SUMMARY
Interleukin (IL)-27 is a key immunosuppressive cytokine that counters T helper 17 (Th17) cell-mediated pathology. To identify mechanisms by which IL-27 might exert its immunosuppressive effect, we analyzed genes in T cells rapidly induced by IL-27. We found that IL-27 priming of naïve T cells upregulated expression of programmed death ligand 1 (PD-L1) in a signal transducer and activator of transcription (STAT)1-dependent manner. When co-cultured with naïve CD4+ T cells, IL-27-primed T cells inhibited the differentiation of Th17 cells in trans through a PD-1-PD-L1 interaction. In vivo, co-administration of naïve TCR transgenic T cells (2D2 T cells) with IL-27-primed T cells expressing PD-L1 inhibited the development of Th17 cells and protected from severe autoimmune encephalomyelitis. Thus, these data identify a suppressive activity of IL-27, by which CD4+ T cells can restrict differentiation of Th17 cells in trans.
INTRODUCTION
Interleukin (IL)-27 is a member of a family of heterodimeric cytokines with critical immunoregulatory properties (Cox et al., 2011; Cua et al., 2003; Villarino et al., 2003). IL-27 was initially identified as being important for inducing expression of T-bet and enhancing Th1 cell differentiation. Later, it was appreciated that IL-27, which shares the EBI3 subunit with IL-35, has essential, nonredundant immunosuppressive actions (Collison et al., 2010; Owaki et al., 2005; Tong et al., 2010; Yoshida et al., 2001). Specifically, mice lacking the IL-27 receptor (IL-27R) develop lethal inflammation upon infection with a variety of pathogens. For instance, during infection with Trypanosoma cruzi, Il27ra−/− mice show severe pathological changes due to enhanced Th1 and Th2 responses (Hamano et al., 2003). Similarly, Il27ra−/− mice infected with Toxoplasma gondii develop uncontrolled tissue damage mediated by activated Th1 cells and increased IL-17 production (Stumhofer et al., 2006; Villarino et al., 2003).
Various mechanisms of IL-27 have been suggested to explain its immunosuppressive potency. IL-27 can directly inhibit inflammatory cytokine production in activated T cells in vitro (Villarino et al., 2006; Yoshimura et al., 2006). In particular, IL-27 has been suggested to diminish Th17 cell differentiation by inhibiting the expression of RORγt (Diveu et al., 2009). In experimental autoimmune encephalomyelitis (EAE), mice deficient in IL-27R develop more severe Th17 cell-associated neuropathology, whereas treatment of wild-type mice with IL-27 can constrain Th17 cell differentiation and abolish development of EAE (Batten et al., 2006; Fitzgerald et al., 2007).
In addition to inhibiting the production of IL-17, IL-27 enhances the production of the immunosuppressive cytokine IL-10 in various T cell subsets (Batten et al., 2008; Diveu et al., 2009; Stumhofer et al., 2007). Importantly, IL-27 works together with TGF-β to drive the differentiation of IL-10-producing regulatory type 1 T cells (Tr1) through induction of aryl hydrocarbon receptor (AhR), c-Maf, IL-21 and ICOS (Apetoh et al., 2010; Awasthi et al., 2007; Murugaiyan et al., 2009; Pot et al., 2009). IL-27 has also been reported to amplify TGF-β-induced FoxP3 expression in a STAT1-dependent manner (Ouaked et al., 2009); however, other work has argued that IL-27 negatively regulates FoxP3 expression (Neufert et al., 2007). Adoptive transfer of Il27ra−/− T cells was associated with reduced pathology and enhanced Treg differentiation in a model of autoimmune colitis (Cox et al., 2011).
IL-27 also has different effects on non-T cell lineages. For instance, IL-27 can enhance IFN-γ expression in NK cells (Chiyo et al., 2005), whereas, IL-27 can suppress LPS-induced cytokine production by dendritic cells (DCs) in vitro. However, the underlying mechanism of the latter has not been elucidated (Wang et al., 2007).
Despite its important actions, relatively little was known about the genes that are directly regulated by IL-27. Herein, we show that naïve T cells express receptors of IL-27. Among the genes upregulated by IL-27 was Cd274, which encodes PD-L1. IL-27-dependent induction of PD-L1 on naïve cells inhibited IL-17 production in an untreated population of differentiating Th17 cells and the development of Th17 cells-mediated autoimmune encephalomyelitis was ameliorated by co-administration of IL-27-treated naïve T cells that express PD-L1. Thus, these data point to a mechanism by which IL-27 can exert its ability to suppress IL-17-mediated pathology.
RESULTS
IL-27 inhibits early IL-17 induction
Previous work has shown that IL-27 inhibits Th17 cell differentiation (Batten et al., 2006; Diveu et al., 2009; Stumhofer et al., 2006). Consistent with these findings, we found that IL-17 production was suppressed when IL-27 was present early in Th17 cell differentiation (Figure S1A). However, when added 24 hours or later after initial activation, it was ineffective (Figure S1A). To explain these results, we first considered that the effects of IL-27 might be a reflection of receptor expression.
IL-27R comprises a heterodimer consisting of two subunits encoded by the Il27ra gene and the Il6st gene (Pflanz et al., 2004; Pflanz et al., 2002). Consistent with previous work (Chen et al., 2000), naïve T cells express both subunits and Il27ra was downregulated upon activation of CD4+ T cells (Figure S1B). This IL-27R expression was functional as assessed by IL-27-dependent STAT activation (Stumhofer et al., 2007) (Figure S1C-S1E). IL-27 readily induced STAT1 activation in naïve CD4+ T cells but induced relatively less STAT3 phosphorylation. This contrasted with the action of IL-6, which induced roughly equivalent levels of STAT1 and STAT3 phosphorylation. In summary, naïve T cells express the IL-27R and functionally respond to this cytokine.
IL-27-primed naïve T cells inhibit Th17 cell differentiation in trans
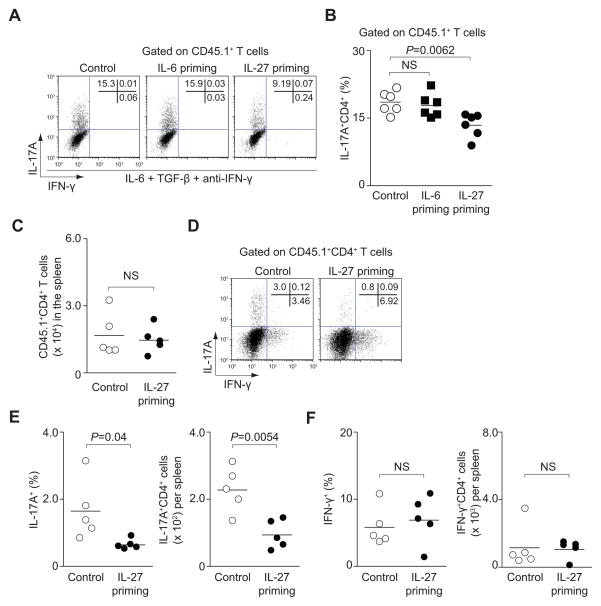
Because IL-27 had the capacity to exert its effect on naïve CD4+ T cells, we wondered if it was necessary for IL-27 to act directly on nascent Th17 cells or if it could modulate naïve cells that might be able to inhibit development of Th17 cells in trans. Therefore, we primed naïve CD45.2+CD4+ T cells with IL-27 for 3 hours, washed the cells to remove the cytokine and then added these cells to naïve CD45.1+CD4+ T cells cultured under Th17 cell-polarizing conditions (Figure S1F). In the presence of untreated naïve CD45.2+CD4+ T cells, more than 15% of non-primed CD45.1+CD4+ T cells became IL-17 producers (Figure 1A). When IL-27-primed CD4+ T cells were added to developing Th17 cells, IL-17 production was significantly inhibited (by 40–60%) (Figure 1A and 1B), regardless of whether the polarizing conditions were IL-6 and TGF-β or IL-6, IL-1β and IL-23 (Figure S1G and S1H). Since we added anti-IFN-γ neutralizing antibody to the Th17 cell-polarizing conditions, this reduction of IL-17 producers was not due to the ability of IL-27 to promote IFN-γ production (Owaki et al., 2005; Yoshida et al., 2001). Because IL-6 can also activate STAT1, we assessed the effect of IL-6-primed naïve T cells in trans. Addition of IL-6-primed naïve T cells had no effect on the production of IL-17 by bystander nascent Th17 cells (CD45.1+CD4+) (Figure 1A and 1B). The inhibition of Th17 cell development by IL-27-primed naïve T cells was also effective when DCs were included (Figure S1I and S1J). However, global T cell activation was not impaired by IL-27-primed naïve cells, as proliferation of bystander CD4+ T cells was unaffected (Figure S1K). Priming with IL-27 did not influence Th1 cell differentiation as evidenced by IFN-γ production in CD45.1+CD4+ T cells (Figure S1L), indicating that the effect was specific for IL-17 production.
Figure 1. IL-27-primed cells inhibit Th17 cell differentiation in trans.
Briefly, naïve CD45.2+CD4+ T cells were primed with the indicated cytokines. After washing, cells were mixed with naïve CD45.1+CD4+ T cells and cultured with IL-6, TGF-β and anti-IFN-γ for 3 days. (A, B) IL-17A and IFN-γ production in CD45.1+CD4+ T cells was assessed by intracellular staining. Representative intracellular staining is shown for Th17 cells (A). Pooled data from six independent experiments are provided in B (mean values, NS, not significant). (C–F) Production of IFN-γ and IL-17A in CD45.1+OT-II CD4+ T cells was analyzed by flow cytometry. Total numbers of CD45.1+OT-II CD4+ T cells are provided in C. Representative results from 2 individual mice are shown in D and pooled data from 2 independent experiments are provided in E and F (mean values, NS, not significant).
Because it has been reported that the inhibitory effect of IL-27 on T cell differentiation can be mediated by DCs (Wang et al., 2007), we wondered whether IL-27 priming of DCs might also constrain Th17 cell differentiation. However, we found that this was not the case. In the absence of IL-27-primed T cells, IL-27 priming of CD11c+ DCs to T cells had no effect on IL-17 production by CD45.1+CD4+ T cells (Figure S1M and Figure S1N). Accordingly, we noted minimal expression of Il27ra on freshly isolated, non-activated CD11c+ cells (Figure S1O). Taken together, naïve T cells primed with IL-27 can inhibit IL-17 production, even if the T cells have not been directly exposed to this cytokine.
IL-27-primed naïve T cells inhibit Th17 cell differentiation in vivo
To assess whether IL-27-primed naïve CD4+ T cells can attenuate the development of Th17 cells in vivo, we primed naïve CD45.2+CD4+ T from OVA-TCR-transgenic (OT-II) mice with either IL-27 or no cytokine for 3 hours and transferred these cells along with unprimed naïve CD45.1+ OT-II T cells into mice. Mice were immunized with OVA peptide and CFA and cytokine expression in directly isolated T cells was measured by intracellular staining 7 days after immunization. Consistent with our results in vitro, we did not find a difference in the absolute number of CD45.1+CD4+ T cells that expanded in vivo, suggesting that proliferation was not impaired (Figure 1C). Importantly though, the relative and absolute numbers of IL-17-producing CD45.1+ T cells were greatly diminished in mice that received IL-27-treated naïve CD45.2+ T cells, but not in immunized mice that received unprimed naïve CD45.2+ T cells (Figure 1D and 1E). The effect of IL-27 priming was limited to Th17 cell differentiation, as there was no difference in the relative or total numbers of CD45.1+CD4+ T cells that produced IFN-γ (Figure 1F). Thus, naïve T cells primed with IL-27 can exert their inhibitor effect on IL-17 production in other T cells in vivo.
Administration of IL-27-primed naïve CD4+ T cells ameliorates development of autoimmune disease
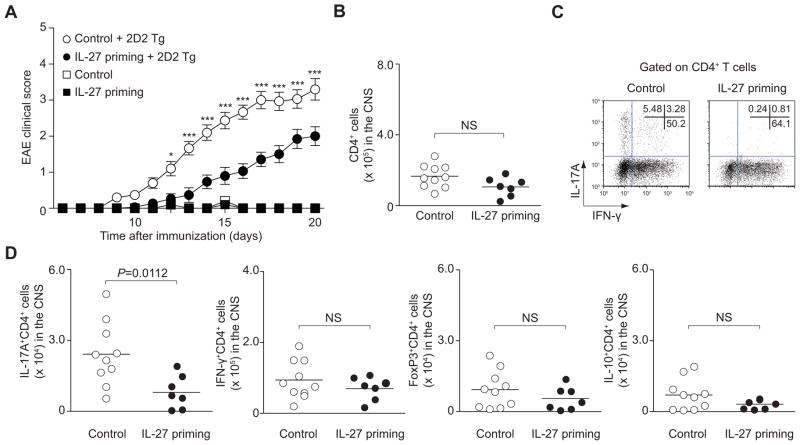
Based on our findings, we reasoned that exposure of naïve Th cells to IL-27 could be another important mechanism by which IL-27 acts to limit IL-17-dependent pathology. Therefore, we next used the model of EAE in which Th17 cells are thought to be key in terms of immunopathogenesis (Langrish et al., 2005). We adoptively transferred untreated or IL-27-primed non-TCR transgenic naïve T cells with or without naïve TCR transgenic T cells that recognize MOG peptide (2D2 T cells), into Rag2−/− mice. After transfer, mice were immunized with MOG and CFA and followed for the development of clinical signs (Figure S2A). As expected, mice that received primed T cells alone without transgenic T cells did not develop disease. Control mice that received unprimed naïve T cells together with naïve 2D2 T cells developed severe disease with an average score of 3.3 (Figure 2A). In sharp contrast, mice that received IL-27-primed naïve T cells together with 2D2 T cells developed a significantly milder disease, manifested by an average score of 2.0 (P<0.001, two-way ANOVA). Cumulative disease scores from each mouse also showed significant decrease in the mice that received IL-27-primed naïve T cells together with 2D2 T cells (Figure S2B, P<0.0001, two-tailed Student’s t-test). To ascertain that IL-27 priming was independent of TCR specificity, we primed OT-ll T cells, transferred along with naïve 2D2 T cells and found that they too limited pathology (Figure S2C and S2D). Again, consistent with the notion that IL-27-cultured naïve cells did not globally suppress immune responses, the absolute numbers of CD4+ T cells infiltrating in the CNS were not significantly different between groups (Figure 2B). Strikingly, the protection from severe EAE in mice, which received IL-27-primed naïve T cells, was associated with significant suppression in the absolute numbers of IL-17+ T cells in the CNS (Figure 2C, 2D and Figure S2E). In contrast, the numbers of IFN-γ+ T cells, IL-10+ T cells or FoxP3 expressing T cells were not different between the two groups (Figure 2C, 2D and Figure S2E). These data suggest that IL-27-primed naïve T cells act to selectively inhibit Th17 cell differentiation in vivo and modulate IL-17-mediated autoimmune disease.
Figure 2. IL-27-primed naïve CD4+ T cells inhibit pathogenicity of T cells in trans.
Briefly, naïve CD4+ T cells were primed with or without IL-27 and transferred with or without sorted naïve TCR transgenic 2D2 CD4+ T cells into Rag2−/− mice. To induce EAE, which were immunized with MOG peptide in CFA. (A) Data provided represent mean ± s.e.m. of the EAE clinical score of 40 mice pooled from 3 independent experiments (***P<0.001, *P<0.05). (B–D) CNS-infiltrating CD4+ T cells from recipient mice of 2 groups from A were analyzed on day 20 after immunization. Absolute numbers (B), representative flow cytometric analysis of IL-17A and IFN-γ (C) and absolute numbers of subsets of CD4+ T cells (D) are provided from 2 independent experiments (mean values, NS, not significant).
IL-27 rapidly induces PD-L1 expression on naïve CD4+ T cells
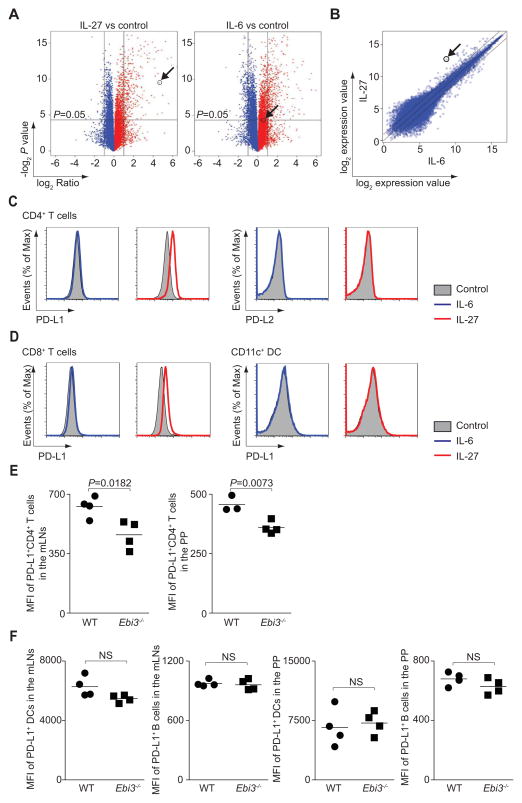
To define potential mechanisms by which IL-27-priming inhibited Th17 cell differentiation of non-primed cells, we analyzed global gene expression in naïve CD4+ T cells stimulated with IL-27 for 3 hours. We found that 375 genes showed a > 2-fold difference in unstimulated versus IL-27-stimulated naïve T cells. Since IL-6-priming was able to activate STAT1 and STAT3, but incapable of inhibiting Th17 cell differentiation in trans, we compared genes induced by IL-27 or IL-6. IL-6 stimulation resulted in 648 genes with a > 2-fold difference compared to unstimulated naïve T cells (Figure 3A). Strikingly, one of the genes significantly increased in IL-27-stimulated cells was Cd274, which encodes PD-L1, a key immunosuppressive molecule that constrains immune responses (Francisco et al., 2010) (Table S1). Of note, this gene was not induced by IL-6 (Figure 3A, 3B and Figure S3A), and the expression of other related co-stimulatory molecules was not affected (Figure S3A). The ability of IL-27 to induce PD-L1 at the level of protein expression was confirmed (Figure 3C).
Figure 3. IL-27 priming rapidly induces PD-L1 on naïve CD4+ T cells.
(A–C) Naïve CD4+ T cells were cultured in medium alone (control), or stimulated with IL-6 or IL-27 for 3 hours before analyzing differential gene expression by microarray. The arrow indicates Cd274 expression. Volcano plots depict differential gene expression induced by IL-27 or IL-6 compared to control (A). Comparison of IL-27- and IL-6-dependent gene expression is shown as a scatter plot (B). (C and D) Naïve CD4+ T cells (C), naïve CD8+ T cells or freshly isolated CD11c+ DCs (D) were stimulated as in A and expression of PD-L1 and PD-L2 was determined by flow cytometry. (E and F) Wild type or Ebi3−/− mice were orally administered T. gondii, and sacrificed on day 9. Mesenteric lymph node (mLN) and Peyers patch (PP) cells were gated on CD4+, TCR-β +, CD8−, and Foxp3− cells. PD-L1 expression is shown as percentages of CD4+ T cells, and by geometric mean channel fluorescence (E). DCs were gated on CD3−, NK1.1−, CD19− and CD11chigh. B cells are gated on CD3−, NK1.1− and CD19+ (F). The experiment shown is representative of 2 separate experiments, (mean values, NS, not significant).
Of note, IL-27 did not induce PD-L1 expression in CD11c+ DCs (Figure 3D), a cell type that lacks the expression of the Il27ra (Figure S1O). In contrast, IL-27 enhanced PD-L1 expression on CD8+ T cells (Figure 3D). In addition, we found no difference in expression of the immunosuppressive cytokines Il10 or Ebi3, a component shared by IL-27 or IL-35, between unstimulated T cells and IL-27-stimulated T cells (Figure S3B). These results indicate that IL-27 rapidly induces PD-L1, affecting naïve T cells, but not other subsets.
Requirement for IL-27 signaling for optimal induction of PD-L1 in T. gondii infection
To assess the relevance of IL-27 in controlling PD-L1 expression in vivo, we employed a model in which IL-27 is important for controlling immunopathology (Villarino et al., 2003). We found that expression of PD-L1 on CD4+ T cells was significantly lower in Ebi3−/− mice (Figure 3E). However, the effect was selective for T cells as PD-L1 expression on other immune cells remained unaffected (Figure 3F).
PD-L1 engagement underlies the immunosuppressive effect of IL-27-primed naïve T cells
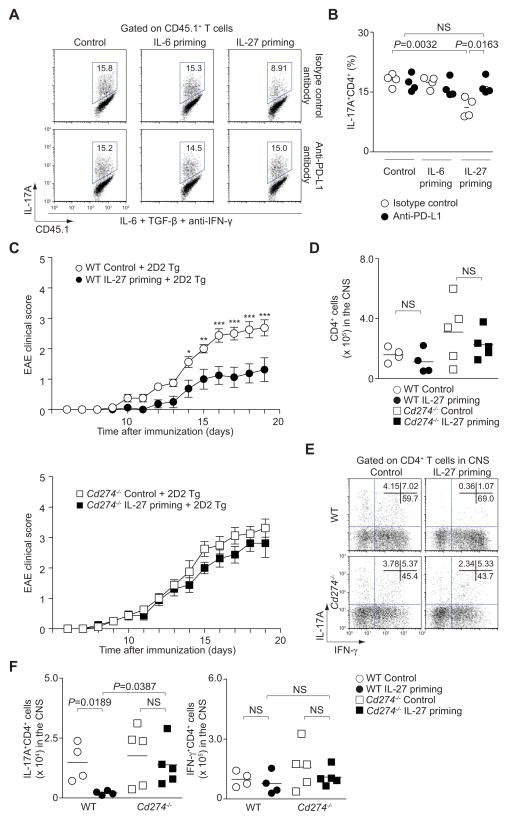
We next sought to examine whether the induction of PD-L1 on IL-27-primed naïve CD4+ T cells was the principal component of the inhibition of bystander Th17 cell differentiation. To this end, we first investigated the effect of the addition of neutralizing anti-PD-L1 antibody to co-cultures of IL-27-primed naïve CD4+ T cells (CD45.2+) added to developing Th17 cells (CD45.1+). The addition of anti-PD-L1 neutralizing antibody (lower panels) completely reversed the effect of IL-27-primed naïve CD4+ T cells to inhibit Th17 cell differentiation in non-primed CD4+ T cells (Figure 4A and 4B).
Figure 4. IL-27-induced expression of PD-L1 on naïve CD4+ T cells inhibits IL-17 production in trans.
(A and B) Naïve CD45.2+CD4+ T cells were primed with IL-6 or IL-27 or cultured in medium alone. After 3 hours, cells were washed and co-cultured with naïve CD45.1+CD4+ T cells under Th17 cell-polarizing conditions with an isotype control antibody (upper panel) or anti-PD-L1 antibody (lower panel). After 3 days IL-17A protein expression of CD45.1+CD4+ T cells was analyzed by intracellular staining. Representative intracellular staining is depicted in A and pooled data from four separate experiments with mean values are shown in B (NS, not significant). (C) Data provided represent mean ± s.e.m. of the EAE clinical score of 32 mice pooled from 2 independent experiments (***P<0.001, **P<0.01, *P<0.05). (D–F) CNS-infiltrating CD4+ T cells from recipient mice of 2 groups from C were analyzed on day 19 after immunization. Absolute numbers (D), representative flow cytometric analysis of IL-17A and IFN-γ (E) and absolute numbers of subsets of CD4+ T cells (F) are provided (mean values, NS, not significant).
To confirm the nonredundant role of PD-L1 in mediating the in trans inhibition of Th17 cell development by IL-27-primed CD4+ T cells in vivo, we assessed the effect of T cells from mice that lack this ligand (Latchman et al., 2004). Specifically, untreated or IL-27-primed CD4+ T cells from wild type or Cd274−/− mice were transferred along with 2D2 T cells into recipient mice that were immunized with MOG peptide. As demonstrated before in figure 2A, IL-27-primed naïve T cells ameliorated EAE severity (Figure 4C, upper panel and Figure S4A). Importantly, this protection by IL-27-primed naïve T cells was completely lost in the absence of PD-L1 (Figure 4C, lower panel and Figure S4A). While similar numbers of absolute CD4+ T cells and IFN-γ-producers were present in the CNS in all groups, the number of IL-17 producers was significantly reduced when IL-27-primed wild type cells were administered (Figure 4D-F). Again, administration of Cd274−/− T cells had no effect on the number of IL-17-producing in the CNS (Figure 4E and 4F).
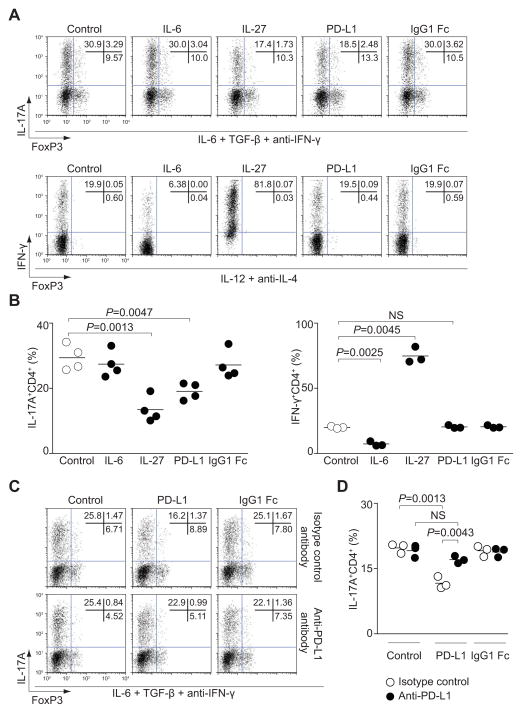
If the immunosuppressive effect of IL-27 priming was mediated by upregulation of PD-L1, it should be possible to mimic this effect simply by adding this ligand to differentiating Th17 cells in vitro. Consistent with our hypothesis, we found that inclusion of soluble PD-L1 fusion protein also resulted in suppression of IL-17 expression comparable to that observed with IL-27 (Figure 5A and 5B). Th17 cell differentiation was not affected by addition of a control Ig fusion protein (IgG1 Fc). The effect of soluble PD-L1 during Th17 cell differentiation was specific for IL-17, since IFN-γ expression in Th1 cells was not affected by exogenous PD-L1 (Figure 5A and 5B). In addition, inhibiting PD-1-PD-L1 interactions with neutralizing antibodies reversed the inhibition of IL-17 production by the PD-L1 fusion protein (Figure 5C and 5D). In summary, the inhibitory effect of IL-27-primed T cells is dependent upon PD-L1 expression.
Figure 5. Soluble PD-L1 affects Th17 cell differentiation.
(A and B) Naïve CD4+ T cells were cultured with IL-6, TGF-β and anti-IFN-γ (Th17 cell-polarizing conditions, upper panel) or IL-12 and anti-IL-4 (Th1 cell-polarizing conditions, lower panel) for 3 days with PD-L1 or IgG1-Fc as a control. IL-27 and IL-6 were added as additional controls. IL-17A, IFN-γ and FoxP3 protein expression were analyzed by intracellular staining. Representative flow cytometry plots are depicted in A and pooled data from 3 (Th1 cell-polarizing condition) or 4 (Th17 cell-polarizing condition) individual experiments with mean values are shown in B (NS, not significant). (C and D) Naïve CD4+ T cells were cultured under Th17 cell-polarizing conditions with PD-L1 or IgG1-Fc in the presence of anti-PD-L1 antibody (lower panel) or isotype control antibody (upper panel) for 3 days. IL-17 and FoxP3 protein expression were analyzed by intracellular staining. Representative intracellular staining is depicted in C and pooled data from 3 individual experiments with mean values are shown in D (NS, not significant).
PD-L1 engagement inhibits Th17 cell differentiation independently of FoxP3
Previous studies have indicated that PD-1-PD-L1 interactions induce expression of FoxP3 in CD4+ T cells (Francisco et al., 2009). We therefore considered the possibility that this might be an explanation underlying the inhibitory effect of PD-L1 on IL-17 production. However, neither IL-27 nor PD-L1 directly affected FoxP3 expression in CD4+ T cells cultured under Th17 cell-polarizing conditions (Figure 5A and Figure S5A). To confirm that PD-L1 exerted its immunoregulatory function independently of FoxP3, we examined whether PD-L1 inhibited IL-17 production in T cells from mice with mutation of Foxp3 (Scurfy). Equivalent inhibitory effects of IL-27 on IL-17 production were evident in T cells from Scurfy mice, arguing for a FoxP3-independent mechanism (Figure S5B and S5C). Similarly, the inhibitory effect of recombinant PD-L1 was the same in cells from wild type and Scurfy mice. Thus, PD-L1 engagement inhibits IL-17 production in a FoxP3-independent manner.
The Cd274 locus is accessible in naïve CD4+ T cells and is directly regulated by STAT1
We next sought to determine how IL-27 might be acting to regulate PD-L1 on naïve CD4+ T cells. First, we hypothesized that the Cd274 gene locus should be accessible in naïve Th cells, as IL-27 rapidly induced PD-L1 expression. Indeed, by chromatin immunoprecipitation and massive parallel sequencing we could identify that the Cd274 promoter was characterized by H3 lysine 4 trimethylation, whereas the Pdcd1Ig2 promoter did not show this positive epigenetic modification (Wei et al., 2009) (Figure S6A).
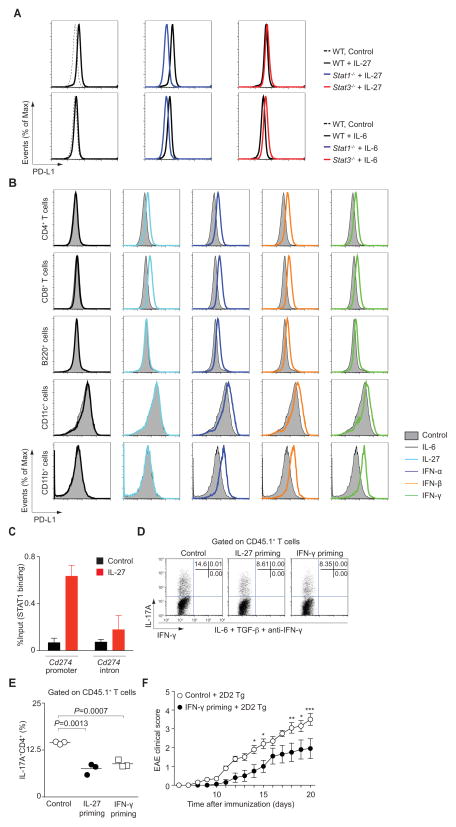
Next, as IL-27 induces phosphorylation of STAT1 and STAT3, we stimulated naïve CD4+ T cells from wild type mice and mice deficient in STAT1 or STAT3. We found that induction of Cd274 by IL-27 was completely abolished by the absence of STAT1, but was unaffected by the absence of STAT3 (Figure 6A (upper panel), Figure S6B and S6D). IL-6 had no effect on PD-L1 in Stat1−/− T cells (Figure S6C). Interestingly though, in Stat3−/− T cells, in which IL-6 induced unopposed activation of STAT1, this cytokine did induce expression of PD-L1 (Figure 6A (lower panel), Figure S6C and S6D). The importance of STAT1 in regulating PD-L1 expression was confirmed by stimulation of cells with other STAT1-activating cytokines. Like IL-27, type I and type II interferons induced PD-L1 expression on naïve CD4+ T cells (Figure 6B). However, the effect of these ligands was not selective; type I and type II interferons (IFNs) also upregulated PD-L1 expression on cells that failed to respond to IL-27, including B cells, DCs and CD11b+ cells (Figure 6B).
Figure 6. Rapid induction of PD-L1 by IL-27 is directly mediated by STAT1.
(A) Naïve CD4+ T cells from Stat1−/−, Stat3−/− or wild type control mice were stimulated with the indicated cytokines for 3 hours. Surface expression of PD-L1 is shown. (B) Naïve CD4+ T cells, freshly isolated CD8+ T cells, B220+ cells, CD11c+ cells and CD11b+ cells were stimulated with indicated cytokines for 3 hours and expression of PD-L1 was determined by flow cytometry. Representative data are provided from 2 separate experiments. (C) Naïve CD4+ T cells were stimulated with IL-27 for 30 minutes. Fixed cells were immunoprecipitated with anti-STAT1 antibody. Eluted DNA was analyzed by quantitative PCR (mean ± s.d.). Representative data are provided from 2 separate experiments. (D, E) Briefly, naïve CD45.2+CD4+ T cells were primed with the indicated cytokines. After washing, cells were mixed with naïve CD45.1+CD4+ T cells and cultured with IL-6, TGF-β and anti-IFN-γ for 3 days. IL-17A and IFN-γ production in CD45.1+CD4+ T cells was assessed by intracellular staining. Representative intracellular staining is shown for Th17 cells (D). Pooled data from three independent experiments are provided in E (mean values). (F) Data show mean ± s.e.m. of the EAE clinical score of 20 mice pooled from 2 independent experiments (***P<0.001, **P<0.01, *P<0.05).
One other important target of STAT1 is Tbx21, which encodes T-bet, the master regulator of Th1 cells (Lighvani et al., 2001). To explore if STAT1 indirectly regulates PD-L1 through induction of T-bet, we first assessed the induction of PD-L1 in cells from mice lacking T-bet. However, we found that this transcription factor had no effect on induction (Figure S6E). We next investigated the possibility that STAT1 directly binds to the Cd274 promoter in naïve T cells. Binding of STAT1 to the Cd274 promoter, which contains GAS and ISRE elements, was noted following stimulation of cells with IL-27. In contrast, minimal binding was observed to the Cd274 intronic region (Figure 6C). Thus, the epigenetic modifications of the Cd274 locus in naïve T cells and the direct binding of IL-27-induced STAT1 help explain the rapid expression and function of PD-L1 on naïve T cells. As IFNs induced PD-L1 expression on naïve CD4+ T cells (Figure 6B), we wondered if IFNs, like IL-27, had the capability to inhibit Th17 cell differentiation in trans. In fact, we found that IFN-γ-priming of T cells inhibited in vitro Th17 cell differentiation to a similar degree as IL-27-priming (Figure 6D and E). To assess whether IFN-γ primed naïve CD4+ T cells can also limit IL-17-dependent pathology in vivo, we adoptively transferred untreated or IFN-γ-primed naïve CD4+ T cells along with 2D2 T cells into Rag2−/− mice, which were immunized with CFA and MOG peptide. Again, control mice that received unprimed naïve T cells together with naïve 2D2 T cells developed severe disease with an average score of 3.5 (Figure 6F and Figure S6F). In contrast, mice that received IFN-γ-primed naïve T cells together with 2D2 T cells developed a significantly milder disease, manifested by an average score of 1.95 (P<0.001, two-way ANOVA).
In summary, STAT1 directly binds the Cd274 promoter region and induces its transcription.
STAT1 is critical for inhibition of Th17 cell differentiation in vivo by IL-27-primed naïve T cells
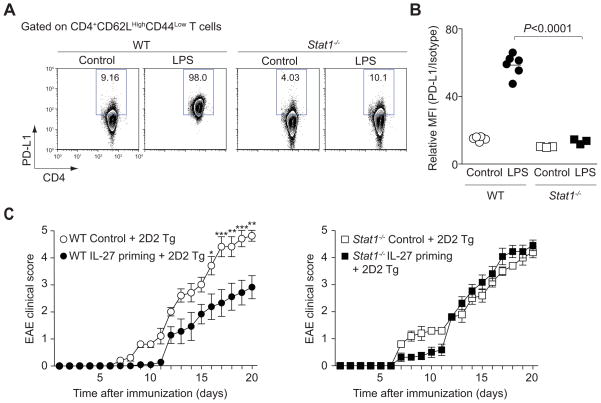
The in vivo physiological relevance of the STAT1-dependent PD-L1 induction on naïve CD4+ T cells was investigated in a model of systemic inflammation in which IFNs and other cytokines are elaborated (Karaghiosoff et al., 2003). To this end, we injected lipopolysaccharide (LPS) into wild type and STAT1-deficient mice and assessed the PD-L1 expression. We found that PD-L1 expression was induced by naïve CD4+ T cells following LPS injection in wild type mice, but the effect was blocked in Stat1−/− mice (Figure 7A and 7B). To confirm the importance of STAT1-dependent PD-L1 regulation, we next examined whether STAT1 was required for the immunosuppressive function of IL-27 primed naïve T cells and their ability to inhibit bystander cells in trans. We therefore adoptively transferred IL-27-primed wild type and Stat1−/− naïve CD4+ T cells along with 2D2 T cells into Rag2−/− mice which were then immunized with CFA and MOG peptide. We found that STAT1 deficiency abrogated the ability of IL-27 priming to attenuate autoimmune disease (Figure 7C and Figure S7A).
Figure 7. STAT1 is critical for induction of PD-L1 and inhibition of Th17 cell differentiation in vivo.
(A, B) Wild type or Stat1−/− mice received an intraperitoneal injection of LPS with PBS. One day after injection, spleens were harvested and CD4+ T cells were analyzed by flow cytometry for PD-L1 expression. Representative results are shown in A and pooled data from 2 independent experiments are provided in B (mean values). (C) Data show mean ± s.e.m. of the EAE clinical score of 32 mice pooled from 2 independent experiments (***P<0.001, **P<0.01, *P<0.05).
In summary, the ability of IL-27 primed T cells to limit immune-mediated pathology is dependent upon STAT1.
DISCUSSION
Previous work has established that IL-27 has critical, non-redundant functions as an immunosuppressive cytokine (Batten et al., 2006; Kastelein et al., 2007; Stumhofer et al., 2006; Villarino et al., 2003). Herein, we report an unexpected, direct action of IL-27 on naïve Th cells that allows primed cells to limit IL-17-mediated pathology in trans. Our data show that IL-27 induces PD-L1 in a STAT1-dependent manner, which in turn, inhibits Th17 cell differentiation in bystander cells. This effect limits IL-17-dependent pathology, but has no effect on global T cell activation or Th1 cell differentiation. Thus, the data point to a new, important mechanism of action of IL-27.
Multiple mechanisms have been identified by which IL-27 can suppress immune responses. It is recognized that IL-27 inhibits Th17 cell differentiation by acting directly upon differentiating CD4+ T cells via induction of T-bet and downregulation of Rorγt (Batten et al., 2006; Diveu et al., 2009; Stumhofer et al., 2006). We found this to be the case as well. There was a trend in reduction of IL-17 expression in CD4+ T cells exposed to IL-27 for 3 hours. But we did not examine this further, as direct versus indirect could not be discerned. While, the mechanism described in the present study is distinct from this mode of inhibition in that differentiating CD4+ T cells were not directly exposed to IL-27 and yet a profound effect was still noted. In the conditions we employed, naïve T cells were exposed to IL-27, after which the cytokine was removed, and thus the inhibitory effect of IL-27 was mediated indirectly.
Another immunosuppressive action of IL-27 is mediated by the production of IL-10 (Batten et al., 2008; Diveu et al., 2009; Stumhofer et al., 2007). IL-27, in combination with TGF-β, has been reported to generate IL-10-producing regulatory T cells characterized by the expression of c-Maf, IL-21, ICOS and AhR (Apetoh et al., 2010; Awasthi et al., 2007; Pot et al., 2009). Our short-term stimulation of IL-27 did not induce IL-10 in naïve CD4+ T cells (Figure S3B); however, this is not to say that IL-27-induced IL-10 production is not an important part of IL-27’s action. Nonetheless, in our system the inhibitory effect of IL-27 was largely reversed by elimination of PD-L1 on T cells, arguing that this is a functionally important mechanism.
One of the striking findings of the present study was that IL-27 rapidly induced PD-L1 on naïve CD4+ and CD8+ T cells. To our knowledge, this has not been recognized previously to be an action of IL-27. Within 3 hours of exposure to this cytokine, mRNA of PD-L1 could be seen. Furthermore we have demonstrated that this is functionally relevant in blocking IL-17 production both in vitro and in vivo. The PD-1-PD-L1 pathway is now recognized to be important in the maintenance of peripheral tolerance (Chen, 2004; Francisco et al., 2010; Freeman et al., 2000). Mice lacking PD-L1 do not develop spontaneous autoimmune disease, but its deficiency exacerbates disease in EAE, autoimmune arthritis and autoimmune diabetes (Chen, 2004; Fife et al., 2006; Hamel et al., 2010; Latchman et al., 2004). The relevance of this pathway in human disease is substantiated by the association of polymorphisms of CD274 (which encodes PD-L1) with Graves’ disease and autoimmune Addison’s disease (Hayashi et al., 2008; Mitchell et al., 2009). Conversely, upregulation of PD-1 in CD8+ T cells contributes to exhaustion of reactive T cells in mice with chronic viral infection (Barber et al., 2006). Similarly, high expression of PD-1 by HIV-specific CD8+ T cells was found in HIV-infected individuals and was associated with high viral load and exhaustion of HIV-specific CD8+ T cells (Trautmann et al., 2006).
In some settings, engagement of PD-L1 with PD-1 generates FoxP3-expressing Tregs (Francisco et al., 2009). However, in our system, we saw no evidence of FoxP3 induction either in naïve cells directly primed with IL-27 or in co-cultured bystander cells under Th17 cell-polarizing condition (data not shown). Multiple stimuli can induce PD-L1 expression on T cells and antigen presenting cells. The promoters of the genes encoding mouse and human PD-L1 exhibit interferon-sensitive response elements, IFN regulatory factor-1 (IRF-1) binding sites, and gamma interferon activated sites (Lee et al., 2006). Accordingly, PD-L1 can be induced in DCs by type I and type II IFNs (Schreiner et al., 2004; Yamazaki et al., 2002), although we found that non-activated DCs do not express IL-27 receptors and thus are not responsive to this cytokine basally. As reported herein, STAT1-activation is of particular importance for PD-L1 upregulation by naïve T cells. These data argue that IL-27 is not unique in its ability to induce PD-L1. In fact, this was the case. We found that IFNs also induced PD-L1 on naïve CD4 and CD8 T cells. Moreover, T cells primed with IFN-γ also inhibited Th17 cell induction in trans, just as cells primed with IL-27. However, it is notable that the effect of IL-27 is more circumscribed compared to type I and type II IFNs. In contrast to IL-27, which acts predominantly on CD4+ and CD8+ T cells, IFNs induce PD-L1 expression on a wide variety of cells.
Activation of T cells via the antigen receptor also upregulates PD-L1 expression (Yamazaki et al., 2002). However, the findings presented in the present study argue that in the course of an inflammatory immune response, IL-27 can induce PD-L1 upregulation in T cells that are not activated by cognate antigen and thereby limit immunopathology. With respect to the relative proportion of bystander cells expressing PD-L1 needed to attenuate Th17 cell differentiation of activated CD4 T cells, it is obviously difficult to directly extrapolate the relevance of in vitro conditions. Nonetheless, the proportion of naïve CD4+ and CD8+ T cells that can upregulate PD-L1 in response to IL-27 greatly exceed the proportion of antigen-specific CD4 cells. We also found that the Cd274 promoter was accessible in naïve Th cells, as characterized by the presence of H3 lysine 4 trimethylation associated with the absence of H3 lysine 27 trimethylation, further supporting the notion that PD-L1 can be rapidly induced. Moreover, our results with T. gondii infection substantiate the claim that this mechanism is relevant in vivo. However, this is not to say that induction of PD-L1 on other cells by other cytokines is irrelevant. This is not the case at all, as PD-L1 is inducible in a wide variety of cells where it has critical actions. The present study simply points out that cytokine-dependent induction of PD-L1 on T cells can influence the differentiation of antigen-activated CD4+ T cells.
In summary, our study highlights that naïve PD-L1+ CD4+ T cells induced by IL-27 can limit the effect of pathogenic IL-17 producing Th17 cells in vitro and in vivo. It is conceivable that the strategy of IL-27-pretreatment of naïve T cells ex vivo with subsequent adoptive transfer might be used therapeutically to attenuate autoimmune disease.
EXPERIMENTAL PROCEDURES
Mice and media
C57BL/6J, B6.Cg-Tg(TcraTcrb)425Cbn/J (OT-ll), C57BL/6-Tg (Tcra2D2, Tcrb2D2)1Kuch/J (TCR(2D2)) and B6.129S6-Tbx21tm1Glm/J (Tbx21−/−) mice were purchased from Jackson Laboratory, B6.129S6-Rag2tm1Fwa (Rag2−/−) mice, B6.SJL-Ptprca/BoyAiTac (CD45.1) mice and Foxp3sf mice from Taconic, Stat3fl/fl mice were from D. Levy (Lee et al., 2002) and bred with CD4-Cre+ Tg mice. Stat1−/− mice were also from D. Levy (Durbin et al., 1996). OT-ll Tg mice were bred with B6.SJL-Ptprca/BoyAiTac (CD45.1) mice in our laboratory. FoxP3 enhanced green fluorescent protein (eGFP) reporter mice have been reported previously (Bettelli et al., 2006). EBI3-deficient (Ebi3−/−) mice were from Lexicon Pharmaceuticals, Inc. (The Woodlands, TX). They were generated by Lexicon Pharmaceuticals, Inc. for Centocor Research and Development, Inc. and provided by Centocor. PD-L1 deficient (Cd274−/−) mice were generated by Dr. Arlene Sharpe (Latchman et al., 2004). All animal studies were performed according to the NIH guidelines for the use and care of live animals and were approved by the Institutional Animal Care and Use Committee of NIAMS. All cells were cultured in RPMI medium with 10% (vol/vol) FCS, 2mM glutamine, 100IU ml−1 of penicillin, 0.1 mg ml−1 of streptomycin and 20mM HEPES buffer, pH 7.2–7.5 (all from Invitrogen) and 2mM β–mercaptoethanol (Sigma-Aldrich).
Cell isolation and differentiation
CD4+ T cells from spleens and lymph nodes of 6–8 week-old mice were purified by negative selection and magnetic separation (Miltenyi Biotec) followed by sorting of naïve CD4+CD62L+CD44−CD25− population using FACSAria II (BD). For natural T regulatory population, GFP+ cell were sorted using FACSAria II (BD). CD8+ T cells, B220+ cells, CD11c+ DCs or CD11b+ macrophages from spleens of 6–8 week-old mice were purified by magnetic separation (Miltenyi Biotec). In some experiments, those cells were stimulated with IL-6 (20 ng ml−1), IL-27 (20 ng ml−1), IFN-α (10000U ml−1), IFN-β (10000U ml−1) or IFN-γ (20ng ml−1) for 3 hours (all from R & D). Naïve CD4+ T cells were activated by plate-bound anti-CD3 (10 μg ml−1; eBioscience) and soluble anti-CD28 (1 μg ml−1; eBioscience) in media for 3 or 4 days either under neutral conditions or with IL-6 (20 ng ml−1, R&D Systems) plus human TGF-β1 (2 ng ml−1, R&D Systems) and anti-IFN-γ neutralizing antibodies (10 μg ml−1, BDPharmingen) for Th17 cell-polarizing condition or IL-12 (20 ng ml−1, R&D Systems) and anti-IL-4 neutralizing antibodies (10 μg ml−1, BioXCell) for Th1 cell-polarizing condition. For co-culture experiments, we incubated naïve CD45.2+CD4+ T cells with CD45.1+ T cells at a ratio of one to one, as we found in preliminary experiments that this was optimal. In some experiments, we added IL-6 (20 ng ml−1), IL-27 (20 ng ml−1), PD-L1 (5 μg ml−1), recombinant human IgG1 Fc (5 μg ml−1) (all from R & D), anti-PD-L1 neutralizing antibody (10 μg ml−1, Abcam (MIH6)) or isotype control antibody (10 μg ml−1, Abcam).
Flow cytometry
Expression of cytokines and transcription factors was assessed by intracellular staining. The following antibodies were used. For cell surface staining: anti-CD4-PerCPCy5.5, anti-CD4-APC, anti-CD44-FITC or -PE, anti-CD25-PE, or -APC, anti-Vβ5.1/5.2-FITC, anti-Vα2-PE, anti-Vβ11-FITC, CD45.1-PerCPCy5.5, anti-PD-L1-PE, anti-PD-L2-PE anti-CD3-FITC (all BD). Anti-CD62L-PE-Cy7, anti-CD11c-PECy7, anti-CD19-eFlour450® and anti-NK1.1-FITC were purchased from eBioscience. Anti-TCR-β-Alexa Fluor700® was purchased from BioLegend. Anti-CD8a-PE-Texas Red® was purchased from Abcam. For intracellular staining: anti-IFNγ-FITC, anti-IL-17A-APC, Foxp3 eFluor450® and anti-FoxP3-FITC (all eBioscience). Anti-IL-4-APC, anti-IL-10-PE, anti-IFNγ-PE, anti-pSTAT1-Alexa-Fluor 647 and anti-pSTAT3-Alexa-Fluro 647 were purchased from BD. For intracellular staining, cells were stimulated for 4 hours with PMA and ionomycin with the addition of brefeldin A (GolgiPlug; BD). Afterwards, cells were fixed with 4% formyl saline, permeabilized with 0.1% saponin buffer and stained with fluorescent antibodies before analyzing on a FACS Calibur (BD). Events were collected and analyzed with Flow Jo software (Tree Star).
In vivo generation of Th17 cells
Sorted naïve CD45.2+ OT-ll CD4+ T cells were primed with or without IL-27 (20 ng ml−1) for 3 hours. After washing 5.0 × 105 cells were transferred together with sorted naïve CD45.1+ OT-ll CD4+ T cells (1.0 × 105 cells) intravenously into C57BL/6J mice. After adoptive transfer recipient mice were immunized subcutaneously with 50μg OVA323-339 peptide (Peptides International) in CFA. Seven days after immunization, spleens were harvested and T cells were analyzed by flow cytometry for surface marker and intracellular cytokine production.
Experimental autoimmune encephalomyelitis (EAE)
Sorted naïve T cells from C57BL/6J mice, TCR(OT-ll) transgenic, Cd274−/− or Stat1−/− mice were primed with or without IL-27 (20ng ml−1) or IFN-γ (20ng ml−1) for 3 hours. After washing 2.5 × 106 cells were transferred intravenously together with sorted naïve Vβ11+ T cells (5.0 × 105 cells) into Rag2−/− mice. After adoptive transfer recipient mice were immunized subcutaneously with 100μg MOG35-55 peptide (Peptides international) in CFA on day 0. These mice were scored daily according to the criteria as mentioned before (Ghoreschi et al., 2010). On day 19 or 20, mononuclear cells were isolated from the spinal cord of a subset of mice, counted and analyzed for cytokine expression. Cumulative disease scores were calculated as the area under the curve for clinical score of each individual mouse as described previously (Greve et al., 2004).
Microarray data collection and analysis
The microarray was performed and analyzed as described previously (Ghoreschi et al., 2010). Approximately 10μg of RNA was labeled and hybridized to GeneChip Mouse Genome 430 2.0 arrays (Affymetrix) according to the manufacturer’s protocols. Expression values were determined using GeneChip Operating Software (GCOS) v1.1.1 and MAS5 method. Downstream analyses were performed with Partek Genomics Suite software and the statistical package R.
T. gondii infections
The ME49 strain of T. gondii was maintained in Swiss Webster and CBA/CaJ, and tissue cysts were prepared as described (Villarino et al., 2003). For infections, mice were administered 10 tissue cysts orally, and sacrificed within 14 days post infection. All experiments were conducted following the guidelines of the University of Pennsylvania Institutional Animal Care and Use Committee.
Chromatin immunoprecipitation (ChIP)
ChIP was performed as described previously (Ghoreschi et al., 2010). Sorted naïve CD4+ T cells were activated with or without IL-27 (20 ng ml−1) for 30 minutes followed by cross-linking for 10 minutes with 1% formaldehyde. The cells were harvested and lysed by sonication. After pre-clearing with protein A agarose beads (Upstate), cell lysates were immunoprecipitated with anti-STAT1 (Cell Signaling) overnight at 4 °C. After washing and elution, cross-link were reversed at 65 °C for 4h. The eluted DNA was purified, samples analyzed by quantitative-PCR with custom-designed primers and probes using a 7500 real-time PCR system (Applied Biosystems). Primers spanning the promoter region of Cd274 and the intronic region of Cd274 are described in Supplemental experimental procedures. The Ct value of each sample was normalized to the corresponding input value.
LPS challenge
Mice received an intraperitoneal injection of 1μg of LPS (Escherichia coli 0111:B4 LPS; Sigma L2630) in 200 μL of PBS. 18 hours after injection, T cells isolated from spleens were analyzed by flow cytometry for surface marker.
Statistics
For statistical analysis, all P values in clinical scores of EAE experiments were calculated with a two-way ANOVA. All of other were calculated with a two-tailed Student’s t-test.
Supplementary Material
HIGHLIGHTS.
IL-27 priming of naïve T cells inhibits Th17 cell differentiation in trans.
IL-27 rapidly induces gene expression in naïve T cells.
IL-27 upregulates PD-L1 in naïve CD4+ T cells through STAT1.
Induction of PD-L1 by IL-27 is an important means of inhibiting IL-17.
Acknowledgments
We thank M. Pelletier (Autoimmunity Branch, NIAMS), J. Simone, J. Lay (Flow Cytometry Section, NIAMS) and the NIAMS LACU staff for their excellent technical support. We thank A. Villarino for critically reading this manuscript and providing helpful suggestions. This work was supported by the Intramural Research Programs of NIAMS, NIAID, the JSPS Research Fellowship for Japanese Biomedical and Behavioral Researchers at NIH (K.H. and H.T.) and Istituto Pasteur-Fondazione Cenci-Bolognetti (G.S.), NIH R01 AI 42334 (C.A.H.), NIH R01 AI 40614 (A.H.S.) and NIH R37 AI 38310 (A.H.S.).
Footnotes
Author contributions. K.H. and K.G. designed, performed, analyzed and interpreted all the experiments and wrote the manuscript. X.-P.Y., H.T., A.L., G.S., A.O’H.H., C.D., L.M.F., Q.C. and M.T. helped with performing experiments. H.-W.S. and G.V. interpreted the microarray experiments and ChIP-seq data. Y.K., A.H.S. and C.A.H. contributed to the experimental design, data interpretation and made helpful suggestions. J.J.O’S contributed to design experiments, analyzed and interpreted all acquired data and helped to write the manuscript.
Competing financial interests. The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Apetoh L, Quintana FJ, Pot C, Joller N, Xiao S, Kumar D, Burns EJ, Sherr DH, Weiner HL, Kuchroo VK. The aryl hydrocarbon receptor interacts with c-Maf to promote the differentiation of type 1 regulatory T cells induced by IL-27. Nat Immunol. 2010;11:854–861. doi: 10.1038/ni.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasthi A, Carrier Y, Peron JP, Bettelli E, Kamanaka M, Flavell RA, Kuchroo VK, Oukka M, Weiner HL. A dominant function for interleukin 27 in generating interleukin 10-producing anti-inflammatory T cells. Nat Immunol. 2007;8:1380–1389. doi: 10.1038/ni1541. [DOI] [PubMed] [Google Scholar]
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- Batten M, Kljavin NM, Li J, Walter MJ, de Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. J Immunol. 2008;180:2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- Batten M, Li J, Yi S, Kljavin NM, Danilenko DM, Lucas S, Lee J, de Sauvage FJ, Ghilardi N. Interleukin 27 limits autoimmune encephalomyelitis by suppressing the development of interleukin 17-producing T cells. Nat Immunol. 2006;7:929–936. doi: 10.1038/ni1375. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Chen L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat Rev Immunol. 2004;4:336–347. doi: 10.1038/nri1349. [DOI] [PubMed] [Google Scholar]
- Chen Q, Ghilardi N, Wang H, Baker T, Xie MH, Gurney A, Grewal IS, de Sauvage FJ. Development of Th1-type immune responses requires the type I cytokine receptor TCCR. Nature. 2000;407:916–920. doi: 10.1038/35038103. [DOI] [PubMed] [Google Scholar]
- Chiyo M, Shimozato O, Yu L, Kawamura K, Iizasa T, Fujisawa T, Tagawa M. Expression of IL-27 in murine carcinoma cells produces antitumor effects and induces protective immunity in inoculated host animals. Int J Cancer. 2005;115:437–442. doi: 10.1002/ijc.20848. [DOI] [PubMed] [Google Scholar]
- Collison LW, Chaturvedi V, Henderson AL, Giacomin PR, Guy C, Bankoti J, Finkelstein D, Forbes K, Workman CJ, Brown SA, et al. IL-35-mediated induction of a potent regulatory T cell population. Nat Immunol. 2010;11:1093–1101. doi: 10.1038/ni.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JH, Kljavin NM, Ramamoorthi N, Diehl L, Batten M, Ghilardi N. IL-27 promotes T cell-dependent colitis through multiple mechanisms. J Exp Med. 2011;208:115–123. doi: 10.1084/jem.20100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cua DJ, Sherlock J, Chen Y, Murphy CA, Joyce B, Seymour B, Lucian L, To W, Kwan S, Churakova T, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- Diveu C, McGeachy MJ, Boniface K, Stumhofer JS, Sathe M, Joyce-Shaikh B, Chen Y, Tato CM, McClanahan TK, de Waal Malefyt R, et al. IL-27 blocks RORc expression to inhibit lineage commitment of Th17 cells. J Immunol. 2009;182:5748–5756. doi: 10.4049/jimmunol.0801162. [DOI] [PubMed] [Google Scholar]
- Durbin JE, Hackenmiller R, Simon MC, Levy DE. Targeted disruption of the mouse Stat1 gene results in compromised innate immunity to viral disease. Cell. 1996;84:443–450. doi: 10.1016/s0092-8674(00)81289-1. [DOI] [PubMed] [Google Scholar]
- Fife BT, Guleria I, Gubbels Bupp M, Eagar TN, Tang Q, Bour-Jordan H, Yagita H, Azuma M, Sayegh MH, Bluestone JA. Insulin-induced remission in new-onset NOD mice is maintained by the PD-1-PD-L1 pathway. J Exp Med. 2006;203:2737–2747. doi: 10.1084/jem.20061577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald DC, Ciric B, Touil T, Harle H, Grammatikopolou J, Das Sarma J, Gran B, Zhang GX, Rostami A. Suppressive effect of IL-27 on encephalitogenic Th17 cells and the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;179:3268–3275. doi: 10.4049/jimmunol.179.5.3268. [DOI] [PubMed] [Google Scholar]
- Francisco LM, Sage PT, Sharpe AH. The PD-1 pathway in tolerance and autoimmunity. Immunol Rev. 2010;236:219–242. doi: 10.1111/j.1600-065X.2010.00923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, Sharpe AH. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206:3015–3029. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, Yang XP, Tato CM, McGeachy MJ, Konkel JE, Ramos HL, Wei L, Davidson TS, Bouladoux N, et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve B, Vijayakrishnan L, Kubal A, Sobel RA, Peterson LB, Wicker LS, Kuchroo VK. The diabetes susceptibility locus Idd5.1 on mouse chromosome 1 regulates ICOS expression and modulates murine experimental autoimmune encephalomyelitis. J Immunol. 2004;173:157–163. doi: 10.4049/jimmunol.173.1.157. [DOI] [PubMed] [Google Scholar]
- Hamano S, Himeno K, Miyazaki Y, Ishii K, Yamanaka A, Takeda A, Zhang M, Hisaeda H, Mak TW, Yoshimura A, Yoshida H. WSX-1 is required for resistance to Trypanosoma cruzi infection by regulation of proinflammatory cytokine production. Immunity. 2003;19:657–667. doi: 10.1016/s1074-7613(03)00298-x. [DOI] [PubMed] [Google Scholar]
- Hamel KM, Cao Y, Wang Y, Rodeghero R, Kobezda T, Chen L, Finnegan A. B7-H1 expression on non-B and non-T cells promotes distinct effects on T- and B-cell responses in autoimmune arthritis. Eur J Immunol. 2010;40:3117–3127. doi: 10.1002/eji.201040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Kouki T, Takasu N, Sunagawa S, Komiya I. Association of an A/C single nucleotide polymorphism in programmed cell death-ligand 1 gene with Graves’ disease in Japanese patients. Eur J Endocrinol. 2008;158:817–822. doi: 10.1530/EJE-07-0649. [DOI] [PubMed] [Google Scholar]
- Karaghiosoff M, Steinborn R, Kovarik P, Kriegshauser G, Baccarini M, Donabauer B, Reichart U, Kolbe T, Bogdan C, Leanderson T, et al. Central role for type I interferons and Tyk2 in lipopolysaccharide-induced endotoxin shock. Nat Immunol. 2003;4:471–477. doi: 10.1038/ni910. [DOI] [PubMed] [Google Scholar]
- Kastelein RA, Hunter CA, Cua DJ. Discovery and biology of IL-23 and IL-27: related but functionally distinct regulators of inflammation. Annu Rev Immunol. 2007;25:221–242. doi: 10.1146/annurev.immunol.22.012703.104758. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latchman YE, Liang SC, Wu Y, Chernova T, Sobel RA, Klemm M, Kuchroo VK, Freeman GJ, Sharpe AH. PD-L1-deficient mice show that PD-L1 on T cells, antigen-presenting cells, and host tissues negatively regulates T cells. Proc Natl Acad Sci U S A. 2004;101:10691–10696. doi: 10.1073/pnas.0307252101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CK, Raz R, Gimeno R, Gertner R, Wistinghausen B, Takeshita K, DePinho RA, Levy DE. STAT3 is a negative regulator of granulopoiesis but is not required for G-CSF-dependent differentiation. Immunity. 2002;17:63–72. doi: 10.1016/s1074-7613(02)00336-9. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Jang BC, Lee SW, Yang YI, Suh SI, Park YM, Oh S, Shin JG, Yao S, Chen L, Choi IH. Interferon regulatory factor-1 is prerequisite to the constitutive expression and IFN-gamma-induced upregulation of B7-H1 (CD274) FEBS Lett. 2006;580:755–762. doi: 10.1016/j.febslet.2005.12.093. [DOI] [PubMed] [Google Scholar]
- Lighvani AA, Frucht DM, Jankovic D, Yamane H, Aliberti J, Hissong BD, Nguyen BV, Gadina M, Sher A, Paul WE, O’Shea JJ. T-bet is rapidly induced by interferon-gamma in lymphoid and myeloid cells. Proc Natl Acad Sci U S A. 2001;98:15137–15142. doi: 10.1073/pnas.261570598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AL, Cordell HJ, Soemedi R, Owen K, Skinningsrud B, Wolff AB, Ericksen M, Undlien D, Husebye E, Pearce SH. Programmed death ligand 1 (PD-L1) gene variants contribute to autoimmune Addison’s disease and Graves’ disease susceptibility. J Clin Endocrinol Metab. 2009;94:5139–5145. doi: 10.1210/jc.2009-1404. [DOI] [PubMed] [Google Scholar]
- Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD4+ T cells. J Immunol. 2009;183:2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufert C, Becker C, Wirtz S, Fantini MC, Weigmann B, Galle PR, Neurath MF. IL-27 controls the development of inducible regulatory T cells and Th17 cells via differential effects on STAT1. Eur J Immunol. 2007;37:1809–1816. doi: 10.1002/eji.200636896. [DOI] [PubMed] [Google Scholar]
- Ouaked N, Mantel PY, Bassin C, Burgler S, Siegmund K, Akdis CA, Schmidt-Weber CB. Regulation of the foxp3 gene by the Th1 cytokines: the role of IL-27-induced STAT1. J Immunol. 2009;182:1041–1049. doi: 10.4049/jimmunol.182.2.1041. [DOI] [PubMed] [Google Scholar]
- Owaki T, Asakawa M, Morishima N, Hata K, Fukai F, Matsui M, Mizuguchi J, Yoshimoto T. A role for IL-27 in early regulation of Th1 differentiation. J Immunol. 2005;175:2191–2200. doi: 10.4049/jimmunol.175.4.2191. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Hibbert L, Mattson J, Rosales R, Vaisberg E, Bazan JF, Phillips JH, McClanahan TK, de Waal Malefyt R, Kastelein RA. WSX-1 and glycoprotein 130 constitute a signal-transducing receptor for IL-27. J Immunol. 2004;172:2225–2231. doi: 10.4049/jimmunol.172.4.2225. [DOI] [PubMed] [Google Scholar]
- Pflanz S, Timans JC, Cheung J, Rosales R, Kanzler H, Gilbert J, Hibbert L, Churakova T, Travis M, Vaisberg E, et al. IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4(+) T cells. Immunity. 2002;16:779–790. doi: 10.1016/s1074-7613(02)00324-2. [DOI] [PubMed] [Google Scholar]
- Pot C, Jin H, Awasthi A, Liu SM, Lai CY, Madan R, Sharpe AH, Karp CL, Miaw SC, Ho IC, Kuchroo VK. Cutting edge: IL-27 induces the transcription factor c-Maf, cytokine IL-21, and the costimulatory receptor ICOS that coordinately act together to promote differentiation of IL-10-producing Tr1 cells. J Immunol. 2009;183:797–801. doi: 10.4049/jimmunol.0901233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiner B, Mitsdoerffer M, Kieseier BC, Chen L, Hartung HP, Weller M, Wiendl H. Interferon-beta enhances monocyte and dendritic cell expression of B7-H1 (PD-L1), a strong inhibitor of autologous T-cell activation: relevance for the immune modulatory effect in multiple sclerosis. J Neuroimmunol. 2004;155:172–182. doi: 10.1016/j.jneuroim.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Laurence A, Wilson EH, Huang E, Tato CM, Johnson LM, Villarino AV, Huang Q, Yoshimura A, Sehy D, et al. Interleukin 27 negatively regulates the development of interleukin 17-producing T helper cells during chronic inflammation of the central nervous system. Nat Immunol. 2006;7:937–945. doi: 10.1038/ni1376. [DOI] [PubMed] [Google Scholar]
- Stumhofer JS, Silver JS, Laurence A, Porrett PM, Harris TH, Turka LA, Ernst M, Saris CJ, O’Shea JJ, Hunter CA. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nat Immunol. 2007;8:1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- Tong H, Miyazaki Y, Yamazaki M, Hara H, Waldmann H, Hori S, Yoshida H. Exacerbation of delayed-type hypersensitivity responses in EBV-induced gene-3 (EBI-3)-deficient mice. Immunol Lett. 2010;128:108–115. doi: 10.1016/j.imlet.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- Villarino A, Hibbert L, Lieberman L, Wilson E, Mak T, Yoshida H, Kastelein RA, Saris C, Hunter CA. The IL-27R (WSX-1) is required to suppress T cell hyperactivity during infection. Immunity. 2003;19:645–655. doi: 10.1016/s1074-7613(03)00300-5. [DOI] [PubMed] [Google Scholar]
- Villarino AV, Stumhofer JS, Saris CJ, Kastelein RA, de Sauvage FJ, Hunter CA. IL-27 limits IL-2 production during Th1 differentiation. J Immunol. 2006;176:237–247. doi: 10.4049/jimmunol.176.1.237. [DOI] [PubMed] [Google Scholar]
- Wang S, Miyazaki Y, Shinozaki Y, Yoshida H. Augmentation of antigen-presenting and Th1-promoting functions of dendritic cells by WSX-1(IL-27R) deficiency. J Immunol. 2007;179:6421–6428. doi: 10.4049/jimmunol.179.10.6421. [DOI] [PubMed] [Google Scholar]
- Wei G, Wei L, Zhu J, Zang C, Hu-Li J, Yao Z, Cui K, Kanno Y, Roh TY, Watford WT, et al. Global mapping of H3K4me3 and H3K27me3 reveals specificity and plasticity in lineage fate determination of differentiating CD4+ T cells. Immunity. 2009;30:155–167. doi: 10.1016/j.immuni.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, Shin T, Tsuchiya H, Pardoll DM, Okumura K, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169:5538–5545. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Hamano S, Senaldi G, Covey T, Faggioni R, Mu S, Xia M, Wakeham AC, Nishina H, Potter J, et al. WSX-1 is required for the initiation of Th1 responses and resistance to L. major infection. Immunity. 2001;15:569–578. doi: 10.1016/s1074-7613(01)00206-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Takeda A, Hamano S, Miyazaki Y, Kinjyo I, Ishibashi T, Yoshimura A, Yoshida H. Two-sided roles of IL-27: induction of Th1 differentiation on naive CD4+ T cells versus suppression of proinflammatory cytokine production including IL-23-induced IL-17 on activated CD4+ T cells partially through STAT3-dependent mechanism. J Immunol. 2006;177:5377–5385. doi: 10.4049/jimmunol.177.8.5377. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.