SUMMARY
S100 proteins are calcium-activated signaling proteins that interact with target proteins to modulate biological processes. Our present studies compare the level of expression, and cellular localization of S100A7, S100A8, S100A9, S100A10, and S100A11 in normal and psoriatic epidermis. S100A7 and S100A11 are present in the basal and spinous layers in normal epidermis. These proteins appear in the nucleus and cytoplasm in basal cells but are associated with the plasma membrane in spinous cells. S100A10 is present in basal and spinous cells, in the cytoplasm, and is associated with the plasma membrane. S100A8 and S100A9 are absent or are expressed at minimal levels in normal epidermis. In involved psoriatic tissue, S100A10 and S100A11 levels remain unchanged, whereas, S100A7, S100A8, and S100A9 are markedly overexpressed. The pattern of expression and subcellular localization of S100A7 is similar in normal and psoriatic tissue. S100A8 and S100A9 are strongly expressed in the basal and spinous layers in psoriasis-involved tissue. In addition, we demonstrate that S100A7, S100A10, and S100A11 are incorporated into detergent and reducing agent-resistant multimers, suggesting that they are in vivo transglutaminase substrates. S100A8 and S100A9 did not form these larger complexes. These results indicate that S100 proteins localize to the plasma membrane in differentiated keratinocytes, suggesting a role in regulating calcium-dependent, membrane-associated events. These studies also indicate, as reported previously, that S100A7, S100A8, and S100A9 expression is markedly altered in psoriasis, suggesting a role for these proteins in disease pathogenesis.
Keywords: S100, psoriasis, epidermis, differentiation, keratinocyte, calcium-binding protein, psoriasin, S100A8
Calcium acts as a second messenger to regulate keratinocyte cell proliferation and differentiation (Boyce and Ham 1983; Menon et al. 1985; Pillai et al. 1990; Chakravarthy et al. 1995; Ahn et al. 1999). The calcium stimulus is mediated by calcium-binding proteins that couple changes in intracellular and extracellular calcium to cell function. These proteins include the calcium receptor, calmodulin, troponin C, parvalbumin, and S100 proteins (Donato 1986; Heizmann and Cox 1998; Tu et al. 2001). Because of their signal transduction role, and role as chemoattractants, S100 proteins are proposed to have an important role in keratinocyte differentiation and in the pathogenesis of psoriasis (Jinquan et al. 1996). There are 18 identified S100 family proteins. Thirteen S100 protein genes are located within the epidermal differentiation complex on human chromosome 1q21 (Engelkamp et al. 1993; Wicki et al. 1996).
Although eight S100 proteins (S100A2, S100A7, S100A8, S100A9, S100A10, S100A11, S100A12, and S100β) are expressed in the human epidermis (Boni et al. 1997; Xia et al. 1997; Robinson and Hogg 2000), the function of these proteins is not well understood. In particular, information is lacking regarding their subcellular distribution as a function of keratinocyte differentiation and how their distribution changes in disease states. In this study we characterize several new antibodies and examine the pattern of expression of multiple S100 proteins in normal and psoriatic human epidermis. Our study identifies definite patterns of expression and specific changes in subcellular localization of individual S100 proteins during differentiation. In addition, we confirm previous reports showing that several of the S100 proteins are expressed at markedly higher levels in involved psoriatic epidermis.
Materials and Methods
Antibodies
Rabbit polyclonal anti-S100 antibodies specific for human S100A7, human S100A10, and human S100A11 were produced in our laboratory. Recombinant human S100A7, S100A10, and S100A11 proteins were produced in Escherichia coli (E. coli BL21 DE3) (Robinson et al. 1997; Ruse et al. 2001). For example, bacteria were transformed with pET28a+-S100A7, which encodes polyhistidine–S100A7 fusion protein, and His–S100A7 production was induced by addition of IPTG. After protein purification by chromatography on a His–Bind column (Novagen; Madison, WI), the polyhistidine track was removed using thrombin. The final S100 protein retains a three-amino-acid extension (Gly-Ser-His) at the amino terminus. S100A10 and S100A11 were prepared using an identical procedure. Rabbit anti-human S100 antibody (anti-S100A7, anti-S100A10, and anti-S100A11) was prepared by immunizing rabbits with human recombinant S100 protein three times over a period of 2 months (each at 1 mg per animal). Immune serum was collected from each rabbit and the IgG fraction was isolated by a salting-out procedure (50% ammonium sulfate). As outlined here, the specificity of each antibody was confirmed by immunoblotting. The optimal antibody concentration for immunohistochemistry (1:100), immunofluorescence (1:1000), and immunoblotting (1:1000) was determined by serial dilution.
Goat anti-human S100A8 (calgranulin A, sc-8112) and goat anti-human S100A9 (calgranulin B, sc-8114) were purchased from Santa Cruz Biologicals (Santa Cruz, CA). Each antibody maps an epitope near the carboxy terminus of the S100 of human origin. The blocking peptides for anti-S100A8 (sc-8112P) and anti-S100A9 (sc-8114P) were also purchased from Santa Cruz. Serial dilution competition assays were done in the absence and presence of blocking peptide to confirm the antibody specificity using both immunohistochemistry and immunoblot analysis. Anti-β-actin was obtained from Sigma (St Louis, MO) (A5411).
Immunohistochemistry
Keratomes, excised from normal and psoriatic patients, were fixed overnight at 4C in 2% paraformaldehyde, processed, and sectioned onto microscope slides. Tissue was obtained as discarded tissue samples from the dermatology clinic in compliance with an Institutional Review Board-approved protocol. Sections were deparaffinized, ethanol-rehydrated, and rinsed with PBS. Nonspecific antibody binding was blocked by incubating sections for 30 min at 25C with 1.5% serum (goat S-1000, or rabbit S-5000; Vector Laboratories, Burlingame, CA) in PBS. Blocked sections were then incubated with S100 isoform-specific antibodies, diluted 1:100 in PBS, for 1 hr at 25C. In some cases, as indicated in figure legends, epitope blocking peptides were included in the incubation mixture to assess specificity of staining. The sections were then rinsed three times with PBS and incubated with biotin-labeled secondary antibody (goat anti-rabbit IgG, PK-6101, or rabbit anti-goat IgG, PK-6105; Vector Laboratories) for 1 hr at 25C in PBS. After washing, the sections were incubated for 30 min with avidin–biotin–peroxidase complex (Vectastain ABC Kit; Vector Laboratories). The sections were rinsed, stained with 3,3′-diaminobenzidine (DAB, SK-4100; Vectastain Kit) and counterstained with hematoxylin.
Immunoblotting Analysis
Equivalent amounts of extract (50 μg protein/lane), prepared from rapidly frozen keratome samples, were electrophoresed on denaturing and reducing 12% polyacrylamide gels. The fractionated proteins were then transferred to nitrocellulose membranes. The blots were blocked with 1% BSA for 1 hr at room temperature with gentle shaking, washed, and incubated for 1 hr at 25C with primary antibody at a dilution of 1:1000. After washing, the blot was incubated for 1 hr at 25C with HRP-conjugated donkey anti-rabbit IgG diluted 1:10,000 (NA934; Amersham Pharmacia, Poole, UK) or HRP-conjugated donkey anti-goat IgG diluted 1:5000 (sc-2033; Santa Cruz Biotechnology). After repeated washing, the blots were immersed in ECL Western blotting detection reagent (Amersham Pharmacia) for 1 min. Chemiluminescence was visualized by exposure of the blot on Kodak X-OMAT AR imaging film (Eastman Kodak; Rochester, NY).
Results
Antibodies Against S100A7, S100A10, and S100A11 Are Specific
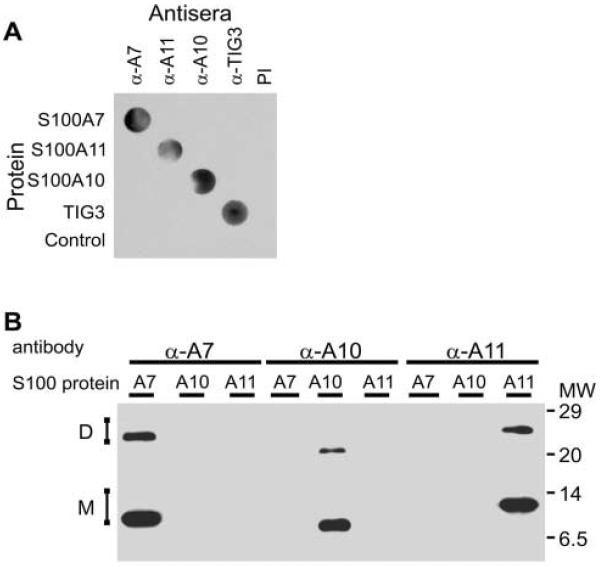
Antibodies developed in our laboratory against recombinant human S100A7, S100A10, and S100A11 proteins were tested for specificity using both dot-blot and gel immunoblotting analysis. First, 0.1 μg of each recombinant human S100 protein was dot-blotted onto nitrocellulose and assayed for reactivity against each individual antibody (Figure 1A). Anti-S100A7, raised in rabbits, recognized only S100A7 protein. Likewise, rabbit anti-S100A10 and anti-S100A11 detected only S100A10 and S100A11, respectively. We also confirmed that none of the antibodies detect an unrelated protein, TIG3 (DiSepio et al. 1998; Deucher et al. 2000), and that the preimmune serum (PI) did not detect these proteins. Next, each antibody was assessed for specificity by gel immunoblotting assay (Figure 1B). Recombinant human S100A7, S100A10, and S100A11 protein (0.1 μg/lane) were electrophoresed on a 12% gel under denaturing conditions, transferred to nitrocellulose, and reacted with each antibody. Each antibody recognized only the corresponding S100 protein. Because S100 proteins are known to be efficient transglutaminase substrates (Robinson et al. 1997; Robinson and Eckert 1998; Ruse et al. 2001), the finding that covalently linked S100 protein dimers are detected in Figure 1B suggests that some of the S100 protein may serve as a substrate for bacterial transglutaminases (Sato et al. 2001; Seitz et al. 2001).
Figure 1.
Specificity of S100 antibodies. (A) Recombinant human S100 protein (S100A7, S100A10, S100A11, 0.1 μg each) and an unrelated protein, TIG3 (DiSepio et al. 1998; Deucher et al. 2000), were bound to nitrocellulose strips at 0.1 μg/dot in rows of five dots. The membrane was then cut vertically to yield strips containing each protein, and each strip was incubated with a single antibody (anti-S100A7, anti-S100A10, anti-S100A11, or anti-TIG3) at a dilution of 1:1000 (determined by titration). All antibodies were raised in rabbits. An additional strip was incubated with rabbit preimmune serum (PI). The blot was washed and then incubated with HRP-conjugated goat anti-rabbit IgG for 1 hr at 25C in PBS. After washing, the sections were visualized using chemiluminescent reagents. (B) Recombinant human S100 proteins (S100A7, S100A11, S100A10, 0.1 μg each) were electrophoresed on a denaturing 12% acrylamide gel, transferred to nitrocellulose, and strips were incubated with anti-S100A7, anti-S100A10, or anti-S100A11. The blots were then incubated with secondary reagents and chemiluminescent detection reagents. Migration of monomers (M) and dimers (D) of each protein are indicated. The dimers may arise from residual transglutaminase-like activity in bacterial extracts. No signal was observed when a blot (not shown) was incubated with preimmune serum. Molecular weights (MW) are indicated in kD.
S100A7 Redistributes During Keratinocyte Differentiation
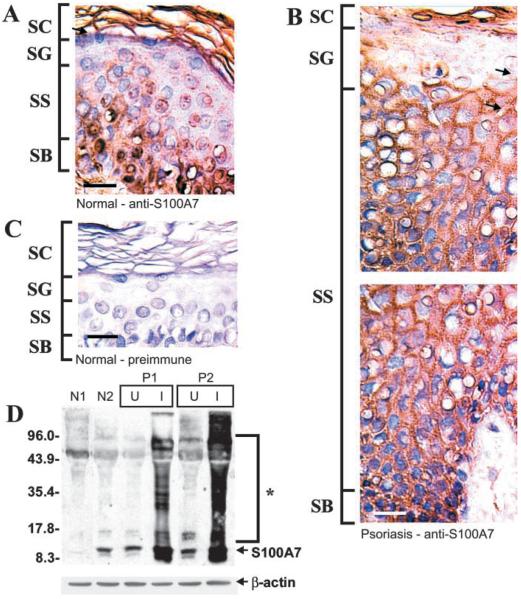
We assayed S100A7 distribution and level in epidermis during normal and psoriatic differentiation. Immunohistological staining of normal epidermis identifies S100A7 in the basal and spinous layers (Figure 2A). At the subcelllar level, in basal cells, S100A7 is localized in the nuclei and cytoplasm. In contrast, in the spinous cell layer, much of the S100A7 immunoreactivity is observed localized with the plasma membrane (Figure 2B, arrow). A similar overall pattern of staining is observed in involved psoriatic epithelium (Figure 2B) and in non-involved epidermis from psoriatic patients (not shown). However, in involved psoriatic tissue, the membrane-associated staining is particularly pronounced in the spinous layer (Figure 2B, arrows). No staining is observed in a control section stained with preimmune serum (Figure 2C). To obtain an estimate of the overall level of S100A7 expression in normal vs involved psoriatic epidermis, we assayed levels by immunoblotting. Figure 2D shows an analysis of tissue extracts prepared from epidermis of two normal subjects (N1, N2) and two psoriatic patients (P1, P2). S100A7 levels are comparable in normal epidermis (N1, N2) and uninvolved epidermis from both psoriatic patients (U). However, the level is markedly increased in involved (I) psoriatic epidermis. This is consistent with previous reports (Madsen et al. 1991). In addition, in all samples, a significant amount of S100A7 migrates slowly, suggesting that it is cross-linked to itself and other proteins (indicated by asterisk), a finding that is consistent with our previous reports showing that S100 proteins are transglutaminase substrates (Robinson et al. 1997; Robinson and Eckert 1998; Ruse et al. 2001).
Figure 2.
Distribution and expression of S100A7 protein in normal and psoriatic epidermis. Epithelial biopsy specimens were excised from normal and from uninvolved and involved psoriatic epidermis. The specimens were fixed, processed, and stained with rabbit anti-human S100A7 serum (1:100 dilution) or goat preimmune serum (Robinson and Eckert 1998). (A) Normal epidermis stained with anti-S100A7. Arrow indicates membrane-associated S100A7. In this and all immunostaining figures, SC is stratum corneum, SG is stratum granulosum, SS is stratum spinosum, and SB is stratum basalis. (B) Involved psoriatic epidermis stained with anti-S100A7. Arrows indicate membrane-associated S100A7 immunoreactivity. (C) Normal epidermis stained with preimmune serum. (D) Equivalent quantities of whole-cell extract (based on protein) prepared from normal epidermis (patients N1, N2), and psoriatic epidermis (patients P1, P2) were electrophoresed on a 12% acrylamide gel. Samples from psoriasis patients include uninvolved (U) and involved (I) tissue. The fractionated samples were transferred to nitrocellulose and incubated with anti-S100A7. Primary antibody binding was visualized using an appropriate secondary antibody and chemiluminescence detection agents. β-Actin was detected as a control to confirm equal loading. No immunoreactivity was observed when the primary antibody was omitted from the incubation (not shown). Arrow indicates the S100A7 monomer. Asterisk indicates high molecular weight anti-S100A7 immunoreactivity (Ruse et al. 2001). The molecular weight markers are indicated in kD. Bar = 10 μm.
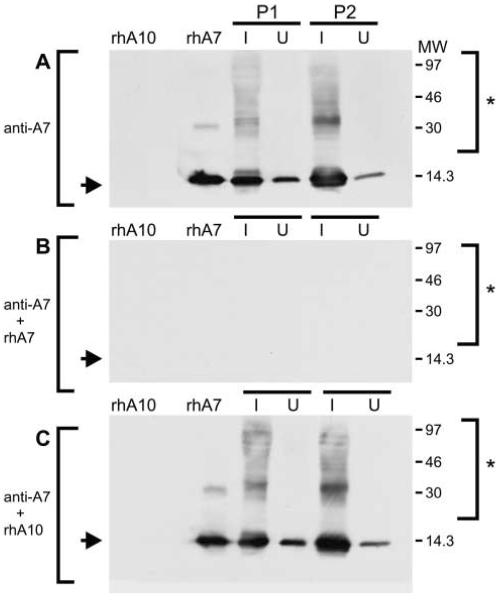
To confirm that the slowly migrating bands shown in Figure 2 represent authentic S100A7-containing complexes, recombinant human S100A7, recombinant human S100A10, and psoriatic cell extract from two patients (P1 and P2) were electrophoresed on a denaturing and reducing 12% polyacrylamide gel and immunoblotted. Figure 3A shows that incubation of the blot with anti-S100A7 detects a band corresponding to rhS100A7 and also immunoreactive material in the extracts from psoriatic patients. This immunoreactive material includes S100A7 monomer (Figure 3, arrows) and high molecular weight S100A7-containing complexes (Figure 3, asterisks). rhS100A10, in contrast, is not detected. Addition of an excess of rhS100A7 protein to the antibody mixture, (Figure 3B) eliminates all anti-S100A7 binding. In contrast, addition of excess rhS100A10 to the anti-S100A7 mixture does not inhibit binding (Figure 3C). Therefore, the high molecular weight forms represent authentic S100A7-containing complexes.
Figure 3.
Presence of multiple S100A7-containing complexes in psoriatic epidermis. Equivalent quantities of protein (20 μg/lane), prepared from uninvolved (U) and involved (I) psoriatic epidermis (patients P1 and P2), was electrophoresed on a denaturing 12% polyacrylamide gel, transferred to nitrocellulose, and incubated with anti-S100A7. Parallel lanes include electrophoresis of 0.2 μg of recombinant human S100A7 (rhA7) or recombinant human S100A10 (rhA10). (A) Blot was incubated with anti-S100A7 (1 μg/incubation). (B) Blot was incubated with 1 μg anti-S100A7 per incubation in the presence of fivefold excess (by weight) of recombinant human S100A7 (rhA7). (C) Blot was incubated with 1 μg anti-S100A7 per incubation in the presence of fivefold excess (by weight) of recombinant human S100A10 (rhA10). Arrows indicate the migration of S100A7 monomer. (B) Arrow marks the expected location of S100A7 monomer. The higher molecular weight immunoreactive complexes are indicated by the asterisk. MW markers are listed in kD.
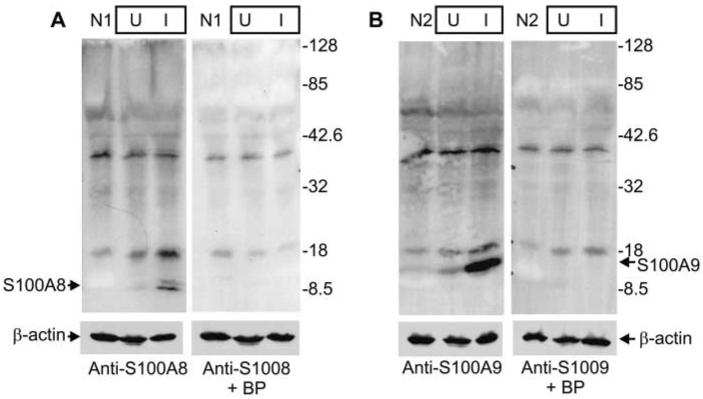
Altered Expression Patterns of S100A8 and S100A9 in Psoriasis
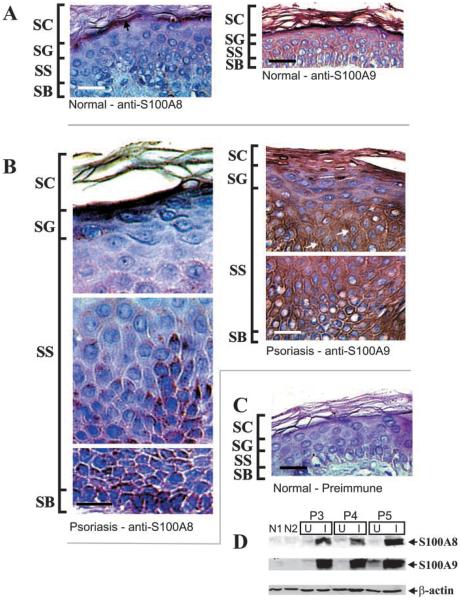
In agreement with previous reports (Kelly et al. 1989; Kunz et al. 1992; Schmidt et al. 2001), we find that S100A8 and S100A9 are absent or are expressed at barely detectable levels in normal epidermis. For example, as shown in Figure 4A, some expression is occasionally detectable in the granular layer (arrow). S100A9 is also present in very limited quantities in normal epidermis; barely visible granular layer staining is observed. In involved psoriatic tissue, however, both S100A8 and S100A9 are expressed at high levels (Figure 4B). In addition to granular layer staining, S100A8 is expressed at high levels in localized regions surrounding positively stained basal cells, and S100A9 is expressed in all epidermal layers. S100A8 is localized in the peripheral cytoplasm, and S100A9 is detected within the peripheral cytoplasm and associated with the plasma membrane. Membrane-associated S100A9 is indicated by the arrows. No expression is detected when epitope-specific blocking peptides are included in the incubations, or preimmune serum is substituted for S100-specific primary antibody (Figure 4C).
Figure 4.
Distribution and expression of S100A8 and S100A9 in normal and psoriatic epidermis. Epithelial biopsies were excised from normal, uninvolved psoriatic, and involved psoriatic epidermis and were stained with goat anti-S100A8 or anti-S100A9 (1:100 dilution), or rabbit preimmune serum. (A) Normal epidermis stained with anti-S100A8 or anti-S100A9. Arrow indicates occasional low-level staining observed for S100A8. (B) Involved psoriatic epidermis stained with anti-S100A8 or anti-S100A9. Arrows indicate areas of possible membrane association. (C) Normal epidermis stained with preimmune serum. In addition, inclusion of 2 μg of S100A8 epitope-specific blocking peptide per 1 μg of anti-S100A8 eliminated S100A8 staining. Inclusion of S100A9 blocking peptide with anti-S100A9 blocked S100A9 staining (not shown). Bars = 10 μm. (D) Equivalent amounts of whole-cell extract (based on protein) prepared from normal epidermis (patients N1, N2), and psoriatic epidermis (patients P3, P4, P5) were electrophoresed on a 12% acrylamide gel. Samples from psoriasis patients include uninvolved (U) and involved (I) tissue. The fractionated samples were transferred to nitrocellulose and then incubated with anti-S100A8, anti-S100A9, or anti-β-actin. Primary antibody binding was visualized as in Figure 2. No immunoreactivity was detected when the primary antibody was omitted (not shown).
Consistent with the immunohistological findings, immunoblot detection of S100A8 and S100A9 reveals minimal expression in normal tissue or uninvolved psoriatic tissue, but elevated expression in involved psoriatic tissue (Figure 4D). To assess whether S100A8 or S100A9 forms covalent high molecular weight complexes, cell extracts prepared from normal epidermis (patient N1) or from uninvolved (U) or involved (I) psoriatic epidermis (patient P3) were electrophoresed, blotted to nitrocellulose, and incubated with anti-S100A8 or anti-S100A9. Figure 5A shows that anti-S100A8 identifies several bands in extracts from normal epidermis and from uninvolved and involved psoriatic epidermis. Competition with the anti-S100A8 blocking peptide (+BP) reveals specific antibody binding to an 8-KD band corresponding to the size of the S100A8 monomer. Comparison of the anti-S100A9 blot in the absence and presence of blocking peptide, (Figure 5B) reveals a specifically competed 14-KD band corresponding to the molecular weight of the S100A9 monomer. The bands detected at 18 and 40 KD are nonspecific. These blots suggest that S100A8 and S100A9 do not form covalently crosslinked high molecular weight species. In addition, these blots reveal that these antibodies do not crossreact, i.e., anti-S100A8 does not detect S100A9, and anti-S100A9 does not detect S100A8.
Figure 5.
S100A8 and S100A9 do not form crosslinked multimers. (A) Equivalent quantities of protein (20 μg/lane), prepared from normal epidermis (patient N1) or from uninvolved and involved psoriatic epidermis (patient P3), were electrophoresed on a denaturing 12% polyacrylamide gel, transferred to nitrocellulose, and incubated with anti-S100A8 (1 μg/incubation) in the absence or presence of S100A8 epitope-specific blocking peptide (5 μg/incubation). (B) An identical parallel blot was incubated with anti-S100A9 (1 μg/incubation) in the presence or absence of S100A9 epitope-specific blocking peptide (5 μg/incubation). β-Actin levels were monitored to normalize gel loading. Arrows indicate the migration of the S100A8 (A) or S100A9 (B) monomer.
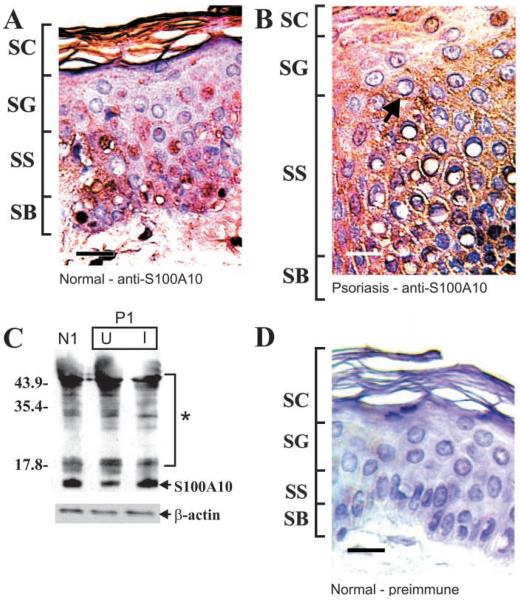
Localization of S100A10
S100A10 is principally localized in the nucleus and cytoplasm of basal and spinous cells in normal epidermis, although some plasma membrane-associated staining is also visible (Figure 6A). In involved psoriatic tissue, faint staining is observed in the cytoplasm of basal and spinous cells, with substantial plasma membrane-associated staining (Figure 6B, arrow). In Figure 6D, no signal is detected in normal skin samples stained with preimmune serum. As shown in Figure 6C, no change in S100A10 level is detected in extracts derived from normal, psoriasis-uninvolved, and psoriasis-involved epidermis. As with S100A7, we detect a substantial number of higher molecular weight S100A10 immunoreactive bands (Figure 6C, asterisk). Anti-S100A10 detection of this material was eliminated by inclusion of recombinant human S100A10 in the antibody incubation mixture (not shown). This suggests that the high molecular weight material represents covalently crosslinked S100A10-containing complexes (Robinson and Eckert 1998).
Figure 6.
S100A10 in human epidermis. Epithelial biopsies from normal and from uninvolved and involved psoriatic epidermis were fixed, processed, and stained with rabbit anti-S100A10 and/or goat preimmune serum (Robinson and Eckert 1998). (A) Normal epidermis stained with anti-S100A10. (B) Involved psoriatic epidermis stained with anti-S100A10. Arrow shows S100A10, which appears to be membrane-associated. (C) Equivalent amounts of whole-cell extract (based on protein) prepared from normal epidermis (patient N1), and psoriatic epidermis (patient P1) were electrophoresed on a 12% acrylamide gel. Samples from the psoriasis patient include uninvolved (U) and involved (I) tissue. Primary antibody binding was visualized using an appropriate secondary antibody and chemiluminescence detection agents. β-Actin was detected as a control to confirm equal loading. No S100A10 immunoreactivity was observed when the primary antibody was replaced with preimmune serum or the antibody mixture contained an excess of S100A10. Asterisk indicates high molecular weight anti-S100A10 immunoreactive bands (Ruse et al. 2001). (D) Normal epidermis stained with preimmune serum. Bar = 10 μm.
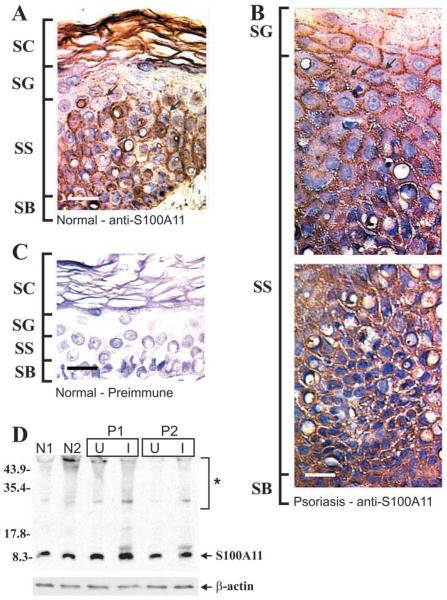
S100A11 Localizes at the Plasma Membrane in Differentiated Keratinocytes
Immunohistological staining of normal human epidermis using rabbit anti-human S100A11 (Robinson and Eckert 1998) reveals S100A11 in the nuclei and cytosol of basal cells (Figure 7A). In spinous cells, S100A11 is localized in the cytoplasm and plasma membrane. Plasma membrane staining is indicated by the arrows. In contrast, in psoriasis there appears to be less S100A11 expression in the nuclei of basal cells (Figure 7B). Instead, S100A11 is found in the cytoplasm of basal and spinous keratinocytes. In the upper spinous layers, most of the S100A11 immunoreactivity appears to associate with the plasma membrane (arrows). Figure 7C shows an absence of signal in normal skin samples stained with preimmune serum. In addition, incubation of sections with anti-S100A11 in the presence of an excess of recombinant human S100A11 is indistinguishable from the staining observed with preimmune serum (not shown). Immunoblotting analysis of S100A11 protein levels in extracts prepared from normal and from uninvolved and involved psoriatic tissue reveals no change in expression level (Figure 7D). The asterisk indicates anti-S100A11 reactive bands of high molecular weight. Anti-S100A11 detection of these bands was eliminated by inclusion of recombinant human S100A11 in the antibody incubation mixture (not shown). This suggests that these bands represent covalently crosslinked S100A11-containing complexes (Ruse et al. 2001).
Figure 7.
S100A11 in epidermis. Epidermal sections were prepared and stained with rabbit anti-human S100A11. (A) Normal epidermis stained with anti-S100A11. (B) Involved psoriatic epidermis stained with anti-S100A11. (C) Normal epidermis stained with preimmune serum. (D) Equivalent amounts of whole-cell extract (based on protein) prepared from normal epidermis (patients N1, N2), and psoriatic epidermis (patients P1, P2) were electrophoresed on a 12% acrylamide gel. Samples from psoriasis patients include uninvolved (U) and involved (I) tissue. Primary antibody binding was visualized using an appropriate secondary antibody and chemiluminescence detection agents. β-Actin was detected as a control to confirm equal loading. Asterisk indicates high molecular weight anti-S100A11 immunoreactive bands (Ruse et al. 2001). Arrows (A,B) indicate membrane-associated S100A11. Bar = 10 μm.
Discussion
S100 Proteins in Epidermis
S100 proteins are calcium sensor proteins that exist in cells as homo- and heterodimers. In response to calcium concentrations in the millimolar range, S100 proteins undergo a conformational change that exposes a target protein-interacting domain. This domain binds to target protein(s) and alters target protein function to produce a biological response (Garbuglia et al. 1996; Wu et al. 1997; Rety et al. 1999,2000). Subcellular localization is known to have important effects on signal transduction protein function. However, information is limited regarding the subcellular distribution of S100 proteins in differentiating epidermis.
S100A7
S100A7 is expressed in both normal and psoriatic epidermis. S100A7 is present in the cytosol in basal cells, but appears to be plasma membrane-associated in spinous layer cells. This suggests that S100A7 performs a function at the plasma membrane. Some may also be released into the extracellular space, a finding that would be consistent with a role in chemotaxis (Hoffmann et al. 1994). In addition, S100A7 levels are markedly increased in psoriatic epidermis (Madsen et al. 1992) (see Table 1), suggesting that S100A7 may play an important functional role in the genesis of the psoriatic state. Perhaps this relates to the cytokine function of S100A7 that has been previously described (Di Nuzzo et al. 2000). S100A7 also appears to form a complex with epidermal fatty acid binding protein (EFABP), although the importance of this interaction is not well understood (Hagens et al. 1999). Our unpublished studies suggest that a S100A7/EFABP complex moves to the plasma membrane in calcium-treated cultured keratinocytes (Ruse and Eckert, unpublished). This may alter lipid metabolism and cell differentiation.
Table 1.
S100 protein expression in normal and psoriatic epidermisa
| Normal epidermis |
Psoriasis |
|||
|---|---|---|---|---|
| S100 protein | Differentiation/subcellular | Level | Differentiation/subcellular | Level |
| S100A7 | Spinous, PM | + | Spinous, PM | +++++ |
| Basal, nuclear, cytosol | Basal, cytosol | |||
| S100A8 | Very low-level expression | −/+ | Granular, PM | +++++ |
| Spinous, PM | ||||
| Basal, cytosol, PM | ||||
| S100A9 | Very low-level expression | −/+ | Granular, cytosol, PM | +++++ |
| Spinous, cytosol, PM | ||||
| Basal, cytosol, PM | ||||
| S100A10 | Spinous, cytosol, PM | ++ | Spinous, cytosol, PM | ++ |
| Basal, nuclear, cytosol, PM | Basal, cytosol, PM | |||
| S100A11 | Spinous, PM | ++ | Spinous, PM | ++ |
| Basal, nuclear, cytosol | Basal, cytosol | |||
−/+, absent or barely detectable; +, present at low levels; ++, present at moderate levels; +++++, highly expressed.
S100A8 and S100A9
S100A8 and S100A9 are expressed at minimal levels in normal epidermis, although occasional expression is observed in the granular layer. Co-expression is expected, because these S100 proteins function as a heterodimer (Teigelkamp et al. 1991). Both proteins are highly overexpressed in psoriasis. Both proteins are expressed in the granular layer and in the basal and spinous layers. S100A8 and S100A9 may have a role in promoting and/or responding to the hyperproliferative state observed in psoriasis, because S100A8 and S100A9 expression is strongly induced within the first week after epidermal injury (Thorey et al. 2001). In addition, significant S100A8 and S100A9 staining is observed at the plasma membrane, which is consistent with the idea that some may be secreted (Katz and Taichman 1999).
S100A10
Our studies suggest that S100A10 is present in both cytosol and plasma membrane in the basal and spinous layers. S100A10 is known to be associated as a covalently linked component of the keratinocyte cornified envelope (Mischke et al. 1996; Robinson et al. 1997), suggesting that it has a role at the membrane during the terminal stages in keratinocyte differentiation. S100A10 forms homodimers that, in turn, bind two copies of annexin II, in a calcium-independent manner, to form a heterotetramer called calpactin I (Nakata et al. 1990; Pigault et al. 1990). This complex localizes to the endosome and plasma membranes (Harder and Gerke 1993). Annexin II has been reported to localize to the plasma membrane in differentiated keratinocytes (Ma and Ozers 1996) and has been identified in the extracellular space (Ma et al. 1994). Therefore, the membrane-associated calpactin I complex may have a role in regulating keratinocyte differentiation.
S100A11
Our studies suggest that S100A11 localizes in the cytoplasm in basal cells and in the cell periphery/plasma membrane in spinous layer cells. Association with the plasma membrane is consistent with our previous observation that S100A11 becomes crosslinked as a component of the corneocyte, via the action of a plasma membrane-localized enzyme, type I transglutaminase. Moreover, it is associated at this location with annexin I (Robinson et al. 1997; Robinson and Eckert 1998; Rety et al. 2000). Our unpublished in vitro results suggest that S100A11 moves to the cell periphery in cultured keratinocytes after calcium challenge. Therefore, the change in distribution observed in vivo may represent differentiation-associated, calcium-dependent movement. S100A11 interacts with annexin I in a calcium-dependent manner (Seemann et al. 1997). Although the precise function of the S100A11/annexin I complex is not known, it may be involved in membrane transport processes (Futter et al. 1993). Annexin I is partly localized on early endosomes and this localization also targets S100A11 to endocytotic organelles (Seemann et al. 1997). Perhaps the S100A11/annexin I functions to alter plasma membrane transport processes during keratinocyte differentiation.
In summary, our results suggest that a substantial fraction of the cellular contents of each S100 protein family member becomes associated with the plasma membrane in terminally differentiated keratinocytes. This provides considerable new insight because it indicates that the plasma membrane constitutes an ultimate target site. Once the proteins are localized at the plasma membrane they may, as discussed previously, facilitate calcium- and differentiation-dependent, membrane-associated responses (Robinson and Eckert 1998).
Covalent Crosslinking S100-associated Complexes
Our present results indicate that S100A7, S100A10, and S100A11 are present in epidermal extracts as part of high molecular weight complexes. These complexes are stable in the presence of reducing agent and detergent and are likely to form by covalent attachment of a particular S100 protein to other cellular proteins. We have previously shown that fragments of S100A11 and S100A10 can be extracted from the keratinocyte cornified envelope, a covalent complex of proteins that is assembled via the action of transglutaminase (Robinson et al. 1997). We also showed, using in vitro crosslinking reactions, that S100A7, S100A10, and S100A11 are transglutaminase substrates, and that the transglutaminase-reactive residues are located within accessible regions of the proteins at the amino and carboxy termini (Robinson and Eckert 1998; Ruse et al. 2001). Therefore, on the basis of our present findings, we propose that S100A7, S100A10 and S100A11 serve as transglutaminase substrates in vivo and that this action accounts for the formation of the S100 protein-containing complexes. One possibility is that S100 proteins localize at the plasma membrane to accomplish specific goals related to, e.g., cell signaling/membrane transport, and that, once these functions are completed, they become incorporated as cornified envelope components.
Acknowledgments
This work utilized the facilities of the Skin Diseases Research Center of Northeast Ohio (NIH, AR39750) and was supported by a grant from the National Institutes of Health (RLE). Dr. Broome's salary was supported by the Developmental Biology Training Program (NIH HD07104-25).
Literature Cited
- Ahn SK, Hwang SM, Jiang SJ, Choi EH, Lee SH. The changes of epidermal calcium gradient and transitional cells after prolonged occlusion following tape stripping in the murine epidermis. J Invest Dermatol. 1999;113:189–195. doi: 10.1046/j.1523-1747.1999.00650.x. [DOI] [PubMed] [Google Scholar]
- Boni R, Burg G, Doguoglu A, Ilg EC, Schafer BW, Muller B, et al. Immunohistochemical localization of the Ca2+ binding S100 proteins in normal human skin and melanocytic lesions. Br J Dermatol. 1997;137:39–43. [PubMed] [Google Scholar]
- Boyce ST, Ham RG. Calcium-regulated differentiation of normal human epidermal keratinocytes in chemically defined clonal culture and serum-free serial culture. J Invest Dermatol. 1983;81:33s–40s. doi: 10.1111/1523-1747.ep12540422. [DOI] [PubMed] [Google Scholar]
- Chakravarthy BR, Isaacs RJ, Morley P, Durkin JP, Whitfield JF. Stimulation of protein kinase C during Ca(2+)-induced keratinocyte differentiation. Selective blockade of MARCKS phosphorylation by calmodulin. J Biol Chem. 1995;270:1362–1368. doi: 10.1074/jbc.270.3.1362. [DOI] [PubMed] [Google Scholar]
- Deucher A, Nagpal S, Chandraratna RA, Di Sepio D, Robinson NA, Dashti SR, et al. The carboxy-terminal hydrophobic domain of TIG3, a class II tumor suppressor protein, is required for appropriate cellular localization and optimal biological activity. Int J Oncol. 2000;17:1195–1203. doi: 10.3892/ijo.17.6.1195. [DOI] [PubMed] [Google Scholar]
- Di Nuzzo S, Sylva–Steenland RM, Koomen CW, de Rie MA, Das PK, Bos JD, et al. Exposure to UVB induces accumulation of LFA-1+ T cells and enhanced expression of the chemokine psoriasin in normal human skin. Photochem Photobiol. 2000;72:374–382. doi: 10.1562/0031-8655(2000)072<0374:etuiao>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- DiSepio D, Ghosn C, Eckert RL, Deucher A, Robinson N, Duvic M, et al. Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc Natl Acad Sci USA. 1998;95:14811–14815. doi: 10.1073/pnas.95.25.14811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato R. S-100 proteins. Cell Calcium. 1986;7:123–145. doi: 10.1016/0143-4160(86)90017-5. [DOI] [PubMed] [Google Scholar]
- Engelkamp D, Schafer BW, Mattei MG, Erne P, Heizmann CW. Six S100 genes are clustered on human chromosome 1q21: identification of two genes coding for the two previously unreported calcium-binding proteins S100D and S100E. Proc Natl Acad Sci USA. 1993;90:6547–6551. doi: 10.1073/pnas.90.14.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Felder S, Schlessinger J, Ullrich A, Hopkins CR. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J Cell Biol. 1993;120:77–83. doi: 10.1083/jcb.120.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbuglia M, Verzini M, Dimlich RV, Jamieson GA, Jr, Donato R. Characterization of type III intermediate filament regulatory protein target epitopes: S-100 (beta and/or alpha) binds the N-terminal head domain; annexin II2-p11(2) binds the rod domain. Biochim Biophys Acta. 1996;1313:268–276. doi: 10.1016/0167-4889(96)00099-7. [DOI] [PubMed] [Google Scholar]
- Hagens G, Masouye I, Augsburger E, Hotz R, Saurat JH, Siegenthaler G. Calcium-binding protein S100A7 and epidermal-type fatty acid-binding protein are associated in the cytosol of human keratinocytes. Biochem J. 1999;339(pt 2):419–427. [PMC free article] [PubMed] [Google Scholar]
- Harder T, Gerke V. The subcellular distribution of early endosomes is affected by the annexin II2p11(2) complex. J Cell Biol. 1993;123:1119–1132. doi: 10.1083/jcb.123.5.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heizmann CW, Cox JA. New perspectives on S100 proteins: a multi-functional Ca(2+)-, Zn(2+)- and Cu(2+)-binding protein family. Biometals. 1998;11:383–397. doi: 10.1023/a:1009212521172. [DOI] [PubMed] [Google Scholar]
- Hoffmann HJ, Olsen E, Etzerodt M, Madsen P, Thogersen HC, Kruse T, et al. Psoriasin binds calcium and is upregulated by calcium to levels that resemble those observed in normal skin. J Invest Dermatol. 1994;103:370–375. doi: 10.1111/1523-1747.ep12395202. [DOI] [PubMed] [Google Scholar]
- Jinquan T, Vorum H, Larsen CG, Madsen P, Rasmussen HH, Gesser B, et al. Psoriasin: a novel chemotactic protein. J Invest Dermatol. 1996;107:5–10. doi: 10.1111/1523-1747.ep12294284. [DOI] [PubMed] [Google Scholar]
- Katz AB, Taichman LB. A partial catalog of proteins secreted by epidermal keratinocytes in culture. J Invest Dermatol. 1999;112:818–821. doi: 10.1046/j.1523-1747.1999.00572.x. [DOI] [PubMed] [Google Scholar]
- Kelly SE, Jones DB, Fleming S. Calgranulin expression in inflammatory dermatoses. J Pathol. 1989;159:17–21. doi: 10.1002/path.1711590107. [DOI] [PubMed] [Google Scholar]
- Kunz M, Roth J, Sorg C, Kolde G. Epidermal expression of the calcium binding surface antigen 27E10 in inflammatory skin diseases. Arch Dermatol Res. 1992;284:386–390. doi: 10.1007/BF00372067. [DOI] [PubMed] [Google Scholar]
- Ma AS, Bell DJ, Mittal AA, Harrison HH. Immunocytochemical detection of extracellular annexin II in cultured human skin keratinocytes and isolation of annexin II isoforms enriched in the extracellular pool. J Cell Sci. 1994;107(pt 7):1973–1984. doi: 10.1242/jcs.107.7.1973. [DOI] [PubMed] [Google Scholar]
- Ma AS, Ozers LJ. Annexins I and II show differences in subcellular localization and differentiation-related changes in human epidermal keratinocytes. Arch Dermatol Res. 1996;288:596–603. doi: 10.1007/BF02505262. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Leffers H, Honore B, Celis JE. Molecular cloning and expression of a novel keratinocyte protein (psoriasis-associated fatty acid-binding protein [PA-FABP]) that is highly up-regulated in psoriatic skin and that shares similarity to fatty acid-binding proteins. J Invest Dermatol. 1992;99:299–305. doi: 10.1111/1523-1747.ep12616641. [DOI] [PubMed] [Google Scholar]
- Madsen P, Rasmussen HH, Leffers H, Honore B, Dejgaard K, Olsen E, et al. Molecular cloning, occurrence, and expression of a novel partially secreted protein “psoriasin” that is highly up-regulated in psoriatic skin. J Invest Dermatol. 1991;97:701–712. doi: 10.1111/1523-1747.ep12484041. [DOI] [PubMed] [Google Scholar]
- Menon GK, Grayson S, Elias PM. Ionic calcium reservoirs in mammalian epidermis: ultrastructural localization by ion-capture cytochemistry. J Invest Dermatol. 1985;84:508–512. doi: 10.1111/1523-1747.ep12273485. [DOI] [PubMed] [Google Scholar]
- Mischke D, Korge BP, Marenholz I, Volz A, Ziegler A. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex (“epidermal differentiation complex”) on human chromosome 1q21. J Invest Dermatol. 1996;106:989–992. doi: 10.1111/1523-1747.ep12338501. [DOI] [PubMed] [Google Scholar]
- Nakata T, Sobue K, Hirokawa N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J Cell Biol. 1990;110:13–25. doi: 10.1083/jcb.110.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigault C, Follenius–Wund A, Lux B, Gerard D. A fluorescence spectroscopy study of the calpactin I complex and its subunits p11 and p36: calcium-dependent conformation changes. Biochim Biophys Acta. 1990;1037:106–114. doi: 10.1016/0167-4838(90)90108-r. [DOI] [PubMed] [Google Scholar]
- Pillai S, Bikle DD, Mancianti ML, Cline P, Hincenbergs M. Calcium regulation of growth and differentiation of normal human keratinocytes: modulation of differentiation competence by stages of growth and extracellular calcium. J Cell Physiol. 1990;143:294–302. doi: 10.1002/jcp.1041430213. [DOI] [PubMed] [Google Scholar]
- Rety S, Osterloh D, Arie JP, Tabaries S, Seeman J, Russo–Marie F, et al. Structural basis of the Ca(2+)-dependent association between S100C (S100A11) and its target, the N-terminal part of annexin I. Struct Fold Des. 2000;8:175–184. doi: 10.1016/s0969-2126(00)00093-9. [DOI] [PubMed] [Google Scholar]
- Rety S, Sopkova J, Renouard M, Osterloh D, Gerke V, Tabaries S, et al. The crystal structure of a complex of p11 with the annexin II N-terminal peptide. Nature Struct Biol. 1999;6:89–95. doi: 10.1038/4965. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Hogg N. A comparison of human S100A12 with MRP-14 (S100A9) Biochem Biophys Res Commun. 2000;275:865–870. doi: 10.1006/bbrc.2000.3407. [DOI] [PubMed] [Google Scholar]
- Robinson NA, Eckert RL. Identification of transglutaminase-reactive residues in S100A11. J Biol Chem. 1998;273:2721–2728. doi: 10.1074/jbc.273.5.2721. [DOI] [PubMed] [Google Scholar]
- Robinson NA, Lapic S, Welter JF, Eckert RL. S100A11, S100A10, annexin I, desmosomal proteins, small proline-rich proteins, plasminogen activator inhibitor-2, and involucrin are components of the cornified envelope of cultured human epidermal keratinocytes. J Biol Chem. 1997;272:12035–12046. doi: 10.1074/jbc.272.18.12035. [DOI] [PubMed] [Google Scholar]
- Ruse M, Lambert A, Robinson N, Ryan D, Shon KJ, Eckert RL. S100A7, S100A10, and S100A11 are transglutaminase substrates. Biochemistry. 2001;40:3167–3173. doi: 10.1021/bi0019747. [DOI] [PubMed] [Google Scholar]
- Sato H, Hayashi E, Yamada N, Yatagai M, Takahara Y. Further studies on the site-specific protein modification by microbial transglutaminase. Bioconjug Chem. 2001;12:701–710. doi: 10.1021/bc000132h. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Gillitzer R, Toksoy A, Brocker EB, Rapp UR, Paus R, et al. Selective expression of calcium-binding proteins S100a8 and S100a9 at distinct sites of hair follicles. J Invest Dermatol. 2001;117:748–750. doi: 10.1046/j.0022-202x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- Seemann J, Weber K, Gerke V. Annexin I targets S100C to early endosomes. FEBS Lett. 1997;413:185–190. doi: 10.1016/s0014-5793(97)00911-3. [DOI] [PubMed] [Google Scholar]
- Seitz A, Schneider F, Pasternack R, Fuchsbauer HL, Hampp N. Enzymatic cross-linking of purple membranes catalyzed by bacterial transglutaminase. Biomacromolecules. 2001;2:233–238. doi: 10.1021/bm0056207. [DOI] [PubMed] [Google Scholar]
- Teigelkamp S, Bhardwaj RS, Roth J, Meinardus–Hager G, Karas M, Sorg C. Calcium-dependent complex assembly of the myeloic differentiation proteins MRP-8 and MRP-14. J Biol Chem. 1991;266:13462–13467. [PubMed] [Google Scholar]
- Thorey IS, Roth J, Regenbogen J, Halle JP, Bittner M, Vogl T, et al. The Ca2+-binding proteins S100A8 and S100A9 are encoded by novel injury-regulated genes. J Biol Chem. 2001;276:35818–35825. doi: 10.1074/jbc.M104871200. [DOI] [PubMed] [Google Scholar]
- Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001;276:41079–41085. doi: 10.1074/jbc.M107122200. [DOI] [PubMed] [Google Scholar]
- Wicki R, Marenholz I, Mischke D, Schafer BW, Heizmann CW. Characterization of the human S100A12 (calgranulin C, p6, CAAF1, CGRP) gene, a new member of the S100 gene cluster on chromosome 1q21. Cell Calcium. 1996;20:459–464. doi: 10.1016/s0143-4160(96)90087-1. [DOI] [PubMed] [Google Scholar]
- Wu T, Angus CW, Yao XL, Logun C, Shelhamer JH. P11, a unique member of the S100 family of calcium-binding proteins, interacts with and inhibits the activity of the 85-kDa cytosolic phospholipase A2. J Biol Chem. 1997;272:17145–17153. doi: 10.1074/jbc.272.27.17145. [DOI] [PubMed] [Google Scholar]
- Xia L, Stoll SW, Liebert M, Ethier SP, Carey T, Esclamado R, et al. CaN19 expression in benign and malignant hyperplasias of the skin and oral mucosa: evidence for a role in regenerative differentiation. Cancer Res. 1997;57:3055–3062. [PubMed] [Google Scholar]