 2009;34(10):2275–2284. doi: 10.1038/npp.2009.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parsey RV, Olvet DM, Oquendo MA, et al. Higher 5-HT1A receptor binding potential during a major depressive episode predicts poor treatment response: preliminary data from a naturalistic study. Neuropsychopharmacology. 2006;31(8):1745–1749. doi: 10.1038/sj.npp.1300992. [DOI] [PubMed] [Google Scholar]
- 42.Ogden RT, Tarpey T. Estimation in regression models with externally estimated parameters. Biostatistics. 2006;7(1):115–129. doi: 10.1093/biostatistics/kxi044. [DOI] [PubMed] [Google Scholar]
- 43.Parsey RV, Oquendo MA, Simpson NR, et al. Effects of sex, age, and aggressive traits in man on brain serotonin 5-HT1A receptor binding potential measured by PET using [C-11]WAY-100635. Brain Res. 2002;954(2):173–182. doi: 10.1016/s0006-8993(02)03243-2. [DOI] [PubMed] [Google Scholar]
- 44.Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52(12):1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 45.Neria Y, Olfson M, Gameroff MJ, et al. The mental health consequences of disaster-related loss: findings from primary care one year after the 9/11 terrorist attacks. Psychiatry. 2008;71(4):339–348. doi: 10.1521/psyc.2008.71.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Oquendo MA, Echavarria G, Galfalvy HC, et al. Lower cortisol levels in depressed patients with comorbid post-traumatic stress disorder. Neuropsychopharmacology. 2003;28(3):591–598. doi: 10.1038/sj.npp.1300050. [DOI] [PubMed] [Google Scholar]
- 47.Vythilingam M, Gill JM, Luckenbaugh DA, et al. Low early morning plasma cortisol in posttraumatic stress disorder is associated with co-morbid depression but not with enhanced glucocorticoid feedback inhibition. Psychoneuroendocrinology. 2010;35(3):442–450. doi: 10.1016/j.psyneuen.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 48.Drevets WC, Thase ME, Moses-Kolko EL, et al. Serotonin-1A receptor imaging in recurrent depression: replication and literature review. Nucl Med Biol. 2007;34(7):865–877. doi: 10.1016/j.nucmedbio.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shively CA, Friedman DP, Gage HD, et al. Behavioral depression and positron emission tomography-determined serotonin 1A receptor binding potential in cynomolgus monkeys. Arch Gen Psychiatry. 2006;63(4):396–403. doi: 10.1001/archpsyc.63.4.396. [DOI] [PubMed] [Google Scholar]
- 50.McCrory E, De Brito SA, Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J Child Psychol Psychiatry. 2010;51(10):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- 51.Harvey BH, Naciti C, Brand L, Stein DJ. Endocrine, cognitive and hippocampal/cortical 5HT 1A/2A receptor changes evoked by a time-dependent sensitisation (TDS) stress model in rats. Brain Res. 2003;983(1-2):97–107. doi: 10.1016/s0006-8993(03)03033-6. [DOI] [PubMed] [Google Scholar]
- 52.Vicentic A, Francis D, Moffett M, et al. Maternal separation alters serotonergic transporter densities and serotonergic 1A receptors in rat brain. Neuroscience. 2006;140(1):355–365. doi: 10.1016/j.neuroscience.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Lambas-Senas L, Mnie-Filali O, Certin V, et al. Functional correlates for 5-HT(1A) receptors in maternally deprived rats displaying anxiety and depression-like behaviors. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33(2):262–268. doi: 10.1016/j.pnpbp.2008.11.017. [DOI] [PubMed] [Google Scholar]
- 54.Lemonde S, Turecki G, Bakish D, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. J Neurosci. 2003;23(25):8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biol Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 56.Koseki H, Matsumoto M, Togashi H, et al. Alteration of synaptic transmission in the hippocampal-mPFC pathway during extinction trials of context-dependent fear memory in juvenile rat stress models. Synapse. 2009;63(9):805–813. doi: 10.1002/syn.20657. [DOI] [PubMed] [Google Scholar]
- 57.Milad MR, Orr SP, Lasko NB, et al. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42(7):515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murrough JW, Huang Y, Hu J, et al. Reduced amygdala serotonin transporter binding in posttraumatic stress disorder. Biol Psychiatry. 2011;70(11):1033–1038. doi: 10.1016/j.biopsych.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Murrough JW, Czermak C, Henry S, et al. The effect of early trauma exposure on serotonin type 1B receptor expression revealed by reduced selective radioligand binding. Arch Gen Psychiatry. 2011;68(9):892–900. doi: 10.1001/archgenpsychiatry.2011.91. [DOI] [PMC free article] [PubMed] [Google Scholar]