Abstract
Objective
The concept of disinhibition as a behavioral and biological trait has been considered to be involved in the etiology of alcoholism and its co-existing disorders. The magnitude and functional mapping of event-related potential P3(00) components were analyzed, in order to examine the possible response inhibition deficits in the offspring of alcoholics.
Method
The P3 components were compared between 50 offspring of alcoholics (OA) and a matched normal control group (NC) using a visual Go/NoGo task. The low-resolution electromagnetic tomography (LORETA) was used to analyze the functional brain mapping between groups.
Results
The results indicated that the OA group manifested decreased P3 amplitude during the NoGo but not the Go condition compared to the NC group. The voxel-by-voxel analysis in LORETA showed group differences at several brain regions including prefrontal areas during the processing of NoGo but not Go signals.
Conclusions
The decreased NoGo-P3 suggests that cognitive and neural disinhibition in offspring of alcoholics may serve as a neurocognitive index for a phenotypic marker in the development of alcoholism and related disorders.
Significance
Dysfunctional neural and response inhibition in the offspring of alcoholics perhaps provides an endophenotypic marker of risk for the development of alcoholism and related disorders.
Keywords: P300, Go/NoGo, Inhibitory control, Offspring of Alcoholics, LORETA, Endophenotype
1. Introduction
Alcoholism is a complex and heterogeneous disorder with genetic and environmental variability. Genetic vulnerability for alcoholism is associated with collective variations in many different genes, which, along with interaction with the environment, can cause the risk for the disorder. In order to understand the risk factors involved in alcoholism, research has been directed at identifying the characteristic traits and behaviors (i.e., phenotypes) in affected alcoholics and their pedigrees. A phenotype generally represents the observable characteristics of an organism, which are the joint product of both genotypic and environmental influences (Gottesman and Gould, 2003). As the genetic complexity of alcoholism involves various phenotypes including electroencephalograms (EEGs), event-related potentials (ERPs), and event-related oscillations (EROs), the analysis of such electrophysiological data in alcoholics and their pedigrees is essential to identify and quantify the phenotypic markers for alcoholism and other co-existing disinhibitory disorders (Begleiter and Porjesz, 1990, 1999; Porjesz and Begleiter, 1991; Porjesz et al., 1996, 1998; Reich, 1996; Iacono et al., 2000; Limosin et al., 2000).
The ERP techniques offer a unique approach for assessing the level of brain functioning, as they permit a non-invasive and simultaneous observation of brain signaling and cognition. Further, the ERP is sensitive to sensory, cognitive, and motor aspects of information processing, and it can be a valuable tool in studying the genetics of alcoholism (Porjesz and Begleiter, 1991). In the ERP literature on alcoholism, the reduced P3 amplitude is a consistent finding that seems to characterize people at risk for alcoholism and may serve as a phenotypic marker for alcoholism and related disorders (Porjesz et al., 1998; Begleiter and Porjesz, 1999). Begleiter et al. (1984) reported that the sons of alcoholic fathers, who had no prior exposure to alcohol, showed lower P3 amplitudes without an alcohol challenge. This finding has been replicated in many different experimental conditions in male as well as female offspring of alcoholics (O’Connor et al., 1986, 1987; Begleiter et al., 1987; Porjesz and Begleiter, 1990a; Hill et al., 1990, 1995; Whipple et al., 1991; Berman et al., 1993; Hill and Steinhauer, 1993; Benegal et al., 1995; Ramachandran et al., 1996; Cohen et al., 1997b; Van der Stelt et al., 1998; Rodriguez Holguin et al., 1999; Hill et al., 2000; Ehlers et al., 2001; Ratsma et al., 2001; Hill and Shen, 2002; Ehlers et al., 2003).
This reduction in P3 amplitude is not only observed in alcoholism, but for a spectrum of disinhibitory disorders, such as conduct disorder (CD), attention-deficit hyperactivity disorder (ADHD), oppositional defiant disorder (ODD), and antisocial personality disorder (ASPD) (e.g., Carlson et al., 1999; Bauer et al., 1994; Kiehl et al., 1999, 2000; Kim et al., 2001; Justus et al., 2001; Iacono et al., 2002). In recent years, alcohol/drug dependence is considered to be part of the disinhibitory/externalizing spectrum (Kendler et al., 2003) as these disorders co-exist in their clinical presentation, and share similar electrophysiological indices (i.e. reduced P3 amplitude) (Lewis and Bucholz, 1991; Sher and Trull, 1994; Myers et al., 1995; Reebye et al., 1995; Kuperman et al., 2001; Bauer, 2001). Further, it has been suggested that the production of P3, irrespective of the task and modality, is associated with widespread cortical inhibition (Nash and Williams, 1982; Elbert and Rockstroh, 1987; Woodward et al., 1991; Rockstroh et al., 1992; Roberts et al., 1994; Coenen, 1995; Nash and Fernandez, 1996; Tomberg and Desmedt, 1998), and hence the low P3 amplitude would indicate a state of disinhibition (Begleiter and Porjesz, 1999; McGue et al., 2001; Iacono et al., 2002). Genetically mediated CNS disinhibition as indexed by such electrophysiological anomalies formed the core of the model for alcoholism as proposed by Begleiter and Porjesz (1999).
It has been suggested that the electrophysiological features, especially the ERP components, during a Go/NoGo task could provide direct measures of frontal inhibitory control and thus can serve as biological markers for cognitive and/or neural disinhibition in several disorders including alcoholism (Kok, 1986; Eimer, 1993; Schroger, 1993; Falkenstein et al., 1995; Kopp et al., 1996; Falkenstein et al., 1999; Filipovic et al., 2000; Bokura et al., 2001; Yamanaka et al., 2002; Kamarajan et al., 2004, in press). The ERP studies of the Go/NoGo paradigm mainly focus on the NoGo condition, as it involves active inhibition of prepared responses, whereas the Go condition accounts for the response execution processes. These studies have identified two major markers (during the NoGo condition) for response inhibition: (1) the N2, a negative deflection with a frontocentral maximum around 200–300 ms, and (2) the NoGo-P3, an augmented positive-going peak usually peaking between 300 and 600 ms (Pfefferbaum et al., 1985; Jodo and Inoue, 1990; Jodo and Kayama, 1992; Eimer, 1993; Kopp et al., 1996). This anteriorly distributed NoGo-P3 has markedly reduced amplitude in alcoholic subjects (Cohen et al., 1997a; Kamarajan et al., in press) as well as in individuals at high risk to develop alcoholism (Cohen et al., 1997b), indicating impaired inhibitory control in such populations.
Although these electrophysiological signatures contain valid functional information in the time domain, they do not provide adequate spatial resolution. In other words, one fundamental limitation of these extracranial measurements of EEG/ERPs is that they do not contain sufficient information on the three-dimensional (3D) distribution of neuronal electric activity (Pascual-Marqui et al., 2002). Therefore, the localization of one or more generators of these brain potentials (i.e., the inverse problem) is possible only by using additional (neuroanatomical) constraints (Luck and Girelli, 1998; Winterer and Goldman, 2003). The recently developed method of low resolution electromagnetic tomography (LORETA) (Pascual-Marqui et al., 2002) overcomes these problems by incorporating the neurophysiological observations that measurable EEG-fields on the scalp reflect synchronized neuronal mass activity while close but opposing sources produce no scalp EEG. Therefore, combining ERP mapping with LORETA can characterize the type, timing, and source configuration of neural processing. LORETA has been applied to study different task-related cognitive processing in normal subjects (Schairer et al., 2001; Bokura et al., 2001; Hamm et al., 2002) and in various disorders (van Leeuwen et al., 1998; Berg et al., 2001; Brandeis et al., 2002; Gallinat et al., 2002) including alcoholism (Prabhu et al., 2001; Saletu et al., 2002). Further, this method has also been employed to examine the phenotype-genotype relationship of gene variants in association with event-related activity (Winterer et al., 2000).
In our laboratory, using ERP measures of a Go/NoGo paradigm, we demonstrated that alcoholics as well as individuals who were at high risk for alcoholism showed impairments in response inhibition as well as response production (Cohen et al., 1997a, 1997b; Kamarajan et al., in press). We also studied brain oscillations during the Go/NoGo task, and found that alcoholics had lower band power in delta and theta activity during a NoGo condition, indicating poor inhibitory mechanisms (Kamarajan et al., 2004). In the present study, along with ERPs, spatial-anatomical mapping using LORETA has been implemented in order to study the inhibitory processes in the offspring of alcoholics (OA) as compared to normal controls (NC). Moreover, as the genetics of alcoholism is strongly associated with the concept of disinhibition (Begleiter and Porjesz, 1999), the present study is an attempt to elucidate the magnitude, temporal and spatial characteristics of the ERP features related to response inhibition in both groups. We hypothesized that if the OA group showed processing dysfunctions associated with response inhibition, this could possibly reflect cognitive and neural disinhibition as a risk marker for the development of alcoholism. We also expected that the functional imaging (through LORETA) would exhibit a lower activation in OA subjects in several brain regions (including frontal lobes) during response inhibition.
2. Methods
2.1. Subjects
A sample of 50 offspring of alcoholics (OA) consisting of 29 males and 21 females with an age-range of 18–25 years (Mean = 20.72; S.D. = 2.06), and 50 normal controls (NC) matched for age (Mean = 20.34; S.D. = 1.93), gender, and education were selected. All subjects were right-handed and were recruited through newspaper advertisements and notices. The OA subjects had at least one of their biological parents diagnosed to have alcohol dependence. The initial screening was done using a questionnaire that included the details of alcohol and drug use, medical and psychiatric histories of the subject and his/her relatives. The individuals with major medical, neurological, and psychiatric conditions inclusive of alcohol/drug dependence, and/or with concurrent psychotropic medications were excluded from the study. However, the OA subjects with concurrent or past history of externalizing disorders (such as CD, ASPD, ODD, ADHD) were included in the study. All the subjects were screened for organicity (gross brain damage), using the Mini Mental State Examination (MMSE; Folstein et al., 1975). The subjects were also excluded for their recent (i.e., past few days) drug/alcohol use, based on Breath-analyzer and urine screen. No subjects had hearing or visual impairments. Informed consent was obtained from each individual, and the experimental procedures and ethical guidelines were in accordance with the Institutional Review Board (IRB).
2.2. Experimental Paradigm
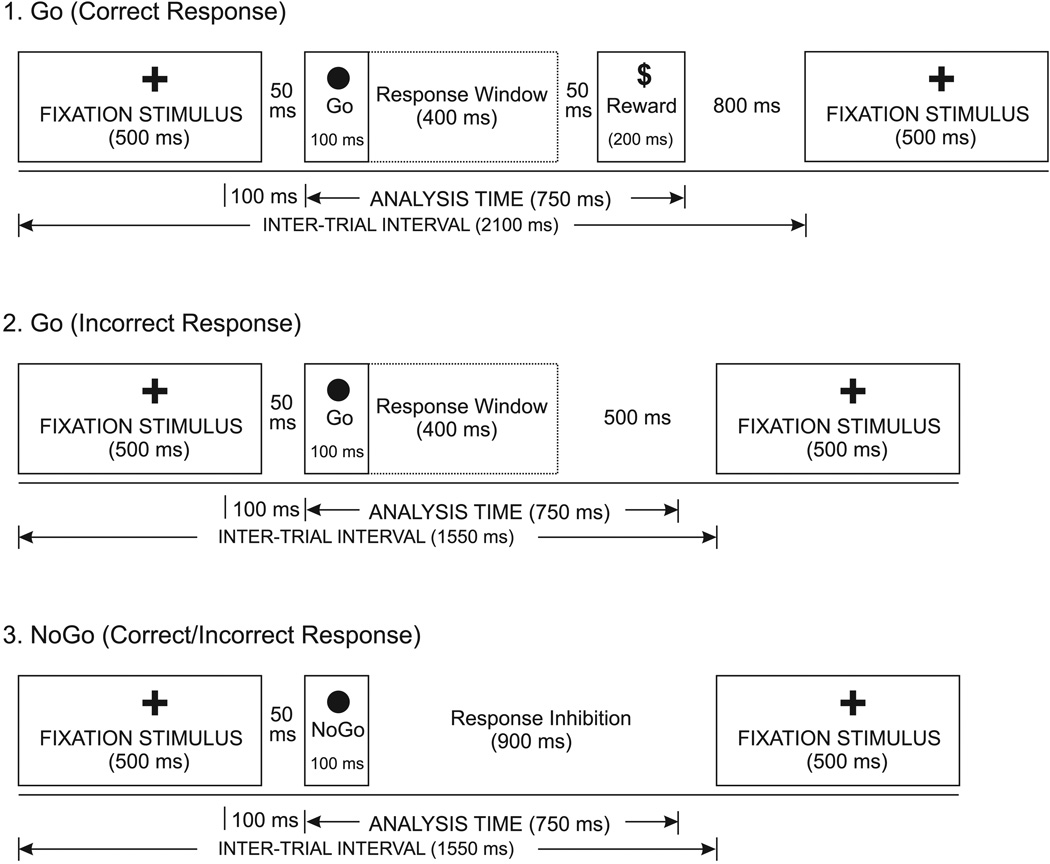
The experimental paradigm is identical to our previous studies (Kamarajan et al., 2004, in press). The stimulus features of the Go/NoGo task are illustrated in Fig. 1. There were three visual stimuli in the task: (i) a cross (fixation stimulus), (ii) a circle (Go or NoGo stimulus), and (iii) a dollar sign (reinforcement sign). These stimuli subtended a visual angle of approximately 1°, and were presented on a computer monitor. The Go and NoGo stimuli were always preceded by a fixation stimulus that appeared at the center of the monitor. The circles that appeared at the top right and bottom left corners served as Go stimuli, to which the subjects had to respond by pressing a button as quickly as possible. The NoGo stimuli, to which the subjects were asked to withhold their response, appeared at the top left and bottom right corners. The dollar-sign appeared whenever there was a correct button-press response to indicate a reward. The probabilities of occurrence of Go and NoGo stimuli were equal (50/50), and the order of these stimuli was randomized.
Figure 1.
Illustration of Go/NoGo task, showing (1) Correct response for the Go condition, (2) Incorrect response for the Go condition, and (3) Correct/incorrect responses for the NoGo condition.
The experiment consisted of a practice phase and a recording phase. The practice phase consisted of twenty Go and NoGo trials, respectively. The subjects were instructed to press a button as quickly as possible whenever they saw a circle in either the top right or bottom left corner. A feedback signal (i.e., a beep) was given whenever the subject’s button-press response was wrong; the practice phase did not accrue any reward. The EEG activity was recorded only during the recording phase which consisted of 100 trials (50 Go and 50 NoGo stimuli). The appearance of a dollar sign in this phase indicated a reward of 25 cents for each correct button-press response, while there was no feedback signal provided for the incorrect responses. The total amount gained as reward was not displayed during the stimulus presentation1.
2.3. EEG Data Acquisition and Signal Analysis
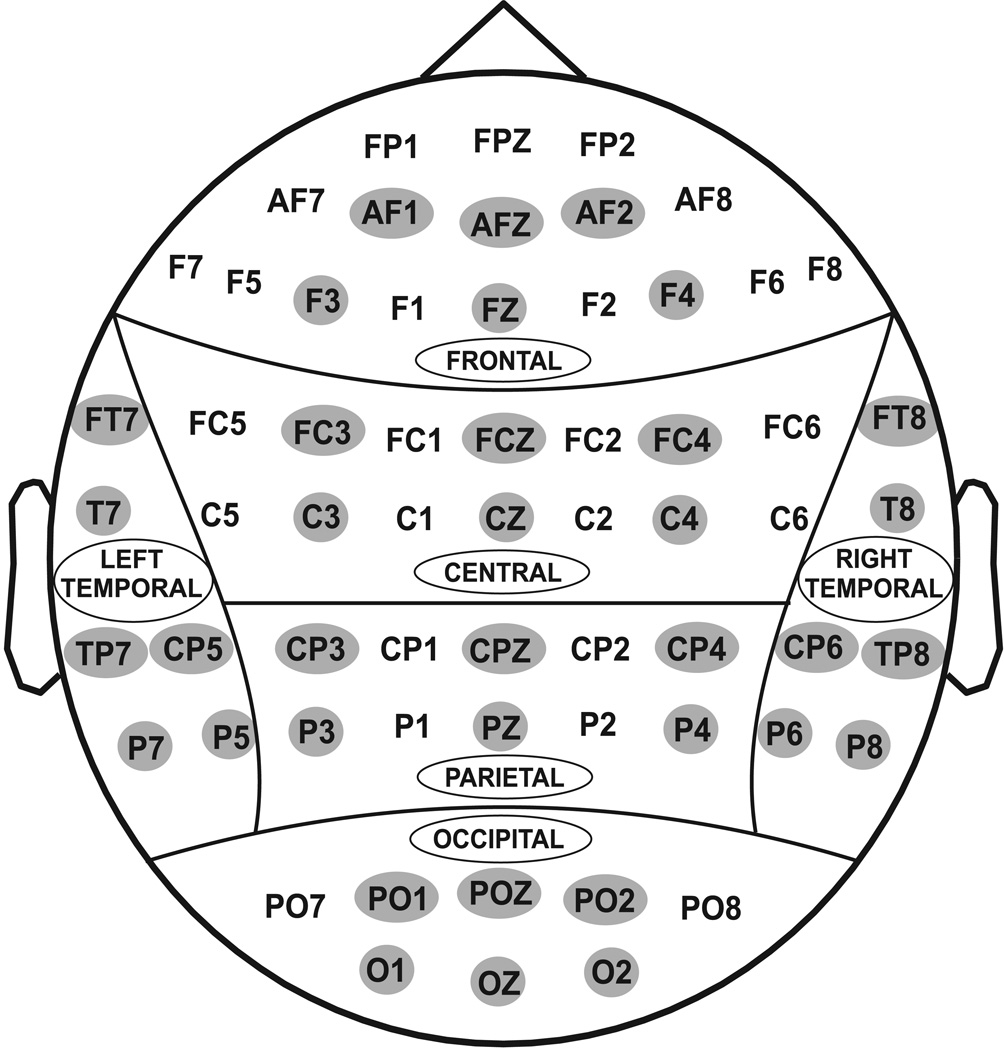
The subjects were seated in a comfortable chair located in a dimly-lit sound-attenuated RF-shielded room (IAC, Industrial Acoustics, Bronx, NY) in front of the task computer placed 1m away. EEG activity was recorded on a Neuroscan system (Version 4.1) (Neurosoft, Inc., El Paso, TX) using a 61-channel electrode cap (Electro-cap International, Inc., Eaton, OH), which included 19 electrodes of the 10–20 International System and 42 additional electrode sites (Electrode Position Nomenclature, American Electroencephalographic Association, 1991) as shown in Fig. 2. The electrodes were referenced to the tip of the nose and the ground electrode was at the forehead (frontal midline). A supraorbital vertical lead and a horizontal lead on the external canthus of the left eye recorded the eye movements. Electrode impedance was maintained below 5 kΩ. The EEG signals were recorded continuously with a bandpass at 0.02–100 Hz and amplified 10,000 times using a set of amplifiers (Sensorium, Charlotte, VT). The data consisted of sampling rates of either 256 or 512 Hz, and were resampled at 256 Hz during the signal analysis for the sake of uniformity.
Figure 2.
Regional grouping of electrodes: (1) Frontal, (2) Central, (3) Parietal, (4) Occipital, (5) Left-temporal, and (6) Right-temporal. The representative channels in each region are highlighted.
The continuous EEG was segmented into epochs of 100 ms pre-stimulus to 750 ms post-stimulus after digital low-pass filtering at 32 Hz. All segments exceeding ± 75 µV threshold were rejected as artifacts. After excluding the trials with eye-movement, the averaged segments for each individual were screened visually for further artifact rejection. Only the trials with correct response (button press) for the Go condition and correct inhibition (no button press) for the NoGo condition were averaged. Using a semi-automatic peak-picking program, the P3 amplitude was measured as the voltage difference from the pre-stimulus baseline to the largest positive going peak in the latency window 300–600 ms after stimulus onset. A minimum of 20 trials was available for each subject in both conditions. The statistical analyses were performed on the P3 amplitude and latency data that were derived separately for Go and NoGo conditions for each subject.
2.4. Statistical Analyses
All 61 electrodes were grouped into six scalp regions for the statistical analyses as shown in Fig. 2. The behavioral data were analyzed using t-test. Initially, the Repeated Measures Analysis of Variance (RMANOVA) was performed by having regions, electrodes, and task condition as within-subject variables and group and gender as between-subject variables. Only 6 representative electrodes from each of the regions were taken into analysis (Fig. 2). As a second stage of analysis, the Multivariate Analysis of Variance (MANOVA) for between groups was performed for each of the regions separately by including all the electrodes of the specific region. The Bonferroni correction for multiple comparisons was implemented by adjusting the resulting p-values.
2.5. LORETA Analyses
The LORETA is a functional imaging method based on certain electrophysiological and neuroanatomical constraints (Pascual-Marqui, 1999, Pascual-Marqui et al., 2002). The cortex has been modeled as a collection of volume elements (voxels) in the digitized Talairach atlas provided by the Brain Imaging Center, Montreal Neurological Institute (MNI). The LORETA algorithm solves the inverse problem by assuming related orientations and strengths of neighboring neuronal sources (represented by adjacent voxels). LORETA has been identified as an efficient tool for functional mapping, since it is consistent with physiology and capable of correct localization (Pascual-Marqui et al., 2002). Along with a comprehensive experimental validation, independent validation of the localization properties of LORETA has been replicated by Yao and He (2001) and by Phillips et al. (2002). The version of LORETA employed here to study the current density and source localization (of the generators of ERP components) was made available at http://www.unizh.ch/keyinst/NewLORETA/LORETA01.htm.
Initially, the voxel-based (2394 voxels per time frame with a spatial resolution of 7 mm) data were created from the ERP data from 61 scalp electrodes for a single time frame that corresponded to the peak value of P3 in each group for both Go and NoGo conditions. The current density (at each voxel) was computed as a linear, weighted sum of the scalp electric potentials scaled to amperes per square meter (A/m2). The current density data created for each of the individuals in both groups were statistically analyzed using the built-in voxelwise independent t-tests with 5000 permutations and corrected for multiple comparisons (Holmes et al., 1996). The voxels with significant differences (p < 0.01) between NC and OA groups were identified in terms of specific brain regions and Brodmann areas (BA) as provided at http://www.unizh.ch/keyinst/NewLORETA/Software/Software.htm.
3. Results
The focus of the current study is to compare ERP features in NC and OA subjects. Therefore, we report the statistical as well as LORETA results only for between-group comparisons.
3.1. Behavioral data
The behavioral performance scores between NC and OA have been shown in Table 1. It was observed that the subjects in OA group tended to commit more errors and have longer reaction times than subjects in the NC group, that approached significance (p<0.10) on both measures.
Table 1.
The performance scores between NC and OA group.
| VARIABLE | NC | OA | t-value | p-value | ||
|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | |||
| MMSE score | 28.74 | 1.94 | 28.10 | 2.04 | 1.608 | 0.111 |
| Reaction time | 301.69 | 27.89 | 311.68 | 31.35 | −1.683 | 0.096 |
| Error (Go) | 4.56 | 2.49 | 6.04 | 5.07 | − 1.852 | 0.067 |
| Error (NoGo) | 1.70 | 1.59 | 1.94 | 1.77 | −0.713 | 0.477 |
| Error (Total) | 6.26 | 2.97 | 7.98 | 5.45 | −1.958 | 0.053 |
3.2. ERP data
The Go/NoGo paradigm used in the present study elicited robust P3 components and also yielded significant statistical differences between groups. Other components of the ERP waveforms (i.e. N1, P2, and N2) did not elicit observable differences and hence were not analyzed. All 61 electrodes were included in the analyses. The RMANOVA model included 2 conditions (Go and NoGo), 6 regions and 6 representative electrodes (Fig. 2) as within-subject factors, and Group and Gender as between-subject factors. It was found that Group main effect (F = 36.09; p = 0.0000) and Group × Condition interaction (F = 13.278; p = 0.0004) were significant. However, the Gender main effect (F = 1.092; p = 0.2985), the Gender × Group interaction (F = 0.481; p = 0.4896), and the Gender × Condition interaction (F = 0.318; p = 0.5744) were not significant. Further, significant main and interaction effects were also observed in Region (F = 98.19; p = 0.0000), Electrode (F = 41.96; p = 0.0000), Group × Region (F = 3.33; p = 0.0083), Group × Electrode (F = 2.81; p = 0.0208), Condition × Region (F = 56.70; p = 0.0000), Condition × Electrode (F = 38.50; p = 0.0000), Region × Electrode (F = 27.56; p = 0.0000), Condition × Region × Electrode (F = 19.83; p = 0.0000). Other interactions were not significant. There were no significant differences observed in P3 latency.
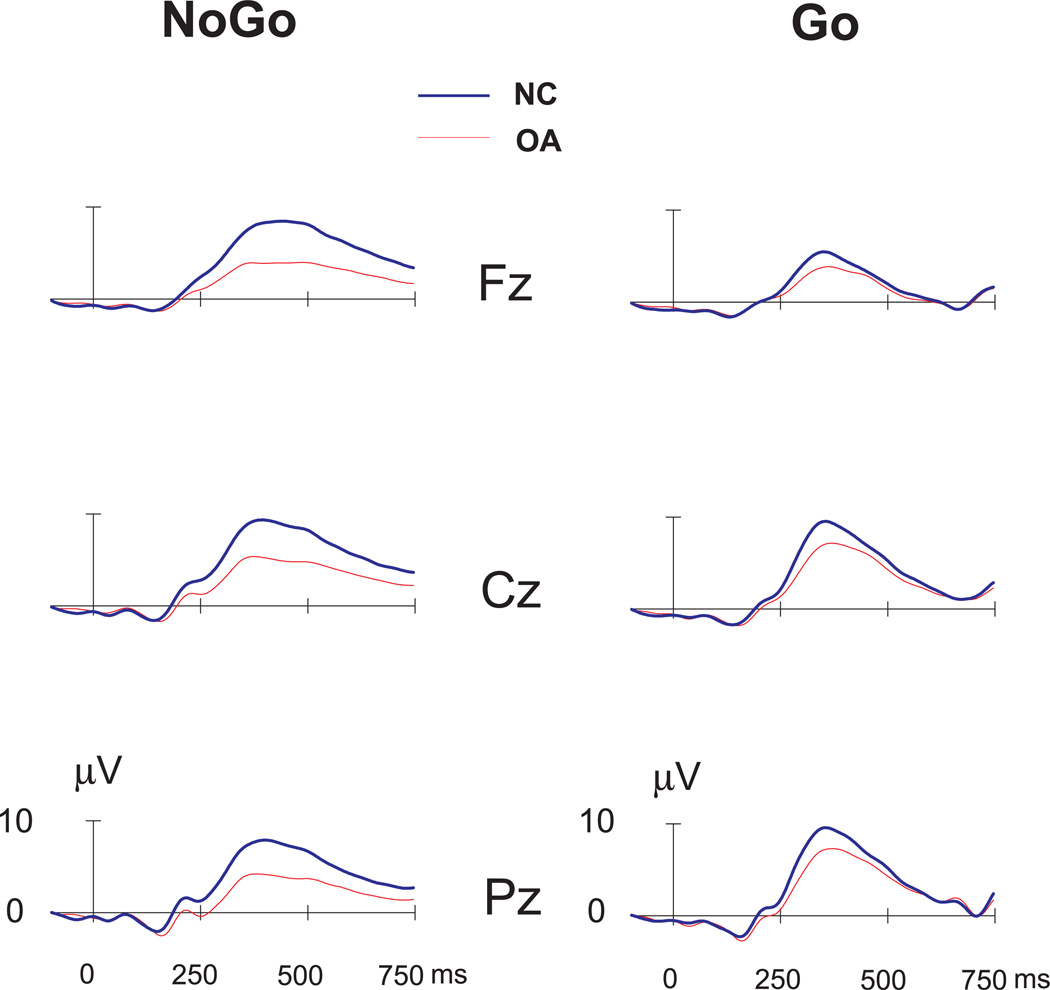
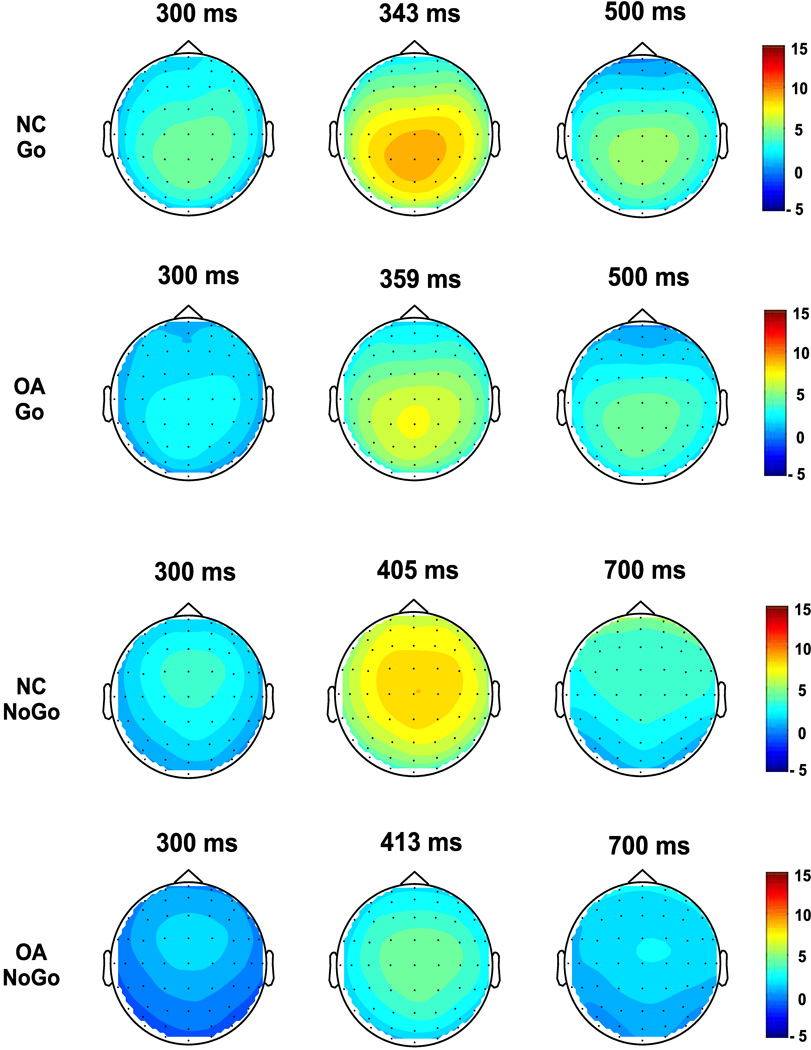
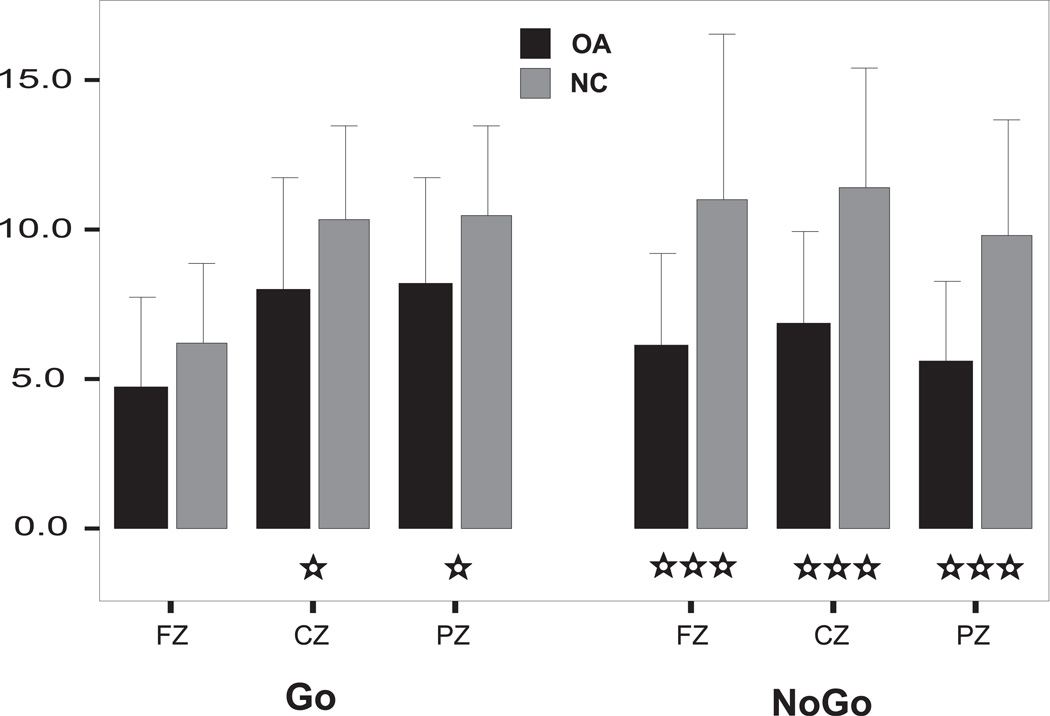
The ERP waveforms and the topography of P3 amplitude in NC and OA groups during NoGo as well as Go conditions are illustrated in (Figs. 3 and 4 respectively. In the NoGo condition, the maximum amplitude was observed in the central region, whereas the Go condition showed a parietal maximum. The region-wise analysis (of MANOVA) showed that the OA group had significantly lower amplitudes in each region during the NoGo but not in Go condition (Table 2). The post-hoc comparison (adjusted for multiple comparisons) between NC and OA groups showed that the significance was more robust in the NoGo condition as compared to Go condition for each of the electrodes (Fig. 5).
Figure 3.
The ERP waveforms of NC versus OA groups during NoGo and Go conditions.
Figure 4.
The spatial distribution of ERP amplitudes (in µV) at three time intervals of P3 component in NC and OA groups during NoGo and Go conditions.
Table 2.
The comparison of P3 amplitude (in µV) between NC and OA groups during the NoGo condition (using MANOVA).
| REGION | NC | OA | F-value (df = 1, 98) |
p-value† | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Frontal | 11.04 | 7.49 | 5.80 | 3.66 | 3.396 | 0.0006*** |
| Central | 10.45 | 4.34 | 6.04 | 2.64 | 3.639 | 0.0006*** |
| Parietal | 9.68 | 3.82 | 5.42 | 2.62 | 4.628 | 0.0002*** |
| Occipital | 7.25 | 3.65 | 3.78 | 2.22 | 5.103 | 0.0002*** |
| Left-Temporal | 7.82 | 4.11 | 4.00 | 2.20 | 6.450 | 0.0001*** |
| Right-Temporal | 8.18 | 3.91 | 4.32 | 2.20 | 8.603 | 0.0000*** |
Bonferroni corrected
p < 0.001
Figure 5.
The mean P3 amplitude (in µV) between NC and OA groups during NoGo and Go trails at FZ, CZ, and PZ electrodes (error bars represent 1 SD). The significance levels represented by star marks are based on independent t-values corrected for multiple comparisons (*p < 0.05; ***p < 0.001).
3.3. LORETA findings
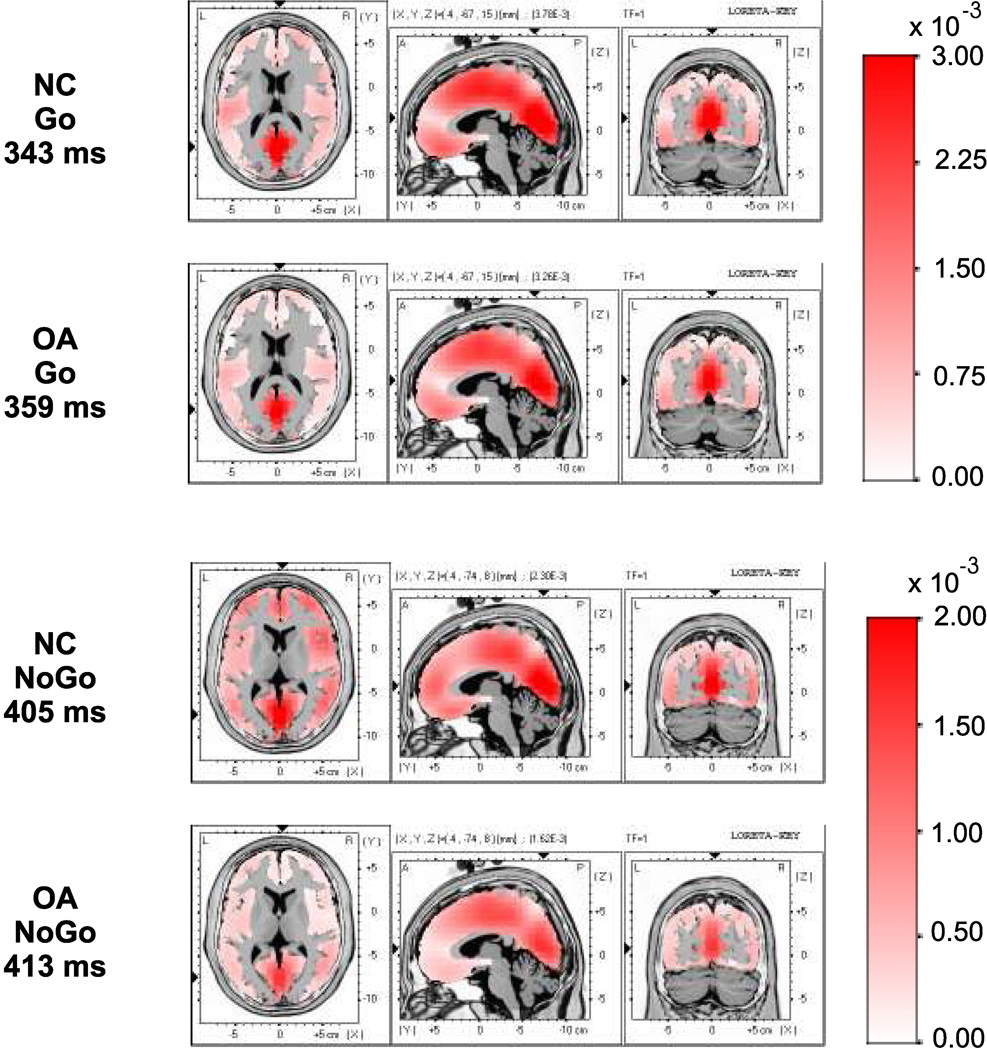
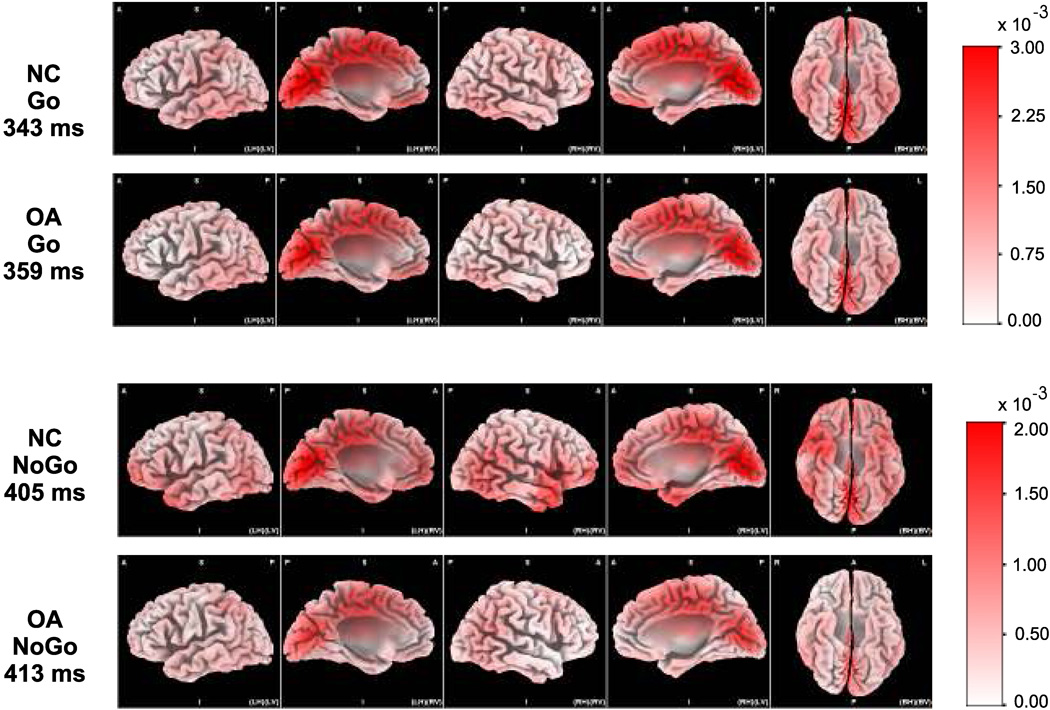
The LORETA images comparing NC and OA groups for Go and NoGo conditions are illustrated in Figs 6 & 7 Statistical analyses revealed that the OA group manifested a significant (p < 0.05) reduction in brain activations in 136 areas (voxels), which also involved 34 specific regions of bilateral frontal lobes such as bilateral anterior cingulate, right inferior frontal gyrus, right middle frontal gyrus, bilateral medial frontal gyri, rectal gyrus (area 11), subcallosal gyrus and left precentral gyrus. These differences in frontal activity were more evident in the right than in the left hemisphere. Other brain regions that showed weaker activations included the bilateral temporal, left parietal, right occipital, limbic, sub-lobar, and hippocampal areas. Visual inspection of LORETA images confirms the findings that OA subjects exhibit reduced activation in frontal, anterior cingulate and tempero-parietal regions during the NoGo condition. On the other hand, there was no significant difference observed in the Go condition in any of the 2394 voxels at the P3 peak, either in the statistical analysis or in the qualitative analysis of LORETA images.
Figure 6.
The LORETA images of three orthogonal (axial, saggital, and coronal) views showing the current density (in amperes per square meter, A/m2) during the peaks of the P3 component in NC and OA groups during the NoGo and Go conditions (NC = Normal Controls; OA = Offspring of Alcoholics).
Figure 7.
The LORETA images of 3-dimensional views showing the current density (in A/m2) during the peaks of the P3 component in NC and OA groups during the NoGo and Go conditions (NC = Normal Controls; OA = Offspring of Alcoholics; LH = Left Hemisphere; RH = Right Hemisphere; BH = Both Hemispheres; A = Anterior; P = Posterior; S = Superior; I = Inferior; LV = Left View; RV = Right View; BV = Bottom view).
4. Discussion
In the present study, the ERP and current density were analyzed in NC and OA groups. In the region-wise analysis, it was found that OA subjects manifested significantly decreased P3 amplitude in the NoGo but not in the Go condition. This finding was further confirmed by the LORETA analyses which revealed that the OA group exhibited a lower activation during the NoGo (but not Go) processing at several brain regions, including frontal and prefrontal areas. These dysfunctions of inhibitory response control in OA group are explained in terms of cognitive and neural disinhibition that is perhaps genetically mediated in causing alcoholism and related disinhibitory disorders. The discussion of the results focuses around three key topics: 1) P3(00) and cognitive processes in the offspring of alcoholics and the genetic implications, 2) different perspectives on the concept of inhibition, and the correlates of disinhibition (especially the electrophysiological indices), and 3) the NoGo-P3 as a potential endophenotypic marker in alcoholism.
4.1. P3 correlates in offspring of alcoholics
Electrophysiological aberrations, using diverse ERP paradigms, have been widely studied in abstinent alcoholics (for reviews, Porjesz and Begleiter, 1983, 1985, 1990b, 1993, 1996). Among the ERP features, the component most frequently studied in alcoholism research is P3(00), a positive going peak that occurs around 300 ms after the stimulus onset (e.g., Porjesz and Begleiter, 1993). The finding that the children of alcoholics have a decreased P3 amplitude has been widely reported, and a meta-analysis of P3 studies in high-risk individuals concluded that the P3 can be a useful investigative tool as an index of vulnerability for alcoholism (Polich et al., 1994). These findings strengthened the view that P3 amplitude can serve as a phenotypic marker for alcoholism. The finding of the present study that the OA group (which also had subjects with co-existing disinhibitory disorders) displayed significantly lower P3 amplitude as compared to that of the (age, gender, handedness, and education matched) NC group is supportive of the notion that P3 amplitude is an index of genetic vulnerability towards the development of alcoholism and related disinhibitory disorders. While P3 amplitude of the Go condition is larger in the NC than OA group, the absence of a significant group difference in the present paradigm may require further explanation, as the Go stimulus cannot be equated with the target stimulus of the Oddball paradigm, where many authors have reported effects of a family history of alcoholism. There are several differences between the target stimulus of the Oddball paradigm and the Go stimulus of the Go/NoGo task used in this study. In general, the task characteristics and instructions are different for the Oddball paradigm and the Go/NoGo task. In the oddball paradigm, attention is directed to the target stimulus, whereas in the Go/NoGo paradigm additional emphasis is placed on inhibiting the NoGo stimuli. Further, in the oddball paradigm, the standard stimuli act as passive signals, whereas in the Go/NoGo task, the NoGo condition involves the active inhibition or suppression of prepotent/prepared responses. While the stimulus probability of Go and NoGo stimuli can vary within paradigms, typically in the “oddball” paradigms the Go (target) is rare and the NoGo is frequent (standard); in the Go/NoGo task they are equiprobable or the NoGo is rare and the Go is frequent. Because probabilities are different, NoGo is more active in Go/NoGo task than oddball where “Go” is a rare occurrence.
Our finding on functional mapping (through LORETA) demonstrated that OA subjects showed less activation only during the NoGo condition in many brain regions including the areas of the prefrontal cortices such as anterior cingulate, orbitofrontal cortex and medial frontal gyri. This reduced activity was more prominent in the right hemisphere than in the left. The possible explanations may include the hypothesis and findings that the right hemisphere is more affected than the left in alcoholics (Ellis and Oscar-Berman, 1989). Imaging studies (e.g. Konishi et al., 1999) as well as ERP studies (e.g. Fallgatter et al., 1998) have shown more prominent right hemispheric activation during the NoGo condition. Taken together, these findings suggest that the cognitive dysfunction in high-risk individuals may be attributable to a dysfunctional response inhibition mechanism which is perhaps genetically mediated. This finding is also supportive of the prefrontal network systems model that was proposed to explain the neuro-cognitive and genetic aspects in the development of alcoholism (Kamarajan et al., 2004, in press). In an fMRI study, Rangaswamy et al. (2004) reported that a dysfunctional fronto-parietal circuit may underlie the low P3 responses seen in children of alcoholics. In addition, the findings of neuropsychological deficits in high risk individuals (Schaeffer et al., 1984; Drejer et al., 1985; Tarter et al., 1989; Peterson et al., 1992; Knop et al., 1993) may serve as valid evidence for the hypothesis that the cognitive deficits may have preceded their alcohol use. Further evidence for the genetic hypothesis of alcoholism came from the observation that the cognitive functions of prefrontal lobe are highly heritable (for a review, Winterer and Goldman, 2003), including that of frontal executive functions (Ando et al., 2001; Swan and Carmelli, 2002; Winterer and Goldman, 2003) and attentional networks (Fan et al., 2001; Fossella et al., 2002). Therefore, it can be suggested that the findings of the present study are suggestive of strong genetic mediation in predisposing the risk to develop alcoholism, as evidenced by cognitive and neurophysiological dysfunctions that are elicited by decreased P3 amplitude and weaker activation of brain areas during response inhibition in OA subjects.
4.2. Inhibition/disinhibition: concepts and correlates
At the behavioral level, however, inhibitory control refers to the ability of the organism to withhold a planned response, to interrupt a response that has been started, to protect an ongoing activity from interfering activity, and to delay a response (Rubia et al., 1998). On the other hand, various neuro-cognitive models suggest that inhibitory control is subserved by the frontal lobe circuits (Giancola and Moss, 1998; Casey et al., 2001, 2002; Goldstein and Volkow, 2002; Chambers et al., 2003; Kamarajan et al., 2004). However, the central concept of inhibition-excitation shares a parallel with Gray’s theory of Behavioral Activation and Inhibition systems (BAS and BIS respectively; Gray, 1972, 1987, 1990). The BAS and BIS are construed as bio-behavioral traits representing the psychobiological systems responsible for arousal/inhibition in humans (Gray, 1988, 1994; Carver and White, 1994), and are linked to frontal activity levels (Harmon-Jones and Allen, 1997; Sutton and Davidson, 1997; Coan and Allen, 2003) and to various forms of child psychopathology (Kooijmans et al., 2000). Further, Finn et al. (1994) found that the subjects who are at high-risk to develop alcoholism had significantly smaller skin conductance responses to the conditioned stimulus for punishment, possibly reflecting weak behavioral inhibition system processes.
It has been theorized that disinhibition as a cognitive and neural construct is involved in predisposing to alcoholism (Begleiter and Porjesz, 1999) and other disinhibitory disorders (Iacono et al., 1999, 2002, 2003). It has been reasoned that the production of P3, irrespective of the task and modality, is associated with widespread cortical inhibition (e.g., Nash and Williams, 1982; Roberts et al., 1994; Tomberg and Desmedt, 1998). Further, the P3 amplitude was found to be significantly decreased in various externalizing disorders (Kendler et al., 2003) such as ASPD (Hesselbrock et al., 1993; Kiehl et al., 1999, 2000; Costa et al., 2000; Justus et al., 2001; Iacono et al., 2002), CD (Bauer and Hesselbrock, 1999a, 1999b, 2003; Iacono et al., 2002), ADHD (Jonkman et al., 1997; Overtoom et al., 1998; Steger et al., 2000; van der Stelt et al., 2001; Banaschewski et al., 2003) and alcohol/drug dependence (e.g., Begleiter and Porjesz, 1999; Hill et al., 1999; Iacono et al., 1999, 2002). Fallgatter and Hermann (2001) explain that features of the NoGo-P3 are valid measures of the brain electrical basis of impulsive behavior and cognitive response control, and hence the impaired NoGo-P3 may also indicate ‘emotional disinhibition’. In this context, alcoholism is considered to be a part of the disinhibitory spectrum, owing to the suppressed P3 amplitude in alcoholics and individuals at risk. Our finding that the OA group showed lower P3 amplitude as well as lower activation of brain regions including frontal areas during response inhibition strongly supports the notion that disinhibition is perhaps the core feature in the predisposition for the development of alcoholism and other disinhibitory disorders.
4.3. NoGo-P3 as an endophenotypic marker for alcoholism
An endophenotype-based approach can facilitate the process of genetic analyses of psychiatric disorders (Gottesman and Gould, 2003). A heritable biological endophenotype could identify those individuals at genetic risk in the absence of overt manifest symptoms (Begleiter and Porjesz, 1999). By citing evidence, Porjesz et al. (1996, 1998) demonstrated that P3 amplitude meets all the criteria to be considered as a phenotypic marker for alcoholism. Since the ERP features and EEG oscillations are highly heritable (e.g., Begleiter et al., 1998; van Beijsterveldt et al., 1996, 1998, 2001, Porjesz et al., 2002; Winterer and Goldman, 2003), dysfunction in these measures in clinical groups, especially in high-risk individuals, suggests a genetic vulnerability for the disorder(s).
In the present study, we observed that the OA group displayed lowered activation in several brain regions during P300 processing of the NoGo (but not Go) condition as elicited by functional imaging through LORETA, reflecting a weaker neural inhibition system in the vulnerable population. This dysfunction may have been genetically mediated, as reported by Jones et al. (2004) who found that the frontal theta band elicited during the P300 time window (300–700 msec) target condition of the visual oddball task was associated with single nucleotide polymorphisms (SNPs) in the cholinergic muscarinic receptor gene (CHRM2) on chromosome 7. Further, this fronto-centrally focused theta activity was reportedly deficient in both alcoholics (Kamarajan et al., 2004) and their offspring (Kamarajan et al. in preparation). The phenomenon of reduced “NoGo-P3”, along with poorer brain activation during NoGo processing, has therefore been supported with considerable evidence to suggest a genetic mediation of inhibition/disinhibition in causing predisposition to develop alcoholism and other disinhibitory disorders in high risk individuals. Thus it can be concluded that the “NoGo-P3” can be considered to be a potential endophenotypic marker for alcoholism and other co-existing disinhibitory disorders, which can be elicited in high-risk individuals even before the onset/manifestation of the disorder. However, further studies using different task paradigms to measure inhibition/disinhibition in different disinhibitory disorders are essential in order to replicate and confirm the findings of the present study.
Acknowledgments
We thank Aquanette Sass, Aleksey Dumer, Lakshmi Krishnamurthy, Glenn Murawski, Tracy Crippen, Carlene Haynes, and Joyce Alonzia for their valuable technical support. This study was supported by the NIH grants # 5 RO1AA002686 and # 5 RO1AA005524 from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). We are grateful to R.D. Pascual-Marqui for the LORETA software used in this study.
Footnotes
However, based on ethical considerations, the subjects received the full amount (without deductions for incorrect responses) at the end of the experiment, although they were not informed of this while performing the experiment.
References
- Ando J, Ono Y, Wright MJ. Genetic structure of spatial and verbal working memory. Behav Genet. 2001;31:615–624. doi: 10.1023/a:1013353613591. [DOI] [PubMed] [Google Scholar]
- Banaschewski T, Brandeis D, Heinrich H, Albrecht B, Brunner E, Rothenberger A. Association of ADHD and conduct disorder--brain electrical evidence for the existence of a distinct subtype. J Child Psychol Psychiatry. 2003;44:356–376. doi: 10.1111/1469-7610.00127. [DOI] [PubMed] [Google Scholar]
- Bauer LO. Antisocial personality disorder and cocaine dependence: their effects on behavioral and electroencephalographic measures of time estimation. Drug Alcohol Depend. 2001;63:87–95. doi: 10.1016/s0376-8716(00)00195-2. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. P300 decrements in teenagers with conduct problems: implications for substance abuse risk and brain development. Biol Psychiatry. 1999a;46:263–272. doi: 10.1016/s0006-3223(98)00335-7. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Subtypes of family history and conduct disorder: effects on P300 during the stroop test. Neuropsychopharmacology. 1999b;21:51–62. doi: 10.1016/S0893-133X(98)00139-0. [DOI] [PubMed] [Google Scholar]
- Bauer LO, Hesselbrock VM. Brain maturation and subtypes of conduct disorder: interactive effects on p300 amplitude and topography in male adolescents. J Am Acad Child Adolesc Psychiatry. 2003;42:106–115. doi: 10.1097/00004583-200301000-00017. [DOI] [PubMed] [Google Scholar]
- Bauer LO, O’Connor S, Hesselbrock VM. Frontal P300 decrements in antisocial personality disorder. Alcohol Clin Exp Res. 1994;18:1300–1305. doi: 10.1111/j.1530-0277.1994.tb01427.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. Neuroelectric processes in individuals at risk for alcoholism. Alcohol Alcohol. 1990;25:251–256. doi: 10.1093/oxfordjournals.alcalc.a044998. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B. What is inherited in the predisposition toward alcoholism? A proposed model. Alcohol Clin Exp Res. 1999;23:1125–1135. doi: 10.1111/j.1530-0277.1999.tb04269.x. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Bihari B, Kissin B. Event-related brain potentials in boys at risk for alcoholism. Science. 1984;225:1493–1496. doi: 10.1126/science.6474187. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Rawlings R, Eckardt M. Auditory recovery function and P3 in boys at high risk for alcoholism. Alcohol. 1987;4:315–321. doi: 10.1016/0741-8329(87)90029-2. [DOI] [PubMed] [Google Scholar]
- Begleiter H, Porjesz B, Reich T, Edenberg HJ, Goate A, Blangero J, Almasy L, Foroud T, Van Eerdewegh P, Polich J, Rohrbaugh J, Kuperman S, Bauer LO, O’Connor SJ, Chorlian DB, Li TK, Conneally PM, Hesselbrock V, Rice JP, Schuckit MA, Cloninger R, Nurnberger J, Jr, Crowe R, Bloom FE. Quantitative trait loci analysis of human event-related brain potentials: P3 voltage. Electroencephalogr Clin Neurophysiol. 1998;108:244–250. doi: 10.1016/s0168-5597(98)00002-1. [DOI] [PubMed] [Google Scholar]
- Benegal V, Jain S, Subbukrishna DK, Channabasavanna SM. P300 amplitudes vary inversely with continuum of risk in first degree male relatives of alcoholics. Psychiatr Genet. 1995;5:149–156. doi: 10.1097/00041444-199524000-00001. [DOI] [PubMed] [Google Scholar]
- Berg D, Herrmann MJ, Muller TJ, Strik WK, Aranda D, Koenig T, Naumann M, Fallgatter AJ. Cognitive response control in writer’s cramp. Eur J Neurol. 2001;8:587–594. doi: 10.1046/j.1468-1331.2001.00298.x. [DOI] [PubMed] [Google Scholar]
- Berman SM, Whipple SC, Fitch RJ, Noble EP. P3 in young boys as a predictor of adolescent substance use. Alcohol. 1993;10:69–76. doi: 10.1016/0741-8329(93)90055-s. [DOI] [PubMed] [Google Scholar]
- Bokura H, Yamaguchi S, Kobayashi S. Electrophysiological correlates for response inhibition in a Go/NoGo task. Clin Neurophysiol. 2001;112:2224–2232. doi: 10.1016/s1388-2457(01)00691-5. [DOI] [PubMed] [Google Scholar]
- Brandeis D, Banaschewski T, Baving L, Georgiewa P, Blanz B, Warnke A, Steinhausen HC, Rothenberger A, Scheuerpflug P. Multicenter P300 brain mapping of impaired attention to cues in hyperkinetic children. J Am Acad Child Adolesc Psychiatry. 2002;41:990–998. doi: 10.1097/00004583-200208000-00018. [DOI] [PubMed] [Google Scholar]
- Carlson SR, Katsanis J, Iacono WG, Mertz AK. Substance dependence and externalizing psychopathology in adolescent boys with small average or large P300 event-related potential amplitude. Psychophysiology. 1999;36:583–590. [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol. 1994;67:319–333. [Google Scholar]
- Casey BJ, Durston S, Fossella JA. Evidence for a mechanistic model of cognitive control. Clin Neurosci Res. 2001;1:267–282. [Google Scholar]
- Casey BJ, Tottenham N, Fossella J. Clinical imaging lesion and genetic approaches toward a model of cognitive control. Dev Psychobiol. 2002;40:237–254. doi: 10.1002/dev.10030. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Taylor JR, Potenza MN. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. Am J Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan JA, Allen JJ. Frontal EEG asymmetry and the behavioral activation and inhibition systems. Psychophysiology. 2003;40:106–114. doi: 10.1111/1469-8986.00011. [DOI] [PubMed] [Google Scholar]
- Coenen AM. Neuronal activities underlying the electroencephalogram and evoked potentials of sleeping and waking: implications for information processing. Neurosci Biobehav Rev. 1995;19:447–463. doi: 10.1016/0149-7634(95)00010-c. [DOI] [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neurophysiological correlates of response production and inhibition in alcoholics. Alcohol Clin Exp Res. 1997a;21:1398–1406. [PubMed] [Google Scholar]
- Cohen HL, Porjesz B, Begleiter H, Wang W. Neuroelectric correlates of response production and inhibition in individuals at risk to develop alcoholism. Biol Psychiatry. 1997b;42:57–67. doi: 10.1016/S0006-3223(96)00221-1. [DOI] [PubMed] [Google Scholar]
- Costa L, Bauer L, Kuperman S, Porjesz B, O’Connor S, Hesselbrock V, Rohrbaugh J, Begleiter H. Frontal P300 decrements alcohol dependence and antisocial personality disorder. Biol Psychiatry. 2000;47:1064–1071. doi: 10.1016/s0006-3223(99)00317-0. [DOI] [PubMed] [Google Scholar]
- Drejer K, Theilgaard A, Teasdale TW, Schulsinger F, Goodwin DW. A prospective study of young men at high risk for alcoholism: neuropsychological assessment. Alcohol Clin Exp Res. 1985;9:498–502. doi: 10.1111/j.1530-0277.1985.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Phillips E, Sweeny A, Slawecki CJ. Event-related potential responses to alcohol-related stimuli in african-american young adults: relation to family history of alcoholism and drug usage. Alcohol Alcohol. 2003;38:332–338. doi: 10.1093/alcalc/agg080. [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Wall TL, Garcia-Andrade C, Phillips E. Visual P3 findings in Mission Indian youth: relationship to family history of alcohol dependence and behavioral problems. Psychiatry Res. 2001;105:67–78. doi: 10.1016/s0165-1781(01)00313-4. [DOI] [PubMed] [Google Scholar]
- Eimer M. Effects of attention and stimulus probability on ERPs in a Go/Nogo task. Biol Psychol. 1993;35:123–138. doi: 10.1016/0301-0511(93)90009-w. [DOI] [PubMed] [Google Scholar]
- Elbert T, Rockstroh B. Threshold regulation-a key to the understanding of the combined dynamics of EEG and event related potentials. J Psychophysiol. 1987;4:317–333. [Google Scholar]
- Ellis RJ, Oscar-Berman M. Alcoholism aging and functional cerebral asymmetries. Psychol Bull. 1989;106:128–147. doi: 10.1037/0033-2909.106.1.128. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol (Amst) 1999;101:267–291. doi: 10.1016/s0001-6918(99)00008-6. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Koshlykova NA, Kiroj VN, Hoormann J, Hohnsbein J. Late ERP components in visual and auditory Go/Nogo tasks. Electroencephalogr Clin Neurophysiol. 1995;96:36–43. doi: 10.1016/0013-4694(94)00182-k. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Herrmann MJ. Electrophysiological assessment of impulsive behavior in healthy subjects. Neuropsychologia. 2001;39:328–333. doi: 10.1016/s0028-3932(00)00115-9. [DOI] [PubMed] [Google Scholar]
- Fallgatter AJ, Wiesbeck GA, Weijers HG, Boening J, Strik WK. Event-related correlates of response suppression as indicators of novelty seeking in alcoholics. Alcohol Alcohol. 1998;33:475–481. doi: 10.1093/alcalc/33.5.475. [DOI] [PubMed] [Google Scholar]
- Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neurosci. 2001;2:14. doi: 10.1186/1471-2202-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovic SR, Jahanshahi M, Rothwell JC. Cortical potentials related to the nogo decision. Exp Brain Res. 2000;132:411–415. doi: 10.1007/s002210000349. [DOI] [PubMed] [Google Scholar]
- Finn PR, Kessler DN, Hussong AM. Risk for alcoholism and classical conditioning to signals for punishment: evidence for a weak behavioral inhibition system? J Abnorm Psychol. 1994;103:293–301. doi: 10.1037//0021-843x.103.2.293. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallinat J, Mulert C, Bajbouj M, Herrmann WM, Schunter J, Senkowski D, Moukhtieva R, Kronfeldt D, Winterer G. Frontal and temporal dysfunction of auditory stimulus processing in schizophrenia. Neuroimage. 2002;17:110–127. doi: 10.1006/nimg.2002.1213. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Moss HB. Executive cognitive functioning in alcohol use disorders. Recent Dev Alcohol. 1998;14:227–251. doi: 10.1007/0-306-47148-5_10. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion: A modification of Eusenck’s theory. In: Nebylitsyn VD, Gray JA, editors. The biological bases of individual behavior. San Diego: Academic Press; 1972. pp. 182–205. [Google Scholar]
- Gray JA. The psychology of fear and stress. Cambridge, England: Cambridge University Press; 1987. [Google Scholar]
- Gray JA. Behavioural and neural-system analyses of the actions of anxiolytic drugs. Pharmacol Biochem Behav. 1988;29:767–769. doi: 10.1016/0091-3057(88)90203-1. [DOI] [PubMed] [Google Scholar]
- Gray JA. Brain systems that mediate both emotion and cognition. Cognition and Emotion. 1990;4:269–288. [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Ekman P, Davidson RJ, editors. The nature of emotion. New York: Oxford University Press; 1994. pp. 243–247. [Google Scholar]
- Hamm JP, Johnson BW, Kirk IJ. Comparison of the N300 and N400 ERPs to picture stimuli in congruent and incongruent contexts. Clin Neurophysiol. 2002;113:1339–1350. doi: 10.1016/s1388-2457(02)00161-x. [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E, Allen JJ. Behavioral activation sensitivity and resting frontal EEG asymmetry: covariation of putative indicators related to risk for mood disorders. J Abnorm Psychol. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. [DOI] [PubMed] [Google Scholar]
- Hesselbrock V, Bauer L, O’Connor S, Gillen R. Reduced P300 amplitude in relation to family history of alcoholism and antisocial personality disorder among young men at risk for alcoholism. Alcohol Alcohol Suppl. 1993;2:95–100. [PubMed] [Google Scholar]
- Hill SY, Lowers L, Locke J, Snidman N, Kagan J. Behavioral inhibition in children from families at high risk for developing alcoholism. J Am Acad Child Adolesc Psychiatry. 1999;38:410–417. doi: 10.1097/00004583-199904000-00013. [DOI] [PubMed] [Google Scholar]
- Hill SY, Shen S. Neurodevelopmental patterns of visual P3b in association with familial risk for alcohol dependence and childhood diagnosis. Biol Psychiatry. 2002;51:621–631. doi: 10.1016/s0006-3223(01)01301-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SY, Shen S, Lowers L, Locke J. Factors predicting the onset of adolescent drinking in families at high risk for developing alcoholism. Biol Psychiatry. 2000;48:265–275. doi: 10.1016/s0006-3223(00)00841-6. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Lowers L, Locke J. Eight-year longitudinal follow-up of P300 and clinical outcome in children from high-risk for alcoholism families. Biol Psychiatry. 1995;37:823–827. doi: 10.1016/0006-3223(95)00041-E. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer S, Park J, Zubin J. Event-related potential characteristics in children of alcoholics from high density families. Alcohol Clin Exp Res. 1990;14:6–16. doi: 10.1111/j.1530-0277.1990.tb00438.x. [DOI] [PubMed] [Google Scholar]
- Hill SY, Steinhauer SR. Assessment of prepubertal and postpubertal boys and girls at risk for developing alcoholism with P300 from a visual discrimination task. J Stud Alcohol. 1993;54:350–358. doi: 10.15288/jsa.1993.54.350. [DOI] [PubMed] [Google Scholar]
- Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. doi: 10.1097/00004647-199601000-00002. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM. Identifying a multivariate endophenotype for substance use disorders using psychophysiological measures. Int J Psychophysiol. 2000;38:81–96. doi: 10.1016/s0167-8760(00)00132-x. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Malone SM, McGue M. P3 event-related potential amplitude and the risk for disinhibitory disorders in adolescent boys. Arch Gen Psychiatry. 2002;59:750–757. doi: 10.1001/archpsyc.59.8.750. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: findings from the Minnesota Twin Family Study. Dev Psychopathol. 1999;11:869–900. doi: 10.1017/s0954579499002369. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Malone SM, McGue M. Substance use disorders, externalizing psychopathology, and P300 event-related potential amplitude. Int J Psychophysiol. 2003;48:147–178. doi: 10.1016/s0167-8760(03)00052-7. [DOI] [PubMed] [Google Scholar]
- Jodo E, Inoue K. Effects of practice on the P300 in a Go/NoGo task. Electroencephalogr Clin Neurophysiol. 1990;76:249–257. doi: 10.1016/0013-4694(90)90019-g. [DOI] [PubMed] [Google Scholar]
- Jodo E, Kayama Y. Relation of a negative ERP component to response inhibition in a Go/No-go task. Electroencephalogr Clin Neurophysiol. 1992;82:477–482. doi: 10.1016/0013-4694(92)90054-l. [DOI] [PubMed] [Google Scholar]
- Jones KA, Porjesz B, Almasy L, Bierut L, Goate A, Wang JC, Dick DM, Hinrichs A, Kwon J, Rice JP, Rohrbaugh J, Stock H, Wu W, Bauer LO, Chorlian DB, Crowe RR, Edenberg HJ, Foroud T, Hesselbrock V, Kuperman S, Nurnberger J, Jr, O’Connor SJ, Schuckit MA, Stimus AT, Tischfield JA, Reich T, Begleiter H. Linkage and linkage disequilibrium of evoked EEG oscillations with CHRM2 receptor gene polymorphisms: implications for human brain dynamics and cognition. Int J Psychophysiol. 2004;53:75–90. doi: 10.1016/j.ijpsycho.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Jonkman LM, Kemner C, Verbaten MN, Koelega HS, Camfferman G, vd Gaag RJ, Buitelaar JK, van Engeland H. Effects of methylphenidate on event-related potentials and performance of attention-deficit hyperactivity disorder children in auditory and visual selective attention tasks. Biol Psychiatry. 1997;41:690–702. doi: 10.1016/s0006-3223(96)00115-1. [DOI] [PubMed] [Google Scholar]
- Justus AN, Finn PR, Steinmetz JE. P300 disinhibited personality and early-onset alcohol problems. Alcohol Clin Exp Res. 2001;25:1457–1466. doi: 10.1097/00000374-200110000-00008. [DOI] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. The role of brain oscillations as functional correlates of cognitive systems: a study of frontal inhibitory control in alcoholism. Int J Psychophysiol. 2004;51:155–180. doi: 10.1016/j.ijpsycho.2003.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamarajan C, Porjesz B, Jones KA, Choi K, Chorlian DB, Padmanabhapillai A, Rangaswamy M, Stimus AT, Begleiter H. Alcoholism is a disinhibitory disorder: neurophysiological evidence from a Go/No-Go Task. Biol Psychol. doi: 10.1016/j.biopsycho.2004.08.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiatry. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Hare RD, Liddle PF, McDonald JJ. Reduced P300 responses in criminal psychopaths during a visual oddball task. Biol Psychiatry. 1999;45:1498–1507. doi: 10.1016/s0006-3223(98)00193-0. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Smith AM, Hare RD, Liddle PF. An event-related potential investigation of response inhibition in schizophrenia and psychopathy. Biol Psychiatry. 2000;48:210–221. doi: 10.1016/s0006-3223(00)00834-9. [DOI] [PubMed] [Google Scholar]
- Kim MS, Kim JJ, Kwon JS. Frontal P300 decrement and executive dysfunction in adolescents with conduct problems. Child Psychiatry Hum Dev. 2001;32:93–106. doi: 10.1023/a:1012299822274. [DOI] [PubMed] [Google Scholar]
- Knop J, Goodwin DW, Jensen P, Penick E, Pollock V, Gabrielli W, Teasdale TW, Mednick SA. A 30-year follow-up study of the sons of alcoholic men. Acta Psychiatr Scand Suppl. 1993;370:48–53. doi: 10.1111/j.1600-0447.1993.tb05360.x. [DOI] [PubMed] [Google Scholar]
- Kok A. Effects of degradation of visual stimulation on components of the event-related potential (ERP) in go/nogo reaction tasks. Biol Psychol. 1986;23:21–38. doi: 10.1016/0301-0511(86)90087-6. [DOI] [PubMed] [Google Scholar]
- Konishi S, Nakajima K, Uchida I, Kikyo H, Kameyama M, Miyashita Y. Common inhibitory mechanism in human inferior prefrontal cortex revealed by event-related functional MRI. Brain. 1999;122(Pt 5):981–991. doi: 10.1093/brain/122.5.981. [DOI] [PubMed] [Google Scholar]
- Kooijmans R, Scheres A, Oosterlaan J. Response inhibition and measures of psychopathology: a dimensional analysis. Neuropsychol Dev Cogn Sect C Child Neuropsychol. 2000;6:175–184. doi: 10.1076/chin.6.3.175.3154. [DOI] [PubMed] [Google Scholar]
- Kopp B, Mattler U, Goertz R, Rist F. N2, P3 and the lateralized readiness potential in a nogo task involving selective response priming. Electroencephalogr Clin Neurophysiol. 1996;99:19–27. doi: 10.1016/0921-884x(96)95617-9. [DOI] [PubMed] [Google Scholar]
- Kuperman S, Schlosser SS, Kramer JR, Bucholz K, Hesselbrock V, Reich T, Reich W. Developmental sequence from disruptive behavior diagnosis to adolescent alcohol dependence. Am J Psychiatry. 2001;158:2022–2026. doi: 10.1176/appi.ajp.158.12.2022. [DOI] [PubMed] [Google Scholar]
- Lewis CE, Bucholz KK. Alcoholism, antisocial behavior and family history. Br J Addict. 1991;86:177–194. doi: 10.1111/j.1360-0443.1991.tb01768.x. [DOI] [PubMed] [Google Scholar]
- Limosin F, Gorwood P, Ad inverted question markes J Relationships between antisocial personality and alcoholism: genetic hypotheses. Eur Psychiatry. 2000;15:123–128. doi: 10.1016/s0924-9338(00)00202-9. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Girelli M. Electrophysiological approaches to the study of selective attention. In: Parasuraman R, editor. The attentive brain. Cambridge MA: MIT Press; 1998. [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I. Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Myers MG, Brown SA, Mott MA. Preadolescent conduct disorder behaviors predict relapse and progression of addiction for adolescent alcohol and drug abusers. Alcohol Clin Exp Res. 1995;19:1528–1536. doi: 10.1111/j.1530-0277.1995.tb01019.x. [DOI] [PubMed] [Google Scholar]
- Nash AJ, Fernandez M. P300 and allocation of attention in dual-tasks. Int J Psychophysiol. 1996;23:171–180. doi: 10.1016/s0167-8760(96)00049-9. [DOI] [PubMed] [Google Scholar]
- Nash AJ, Williams CS. Effects of preparatory set and task demands on auditory event-related potentials. Biol Psychol. 1982;15:15–31. doi: 10.1016/0301-0511(82)90028-x. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Hesselbrock V, Tasman A. Correlates of increased risk for alcoholism in young men. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:211–218. doi: 10.1016/0278-5846(86)90075-8. [DOI] [PubMed] [Google Scholar]
- O’Connor S, Hesselbrock V, Tasman A, DePalma N. P3 amplitudes in two distinct tasks are decreased in young men with a history of paternal alcoholism. Alcohol. 1987;4:323–330. doi: 10.1016/0741-8329(87)90030-9. [DOI] [PubMed] [Google Scholar]
- Overtoom CC, Verbaten MN, Kemner C, Kenemans JL, van Engeland H, Buitelaar JK, Camfferman G, Koelega HS. Associations between event-related potentials and measures of attention and inhibition in the Continuous Performance Task in children with ADHD and normal controls. J Am Acad Child Adolesc Psychiatry. 1998;37:977–985. doi: 10.1097/00004583-199809000-00018. [DOI] [PubMed] [Google Scholar]
- Pascual-Marqui RD. Review of methods for solving the EEG inverse problem. Int J Bio-chromatogr. 1999;1:75–86. [Google Scholar]
- Pascual-Marqui RD, Esslen M, Kochi K, Lehmann D. Functional imaging with low-resolution brain electromagnetic tomography (LORETA): a review. Methods Find Exp Clin Pharmacol. 2002;24(Suppl C):91–95. [PubMed] [Google Scholar]
- Peterson JB, Finn PR, Pihl RO. Cognitive dysfunction and the inherited predisposition to alcoholism. J Stud Alcohol. 1992;53:154–160. doi: 10.15288/jsa.1992.53.154. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Ford JM, Weller BJ, Kopell BS. ERPs to response production and inhibition. Electroencephalogr Clin Neurophysiol. 1985;60:423–434. doi: 10.1016/0013-4694(85)91017-x. [DOI] [PubMed] [Google Scholar]
- Phillips C, Rugg MD, Fristont KJ. Systematic regularization of linear inverse solutions of the EEG source localization problem. Neuroimage. 2002;17:287–301. doi: 10.1006/nimg.2002.1175. [DOI] [PubMed] [Google Scholar]
- Polich J, Pollock VE, Bloom FE. Meta-analysis of P300 amplitude from males at risk for alcoholism. Psychol Bull. 1994;115:55–73. doi: 10.1037/0033-2909.115.1.55. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Almasy L, Edenberg HJ, Wang K, Chorlian DB, Foroud T, Goate A, Rice JP, O’Connor SJ, Rohrbaugh J, Kuperman S, Bauer LO, Crowe RR, Schuckit MA, Hesselbrock V, Conneally PM, Tischfield JA, Li TK, Reich T, Begleiter H. Linkage disequilibrium between the beta frequency of the human EEG and a GABAA receptor gene locus. Proc Natl Acad Sci U S A. 2002;99:3729–3733. doi: 10.1073/pnas.052716399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Brain dysfunction and alcohol. In: Kissin B, Begleiter H, editors. The pathogenesis of alcoholism: biological factors. New York: Plenum Press; 1983. pp. 415–483. [Google Scholar]
- Porjesz B, Begleiter H. Human brain electrophysiology and alcoholism. In: Tarter RD, Van Thiel D, editors. Alcohol and the brain. New York: Plenum Press; 1985. pp. 139–182. [Google Scholar]
- Porjesz B, Begleiter H. Event-related potentials in individuals at risk for alcoholism. Alcohol. 1990a;7:465–469. doi: 10.1016/0741-8329(90)90033-9. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Individuals at risk for alcoholism: Neurophysiologic processes. In: Cloninger CR, Begleiter H, editors. Genetics and biology of alcoholism. New York: Cold Spring Harbor Laboratory Press; 1990b. pp. 137–182. [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors in individuals at risk for alcoholism. Recent Dev Alcohol. 1991;9:53–67. [PubMed] [Google Scholar]
- Porjesz B, Begleiter H. Neurophysiological factors associated with alcoholism. In: Nixon SJ, Hunt WA, editors. Alcohol-induced brain damage. Rockville, MD: NIH Publication; 1993. pp. 89–120. [Google Scholar]
- Porjesz B, Begleiter H. Effects of alcohol on electrophysiological activity of the brain. In: Begleiter H, Kissin B, editors. Alcohol and Alcoholism: The Pharmacology of Alcohol and Alcohol Dependence, Vol 2. New York: Oxford University Press; 1996. pp. 207–247. [Google Scholar]
- Porjesz B, Begleiter H, Litke A, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Visual P3 as a potential phenotypic marker for alcoholism: Evidence from the COGA national project. In: Ogura C, Koga Y, Shimokochi M, editors. Recent Advances in Event-Related Brain Potential Research. Holland: Elsevier Science; 1996. pp. 539–549. [Google Scholar]
- Porjesz B, Begleiter H, Reich T, Van Eerdewegh P, Edenberg HJ, Foroud T, Goate A, Litke A, Chorlian DB, Stimus A, Rice J, Blangero J, Almasy L, Sorbell J, Bauer LO, Kuperman S, O’Connor SJ, Rohrbaugh J. Amplitude of visual P3 event-related potential as a phenotypic marker for a predisposition to alcoholism: preliminary results from the COGA Project. Collaborative Study on the Genetics of Alcoholism. Alcohol Clin Exp Res. 1998;22:1317–1323. [PubMed] [Google Scholar]
- Prabhu VR, Porjesz B, Chorlian DB, Wang K, Stimus A, Begleiter H. Visual p3 in female alcoholics. Alcohol Clin Exp Res. 2001;25:531–539. [PubMed] [Google Scholar]
- Ramachandran G, Porjesz B, Begleiter H, Litke A. A simple auditory oddball task in young adult males at high risk for alcoholism. Alcohol Clin Exp Res. 1996;20:9–15. doi: 10.1111/j.1530-0277.1996.tb01035.x. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Ratsma JE, van der Stelt O, Schoffelmeer AN, Westerveld Boudewijnv W. P3 event-related potential, dopamine D2 receptor A1 allele, and sensation-seeking in adult children of alcoholics. Alcohol Clin Exp Res. 2001;25:960–967. [PubMed] [Google Scholar]
- Reebye P, Moretti MM, Lessard JC. Conduct disorder and substance use disorder: comorbidity in a clinical sample of preadolescents and adolescents. Can J Psychiatry. 1995;40:313–319. doi: 10.1177/070674379504000606. [DOI] [PubMed] [Google Scholar]
- Reich T. A genomic survey of alcohol dependence and related phenotypes: results from the Collaborative Study on the Genetics of Alcoholism (COGA) Alcohol Clin Exp Res. 1996;20:133A–137A. doi: 10.1111/j.1530-0277.1996.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Roberts LE, Rau H, Lutzenberger W, Birbaumer N. Mapping P300 waves onto inhibition: Go/No-Go discrimination. Electroencephalogr Clin Neurophysiol. 1994;92:44–55. doi: 10.1016/0168-5597(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Rockstroh B, Müller MM, Cohen R, Elbert T. Probing the functional brain state during P300-evocation. Journal of Psychophysiology. 1992;6:175–184. [Google Scholar]
- Rodriguez Holguin S, Porjesz B, Chorlian DB, Polich J, Begleiter H. Visual P3a in male subjects at high risk for alcoholism. Biol Psychiatry. 1999;46:281–291. doi: 10.1016/s0006-3223(98)00247-9. [DOI] [PubMed] [Google Scholar]
- Rubia K, Oosterlaan J, Sergeant JA, Brandeis D, v Leeuwen T. Inhibitory dysfunction in hyperactive boys. Behav Brain Res. 1998;94:25–32. doi: 10.1016/s0166-4328(97)00166-6. [DOI] [PubMed] [Google Scholar]
- Saletu B, Anderer P, Saletu-Zyhlarz GM, Pascual-Marqui RD. EEG topography and tomography in diagnosis and treatment of mental disorders: evidence for a key-lock principle. Methods Find Exp Clin Pharmacol. 2002;24(Suppl D):97–106. [PubMed] [Google Scholar]
- Schaeffer KW, Parsons OA, Yohman JR. Neuropsychological differences between male familial and nonfamilial alcoholics and nonalcoholics. Alcohol Clin Exp Res. 1984;8:347–351. doi: 10.1111/j.1530-0277.1984.tb05678.x. [DOI] [PubMed] [Google Scholar]
- Schairer KS, Gould HJ, Pousson MA. Source generators of mismatch negativity to multiple deviant stimulus types. Brain Topogr. 2001;14:117–130. doi: 10.1023/a:1012992829580. [DOI] [PubMed] [Google Scholar]
- Schroger E. Event-related potentials to auditory stimuli following transient shifts of spatial attention in a Go/Nogo task. Biol Psychol. 1993;36:183–207. doi: 10.1016/0301-0511(93)90017-3. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Steger J, Imhof K, Steinhausen H, Brandeis D. Brain mapping of bilateral interactions in attention deficit hyperactivity disorder and control boys. Clin Neurophysiol. 2000;111:1141–1156. doi: 10.1016/s1388-2457(00)00311-4. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and inhibition systems. Psychol Sci. 1997;8:204–210. [Google Scholar]
- Swan GE, Carmelli D. Evidence for genetic mediation of executive control: a study of aging male twins. J Gerontol B Psychol Sci Soc Sci. 2002;57:P133–P143. doi: 10.1093/geronb/57.2.p133. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Jacob T, Bremer DA. Cognitive status of sons of alcoholic men. Alcohol Clin Exp Res. 1989;13:232–235. doi: 10.1111/j.1530-0277.1989.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Tomberg C, Desmedt JE. Human perceptual processing: inhibition of transient prefrontal-parietal 40 Hz binding at P300 onset documented in non-averaged cognitive brain potentials. Neurosci Lett. 1998;255:163–166. doi: 10.1016/s0304-3940(98)00740-x. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Heritability of human brain functioning as assessed by electroencephalography. Am J Hum Genet. 1996;58:562–573. [PMC free article] [PubMed] [Google Scholar]
- van Beijsterveldt CE, Molenaar PC, de Geus EJ, Boomsma DI. Individual differences in P300 amplitude: a genetic study in adolescent twins. Biol Psychol. 1998;47:97–120. doi: 10.1016/s0301-0511(97)00025-2. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC, Molenaar PC, Boomsma DI, de Geus EJ. Stability of genetic and environmental influences on P300 amplitude: a longitudinal study in adolescent twins. Behav Genet. 2001;31:533–543. doi: 10.1023/a:1013389226795. [DOI] [PubMed] [Google Scholar]
- Van der Stelt O, Geesken R, Gunning WB, Snel J, Kok A. P3 scalp topography to target and novel visual stimuli in children of alcoholics. Alcohol. 1998;15:119–136. doi: 10.1016/s0741-8329(97)00106-7. [DOI] [PubMed] [Google Scholar]
- van der Stelt O, van der Molen M, Boudewijn Gunning W, Kok A. Neuroelectrical signs of selective attention to color in boys with attention-deficit hyperactivity disorder. Brain Res Cogn Brain Res. 2001;12:245–264. doi: 10.1016/s0926-6410(01)00055-6. [DOI] [PubMed] [Google Scholar]
- van Leeuwen TH, Steinhausen HC, Overtoom CC, Pascual-Marqui RD, van’t Klooster B, Rothenberger A, Sergeant JA, Brandeis D. The continuous performance test revisited with neuroelectric mapping: impaired orienting in children with attention deficits. Behav Brain Res. 1998;94:97–110. doi: 10.1016/s0166-4328(97)00173-3. [DOI] [PubMed] [Google Scholar]
- Whipple SC, Berman SM, Noble EP. Event-related potentials in alcoholic fathers and their sons. Alcohol. 1991;8:321–327. doi: 10.1016/0741-8329(91)90497-k. [DOI] [PubMed] [Google Scholar]
- Winterer G, Goldman D. Genetics of human prefrontal function. Brain Res Brain Res Rev. 2003;43:134–163. doi: 10.1016/s0165-0173(03)00205-4. [DOI] [PubMed] [Google Scholar]
- Winterer G, Smolka M, Samochowiec J, Mulert C, Ziller M, Mahlberg R, Wuebben Y, Gallinat J, Rommelspacher H, Herrman WM, Sander T. Association analysis of GABAAbeta2 and gamma2 gene polymorphisms with event-related prefrontal activity in man. Hum Genet. 2000;107:513–518. doi: 10.1007/s004390000401. [DOI] [PubMed] [Google Scholar]
- Woodward SH, Brown WS, Marsh JT, Dawson ME. Probing the time-course of the auditory oddball P3 with secondary reaction time. Psychophysiology. 1991;28:609–618. doi: 10.1111/j.1469-8986.1991.tb01003.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka K, Kimura T, Miyazaki M, Kawashima N, Nozaki D, Nakazawa K, Yano H, Yamamoto Y. Human cortical activities during Go/NoGo tasks with opposite motor control paradigms. Exp Brain Res. 2002;142:301–307. doi: 10.1007/s00221-001-0943-2. [DOI] [PubMed] [Google Scholar]
- Yao D, He B. A self-coherence enhancement algorithm and its application to enhancing three-dimensional source estimation from EEGs. Ann Biomed Eng. 2001;29:1019–1027. doi: 10.1114/1.1415526. [DOI] [PubMed] [Google Scholar]