Abstract
Large-gap peripheral nerve injuries present a significant challenge for nerve regeneration due to lack of suitable grafts, insufficient cell penetration, and repair. Biomimetic nanofibrous scaffolds, functionalized on the surface with extracellular matrix proteins, can lead to novel therapies for repair and regeneration of damaged peripheral nerves. Here, nanofibrous scaffolds electrospun from blends of poly(caprolactone) (PCL) and chitosan were fabricated. Taking advantage of the amine groups on the chitosan, the surface of the scaffolds were functionalized with laminin by carbodiimide based crosslinking. Crosslinking allowed laminin to be attached to the surfaces of the PCL-chitosan nanofibers at relatively high concentrations that were not possible using conventional adsorption methods. The nanofibrous meshes were tested for wettability, mechanical properties and cell attachment and proliferation. Blending of chitosan with PCL provided more favorable surfaces for attachment of Schwann cells due to the reduction of the contact angle in comparison to neat PCL. Proliferation rates of Schwann cells grown on PCL-chitosan scaffolds with crosslinked laminin were significantly higher than the rates for PCL-chitosan nanofibrous matrices with adsorbed laminin. PCL-chitosan scaffolds with modified surfaces via crosslinking of laminin could potentially serves as versatile substrates with excellent mechanical and surface properties for in vivo cell delivery for nerve tissue engineering applications.
Keywords: Electrospun Nanofibers, Laminin, Nerve Tissue Engineering, Chitosan
1. Introduction
Peripheral nerve injuries affect 200,000 people every year around the globe and are classified into 2 genres; the small gap (less than 4mm) and the large gap (greater than 4 mm).1 The repair of the small gap injuries is easier and the current gold standards, the autografts, are most suited to such applications. However, the success of autografts to regenerate large gaps over 20 mm, in humans, is fairly limited, owing to shortage of graft sizes and the subsequent loss of function at the donor site.2 Although allografts are effective in some cases, they have a potential to cause immune reactions3,4 and are undesirable due to the possibility of disease transmission.5,6 Tissue engineering serves as an alternative to regenerate large gaps owing to their ease of fabrication and adaptability for generation of custom sized grafts.
Electrospinning allows for the fabrication of scaffolds with an architecture that mimics native extracellular matrix (ECM)7 and can be used to spin many polymers8 including natural polymers like proteins,9 carbohydrates, or even cells.10 Several studies have shown the application of electrospun polycaprolactone (PCL) based scaffolds for tissue engineering applications.11,12,13 These scaffolds mimic the native collagen like architecture, provide increased surface area supporting increased attachment of cells to the scaffolds, and exhibit mechanical properties which are comparable to those of native acellular nerve grafts.14,15 However, PCL based scaffolds lack active chemical moieties on their surfaces to enable the attachment of extracellular matrix (ECM) proteins, such as laminin.16,17 Contact guidance cues of ECM proteins like laminin play critical roles in the regeneration of large gaps in peripheral nerve tissues and were shown to enhance regeneration capabilities if included in scaffolds.18,19 Laminin containing scaffolds have been shown to promote Schwann cell proliferation and migration, which could potentially provide guidance cues to the ensuing axons, migrating from the proximal to the distal ends.20
Several groups have fabricated functionalized scaffolds by adsorbing laminin on the surfaces of scaffolds to improve regeneration capacity21,22. However, inherent to such methods is the typical uneven coating of the laminin on the surface as well as desorption of the laminin from the surface of scaffolds. Several research groups have also functionalized PCL based scaffolds to produce active groups on the surface, using methods like grafting, blending, plasma treatment and even chemical modifications using agents NaOH or 1,6, hexane diamine.23,24 Such treatments modify the surface morphology of the scaffolds along with adding functionality. Some of the most common grafting agents for PCL are gelatin25 or collagen.20 Both gelatin and collagen would leave the matrix earlier than desired, unless they are crosslinked to the surface, usually with cytotoxic agents like paraformaldehyde, or glutaraldehyde.22
On the other hand, addition of chitosan, a natural biopolymer, to PCL provides a promising approach to modify the surface and provide active amine groups, which are available for protein attachment and subsequent functionalization. 26,27 Chitosan has also been electrospun by itself as a scaffold for peripheral nerve regeneration, but the scaffold is very brittle under physiological conditions hence it is deemed unsuitable for nerve tissue engineering.28 We focus here on the fabrication of nanofibrous scaffolds, which are electrospun from blends of PCL-chitosan and evaluate the ability of such nanofibers to provide a medium to crosslink ECM proteins like laminin. Furthermore, we aim to understand the effects of chitosan addition to PCL25 and evaluate surface wetting and attachment and proliferation of Schwann cells on the scaffolds.
2. Experimental Details
2.1 Morphological Characterization
2.1.1 Electrospinning
Polycaprolactone and chitosan were purchased in pellet and powdered forms, respectively, from Sigma Aldrich and were used without any further purification. An 8% (w/v) of PCL and 0.8% (w/v) of chitosan were prepared in hexafluoroisopropanol (HFIP, Oakwood products) and were stirred overnight to obtain clear solutions. They were then mixed in a 1% (vol/vol of chitosan to PCL) and stirred over night to form a uniform clear solution. The electrospinning conditions were set at 10 μl/min, 10 cm distance and an operating voltage of 12kV. The fibers were collected on a grounded aluminum foil until the mesh thickness reached 500 micrometers. Scaffolds measuring about 1 inch by 1 inch were cut, removed from the backing aluminum foil and transferred to sterile 6-well plates.
2.1.2 Scanning Electron Microscopy
The surface morphology of the polymeric scaffolds was qualitatively and quantitatively analyzed with a Philips Leo 982 FEG SEM. All SEM specimens were dried in a vacuum desiccator overnight and gold coated, prior to loading into the sample holder of SEM. All samples were imaged at an accelerating voltage of 5 kV and a working distance of 20 mm. Images were obtained at 5000 X to evaluate the quality of the fiber coatings obtained as well as for the determination of the diameters of the fibers obtained. In order to determine the diameter distributions of the nanofibers, at least 25 nanofibers, were characterized from different locations of the electrospun meshes.
2.3 Confocal Microscopy
In order to confirm the presence of chitosan and visualize its distribution, the scaffolds were stained with a 1:1000 solution of Texas red isothiocyanide (TRITC) in dimethylsulfoxide (DMSO) / ethanol mixture (50:50) and allowed to crosslink for an hour. During this process the thiourea bond forms between the NH2 groups from chitosan and the thiocyanide groups from the TRITC. In order to confirm this, plain PCL scaffolds without any chitosan were also used as controls. All samples were imaged using a Nikon Eclipse TE 1000 microscope equipped with confocal optics.
2.4 Water Contact Angle Measurements
The water contact angles of the nanofibers of PCL, chitosan and PCL chitosan nanofibers were measured using a sessile drop method and deionized water. For this experiment, pure PCL, pure chitosan and two different concentrations of chitosan and PCL blends (1 % and 10% vol/vol) were prepared. The contact angles of deionized water on at least 3 samples per scaffold type were characterized to investigate the effects of the blending of chitosan into PCL on the wettability of the scaffolds.
2.5 Differential Scanning Calorimetry
In differential scanning calorimetry (DSC) a scanning rate of 10 °C/min was used to heat electrospun samples from 25 to 400 °C. Nitrogen gas at the rate of 20 mL/min was used as purge gas. Approximately, 10 mg specimens of nanofibrous PCL, PCL-chitosan (0.1% wt/wt) and pure chitosan scaffolds were loaded onto aluminum pans. The nominal melting temperature of PCL was taken as the temperature at which the endothermic peak occurred.
2.6 Tensile properties of PCL-chitosan scaffolds
Tensile properties of the electrospun nanofibrous scaffolds were measured using a Rheometrics Solid Analyzer (RSA III) at an extension rate of 0.10 mm/sec. All the samples were tested at 15 mm gauge length. The specimens consisted of thin sheets, which were 20 mm in length and 10 mm in width. Data were collected and analyzed using TA Orchestrator. At least 5 samples per scaffold type were tested and the data were reported as mean ± standard deviation.
2.7 Crosslinking of Laminin onto PCL-chitosan nanofibers
The key to the functionalization experiments was the crosslinking or binding of the ECM proteins and biologically relevant macromolecules to the free amine groups expressed on the polymer surface. The amine groups being provided by the chitosan polymer configuration furnish the necessary sites for protein crosslinking.29 Solutions of laminin 1:100 (v/v) (1 mg/ml) and PBS were prepared and mixed with 10 mM of ethylene diaminecarbodiimide (EDC) and n – hydroxysuccinimide (NHS). The resulting solution was allowed to sit for one hour under room conditions. The solution was transferred to the fibers and allowed to crosslink overnight, at 4 °C. The scaffolds were then washed with sterile PBS thrice to obtain functionalized scaffolds. As a control, scaffolds without any modifications (plain PCL-chitosan and plain PCL) and laminin adsorbed PCL-chitosan scaffolds were also prepared.
2.8 Evaluation of Laminin Coating on Nanofibrous Scaffolds
To assess the efficiency of the crosslinking of laminin and to determine the amount of laminin crosslinked onto PCL-chitosan scaffolds via EDC, an assay similar to ELISA was developed.30 PCL-chitosan scaffolds with adsorbed laminin, pure PCL-chitosan, and PCL scaffolds with laminin along with EDC were used as controls. At least 3 samples of each type, measuring 1 inch by 1 inch were evaluated for protein attachment studies. The scaffolds were loaded with laminin as described above and soaked thoroughly overnight in PBS, to remove unreacted or un-adsorbed laminin. The scaffolds were then transferred to micro-centrifuge tubes containing 250 μL of PBS and 250 μL of DCM. The PCL component of the scaffolds would dissolve in the DCM and move to the organic phase. Meanwhile the chitosan and the laminin would migrate to the PBS phase, upon vortexing for 1 minute followed by centrifugation at 10000 RPM for 10 minutes. Two distinct layers were formed during this step, and the top layer was extracted for analysis using Bradford protein assay kit, as per the manufacturer’s protocols. Both chitosan and laminin interact with BCA. PCL-chitosan scaffolds without bound laminin were also analyzed and absorbance values were subtracted to obtain the actual laminin content bound to the scaffolds.
2.9 Cell Culture
The testing of cell adhesion and proliferation was conducted using rat Schwann cells obtained from ATCC. Cells were maintained in Dulbecco’s modified eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin streptomycin. The scaffolds were sterilized using ethanol and exposure to ultraviolet light. Cells were seeded at a density of 1•105 cells per scaffold. The scaffolds were then left in an incubator at 37 °C under 5% CO2 for 1 and 4 days before being analyzed using tetrazolium compound (3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt) (MTS) assay. At least 3 scaffolds per time point per scaffold type were evaluated for cell proliferation.
2.10 Statistical analysis
All quantitative data are reported as mean ± standard deviation. At least 3 samples per time point per scaffold type were evaluated for statistical analysis. Statistical differences among the groups of scaffolds were determined by performing a Student’s t-test. A confidence interval of p<0.05 was considered to be statistically significant.
3. Results
3.1 Electrospinning and Morphological Characterization
Parameters such as flow rate and voltage were optimized to obtain continuous fibers of PCL, chitosan and PCL-chitosan blends. Figure 1 shows the SEM images of PCL (Fig. 1(A)), chitosan (Fig. 1(B)) and PCL-chitosan blend (Fig. 1(C)). This proves that continuous non-beaded fibers could be obtained. The distributions of the diameters of the nanofibers were measured. PCL nanofibers had diameters averaging 636.1± 94.8 nm. Meanwhile, the chitosan nanofibers were much thinner with an average diameter of 262.6 ± 161.1 nm. The blended PCL-chitosan nanofibers had an average diameter of 494.5 ± 114.9 nm, which is closer to the diameters of PCL nanofibers, owing to the fact that a major component in the nanofibers was PCL.
Fig. 1.
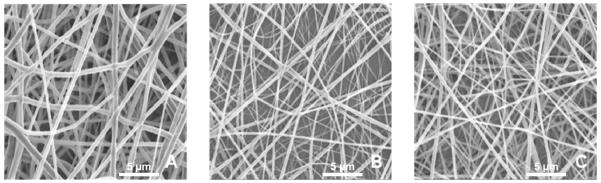
Electrospun nanofibers of (A) PCL nanofibers (B) chitosan nanofibers (C) PCL-chitosan blended nanofibers. Scale bar = 5 microns.
3.2 Confocal Microscopy
The confocal images were obtained to confirm the presence of surface active chitosan on the PCL nanofibers (Fig. 2). Texas Red has the ability to bind to primary amines, present in chitosan, to form a permanent isothiourea linkage. Chitosan was well spread on the surfaces of PCL nanofibers (Fig. 2(A)). Additionally, no beading or phase separation, which would have indicated the non-uniform loading of chitosan in the nanofibers, was observed. In the case of PCL nanofibers (Fig. 2(B)), no significant fluorescence was detected, indicating that active amine groups were not present at the surfaces of the PCL nanofibers.
Fig. 2.
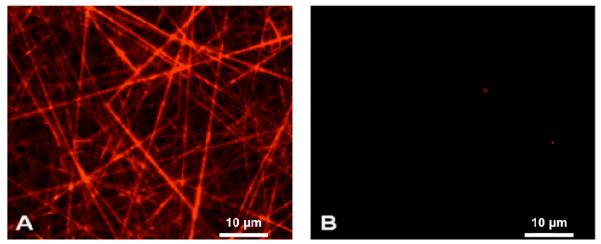
Confocal images of (A) PCL-chitosan and (B) PCL scaffolds stained with amine reactive Texas red dye. Scale Bar = 5 microns.
3.3 Contact Angle Measurements
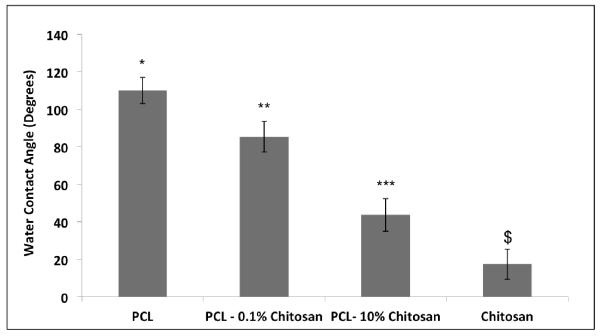
It is known that hydrophilic surfaces promote attachment of cells on the surface. 31,32,33 Therefore, it was very important to control the surface wettability. In order to achieve this, scaffolds were fabricated with varying quantities or ratios of chitosan to PCL. Figure 3 shows the effect of increasing chitosan concentration on the wettability of the scaffolds. PCL provides scaffolds with maximum hydrophobicity, with a water contact angle of 110 ± 7 degrees. Meanwhile, chitosan containing scaffolds showed reduced hydrophobicity, with pure chitosan generating a contact angle of 17.33± 8.58 degrees. PCL and chitosan blends at different ratios, 1% and 10 % (w/w, chitosan to PCL) showed reduced water contact angles in comparison to pure PCL. The reduction was especially important at 10% chitosan. Increased chitosan content has been shown to increase the brittleness of the scaffold34; thus, the composition of PCL-0.1% chitosan was chosen for further cell proliferation and laminin binding studies.
Fig. 3.
Water Contact angle measurements of nanofibrous PCL and chitosan scaffolds. * indicates significant difference (p<0.05) in wettability as compared to scaffolds containing chitosan. ** indicates significant difference in wettability as compared to PCL, PCL-10% chitosan and chitosan scaffolds. *** indicates significant reduction in wettability as compared to PCL, PCL-0.1% chitosan scaffolds. $ indicates significant difference in wettability as compared to scaffolds containing PCL.
3.4 Differential Scanning Calorimetry
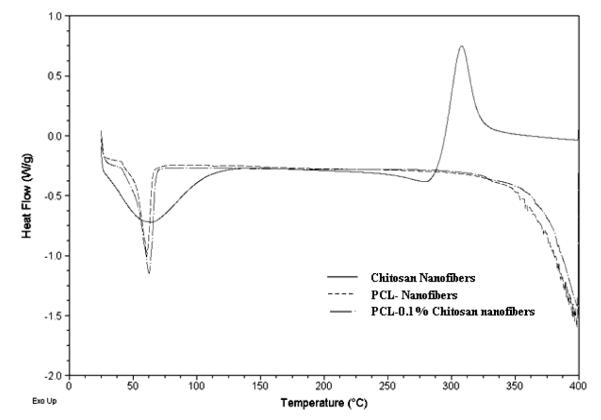
PCL has a melting point around 57 °C and the addition of chitosan to PCL slightly increased the melting point as determined from the peak temperature or from the highest temperature at which the last trace of crystallinity disappears (Fig. 4). However, since most of the nanofibers were composed of PCL and chitosan accounts for only 1% of the fiber chemical composition, the nominal melting temperature (peak temperature of the endotherm) increased only slightly to around 58.5°. At temperatures > 300 °C the degradation of PCL and chitosan was observed.
Fig. 4.
Differential scanning calorimetric evaluation of PCL and chitosan blends.
3.5 Mechanical Properties of PCL chitosan nanofibers
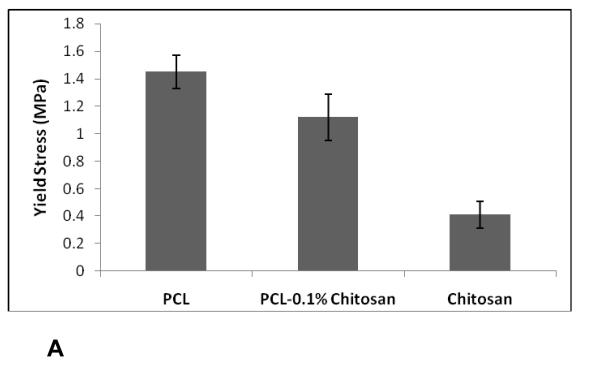
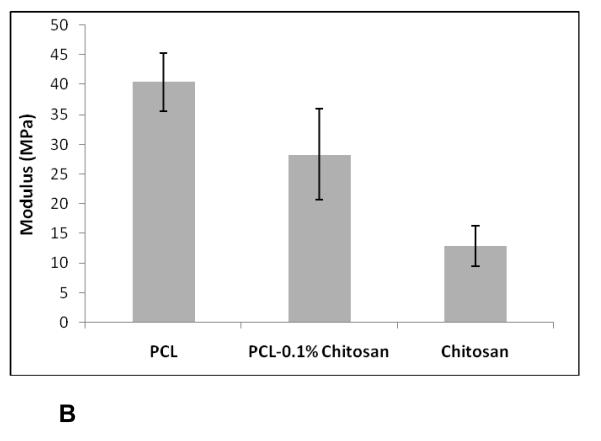
In order to evaluate the mechanical properties of the scaffolds under tensile load, thin films of nanofibrous scaffolds were tested. Figure 5(A)(B) shows histograms of yield stress and modulus of PCL, chitosan, and PCL-0.1% chitosan nanofibrous scaffolds under tensile loading. The ultimate tensile stress was calculated from the point of the first failure or change in slope. The Young’s modulus was calculated from the slope of the linear region of the curve tensile stress versus strain curve below the yield stress. For pure PCL scaffolds, the average tensile strength was 1.44 ± 0.12 MPa, with a Young’s modulus of 40.41 ± 4.78 MPa. The chitosan nanofibers had yield strength of 0.41 ± 0.09 MPa and a modulus of 12.88 ± 3.42 MPa, indicative of brittleness and failure under tensile conditions. However, PCL-chitosan exhibited similar properties to those of PCL nanofibrous scaffolds, with an ultimate stress of 1.12 ± 0.17 MPa and a modulus of 28.31 ± 7.64MPa.
Fig. 5A.
Yield Stress of PCL-chitosan blends under tensile loading
Fig. 5B.
Modulus of PCL-chitosan blends under tensile loading
3.6 Laminin Crosslinked Scaffolds and Evaluation of amount of Laminin available
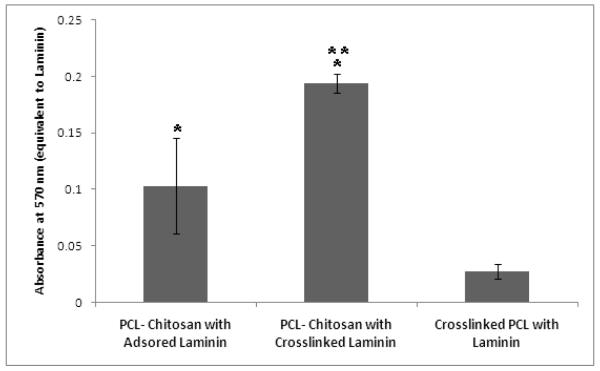
An ELISA-like assay was employed to study the amount of laminin available at the surfaces of the nanofibrous meshes after a thorough washing overnight in PBS. It can be observed from Fig. 6 that, a significantly higher amount of laminin was available at the surface of PCL–chitosan scaffolds in comparison to the amount of laminin found at the surfaces of the fibers of pure PCL. The EDC crosslinked laminin on the scaffolds had significantly higher (p<0.05) amount of laminin on the surface as compared to adsorbed scaffolds. Therefore, the laminin present was irreversibly bound to the surface, which was not the case with adsorbed scaffolds.
Fig 6.
Laminin-crosslinked PCL-chitosan scaffolds. * indicates significantly increased availability of laminin on the scaffolds as compared to pure PCL scaffolds and ** indicates significantly increased laminin present on the scaffolds as compared to PCL-chitosan scaffolds with laminin adsorbed on the scaffolds.
3.7 Schwann cell attachment and proliferation
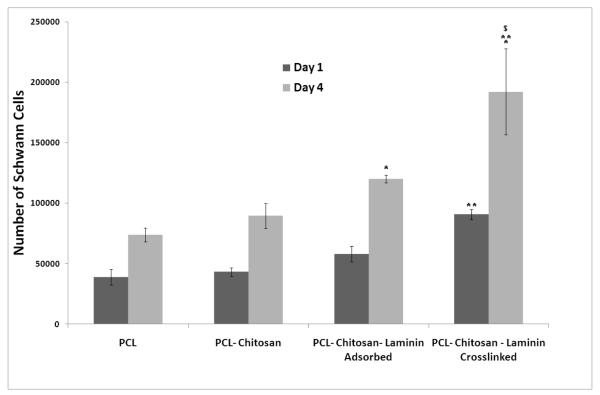
The attachment and proliferation of Schwann cells on scaffolds plays a vital role on the performance of the scaffolds in vivo. PCL-chitosan scaffolds coupled with laminin were evaluated for enhanced Schwann cell attachment and proliferation at 2 time points, days 1 and 4. In Figures 6 and 7 it is shown that the Schwann cells attached and proliferated on all scaffolds; however, the number of cells attached and the rate of proliferation on the laminin-coated scaffolds were higher than those of pure PCL and PCL-chitosan scaffolds. Furthermore, laminin crosslinked scaffolds showed significantly higher attachment and proliferation rates as compared to PCL-chitosan scaffolds with laminin adsorbed on them. By comparing the growth rates through cell number ratios it can be concluded that PCL-chitosan scaffolds with crosslinked laminin had a 2.7-fold increase as compared to the 2.1-fold case increase observed in the case of PCL-chitosan scaffolds with adsorbed laminin. Meanwhile, Schwann cell growth on PCL and PCL-chitosan attained only about 1.5 and 1.7-fold increases, respectively.
Fig. 7.
Schwann cell attachment (day 1) and proliferation (day 4) on PCL-chitosan based scaffolds. * indicates significantly higher Schwann cell numbers as compared to PCL and PCL-chitosan scaffolds (without laminin) ** indicates significantly higher Schwann cell attachment as compared to PCL and PCL-chitosan scaffolds with and without adsorbed laminin $ indicates significantly higher Schwann cell proliferation as compared to PCL-chitosan scaffolds with and without adsorbed laminin
Discussion
Cell attachment and associated interactions with scaffolds are essential to a complete and uniform matrix and tissue development during the process of repair and regeneration of damaged nerve tissue. Scaffolds that mimic the native extracellular matrix (ECM) in terms of their surface topographies, optimal mechanical properties, and more importantly their biological and chemical composition can be advantageous in the process of regeneration. They can promote enhanced cell-material interactions at the micro- scale. Such scaffolds can alleviate drawbacks associated with contemporary techniques and can hasten the process and quality of regeneration.
Cell attachment is a mandatory step in regenerating tissues and populating scaffold with desired cell type. To ensure Schwann cells’ survival in the graft attachment is a requirement for survival and proliferation. 35,36 Attachment studies done by Vleggeert-Lankamp et al. on human Schwann cells showed that 1 hour after seeding most hSCs adhered to laminin coated coverslips as cells displayed small lamellipodial extensions.37 PCL-collagen nanofibers-blends, in comparison to PCL meshes, significantly improved attachment of Schwann cells to PCL-collagen meshes as well as increased proliferation rate between days 1 and 7.38 Proliferation of Schwann cells between 0-3 days on laminin-coated glass was also comparable to those surfaces coated with other substrates such as collagen, fibronectin, or gelatin. However, the proliferation rate in subsequent days (3-12 days) remained unchanged or decreased.37 Similarly, Schwann cell proliferation studies on PCL and PCL-chitosan nanofibers by Prabhakaran et al. have shown increased proliferation rates within first 4 days; nevertheless, these rates stabilized during subsequent day 6 and day 8 MTS measurements.29 Proliferation rates of Schwann cells on PCL-chitosan nanofibers, with increased surface area as compared to glass coverslips, confirmed that this substrate provides a superior niche for Schwann cell growth. Based on previous studies, time points of days 1 and day 4 were chosen specifically to show if laminin content along with increased surface area enhanced proliferation of Schwann cells. Studying cell proliferation during longer in vitro cultures has shown cessation of proliferation as the cell density increased. This phenomenon is ascribed to contact-mediated growth inhibition.39
Addition of chitosan to PCL scaffolds provided functionality, without significantly deteriorating the mechanical properties of the scaffolds. These results are comparable to the results obtained by Ramakrishna et al, where the diameters of PCL and PCL-chitosan fibers range from 190±26nm and 630±40nm respectively. The difference can be accounted by use of TFA/DCM as a solvent and PCL-chitosan ratio of 75:25.25 Confocal microscopy experiments confirmed that PCL-chitosan nanofibers have accessible primary amine groups on the surface available for binding proteins such as laminin. The availability of laminin, a haptotactic heterotrimeric ECM protein composed of α, β and γ chains, is known to be synthesized after the occurrence of nerve damage and provide surface cues for enhanced neurite outgrowth17. Additionally, PCL-0.1% chitosan scaffolds exhibited significantly reduced water contact angles, below 95 degrees. It has been shown that with increasing chitosan content, generally brittleness of the blend increases.34 However, our investigation revealed that PCL-chitosan could exhibit similar properties to those of PCL nanofibrous scaffolds with respect to their ultimate stress and modulus values, provided that the concentration of the chitosan is kept relatively low.
Significantly higher amounts of laminin were present at the surfaces of PCL–chitosan scaffolds as compared to other scaffolds. This can be attributed to the positive charges associated with the presence of amine groups on the surface. Similar observations have been made by Ramakrishna et.al.25 Crosslinking laminin with chitosan component of scaffolds was shown to influence cell material interactions and enhance cell attachment on the scaffolds as compared to pure PCL or PCL-chitosan with adsorbed laminin. However, Ramakrishna et al. showed that the process of blending of laminin with the nanofibers gave rise to a greater concentration of laminin in the nanofibers in comparison to laminin crosslinked at the surface.40 They also indicated that laminin present on the surface as compared to the blending approach enhanced scaffold performance in vitro with a PC-12 based assay. This can be attributed to the fact that laminin, an ECM protein, has to be crosslinked to the surface to provide contact guidance and not diffusional cues for regeneration as in the case of growth factors. The crosslinked laminin on the surface of PLLA fibers showed enhanced performance as compared to scaffolds with laminin adsorbed or scaffolds without laminin, which are similar to our observations.
Enhanced rates of proliferation can potentially reduce the time needed for a graft to be implanted back into the patient and give rise to an enhanced nerve regeneration process. Addition of laminin onto PCL-chitosan scaffolds increased the rate of Schwann cell proliferation as compared to other scaffolds; furthermore, laminin crosslinking further enhanced this effect. Ramkrishna et al. compared the rate of cell growth on scaffolds fabricated with or without chitosan and identified that chitosan-containing scaffolds enhanced Schwann cell proliferation on the scaffolds. They attributed this fact to the surface being more wettable owing to the inclusion of chitosan.25 Similar observations have been made by Suwantong et al., where they identified that a more hydrophilic surface (polyhydroxybutyrate) (PHBT) promoted Schwann cell attachment and proliferation as compared to hydrophobic polyhydroxybutyrate-valerate.41 In experiments with other cell types, Madihally et al., showed that blending chitosan with polycaprolactone, enhanced response with mouse embryonic fibroblasts.42
Nonetheless, these authors used a different molecular weight of chitosan, which could account for variability in responses as compared to those reported here. Specifically, these investigations determined that as the ratio of chitosan to PCL increased, cell numbers on their scaffolds were reduced. This can be explained by the fact that a slightly hydrophobic surface supports improved cell proliferation and migration as compared to a very hydrophilic surface. Additionally, Howling et al. have shown that a low-molecular weight chitosan promoted increased cell proliferation in comparison to that of a chitosan with a higher molecular weight.43 Yang et al., have also shown that the inclusion of chitosan in the PCL matrix has increased the rates of osteoblast attachment and proliferation as compared to scaffolds constructed out of pure PCL or pure chitosan.44 Moreover, scaffolds composed of PCL and chitosan gave rise to the osteoblasts forming a more mineralized matrix and increased differentiation.
Additional functionalities can be gained for repair and regeneration of peripheral nerve damage if the nanofibrous scaffolds are to be functionally graded to generate distributions of types of bioactives and bioactive concentrations akin to the fabrication of scaffolds for bone tissue engineering applications using twin screw extrusion based novel processing methods.45,46,47, 48, 49 Such scaffolds can be graded, i.e., tailored for distributions of topographical and biochemical cues for enhancement of nerve regeneration and will be the subject of our future investigations.
Conclusions
Contact guidance cues on the scaffolds play vital roles in the regeneration process, especially in the case of large-nerve gap injuries. Scaffolds that contain ECM proteins, specifically laminin, could potentially enhance Schwann cell migration into the scaffolds and therefore enhance the quality of regeneration, post injury. Scaffolds fabricated with conventional scaffold preparation techniques and their respective biodegradable polymers generally lack active moieties on their surfaces that allow the binding of ECM proteins such as laminin. To accommodate such concerns in this investigation nanofibrous scaffolds composed of polycaprolactone and chitosan were fabricated. The effects of chitosan incorporation on the wettability and mechanical properties were investigated and a suitable composition for studying nerve regeneration process was identified. Scaffolds composed of 0.1% (w/w) of chitosan blended into PCL were determined to be optimal for further investigations of binding of laminin onto the surfaces of the scaffolds. Scaffolds with crosslinked laminin were also fabricated. Rates of Schwann cell attachment and proliferation on these laminin-crosslinked chitosan/PCL scaffolds were determined and compared to the rates on scaffolds adsorbed laminin or without laminin. Enhanced cell attachment and proliferation rates were observed on laminin crosslinked to PCL-chitosan scaffolds in comparison to other scaffold types. A uniform and complete coverage of Schwann cells on the scaffolds was achieved on day 4, indicative of the potential of the laminin-crosslinked chitosan/PCL scaffolds to promote enhanced Schwann cell proliferation rates. This study demonstrates the potential and versatility of tissue engineering scaffolds constructed out of PCL-chitosan and crosslinked laminin to serve as a good alternative to autografts for repair of large-gap nerve defects.
Acknowledgements
The work was supported by U.S. National Institutes of Health grant RO3NS058595. We would like to thank Dr. Seher Ozkan and Dr. Asli Ergun for assistance with water contact angle measurement, differential scanning calorimetry and thermogravimetric analysis. This research effort used microscopy resources that were partially funded by the National Science Foundation through NSF Grant DMR-0922522.
References
- 1.Li ST, Archibald SJ, Krarup C, Madison RD. Peripheral nerve repair with collagen conduits. Clin. Materials. 1992;9:195. doi: 10.1016/0267-6605(92)90100-8. [DOI] [PubMed] [Google Scholar]
- 2.Steed MB, Mukhatyar V, Valmikinathan C, Bellamkonda RV. Advances in bioengineered conduits for peripheral nerve regeneration. Atlas Oral Maxillofac Surg Clin North Am. 2011;19(1):119. doi: 10.1016/j.cxom.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Bain JR. Peripheral nerve and neuromuscular allotransplantation: current status. Microsurgery. 2000;20:384. doi: 10.1002/1098-2752(2000)20:8<384::aid-micr7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 4.Zalwski AA, Gulati AK. Rejection of nevre allografts after cession of immunosuppression with cyclosporine. Transplantation. 1981;31:88. doi: 10.1097/00007890-198101000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Millesi H. Indications and techniques of nerve grafting. In: Gelbertman RH, Lippincott JB, editors. Operative Nerve repair and Reconstruction. Philadelphia: 1991. pp. 525–544. [Google Scholar]
- 6.Terzis JK, Sun DD, Thanos PK. History and basic science review; past, present, and future of nerve repair. J. Reconstr. Microsurg. 1997;13:215. doi: 10.1055/s-2007-1006407. [DOI] [PubMed] [Google Scholar]
- 7.Ma ZW, Kotaki M, Inai R, Ramakrishna S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Eng. 2005;11:101. doi: 10.1089/ten.2005.11.101. [DOI] [PubMed] [Google Scholar]
- 8.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 9.Valmikinathan CM, Defroda S, Yu X. Polycaprolactone and Bovine Serum Albumin Based Nanofibers for Controlled Release of Nerve Growth Factor. Biomacromolecules. 2009;10(5):1084. doi: 10.1021/bm8012499. [DOI] [PubMed] [Google Scholar]
- 10.Jayasinghe S, Irvine S, McEwan JR. Cell electrospinning highly concentrated cellular suspensions containing primary living organisms into cell-bearing threads and scaffolds. Nanomedicine. 2007;2(4):555. doi: 10.2217/17435889.2.4.555. [DOI] [PubMed] [Google Scholar]
- 11.Valmikinathan CM, Hoffman J, Yu X. Impact of Scaffold Micro and Macro Architecture on Schwann Cell Proliferation under Dynamic Conditions in a Rotating Wall Vessel Bioreactor. Mater Sci Eng C Mater Biol Appl. 2011;31(1):22. doi: 10.1016/j.msec.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta D, Venugopal J, Prabhakaran MP, Dev VR, Low S, Choon AT, Ramakrishna S. Aligned and random nanofibrous substrate for the in vitro culture of Schwann cells for neural tissue engineering. Acta Biomat. 2009;5(7):2560. doi: 10.1016/j.actbio.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Wang J, Valmikinathan CM, Liu W, Laurencin CT, Yu X. Spiral-structured, nanofibrous, 3D scaffolds for bone tissue engineering. J Biomed Mater Res A. 2010;93(2):753. doi: 10.1002/jbm.a.32591. [DOI] [PubMed] [Google Scholar]
- 14.Li WJ, Danielson KG, Alexander PG, Tuan RS. Biological responses of chondrocytes cultures in three-dimensional nanofibers poly(ε-caprolactone) scaffolds. J. Biomed. Mater. Res. 2003;67A:1105. doi: 10.1002/jbm.a.10101. [DOI] [PubMed] [Google Scholar]
- 15.Li WJ, Tuli R, Okafor C, Derfoul A, Danielson KG, Hall DJ, Tuan RS. A three-dimensional nanofibrous scaffold for cartilage tissue engineering using human mesenchymal stem cells. Biomaterials. 2005;26:599. doi: 10.1016/j.biomaterials.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 16.Cruz DM, Ribelles JL, Sanchez MS. Blending Polysaccharides with biodegradable Polymers. I. Properties of chitosan/polycarolactone blends. J. Biomed Mater. Res. Appl. Biomater. 2007;85B:303. doi: 10.1002/jbm.b.30947. [DOI] [PubMed] [Google Scholar]
- 17.McDonald D, Cheng C, Chen Y, Zochodne D. Early events of peripheral nerve regeneration. Neuron. Glia. Biol. 2006;2:139. doi: 10.1017/S1740925X05000347. [DOI] [PubMed] [Google Scholar]
- 18.Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin – a glucoprotein from basement membranes. J. Biol. Chem. 1979;254:9933. [PubMed] [Google Scholar]
- 19.Rutka JT, Apodaca G, Stern R, Rosenblum M. The extracellular-matrix of the central and peripheral nervous systems – structure and function. J. Neurosurg. 1988;69:155. doi: 10.3171/jns.1988.69.2.0155. [DOI] [PubMed] [Google Scholar]
- 20.Luckenbill-Edds L. Laminin and the mechanism of neuronal outgrowth. Brain Res. Rev. 1997;23:1. doi: 10.1016/s0165-0173(96)00013-6. [DOI] [PubMed] [Google Scholar]
- 21.Milner R, Wilby M, Nishimura S, Boylen K, Edwards G, Fawcett J, Streuli C, Pytela R, French-Constant C. Division of labor of Schwann cell integrins during migration on peripheral nerve extracellular matrix ligands. Developmental Biol. 1997;185:215. doi: 10.1006/dbio.1997.8547. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZL, Strickland S. Laminin gamma 1 is critical for Schwann cell differentiation, axon myelination, and regenration in the peripheral nerve. J. Cell Biol. 2003;163:889. doi: 10.1083/jcb.200307068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, Lee CN, Teoh SH. Nanofibrous modification on ultra-thin poly(e-caprolactone) membrane via electrospinning. Mater. Sci. Eng. 2007;27(2C):325. [Google Scholar]
- 24.Zhu Y, Gao C, Liu X, Shen J. Surface Modification of Polycaprolactone Membrane via Aminolysis and Biomacromolecule Immobilization for Promoting Cytocompatibility of Human Endothelial Cells. Biomacromolecules. 2002;3(6):1312. doi: 10.1021/bm020074y. [DOI] [PubMed] [Google Scholar]
- 25.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M, Nasr-Esfahani MH, Ramakrishna S. Electrospun poly(ε-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials. 2008;29(34):4532. doi: 10.1016/j.biomaterials.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 26.Valmikinathan CM, Mukhatyar VJ, Jain A, Karumbaiah L, Dasari M, Bellamkonda RV. Photocrosslinkable chitosan based hydrogels for neural tissue engineering. Soft Matter. 2012;8:1964. doi: 10.1039/c1sm06629c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kato K, Utani A, Suzuki N, Mochizuki M, Yamada M, Nomizu M. Identification of Neurite Outgrowth Promoting Sites on the Laminin α3 Chain G Domain. Biochemistry. 2002;41(35):10747. doi: 10.1021/bi020180k. [DOI] [PubMed] [Google Scholar]
- 28.Sarasam AR, Krishnaswamy RK, Madihally SV. Blending chitosan with polycaprolactone: effects on the physicochemical and antibacterial properties. Biomacromolecules. 2006;7:1131. doi: 10.1021/bm050935d. [DOI] [PubMed] [Google Scholar]
- 29.Ghasemi-Mobarakeh L, Prabhakaran MP, Morshed M M, Nasr-Esfahani MH, Ramakrishna S. Electrospun Biocomposite nanofibrous Scaffolds for Neural Tissue Engineering. Tissue Eng. 2008;14(11):1787. doi: 10.1089/ten.tea.2007.0393. [DOI] [PubMed] [Google Scholar]
- 30.Valmikinathan CM, Wang J, Smiriglio S, Golwala N, Yu X. Magnetically Induced Protein Gradients on Electrospun Nanofibers. Comb Chem High Throughput Screen. 2009;12(7):656. doi: 10.2174/138620709788923683. [DOI] [PubMed] [Google Scholar]
- 31.Xu CY, Inai R, Kotaki M, Ramakrishna S. Aligned biodegradable nanofibrous structure: a potential scaffold for blood vessel engineering. Biomaterials. 2004;25:877. doi: 10.1016/s0142-9612(03)00593-3. [DOI] [PubMed] [Google Scholar]
- 32.van Wachem PB, Beugeling T, Feijen J, Bantjes A, Detmers JP, van Aken WG. Interaction of cultured human endothelial cells with polymeric surfaces of different wettabilities. Biomaterials. 1985;6:403. doi: 10.1016/0142-9612(85)90101-2. [DOI] [PubMed] [Google Scholar]
- 33.Clark PP, Moores GR. Cell guidance by micropatterned adhesiveness in vitro. J. Cell Sc. 1992;103:287. doi: 10.1242/jcs.103.1.287. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Song G, Lou T, Peng W. Fabrication of Nano-fibrous PLLA Scaffold Reinforced with Chitosan Fibers. J Biomater Sci Polym Ed. 2009;20(14):1995. doi: 10.1163/156856208X396083. [DOI] [PubMed] [Google Scholar]
- 35.Kleinman HK, Klebe RJ, Martin GR. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981;88:473. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Keilhoff G, Fansa H, Smalla KH, Schneider W, Wolf G. Neuroma: a donor-age independent source of human Schwann cells for tissue engineered nerve grafts. Neuroreport. 2000;11:3805. doi: 10.1097/00001756-200011270-00042. [DOI] [PubMed] [Google Scholar]
- 37.Vleggeert-Lankamp CL, Pêgo AP, Lakke EA, Deenen M, Marani E, Thomeer RT. Adhesion and proliferation of human Schwann cells on adhesive coatings. Biomaterials. 2004;25(14):2741. doi: 10.1016/j.biomaterials.2003.09.067. [DOI] [PubMed] [Google Scholar]
- 38.Schnell E, Klinkhammer K, Balzer S, Brook G, Klee D, Dalton P, Mey J. Guidance of glial cell migration and axonal growth on electrospun nanofibers of poly-epsilon-caprolactone and a collagen/poly-epsilon-caprolactone blend. Biomaterials. 2007;28(19):3012. doi: 10.1016/j.biomaterials.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Casella GT, Wieser R, Bunge RP, Margitich IS, Katz J, Olson L, Wood PM. Density dependent regulation of human Schwann cell proliferation. Glia. 2000;30(2):165. doi: 10.1002/(sici)1098-1136(200004)30:2<165::aid-glia6>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 40.Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29(26):3574. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Suwantong O, Waleetorncheepsawat S, Sanchavanakit N, Pavasant P, Cheepsunthorn P, Bunaprasert T, Supaphol P. In vitro biocompatibility of electrospun poly(3-hydroxybutyrate-co-3-hydroxyvalerat) fiber mats. Int. J. Boil. Macromol. 2007;40:217. doi: 10.1016/j.ijbiomac.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 42.Sarasam A, Madihally SV. Characterization of chitosan–polycaprolactone blends for tissue engineering applications. Biomaterials. 2005;26(27):5500. doi: 10.1016/j.biomaterials.2005.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Howling GI, Dettmar PW, Goddard PA, Hampson FC, Dornish M, Wood EJ. The effect of chitin and chitosan on the proliferation of human skin fibroblasts and keratinocytes in vitro. Biomaterials. 2001;22:2959. doi: 10.1016/s0142-9612(01)00042-4. [DOI] [PubMed] [Google Scholar]
- 44.Yang X, Chen X, Wang H. Acceleration of Osteogenic Differentiation of Preosteoblastic Cells by Chitosan Containing Nanofibrous Scaffolds. Biomacromolecules. 2009;10(10):2772. doi: 10.1021/bm900623j. [DOI] [PubMed] [Google Scholar]
- 45.Ozkan S, Kalyon D, Yu X. Functionally graded β-TCP/PCL nanocomposite scaffolds for bone tissue engineering: In vitro evaluation with human fetal osteoblast cells. J Biomed Mater Res A. 2010;92(3):1007. doi: 10.1002/jbm.a.32425. [DOI] [PubMed] [Google Scholar]
- 46.Ozkan S, Kalyon DM, Yu X, McKelvey C, Lowinger M. Multifunctional protein-encapsulated polycaprolactone scaffolds: Fabrication and in vitro assessment for tissue engineering. Biomaterials. 2009;30:4336. doi: 10.1016/j.biomaterials.2009.04.050. [DOI] [PubMed] [Google Scholar]
- 47.Erisken C, Kalyon D, Wang H. A hybrid twin screw extrusion/electrospinning method to process nanoparticle-incorporated electrospun nanofibers. Nanotechnology. 2008;19:165302. doi: 10.1088/0957-4484/19/16/165302. [DOI] [PubMed] [Google Scholar]
- 48.Erisken C, Kalyon D, Ornek C, Wang H, Xu J. Osteochondral tissue formation through adipose-derived stem cell differentiation using biomimetic tissue scaffolds with graded stimulator concentrations. Tissue Eng Part A. 2011;17(9-10):1239. doi: 10.1089/ten.TEA.2009.0693. [DOI] [PubMed] [Google Scholar]
- 49.Ergun A, Yu X, Valdevit A, Ritter A, Kalyon DM. Radially and axially-graded multi-zonal scaffolds targeting critical-sized bone defects from polycaprolactone/hydroxyapatite/tricalcium phosphate. Tissue Eng Part A. 2012;18(23-24):2426. doi: 10.1089/ten.tea.2011.0625. [DOI] [PMC free article] [PubMed] [Google Scholar]