Abstract
Objective
To evaluate the relationship between maternal preeclampsia resulting in premature delivery and adiposity in the offspring during adolescence.
Study design
The 172 study participants were 14 years old and had very low birth weight. We compared height, weight, body mass index (BMI), percent fat, waist circumference, and triceps and subscapular skin fold thicknesses between those born prematurely secondary to preeclampsia (n = 51; 22 male) and those born prematurely after nor-motensive pregnancies (n = 121; 55 male). Multiple linear regression analysis was used to adjust for potential con-founders (maternal BMI, antenatal steroid exposure, and race) and to evaluate potential explanatory variables (fetal, infancy, and childhood weight gain, and caloric intake, level of fitness, and physical activity at 14 years).
Results
When adjusted for potential prenatal confounders (antenatal steroid exposure and race), adolescent male offspring of preeclamptic pregnancies had higher BMI (4.0 kg/m2 [1.5, 6.6]) (mean difference [95% CI]), waist circumference (11.8 cm [3.8, 19.7]), triceps (4.6 mm [0.6, 8.6]) and subscapular skinfold thicknesses (6.2 mm [1.5, 10.9]), and percent body fat (4.1% [−0.1, 8.3]). Adjusting for infancy and childhood weight gain attenuated these group differences. There were no group differences among females.
Conclusion
Male adolescent offspring born prematurely of women with preeclampsia have higher measures of adiposity than those born prematurely of normotensive pregnancies. (J Pediatr 2012; ■:■-■).
Offspring of pregnancies complicated by preeclampsia are at increased risk for adverse cardiovascular outcomes.1,2 Alterations in adiposity might contribute to this increased cardiovascular risk. A recent meta-analysis reported a modest but significant increase in body mass index (BMI) in the offspring of mothers with preeclampsia, compared with normotensive pregnancies.3 Potential mechanisms linking maternal preeclampsia to later differences in offspring body composition include preeclampsia-induced alterations in the intrauterine environment, restricted fetal growth followed by rapid childhood weight gain, and genetic and environmental factors shared by the mother and her offspring that increase both maternal and offspring body size.
Children born very preterm have not been well represented in studies of whether preeclampsia predisposes the offspring to increased adiposity. Preeclampsia that leads to very preterm birth is likely to provide a different intrauterine exposure than preeclampsia resulting in a term or near term delivery.4 Thus, infants born prematurely might be at particularly high risk for the potential programming effects of the intrauterine environment specific to preeclampsia. Although there is evidence that male and female fetuses respond differently to an adverse intrauterine environment,5 most studies have not reported analyses stratified by sex.
Our objective was to evaluate the relationship between maternal preeclampsia resulting in premature delivery of a very low birth weight (VLBW; ≤1500 g) infant and adiposity in the offspring during adolescence and to determine if this relationship varies by sex. We studied a cohort of VLBW infants at 14 years of age and compared BMI, waist circumference, triceps and subscapular skin fold thicknesses, and percent body fat between those born prematurely secondary to preeclampsia and those born prematurely from normotensive pregnancies.
Methods
The study sample was derived from a cohort of consecutive VLBW births at the regional perinatal center in Forsyth County, North Carolina from January 1,1992-June 30, 1996 (Figure; available at www.jpeds.com). Eligible infants (n = 479) were singleton at birth, had no major congenital malformation, and returned to the medical center for evaluation at 1-year corrected age. Among eligible children, 312 were located and 193 were studied at 14 years of age. Twenty-one participants were excluded from this analysis because of presence of maternal hypertension but not preeclampsia during pregnancy (16), twin gestation (2), chromosomal abnormality (1), polycystic kidney disease (1), and severe cerebral palsy (1).
Figure.
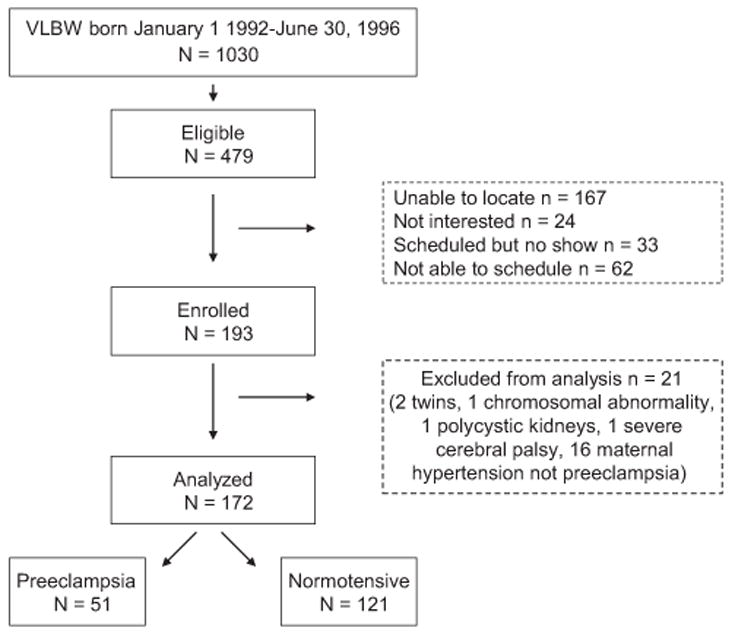
Progress of the participants through the observational follow-up study.
This study was approved by the Wake Forest School of Medicine and the Forsyth Medical Center Institutional Review Boards. Informed consent was obtained from parents or legal guardians, and participants gave assent.
We reviewed medical records and delivery logs to identify participants whose mothers were diagnosed as having preeclampsia/eclampsia, pregnancy-induced hypertension, or chronic hypertension. When available, a first trimester ultrasound was used to estimate gestational age. Otherwise, we used, in order of priority, the mother’s last menstrual period or a clinical assessment of the newborn infant. From infant medical records, we obtained birth weight and weight at 1 year of age adjusted for prematurity. As an index of fetal growth we used birth weight z score, calculated using US natality data.6 Z scores for measurements made at 1 year of age corrected for prematurity, and at 14 years were derived using Epi Info (Centers for Disease Control, Atlanta, Georgia).7 Race was categorized as black or non-black, based on participants’ self-report. From information obtained up to 1 year corrected age, we calculated the social disadvantage score (1-3), by assigning 1 point each for maternal black race, single marital status, and completion of less than a high school education.8 Maternal prepregnancy BMI was calculated from prepregnancy weight determined from medical record review and height obtained by maternal report at the follow-up visit. BMI percentile ≥85th to <95th was considered overweight, and BMI percentile ≥95th, obese.9 Waist circumference percentiles were determined using US normative data.10
Measurements
Anthropometrics/Body Composition
Anthropometric measurements were made in triplicate with participants wearing light clothing and no shoes. Height was measured using a wall-mounted stadiometer. Weight was measured using a digital platform scale. Abdominal circumference was assessed with a measuring tape according to procedures of the third National Health and Nutrition Examination Survey.11 Triceps, subscapular, and suprailiac skin fold thicknesses were measured using a Lange skinfold caliper.12 Percent body fat and lean mass were assessed using a Delphi dual energy X-ray absorptiometer (Hologic, Bedford, Massachusetts) equipped with pediatric software.
Aerobic Fitness and Physical Activity
Aerobic fitness was determined from a graded exercise test to exhaustion on an electronically-braked cycle ergometer following the Godfrey Protocol.13 Expired gases were analyzed using a Viasys Vmax Encore metabolic cart (CareFusion, Yorba Linda, California). Peak oxygen uptake was taken from the highest 20 seconds of exercise and expressed per kilogram body mass. Subjects were verbally encouraged to give a maximal effort. Habitual physical activity was assessed using Kriska’s Modifiable Activity Questionnaire and expressed as average total hours of physical activity per week for the past year.14
Dietary Intake
Subjects were provided with standard measuring cups, spoons, and a ruler, and were instructed in the proper use of these items for measuring food portions by a registered dietician. The food record was completed for 3 days. Nutrient content was calculated using the Nutrition Data System for Research software developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, Minnesota.15
Statistical Analyses
Group means were compared by the 2-sample independent t test, and proportions were compared by the χ2 or Fisher exact test. We defined potential confounders (birth weight, Cesarean delivery, race, sex, social disadvantage score, antenatal steroid treatment of the mother, and mother’s prepregnancy BMI) as those factors associated with both maternal preeclampsia and an obesity-related outcome using a P value of ≤0.2. As we hypothesized sex differences, we stratified the analyses for evaluating the effects of maternal preeclampsia by sex of the child. Thus, for each outcome, there were 2 prespecified subgroups for analysis and twice the expectation of results reaching statistical significance on the basis of chance alone. Multiple linear regression was used to evaluate the relationship of maternal preeclampsia and the obesity-related outcomes adjusting for potential confounders. Similar results were obtained in models that adjusted for race or social disadvantage score; we report here only the results from models that included race. When associations were observed between maternal preeclampsia and obesity-related outcomes, we were interested in whether these associations might involve, in the causal pathway, weight gain during gestation (birth weight z score), infancy (weight z score at 1-year corrected age – birth weight z score), and childhood (weight z score at 14 years – weight z score at 1-year corrected age), estimated daily caloric intake at 14 years, aerobic fitness at 14 years, and physical activity at 14 years. We examined this possibility with regression models that adjusted for each of these factors. Statistical significance was defined as P < .05. No correction for multiple testing was applied to outcomes, as those selected for analysis were closely related and were used to evaluate consistency across findings. Analyses were performed using SAS for Windows v. 9.2 (SAS Institute Inc, Cary, North Carolina).
Results
The participants whose data are included in the study had slightly lower gestational age and higher birth weight z score than those eligible but not included (Table I). Characteristics of the 51 participants who were born to mothers with preeclampsia (offspring of preeclamptic pregnancies [PreE] group) and 121 who were born to normotensive mothers (offspring of normotensive pregnancies [No HTN] group) are shown in Table II. The PreE group (males and females) had greater gestational age and lower birth weight z score, and were more likely to have been delivered by Cesarean section and exposed to antenatal steroids when compared with the No HTN group. PreE males were more likely to be born to mothers with higher prepregnancy BMI, and PreE females were more likely to be non-black and had lower social disadvantage scores (less disadvantaged) when compared with their No HTN peers. At 1-year corrected age, PreE females had lower weight z scores when compared with the No HTN females. The PreE males had increased weight gain during infancy and the PreE females had increased weight gain in childhood compared with the their respective No HTN group. A subset of participants had reliable data for aerobic fitness (No HTN = 99, PreE = 43), physical activity (No HTN = 115, PreE = 48), and caloric intake (No HTN = 86, PreE = 43) at age 14 years. No group differences were found for aerobic fitness (39.2 ± 10.3 vs 35.9 ± 10.6 mL/kg/min, P = .08; mean ± SD, P value for No HTN vs PreE, respectively), physical activity (11.6 ± 12.6 vs 10.3 ± 9.4 h/wk, P = .46), or caloric intake (2184 ± 814 vs 2118 ± 787 kcal/d, P = .66).
Table I.
Comparison of neonatal characteristics of those included with those not included at 14 years of age
| Included
|
Not included
|
P value | |
|---|---|---|---|
| n = 172 | n = 307 | ||
| Gestational age, wk | 27.7 ± 2.6 | 28.3 ± 2.5 | .01 |
| Birth weight, g | 1040 ± 268 | 1083 ± 260 | .09 |
| Birth weight Z score | −0.25 ± 0.79 | −0.42 ± 0.85 | .03 |
| Cesarean delivery, n (%) | 86 (51) | 168 (55) | .31 |
| Male, n (%) | 77 (45) | 154 (50) | .26 |
| Black race, n (%) | 72 (42) | 121 (39) | .60 |
Values are mean ± SD or n (%).
Table II.
Characteristics of participants expressed as mean ± SD or n (%)
| Entire group
|
Males
|
Females
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No HTN
|
PreE
|
P value | No HTN
|
PreE
|
P value | No HTN
|
PreE
|
P value | |
| n = 121 | n = 51 | n = 55 | n = 22 | n = 66 | n = 29 | ||||
| Birth | |||||||||
| Gestational age, wk | 27.1 ± 2.3 | 29.0 ± 2.7 | <.01 | 26.9 ± 2.3 | 29.5 ± 2.4 | <.01 | 27.2 ± 2.4 | 28.7 ± 2.8 | .01 |
| Birth weight, g | 1033 ± 270 | 1059 ± 267 | .55 | 1028 ± 267 | 1154 ± 230 | .06 | 1036 ± 274 | 988 ± 274 | .43 |
| Birth weight z score | 0.01 ± 0.66 | −0.87 ± 0.74 | <.01 | −0.04 ± 0.73 | − 0.89 ± 0.73 | <.01 | 0.06 ± 0.60 | − 0.85 ± 0.76 | <.01 |
| Cesarean delivery, n (%) | 42 (35) | 45 (88) | <.01 | 23 (42) | 21 (95) | <.01 | 19 (29) | 24 (83) | <.01 |
| Antenatal steroid exposure, n (%) | 54 (45) | 32 (63) | .03 | 29 (53) | 11 (50) | .83 | 25 (38) | 21 (72) | <.01 |
| Male, n (%) | 55 (45) | 22 (43) | .78 | - | - | - | - | ||
| Black race, n (%) | 54 (45) | 15 (29) | .06 | 25 (45) | 9 (41) | .72 | 29 (44) | 6 (21) | .03 |
| Social disadvantage score | 1.20 ± 1.06 | 0.75 ± 0.87 | <.01 | 1.21 ± 1.01 | 0.91 ± 0.88 | .25 | 1.18 ± 1.11 | 0.62 ± 0.86 | .02 |
| Maternal prepregnancy BMI* | 25.6 ± 6.8 | 28.6 ± 6.8 | .04 | 25.5 ± 6.1 | 30.8 ± 8.5 | .02 | 25.7 ± 7.3 | 27.1 ± 5.1 | .45 |
| 1-y corrected age | |||||||||
| Age at follow-up, mo | 12.8 ± 1.3 | 12.7 ± 1.4 | .73 | 12.6 ± 1.1 | 12.8 ± 2.1 | .68 | 12.9 ± 1.4 | 12.6 ± 0.5 | .15 |
| Weight z score | −1.04 ± 1.38 | −1.37 ± 1.11 | .13 | −1.31 ± 1.45 | −1.18 ± 1.22 | .71 | −0.83 ± 1.29 | −1.52 ± 1.01 | .01 |
| Height z score | −0.11 ± 1.33 | −0.35 ± 1.52 | .31 | −0.31 ± 1.30 | −0.30 ± 1.60 | .99 | 0.06 ± 1.34 | −0.38 ± 1.49 | .16 |
| Weight trajectories (change in weight z score) | |||||||||
| Birth-1-y corrected age | −1.05 ± 1.39 | -0.50 ± 1.11 | .01 | −1.26 ± 1.47 | −0.28 ± 0.80 | <.01 | − 0.88 ± 1.32 | −0.67 ± 1.29 | .47 |
| 1-y corrected age-14 y | 1.26 ± 1.43 | 1.94 ± 1.29 | <.01 | 1.44 ± 1.64 | 2.11 ± 1.56 | .11 | 1.10 ± 1.23 | 1.81 ± 1.07 | <.01 |
n = 78 No HTN, n = 30 PreE.
Male participants in the PreE group had higher, BMI, waist circumference, subscapular skinfold thickness, and triceps skin-fold thickness, and a trend toward higher percent body fat. These differences were not found among females (Tables III and IV; Table IV is available at www.jpeds.com online). Mean differences (increment in adiposity measure for offspring of mothers with preeclampsia vs normotensive) among males persisted when adjusted for antenatal steroid exposure and race (Table V). The association of preeclampsia and obesity-related outcomes persisted when adjusted for mother’s prepregnancy BMI in the subset of 104 children for whom we had data about this factor (data not shown).
Table III.
Comparison of anthropometric measures in offspring of preeclamptic and normotensive pregnancies at age 14 y expressed as mean ± SD or n (%)
| Entire Group
|
Males | Females
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
| No HTN
|
PreE
|
p | No HTN
|
PreE
|
P | No HTN
|
PreE
|
P | |
| n = 121 | n = 51 | n = 55 | n = 22 | n = 66 | n = 29 | ||||
| Weight, kg | 58 ± 15 | 62 ± 19 | .07 | 57 ± 13 | 71 ± 22 | <.01 | 58 ± 17 | 56 ± 13 | .92 |
| Weight Z score | 0.21 ± 1.21 | 0.56 ± 1.38 | .10 | 0.14 ± 1.14 | 0.93 ± 1.73 | <.01 | 0.27 ± 1.26 | 0.29 ± 0.98 | .96 |
| Height, cm | 161 ± 8 | 161 ± 10 | .83 | 165 ± 9 | 167 ± 11 | .26 | 158 ± 7 | 156 ± 7 | .32 |
| Height Z score | −0.37 ± 1.04 | −0.43 ± 1.23 | .73 | −0.20 ± 1.04 | −0.01 ± 1.26 | .34 | −0.51 ± 1.02 | −0.75 ± 1.13 | .34 |
| BMI, kg/m2 | 22 ± 6 | 24 ± 6 | .06 | 20 ± 4 | 25 ± 7 | <.01 | 23 ± 6 | 23 ± 5 | .68 |
| BMI Z score | 0.34 ± 1.13 | 0.73 ± 1.23 | .04 | 0.13 ± 1.16 | 0.91 ± 1.44 | <.01 | 0.51 ± 1.09 | 0.60 ± 1.05 | .66 |
| Overweight, BMI 85-<95th percentile | 14 (12) | 10 (20) | .03 | 5 (9) | 4 (18) | <.01 | 9 (14) | 6 (21) | .55 |
| Obese, BMI ≥95th percentile | 18 (15) | 14 (27) | 6 (11) | 8 (36) | 12 (18) | 6 (21) | |||
| Percent body fat* | 24 ± 9 | 27 ± 9 | .09 | 18 ± 7 | 22 ± 10 | .05 | 30 ± 7 | 31 ± 7 | .59 |
| Waist circumference, cm* | 77 ± 14 | 82 ± 16 | .07 | 74.9 ± 12.9 | 87.1 ± 20.8 | .01 | 79.3 ± 14.8 | 78.1 ± 10.8 | .79 |
| Waist circumference >90th percentile* | 19 (17) | 13 (27) | .20 | 7 (14) | 8 (38) | 03 | 12 (20) | 5 (18) | .99 |
| Subscapular skinfold thickness, mm† | 15 ± 10 | 19 ± 10 | .04 | 12 ± 8 | 18 ± 12 | 02 | 18 ± 10 | 19 ± 9 | .52 |
| Triceps skinfold thickness, mm* | 15 ± 8 | 18 ± 9 | .03 | 11 ± 7 | 16 ± 10 | .04 | 19 ± 8 | 20 ± 8 | .41 |
Female = 88 (No HTN = 60, PreE = 28); male = 72 (No HTN = 51, PreE = 21).
Female = 87 (No HTN = 59, PreE = 28); male = 72 (No HTN = 51, PreE = 21).
Table IV.
Mean differences (preeclampsia-normotensive, 95% CI and P values) for multivariate analyses relating preeclampsia to measures of adiposity in female adolescent offspring who were born prematurely
| Model | BMI, kg/m2
|
Abdominal circumference, cm
|
Triceps skinfold thickness, mm
|
Subscapular skinfold thickness, mm
|
Percent body fat
|
|---|---|---|---|---|---|
| n = 95 | n = 88 | n = 88 | n = 87 | n = 88 | |
| Unadjusted | −0.1 (−2.7, 2.5) 0.97 | −1.2 (−7.4, 5.1) 0.71 | 1.4 (−2.1, 4.9) 0.42 | 1.0 (−3.6, 5.5) 0.67 | 1.0 (−2.3, 4.3) 0.56 |
| Adjusted for prenatal confounders* | 0.7 (−2.0, 3.4) 0.60 | −0.3 (−6.9, 6.4) 0.94 | 2.4 (−1.2, 6.1) 0.19 | 2.8 (−1.9, 7.4) 0.24 | 1.0 (−2.6, 4.5) 0.59 |
| Prenatal confounders + Birth weight z score | 0.6 (−2.7, 3.8) 0.73 | −0.9 (−8.8, 7.0) 0.82 | 2.5 (−1.9, 6.8) 0.26 | 2.3 (−3.2, 7.8) 0.41 | 0.5 (−3.8, 4.7) 0.83 |
| Prenatal confounders + Birth weight z score + Infancy weight gain† | 1.1 (−2.2, 4.2) 0.51 | 1.4 (−5.9, 8.7) 0.71 | 3.3 (−1.0, 7.6) 0.13 | 3.3 (−2.2, 8.8) 0.23 | 1.2 (−2.9, 5.3) 0.56 |
| Prenatal confounders + Birth weight z score + Infancy weight gain + Childhood weight gain‡ | −0.4 (−2.1, 1.3) 0.63 | −1.9 (−6.1, 2.3) 0.38 | 1.5 (−1.3, 4.4) 0.29 | 1.4 (−2.5, 5.3) 0.48 | −0.3 (−3.1, 2.6) 0.86 |
Prenatal confounders = antenatal steroid exposure and race.
Infancy weight gain = weight z score at 1-y corrected age–birth weight z score.
Childhood weight gain = weight z score at 14 y–weight z score at 1-y corrected age.
Table V.
Mean differences (preeclampsia-normotensive, 95% CI and P values) for multivariate analyses relating preeclampsia to measures of adiposity in male adolescent offspring who were born prematurely
| Model | BMI, kg/m2
|
Abdominal circumference, cm
|
Triceps skinfold thickness, mm
|
Subscapular skinfold thickness, mm
|
Percent body fat
|
|---|---|---|---|---|---|
| n = 77 | n = 72 | n = 72 | n = 72 | n = 72 | |
| Unadjusted | 4.1 (1.5, 6.7) 0.002 | 12.2 (4.1, 20.2) 0.004 | 4.8 (0.7, 8.9) 0.02 | 6.4 (1.6, 11.1) 0.01 | 4.3 (0.1, 8.6) 0.05 |
| Adjusted for prenatal confounders* | 4.0 (1.5, 6.6) 0.002 | 11.8 (3.8, 19.7) 0.004 | 4.6 (0.6, 8.6) 0.02 | 6.2 (1.5, 10.9) 0.01 | 4.1 (−0.1, 8.3) 0.05 |
| Prenatal confounders + Birth weight z score | 4.5 (1.5, 7.4) 0.003 | 13.7 (4.4, 22.9) 0.004 | 4.5 (−0.2, 9.1) 0.058 | 5.8 (0.3, 11.3) 0.04 | 3.7 (−1.2, 8.5) 0.13 |
| Prenatal confounders + Birth weight z score + Infancy weight gain† | 3.9 (0.9, 6.9) 0.01 | 11.6 (2.3, 20.9) 0.02 | 3.5 (−1.2, 8.1) 0.14 | 4.9 (−0.7, 10.4) 0.09 | 2.9 (−2.0, 7.9) 0.24 |
| Prenatal confounders + Birth weight z score + Infancy weight gain + Childhood weight gain‡ | 1.0 (−0.7, 2.7) 0.24 | 2.6 (−2.4, 7.5) 0.30 | −0.2 (−3.7,3.4) 0.93 | 0.4 (−3.8, 4.6) 0.84 | −0.3 (−4.5, 3.9) 0.88 |
Prenatal confounders = antenatal steroid exposure and race.
Infancy weight gain = weight z score at 1-y corrected age–birth weight z score.
Childhood weight gain = weight z score at 14 y–weight z score at 1-y corrected age.
Entering birth weight z score into regression models for males resulted in only small changes in the estimated mean difference (Table V). Addition of measures of infancy and childhood weight gain into the models attenuated these relationships (Table V). Similarly, including aerobic fitness in regression models slightly attenuated differences between the PreE group and children of normotensive mothers, but inclusion of physical activity and caloric intake did not (data not shown).
Results similar to those described above were obtained from analyses that included 12 additional participants whose mothers’ pregnancies were complicated by a hypertensive disorder other than preeclampsia (data not shown).
Discussion
In a cohort of adolescents born prematurely with VLBW, male offspring of women with preeclampsia had higher measures of adiposity, including BMI, abdominal circumference, and triceps and subscapular skinfold thicknesses, than male offspring of mothers who did not have preeclampsia. We did not find this association among female offsprings. Among males, rate of postnatal weight gain, as well as aerobic fitness at 14 years of age, were identified as potentially influencing the relationship of preeclampsia to offspring adiposity.
We found that the association of maternal preeclampsia and greater adiposity in the male offspring persisted when adjusted for birth weight z score, suggesting that there may be direct effects of the preeclamptic intrauterine environment that program obesity independent of fetal growth restriction. This finding agrees with studies of PreE born at term.16,17 Male No HTN had a nearly normal BMI (BMI z score = 0.1) in contrast to females for whom the average BMI z score was 0.5. Thus, the difference we found in males could reflect both an increased BMI among male PreE and a decreased BMI in No HTN.
In the only study to focus on those born prematurely, Laz-dam et al found no difference between young adult offspring of hypertensive mothers (85% with preeclampsia) and normotensive mothers, in height, weight, BMI, or waist to hip ratio.18 These individuals were born later in gestation (at a mean of 30 weeks) than those whom we studied, and the sample was smaller (19 offspring of hypertensive mothers and 52 of normotensive mothers). In addition, the children were born 1982-1985, prior to the recent “obesity epidemic,” which may have influenced postnatal weight gain in our participants. In 2 other cohorts that included 17 and 25 prematurely born offspring of mothers with preeclampsia, no difference in body size was found between offspring of mothers with and without preeclampsia.19,20 All 3 prior studies that included prematurely born children had small sample sizes and studied children born closer to term compared with the present study.
Our finding of greater adiposity among prematurely born male offspring of mothers with preeclampsia agrees with a study that included term and near term born individuals who were evaluated at 17 years of age.21 However, in 2 Nor-wegian cohorts, female PreE had increased BMI at 10-19 years of age.16,17 Contrasting with those studies and ours, are findings from studies of younger children (6-9 years of age)22-24 in which preeclampsia was not associated with adiposity in the offspring, a finding, which might emerge at older ages. A recent meta analysis3 described an 0.83 kg/m2 increase in BMI for male PreE compared with our finding that BMI was increased by 4 kg/m2. This difference could be due to an accentuation of the effect of preeclampsia when it is severe enough to lead to very preterm delivery, as well as an accentuation among males. Thomas et al reported young adult males, but not females, born prematurely (≤33 weeks gestation), have increased internal adipose tissue compared with those born at term.25 Although BMI is not a measure of internal adiposity, in our cohort, males of preeclamptic pregnancies had increased abdominal circumference compared with No HTN.
Our finding that a prenatal exposure was associated with outcomes in the offspring 14 years later could be explained by developmental programming. We speculate that systems such as leptin regulation might be altered in male offspring who experience preterm preeclampsia. In human pregnancies, preeclampsia is associated with increased expression of obesity-related genes, increased leptin synthesis in the placenta, and increased levels of leptin in cord blood of preterm infants.26,27 Leptin resistance and obesity has been described in experimental intrauterine growth restriction followed by rapid postnatal growth.28 In preterm infants postnatal growth during the first 2 years is related to leptin levels.29 Sexual dimorphism has been described in fetal and childhood leptin levels, providing a potential biologic basis to our finding that the association between preeclampsia and increased adiposity in the offspring was limited to males.30,31
Two limitations of our study should be noted. First, about 60% of our inception cohort was not located or did not agree to participate. Second, data about maternal BMI were missing for 41% of the cohort, limiting our ability to assess potential confounding by this factor.
In summary, we found males born prematurely to mothers with preeclampsia have increased adiposity in adolescence when compared with those born to normotensive mothers. We speculate that processes that determine postnatal adiposity are altered by developmental programming. Research is needed to determine the mechanisms regulating postnatal growth after intrauterine adversity and to evaluate whether interventions to modify early weight gain or level of fitness can prevent the higher adiposity of prematurely born male offspring of mothers with preeclampsia.■
Acknowledgments
Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (PO1 HD047584), the Clinical Research Unit of Wake Forest Baptist Medical Center (MO1-RR07122), Forsyth Medical Center, and the Wake Forest School of Medicine Department of Pediatrics research funds. The authors declare no conflicts of interest.
Glossary
- BMI
Body mass index
- No HTN
Offspring of normotensive pregnancies
- PreE
Offspring of preeclamptic pregnancies
- VLBW
Very low birth weight
References
- 1.Ferreira I, Peeters LL, Stehouwer CD. Preeclampsia and increased blood pressure in the offspring: meta-analysis and critical review of the evidence. J Hypertens. 2009;27:1955–9. doi: 10.1097/HJH.0b013e328331b8c6. [DOI] [PubMed] [Google Scholar]
- 2.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJP. Preeclampsia is associated with increased risk of stroke in the adult offspring. Stroke. 2009;40:1176–80. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 3.Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012;129:e1552–e61. doi: 10.1542/peds.2011-3093. [DOI] [PubMed] [Google Scholar]
- 4.Phillips JK, Janowiak M, Badger GJ, Bernstein IM. Evidence for distinct preterm and term phenotypes of preeclampsia. J Matern Fetal Neonatal Med. 2010;23:622–6. doi: 10.3109/14767050903258746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(Suppl A):S33–9. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Oken E, Kleinman KP, Rich-Edwards J, Gillman MW. A nearly continuous measure of birth weight for gestational age using a United States national reference. BMC Pediatr. 2003;3:6. doi: 10.1186/1471-2431-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McDowell MA, Fryar CD, Hirsch R, Ogden CL. Anthropometric reference data for children and adults: U.S. population, 1999-2002. Adv Data. 2005;361:1–5. [PubMed] [Google Scholar]
- 8.Hack M, Taylor HG, Klein N, Eiben R, Schatschneider C, Mercuri-Minich N. School-age outcomes in children with birth weights under 750 g. N Engl J Med. 1994;331:753–9. doi: 10.1056/NEJM199409223311201. [DOI] [PubMed] [Google Scholar]
- 9.Barlow SE. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics. 2007;120:S164–92. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 10.McDowell MA, Fryar CD, Ogden CL, Flegal KM. National health statistics reports. Vol. 10. Hyattsville, MD: National Center for Health Statistics; 2008. Anthropometric reference data for children and adults: United States, 2003-2006. [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. NHANES III Anthropometric Procedures Video. Washington, DC: U.S. Government Printing Office; 2012. [Stock Number 017-022-01335-5] 1996. [Google Scholar]
- 12.Lohman TG, Roche AF, Martorell R. Skinfold thicknesses. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 13.Godfrey S. Exercise testing in children. Philadelphia: W. B. Saunders; 1974. [Google Scholar]
- 14.Aaron DJ, Kriska AM, Dearwater SR, Cauley JA, Metz KF, LaPorte RE. Reproducibility and validity of an epidemiologic questionnaire to assess past year physical activity in adolescents. Am J Epidemiol. 1995;142:191–201. doi: 10.1093/oxfordjournals.aje.a117618. [DOI] [PubMed] [Google Scholar]
- 15.Schakel SF, Sievert YA, Buzzard IM. Sources of data for developing and maintaining a nutrient database. J Am Diet Assoc. 1988;88:1268–71. [PubMed] [Google Scholar]
- 16.Vatten LJ, Romundstad PR, Holmen TL, Hsieh CC, Trichopoulos D, Stuver SO. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet Gynecol. 2003;101:529–33. doi: 10.1016/s0029-7844(02)02718-7. [DOI] [PubMed] [Google Scholar]
- 17.Ogland B, Vatten LJ, Romundstad PR, Nilsen ST, Forman MR. Pubertal anthropometry in sons and daughters of women with preeclamptic or normotensive pregnancies. Arch Dis Child. 2009;94:855–9. doi: 10.1136/adc.2008.150870. [DOI] [PubMed] [Google Scholar]
- 18.Lazdam M, de la Horra A, Pitcher A, Mannie Z, Diesch J, Trevitt C, et al. Elevated blood pressure in offspring born premature to hypertensive pregnancy: is endothelial dysfunction the underlying vascular mechanism? Hypertension. 2010;56:159–65. doi: 10.1161/HYPERTENSIONAHA.110.150235. [DOI] [PubMed] [Google Scholar]
- 19.Tenhola S, Rahiala E, Martikainen A, Halonen P, Voutilainen R. Blood pressure, serum lipids, fasting insulin, and adrenal hormones in 12-year-old children born with maternal preeclampsia. J Clin Endocrinol Metab. 2003;88:1217–22. doi: 10.1210/jc.2002-020903. [DOI] [PubMed] [Google Scholar]
- 20.Lawlor DA, Donald-Wallis C, Fraser A, Nelson SM, Hingorani A, Davey Smith G, et al. Cardiovascular biomarkers and vascular function during childhood in the offspring of mothers with hypertensive disorders of pregnancy: findings from the Avon Longitudinal Study of Parents and Children. Eur Heart J. 2012;33:335–45. doi: 10.1093/eurheartj/ehr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seidman DS, Laor A, Gale R, Stevenson DK, Mashiach S, Danon YL. Preeclampsia and offspring’s blood pressure, cognitive ability and physical development at 17-years-of-age. Br J Obstet Gynaecol. 1991;98:1009–14. doi: 10.1111/j.1471-0528.1991.tb15339.x. [DOI] [PubMed] [Google Scholar]
- 22.Geelhoed JJ, Fraser A, Tilling K, Benfield L, Davey Smith G, Sattar N, et al. Preeclampsia and gestational hypertension are associated with childhood blood pressure independently of family adiposity measures: the Avon Longitudinal Study of Parents and Children. Circulation. 2010;122:1192–9. doi: 10.1161/CIRCULATIONAHA.110.936674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kvehaugen AS, Andersen LF, Staff AC. Anthropometry and cardiovascular risk factors in women and offspring after pregnancies complicated by preeclampsia or diabetes mellitus. Acta Obstet Gynecol Scand. 2010;89:1478–85. doi: 10.3109/00016349.2010.500368. [DOI] [PubMed] [Google Scholar]
- 24.Palti H, Rothschild E. Blood pressure and growth at 6 years of age among offsprings of mothers with hypertension of pregnancy. Early Hum Dev. 1989;19:263–9. doi: 10.1016/0378-3782(89)90061-3. [DOI] [PubMed] [Google Scholar]
- 25.Thomas EL, Parkinson JR, Hyde MJ, Yap IK, Holmes E, Dore CJ, et al. Aberrant adiposity and ectopic lipid deposition characterize the adult phenotype of the preterm infant. Pediatr Res. 2011;70:507–12. doi: 10.1203/PDR.0b013e31822d7860. [DOI] [PubMed] [Google Scholar]
- 26.Reimer T, Koczan D, Gerber B, Richter D, Thiesen HJ, Friese K. Micro-array analysis of differentially expressed genes in placental tissue of preeclampsia: up-regulation of obesity-related genes. Mol Hum Reprod. 2002;8:674–80. doi: 10.1093/molehr/8.7.674. [DOI] [PubMed] [Google Scholar]
- 27.Hytinantti T, Koistinen HA, Koivisto VA, Karonen SL, Rutanen EM, Andersson S. Increased leptin concentration in preterm infants of preeclamptic mothers. Arch Dis Child Fetal Neonatal Ed. 2000;83:F13–6. doi: 10.1136/fn.83.1.F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Coupe B, Grit I, Hulin P, Randuineau G, Parnet P. Postnatal growth after intrauterine growth restriction alters central leptin signal and energy ho-meostasis. PLoS One. 2012;7:e30616. doi: 10.1371/journal.pone.0030616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel L, Cavazzoni E, Whatmore AJ, Carney S, Wales JK, Clayton PE, et al. The contributions of plasma IGF-I, IGFBP-3 and leptin to growth in extremely premature infants during the first two years. Pediatr Res. 2007;61:99–104. doi: 10.1203/01.pdr.0000250036.34522.f1. [DOI] [PubMed] [Google Scholar]
- 30.Hassink SG, Sheslow DV, de LE, Opentanova I, Considine RV, Caro JF. Serum leptin in children with obesity: relationship to gender and development. Pediatrics. 1996;98:201–3. [PubMed] [Google Scholar]
- 31.Ong KK, Ahmed ML, Sherriff A, Woods KA, Watts A, Golding J, et al. Cord blood leptin is associated with size at birth and predicts infancy weight gain in humans. ALSPAC Study Team. Avon Longitudinal Study of Pregnancy and Childhood. J Clin Endocrinol Metab. 1999;84:1145–8. doi: 10.1210/jcem.84.3.5657. [DOI] [PubMed] [Google Scholar]


