Abstract
Reactions of nitric oxide (NO) with hemoglobin (Hb) are important elements in protection against nitrosative damage.NO in the vasculature is depleted by the oxidative reaction with oxyHb or by binding to deoxy Hb to generate partially nitrosylated Hb (Hb–NO). Many aspects of the formation and persistence of Hb–NO are yet to be clarified. In this study, we used a combination of EPR and visible absorption spectroscopy to investigate the interactions of partially nitrosylated Hb with O2. Partially nitrosylated Hb samples had predominantly hexacoordinate NO–heme geometry and resisted oxidation when exposed to O2 in the absence of anionic allosteric effectors. Faster oxidation occurred in the presence of 2,3-diphosphoglycerate (DPG) or inositol hexaphosphate (IHP), where the NO–heme derivatives had higher levels of pentacoordinate heme geometry. The anion-dependence of the NO–heme geometry also affected O2 binding equilibria. O2-binding curves of partially nitrosylated Hb in the absence of anions were left-shifted at low saturations, indicating destabilization of the low O2 affinity T-state of the Hb by increasing percentages of NO–heme,much as occurs with increasing levels of CO–heme. Samples containing IHP showed small decreases in O2 affinity, indicating shifts toward the low-affinity T-state and formation of inert α-NO/β-met tetramers. Most remarkably, O2-equilibria in the presence of the physiological effector DPG were essentially unchanged by up to 30% NO–hemein the samples. As will be discussed, under physiological conditions the interactions of Hb with NO provide protection against nitrosative damage without impairing O2 transport by Hb's unoccupied heme sites. This article is part of a Special Issue entitled: Oxygen Binding and Sensing Proteins.
Keywords: Nitric oxide, Oxygen binding curve, Allostery, Pentacoordinate
1. Introduction
Nitric oxide (NO) is a short-lived free radical gas that is highly reactive. Because of its high reactivity and short half-life (~0.1–5 s), it wasn't until 1977 that Murad and coworkers documented the important role of NO in the activation of guanylate cyclase in tissues [1]. It was a decade later that Moncada and Ignarro and coworkers independently identified NO as the elusive endothelium-derived relaxing factor (EDRF) that controls blood pressure by smooth muscle relaxation and associated vasodilation [2–4]. Following discovery of its surprising role in blood pressure regulation, NO was found to be a neurotransmitter [5] and to play an essential role in the inflammatory response and in host immunity [6,7]. These beneficial functions of NO are controlled uses of a highly reactive molecule that has the potential for indiscriminate killing of cells and for chemical modification of biologically important molecules via damaging nitrosative reactions [8].
Reactions of NO with hemoglobin (Hb) in circulating erythrocytes can be a critical determinant of the success or failure of new and emerging NO-dependent therapies. These interactions protect against damaging nitrosative reactions (nitrosative stress) and make it possible for inhaled NO to aid in the survival of newborn infants with pulmonary hypertension or hypoxemic respiratory failure. Success in this use of inhaled NO is being expanded for other clinical indications in both infants and adults [9,10].
The exciting prospects of benefiting human health by pharmacological manipulation of NO-dependent reactions must be regarded with a degree of caution in light of potentially damaging nitrosative reactions. Use of the redox reactions of oxy Hb and NO to convert the highly reactive NO molecule to nitrate, its less reactive oxidized state, is a well-documented survival strategy for invading microbes that are subject to host-initiated nitrosative stress [11,12]. Rapid NO conversion to nitrate by reaction with human oxy Hb is also well documented [13,14], but is less well recognized for its protective function.
Since the discovery of the bioactive nature of NO, the nature and chemistry of interactions between NO and Hb in red blood cells has been the subject of intensive investigation and on-going controversy. Mammalian blood contains small amounts (~0.05 µM, ~0.0002%) of circulating NO–Hb in vivo [15–18]. Most of the NO–Hb within red blood cells is derived from the reaction between NO produced in the vasculature endothelium and vacant heme sites on deoxy Hb that generates the highly stable (~10−12 M) Hb–NO complex. Alternatively, NO may react with oxy Hb to generate met (ferric) Hb and nitrate. The very fast reaction of either oxy (3.7 × 107 M−1 s−1) [13] or deoxy Hb (1.4 × 107 M−1 s−1) [19] with NO plays a major role in NO catabolism and in keeping NO at low physiological levels in vivo.
A major debate has centered on whether erythrocytic Hb consumes or conserves NO bioactivity [20,21]. Transnitrosation reactions, which transfer NO+ between S-nitrosothiols and Hb's β93Cys groups [22], can sequester NO+ equivalents within red blood cells and have been proposed as a source of bioactive NO [23,24]. Moreover, recent studies have shown that nitrite can be converted to NO via the nitrite reductase function of deoxy Hb, and can also add to the levels of erythrocytic NO–heme and bioactive NO [16,25].
Basic principles dictate that the ratio of oxy to deoxy Hb exerts a profound effect on the amount of NO–heme formed when NO passes through the red cell membrane. The higher the oxygenation level, the more probable is the formation of met Hb and nitrate from the encounter of NO with an oxy Hb molecule. Notably, inhaled NO is largely degraded in the pulmonary system due to reactions with oxy Hb, protecting the rest of the circulatory system from nitrosative damage [10].
This report and some of the prior studies from our laboratories have addressed relevant aspects of the multifaceted interactions of Hb with NO [21,26,27]. By binding preferentially to the T quaternary structure of the Hb, allosteric anionic effectors, such as chloride, 2,3-diphosphoglycerate (DPG) and inositol hexaphosphate (IHP), alter the position of the allosteric equilibrium between the low-affinity T and the high-affinity R structure and have profound effects on the reactions of Hb with NO and O2. In this report we address as yet unresolved aspects of the fate and effects of NO in vivo by documenting the anion-dependent effects of NO on O2 binding by Hb.
We prepared Hb samples containing various levels of NO–heme (up to ~40%) by adding sub-stoichiometric amounts of gaseous NO to deoxy Hb in tonometers. After anaerobic equilibration, the samples were exposed to a cycle of oxygenation and deoxygenation, simulating the arterial-venous circuit of red blood cells in vivo.We used EPR spectroscopy to determine changes in NO–heme hexacoordination and pentacoordination states and met formation when samples containing varied levels of NO–heme and anionic effectors were exposed to air. In other experiments,we used visible absorption spectroscopy to measure O2 equilibria of partially nitrosylated Hb under varied anionic conditions while following changes in levels of heme derivatives (deoxy, oxy, NO and met) when changing O2 tensions.
We found that the affinity of Hb for O2 under physiological conditions, e.g. in solutions where the physiological Hb cofactor DPG was present, was only slightly affected despite large increases in the levels of heme nitrosylation. This was a surprising result, unlike that observed when increasing levels of carbon monoxide (CO) are bound to Hb's active sites. As will be discussed, these results show that while interactions of NO with oxy Hb provide protection against nitrosative stress, interactions with deoxy Hb can occur without impairing O2 transport by Hb's unoccupied active sites.
2. Materials and methods
2.1. Preparation of partially nitrosylated Hb
All studies of Hb's properties reported here made use of purified adult human Hb (HbA0). The Hb was prepared by ammonium sulfate precipitation, stripped of endogenous organic phosphates and purified by anion-exchange FPLC as previously described [28]. Hb samples with varying partial NO–heme occupancy were prepared anaerobically by injection of sub-stoichiometric amounts of purified gaseous NO (to achieve a range of ~10–40% NO saturation) into tonometers containing deoxy Hb (0.5 ml, 5 mM heme, 0.05 M bistris buffer pH 7.5, 0.5 mM EDTA), and by incubation for 1 h at room temperature, as previously described [26]. Aliquots of partially nitrosylated Hb were then shortly (<10 min) exposed to air when transferred into four different tonometers containing 0.05 M bistris buffer, pH 7.5, 0.5 mM EDTA in the absence (stripped) and presence of either 0.1 M NaCl, 0.5 mM DPG or 0.15 mM IHP, respectively, and degassed prior to O2-binding equilibrium experiments, as described below. Final heme concentration was 0.06 mM. To minimize oxidation into met Hb during O2-binding equilibria and to mimic conditions existing within red blood cells, the enzymatic met-reducing system [29] was added throughout.
2.2. O2 binding experiments
O2 equilibrium curves were determined tonometrically at 20 °C [30]. After deoxygenation by three cycles of exposure to N2 and vacuum, a gastight syringe was used to inject measured volumes of room air through the rubber septum of a tonometer containing the Hb sample, ending with an equilibration with 100% air. After each air addition (each corresponding to a PO2 value) the tonometers were rotated in a water bath for 10 min before an absorbance spectrum (450–700 nm) was measured. Each O2 equilibrium experiment took approximately 1 h. At each equilibration step the observed absorption spectrum was used to calculate the corresponding fraction of hemes bound to O2 (Y, oxy/(oxy + deoxy)), NO (NO/(NO + deoxy + oxy + met)) or in the ferric state (met/(met + NO + deoxy + oxy)) by spectral deconvolution using reference absorbance spectra for pure oxy, deoxy, met and NO derivatives measured under the various buffer condition used as previously described [26]. Absorbance spectra were measured using a HP 8543 diode array spectrophotometer and analyzed for spectral deconvolution by least-square fitting procedures. Values of P50 (O2 tension at half-saturation) and n50 (degree of cooperativity) were interpolated from the zero intercept and slope of Hill plots, log(Y / (1 − Y)) vs logPO2, obtained by linear regression (r2 > 0.99) of data points within the saturation interval ~0.3–0.7. At the end of experiments, the levels of S-nitrosated Hb (SNO-Hb) formed were measured by spectral deconvolution after addition of 4 mg/ml sodium dithionite (that cleaves the S–NO linkage and generates NO that is captured by ferrous heme), as previously described [28].
2.3. EPR measurements
X-band (9.65 GHz) EPR spectra were recorded on a Bruker ESP 300 spectrometer equipped with an Oxford ESR 910 cryostat for low temperature measurements. The microwave frequency was calibrated by a frequency counter, and the magnetic field was calibrated with a NMR gaussmeter. The temperature was calibrated with resistors (CGR-1–1000) from LakeShore. A modulation frequency of 100 kHz was used for all EPR spectra. All experimental data were collected under nonsaturating conditions. Analysis of the EPR spectra used diagonalization of the standard spin Hamiltonian. The simulations were generated with consideration of all intensity factors relative to a spin standard (CuEDTA), and thus allowed a quantitative determination of protein signal intensities from simulations. The Windows software package (SpinCount) can be obtained from Dr. Hendrich (hendrich@andrew.cmu.edu). The total concentration of S = 1/2 species was determined by double integration. The concentration of the pentacoordinate heme-NO species was determined by double integration after a subtraction which minimizes the hexacoordinate His-heme-NO signal. The high-spin (S = 5/2) heme signals were quantified from simulations of the spectra using D = 10 cm−1, and E/D = 0. Overall apparent rates of NO decrease and met increase when fully nitrosylated HbNO was exposed to air were obtained by monoexponential fitting of EPR data collected at varied time and expressed as mean ± sem. In these experiments, the enzymatic met-reducing system was not present. In the EPR samples there was never more than ~1% met Hb present at the start, confirming the anaerobic integrity of the sample manipulations.
3. Results
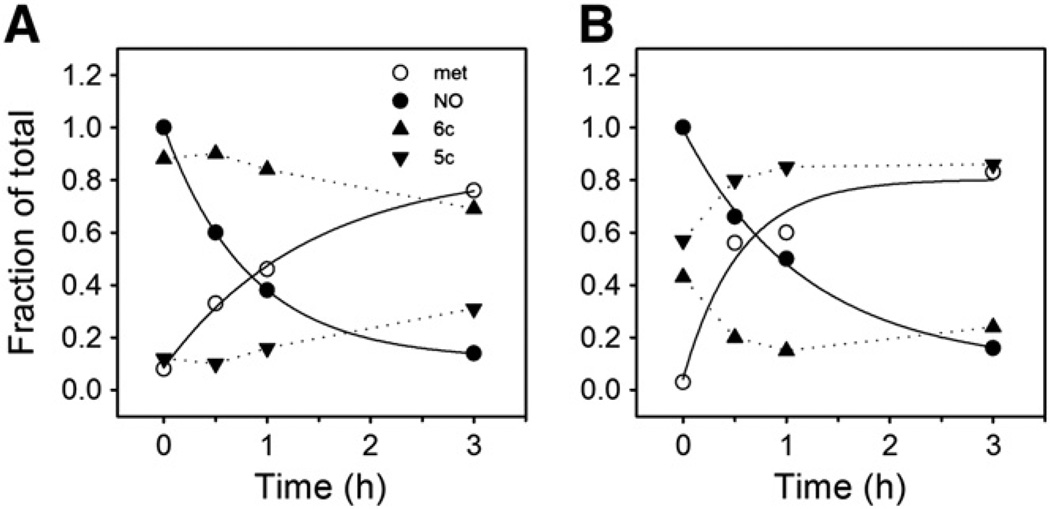
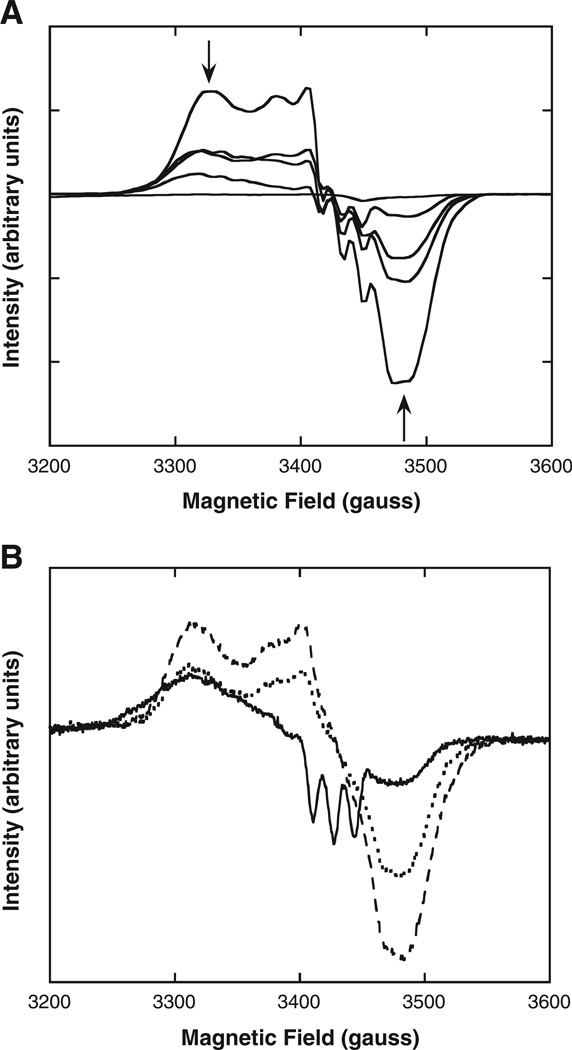
We determined by EPR spectroscopy the stability of fully nitrosylated Hb (HbNO) toward oxidation and the ratios of penta- to hexacoordinate NO–heme geometries upon exposure to air under various buffer conditions. Besides forming hexacoordinate NO–heme (analogous to hexacoordinate O2–heme and CO–heme), NO bound to α-hemes can become pentacoordinate by disruption of the heme linkage to the proximal His on the F-helix of the globin [31]. EPR spectra show sharp triplet 14N hyperfine structures around gz = 2.009 [15,31] that make it possible to monitor levels of pentacoordinate α-NO heme. Changes in levels of NO–heme and met–heme derivatives upon air exposure are reported in Table 1 and show a strong dependence on pH and on the anions present in solution. In the presence of IHP, the levels of pentacoordinate heme increased slowly at the physiological pH of 7.4 (Fig. 1A). In contrast, at pH 6.5 half of the NO–hemes (i.e. the α-hemes) became immediately pentacoordinate (see time zero in Fig. 1B and Table 1), with pentacoordinate α-NO heme becoming gradually the dominant NO species as the hexacoordinate β-NO– heme disappeared (Fig. 1B). These time dependent changes in the EPR spectrum are shown in Fig. 2A. These changes are consistent with the previously hypothesized mechanism whereby Hb–NO oxidation to met Hb and nitrate proceeds via a slow and pH-dependent dissociation of NO as the initial rate limiting step, with subsequent fast reaction of NO with oxygenated heme [32]. We found similar apparent rates of ~10−4 s−1 for Hb–NO decrease and met increase (Table 1), in accord with prior studies of the reaction of Hb–NO with O2 made under similar conditions [32,33]. In the presence of IHP, calculated rate constants for Hb–NO decrease were 3.4 × 10−4 ± 9.4 × 10−7 and 2.3 × 10−4 ± 3.7 × 10−5 s−1 at pH 7.4 and 6.5, respectively, and for met increase the rate constants were 2.0 × 10−4 ± 3.1 × 10−5 and 5.1 × 10−4 ± 2.3 × 10−4 s−1 at pH 7.4 and 6.5, respectively (Fig. 1). Met Hb formation was somewhat faster at low pH with IHP (5.1 × 10−4 ± 2.3 × 10−4 s−1) than NO–Hb disappearance (2.3 × 10−4 ± 3.7 × 10−5 s−1), suggesting a higher tendency of the heme to autoxidize. Compared to the levels with IHP, less pentacoordinate α-NO heme was formed when partially nitrosylated Hb samples contained DPG. Under these conditions, the three-line hyperfine EPR spectra characteristic of pentacoordinate NO–heme was less pronounced than in the presence of IHP (Fig. 2B).
Table 1.
Pentacoordinate to hexacoordinate NO–heme initial ratios (5:6 coord.), apparent rate constants of met increase and NO decrease (kapp) of fully nitrosylated Hb–NO after exposure to air obtained from EPR spectra under varied buffer conditions at 20 °C. Hb–NO (0.06 mM heme) was in 0.05 M bis–Tris (pH 7.4 or 6.5) or Tris (pH 8.5) buffer in the absence or presence of 0.15 mM IHP, as indicated. Rates were determined by monoexponential fitting of traces, as shown in Fig. 1 (n.d., not determined).
| pH 8.5 | pH 7.4 | pH 6.5 | |||
|---|---|---|---|---|---|
| IHP | − | − | + | − | + |
| 5:6 coord. | 0:100 | 0:100 | 12:88 | 22:78 | 57:43 |
| kapp met (s−1) | n.d. | 3.2 × 10−4 | 2.0 × 10−4 | 2.5 × 10−4 | 5.1 × 10−4 |
| kapp NO (s−1) | n.d. | 3.2 × 10−4 | 3.4 × 10−4 | 2.5 × 10−4 | 2.3 × 10−4 |
Fig. 1.
Oxidation of fully nitrosylated Hb followed by EPR spectroscopy. Fully nitrosylated HbA samples, 0.06–0.07 mM in heme, in 0.05 M Tris buffer, 0.05 M EDTA, 0.15 M IHP at either A) pH 7.4 or B) pH 6.4 were exposed to air at 20 °C. Samples were frozen at 0, 0.5, 1, and 3 h for running EPR spectroscopy (x-band, 20 K) at a later time. EPR conditions: 9.8 G modulation amplitude, 63 µW microwave power. The fraction of the sample as determined by EPR spectroscopy to be in the met Hb (ferric), total nitrosylated Hb and the penta- or hexacoordinate nitrosylated Hb forms is plotted versus time. Monoexponential fits of the metHb increase or the total nitrosylated Hb decrease are indicated by solid lines and the derived rates are given in Table 1.
Fig. 2.
Comparison of EPR (x-band, 20 K) spectral changes for fully and partially nitrosylated Hb in the presence of various anions after exposure to air. EPR conditions: 9.8 G modulation amplitude, 63 µW microwave power. A) Fully nitrosylated HbA (60 µMin heme, containing 0.15 mM IHP) sampled at 0, 0.5, 1, 2.5 and 25 h during exposure to air at 20 °C. Levels of pentacoordinate nitrosylated Hb, indicated by 3-line hyperfine, increase (see Fig. 1) relative to total levels of nitrosylated Hb decrease, indicated by arrows. B) Anion dependence of EPR spectral lineshapes following air exposure (~15 min.) for 40% nitrosylated HbA (70 µM in heme) samples containing IHP (0.15 mM, solid line), DPG (0.5 mM, dotted line) and NaCl (0.1 M, dashed line). Only samples containing IHP have appreciable levels of the 3-line hyperfine indicative of pentacoordinate nitrosylated Hb.
Taken together, these data show that Hb–NO lifetime in air reflects the pH and anion-dependence of the equilibrium between pentacoordinate and hexacoordinate NO–heme geometry. Moreover, the allosteric effects that promote pentacoordinate NO–heme geometry on the α chains simultaneously poise the β chains for rapid NO release and subsequent heme oxidation. HbNO oxidation in air thus generates pentacoordinate α-NO heme and β-met heme as the most prevalent species at equilibrium.
Although we did not monitor in detail the rapid phase of alterations induced by IHP (Fig. 1B), our EPR data are consistent with early reports of a rapid NO migration among subunits under anaerobic conditions, with formation of the pentacoordinate α-NO species at equilibrium [31,34]. Yonetani and coworkers [15] showed that the tetranitrosyl species α(Fe–NO)2β(Fe–NO)2 oxidizes faster than the α(Fe–NO)2β(Fe–O2)2 species in air, consistent with our observations of NO–heme oxidation.
The EPR data show that, at the physiological pH 7.4, partially nitrosylated Hb is relatively stable toward oxidation or NO loss when exposed to air under the anionic conditions investigated (Fig. 1A, Table 1). Therefore, this pH was suitable for determination of O2 equilibrium curves.
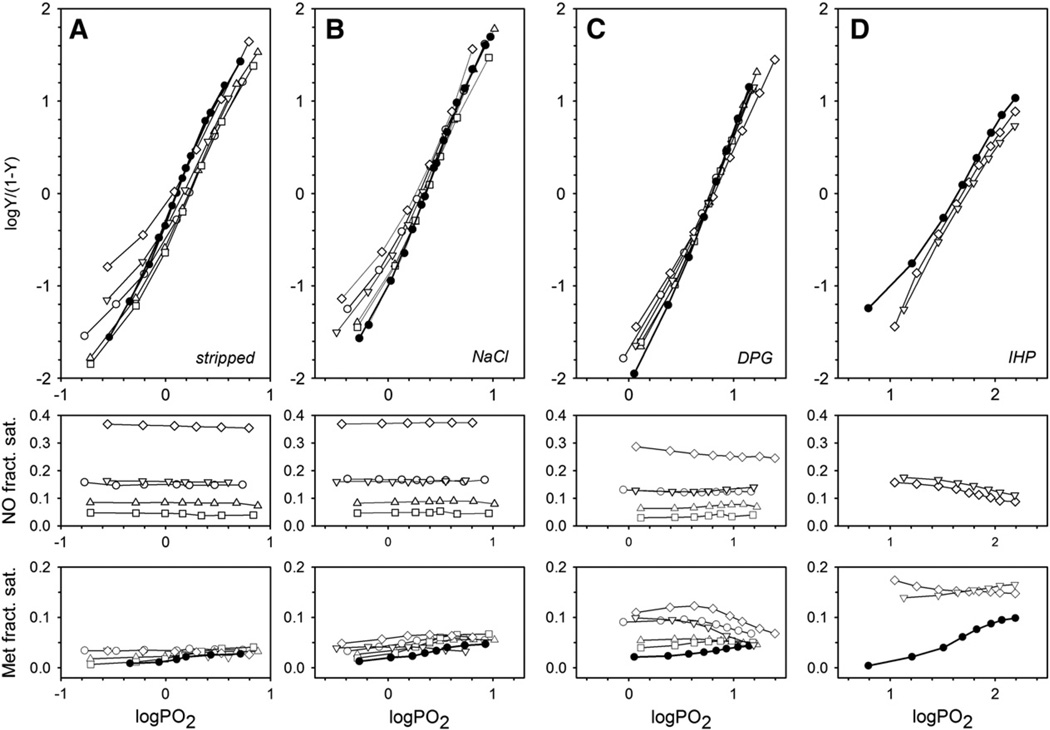
Fig. 3 shows how variable partial occupancy of heme sites by NO affects subsequent O2 binding under different anionic conditions. As shown, the levels of NO saturation stayed remarkably stable during O2-binding experiments (Fig. 3, middle panels) and the apparent levels of met Hb (Fig. 3, lower panels) were low in the absence of organic phosphates (<4.1% for stripped and <6.7% for NaCl) and higher in the presence of DPG or IHP (<12.3% and <17.4%, respectively). As these experiments were all performed in the presence of the met Hb enzymatic reducing system (see Materials and methods), the apparent levels of met Hb include the steady-state balance between met heme generated and reduced, as well as any pentacoordinate α-NO heme, which has a met-like spectral character [26].
Fig. 3.
Anion effects on O2 binding by normal Hb and samples with varied levels of NO–heme. O2 equilibrium curves (Hill plots, upper panels) of normal (solid circles, thick lines) and partially nitrosylated adult human Hb (open symbols, thin lines) are shown. All samples were 0.06 mMin heme, in 0.05 Mbis–Tris buffer, pH 7.5, 0.5 m MEDTA, 20 °C, with met Hb reductase system (see Materials and methods). A) Stripped, anion-free Hb; B) 0.1 M NaCl; C) 0.5 mM DPG and D) 0.15 mM IHP. Middle and lower panels show fractional saturation levels of nitrosylation and met heme at the corresponding oxygen tension values, respectively, measured by spectral deconvolution. Identical symbols indicate identical initial NO– heme levels before air exposure and deoxygenation.
For Hb stripped of anionic effectors (Fig. 3A), the Hill plots for O2 binding to partially NO-liganded Hb were left-shifted compared to unmodified hemoglobin in the initial phase of O2 binding. Although a thermodynamic analysis of the O2 binding curves according to the two state model [40] was not possible because of lack of sufficient data points, such left-shifted curves suggest an overall shift toward the R state conformation with increasing NO–heme occupancy. These changes closely resemble the changes induced by low levels of HbCO or met Hb. These results are in accord with the homotropic allosteric effects for binding of an alternative heme ligand. Albeit less pronounced, the right-shifts of the upper part of the Hill plots for samples with low (less than ~16%) levels of NO–heme are indicative of a reduced affinity for O2. This result indicates that the R-state O2 affinity is lower when some of the hemes carry NO. In the presence of chloride (Fig. 3B), the left-shifts of the lower part of the Hill plots are still present, but less pronounced than in the Hb samples that are stripped of anionic effectors.
In contrast, the O2 binding equilibria in the presence of the physiological allosteric effector DPG were found to be largely unaffected by NO–heme ligation levels up to ~30% (Fig. 3C). This surprising result, which is very different from the left shifts that accompany increasing CO–heme or met–heme levels, is a key finding of this study. In these experiments, met heme levels appeared to decrease at elevated O2 tensions, reflecting the reversible change from penta-to hexacoordinate α-NO heme induced by progressive oxygenation and the associated allosteric shift from the T to the R state. We have previously documented this phenomenon and noted the met-like absorbance spectral character of pentacoordinate α-NO [26].
In the presence of the potent allosteric anionic effector IHP (Fig. 3D), O2 binding curves were slightly shifted to the right, indicating an overall lower O2 affinity for the partially nitrosylated Hb under this condition. Met Hb levels increased in the unmodified Hb in the presence of IHP, indicating a higher tendency for heme to autoxidize. In the presence of IHP, samples were found to contain similar initial levels of met and NO (~15%; Fig. 3D), suggesting that IHP had induced formation of hybrid α-NO/β-met tetramers prior to O2 equilibrium curve determination, consistent with the EPR results (Fig. 1). These hybrid tetramers would be inactive with regard to reaction with O2, which may explain why heme nitrosylation has such a small effect on O2 equilibria under our measuring conditions.
Whereas, under all conditions examined, partial heme nitrosylation led to only small variations in the overall O2 affinity at 50% saturation (Fig. 3), cooperativity in O2 binding as expressed by the Hill coefficient n50was decreased as a consequence of partial nitrosylation (see Supplemental Table S1). This effect was most evident in the stripped and chloride-containing samples, indicative of the progressive shift of quaternary allosteric equilibrium toward the R state with increased heme occupancy by NO.
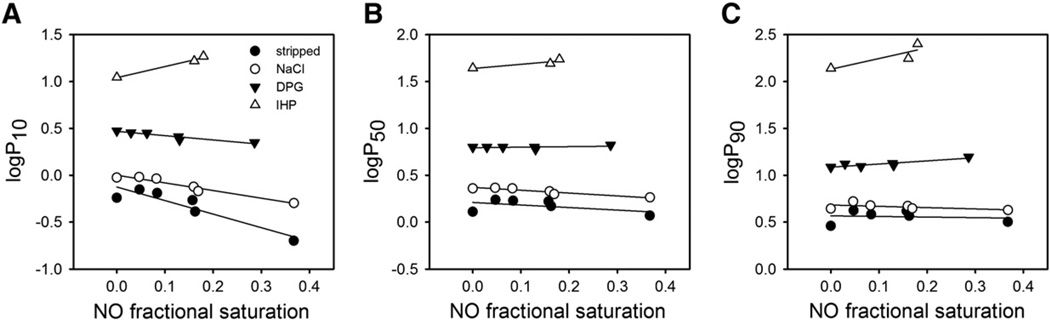
Fig. 4 shows the effect of the different types of anions examined on the changes in O2 saturations at 10%, 50% and 90% (expressed as logP10, logP50 and logP90, respectively) at increasing levels of NO occupancy. The general increase in the O2 tension to achieve a given O2 saturation (in the absence and presence of NO) reflects the increase in T-state stabilization in the order chloride < DPG < IHP. Furthermore, the decrease in logP10 with increasing levels of Hb nitrosylation (Fig. 4A) indicated a progressive destabilization of the T state with increasing NO ligation, particularly in anion-stripped samples. In contrast, in IHP-containing samples, the apparent O2 affinity progressively decreased with increasing NO saturation (Fig. 4). This change was expected in light of the studies of Yonetani and coworkers who showed that α(Fe–NO)2β(Fe)2 tetramers have reduced O2 affinity in the presence of IHP [15]. Heme nitrosylation had only little effect on O2 tension values at 50% or 90% saturation (Fig. 4B,C), confirming that the major changes occur in the lower part of the O2 equilibrium curve.
Fig. 4.
Anion effects on changes in Hill plots brought about by the presence of NO–heme. Values of O2 tension at A) 10%, B) 50% and C) 90% O2 saturation as a function of fractional NO saturation. Data are derived from the O2 equilibrium curves shown in Fig. 3.
In contrast to results obtained in the presence of a strong organic phosphate effector like IHP, a random equilibrium distribution of NO– heme, on either the α or β subunits, is favored under anion-free experimental conditions or in the presence of weak effectors (e.g. chloride), where the T-state is less stabilized and hexacoordinate NO–heme prevails (see Fig. 2B).WhenNO–hemeis randomly distributed, the changes due to partial NO-saturation occur mainly in the lower part of the O2 equilibrium curves (Figs. 3A,B; 4A), with shifts like those observed for partial CO-saturation.
In contrast, NO binding decreases the O2 affinity of unliganded heme sites when the protein is in the presence of IHP (Fig. 3D) and pentacoordinate α NO heme is favored (Fig. 2A), a condition that at the same time promotes formation of met hemes in the same Hb tetramer (Fig. 1A). Such effects are distinctive properties of NO–heme but not of CO or ferric heme, which are both incapable of generating pentacoordinate heme.
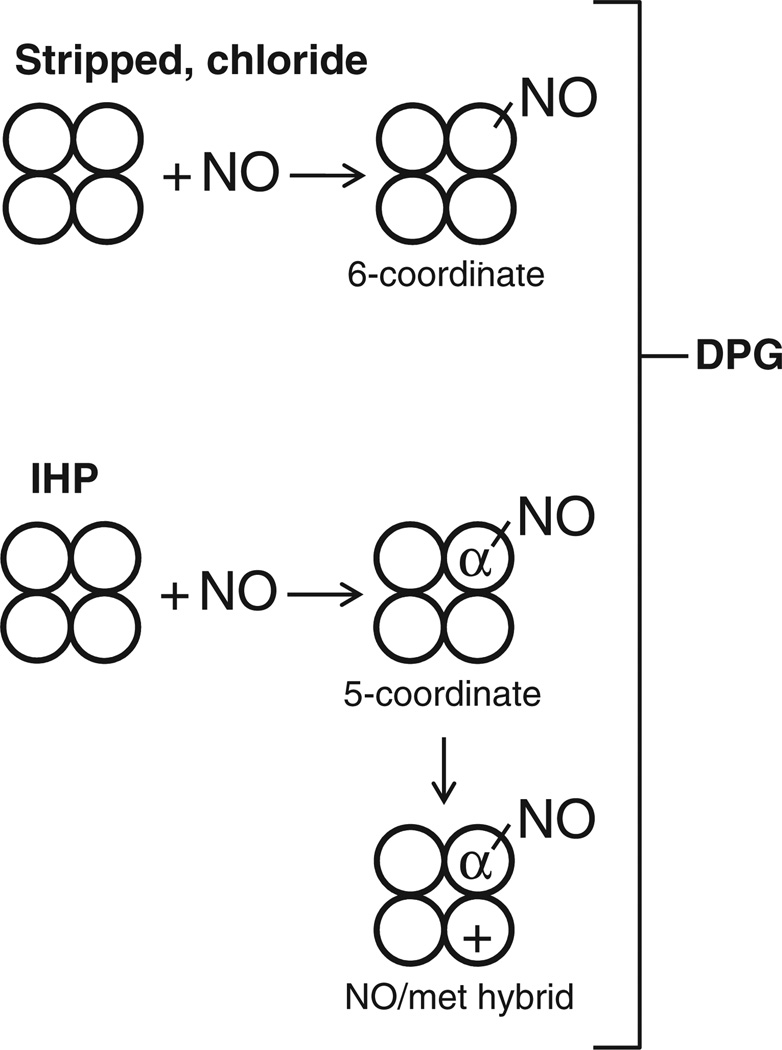
Interestingly, among the cofactors investigated, only the physiological effector DPG favors a mixture of high- and low-O2 affinity nitrosylated Hb, with the consequence of leaving the O2 equilibrium curve essentially unaffected by the level of NO occupancy (Fig. 3C). This result is due to the fact that DPG also promotes formation of pentacoordinate α-NO, but to a lower extent than IHP (Fig. 2B) [15]. A schematic overview of the main heme derivatives in solutions containing partially nitrosylated Hb is provided in Fig. 5.
Fig. 5.
Proposed scheme for anion-dependent changes in NO-coordination state. Samples stripped of anions or with chloride (upper part) favor hexacoordinate NO–heme formation on either α or β chain, with consequent increase in O2 affinity associated with NO-induced shift toward the high-affinity R-state. Samples containing the strong allosteric effector IHP (lower part) favor formation of Hb tetramers containing pentacoordinate α-NO hemes with low O2 affinity or oxidation of the remaining β subunits in air (NO/met hybrid), forming tetramers that show a decrease in O2 affinity or are inert toward oxygenation, respectively. The physiological effector DPG (with intermediate strength) favors an intermediate combination of these effects, creating both high- and low-O2-affinity tetramers (containing predominantly hexacoordinate and pentacoordinate NO–heme, respectively), whereby partial heme nitrosylation has a minimal effect on O2 binding. For simplicity, only one type of heme NO or met derivative per tetramer is illustrated.
Samples of partially nitrosylated Hb were tested to determine if the protocol used in this study (varied levels of NO–heme formed under deoxy conditions, air-exposure, deoxygenation, and O2-affinity determinations) formed appreciable SNO-Hb. Under all the conditions examined, the amount of SNO-Hb was below the detection level of the method used here (~5% SNO-Hb). Although it has previously been reported that when partially nitrosylated Hb becomes exposed to O2, some nitrosation at Cysβ93 occurs that generates SNO-Hb [35], no detectible SNO-Hb was formed under our experimental conditions. Our result is similar to that of Herold and Röck [36], who reported low yields of SNO-Hb (~1–2%) after air-exposure of 10% nitrosylated Hb. It is possible that the enzymatic met Hb reducing system used in our experiments may have affected our results [37]. In any case, the low levels of SNO-Hb that could have been generated at the range of NO–heme saturation used in our studies would have had a negligible effect on O2 equilibria [28]. Therefore, the functional consequences reported in Figs. 3 and 4 (see also Supplemental Table S1) are ascribable to changes in heme ligation and coordination state, without significant interference from S-nitrosation effects.
4. Discussion
Many aspects of the formation and persistence of partially nitrosylated Hb that results from the reaction of NO with deoxy Hb are yet to be clarified. In this study, we prepared partially nitrosylated Hb samples and monitored their oxidation and oxygenation reactions upon exposure to O2 in the presence of varied allosteric effectors. The samples exhibited changes in NO–heme-coordination state and variations in rates of NO dissociation, depending on the pH and anion present in solution, and ultimately on the position of the T–R allosteric equilibrium.We demonstrated that under physiological conditions, exposure of Hb to increasing levels of NO results in increased levels of both oxidized and nitrosylated hemes, which decreases the total amount of heme available for O2 transport, i.e. the total O2 carrying capacity. Remarkably, however, under physiological conditions, the sites that were not oxidized or nitrosylated were able to bind O2 with relatively unaltered affinity, even at high NO–heme levels. This surprising finding shows NO to be a unique and non-toxic heme ligand.
Our findings add to prior results froma long history of investigation of NO–hemecomplexes. The surprising results of this report on O2 binding by partially nitrosylated Hb are attributable to the distinctive features of NO–heme complexes in Hb that allow for redox-mediated protection against nitrosative stress and for allosteric modulation of heme coordination in ways that do not apply to heme ligation by CO or O2. These distinctive features of NO as a heme ligand affect the extent of formation of NO–heme in erythrocytes and its persistence in vivo.
Although binding of NO to Hb occurs with much higher affinity than CO or O2 [38,39], the governing allosteric controls can be conveniently viewed in terms of the two-state allosteric model of Hb function [40] as operating on the regulation of the T–R equilibrium by allosteric effectors, such as chloride and organic phosphates [38,41]. According to the two-state model, progressive heme occupancy by O2, CO or NO shifts the allosteric equilibrium toward the high-affinity R state. In the reaction of Hb with CO, the consequence is poisoning of Hb oxygenation, in part because CO and O2 are competitive heme ligands and in part because CO occupancy of binding sites on the Hb tetramer has the effect of increasing O2 affinity, particularly at low O2 tensions. Sub-stoichiometric levels of CO that create partially CO-liganded Hb thus inhibit the release of O2 from Hb to respiring tissues and severely impair O2 transport [42,38].
In having a ~105-fold higher affinity for Hb than CO [38,39], NO would be predicted to have an even larger inhibitory effect on Hb oxygenation. However, as we show here, this is not the case. Furthermore, unlike CO, NO reacts very rapidly with oxy Hb. This oxygenase reaction, which oxidizes NO to nitrate, is protective against respiratory poisoning and nitrosative stress. Although met Hb (that cannot transport O2) is also formed, met Hb reductase within red blood cells can restore O2-binding functionality to the oxidized sites.
NO has other distinctive characteristics as a heme ligand besides the ability to enter into redox reactions. Notably, NO can form hexacoordinate NO–heme in a fashion similar to O2–heme and CO– heme, but can also, under certain buffer conditions, assume pentacoordinate geometry as a result of disruption of the heme linkage to the proximal His onthe F-helix of the globin [31]. This conformational equilibrium is distinctive to NO–heme and confers unexpected anion-dependent oxygen-binding properties to partially nitrosylated Hb.
Formation of pentacoordinate α-NO heme, which is favored by multivalent anionic effectors, is most pronounced at low levels of ligand binding where the T-quaternary conformation is favored [15,26,31]. The formation of pentacoordinate NO–heme on the α-chains can be reversed by shifts to the R-state, inducible by increases in the degree of ligation of the remaining heme groups by binding of either O2 [15,26] or NO [31]. Using in vitro reconstituted Hb tetramers of nitrosylated and non-nitrosylated purified α and β subunits, Yonetani and coworkers found a right-shift of the O2 binding curve of α(Fe–NO)2β(Fe)2 tetramers in the presence of IHP, indicative of a T-quaternary state that the authors named low-affinity extreme, whereas less pronounced shifts were observed with DPG, consistent with the lower levels of pentacoordinate α-NO formed with this effector [15] (Fig. 2B). Our studies, carried out at variable NO–heme levels, confirmed that IHP induces a similar, albeit smaller, right-shift in the O2 binding curve (Fig. 3D) that is not seen in the presence of DPG (Fig. 3C). A major difference between our study and that of Yonetani and coworkers is that α-NO–hemes were not exclusively formed in our experiments, where heme nitrosylation was achieved by equilibration of deoxy Hb and NO gas with consequent formation of other possible structures, including hybrid α-NO/β-met tetramers that formed when samples were subsequently exposed to air (Fig. 5). Nevertheless, both studies support the conclusion that because of the unique mode of interaction between Hb and NO, “Hb will not be poisoned in the presence of low concentrations of NO” [15].
In conclusion, when NO–heme geometry was predominantly hexacoordinate in the absence of anionic effectors, NO binding, like CO binding, shifted the R–T equilibrium toward the R state and subsequent O2 binding to vacant sites on the Hb tetramer occurred with elevated affinity. Under these conditions the slow NO dissociation prevents heme oxidation in air. Consistent with our results, other researchers have reported that levels of NO–heme remain stable in blood or purified Hb samples devoid of organic phosphates after air exposure, deoxygenation and subsequent reoxygenation, but decrease in red blood cells containing endogenous DPG [43]. In contrast, anions that more strongly favor the T-state (IHP in particular) were found to promote pentacoordinate α NO–heme geometry, increased heme oxidation and decreased O2 affinity. Increasing NO–heme levels in samples containing IHP resulted in small shifts in O2 equilibria toward the low O2 affinity T-state. Increases in oxidized (met) Hb accompanied rises in NO–heme, suggesting that part of the reason for the very small effect observed was the formation of inert hybrid α-NO/β-met tetramers. Most remarkably, O2 equilibria were essentially unchanged by variations from 0% to 30% NO–heme in samples containing DPG, the physiological effector of human red blood cells. DPG was found to promote formation of a favorable proportion of pentacoordinate and hexacoordinate NO–hemes, so that the protein is neither constrained in the T state nor shifted toward the R-state by increasing the percentage of NO ligation. In addition to the protective Hb-mediated redox reactions with NO that are summarized above, the ability of NO–heme to exist in varied geometries further explains why exposure to low levels of NO–heme do not poison O2 delivery to tissues in the normal human physiological condition where DPG is present as an allosteric effector.
Supplementary Material
Acknowledgment
The authors wish to thank Giulia Ferruzzi for the expert technical assistance. This work was supported by the Danish Council for Independent Research, Natural Sciences (grant 10-084565 to AF), the National Institute of Health (HL61411 and NS06732 to JP and LLP), the National Science Foundation Shared Instrument Award (CHE1126268 to MPH) and the National Science Foundation Grant CHE 0809466 (to ALC). We thank Duke University for the support provided to ALC and CB.
Abbreviations
- NO
nitric oxide
- Hb
hemoglobin
- EDRF
endothelium-derived relaxing factor
- EPR
electron paramagnetic resonance
- DPG
2,3-diphosphoglycerate
- IHP
inositol hexaphosphate
- SNO-Hb
S-nitrosated hemoglobin
Footnotes
This article is part of a Special Issue entitled: Oxygen Binding and Sensing Proteins.
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.bbapap.2013.04.017.
References
- 1.Arnold WP, Mittal CK, Katsuki S, Murad F. Nitric oxide activates guanylate cyclase and increases guanosine 3′:5′-cyclic monophosphate levels in various tissue preparations. Proc. Natl. Acad. Sci. U. S. A. 1977;74:3203–3207. doi: 10.1073/pnas.74.8.3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 3.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc. Natl. Acad. Sci. U. S. A. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palmer RM, Ferrige AG, Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987;327:524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- 5.Garthwaite J, Charles SL, Chess-Williams R. Endothelium-derived relaxing factor release on activation of NMDA receptors suggests role as intercellular messenger in the brain. Nature. 1988;336:385–388. doi: 10.1038/336385a0. [DOI] [PubMed] [Google Scholar]
- 6.Hibbs JB, Jr, Taintor RR, Vavrin Z. Macrophage cytotoxicity: role for l-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987;235:473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- 7.Iyengar R, Stuehr DJ, Marletta MA. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc. Natl. Acad. Sci. U. S. A. 1987;84:6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moncada S, Palmer RMJ, Higgs EA. Biosynthesis of nitric oxide from l-arginine: a pathway for the regulation of cell function and communication. Biochem. Pharmacol. 1989;38:1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- 9.Cannon RO, III, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, Shelhamer JH, Gladwin MT. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J. Clin. Invest. 2001;108:279–287. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ichinose F, Roberts JD, Zapol WM. Inhaled nitric oxide: a selective pulmonary vasodilator: current uses and therapeutic potential. Circulation. 2004;109:3106–3111. doi: 10.1161/01.CIR.0000134595.80170.62. [DOI] [PubMed] [Google Scholar]
- 11.Forrester MT, Foster MW. Protection from nitrosative stress: a central role for microbial flavohemoglobin. Free Radical Biol. Med. 2012;52:1620–1633. doi: 10.1016/j.freeradbiomed.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 12.Hausladen A, Stamler JS. Is the flavohemoglobin a nitric oxide dioxygenase? Free Radical Biol. Med. 2012;53:1209–1210. doi: 10.1016/j.freeradbiomed.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 13.Doyle MP, Hoekstra JW. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 1981;14:351–358. doi: 10.1016/s0162-0134(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 14.Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr, Olson JS. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry. 1996;35:6976–6983. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 15.Yonetani T, Tsuneshige A, Zhou Y, Chen X. Electron paramagnetic resonance and oxygen binding studies of α-nitrosyl hemoglobin. A novel oxygen carrier having NO-assisted allosteric functions. J. Biol. Chem. 1998;273:20323–20333. doi: 10.1074/jbc.273.32.20323. [DOI] [PubMed] [Google Scholar]
- 16.Cosby K, Partovi KS, Crawford JH, Patel RP, Reiter CD, Martyr S, Yang BK, Waclawiw MA, Zalos G, Xu X, Huang KT, Shields H, Kim-Shapiro DB, Schechter AN, Cannon RO, Gladwin MT. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 2003;9:1498–1505. doi: 10.1038/nm954. [DOI] [PubMed] [Google Scholar]
- 17.Bryan NS, Rassaf T, Rodriguez J, Feelisch M. Bound NO in human red blood cells: fact or artifact? Nitric Oxide. 2004;10:221–228. doi: 10.1016/j.niox.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Dejam A, Hunter CJ, Pelletier MM, Hsu LL, Machado RF, Shiva S, Power GG, Kelm M, Gladwin MT, Schechter AN. Erythrocytes are the major intravascular storage sites of nitrite in human blood. Blood. 2005;106:734–739. doi: 10.1182/blood-2005-02-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cassoly R, Gibson Q. Conformation, co-operativity and ligand binding in human hemoglobin. J. Mol. Biol. 1975;91:301–313. doi: 10.1016/0022-2836(75)90382-4. [DOI] [PubMed] [Google Scholar]
- 20.Hobbs AJ, Gladwin MT, Patel RP, Williams DL, Butler AR. Haemoglobin: NO transporter, NO inactivator or NOne of the above? Trends Pharmacol. Sci. 2002;23:406–411. doi: 10.1016/s0165-6147(02)02067-9. [DOI] [PubMed] [Google Scholar]
- 21.Bonaventura C, Henkens R, Alayash AI, Banerjee S, Crumbliss AL. Molecular controls of the oxygenation and redox reactions of hemoglobin. Antioxid. Redox Signal. 2013;18:2298–2313. doi: 10.1089/ars.2012.4947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia L, Bonaventura C, Bonaventura J, Stamler JS. S-nitrosohaemoglobin: a dynamic activity of blood involved in vascular control. Nature. 1996;380:221–226. doi: 10.1038/380221a0. [DOI] [PubMed] [Google Scholar]
- 23.Stamler JS, Jia L, Eu JP, McMahon TJ, Demchenko IT, Bonaventura J, Gernert K, Piantadosi CA. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science. 1997;276:2034–2037. doi: 10.1126/science.276.5321.2034. [DOI] [PubMed] [Google Scholar]
- 24.McMahon TJ, Moon RE, Luschinger BP, Carraway MS, Stone AE, Stolp BW, Gow AJ, Pawloski JR, Watke P, Singel DJ, Piantadosi CA, Stamler JS. Nitric oxide in the human respiratory cycle. Nat. Med. 2002;8:711–717. doi: 10.1038/nm718. [DOI] [PubMed] [Google Scholar]
- 25.Crawford JH, Isbell TS, Huang Z, Shiva S, Chacko BK, Schechter AN, Darley-Usmar VM, Kerby JD, Lang JD, Jr, Kraus D, Ho C, Gladwin MT, Patel RP. Hypoxia, red blood cells, and nitrite regulate NO-dependent hypoxic vasodilation. Blood. 2006;107:566–574. doi: 10.1182/blood-2005-07-2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fago A, Crumbliss AL, Peterson J, Pearce LL, Bonaventura C. The case of the missing NO-hemoglobin: spectral changes suggestive of heme redox reactions reflect changes in NO-heme geometry. Proc. Natl. Acad. Sci. U. S. A. 2003;100:12087–12092. doi: 10.1073/pnas.2032603100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bonaventura C, Fago A, Henkens R, Crumbliss AL. Critical redox and allosteric aspects of nitric oxide interactions with hemoglobin. Antioxid. Redox Signal. 2004;6:979–991. doi: 10.1089/ars.2004.6.979. [DOI] [PubMed] [Google Scholar]
- 28.Bonaventura C, Ferruzzi G, Tesh S, Stevens RD. Effects of S-nitrosation on oxygen binding by normal and sickle cell hemoglobin. J. Biol. Chem. 1999;274:24742–24748. doi: 10.1074/jbc.274.35.24742. [DOI] [PubMed] [Google Scholar]
- 29.Hayashi A, Suzuki T, Shin M. An enzymic reduction system for metmyoglobin and methemoglobin, and its application to functional studies of oxygen carriers. Biochim. Biophys. Acta. 1973;310:309–316. doi: 10.1016/0005-2795(73)90110-4. [DOI] [PubMed] [Google Scholar]
- 30.Riggs AF, Wolbach RA. Sulfhydryl groups and the structure of hemoglobin. J. Gen. Physiol. 1956;39:585–605. doi: 10.1085/jgp.39.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hille R, Olson JS, Palmer G. Spectral transitions of nitrosyl hemes during ligand binding to hemoglobin. J. Biol. Chem. 1979;254:12110–12120. [PubMed] [Google Scholar]
- 32.Herold S, Rock G. Mechanistic studies of the oxygen-mediated oxidation of nitrosylhemoglobin. Biochemistry. 2005;44:6223–6231. doi: 10.1021/bi0475929. [DOI] [PubMed] [Google Scholar]
- 33.Azizi F, Kielbasa JE, Adeyiga AM, Maree RD, Frazier M, Yakubu M, Shields H, King SB, Kim-Shapiro DB. Rates of nitric oxide dissociation from hemoglobin. Free Radical Biol. Med. 2005;39:145–151. doi: 10.1016/j.freeradbiomed.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 34.Taketa F, Antholine WE, Chen JY. Chain nonequivalence in binding of nitric oxide to hemoglobin. J. Biol. Chem. 1978;253:5448–5451. [PubMed] [Google Scholar]
- 35.Gow AJ, Stamler JS. Reactions between nitric oxide and haemoglobin under physiological conditions. Nature. 1998;391:169–173. doi: 10.1038/34402. [DOI] [PubMed] [Google Scholar]
- 36.Herold S, Rock G. Reactions of deoxy-, oxy-, and methemoglobin with nitrogen monoxide. Mechanistic studies of the S-nitrosothiol formation under different mixing conditions. J. Biol. Chem. 2003;278:6623–6634. doi: 10.1074/jbc.M210275200. [DOI] [PubMed] [Google Scholar]
- 37.Gladwin MT, Wang X, Reiter CD, Yang BK, Vivas EX, Bonaventura C, Schechter AN. S-Nitrosohemoglobin is unstable in the reductive erythrocyte environment and lacks O2/NO-linked allosteric function. J. Biol. Chem. 2002;277:27818–27828. doi: 10.1074/jbc.M203236200. [DOI] [PubMed] [Google Scholar]
- 38.Antonini E, Brunori M. Hemoglobin and Myoglobin in their Reactionswith Ligands. Amsterdam: North-Holland Publishing Company; 1971. [Google Scholar]
- 39.Tsai AL, Berka V, Martin E, Olson JS. A “sliding scale rule” for selectivity among NO, CO and O2 by heme protein sensors. Biochemistry. 2012;51:172–186. doi: 10.1021/bi2015629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Monod J, Wyman J, Changeux JP. On the nature of allosteric transitions: a plausible model. J. Mol. Biol. 1965;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- 41.Perutz MF, Wilkinson AJ, Paoli M, Dodson GG. The sterochemical mechanism of the cooperative effects in hemoglobin revisited. Annu. Rev. Biophys. Biomol. Struct. 1998;27:1–34. doi: 10.1146/annurev.biophys.27.1.1. [DOI] [PubMed] [Google Scholar]
- 42.Roughton FJW. Transport of oxygen and carbon dioxide. In: Fenn WO, Rahn H, editors. Respiration, Handbook of Physiology, Am. Phys. Soc. Washington, DC: 1964. pp. 767–825. [Google Scholar]
- 43.Xu X, Cho M, Spencer NY, Patel N, Huang Z, Shields H, King SB, Gladwin MT, Hogg N, Kim-Shapiro DB. Measurements of nitric oxide on the heme iron and β-93 thiol of human hemoglobin during cycles of oxygenation and deoxygenation. Proc. Natl. Acad. Sci. U. S. A. 2003;100:11303–11308. doi: 10.1073/pnas.2033883100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.