Abstract
Objective
To test whether cancer patients’ expectations for cure prior to interacting with their oncologist influence their decisions to follow treatment recommendations. Further, to test whether patients’ expectations for cure are affected by the strength of the oncologist–patient alliance or the extent to which companions (if present) share patients’ expectations for cure.
Methods
Interactions of 101 patients (and 114 companions) with oncologists about treatment were coded for the strength of the oncologist–patient alliance. Prior to the interaction, patients and companions reported expectations about whether the patient would be cured of cancer. After the interaction, patients reported whether they intended to follow the recommended treatment.
Results
Patients who expected a cure were more likely to report an intention to follow oncologists’ treatment recommendation when the strength of their alliance with their oncologist was weaker (B = −0.51, p < .05). Patients whose expectations for cure matched their companions’ expectations were less likely to report intentions to follow treatment recommendations (B = −0.28, p < .05).
Conclusion
Patients who have an expectation of being cured of cancer prior to meeting with their oncologist are more likely to intend to follow recommended treatment when their alliance with the oncologist is weaker and their companions do not believe they will be cured.
Practice implications
To better understand patient treatment decisions and improve overall cancer care, oncologists should be aware of the complex ways that patients’ expectations about cure influence treatment choices.
Keywords: Cancer, Oncology, Expectations, Cure, Physician-patient interactions, Treatment decisions
1. Introduction
Expectations are subjective beliefs about what will occur in the future [1–6]. In the context of cancer, expectations are critical because they affect the way individuals experience and interpret their disease [7–9]. For instance, patients with optimistic expectations regarding their cancer prognosis are generally able to cope and have less distress than other patients [10–14]. However, little is known about the effect context-specific expectations – such as the belief that one will be cured of cancer (regardless of the physician’s prognosis) – have on patients’ treatment decisions. The influence of patient beliefs in whether they will be cured, independent of their actual prognosis, is of particular interest given that research has demonstrated that patients often hold prognostic views which contradict those of their oncologist even after discussion about prognosis which may in turn affect their treatment decisions [15,16].
Previous research suggests patients’ treatment decisions are affected by the quality of the interactions they have with their oncologists [17–23]. For instance, when oncologists discuss clinical trials in a communication style that is informative, warm, responsive, and caring, patients are more likely to agree to join the trial [24,25]. Although research shows that expectations influence how individuals interpret information and behavior [26,27], questions remain regarding the extent to which cancer patients’ expectations for cure interact with the strength of their alliance with their oncologist to influence treatment decisions. In other words, although the quality of physician–patient communication is related to patient adherence to treatment recommendations, the role of patients’ expectations for cure in this relationship is unknown.
Further, the presence of a companion during the interaction may add to the complexity of patient decision making processes. Previous research has shown that companions are not only frequently present during oncology outpatient visits, but also are active participants in the interactions[24,28–31] and treatment decisions[32,33]. Although not yet empirically studied, it is likely that companions, like patients, have their own expectations about whether the patient will be cured, and these expectations may or may not match those of the patient. Further, little is known about how companion expectations may influence patient decisions about treatment. Studies show that being with others who share similar attitudes/beliefs to one’s own makes one less open to adjusting or altering that shared attitude/belief [34]; suggesting that consistent expectations between patients and companions may influence patient decision-making. However, it is not yet known whether consistent (i.e., matched) expectations between patients and companions influence patients’ decision to follow their doctor’s treatment recommendations, and further, whether the direction of the match (i.e., do patients and companions agree that the patient will be cured or do they agree that the patient will not be cured) influences patients’ decision to follow treatment recommendations.
Thus, this research addresses the following questions:
Do patients’ expectations for cure prior to oncologist–patient interactions have a direct effect on patients’ subsequent decisions to follow the treatment recommended by oncologists?
Does the strength of the alliance between patients and their oncologists (i.e., the objectively observed quality of rapport, closeness, trust, hope, responsiveness, organization, and the amount and clarity of information provided during the interaction) moderate the effect of patients’ expectations for cure on patients’ decisions to follow oncologists’ treatment recommendations?
-
For patients with companions during interactions:
Do patients who are accompanied to the visit have expectations for cure which match their companions’ expectations?
Does a match between patients and companions expectations for cure influence patients’ decisions to follow treatment recommendations?
2. Methods
Data for this study were collected as part of a larger investigation of oncologist–patient communication [24,35]. The study occurred between April 2002 and March 2006 and consisted of 235 patient/companion–oncologist interactions in the multi-disciplinary outpatient oncology clinics at two National Cancer Institute-designated comprehensive cancer centers. The participation rate was unavailable at one site; the other had a participation acceptance rate of 72%. Oncology patients and their companions (>18 years old) were eligible for participation if (a) their oncologist was participating in the research project, (b) patients were potentially eligible for a Phase II or III clinical trial, and (c) patients (and companions if present) were able to speak and read English.
2.1. Procedure
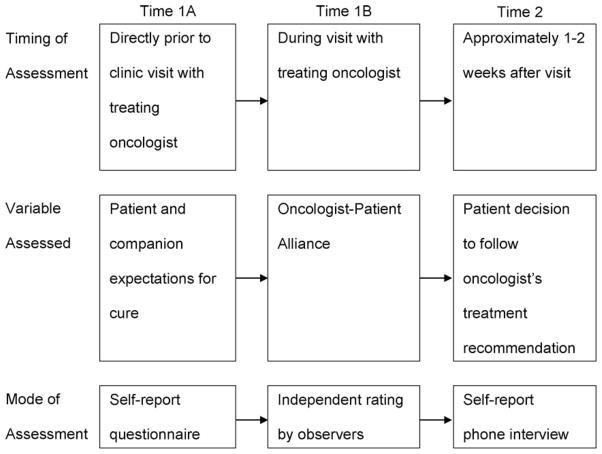
Eligible patients (and companions, if present) were approached by a research assistant who explained the procedures of the study and invited them to participate. Patients and companions gave written consent and completed a background questionnaire while they waited for their appointment to see the oncologist (Time1A). The questionnaire assessed socio-demographic information and expectations about whether the patient’s cancer would be cured. The interaction with the oncologist was then video recorded (Time1B; procedures are described below). Approximately two weeks after the interaction, a brief telephone interview was conducted with patients about their perceptions of the interaction and whether they had decided to follow the treatment recommended by their oncologist (Time2) (see Fig. 1).
Fig. 1.
Study design.
Patients (n = 101; 77 of whom were accompanied by at least one person) were selected from the parent study if they fulfilled requirements for all three assessments: (1) completed the background questionnaire (patient and any present companions), (2) allowed their interaction to be videotaped, and (3) was available by phone and completed the follow-up interview. Any exclusion was due to failure of the videotaping equipment preventing coding of the patient/oncologist interaction, or patients being unreachable by telephone for the follow-up survey either because they had moved, declined to participate, or were unable to participate due to illness or having passed away. Patients included in the study were seen by one of 10 medical oncologists at the first cancer center or one of 11 from the second center. The patients were diagnosed with various cancers: Lung (32%), Colorectal (15%), Breast (7%), GI Stromal Tumor (7%), Multiple Myeloma (6%), Lymphoma (5%), Head/Neck (5%), Prostate (4%), Leukemia (3%), Liver (2%), Pancreatic (2%), Esophageal (1%), Ovarian (1%), Testicular (1%), Mesothelioma (1%), Other Cancers (6%), Unidentified (2%). Although all patients were judged as being potentially eligible for a clinical trial prior to their appointment, actual offers of clinical trials were only made to 33 patients (33%) by their oncologist during the treatment interaction [for more detail on clinical trial offers in this sample see Albrecht et al. (2008)]. In the current study, patients were included regardless of whether or not they received an actual offer of a clinical trial. A majority (85%) of patients were visiting the cancer centers for the first time and a variable controlling for whether the visit was an initial or return visit was entered in all analyses to ensure that familiarity with the oncologist was not a confounding variable.
2.2. Informed consent
The study was approved by the Institutional Review Boards of two universities and underwent protocol review at both cancer centers. All oncologists, patients, and companions (and other medical providers if present) signed consent forms. Patients also signed HIPAA release forms.
2.3. Video recording procedures
Interactions with the oncologist were recorded using a custom-designed, remote-controlled digital video recording system, which includes two high-resolution digital video cameras, two external microphones, and remote monitoring and recording capabilities [36]. Previous research using this video recording equipment has shown the cameras to be unobtrusive and virtually unnoticeable (producing minimal, if any, reactance) by oncologists, patient, or companion(s) [37]. After placing the camera cylinder in the examination room, investigators monitored the video recording from a secured, remote location in the clinic
2.4. Measures
2.4.1. Expectations for cure
Prior to interacting with the oncologist, patients and companions were independently asked to endorse one of four statements that best reflected their expectations regarding the patient’s prognosis. These included (for the patient): “I expect to be cured of my cancer,” “I expect my cancer will not worsen, but I will not be cured,” “I expect my symptoms will be relieved, but I will not be cured,” and “I do not know what to expect.” Participants who believed that the cancer would worsen and that their symptoms would not be relieved would have to choose the option “I do not know what to expect.” Forty-seven patients reported expectations of being cured of their cancer; 10 expected their cancer not to worsen, but did not expect a cure; 8 expected symptom relief without a cure; and 35 did not know what to expect regarding cure. The first statement (“I expect to be cured of my cancer”) was effect coded as having a positive expectation of a cure (cure = 0.5); the remaining three statements were effect coded as negative expectations for cure (i.e., not expecting a cure; cure = −0.5). The simplification of expectations categories is supported by data analyses in which all possible expectations were analyzed, and the three categories indicating a negative expectation produced consistent results (i.e., all three negative expectations were associated with the same direction of found effects).
A chi-square analysis of the extent to which expectations about cure were related to patients’ cancer diagnoses indicated that lung cancer patients were less likely to expect a cure than those with other types of cancer; other cancer diagnoses were unrelated to expectations.
2.4.2. Matched expectations
Matched patient–companion expectations were defined as a consistent expectation (i.e., both reported “cure” or both reported “no cure”) between the patient and companion. If more than one companion was present, a match was determined by whether all companions as a group were matched with patients on cure expectations. A mismatch was defined as an inconsistency in expectations as reported by the patient and any one of the present companions. Given that there were three options which corresponded to “no cure”, it was perhaps easier for patients and companions to agree on not expecting a cure, however, when more stringent matching criteria were used (patients and companions had to pick exactly the same “no cure” response) the pattern of results were again consistent with that of the simplified coding.
2.4.3. Oncologist–Patient Alliance
The Oncologist-Patient Alliance is a subscale of the global judgment section of the Karmanos Accrual Assessment System (KAAS) [25]. The KAAS is a 14-item revised version of the Moffitt Accrual Assessment System (MAAS [24]) in which three independent, trained observers rate several aspects of the videotaped oncologist–patient interactions. Any disagreements between coders were resolved through discussion. Principal components factor analyses with varimax (orthogonal) rotations were conducted on the items. Using the criteria of eigenvalues >1, visual inspection of plots of these values (i.e., scree criteria) and theoretical coherence of the factors, the subscale for the Oncologist–Patient Alliance was identified (which accounted for 31% of the variance across items with an internal reliability of alpha = 0.82). The Oncologist–Patient Alliance is measured by the following 8 items: “rapport with the oncologist,” “closeness to the oncologist,” “trust with the oncologist,” “level of information provided by oncologist (whether too much or too little),” “oncologist responsiveness,” “the extent to which the oncologist appears organized,” “clarity and use of explanatory examples by the oncologist,” and “amount of hope provided by the oncologist.” The eight items were combined into a single aggregate score using a 0–6 rating scale; higher scores reflected a stronger oncologist–patient alliance. The scale approximated a normal distribution with a mean of 4.33 (SD = 0.57) and was centered in all analyses to facilitate interpretation of analytic results (i.e., the intercept was set as the starting value for those who experienced the mean level of Oncologist–Patient Alliance). See Albrecht et al. (2008) for a more detailed presentation of the scale development and characteristics.
2.4.4. Treatment decision
During the follow-up telephone interview, patients were asked what treatment they believed was being recommended by their oncologists and whether they intended to follow it. Treatment decision was determined by their response. (0 = no intention to follow recommendations, 1 = some intention to follow recommendations, and 2 = intention to follow recommendations). Treatment decision is evaluated from the subjective viewpoint of the patient based on both what the patient thinks the oncologist recommended (which could have involved multiple options including a clinical trial) and whether the patient intends to follow what the patient feels was recommended.
Table 1 provides a breakdown of the expectations of each patient, whether they held matched expectations with present companions, and whether they reported that they intended to follow treatment recommendations.
Table 1.
Breakdown of patient expectations about cure, whether companion(s) matched those expectations, and what treatment intentions patients expressed (PT = patient; CP = companion)
| Cure | Did not expect to be cured | |
|---|---|---|
| Patients with companion(s) (n = 77) | 34 (44%) | 43 (56%) |
| Number of PT whose CP agreed with them | 21 (62%) | 30 (70%) |
| Number of PT who followed recommendations | 26 (76%) | 33 (77%) |
| No companion (n = 24) | 13 (54%) | 11 (46%) |
| Number of PT who followed recommendations | 7 (54%) | 8 (73%) |
2.5. Data preparation and analysis
Analyses were conducted using the GENMOD procedure with GEE (general estimating equations) in SAS 9.1 software for Windows (2003). The general linear models used are based on a regression framework that adjusts for dependency within the data (i.e., the effect of one oncologist treating multiple patient participants). The criterion for statistical significance for all analyses was set at p ≤ .05.
3. Results
Seventy-six percent of the patients (n = 77) were accompanied by at least one companion during the clinic visit. The total number of companions was 114; the largest percentage were spouses (n = 54; 47%), followed by adult children (n = 32; 28%), “other” relationships (n = 10; 9%), friends (n = 7; 6%), siblings, (n = 6; 5%), and parents (n = 5; 4%). Thirty-two percent of patients (n = 25) had more than one companion present (the maximum number of companions with a single patient was 4). Average patient age was 59 (range = 24–89); average companion age was 53 (range = 22–81). Fifty-eight percent (n = 59) of patients were male, 29% (n = 33) of companions were male. Seventy-seven percent of patients were White (non-Hispanic), 15% Black (non-Hispanic), 2% Hispanic, 1% Asian, and 5% did not identify themselves; 89% of companions were White (non-Hispanic), 7% Black (non-Hispanic), 2% Asian, 1% Native American, and 1% did not identify themselves. More than half of patients (63%) had some secondary education. Average household income of the patients varied: 17% <$20,000/year, 33% between $20,000 and $60,000/year, and 35% >$60,000/year (15% were unspecified). None of these demographic variables were found to have an influence on the outcome of interest: patient decision to follow oncologists’ treatment decisions.
Research question #1 Influence of patient expectations for cure on patient decisions to follow treatment as recommended by the oncologist
The majority of patients (54%; n = 54) did not expect their cancer would be cured. A general linear model used patient expectation for cure as the predictor variable and patient decision to follow treatment recommendations as the outcome (whether this visit was the first visit was included as a control). Surprisingly, patient expectations for cure did not directly influence patients’ decision to follow treatment recommendations (Intercept = 1.64, p < .01, Bexpectation = −0.01, ns, Bvisit = 0.01, ns).
Research question #2 Moderating effect of Oncologist–Patient Alliance on patient treatment decision
We tested whether the Oncologist–Patient Alliance moderated the effect of patient expectation for cure on patient treatment decisions. A general linear model used patient expectation for cure, Oncologist–Patient Alliance, and the interaction between these two variables to predict patients’ decision to follow oncologist treatment recommendations (visit number was again included as a control variable). Results showed that the Oncologist–Patient Alliance significantly moderated the effect of patients’ expectation for cure on patients’ decision to follow the recommended treatment (see Table 2 and Fig. 2). Specifically, patients who expected a cure prior to interaction with their oncologist were more likely to follow treatment recommendations when the strength of the Oncologist–Patient Alliance was less strong.
Research question #3 Influence of patient and companion expectations matching on patient treatment decisions
Table 2.
Predicting patient treatment decisions
| Patient decision
|
|||
|---|---|---|---|
| B | SE | p | |
| Research question 2: Does the strength of the Oncologist–Patient Alliance moderate the effect of patients’ expectations for cure on patients’ decisions to follow oncologists’ treatment recommendations (n = 101)? | |||
| Intercept | 1.64 | 0.16 | 0.01 |
| Visit number | 0.01 | 0.19 | ns |
| Patient expectation of cure (A) | −0.02 | 0.10 | ns |
| Observed ratings of interaction (B) | −0.12 | 0.11 | ns |
| A × B | −0.51 | 0.21 | 0.01 |
| Research question 3: Does a match between patients and companions expectations for cure influence patients’ decisions to follow treatment recommendations (n = 77)? | |||
| Intercept | 1.97 | 0.08 | 0.01 |
| Visit number | 0.30 | 0.11 | 0.01 |
| Patient expectation of cure (A) | −0.01 | 0.090 | ns |
| Match between patient and companion with regard to cure (B) | −0.28 | 0.14 | 0.04 |
| A × B | 0.11 | 0.25 | ns |
Fig. 2.
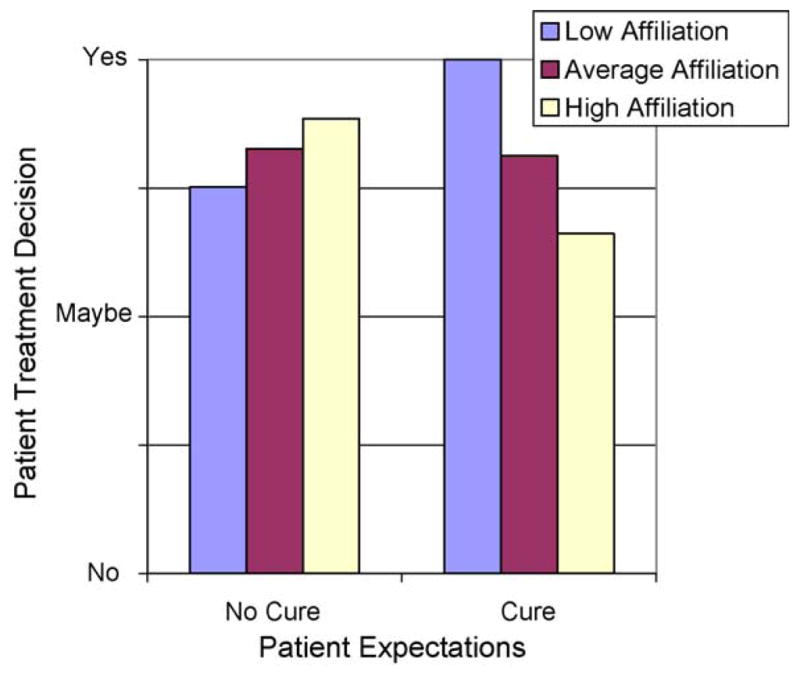
Treatment decision predicted as a function of patient expectations regarding cure and observer rating of the level of affiliation in the oncologist–patient interaction. (Although the results are continuous, to ease interpretation of the predicted results, level of affiliation was divided into low (10th percentile), average (median), and high (90th percentile)).
Matching between patient and companion expectations
First, we analyzed the extent of matching between patients and companions. Similar to patients, 60% of companions (n = 70) believed that the patient would not be cured of cancer. Further, two-thirds of the patient–companion dyads (n = 52) held matched expectations about the patient’s potential for cure. This “matched” group included 40% (n = 21) who expressed an expectation that the patient would be cured, and 60% (n = 31) who did not expect the patient to be cured.
Effect of matching on patient treatment decisions
Next, we tested a general linear model using extent of match in patient–companion expectations, patient expectations, and the interaction between these two variables to predict patients’ decision to follow oncologist treatment recommendations (visit number was again included as a control). The visit number was significant in that when the interaction was not a first visit patients were more likely to follow treatment recommendations. The effect of patient and companion matching (agreement) was significant and negative (see Table 2 and Fig. 3). The interaction between patient expectation for cure and matching was not significant. These two findings together indicate that the important factor is not whether the patients and companions expect a cure or do not except a cure, but rather that they are matched in their expectations for cure. Thus, when patients’ and companions’ expectations were matched in their cure expectations (i.e., agreed regardless of direction of expectation), patients were less likely to report that they intended to follow the doctor’s treatment recommendations.
Fig. 3.
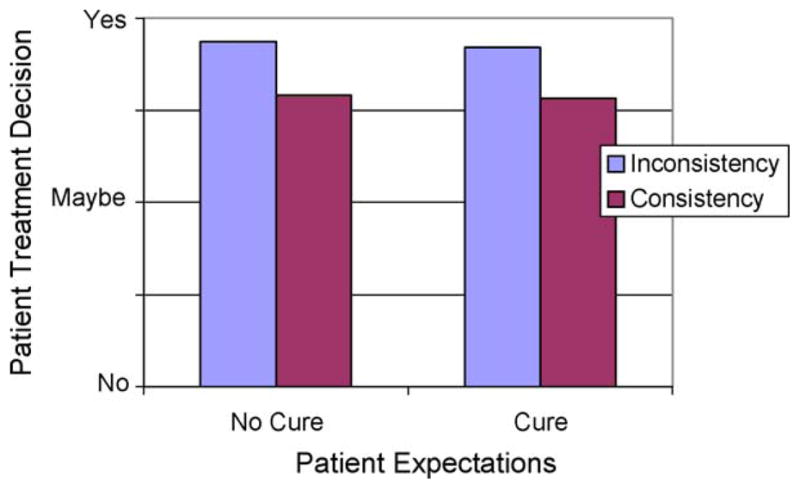
Treatment decision predicted as a function of patient expectations for cure and consistency between patient and companions expectations for cure.
4. Discussion and conclusion
4.1. Discussion
The goal of the study was to investigate whether cancer patients’ expectations for cure prior to meeting with their oncologist influence their decisions to follow their oncologist’s treatment recommendations. Surprisingly, results did not reveal a direct effect of patient expectations for cure on patient treatment decisions; patients’ beliefs about whether they would be cured did not directly influence their decisions about treatment. However, results showed that patient expectations did significantly influ-ence patient decisions when the strength of the Oncologist–Patient Alliance is taken into account. That is, cancer patients who enter the interaction with an expectation of being cured are more likely to decide to follow their oncologist’s treatment recommendations if their alliance with the oncologist is observed to be weaker (i.e., less rapport, responsiveness, trust, organization) during the interaction. Additionally, we found that when patients’ expectations for cure are the same as their companions’ expectations for cure, patients are less likely to follow the oncologists’ treatment recommendations. Taken together, these findings demonstrate that patients’ expectations regarding cure do in fact influence their treatment decisions, but only when considered in the context of the patients’ immediate context, that is their companions’ expectations and the tone of the conversation with the oncologist.
The first finding – that patient expectations interact with the strength of the observed oncologist–patient alliance in the interaction to influence treatment decisions – is perhaps not an expected finding. However, a possible interpretation is that patients who expect a cure prior to meeting with their oncologist may have less of a need for an alliance with their oncologist for their decision-making process. These patients may prefer an interaction that focuses on the details of the task at hand (i.e., treatment) rather than on psychosocial and/or relational aspects of the interaction. Indeed, the psychosocial and/or relational aspects of an interaction may seem less important, and perhaps even distracting, to patients who feel confident about a positive prognosis. Further, this interpretation suggests that patients who do not expect a cure – and therefore may feel a greater burden of uncertainty about the course of their disease – may want and/or need a stronger alliance with their oncologist. A strong alliance may be reassuring and provide a context that enables patients to listen and follow their physician’s advice and perhaps, as a result, allow patients to feel more confident about following recommended treatments. Not expecting a cure and/or feeling uncertain may leave a patient more emotionally vulnerable in the interaction with the oncologist.
Oncologists who are sensitive to these potential dynamics and engage in alliance-building behaviors may help the patient feel reassured, better supported, and thus more confident in their treatment decisions. Indeed, for a patient with an expectation of a poor prognosis, the perception that he/she is relationally aligned with the oncologist in facing the disease and treatment may serve as the basis for following the doctor’s recommendation. In the current study, however, we can only say that patients who expect a cure and have a less strong alliance with their oncologist are more likely to follow treatment recommendations.
Our second finding was that when patients and companions have matched expectations for cure, patients are less likely to follow oncologists’ treatment recommendations. This result occurred regardless of whether both patients and companions held a positive or negative expectation for cure, and further, without knowledge of whether they matched the patients’ actual prognosis. Conversely, when patients’ expectations about cure differed from their companions, patients were more likely to follow the oncologist’s treatment recommendations. These results suggest that when patients and their companions agree on their expectations, the influence of the oncologist may be reduced. Conversely, when patients and companions hold different expectations for cure, patients may be more likely to align with their oncologist, and therefore, follow the treatment recommended by him or her. This finding is in line with previous attitudinal research, which shows that individuals are less likely to change their beliefs when receiving new contrary information when they are with people who share those specific beliefs [31].
4.2. Conclusion
It is clear from this research that it is not patient expectations, companion expectations, or the patient/oncologist interaction alone that influence patient decisions, but rather that a complex interaction of these factors, and likely other factors not included in this study, that influence patient decisions. Future research will likely benefit from considering how patient outcomes are influenced by the interplay between pre-existing expectations, thoughts, and attitudes that patients and companions bring to interactions with oncologists.
It is important to note that in the current study expectations were used to predict a behavioral intention which does not address whether patient expectations remain the same. In the future, research should examine changes in patients’ expectations from prior to the meeting with their oncologists to immediately following and several weeks after the meeting in order to understand how expectations regarding cancer cure might change over time.
Future research should also address several limitations in the current study. For instance, it would be of interest to include the oncologist’s expectation for whether the patient will be cured (the actual medical prognosis) in order to determine the extent to which patients’ expectations reflect medical reality, and in turn, whether this match affects treatment decisions. Although examining questions about cure expectations in a sample of patients with diverse types of cancer is important, as we have done in the current study, it might also be advantageous to investigate these ideas in a sample that was homogenous with respect to diagnosis to explore variations that might be due to particular cancers and/or different stages of that cancer.
4.3. Practice implications
Taken together, these findings suggest that patients who expect a cure are most likely to choose to follow recommended treatment when their companion(s) do not agree with their expectation and when the Oncologist–Patient Alliance during the interaction with the oncologist is less strong. However, it is important to note that our findings come from two separate analyses (i.e., those with and those without companions), and in order to completely understand the full complexity of patient decision-making, further study of the interplay between patient expectations and contextual variables, as studied here, is needed. Additionally, we caution against interpreting these results to suggest that the oncologists’ influence is unimportant or that oncologists should avoid alliance-building behaviors regardless of patient expectations. Rather, we encourage researchers and practitioners to consider the potential dynamics created by the expectations for cure that patients bring with them to oncology interactions—patients and their companions are not blank slates when they visit their oncologist, but instead have previously developed beliefs about many aspects of their illness that may influence their treatment decisions.
Here we have demonstrated the influence that basic beliefs about the possibility of cure have on an important outcome, but patients and their companions likely have expectations about every aspect of their care that will in turn influence how they interpret the information they receive from their practitioner and how they act on that information. In line with work by Back and colleagues [15,16], which suggests that practitioners should question patients about what and how much information they desire, our research suggests that practitioners may want to ask patients and their companions about their expectations for the course of the patient’s disease. Gaining an understanding of the patient and companion expectations may allow practitioners to tailor their messages and behavior to improve communication with patients and their companions.
Footnotes
This research was supported by a grant from the National Cancer Institute (Terrance Albrecht, PI, RO1CA075003) and a postdoctoral training grant from the Agency for Health Quality Research Program on Aging and Urban Health to the Institute of Gerontology, Wayne State University (5T32 HS013819-04).
References
- 1.Cohen L, de Moor C, Amato RJ. The association between treatment-specific optimism and depressive symptomatology in patients enrolled in a Phase I cancer clinical trial. Cancer. 2001;91:1949–55. doi: 10.1002/1097-0142(20010515)91:10<1949::aid-cncr1218>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 2.Kunda Z. Social cognition: making sense of people. Cambridge, MA, US: The MIT Press; 1999. [Google Scholar]
- 3.Lepore SJ, Ituarte PHG. Optimism about cancer enhances mood by reducing negative social interactions. Cancer Res Ther Cont. 1999;8:165–74. [Google Scholar]
- 4.Miller DL, Manne SL, Taylor K, Keates J. Psychological distress and well-being in advanced cancer: the effects of optimism and coping. J Clin Psychol Med S. 1996;3:115–30. doi: 10.1007/BF01996132. [DOI] [PubMed] [Google Scholar]
- 5.Olson JM, Roese NJ, Zanna MP. Expectancies. In: Higgins ET, Kruglanski AW, editors. Social psychology: handbook of basic principles. New York, NY: Guilford Press; 1996. pp. 211–38. [Google Scholar]
- 6.Scheier MF, Weintraub JK, Carver CS. Coping with stress: divergent strategies of optimists and pessimists. J Pers Soc Psychol. 1986;51:1257–64. doi: 10.1037//0022-3514.51.6.1257. [DOI] [PubMed] [Google Scholar]
- 7.Darley JM, Gross PH. A hypothesis-confirming bias in labeling effects. J Pers Soc Psychol. 1983;44:20–33. [Google Scholar]
- 8.Jacobs JE, Eccles JS. The impact of mothers ‘gender-role stereotypic beliefs on mothers’ and children’s ability perceptions. J Pers Soc Psychol. 1992;63:932–44. doi: 10.1037//0022-3514.63.6.932. [DOI] [PubMed] [Google Scholar]
- 9.Rothbart M, Evans M, Fulero S. Recall for confirming events: memory processes and the maintenance of social stereotypes. J Exp Soc Psychol. 1979;15:343–55. [Google Scholar]
- 10.Carver CS, Pozo C, Harris SD, Noriega V, Scheier MF, Robinson DS, et al. How coping mediates the effect of optimism on distress: a study of women with early stage breast cancer. J Pers Soc Psychol. 1993;65:375–90. doi: 10.1037//0022-3514.65.2.375. [DOI] [PubMed] [Google Scholar]
- 11.Fitzgerald TE, Tennen H, Affleck G, Pransky GS. The relative importance of dispositional optimism and control appraisals in quality of life after coronary artery bypass surgery. J Behav Med. 1993;16:25–43. doi: 10.1007/BF00844753. [DOI] [PubMed] [Google Scholar]
- 12.Harper FWK, Schmidt JE, Beacham AO, Salsman JM, Averill AJ, Graves KD, et al. The role of social cognitive processing theory and optimism in positive psychosocial and physical behavior change after cancer diagnosis and treatment. Psycho-oncol. 2007;16:79–91. doi: 10.1002/pon.1068. [DOI] [PubMed] [Google Scholar]
- 13.Scheier MF, Matthews KA, Owens JF, Magovern GJ, et al. Dispositional optimism and recovery from coronary artery bypass surgery: the beneficial effects on physical and psychological well-being. J Pers Soc Psychol. 1989;57:1024–40. doi: 10.1037//0022-3514.57.6.1024. [DOI] [PubMed] [Google Scholar]
- 14.Schulz R, Bookwala J, Knapp JE, Scheier MF, Williamson GM. Pessimism, age, and cancer mortality. Psychol Aging. 1996;11:304–9. doi: 10.1037//0882-7974.11.2.304. [DOI] [PubMed] [Google Scholar]
- 15.Back AL, Arnold RM. Discussing prognosis: how much do you want to know? Talking to patients who do not want information or who are ambivalent. J Clin Oncol. 2006;24:4214–7. doi: 10.1200/JCO.2006.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Back AL, Arnold RM, Baile WF, Tulsky JA, Fryer-Edwards K. Approaching difficult communication tasks in oncology. CA-Cancer J Clin. 2005;55:164–7. doi: 10.3322/canjclin.55.3.164. [DOI] [PubMed] [Google Scholar]
- 17.Arora NK. Interacting with cancer patients: the significance of physicians’ communication behavior. Soc Sci Med. 2003;57:791–806. doi: 10.1016/s0277-9536(02)00449-5. [DOI] [PubMed] [Google Scholar]
- 18.Epstein RM, Alper BS, Quill TE. Communicating evidence for participatory decision making. JAMA-J Am Med Assoc. 2004;291:2359–66. doi: 10.1001/jama.291.19.2359. [DOI] [PubMed] [Google Scholar]
- 19.Ford S, Schofield T, Hope T. What are the ingredients for a successful evidence-based patient choice consultation?: a qualitative study. Soc Sci Med. 2003;56:589–602. doi: 10.1016/s0277-9536(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 20.Lutz GK, Butzlaff ME, Atlas SJ, Keller RB, Singer DE, Deyo RA. The relation between expectations and outcomes in surgery for sciatica. J Gen Intern Med. 1999;14:740–4. doi: 10.1046/j.1525-1497.1999.10417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marschall-Kehrel D, Roberts RG, Brubaker L. Patient-reported outcomes in overactive bladder the influence of perception of condition and expectation for treatment benefit. Urology. 2006;68:29–37. doi: 10.1016/j.urology.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 22.Ruckdeschel JC, Blanchard CG, Albrecht T. Psychosocial oncology research. Where we have been, where we are going, and why we will not get there. Cancer. 1994;74:1458–63. doi: 10.1002/1097-0142(19940815)74:4+<1458::aid-cncr2820741610>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 23.Siminoff LA, Step MM. A communication model of shared decision making: accounting for cancer treatment decisions. Health Psychol. 2005;24:99–105. doi: 10.1037/0278-6133.24.4.S99. [DOI] [PubMed] [Google Scholar]
- 24.Albrecht TL, Blanchard C, Ruckdeschel JC, Coovert M, Strongbow R. Strategic physician communication and oncology clinical trials. J Clin Oncol. 1999;17:3324–32. doi: 10.1200/JCO.1999.17.10.3324. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht TL, Eggly SS, Gleason MEJ, Harper FWK, Foster T, Peterson A, Orom H, Penner LA, Ruckdeschel JC. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26:2666–73. doi: 10.1200/JCO.2007.14.8114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins ET, Bargh JA. Social cognition and social perception. Annu Rev Psychol. 1987;38:369–425. doi: 10.1146/annurev.ps.38.020187.002101. [DOI] [PubMed] [Google Scholar]
- 27.Trope Y. Identification and inferential processes in dispositional attribution. Psychol Rev. 1986;93:239–57. [Google Scholar]
- 28.Blanchard CG, Albrecht TL, Ruckdeschel JC. The crisis of cancer: psychological impact on family caregivers. Oncology. 1997;11:189–94. [PubMed] [Google Scholar]
- 29.Eggly S, Penner LA, Greene M, Harper FW, Ruckdeschel JC, Albrecht TL. Information seeking during “bad news” oncology interactions: question asking by patients and their companions. Soc Sci Med. 2006;63:2974–85. doi: 10.1016/j.socscimed.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 30.Labrecque MS, Blanchard CG, Ruckdeschel JC, Blanchard EB. The impact of family presence on the physician-cancer patient interaction. Soc Sci Med. 1982;33:1253–61. doi: 10.1016/0277-9536(91)90073-l. [DOI] [PubMed] [Google Scholar]
- 31.Lederberg MS. The family of the cancer patient. In: Holland J, editor. Psycho-Oncology. New York: Oxford University Press; 1998. pp. 981–93. [Google Scholar]
- 32.Siminoff LA, Rose JH, Zhang A, Zyzanksi SJ. Measuring discord in treatment decision-making; progress toward development of a cancer communication and decision-making tool. Psycho-oncol. 2006;15:528–40. doi: 10.1002/pon.989. [DOI] [PubMed] [Google Scholar]
- 33.Visser AD, van Leeuwen AF. Clinical decision-making at the end of life: the role of the patient’s wish. Patient Educ Couns. 2003;50:263–4. doi: 10.1016/s0738-3991(03)00046-6. [DOI] [PubMed] [Google Scholar]
- 34.Eagly AH, Chaiken S. The psychology of attitudes. Orlando, FL: Harcourt Brace Jovanovich College Publishers; 1993. [Google Scholar]
- 35.Albrecht TL, Penner LA, Ruckdeschel JC. Understanding patient decisions about clinical trials and the associated communication process: a preliminary report. J Cancer Educ. 2003;18:210–4. doi: 10.1207/s15430154jce1804_8. [DOI] [PubMed] [Google Scholar]
- 36.Albrecht TL, Ruckdeschel JC, Ray FL, 3rd, Pethe BJ, Riddle DL, Strohm J, et al. A portable, unobtrusive device for videorecording clinical interactions. Behav Res Meth. 2005;37:165–9. doi: 10.3758/bf03206411. [DOI] [PubMed] [Google Scholar]
- 37.Penner LA, Orom H, Albrecht TL, Franks M, Foster T, Ruckdeschel JC. Camera-related behaviors during video recorded medical interactions. J Nonverbal Behav. 2007;31:99–117. [Google Scholar]