Abstract
Background
Feline immunodeficiency virus (FIV) is a lentivirus that infects domestic and wild felidae and the course of disease is similar to that of human immunodeficiency virus infection. The thymidine nucleoside analog fozivudine (FZD) tidoxil is a lipid—zidovudine (ZDV) conjugate and member of the family of nucleoside reverse transcriptase (RT) inhibitors (NRTIs).
Hypothesis
FZD administration to cats during acute FIV infection produces antiviral activity with fewer adverse effects than its parent compound ZDV (AZT).
Animals
Male, neutered cats approximately 7 months of age (n = 12).
Methods
FZD (45 mg/kg q12h, n = 6) or placebo (n = 6) was administered PO in a nonblinded trial for 6 weeks to cats infected with the NCSU1 isolate of FIV. Peripheral blood was collected preinfection and at 2, 4, and 6 weeks postinfection for CBC, evaluation of CD4+ and CD8+ cell counts by flow cytometry, and quantification of plasma and cell-associated viremia by real time RT-PCR.
Results
Treatment of cats with FZD during the acute stage of FIV infection decreased plasma and cell-associated viremia during the first 2 weeks of infection, but was not protective against FIV, as all cats were infected by 6 weeks.
Conclusions
At the dosage used in this study, treatment with FZD results in a short-term decrease in viral load with no adverse effects. Further investigation of FZD is warranted to assess pharmacokinetics, optimal dosage, and to directly compare the antiviral activity of FZD to ZDV in naturally infected cats.
Keywords: Acquired immunodeficiency syndrome, Antiretroviral therapy, Lentivirus, Zidovudine
Feline immunodeficiency virus (FIV) is a natural infection of domestic cats and was first described by Pedersen et al.1 FIV is most closely related to the lenti-viruses of small ruminants, but the course of disease is similar to that of human immunodeficiency virus (HIV) infection.2 Although the natural route of transmission is by bite wounds, FIV can be transmitted experimentally by IV and transmucosal routes.1,3,4 Peak plasma viremia occurs 2–6 weeks postinfection (PI) and is accompanied by profound lympholysis resulting in generalized B and T lymphocyte depletion.4,5 During acute lentiviral infections, an early rebound in the CD8+ cell count is observed and the amount of CD8+ antiviral activity corresponds with a decline in plasma viremia.2,6–9
Although many drugs and immunomodulators have been reported anecdotally to be effective against FIV, only 2 drugs, human interferon-α and zidovudine (ZDV, also known as AZT), have demonstrated some benefit in controlled trials in naturally infected cats.10–12 The nucleoside analog ZDV was one of the first drugs shown to be effective against HIV and today is used worldwide for HIV therapy. ZDV also is effective against FIV, lowering viral burden in both acute and chronic FIV infection, and has been shown to transiently improve CD4 :CD8 ratios and clinical signs in chronically infected cats.13–15 The recommended ZDV (AZT) dosage range for treatment of chronically infected FIV-positive cats is 5–10 mg/kg PO or SC q12h.11 Anemia is the most commonly observed adverse effect associated with ZDV administration and resolves upon lowering the dosage or cessation of administration.11,14,16,17 The development of severe anemia precludes with higher dosages of ZDV, although higher dosages may be more effective against FIV and have been used experimentally.13,14
Because of the potential need for more effective drugs with fewer adverse effects for FIV treatment in naturally infected cats, the authors evaluated a compound related to ZDV in a controlled experimental trial. Fozivudine (FZD) and ZDV are members of the nucleoside reverse transcriptase (RT) inhibitor (NRTI) family of drugs, nucleoside analogs that incorporate into a growing DNA strand and bind viral RT, thus halting reverse transcription.18 FZD tidoxil is a thioether lipid—ZDV conjugate originally developed for use against HIV. In vitro studies have demonstrated that FZD undergoes intracellular cleavage to ZDV monophosphate and subsequent phosphorylation to the active form of the drug.19,20 The formulation of FZD theoretically decreases the potential for hematologic toxicity because mononuclear cells (lymphocytes and monocytes) exhibit higher intracellular cleavage activity compared with red blood cells (RBCs) and bone marrow stem cells.19 Because of its unique lipid formulation, FZD potentially may be a better option than AZT for the treatment of FIV, enabling an increased dosage while minimizing the potential for development of anemia. The purpose of this study was to determine FZD’s effectiveness at decreasing the viral burden during experimental acute FIV infection and to record the development of anemia or any other associated adverse effects.
Materials and Methods
Cats
Specific pathogen-free cats were obtained from a commercial vendora at 6 months of age, and housed in the Laboratory Animal Resource Facility at the College of Veterinary Medicine, North Carolina State University. Protocols were approved by the North Carolina State University Institutional Animal Care and Use Committee.
FZD Tidoxil
(HDP 99.0002) FZD tidoxil (3′-azido-3′-deoxy-5′-thymidylic acid, mono[3-(dodecylthio)-2-deoxy-decycloxypropyl] ester, sodium salt) is a ZDV-thioether lipid conjugate. It is a white to off-white powder, which should be stored between 15 and 30°C. For these experiments, FZD was prepared in either 170 or 187 mg gelatin capsules for administration PO at an average dosage of 45 mg/kg twice daily (90 mg/kg/d). The dosage range was 42–46 mg/kg twice daily. Piedmont Pharmaceuticals had conducted previously a preliminary safety trial in which FIV-negative cats were administered FZD at a dosage of 100 mg/kg/d for 28 days.21 Placebo consisted of an equal amount of sucrose prepared in gelatin capsules. All gelatin capsules were prepared at a compounding facilityb and shipped to the NCSU-CVM.
Infection with FIV
The NCSU1 isolate of FIV originally was obtained from a naturally infected cat at the North Carolina State University College of Veterinary Medicine and has been described in detail elsewhere.22 Virus inoculum was grown as a single-tissue culture passage in an IL-2-dependent feline CD4+ cell line (FCD4-E cells) as described previously.23 The cats were inoculated IV with 5×105 TCID50 of cell-free virus culture. The cats used for the study reported here then were maintained and housed at the NCSU-CVM to be utilized later for FIV chronic infection studies.
Sample Collection
Blood was collected on day 0, before infection, and 2, 4, and 6 weeks PI. Blood was collected by jugular venipuncture into EDTA vacutainer tubes and 2 mL were retained for a CBC and lymphocyte subset analysis by multicolored flow cytometry. Plasma was separated from the remaining blood and frozen (−20°C) for subsequent analysis of viral load by real time RT-PCR.
Lymphocyte Subset Analysis
The phenotype of lymphocytes from peripheral blood was determined by multicolored flow cytometric analysis. Peripheral blood mononuclear cells (PBMCs) were stained with anti-CD4-Strep-avidin/PerCp and anti-CD8-PE by an established whole blood lysis protocol.23 The murine monoclonal antifeline CD4 (mAb 30A) and CD8 (mAb 3.357) used to stain PBMCs were produced in the authors’ laboratory.24 For flow cytometric analysis, lymphocytes were gated based on forward versus side scatter, and approximately 20,000 gated events were acquired and stored list-mode fashion for analysis by CellQuest software.
Plasma and Cell-Associated Viremia
Evidence of infection was assessed on each plasma sample by a commercially available snap test ELISAc to detect antibodies against FIV. Quantitative real-time PCR was used to determine viral gag-mRNA loads in each plasma sample and was reported as copies/mL plasma. PBMCs were isolated by Percoll density gradient centrifugation and 1×106 PBMCs then were stored in RNA Protect.d The primer and probe sequences used for FIV-gag have been reported elsewhere.25,26 All PCR testing was performed by an independent laboratory.e
Monitoring
Each cat’s attitude, appetite, activity level, and physical examination findings were recorded on a daily basis. CBCs were performed every 2 weeks to detect any hematologic abnormalities, including anemia. CBCs included manual WBC differential counts and visual evaluation of RBC morphology.
Statistical Analysis
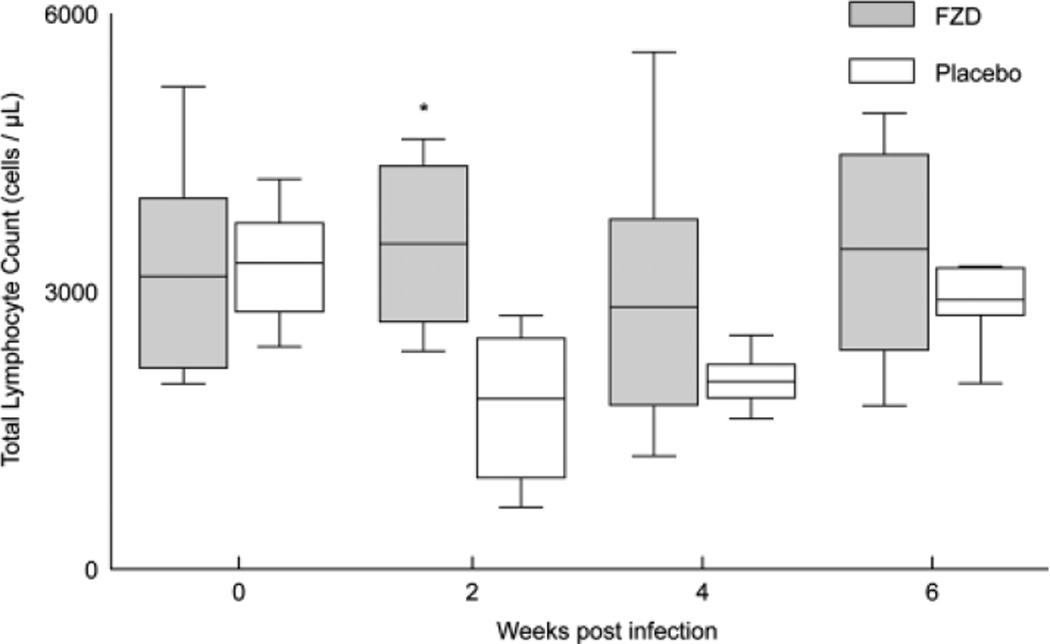
Cats with undetectable plasma and cell-associated viremia were assigned a value of zero (Tables 1–2). In the case of FZD-treated cats at week 2, a value of zero was assigned to 5/6 and 6/6 cats for plasma and cell-associated viremia, respectively. A Fisher exact test was used to calculate the 2-tailed P-value with FZD or placebo cats categorized as either FIV-positive or FIV-negative (by PCR analysis; Tables 1–2). A Student t-test was used to compare total lymphocyte counts from FZD-treated and placebo-treated cats with P < .05 considered significant (Fig 1).
Table 1.
FZD administration during acute FIV infection temporarily reduces plasma viremia.
| (FIV-gag-mRNA [copies/mL plasma]) Week Postinfection (PI) |
|||
|---|---|---|---|
| 2 | 4 | 6 | |
| FZD | |||
| Positive (%) | 17 | 83 | 100 |
| Mean | 167 | 12,918 | 6,891 |
| Median | 0 | 4,767 | 8,691 |
| Range | 0–1,000 | 0–53,271 | 0–9,604 |
| Placebo | |||
| Positive (%) | 100 | 100 | 100 |
| Mean | 17,385 | 35,390 | 19,991 |
| Median | 1,574 | 20,361 | 12,752 |
| Range | 1,000–73,809 | 7,766–98,786 | 1,000–62,529 |
| P-value | .015* | NS | NS |
The percentage of positive cats by PCR analysis is shown in the first row for FZD- and placebo-treated groups. The mean, median, and range of the number of virus copies/mL are listed for each cat at 2, 4, and 6 weeks PI. P < .05.
FIV, feline immunodeficiency virus; FZD, fozivudine; NS, not significant.
Table 2.
FZD administration during acute FIV infection temporarily reduces cell-associated viremia.
| (FIV-gag-mRNA [copies/106 PMBC]) Week Postinfection (PI) |
|||
|---|---|---|---|
| 2 | 4 | 6 | |
| FZD | |||
| Positive (%) | 0 | 50 | 100 |
| Mean | 0 | 500 | 1,553 |
| Median | 0 | 500 | 1,311 |
| Range | 0 | 0–1,000 | 1,009–3,011 |
| Placebo | |||
| Positive (%) | 83 | 100 | 100 |
| Mean | 1,039 | 1,038 | 2,133 |
| Median | 1,013 | 1,000 | 1,141 |
| Range | 0–1,854 | 1,000–1,227 | 1,000–6,722 |
| P-value | .015* | NS | NS |
The percentage of positive cats by PCR analysis is shown in the first row for FZD and placebo groups. The mean, median, and range of the number of virus copies/million PBMCs are listed for each cat at 2, 4, and 6 weeks PI.
P < .05
FIV, feline immunodeficiency virus; FZD, fozivudine; NS, not significant; PBMC, peripheral blood mononuclear cell.
Fig 1.
The total lymphocyte count remains unchanged in fozivudine (FZD)-treated cats but is decreased in placebo-treated cats. The box and whisker plots represent 5th and 95th percentiles (whisker), 25th and 75th percentiles (box), and mean (line) lymphocyte count. *P = .020.
Results
Plasma and Cell-Associated Viremia
FZD or placebo was administered twice daily, starting 1 before FIV challenge, for 6 weeks. Plasma viremia was assessed at 2, 4, and 6 weeks PI by real time RT-PCR for the placebo-treated and FZD-treated cats. There was a reduction in plasma viremia (P = .015) in the FZD-treated cats at week 2 PI and 1/6 (17%) FZD-treated cats had detectable virus by PCR, whereas all 6 placebo-treated cats (100%) had detectable virus (Table 1). By week 4, 83% of the FZD-treated cats had detectable plasma viremia, and although mean plasma viremia (12,918 copies/mL) was approximately 33% that of the placebo group (35,390 copies/mL), the difference between the 2 groups was not statistically significant. Peak plasma viremia also occurred at week 4 in both groups (Table 1). By week 6, 100% of the cats from the FZD and the placebo group had detectable virus by PCR and mean plasma viremia for the FZD group (6,819 copies/mL) remained approximately 33% that of the placebo group (19,991 copies/mL).
Cell-associated viremia was assessed at 2, 4, and 6 weeks PI by real-time RT-PCR for the placebo-treated and FZD-treated cats. At week 2, there was a significant difference in cell-associated viremia (P = .015); none of the FZD-treated cats had detectable cell-associated virus compared with 5/ (83%) placebo-treated cats (Table 2). By week 4, 50% of the FZD-treated cats and all placebo-treated cats had detectable cell-associated viremia. By week 6, 100% of the cats from the FZD and placebo groups had detectable cell-associated viremia.
Clinical and Laboratory Findings
The lymphocyte nadir in the placebo-treated cats occurred at week 2 PI whereas the lymphocyte count in the FZD treatment group remained relatively stable for the duration of the study (Fig 1). Also at 2 weeks PI, the total lymphocyte count in the FZD-treated group (mean, 3,515 cells/µL) was significantly higher than that of the placebo group (mean, 1,842 cells/µL; P = .020).
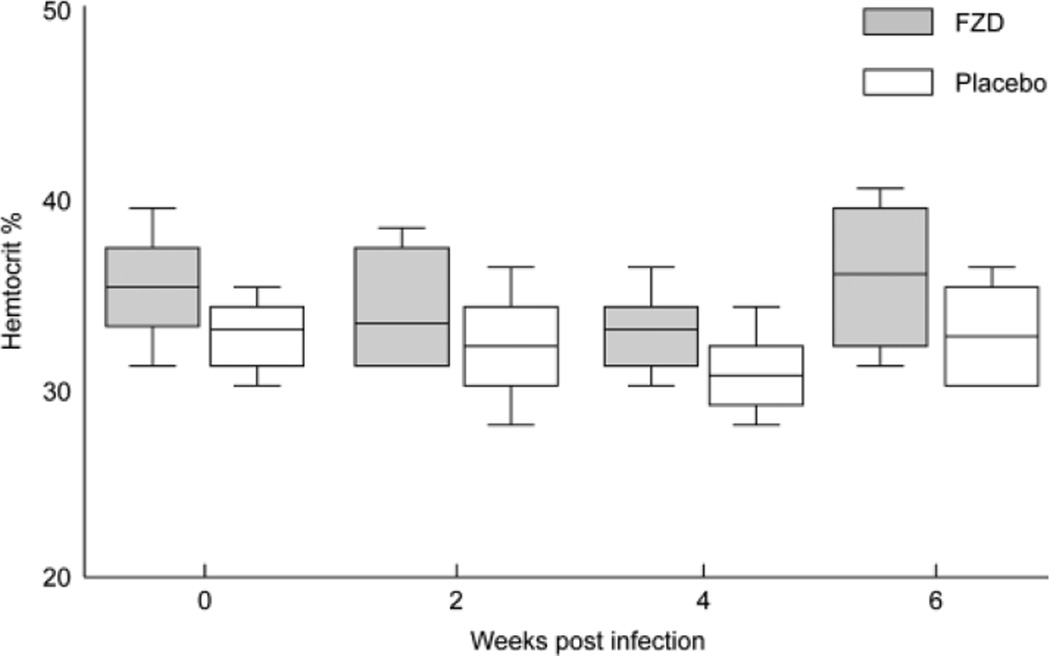
The mean hematocrit was approximately 34% for both the FZD and placebo-treated groups and remained unchanged in both groups for the duration of the study (Fig 2). All other RBC indices and RBC morphology (as determined by blood smear evaluation) remained normal. There was no difference in the CD4 : CD8 ratio between the FZD and placebo-treated groups. No abnormal physical examination findings were associated with FZD administration and physical examination findings in the FZD treatment group paralleled those of the placebo-treated group for the duration of the study.
Fig 2.
Administration of fozivudine (FZD) has no effect on hematocrit values. The box and whisker plots represent 5th and 95th percentiles (whisker), 25th and 75th percentiles (box), and mean (line) hematocrit.
Discussion
Several reports address the in vivo activity of different antiretroviral drugs for the treatment of acute and chronic FIV infection, and these investigations suggest that many of the nucleoside analogs originally developed for HIV therapy exhibit varying degrees of effectiveness against FIV.12–14,27,28 This study was a preliminary investigation to evaluate the in vivo anti-FIV activity of FZD. Data from humans suggest that FZD is effective at lowering virus burden, and administration is associated with fewer adverse effects compared with ZDV.19,29 During the chronic, asymptomatic stage of FIV infection, plasma and cell-associated viremia can be quite low. One investigation by high-dose combination antiretroviral therapy (ZDV/3TC) was unable to demonstrate effectiveness during experimental, chronic FIV infection.14 Therefore, as a 1st step in demonstrating effectiveness against FIV, the authors chose to evaluate FZD’s antiviral activity during the early acute stage of infection, when plasma and cell-associated viremia are higher, making it easier to discern differences between treatment groups.
In an acute infection study performed by Hayes et al28 to assess the effectiveness of ZDV in the FIV model, therapy was initiated 48 hours before acute FIV infection, continued for a total of 4 weeks, and was effective at lowering acute antigenemia but did not prevent infection. In a more recent report, Arai et al14 demonstrated that combination AZT (ZDV)/3TC (lamivudine) therapy at relatively high dosages (100–150 mg/kg/d) was effective against acute FIV infection, but all cats exhibited anemia after 3–4 weeks of therapy. In the study reported here, utilizing FZD as single-agent therapy, only 1/6 FZD-treated cats (17%) had detectable plasma viremia by PCR 2 weeks PI compared with 100% of cats in the placebo-treated group. At 4 weeks PI, 5/6 FZD-treated cats (83%) had detectable plasma viremia. However, mean plasma viremia was approximately 3 times higher in the placebo-treated group (35,390 copies/mL) compared with the FZD-treated group (12,918 copies/mL).
Peak plasma viremia generally is noted during acute FIV infection 2–6 weeks PI, depending on the route and dose of virus, and is associated with concurrent lympholysis.5,25 The initial increase in plasma viral load is responsible for the dissemination of FIV, and early targets for the virus include lymphatic tissue.5,30,31 The data shown in Tables 1–2 and Figure 1 indicate that ZDV is effective at lowering viral load during the first 2 weeks of FIV infection and that FZD may decrease early FIV-associated lympholysis. It is unclear why FZD exhibited the most benefit at week 2 PI, yet the viral load increased by week 4 PI. Although not reaching statistical significance, plasma viremia and cell-associated viremia were lower, and lymphocyte counts higher in the FZD-treated cats for the duration of treatment suggesting that FZD therapy during acute FIV infection may lower the viral setpoint. A single-agent antiviral therapy would not be expected to completely inhibit viral replication because viral replication occurs rapidly during the first 4–6 weeks of infection and NRTIs affect only one part of the viral replication cycle.
FZD and ZDV are members of the NRTI family of drugs (nucleoside analogs) which incorporate into a growing DNA strand and bind viral RT, thus halting reverse transcription.18 FZD undergoes intracellular cleavage to ZDV monophosphate and subsequent phosphorylation to the active form of the drug.18–20 In experimental FIV infection, higher doses of ZDV (AZT) have been effective at decreasing plasma viremia, but higher dosages or long-term therapy in cats is associated with adverse effects such as anemia, neutropenia, and vomiting.14,17,32 High plasma concentrations of ZDV are thought to be responsible for many of ZDV’s adverse effects.19 Studies in humans and animals suggest that despite high plasma concentrations of FZD, there is a very low plasma concentration of its primary metabolite ZDV, which is the active form of the drug.19,33,34 In a previous safety study, no adverse effects were noted with an FZD dosage of 100 mg/kg/d for 28 days in FIV-negative cats.21 Adverse effects associated with ZDV administration in cats, including anemia, were not evident in any of the cats treated with FZD at a dosage of 45 mg/kg q12h for 6 weeks.
The aim of this study was to demonstrate that FZD exhibits antiviral activity against FIV and the results suggest the drug does exhibit antiviral activity. Based on studies in humans, there is the potential that utilizing FZD instead of ZDV (AZT) for the treatment of FIV may allow higher dosages to be administered while avoiding many of the adverse effects associated with high dosages of ZDV in cats. Although these preliminary results are promising, further investigation is needed to assess pharmacokinetics, optimal dosages, and to directly compare the antiviral activity of FZD to ZDV in naturally infected cats. Through continued investigation of novel NRTIs such as FZD, it may be possible to develop new antiviral therapies for the treatment of FIV in a clinical setting.
Acknowledgments
The use of FZD tidoxil was licensed to Piedmont Pharmaceuticals by Heidelberg Pharma AG, Schriesheimer Street 101, Ladenburg 68526, Germany. The authors thank Deb Anderson, Janet Dow, and Lawana Hartsell for their excellent technical assistance, Dr Adam Birkenheuer for suggestions regarding data interpretation, and Dr Maria Correa for assistance with statistical analysis.
Funding/disclosure: This study was funded by Piedmont Pharmaceuticals and by the National Institute of Health (NIH) 1K08AI074445-01A1 (Fogle) and R01 AI080288 (MB Tompkins).
Abbreviations
- FIV
feline immunodeficiency virus
- FZD
fozivudine
- PBMC
peripheral blood mononuclear cell
- PI
postinfection
- ZDV (AZT)
zidovudine
Footnotes
This work was done at North Carolina State University College of Veterinary Medicine, Raleigh, NC.
Liberty Research Inc, Waverly, NY
Stillmeadow Inc, Sugarland, TX
SNAP FIV/FeLV Combo Test, IDEXX Laboratories Inc, West-brook, ME
RNA Protect, Qiagen, Valencia, CA
Zoologix Inc, Chatsworth, CA
Conflict of interest: Two of the authors (Campbell and Sumner) were employed by Piedmont Pharmaceuticals at the time this study was performed.
References
- 1.Pedersen NC, Ho E, Brown ML, Yamamoto JK. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science. 1987;235:790–793. doi: 10.1126/science.3643650. [DOI] [PubMed] [Google Scholar]
- 2.Vahlenkamp TW, Tompkins MB, Tompkins WA. FIV as a model of AIDS pathogenesis studies. In: Friedman H, Specter S, Bendinelli M, editors. In Vivo Models of HIV Disease and Control. New York: Springer; 2006. pp. 239–273. [Google Scholar]
- 3.Burkhard M, Obert LA, O’Neil LL, et al. Mucosal transmission of cell-associated and cell-free feline immunodeficiency virus. AIDS Res Hum Retroviruses. 1997;13:347–355. doi: 10.1089/aid.1997.13.347. [DOI] [PubMed] [Google Scholar]
- 4.Bendinelli M, Pistello M, Lombardi S, et al. Feline immunodeficiency virus: An interesting model for AIDS studies and an important cat pathogen. Clin Microbiol Rev. 1995;8:87–112. doi: 10.1128/cmr.8.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burkhard MJ, Dean GA. Transmission and immunopathogenesis of FIV in cats as a model for HIV. Curr HIV Res. 2003;1:15–29. doi: 10.2174/1570162033352101. [DOI] [PubMed] [Google Scholar]
- 6.Bucci J, English R, Jordan H, et al. Mucosally transmitted feline immunodeficiency virus induces a CD8+ antiviral response that correlates with reduction of cell-associated virus. J Infect Dis. 1998;177:18–25. doi: 10.1086/513822. [DOI] [PubMed] [Google Scholar]
- 7.Willett BJ, Hosie MJ, Callanan JJ, et al. Infection with feline immunodeficiency virus is followed by the rapid expansion of a CD8+ lymphocyte subset. Immunology. 1993;78:1–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Lopez M, Soriano V, Lozano S, et al. No major differences in the functional profile of HIV Gag and Nef-specific CD8+ responses between long-term nonprogressors and typical progressors. AIDS Res Hum Retroviruses. 2008;24:1185–1195. doi: 10.1089/aid.2008.0006. [DOI] [PubMed] [Google Scholar]
- 9.Jagannathan P, Osborne CM, Royce C, et al. Comparisons of CD8+ T cells specific for human immunodeficiency virus, hepatitis C virus, and cytomegalovirus reveal differences in frequency, immunodominance, phenotype, and interleukin-2 responsiveness. J Virol. 2009;83:2728–2742. doi: 10.1128/JVI.02128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pedretti E, Passeri B, Amadori M, et al. Low-dose interferon-alpha treatment for feline immunodeficiency virus infection. Vet Immunol Immunopathol. 2006;109:245–254. doi: 10.1016/j.vetimm.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 11.Levy J, Crawford C, Hartmann K, et al. American Association of Feline Practitioners’ feline retrovirus management guidelines. J Feline Med Surg. 2008;10:300–316. doi: 10.1016/j.jfms.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hartmann K, Donath A, Beer B, et al. Use of two virustatica (AZT, PMEA) in the treatment of FIV and of FeLV seropositive cats with clinical symptoms. Vet Immunol Immunopathol. 1992;35:167–175. doi: 10.1016/0165-2427(92)90129-e. [DOI] [PubMed] [Google Scholar]
- 13.Hayes KA, Phipps AJ, Francke S, Mathes LE. Antiviral therapy reduces viral burden but does not prevent thymic involution in young cats infected with feline immunodeficiency virus. Antimicrob Agents Chemother. 2000;44:2399–2405. doi: 10.1128/aac.44.9.2399-2405.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arai M, Earl DD, Yamamoto JK. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet Immunol Immunopathol. 2002;85:189–204. doi: 10.1016/s0165-2427(01)00426-3. [DOI] [PubMed] [Google Scholar]
- 15.Hart S, Nolte I. Long-term treatment of diseased, FIV-sero-positive field cats with azidothymidine (AZT) Zentralbl Veterinarmed A. 1995;42:397–409. doi: 10.1111/j.1439-0442.1995.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 16.Hartmann K. Antiviral chemotherapy in veterinary medicine. Proc ACVIM Forum. 2008;26:634–636. [Google Scholar]
- 17.Haschek WM, Weigel RM, Scherba G, et al. Zidovudine toxicity to cats infected with feline leukemia virus. Fundamental Appl Tox. 1990;14:764–775. doi: 10.1016/0272-0590(90)90301-y. [DOI] [PubMed] [Google Scholar]
- 18.Furman PA, Fyfe JA, St Clair MH, et al. Phosphorylation of 3′-azido-3′-deoxythymidine and selective interaction of the 5′-triphosphate with human immunodeficiency virus reverse transcriptase. Proc Natl Acad Sci USA. 1986;83:8333–8337. doi: 10.1073/pnas.83.21.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girard PM, Pegram PS, Diquet B, et al. Phase II placebo-controlled trial of fozivudine tidoxil for HIV infection: Pharmacokinetics, tolerability, and efficacy. J Acquir Immune Defic Syndr. 2000;23:227–235. doi: 10.1097/00126334-200003010-00003. [DOI] [PubMed] [Google Scholar]
- 20.Kucera GL, Goff CL, Iyer N, et al. Cellular metabolism in lymphocytes of a novel thioether-phospholipid-AZT conjugate with anti-HIV-1 activity. Antiviral Res. 2001;50:129–137. doi: 10.1016/s0166-3542(01)00137-1. [DOI] [PubMed] [Google Scholar]
- 21.Pharmaceuticals P. Preliminary tolerance of 28-day oral administration of fozivudine to cats. Study Memorandum—Piedmont Pharmaceuticals. :2007–2008. [Google Scholar]
- 22.English RV, Nelson P, Johnson CM, et al. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J Infec Dis. 1994;170:543–552. doi: 10.1093/infdis/170.3.543. [DOI] [PubMed] [Google Scholar]
- 23.Davidson MG, Rottman J, English RV, et al. Feline immunodeficiency virus predisposes cats to acute generalized toxoplasmosis. Am J Pathol. 1993;143:1486–1497. [PMC free article] [PubMed] [Google Scholar]
- 24.Tompkins MB, Gebhard DH, Bingham HR, et al. Characterization of monoclonal antibodies to feline T lymphocytes and their use in the analysis of lymphocyte tissue distribution in the cat. Vet Immunol Immunopathol. 1990;26:305–317. doi: 10.1016/0165-2427(90)90115-9. [DOI] [PubMed] [Google Scholar]
- 25.Mexas AM, Fogle JE, Tompkins WA, Tompkins MB. CD4+CD25+ regulatory T cells are infected and activated during acute FIV infection. Vet Immunol Immunopathol. 2008;126:263–272. doi: 10.1016/j.vetimm.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fogle JE, Mexas AM, Tompkins WA, Tompkins MB. CD4(+)CD25(+) T regulatory cells inhibit CD8(+) IFN-gamma production during acute and chronic FIV infection utilizing a membrane TGF-beta-dependent mechanism. AIDS Res Hum Retroviruses. 2010;26:201–216. doi: 10.1089/aid.2009.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Uckun FM, Chen CL, Samuel P, et al. In vivo antiretroviral activity of stampidine in chronically feline immunodeficiency virus-infected cats. Antimicrob Agents Chemother. 2003;47:1233–1240. doi: 10.1128/AAC.47.4.1233-1240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hayes KA, Lafrado LJ, Erickson JG, et al. Prophylactic ZDV therapy prevents early viremia and lymphocyte decline but not primary infection in feline immunodeficiency virus-inoculated cats. J Acquir Immune Defic Syndr. 1993;6:127–134. [PubMed] [Google Scholar]
- 29.Bogner JR, Roecken M, Herrmann DB, et al. Phase I/II trial with fozivudine tidoxil (BM 21.1290): A 7 day randomized, placebo-controlled dose-escalating trial. Antivir Ther. 1997;2:257–264. [PubMed] [Google Scholar]
- 30.Dow SW, Poss ML, Hoover EA. Feline immunodeficiency virus: A neurotropic lentivirus. J Acquir Immune Defic Syndr. 1990;3:658–668. [PubMed] [Google Scholar]
- 31.English RV, Johnson CM, Gebhard DH, Tompkins MB. In vivo lymphocyte tropism of feline immunodeficiency virus. J Virol. 1993;67:5175–5186. doi: 10.1128/jvi.67.9.5175-5186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hartmann K, Ferk G, North TW, Pedersen NC. Toxicity associated with high dosage 9-[(2R,5R-2,5-dihydro-5-phosphono-methoxy)-2-furanyl]adenine therapy off attempts to abort early FIV infection. Antiviral Res. 1997;36:11–25. doi: 10.1016/s0166-3542(97)00030-2. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann DBJ, Opitz H-G. BM 21.1290: Pharmacological/toxicological evaluation of a novel candidate anti-AIDS drug. AIDS. 1996;1(Suppl 2):S39. [Google Scholar]
- 34.Bogner JR, Boerner D, Muhlhofer A, et al. Single dose, dose-escalating trial with fozivudine tidoxil (BM 211290) Antivir Ther. 1997;2:249–256. [PubMed] [Google Scholar]