Abstract
All organisms must sense and respond to their external environments, and this signal transduction is often done with second messengers such as cyclic nucleotides. Adenosine 3'5'-cyclic AMP is a universal second messenger that is used by diverse forms of life, including mammals, fungi, protozoa and bacteria. In this review, we discuss the many roles of cAMP in bacterial, fungal and protozoan pathogens and its contributions to microbial pathogenesis. These include coordination of intracellular processes such as virulence gene expression with extracellular signals from the host environment, and manipulation of host immunity by increasing cAMP levels in host cells during infection.
All organisms must respond to changing environments, and the signal transduction pathways that allow them to do so on a cellular level are essential for the control of processes ranging from chemotaxis to differentiation and apoptosis1–4. This signaling is mediated by a vast array of small soluble signaling molecules, both within and outside of cells5,6. Some of these molecules, such as the hormone-like acyl homoserine lactone (AHL) autoinducers that control quorum sensing in gram negative bacteria, diffuse across cell membranes from the extracellular millieu to directly control gene expression5. However, many environmental signaling pathways rely on 'second messenger' molecules to relay external signals from membrane receptors to one or more effectors within the cell. Second messengers include such diverse molecules as cyclic nucleotides, (p)ppGpp, Ca2+, inositoltriphosphate and diacylglycerol7,8–9–12.
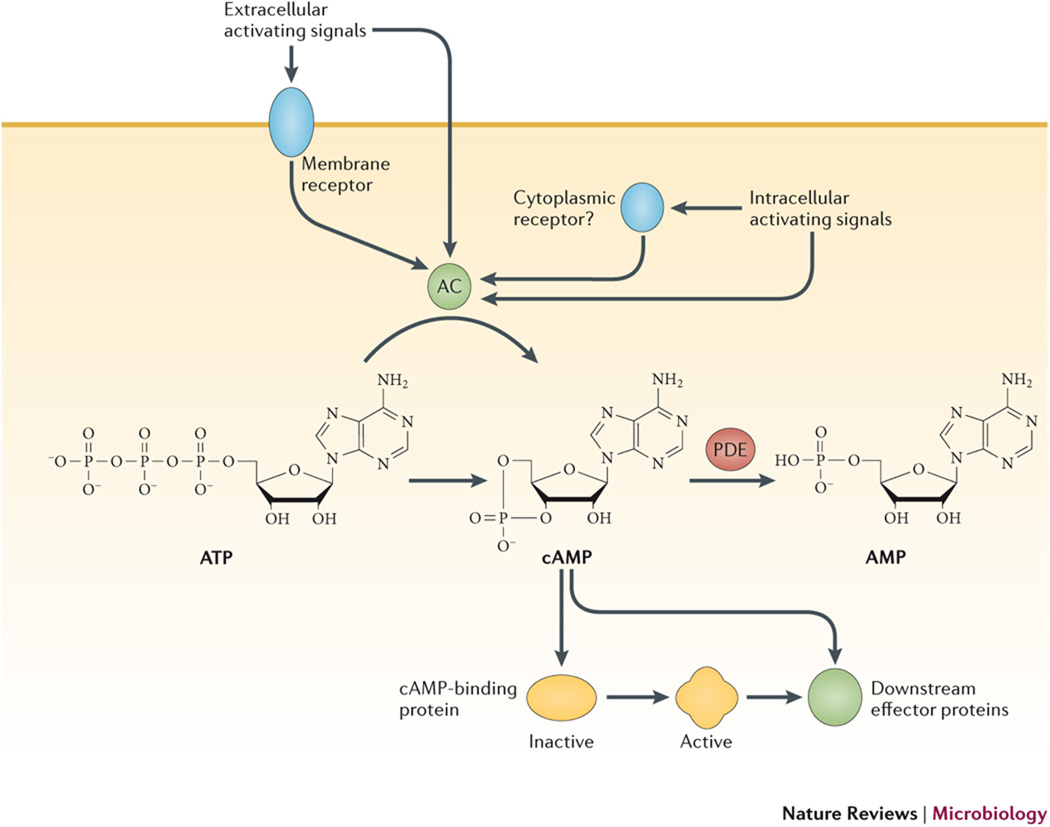
The cyclic nucleotide adenosine 3'-5' cyclic monophosphate (cAMP) was the first second messenger to be described, and it is also the most broadly used by organisms. cAMP is generated from ATP by adenylyl cyclases (ACs), while phosphodiesterases (PDEs) catalyze its hydrolytic degradation (Figure 1). ACs are divided into six classes based on primary amino acid sequences. Class III is the largest and most diverse group of cyclases, and it includes all known ACs from eukaryotes, as well as many bacterial ACs7,13,14,15. However, the well-studied bacterial AC from Escherichia coli belongs to Class I. Downstream regulatory effects of cAMP are mediated through its allosteric interactions with cAMP-binding proteins, whose activation states are altered by conformational changes that are induced upon cAMP binding.
Fig. 1. cAMP relays environmental signals to regulatory outcomes.
Adenylyl cyclases (ACs) can be directly or indirectly activated at the post-translational level by a number of environmental signals. Indirect activation pathways often involve membrane receptors that transmit extracellular signals to the AC by a phosphorylation event, although other cytoplasmic factors (“Factor X” in the figure) may also be required. cAMP then relays the signal to downstream effector proteins (DEP) either directly or indirectly by allosterically activating one or more cAMP-binding proteins. These cAMP-binding proteins may themselves be effector proteins, such as transcription factors or cyclic nucleotide gated channel (CNG) proteins, or they may be intermediaries, such as the regulatory subunits of PKA, which control activation of effector proteins further downstream. The inset shows the synthesis of cAMP by ACs, which catalyze conversion of ATP into cAMP and inorganic pyrophosphate while linking the remaining 5' phosphate with the 3' ribose carbon. cAMP is degraded by phosphodiesterases (PDEs) that catalyze the hydrolysis of the 3' linkage (marked with arrowhead), leaving adenosine 5' phosphate (not shown).
Regulation of gene expression is a major outcome of cAMP signaling, but the mechanisms differ between bacteria and eukaryotic cells16,17. Transcription factors of the cAMP-receptor protein (CRP) family in bacteria are activated by direct binding of cAMP, while cAMP-mediated activation of the transcription factors in eukaryotic cells often requires protein kinase A (PKA) complex as an intermediate. In this case, cAMP binding to regulatory subunits in the PKA complex liberates catalytically active kinase subunits, which activate downstream transcription factors by phosphorylation.
cAMP was first discovered for its role in hormone signal transduction in eukaryotic cells18. Biological processes now known to be controlled by cAMP signaling in eukaryotes range from metabolism to memory formation and innate immunity16,19. Later, cAMP was also discovered in bacteria, and its role in mediating the 'glucose response', or catabolite repression, was extensively studied in E. coli over several decades17,20. During catabolite repression, the presence of glucose reduces cAMP production, which is needed for activation of the lac operon (which codes for proteins that allow lactose to be used as a secondary carbon source) through binding of the cAMP-Crp complex21.
However, increasing recognition of the roles of cAMP in microbial virulence, ranging from potent toxin to master regulator of virulence gene expression, has generated new interest in this second messenger. The near universal use of cAMP signaling in life forms as diverse as bacteria, archaea, fungi, eukaryotic parasites and mammals provides unique opportunities for cAMP-mediated modulation of host-pathogen interactions. However, many of these interactions are only just being discovered. The essential roles of cAMP in eukaryotic signal transduction and bacterial carbon catabolite repression have been covered previously14–17,21. In this review, we focus on the multiple roles of cAMP in pathogen biology, with an emphasis on the importance of cAMP to virulence gene regulation, host-pathogen interactions and pathogen responses to their host environments.
Role of cAMP signaling in pathogenic bacteria
Host-dependent AC toxins, secreted by several pathogens into host cells during infection, provided the first examples of the role of cAMP in bacterial virulence14,22. More recently, it has become evident that cAMP is involved in processes other than catabolite repression and AC toxin action, as disruption of intracellular cAMP signaling attenuates virulence in numerous bacterial pathogens23–27. cAMP has central roles in regulating biofilm formation, type III secretion, carbon metabolism and virulence gene regulation in many pathogens. Several new strategies by which pathogens modulate cAMP levels within their host cells have also recently been discovered28,29.
Glucose depletion induces AC activity in some, but not all, bacteria, and the use of CRP-family transcription factors as a primary means for relaying the message is common. Much of the cAMP signaling diversity within bacteria occurs at the level of the cAMP-associated regulatory targets, which vary between organisms. The effects of this cAMP signaling are amplified in some cases by cAMP-mediated co-regulation of other global regulators. For example, expression of the stress-associated global regulator RpoS is controlled by cAMP-Crp in Vibrio vulnificus, E. coli and Salmonella enterica30–32. In the case of V. vulnificus, transcriptional repression of rpoS occurs by direct binding of cAMP-Crp complex to each of two binding sites in the rpoS promoter30. Similarly, cAMP levels control biofilm production in Vibrio cholerae, partly through regulation of a diguanylate cyclase responsible for production of the second messenger cyclic di-GMP33.
cAMP-CRP also appears to be adept at integrating horizontally acquired plasmid and island-based genes into existing regulatory networks, possibly due to its broad use across many unrelated species. For example, in Yersinia pestis, Crp directly activates expression of pla, a plasmid-encoded virulence gene that facilitates infection following either flea bite or aerosol transmission34. Plasticity is evident in this adaptive process, as cAMP-Crp positively regulates protein secretion from each of three type III secretion systems (T3SSs) in Yersinia. enterocolitica24, but directly down-regulates transcription of an orthologous T3SS, Ysc, in Y. pestis35. Similarly, the horizontally-acquired tcpA-F genes, which encode proteins needed for biogenesis of a virulence-associated toxin-coregulated pilus (TCP), are regulated by Crp in V. cholerae36.
The following three cases provide examples of both the extent and significant diversity of cAMP-associated gene regulation in bacterial pathogens.
Pseudomonas aeruginosa
cAMP has an integral and multifaceted role in P. aeruginosa pathogenesis, as this bacterium produces three ACs: CyaA, CyaB and ExoY. ExoY is a host-activated AC toxin that is exported with several other proteins into host cells through a cAMP-regulated T3SS37,38, and is discussed in a later section. However, CyaA and CyaB remain within the P. aeruginosa cytosol, where they increase intracellular cAMP levels to control virulence gene expression (Figure 2). Low Ca2+ concentration is a signal that leads to cAMP production and expression of all known T3SS components39. CyaB, which produces the bulk of intracellular cAMP in P. aeruginosa, may itself be a membrane sensor protein that detects low Ca2+ concentrations and/or contact with host cells39. Deletion of cyaB attenuates virulence26,40 owing to a reduced expression of T3SS components, as virulence can be recovered by overexpression of a regulatory factor, ExsA, that specifically restores expression of T3SS components26.
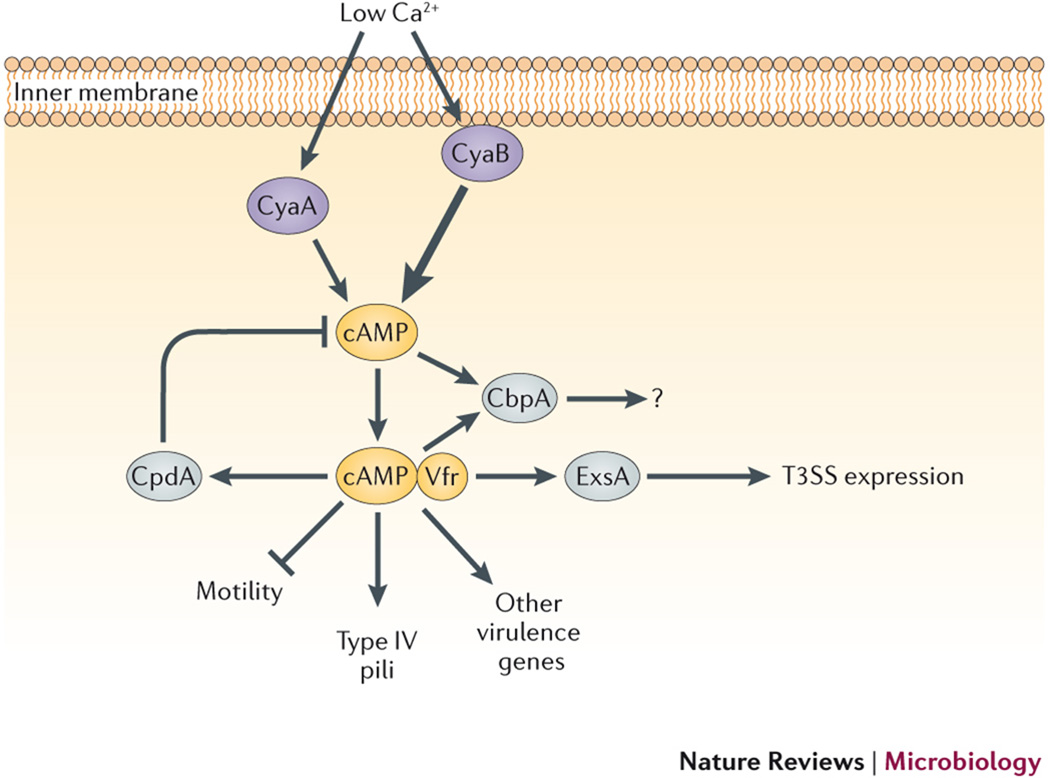
Fig. 2. Effects of cAMP signaling on gene regulation in Pseudomonas aeruginosa.
Two ACs in P. aeruginosa, CyaA and CyaB, are induced by low Ca2+ concentrations, although CyaB is the dominant producer of cAMP, as indicated by the thicker arrow. cAMP binding to the CRP family transcription factor, Vfr (virulence factor regulator), regulates expression of a broad range of phenotypes. Intermediate regulatory factors are noted, where known, including LasI (LuxI-type acyl homoserine lactone (AHL) synthase) and LasR (regulator of the Las quorum sensing system), as well as the transcription regulator ExsA (exoenzyme secretion protein A). Arrows indicate positive regulation, while blunted lines indicate repression.
Transcriptional upregulation of T3SS genes is mediated by the cAMP-Vfr complex in P. aeruginosa. Vfr is a CRP-family transcription factor that is similar to, but not functionally interchangeable with, E. coli Crp41. While vfr complements an inactivating crp mutation in E. coli, E. coli crp cannot substitute for vfr in P. aeruginosa42. Vfr controls expression of many virulence-associated genes in P. aeruginosa, including those encoding exotoxin A, type IV pili, a T3SS, and the las quorum-sensing system43. All of these genes, except the las genes, are regulated by Vfr in a cAMP-dependent fashion. Surprisingly, expression of the las genes requires Vfr, but las regulation is not affected by intracellular cAMP levels43. Rather, Vfr appears to require a cAMP-independent cofactor for las induction, raising the possibility that Vfr functions as a dual regulator that is capable of controlling different gene sets in response to various environmental conditions. The cAMP-Vfr complex also upregulates expression of a 3',5'-cAMP PDE, CpdA44, as well as a second cAMP-binding protein, CbpA, in P. aeruginosa45. CpdA degrades cAMP as a counterbalance to cAMP synthesis by the ACs, and is required for proper expression of virulence factors44. In contrast, the functions of CbpA are unknown, although its cAMP-dependent polar localization in the cell is intriguing and suggests that additional roles for cAMP in P. aeruginosa may be awaiting discovery.
Central role for cAMP signaling in Vibrio cholerae
Cholera toxin (CT) disrupts cAMP signaling in host intestinal epithelial cells during infection, causing the watery diarrhea that characterizes cholera disease (discussed below). However, the extensive role of cAMP signaling within the cells of V. cholerae, throughout the entire infection cycle, is much less appreciated. Recent studies show that cAMP signaling within V. cholerae integrates carbon source availability with population density, biofilm formation, bacteriophage sensitivity and virulence gene expression (Figure 3).
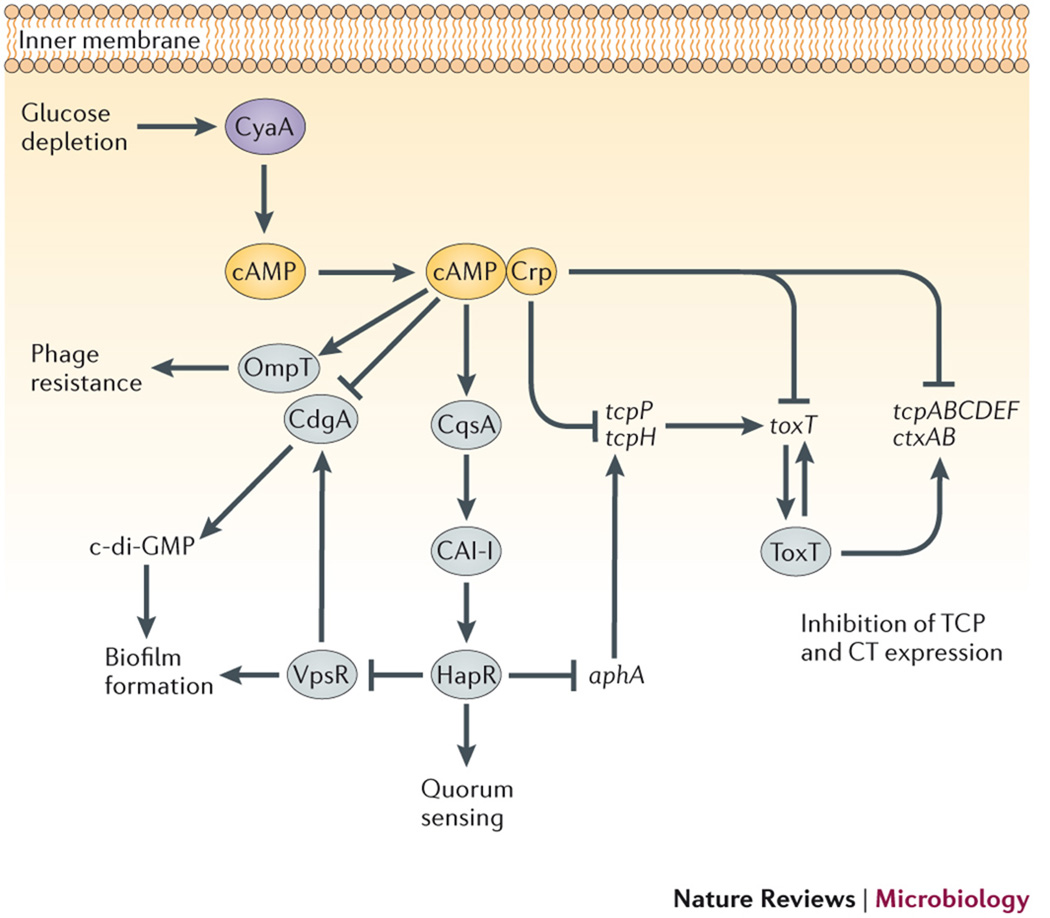
Fig. 3. Effects of cAMP signaling on gene regulation in Vibrio cholerae.
cAMP signaling integrates carbon source information with a wide range of pathogenesis-associated processes in V. cholerae, through its effects on gene regulation. Biofilm formation, quorum sensing, virulence gene expression and phage sensitivity are all regulated either directly or indirectly by cAMP-CRP. cAMP-CRP regulates several individual processes by multiple pathways, emphasizing the likely importance of cAMP signaling throughout the infection cycle. Gene names: ompT, outer membrane protein T; cqsA, CAI-1 synthase ; vpsR, Vibrio polysaccharide regulator; ahpAB, transcriptional regulators; toxT, toxin regulator T; ctxAB, cholera toxin A and B (operon); tcpA-F, toxin-co-regulated pilus genes A to F (operon). Protein names: CyaA, adenylyl cyclase A; CrpVc, cAMP receptor protein; ToxR, toxin regulator R; CdgA, cyclic diguanylate cyclase A; HapR, hemagglutinin protease regulator; ToxT, toxin regulator T. Small molecules: di-cGMP, di-cyclic GMP; CAI-I, cholera autoinducer 1; HCO3−, bicarbonate ion.
Vibrio spp. have a single AC, CyaA, that mediates signaling through the CRP family cAMP-binding transcription factor, Crp46. Glucose limitation stimulates production of cAMP by CyaA47, and this cAMP binds with Crp to form the active transcription factor, cAMP-Crp. cAMP-Crp regulates quorum sensing, biofilm formation and expression of the cholera toxin (CT) and toxin co-regulated pilus (TCP) in V. cholerae by increasing levels of the master quorum sensing regulator, HapR47. HapR up-regulates quorum sensing, while indirectly down-regulating biofilm formation and expression of the ctxAB and tcpA-F genes, which encode CT and TCP proteins respectively48,49. HapR represses expression of the biofilm gene activator, VpsR50. Likewise, HapR reduces CT and TCP production by downregulating expression of AphA, a positive regulator of the coordinately expressed CT and TCP genes51.
HapR expression requires the presence of two autoinducers, CAI-1 (cholera autoinducer 1) and AI-2 (autoinducer 2), which are extracellular signaling molecules secreted by V. cholerae52. In this bacterium, cAMP-Crp promotes CAI-1 production by positively regulating expression of the gene encoding the CAI-1 synthase, CqsA. The key role of cAMP in linking carbon source to population density in V. cholerae was further confirmed by showing that the cell density needed to activate HapR was increased when the intracellular levels of cAMP were decreased by the addition of glucose to the culture53. Surprisingly, the regulation of CqsA expression by cAMP-Crp seems to act at the level of mRNA stability, although the mechanism for this CRP-mediated post-transcriptional regulation has not been determined53.
In addition, cAMP modulates V. cholerae pathogenesis through HapR-independent mechanisms, which may be important in El Tor strains that lack functional HapR due to a frameshift mutation54. cAMP-Crp downregulates expression of CdgA, a cyclic diguanylate cyclase that promotes V. cholerae biofilm formation through its production of c-di-GMP33. Moreover, cAMP-Crp participates with transcription factor ToxT in forming a bistable regulatory switch that controls CT and TCP expression during infection36. This bistable switch may allow a subpopulation of V. cholerae cells in rice water stool to remain in a hyper-infectious state by retaining their expression of CT and TCP for up to five hours outside the host. This strategy would allow increased transmission during epidemics without compromising the fitness of V. cholerae outside the host. The role of cAMP-Crp signaling in promoting resistance of V. cholerae O1 strains to environmental bacteriophages provides another means by which cAMP may contribute to V. cholerae fitness outside the host55. Resistance occurs at the level of phage adsorption, and may be due to loss or masking of a lipid or protein-based phage receptor on the bacterial cell surface. This possibility is consistent with the known role of cAMP-Crp in regulating expression of the outer membrane protein OmpT in V. cholerae. In this case, Crp competes with the ToxR repressor for binding to the ompT promoter, and leads to enhanced transcription of ompT56.
Multiple cyclases and effector proteins in M. tuberculosis
The ACs of mycobacteria and their effector proteins have been recently reviewed28,57, so they will be discussed only briefly here. Mycobacterium tuberculosis has 16 AC-like proteins, 10 of which have confirmed AC activity58,59 (Figure 4). All are Class III cyclases, but the group includes soluble, membrane bound and integral membrane enzymes. Many M. tuberculosis ACs contain functional domains other than the AC catalytic core region, including receptor, inhibitory, DNA-binding and HAMP (histidine kinases, ACs, methyl binding proteins and phosphatases) domains58,60–62. These accessory domains probably regulate the AC activity, and may in some cases add additional functions to the enzyme. Glucose, except when added at very high concentrations, has little effect on M. tuberculosis cAMP levels63,64. Instead, M. tuberculosis ACs are regulated at multiple levels by a range of conditions encountered within the host. For example, the activity of M. tuberculosis ACs is directly affected by pH, fatty acids, and carbon dioxide (CO2), whereas hypoxia and starvation affect the expression of AC-encoding genes28. In particular, macrophage passage stimulates AC activity within M. tuberculosis, raising cAMP levels as much as 50 fold in two hours, as compared to incubation in tissue culture media alone63. Viable mycobacteria also secrete cAMP into their host macrophages during infection, as discussed below. One AC, Rv0386, has been specifically linked to production and secretion of cAMP within macrophages, and deletion of the corresponding gene decreases M. tuberculosis virulence and pathology in a murine infection model65.
Fig. 4. Effects of cAMP signaling on gene regulation in Mycobacterium tuberculosis.
cAMP signaling in M. tuberculosis is complex owing to its large number of adenylyl cyclases (ACs). The 'Rv' numbers listed in top section without boxes refer to the names of ten individual M. tuberculosis ACs that have demonstrated activity. Six additional putative ACs are not shown. Green boxes (top) include signals that activate the specific ACs indicated by arrows. Crp, Cmr and Rv0998 are cAMP-dependent effector proteins, which are activated by association with cAMP. However, a direct binding of cAMP to Cmr has not been demonstrated. CrpMt and Cmr are transcription factors, while Rv0998 is a protein acetylase. The other 'Rv' numbers in yellow boxes (middle section) correspond to seven other putative cAMP binding proteins. Blue boxes indicate the respective functions of specific cAMP binding proteins. Secretion of cAMP into macrophages is indicated by an arrow that connects cAMP (middle) to a blue box. Rv0805 is a phosphodiesterase that has low activity on cAMP. Arrows indicate positive activation, while the blunt ended line indicates degradation.
In addition to its complex array of ACs, M. tuberculosis encodes 10 putative cAMP binding proteins28,57,58. These include two CRP family transcription factors, and a protein lysine acetylase25,66–68. The remaining seven putative cAMP binding proteins have yet to be characterized. One of the two transcription factors, Crp, controls a regulon of over 100 genes and its DNA binding activity is similar to that of E. coli Crp66. Deletion of crp attenuates M. tuberculosis virulence in a murine model25, and current efforts are aimed at determining which of its regulon members specifically contribute to virulence. The second transcription factor, Cmr, controls expression of a different set of genes in response to cAMP levels and macrophage passage67. The biological role of the cAMP-responsive acetylase is not known.
Bacterial manipulation of host cAMP levels
Disruption of signal transduction within host cells is an effective virulence strategy used by many pathogens, and the myriad ways in which cAMP levels affect immune function make cAMP signaling a frequent target within host cells19 (Figure 5). Elevated cAMP levels can suppress innate immune functions by modulating inflammatory mediator expression, dampening the phagocytic response and reducing intracellular killing of ingested pathogens19. Another powerful effect of elevated cAMP levels in host intestinal epithelial cells is the excessive fluid secretion that causes the watery diarrhea associated with cholera and some toxigenic E. coli infections69,70.
Fig. 5. Bacterial manipulation of host cAMP levels.
Bacterial pathogens use numerous strategies to elevate cAMP levels within host cells during infection. Shown are the ADP-ribosylating exotoxins that increase the activity of endogenous host ACs (top, AC Modulating Toxins); the exported host-activated bacterial ACs produced by Bordetella pertussis (CyaA), Bacillus anthracis (EF), Pseudomonas aeruginosa (ExoY) and Yersinia pestis (Yp AC), (middle, AC Toxins); the activation of host ACs through coordinate signaling of complement C5a receptor C5aR (pink) and Toll-like receptor TLR2 (orange) stimulated by Porphyromonas gingivalis; and the direct secretion of cAMP by Mycobacterium tuberculosis (bottom). Red triangles represent calmodulin, red squares represent an unknown host activation factor, a purple diamond is C5a and the yellow diamonds are cAMP. Gαs and Gαi are stimulatory and inhitory subunits of heterotrimeric G proteins, respectively.
The most studied microbial strategy for elevation of host cAMP levels is the production of certain toxins by bacteria40,69,70. These toxins can be ACs themselves, as is the case with the AC toxin of Bordetella pertussis, the edema factor (EF) toxin of Bacillus anthracis and ExoY of P. aeruginosa. Alternatively, toxins such as cholera toxin (CT) of V. cholerae, pertussis toxin (PT) of B. pertussis or labile toxin (LT) of E. coli can modulate the activity of the endogenous host ACs by altering the function of heterotrimeric G proteins that in turn regulate AC activity. Elevation of cAMP concentrations by bacterial pathogens also occurs by toxin-independent mechanisms, as discussed below. Some bacterial pathogens even use multiple simultaneous strategies to elevate host cAMP levels. For example, B. pertussis secretes two toxins (CyaA and PT), each of which increases cAMP synthesis in the host cell by a different mechanism. The end result of such a two-pronged approach with B. pertussis is reduced phagocytosis and decreased production of reactive oxygen intermediates by intoxicated macrophages, along with suppression of neutrophil recruitment to the lungs during infection71,72.
AC toxins
B. anthracis and B. pertussis both produce and export calmodulin-dependent AC toxins, which are active only in their host cells. Activation of these ACs requires binding with the calcium-binding protein calmodulin, a host protein69. P. aeruginosa also exports a host-dependent AC toxin, ExoY, that is activated by an unidentified host factor, different from calmodulin37. A secreted AC has also been identified in Y. pestis, although it has not been characterized73.
The AC toxins from B. pertussis (CyaA) and B. anthracis (EF) have numerous and varied effects on phagocytosis, apoptosis, and migration of leukocytes69,71,74–76. Despite mechanistic similarities, CyaA and EF show differences in protein structure, enzyme kinetics, localization in the host cell and interactions with calmodulin77,78. CyaA is a single polypeptide that inserts into the plasma membrane of target cells79. In contrast, EF is the toxic component of an A-B toxin (ET) that is taken up by receptor-mediated endocytosis and ultimately released into the cytosol, where it typically resides in a perinuclear localization80. CyaA and EF both specifically promote a Th1 to Th2 immunological switch at low levels of toxin, while all T cell differentiation is suppressed at high levels of toxin77. The Th1-to-Th2 shift occurs when the cAMP produced by the toxins activates a PKA pathway that increases production of Th2 cytokines such as IL10 and IL4. However, this same pathway also turns down expression of IL12, a key cytokine involved in development of the kind of Th1-type cell-mediated immune response that is required for clearance of many pathogens. Structural comparisons of EF with host ACs has lead to the identification of two inhibitors that specifically target EF and may be candidates for post-exposure treatment81.
Toxins that upregulate the activity of host ACs
A second approach for increasing host cell cAMP levels is to modify the alpha subunits of heterotrimeric G proteins that regulate the activity of host ACs19,40,70. V. cholerae CT, E. coli LT and B. pertussis PT are ADP ribosylating enzymes that target different regulatory G protein subunits. CT and LT target the stimulatory Gs alpha subunits, effectively locking them into an active mode that causes continuous production of cAMP by host ACs. In contrast, the ADP-ribosylation activity of PT inactivates the inhibitory Gi alpha subunits, preventing them from down-regulating AC activity. In all cases, the result is constitutive AC activity that leads to accumulation of cAMP within targeted cells.
Secretion of cAMP into host cells
M. tuberculosis secretes cAMP directly into host macrophages upon infection. cAMP levels within macrophages increase 2–3 fold upon infection with viable, but not heat-killed M. tuberculosis-complex bacteria28,63,65. Increased levels of cAMP within infected macrophages correlates with decreased phagolysosome fusion82, reduced pathology and attenuated virulence65. The increased cAMP was shown to arise from the bacteria rather than the host macrophages by using [14C]-radiolabeled M. tuberculosis and is largely due to synthesis by a specific AC, Rv038665. The mechanism for cAMP secretion is not known but it is regulated separately from cAMP production63. Albumin inhibits both the production and secretion of cAMP in M. tuberculosis-complex bacteria grown in vitro. Oleic acid restores cAMP production, but not secretion, in these bacteria, resulting in very high levels of cytoplasmic cAMP63. Identification of the secretory mechanism and its regulation is key to unraveling the specific roles of cAMP in tuberculosis infection.
Exploiting host signaling via complement-TLR crosstalk
Porphyromonas gingivalis provides an additional example of a means by which a pathogen can directly increase cAMP levels in host cells. P. gingivalis expresses a protease with C5 convertase-like activity, which cleaves the host complement protein C5 into its active C5a and C5b components. The resulting C5b is degraded to prevent stimulation of an inflammatory response. However, C5a binds to its receptor C5aR while intact P. gingivalis bacteria bind to a Toll-like receptor, TLR2, and/or the chemokine receptor CXCR4 on macrophages. Coordinate stimulation of these two receptors triggers cAMP production in the host cell, with subsequent PKA activation that leads to down-regulation of the iNos inducible nitric oxide killing pathway and a concomitant reduction in the concentration of antimicrobial nitric oxide29.
cAMP signaling in pathogenic fungi
Eukaryotes do not possess CRP-like transcription factors, and protein kinase A (PKA) is the primary cAMP-activated intermediary between ACs and downstream gene regulatory events. Mammalian cells carry two distinct types of ACs: transmembrane ACs (tmAC) are activated by heterotrimeric G proteins in response to signals received through G protein coupled receptors (GPCRs), while soluble ACs (sAC) are directly activated by CO2/HCO3−15. Fungal ACs combine features of both tmAC and sACs, as they are membrane-bound ACs that can be activated by both G proteins and CO2/HCO3−. In contrast to mammalian cells that contain many different ACs, each of which may be controlled by a specific signal, fungi carry only one extremely versatile AC that is capable of processing and integrating environmental signals from a variety of sources. This single AC is required for virulence of many pathogenic fungi, including the human pathogens Cryptococcus neoformans83, Candida albicans84 and Aspergillus fumigatus85, as well as the rice pathogen Magnaporthe oryzae86. The role of cAMP/PKA signaling in virulence has been best studied in C. neoformans and C. albicans83,87.
Cryptococcus neoformans
C. neoformans is a dimorphic basidiomycete that causes fungal meningoencephalitis, particularly in immunocompromised individuals88. Essential virulence determinants of C. neoformans include the antioxidant melanin and an antiphagocytic capsule83. The single AC present in C. neoformans is a membrane-bound protein, Cac1, that is dispensable for in vitro growth but essential for virulence89. Genetic analyses have shown that cAMP production by Cac1 is activated by the heterotrimeric G-protein Gα subunit Gpa1, following stimulation of the GPCR Gpr4 by amino acids90 (Figure 6). Transcriptome analyses have shown that the Ras and cAMP/PKA signaling pathways are largely independent from one another in C. neoformans, although both contribute to antifungal drug susceptibility91. Nonetheless, regulation of mating and hyphal differentiation requires the AC-associated protein Aca1, which is a positive regulator of Cac1, and this may involve input from the Ras signaling pathway.
Fig. 6. cAMP signaling pathways in fungal pathogens.
Both Cryptococcus neoformans (left) and Candida albicans (right) use their highly conserved cAMP/PKA pathways to regulate expression of key virulence determinants. However, the functions of these pathways differ. Matching shapes and colors are used to indicate orthologs in both fungi. Arrows denote positive regulatory effects while flat ended lines indicate inhibitory effects. Protein abbreviations for C. neoformans: Gpr4, G protein receptor; Gpa1, G protein G alpha subunit; Crg2, regulator of G protein signaling; Pkr1, PKA regulatory subunit; Cac1, adenylyl cyclase; Pka1, PKA catalytic subunit; Nrg1, transcription factor ; Can2, carbonic anhydrase; Ras1, guanosine nucleotide binding protein; Aca1, AC-associated protein; Pde1, phosphodiesterase. Protein abbreviations for C. albicans: Gpr1, G protein-coupled receptor; Gpa2, G protein alpha subunit; GAP, GTPase activator protein; Cyr1, adenylyl cyclase; Tpk1 and Tpk2, protein kinases; Nce103, carbonic anhydrase; Ras1, guanosine nucleotide binding protein; Cap1, cyclase associated protein; Pde2, phosphodiesterase; Bcy1, PKA regulatory subunit; Efg1 and Flo8, transcription factors.
In the absence of cAMP, PKA exists as an inactive tetrameric complex that includes two regulatory (Pkr1) and two catalytic (Pka1) subunits. cAMP binding to PKA causes Pkr1 subunits to release active Pka1 that then phosphorylates effector proteins such as the transcription factor Nrg1, which regulates downstream functions such as virulence gene expression92,93. Maintaining homeostasis of the cAMP/PKA system also requires ways to decrease cAMP levels. Such negative inputs include inhibition of Gpa1 by the GTPase activator protein (GAP) Crg2 to reduce cAMP production, and hydrolysis of cAMP by a PDE, Pde183.
Despite the absence of much information on the downstream targets of cAMP-PKA, the essentiality of this signaling pathway for virulence gene regulation in C. neoformans serotype A is clear. Deletion of any of the genes encoding components such as Gpa1, Cac1 and Pka1 of the regulatory cascade results in loss of melanin, capsule production and virulence83,94. Similarly, pkr1-deleted mutants of C. neoformans are hypervirulent89,95.
An alternative way to activate the cAMP/PKA pathway in C. neoformans is with CO2/HCO3−, which is consistent with the role of CO2 in stimulating capsule production96. Cac1 is directly activated by CO2/HCO3−, and this effect is independent of G-protein mediated regulation of Cac1 by Gpa197,98. CO2 exists in equilibrium with HCO3−, and the conversion of low CO2 concentrations into HCO3− is catalyzed by carbonic anhydrases (CAs). C. neoformans produces two CAs, Can1 and Can299. Only Can2 is required for growth in vitro, where CO2 levels are low (~0.033%), and this dependence on Can2 is relieved by high levels of CO2. Palmitate supplementation also rescues in vitro growth of can2-deleted mutants, suggesting a role for Can2 in fatty acid metabolism97,99. However, neither CA is required for virulence, presumably because of the high levels of CO2 (~5%) present within mammalian hosts99. Future studies are needed to address the specific roles of CO2 versus HCO3− in Cac1 activation, and whether the means by which the cAMP/PKA pathway is stimulated confers any specificity on the downstream effector functions that are affected.
Candida albicans
The cAMP/PKA signaling pathway is highly conserved in all fungi, but each system has its own signaling specificities and controls different functions (Figure 6). C. albicans is a commensal ascomycete that causes serious disease in immunocompromised individuals100. The yeast-to-filament transition that is crucial for C. albicans virulence is regulated by the cAMP/PKA pathway, although using G-protein pathways that are different from those used by C. neoformans.
In contrast to C. neoformans, the single AC (Cyr1, previously called Cdc35) of C. albicans is directly activated by the G-protein Ras1 in response to glucose, serum and N-acetylglucosamine, leading to hyphal growth84. This direct activation takes place by Ras1-GTP binding to a Ras-association (RA) domain of Cyr1, and therefore differs from what has been observed in C. neoformans, in which the cAMP/PKA pathway appears to be largely independent of Ras signaling91,101. Two catalytic subunits of PKA, Tpk1 and Tpk2, regulate downstream events in response to cAMP production in C. albicans. Tpk2 is the primary kinase responsible for hyphal growth and virulence, while Tpk1 controls stress responses102,103. Following activation by Tpk2, the Efg1 and Flo8 transcription factors are responsible for virulence gene regulation104. The AC-cAMP signaling pathway in C. albicans can also be activated in vitro by amino acids through the heterotrimeric GPCR Gpr1 and its associated G-protein, Gpa2105. However, the mechanism by which this activation pathway causes hyphal growth in C. albicans has not been described.
In contrast to their differential use of G-protein activation pathways, AC activation by CO2/HCO3− and the importance of CAs at low CO2 concentrations is similar in C. albicans and C. neoformans98,99, as demonstrated by the successful complementation of the hyphal growth deficiency of a C. albicans cyr1-deleted mutant by expressing Cac1 from C. neoformans97. The activation of Cyr1 by CO2/HCO3− is independent of G-protein signaling and occurs by direct binding to the catalytic domain of Cyr1.
CO2 induces the development of pseudohyphae and the invasion of underlying agar in C. albicans but not in non-pathogenic Candida spp., making it a specific biomarker for pathogenicity98. CO2 generated by C. albicans cells can accumulate to levels sufficient to induce invasive hyphal growth, providing numerous ways in which CO2 can contribute to pathogenesis87. While hyphal growth is essential for virulence, the ability of C. albicans to switch between yeast and hyphal forms is also critical for pathogenesis, as mutations that lock cells in either yeast or filamentous forms leads to attenuation106. Similarly, set3-deleted mutants, in which loss of the Set3C histone deacetylase complex causes hypersensitivity to filamentation signals, are avirulent107. The multiple ways in which disruption of the cAMP/PKA signaling pathway attenuates virulence makes it a promising target for antifungal drug development.
Serum is one of the most potent stimulators of hyphal growth in C. albicans, and it was recently shown that muramyl dipeptides (MDP) derived from the breakdown of cell wall peptidoglycan from co-infecting or commensal bacteria are critical for this activation108. MDPs stimulate cAMP production by directly binding leucine-repeat regions (LRR) within Cyr1. Ras1 contributes to Cyr1 activation in response to serum, but full activation of hyphal growth via this pathway also involves the sensing of G-actin pools, as extensive actin cytoskeleton remodeling is required for hyphae development. Cyr1 does not directly bind G-actin, but is able to integrate the serum, MDP and actin signals as part of a tripartite complex with G-actin and the cyclase-associated protein Cap187,109. Cap1 binds both Cyr1 and G-actin, serving as a bridge between them. Discovery of MDPs from commensal and/or co-infecting bacteria as direct enhancers of C. albicans pathogenesis is a reminder of the complexity of the microbial interactions within the human host. It also has important implications for treatment of both bacterial and fungal infections, as antibiotic therapies that increase bacterial cell wall fragmentation may specifically trigger or exacerbate a fungal infection.
cAMP signaling in parasitic protozoa
Parasites also need accurate signaling mechanisms to sense and respond to new conditions as they move between mammalian hosts, insect vectors and the environment. Frequently, a membrane receptor senses the environment and activates signaling pathways to trigger an immediate response, such as motility or exocytosis, but also a delayed response, such as cell division or differentiation into the next developmental stage. cAMP is emerging as a central signaling mediator that regulates essential processes in parasites. Evidence for an evolutionarily conserved cAMP signaling pathway is abundant in protozoa of the genera Plasmodium, Leishmania, Trypanosoma, Toxoplasma and Entamoeba, as well as in the parasitic worm Schistosoma, which together cause the majority of human mortality and morbidity worldwide.
Modulation of cAMP levels in the host cell
Intracellular parasites frequently induce signaling cascades in the host cell that facilitate invasion. An example of this behavior is Trypanosoma cruzi, which stimulates Ca2+ and cAMP signaling in the host cell to induce the recruitment and fusion of host cell lysosomes that the parasite requires for invasion110. Another parasite strategy for survival is the evasion of the host immune system. In this case, cAMP has been implicated in Plasmodium evasion of Kupffer cells, where parasites induce an increase in cAMP that mediates the inhibition of the microbicidal respiratory burst and as a consequence, results in the survival of the parasite111.
Host cell invasion by the malaria parasite
Plasmodium spp., the causative agents of malaria, start infection in the hepatocytes of the host, but continue by invading and replicating in erythrocytes. In both stages, liver and blood, Plasmodium uses cAMP pathways to regulate processes required to establish a successful infection. During the liver stage, activation of an AC, ACα, in Plasmodium sporozoites increases intracellular cAMP and induces exocytosis that results in the exposure of proteins required for hepatocyte invasion112 (Figure 7). The ACα protein of Plasmodium contains a domain with homology to K+ channels, which is thought to be functionally linked to the AC activity. Additionally, motility induced by albumin in sporozoites is regulated by cAMP113.
Fig. 7. Model for cAMP signaling in Plasmodium sporozoites leading to exocytosis.
ACα contains a domain with a putative K+ channel in Plasmodium. Extracellular K+ influx (1) activates AC activity, resulting in the increase of cAMP (2). Elevation of cAMP levels activates PKA (3) and leads to exocytosis of apical granules (micronemes) (4) that carry transmembrane proteins, which are necessary for invasion of host hepatocytes by Plasmodium sporozoites.
In the erythrocytic stage, cAMP signaling pathways have been implicated in intra-erythrocyte survival, regulation of the parasite cell cycle and invasion of host cells. At this stage, Plasmodium replicates in a synchronous way, which is responsible for the cyclical fevers that characterize malaria. The mechanism underlying this phenomenon is a melatonin-activated cAMP-signaling cascade in the parasite, which regulates cell cycle, resulting in the synchronized replication114,115. The permeability of the host erythrocyte membrane, which allows intake of nutrients required for parasite survival and replication, is also regulated by cAMP. A cAMP-dependent PKA regulates anion channel conductance in the infected erythrocyte membrane, a process that controls parasite growth116. Invasion of host erythrocytes is also regulated by PKA, which phosphorylates apical membrane antigen 1 (AMA1), a protein present on the parasite surface that mediates erythrocyte invasion117. Besides the role in host cell invasion, cAMP is also required in Plasmodium for the transition from the asexual form to gametocytes, which appears to be regulated by cAMP signaling pathways118–120. PKA has also been implicated in stage transition from an actively replicating form into the quiescent chronic stage in Toxoplasma gondii121, a parasite related to Plasmodium.
cAMP in stage transition of trypanosomatids and other parasites
cAMP signaling pathways have been described in T. cruzi, which causes Chagas disease, and Trypanosoma brucei, the cause of human African trypanosomiasis. In T. cruzi, cAMP regulates PKA with striking effects on cell survival122 and cell proliferation through the inhibition of DNA, RNA and protein synthesis123. cAMP has also been implicated in the transition of the human-infective form to the insect-infective form of T. cruzi124,125. Additionally, a PDE regulates osmosis in the insect-infective form of this parasite126.
In T. brucei, cAMP regulates parasite virulence127 and induces the cell cycle arrest that occurs in the differentiation from the human-infective stage into the insect-infective stage128. In the related parasite Leishmania, cAMP also appears to regulate stage transition129 and motility130. Entamoeba, the causative agent of amebiasis, is another example of a parasite where cAMP-dependent pathways seem to regulate stage transition, in this case from the human intestinal form into the free-living cyst131. In Schistosoma, a parasitic worm, cAMP-mediated pathways are also present and essential for parasite survival132.
There is growing evidence for the use of cAMP signaling pathways in processes required for parasite survival. Although there is a great diversity in the functional processes involved, transition into a different stage of the life cycle and invasion of the host cell appear as common processes regulated by cAMP. This is not surprising, since both events require a specific response to the environment that will facilitate survival of the parasite. From this point of view, cAMP-regulated pathways, which involve proteins related, but not identical, to the human host, appear as promising pharmacological targets for drug development.
Conclusions and future directions
cAMP signaling controls a surprisingly diverse range of processes in pathogenic bacteria, fungi and protozoa, and the critical roles for cAMP in microbial pathogenesis extend from within the pathogens themselves to their mammalian host cells. cAMP is a key regulator of virulence gene expression in bacteria and fungi, through its influence on the activity of transcription factors. Regulation of virulence gene expression by cAMP-dependent transcription factors, coupled with the environmental control of cAMP production, allows pathogens to coordinate their gene expression programs with the presence of appropriate environmental signals from their hosts. Modulation of cAMP levels within host cells by bacteria and protozoa contributes to their pathogenesis by suppressing immune responses while facilitating pathogen invasion, colonization and transmission. The success of this strategy is evident from the number of bacterial species that have independently evolved such mechanisms, which range from direct secretion of cAMP and bacterial ACs to manipulation of host cAMP production pathways. The frequent integration of horizontally-acquired virulence genes into existing cAMP-controlled regulatory circuits also suggests that cAMP-mediated signaling may facilitate virulence evolution in some pathogenic microbes.
Despite some general themes, e.g., the presence of CRP family transcription factors in bacteria, and cAMP-PKA regulated transcription factors in fungi and protozoa, cAMP signaling pathways are complex, and there exists a great deal of diversity among cAMP-associated regulatory circuits in different pathogens. Much of this diversity likely arises from the participation of regulatory cofactors, and recent evidence suggests that many cofactors involved in the activation of ACs as well as cAMP-dependent gene regulation remain to be discovered. The recent findings of cAMP-regulated transcriptional coactivators (CRTC) and PKA-independent cAMP-responsive EPAC (exchange proteins activated by cAMP) family transcription factors in eukaryotes15,16,75, as well as the presence of cAMP binding proteins other than transcription factors in bacteria such as P. aeruginosa and M. tuberculosis, promise additional levels of complexity that should be investigated. Characterization of these factors and deciphering their regulatory functions is likely to establish new cAMP signaling paradigms in both bacteria and fungi.
cAMP signaling also plays an important role in sensing and responding to host signals. In some, but not all cases, CRP-mediated gene expression is coordinated with carbon source availability in the host. CO2 and Ca2+ are additional signals used by multiple pathogens to sense the host environment through their AC/cAMP signaling systems. Fungi and mycobacteria express CO2-responsive ACs, while ACs from P. aeruginosa, B. pertussis and B. anthracis are Ca2+ sensitive. A further level of cAMP signaling being used by pathogens to sense the host environment is the peptidoglycan sensitivity of C. albicans. This ability of an opportunistic pathogen to sense and exploit a polymicrobial infection is especially intriguing and has important implications for treatment. The possibility that other pathogens may have similar abilities should be investigated.
The specificity of cAMP signaling is a critical issue for pathogens with multiple ACs and for the host cells. M. tuberculosis may be the pathogen that presents the most challenging situation in this regard, as it has so many ACs. Linking individual ACs with specific regulatory outcomes is important for understanding cAMP signal transduction from a mechanistic perspective, as well as tuberculosis pathogenesis.
Perhaps the most important new frontier will be in understanding the effects of pathogen-mediated cAMP increases in host cells. The large number of pathogens that use this strategy, and the diversity of mechanisms that are used, point to the effectiveness of this approach. Understanding the consequences of pathogen-mediated manipulation of host cAMP may provide new antimicrobial targets as well as important new insights into the natural roles of cAMP in regulating innate immunity, as these pathogens can be used to probe the system in health and disease.
The myriad roles of cAMP signaling in microbial pathogenesis have only recently begun to be recognized, but it is clear that the universality of this second messenger provides tremendous opportunities for pathogens to modulate their host interactions during infection. Likewise, investigation of the diverse roles of cAMP in pathogen and host biology holds enormous potential for better understanding the interface between host and pathogen during infection, with the possibility of identifying new antimicrobial interventions.
Glossary
- Second messenger
a small molecule inside cells that relays signals from a receptor to one or more targets. The signal often originates outside the cell, although intracellular signals are also transmitted by second messengers in bacteria
- Effector proteins
the downstream functional targets that are activated by second messengers in response to specific signal transduction events. Effector proteins may also be virulence factors that are injected into host cells by bacterial secretion systems
- CRP (cAMP receptor protein) family transcription factors
global regulatory factors that contain cAMP and DNA binding domains and are associated with positive and negative gene regulation in some bacteria. Binding of cAMP typically induces a conformational change that increases the protein's DNA binding
- Cyclic di-GMP (cyclic diguanylate)
a second messenger used exclusively in bacteria that has been associated with regulation of biofilm formation, motility and expression of virulence factors
- Bistable switch
a regulatory mechanism that allows reversible toggling between two stable states. Such switching can occur in response to a transient signal, allowing stable maintenance of either state without continued presence of the inducer, and the generation of multiple subpopulations in a homogeneous environment
- Regulon
a set of genes or operons whose expression is controlled by a common regulatory factor
- M. tuberculosis-complex
includes five mycobacterial species that are capable of causing tuberculosis (TB) disease. This group includes M. tuberculosis, M. bovis, M. africanum, M. canetti and M. microti
- Ras family G proteins or guanosine-nucleotide-binding proteins
a subfamily of small GTPases that control signaling cascades in eukaryotic cells, resulting in transmission of regulatory signals from outside the cell to the nucleus
- G-actin
or globular actin, subunits polymerize into the long F-actin filaments that contribute to many eukaryotic processes, including cell structure, motility, cell division and cell signaling
- Pseudohyphae
a filamentous form of yeast growth that occurs when budding yeast cells remain connected, forming a string of connected cells. They differ from true hyphae by their method of growth
- Toll-like receptors
a class of transmembrane proteins expressed on the surface of many immune cells. These pattern recognition receptors stimulate innate immune defenses upon recognizing and binding any of a broad range of specific pathogen-derived molecules
- Th1 and Th2 responses
lie at opposite ends of an immunological response spectrum and are driven by the activation of different subsets of helper T cells (Th). A Th1 response promotes killing of pathogens within host cells, while a Th2 response drives production of antibodies to neutralize the impact of toxins or extracellular pathogens. Each pathway amplifies itself while inhibiting the other. Thus, pathogens can evade host immunity by tipping the Th1/Th2 balance towards the type of immune response that is least effective against them
- A-B toxins
are comprised of active (A) and binding (B) protein subunits. The A subunits are responsible for all of the toxic activity, but they require the B subunits for delivery into their target cells
- Sporozoite
Stage of the Plasmodium parasite that is generated in the mosquito and is transmitted to the mammalian host, where it infects hepatocytes;
- Gametocyte
Sexual stage of the Plasmodium parasite that is generated within infected erythrocytes in the mammalian host and it is transmitted to the mosquito.
References
- 1.Baumann A, Lange C, Soppa J. Transcriptome changes and cAMP oscillations in an archaeal cell cycle. BMC Cell Biol. 2007;8:21–40. doi: 10.1186/1471-2121-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beavo JA, Brunton LL. Cyclic nucleotide research -- still expanding after half a century. Nat Rev Mol Cell Biol. 2002;3:710–718. doi: 10.1038/nrm911. [DOI] [PubMed] [Google Scholar]
- 3.Insel PA, Zhang L, Murray F, Yokouchi H, Zambon AC. Cyclic AMP is both a pro-apoptotic and anti-apoptotic second messenger. Acta Physiol (Oxf) 2011 doi: 10.1111/j.1748-1716.2011.02273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tojima T, Hines JH, Henley JR, Kamiguchi H. Second messengers and membrane trafficking direct and organize growth cone steering. Nat Rev Neurosci. 2011;12:191–203. doi: 10.1038/nrn2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Camilli A, Bassler BL. Bacterial small-molecule signaling pathways. Science. 2006;311:1113–1116. doi: 10.1126/science.1121357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rumbaugh KP. Convergence of hormones and autoinducers at the host/pathogen interface. Anal Bioanal Chem. 2007;387:425–435. doi: 10.1007/s00216-006-0694-9. [DOI] [PubMed] [Google Scholar]
- 7.Gomelsky M. cAMP, c-di-GMP, c-di-AMP and now cGMP: bacteria use them all! Mol Microbiol. 2011;79:562–565. doi: 10.1111/j.1365-2958.2010.07514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Romling U. Great times for small molecules: c-di-AMP, a second messenger candidate in Bacteria and Archaea. Sci Signal. 2008;1:e39. doi: 10.1126/scisignal.133pe39. [DOI] [PubMed] [Google Scholar]
- 9.Dalebroux ZD, Svensson SL, Gaynor EC, Swanson MS. ppGpp conjures bacterial virulence. Microbiol Mol Biol Rev. 2010;74:171–199. doi: 10.1128/MMBR.00046-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Potrykus K, Cashel M. (p)ppGpp: still magical? Annu Rev Microbiol. 2008;62:35–51. doi: 10.1146/annurev.micro.62.081307.162903. [DOI] [PubMed] [Google Scholar]
- 11.Springett GM, Kawasaki H, Spriggs DR. Non-kinase second-messenger signaling: new pathways with new promise. Bioessays. 2004;26:730–738. doi: 10.1002/bies.20057. [DOI] [PubMed] [Google Scholar]
- 12.Simeoni L, Smida M, Posevitz V, Schraven B, Lindquist JA. Right time, right place: the organization of membrane proximal signaling. Semin Immunol. 2005;17:35–49. doi: 10.1016/j.smim.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Tang WJ, Yan S, Drum CL. Class III adenylyl cyclases: regulation and underlying mechanisms. Adv Second Messenger Phosphoprotein Res. 1998;32:137–151. doi: 10.1016/s1040-7952(98)80009-8. [DOI] [PubMed] [Google Scholar]
- 14.Botsford JL, Harman JG. Cyclic AMP in prokaryotes. Microbiol Rev. 1992;56:100–122. doi: 10.1128/mr.56.1.100-122.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamenetsky M, et al. Molecular details of cAMP generation in mammalian cells: a tale of two systems. J Mol Biol. 2006;362:623–639. doi: 10.1016/j.jmb.2006.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altarejos JY, Montminy M. CREB and the CRTC co-activators: sensors for hormonal and metabolic signals. Nat Rev Mol Cell Biol. 2011;12:141–151. doi: 10.1038/nrm3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kolb A, Busby S, Buc H, Garges S, Adhya S. Transcriptional regulation by cAMP and its receptor protein. Annu Rev Biochem. 1993;62:749–795. doi: 10.1146/annurev.bi.62.070193.003533. [DOI] [PubMed] [Google Scholar]
- 18.Kresge N, Simoni RD, Hill RLEarl W. Sutherland's discovery of cyclic adenine monophosphate and the second messenger system. Journal of Biological Chemistry. 2005;280:e39–e40. [Google Scholar]
- 19.Serezani CH, Ballinger MN, Aronoff DM, Peters-Golden M. Cyclic AMP: master regulator of innate immune cell function. Am J Respir Cell Mol Biol. 2008;39:127–132. doi: 10.1165/rcmb.2008-0091TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Botsford JL. Cyclic nucleotides in procaryotes. Microbiol Rev. 1981;45:620–642. doi: 10.1128/mr.45.4.620-642.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gorke B, Stulke J. Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol. 2008;6:613–624. doi: 10.1038/nrmicro1932. [DOI] [PubMed] [Google Scholar]
- 22.Masure HR, Shattuck RL, Storm DR. Mechanisms of bacterial pathogenicity that involve production of calmodulin-sensitive adenylate cyclases. Microbiol Rev. 1987;51:60–65. doi: 10.1128/mr.51.1.60-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhan L, et al. The cyclic AMP receptor protein, CRP, is required for both virulence and expression of the minimal CRP regulon in Yersinia pestis biovar microtus. Infect Immun. 2008;76:5028–5037. doi: 10.1128/IAI.00370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petersen S, Young GM. Essential role for cyclic AMP and its receptor protein in Yersinia enterocolitica virulence. Infect Immun. 2002;70:3665–3672. doi: 10.1128/IAI.70.7.3665-3672.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rickman L, et al. A member of the cAMP receptor protein family of transcription regulators in Mycobacterium tuberculosis is required for virulence in mice and controls transcription of the rpfA gene coding for a resuscitation promoting factor. Mol Microbiol. 2005;56:1274–1286. doi: 10.1111/j.1365-2958.2005.04609.x.• This was the first study to implicate a cAMP signaling pathway in M. tuberculosis virulence.
- 26. Smith RS, Wolfgang MC, Lory S. An adenylate cyclase-controlled signaling network regulates Pseudomonas aeruginosa virulence in a mouse model of acute pneumonia. Infect Immun. 2004;72:1677–1684. doi: 10.1128/IAI.72.3.1677-1684.2004.• This paper provides the first direct evidence that cAMP is required for P. aeruginosa virulence through its control of the T3SS, and shows the the Class III cyclase, CyaB, is more critical to this phenotype that the Class I CyaA AC.
- 27.Curtiss R, 3rd, Kelly SM. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987;55:3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bai G, Knapp GS, McDonough KA. Cyclic AMP signalling in mycobacteria: redirecting the conversation with a common currency. Cell Microbiol. 2011;13:349–358. doi: 10.1111/j.1462-5822.2010.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang M, et al. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3 doi: 10.1126/scisignal.2000697. ra11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HJ, Park SJ, Choi SH, Lee KH. Vibrio vulnificus rpoS expression is repressed by direct binding of cAMP-cAMP receptor protein complex to its two promoter regions. J Biol Chem. 2008;283:30438–30450. doi: 10.1074/jbc.M802219200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fuentes JA, Jofre MR, Villagra NA, Mora GC. RpoS- and Crp-dependent transcriptional control of Salmonella Typhi taiA and hlyE genes: role of environmental conditions. Res Microbiol. 2009;160:800–808. doi: 10.1016/j.resmic.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 32.Hengge-Aronis R. Signal transduction and regulatory mechanisms involved in control of the sigma(S) (RpoS) subunit of RNA polymerase. Microbiol Mol Biol Rev. 2002;66:373–395. doi: 10.1128/MMBR.66.3.373-395.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fong JC, Yildiz FH. Interplay between cyclic AMP-cyclic AMP receptor protein and cyclic di-GMP signaling in Vibrio cholerae biofilm formation. J Bacteriol. 2008;190:6646–6659. doi: 10.1128/JB.00466-08.• This study shows that cAMP-Crp Vc controls biofilm formation by regulating expression of a set of genes that encode diguanylate cyclases and phosphodiesterases. This link to cyclic di-GMP second messenger signaling is an example of cAMP signal amplification by its regulation of other global regulators.
- 34.Kim TJ, et al. Direct transcriptional control of the plasminogen activator gene of Yersinia pestis by the cyclic AMP receptor protein. J Bacteriol. 2007;189:8890–8900. doi: 10.1128/JB.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhan L, et al. Direct and negative regulation of the sycO-ypkA-ypoJ operon by cyclic AMP receptor protein (CRP) in Yersinia pestis . BMC Microbiol. 2009;9:178. doi: 10.1186/1471-2180-9-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nielsen AT, et al. A bistable switch and anatomical site control Vibrio cholerae virulence gene expression in the intestine. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001102.• This paper describes how cAMP signaling integrates localized environmenal signals with virulence gene expression in V. cholerae during infection. It also demonstrates that cAMP-Crp controls a bistable switch that allows a subpopulation of bacteria to continue expression of virulence factors for an extended period of time in rice water stools of cholera patients, which may facilitate transmission without decreasing fitness.
- 37.Yahr TL, Vallis AJ, Hancock MK, Barbieri JT, Frank DW. ExoY, an adenylate cyclase secreted by the Pseudomonas aeruginosa type III system. Proc Natl Acad Sci U S A. 1998;95:13899–13904. doi: 10.1073/pnas.95.23.13899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engel J, Balachandran P. Role of Pseudomonas aeruginosa type III effectors in disease. Curr Opin Microbiol. 2009;12:61–66. doi: 10.1016/j.mib.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 39. Wolfgang MC, Lee VT, Gilmore ME, Lory S. Coordinate regulation of bacterial virulence genes by a novel adenylate cyclase-dependent signaling pathway. Dev Cell. 2003;4:253–263. doi: 10.1016/s1534-5807(03)00019-4.• This paper identifies cAMP and the cAMP binding protein Vfr as regulators of virulence gene expression,including the T3SS, in P. aeruginosa
- 40.Lory S, Wolfgang M, Lee V, Smith R. The multi-talented bacterial adenylate cyclases. Int J Med Microbiol. 2004;293:479–482. doi: 10.1078/1438-4221-00297. [DOI] [PubMed] [Google Scholar]
- 41.Suh SJ, et al. Effect of vfr mutation on global gene expression and catabolite repression control of Pseudomonas aeruginosa . Microbiology. 2002;148:1561–1569. doi: 10.1099/00221287-148-5-1561. [DOI] [PubMed] [Google Scholar]
- 42.West SE, Sample AK, Runyen-Janecky LJ. The vfr gene product, required for Pseudomonas aeruginosa exotoxin A and protease production, belongs to the cyclic AMP receptor protein family. J Bacteriol. 1994;176:7532–7542. doi: 10.1128/jb.176.24.7532-7542.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fuchs EL, et al. The Pseudomonas aeruginosa Vfr regulator controls global virulence factor expression through cyclic AMP-dependent and -independent mechanisms. J Bacteriol. 2010;192:3553–3564. doi: 10.1128/JB.00363-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fuchs EL, et al. In vitro and in vivo characterization of the Pseudomonas aeruginosa cyclic AMP (cAMP) phosphodiesterase CpdA, required for cAMP homeostasis and virulence factor regulation. J Bacteriol. 2010;192:2779–2790. doi: 10.1128/JB.00168-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Endoh T, Engel JN. CbpA: a polarly localized novel cyclic AMP-binding protein in Pseudomonas aeruginosa . J Bacteriol. 2009;191:7193–7205. doi: 10.1128/JB.00970-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Skorupski K, Taylor RK. Cyclic AMP and its receptor protein negatively regulate the coordinate expression of cholera toxin and toxin-coregulated pilus in Vibrio cholerae. Proc Natl Acad Sci U S A. 1997;94:265–270. doi: 10.1073/pnas.94.1.265.• This paper provides some of the earliest evidence that cAMP played a role in virulence gene expression in V. cholerae. Recent studies have revealed just how extensive this role is.
- 47.Liang W, Pascual-Montano A, Silva AJ, Benitez JA. The cyclic AMP receptor protein modulates quorum sensing, motility and multiple genes that affect intestinal colonization in Vibrio cholerae . Microbiology. 2007;153:2964–2975. doi: 10.1099/mic.0.2007/006668-0. [DOI] [PubMed] [Google Scholar]
- 48.Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae . Mol Microbiol. 2003;50:101–104. doi: 10.1046/j.1365-2958.2003.03688.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhu J, Mekalanos JJ. Quorum sensing-dependent biofilms enhance colonization in Vibrio cholerae . Dev Cell. 2003;5:647–656. doi: 10.1016/s1534-5807(03)00295-8. [DOI] [PubMed] [Google Scholar]
- 50.Beyhan S, Bilecen K, Salama SR, Casper-Lindley C, Yildiz FH. Regulation of rugosity and biofilm formation in Vibrio cholerae: comparison of VpsT and VpsR regulons and epistasis analysis of vpsT vpsR, and hapR . J Bacteriol. 2007;189:388–402. doi: 10.1128/JB.00981-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Matson JS, Withey JH, DiRita VJ. Regulatory networks controlling Vibrio cholerae virulence gene expression. Infect Immun. 2007;75:5542–5549. doi: 10.1128/IAI.01094-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Miller MB, Skorupski K, Lenz DH, Taylor RK, Bassler BL. Parallel quorum sensing systems converge to regulate virulence in Vibrio cholerae . Cell. 2002;110:303–314. doi: 10.1016/s0092-8674(02)00829-2. [DOI] [PubMed] [Google Scholar]
- 53.Liang W, Sultan SZ, Silva AJ, Benitez JA. Cyclic AMP post-transcriptionally regulates the biosynthesis of a major bacterial autoinducer to modulate the cell density required to activate quorum sensing. FEBS Lett. 2008;582:3744–3750. doi: 10.1016/j.febslet.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J, et al. Quorum-sensing regulators control virulence gene expression in Vibrio cholerae. Proc Natl Acad Sci U S A. 2002;99:3129–3134. doi: 10.1073/pnas.052694299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zahid MS, et al. The cyclic AMP (cAMP)-cAMP receptor protein signaling system mediates resistance of Vibrio cholerae O1 strains to multiple environmental bacteriophages. Appl Environ Microbiol. 2010;76:4233–4240. doi: 10.1128/AEM.00008-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li CC, Merrell DS, Camilli A, Kaper JB. ToxR interferes with CRP-dependent transcriptional activation of ompT in Vibrio cholerae . Mol Microbiol. 2002;43:1577–1589. doi: 10.1046/j.1365-2958.2002.02845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shenoy AR, Visweswariah SS. New messages from old messengers: cAMP and mycobacteria. Trends Microbiol. 2006;14:543–550. doi: 10.1016/j.tim.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 58. McCue LA, McDonough KA, Lawrence CE. Functional classification of cNMP-binding proteins and nucleotide cyclases with implications for novel regulatory pathways in Mycobacterium tuberculosis. Genome Res. 2000;10:204–219. doi: 10.1101/gr.10.2.204.• This study was the first to identify an unusually large number of putative AC genes and cAMP binding proteins in M. tuberculosis, suggesting that cAMP signaling constituted a potentially key, but overlooked, signal transduction system in mycobacteria.
- 59.Shenoy AR, Sreenath NP, Mahalingam M, Visweswariah SS. Characterization of phylogenetically distant members of the adenylate cyclase family from mycobacteria: Rv1647 from Mycobacterium tuberculosis and its orthologue ML1399 from M. leprae . Biochem J. 2005;387:541–551. doi: 10.1042/BJ20041040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Linder JU, Hammer A, Schultz JE. The effect of HAMP domains on class IIIb adenylyl cyclases from Mycobacterium tuberculosis . Eur J Biochem. 2004;271:2446–2451. doi: 10.1111/j.1432-1033.2004.04172.x. [DOI] [PubMed] [Google Scholar]
- 61. Linder JU, Schultz A, Schultz JE. Adenylyl cyclase Rv1264 from Mycobacterium tuberculosis has an autoinhibitory N-terminal domain. J Biol Chem. 2002;277:15271–15276. doi: 10.1074/jbc.M200235200.• This paper provided the first evidence for direct pH-mediated activation of an AC from M. tuberculosis
- 62.Tews I, et al. The structure of a pH-sensing mycobacterial adenylyl cyclase holoenzyme. Science. 2005;308:1020–1023. doi: 10.1126/science.1107642. [DOI] [PubMed] [Google Scholar]
- 63.Bai G, Schaak DD, McDonough KA. cAMP levels within Mycobacterium tuberculosis and Mycobacterium bovis BCG increase upon infection of macrophages. FEMS Immunol Med Microbiol. 2009;55:68–73. doi: 10.1111/j.1574-695X.2008.00500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Padh H, Venkitasubramanian TA. Adenosine 3',5'-monophosphate in Mycobacterium phlei and Mycobacterium tuberculosis H37Ra. Microbios. 1976;16:183–189. [PubMed] [Google Scholar]
- 65. Agarwal N, Lamichhane G, Gupta R, Nolan S, Bishai WR. Cyclic AMP intoxication of macrophages by a Mycobacterium tuberculosis adenylate cyclase. Nature. 2009;460:98–102. doi: 10.1038/nature08123.• This study shows that cAMP produced by a specific bacterial AC during M. tuberculosis infection is secreted into macrophages and contributes to virulence.
- 66.Bai G, McCue LA, McDonough KA. Characterization of Mycobacterium tuberculosis Rv3676 (CRPMt), a cyclic AMP receptor protein-like DNA binding protein. J Bacteriol. 2005;187:7795–7804. doi: 10.1128/JB.187.22.7795-7804.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gazdik MA, Bai G, Wu Y, McDonough KA. Rv1675c (cmr) regulates intramacrophage and cyclic AMP-induced gene expression in Mycobacterium tuberculosis-complex mycobacteria. Mol Microbiol. 2009;71:434–448. doi: 10.1111/j.1365-2958.2008.06541.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nambi S, Basu N, Visweswariah SS. cAMP-regulated protein lysine acetylases in mycobacteria. J Biol Chem. 2010;285:24313–24323. doi: 10.1074/jbc.M110.118398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahuja N, Kumar P, Bhatnagar R. The adenylate cyclase toxins. Crit Rev Microbiol. 2004;30:187–196. doi: 10.1080/10408410490468795. [DOI] [PubMed] [Google Scholar]
- 70.Krueger KM, Barbieri JT. The family of bacterial ADP-ribosylating exotoxins. Clin Microbiol Rev. 1995;8:34–47. doi: 10.1128/cmr.8.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carbonetti NH. Pertussis toxin and adenylate cyclase toxin: key virulence factors of Bordetella pertussis and cell biology tools. Future Microbiol. 2010;5:455–469. doi: 10.2217/fmb.09.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carbonetti NH, Artamonova GV, Andreasen C, Bushar N. Pertussis toxin and adenylate cyclase toxin provide a one-two punch for establishment of Bordetella pertussis infection of the respiratory tract. Infect Immun. 2005;73:2698–2703. doi: 10.1128/IAI.73.5.2698-2703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith N, Kim SK, Reddy PT, Gallagher DT. Crystallization of the class IV adenylyl cyclase from Yersinia pestis . Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62:200–204. doi: 10.1107/S1744309106002855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim C, et al. Antiinflammatory cAMP signaling and cell migration genes co-opted by the anthrax bacillus. Proc Natl Acad Sci U S A. 2008;105:6150–6155. doi: 10.1073/pnas.0800105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Yeager LA, Chopra AK, Peterson JW. Bacillus anthracis edema toxin suppresses human macrophage phagocytosis and cytoskeletal remodeling via the protein kinase A and exchange protein activated by cyclic AMP pathways. Infect Immun. 2009;77:2530–2543. doi: 10.1128/IAI.00905-08.• This study shows that in addition to the inhibition of macrophage function caused through PKA-controlled pathways, cAMP produced by the B. anthracis edema toxin during infection disables macrophages through a PKA-independent pathway that requires newly identified Exchange Protein Activated by Cyclic AMP (Epac) proteins.
- 76.Maldonado-Arocho FJ, Fulcher JA, Lee B, Bradley KA. Anthrax oedema toxin induces anthrax toxin receptor expression in monocyte-derived cells. Mol Microbiol. 2006;61:324–337. doi: 10.1111/j.1365-2958.2006.05232.x. [DOI] [PubMed] [Google Scholar]
- 77. Rossi Paccani S, et al. The adenylate cyclase toxins of Bacillus anthracis and Bordetella pertussis promote Th2 cell development by shaping T cell antigen receptor signaling. PLoS Pathog. 2009;5:e1000325. doi: 10.1371/journal.ppat.1000325.• This paper details the effects on host immunity of the AC toxins from B. anthracis and B. pertussis, and provides evidence that the cAMP produced by these toxins is immunosuppressive and Th2 driving.
- 78.Guo Q, et al. Protein-protein docking and analysis reveal that two homologous bacterial adenylyl cyclase toxins interact with calmodulin differently. J Biol Chem. 2008;283:23836–23845. doi: 10.1074/jbc.M802168200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vojtova J, Kamanova J, Sebo P. Bordetella adenylate cyclase toxin: a swift saboteur of host defense. Curr Opin Microbiol. 2006;9:69–75. doi: 10.1016/j.mib.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 80.Young JA, Collier RJ. Anthrax toxin: receptor binding, internalization, pore formation, and translocation. Annu Rev Biochem. 2007;76:243–265. doi: 10.1146/annurev.biochem.75.103004.142728. [DOI] [PubMed] [Google Scholar]
- 81.Tang WJ, Guo Q. The adenylyl cyclase activity of anthrax edema factor. Mol Aspects Med. 2009;30:423–430. doi: 10.1016/j.mam.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lowrie DB, Aber VR, Jackett PS. Phagosome-lysosome fusion and cyclic adenosine 3':5'-monophosphate in macrophages infected with Mycobacterium microti, Mycobacterium bovis BCG or Mycobacterium lepraemurium . J Gen Microbiol. 1979;110:431–441. doi: 10.1099/00221287-110-2-431. [DOI] [PubMed] [Google Scholar]
- 83.Kozubowski L, Lee SC, Heitman J. Signalling pathways in the pathogenesis of Cryptococcus . Cell Microbiol. 2009;11:370–380. doi: 10.1111/j.1462-5822.2008.01273.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hogan DA, Sundstrom P. The Ras/cAMP/PKA signaling pathway and virulence in Candida albicans . Future Microbiol. 2009;4:1263–1270. doi: 10.2217/fmb.09.106. [DOI] [PubMed] [Google Scholar]
- 85.Brakhage AA, Liebmann B. Aspergillus fumigatus conidial pigment and cAMP signal transduction: significance for virulence. Med Mycol. 2005;(43 Suppl 1):S75–S82. doi: 10.1080/13693780400028967. [DOI] [PubMed] [Google Scholar]
- 86.Ramanujam R, Naqvi NI. PdeH, a high-affinity cAMP phosphodiesterase, is a key regulator of asexual and pathogenic differentiation in Magnaporthe oryzae . PLoS Pathog. 2010;6:e1000897. doi: 10.1371/journal.ppat.1000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hall RA, Muhlschlegel FA. A multi-protein complex controls cAMP signalling and filamentation in the fungal pathogen Candida albicans . Mol Microbiol. 2010;75:534–537. doi: 10.1111/j.1365-2958.2009.06979.x. [DOI] [PubMed] [Google Scholar]
- 88.Casadevall A, Perfect JR. Cryptococcus neoformans. American Society for Microbiology Press; 1998. [Google Scholar]
- 89.Pukkila-Worley R, Alspaugh JA. Cyclic AMP signaling in Cryptococcus neoformans . FEMS Yeast Res. 2004;4:361–367. doi: 10.1016/S1567-1356(03)00241-1. [DOI] [PubMed] [Google Scholar]
- 90.Xue C, Bahn YS, Cox GM, Heitman J. G protein-coupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans . Mol Biol Cell. 2006;17:667–679. doi: 10.1091/mbc.E05-07-0699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Maeng S, et al. Comparative transcriptome analysis reveals novel roles of the Ras and cyclic AMP signaling pathways in environmental stress response and antifungal drug sensitivity in Cryptococcus neoformans. Eukaryot Cell. 2010;9:360–378. doi: 10.1128/EC.00309-09.• This study shows that the cAMP signaling pathway is distinct from the Ras signaling pathway in C. neoformans. It also explores the role of cAMP-dependent gene products on antifungal drug sensitivity.
- 92.Hu G, et al. Transcriptional regulation by protein kinase A in Cryptococcus neoformans . PLoS Pathog. 2007;3:e42. doi: 10.1371/journal.ppat.0030042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pukkila-Worley R, et al. Transcriptional network of multiple capsule and melanin genes governed by the Cryptococcus neoformans cyclic AMP cascade. Eukaryot Cell. 2005;4:190–201. doi: 10.1128/EC.4.1.190-201.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alspaugh JA, Perfect JR, Heitman J. Cryptococcus neoformans mating and virulence are regulated by the G-protein alpha subunit GPA1 and cAMP. Genes Dev. 1997;11:3206–3217. doi: 10.1101/gad.11.23.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.D'Souza CA, et al. Cyclic AMP-dependent protein kinase controls virulence of the fungal pathogen Cryptococcus neoformans. Mol Cell Biol. 2001;21:3179–3191. doi: 10.1128/MCB.21.9.3179-3191.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Granger DL, Perfect JR, Durack DT. Virulence of Cryptococcus neoformans Regulation of capsule synthesis by carbon dioxide. J Clin Invest. 1985;76:508–516. doi: 10.1172/JCI112000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Mogensen EG, et al. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot Cell. 2006;5:103–111. doi: 10.1128/EC.5.1.103-111.2006.• This paper demonstrates that bicarbonate directly activates the AC that is required for virulence in C. neoformans, and that one of two carbonic anhydrases is required for growth of this fungus when CO2 levels are limiting so that bicarbonate can be generated.
- 98.Klengel T, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bahn YS, Cox GM, Perfect JR, Heitman J. Carbonic anhydrase and CO2 sensing during Cryptococcus neoformans growth, differentiation, and virulence. Curr Biol. 2005;15:2013–2020. doi: 10.1016/j.cub.2005.09.047. [DOI] [PubMed] [Google Scholar]
- 100.Leroy O, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006) Crit Care Med. 2009;37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 101.Fang HM, Wang Y. RA domain-mediated interaction of Cdc35 with Ras1 is essential for increasing cellular cAMP level for Candida albicans hyphal development. Mol Microbiol. 2006;61:484–496. doi: 10.1111/j.1365-2958.2006.05248.x. [DOI] [PubMed] [Google Scholar]
- 102.Park H, et al. Role of the fungal Ras-protein kinase A pathway in governing epithelial cell interactions during oropharyngeal candidiasis. Cell Microbiol. 2005;7:499–510. doi: 10.1111/j.1462-5822.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 103.Giacometti R, Kronberg F, Biondi RM, Passeron S. Catalytic isoforms Tpk1 and Tpk2 of Candida albicans PKA have non-redundant roles in stress response and glycogen storage. Yeast. 2009;26:273–285. doi: 10.1002/yea.1665. [DOI] [PubMed] [Google Scholar]
- 104.Harcus D, Nantel A, Marcil A, Rigby T, Whiteway M. Transcription profiling of cyclic AMP signaling in Candida albicans . Mol Biol Cell. 2004;15:4490–4499. doi: 10.1091/mbc.E04-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maidan MM, et al. The G protein-coupled receptor Gpr1 and the Galpha protein Gpa2 act through the cAMP-protein kinase A pathway to induce morphogenesis in Candida albicans . Mol Biol Cell. 2005;16:1971–1986. doi: 10.1091/mbc.E04-09-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kumamoto CA, Vinces MD. Contributions of hyphae and hypha-co-regulated genes to Candida albicans virulence. Cell Microbiol. 2005;7:1546–1554. doi: 10.1111/j.1462-5822.2005.00616.x. [DOI] [PubMed] [Google Scholar]
- 107.Hnisz D, Majer O, Frohner IE, Komnenovic V, Kuchler K. The Set3/Hos2 histone deacetylase complex attenuates cAMP/PKA signaling to regulate morphogenesis and virulence of Candida albicans . PLoS Pathog. 2010;6:e1000889. doi: 10.1371/journal.ppat.1000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Xu XL, et al. Bacterial peptidoglycan triggers Candida albicans hyphal growth by directly activating the adenylyl cyclase Cyr1p. Cell Host Microbe. 2008;4:28–39. doi: 10.1016/j.chom.2008.05.014.• This study demonstrates that muramyl dipeptides, which are fragments of peptidoglycan that arise from commensal or co-infecting bacteria, can directly activate the AC that controls virulence-associated signaling in C. albicans
- 109.Zou H, Fang HM, Zhu Y, Wang Y. Candida albicans Cyr1, Cap1 and G-actin form a sensor/effector apparatus for activating cAMP synthesis in hyphal growth. Mol Microbiol. 2010;75:579–591. doi: 10.1111/j.1365-2958.2009.06980.x. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez A, Martinez I, Chung A, Berlot CH, Andrews NW. cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes. J Biol Chem. 1999;274:16754–16759. doi: 10.1074/jbc.274.24.16754. [DOI] [PubMed] [Google Scholar]
- 111.Usynin I, Klotz C, Frevert U. Malaria circumsporozoite protein inhibits the respiratory burst in Kupffer cells. Cell Microbiol. 2007;9:2610–2628. doi: 10.1111/j.1462-5822.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 112. Ono T, et al. Adenylyl cyclase alpha and cAMP signaling mediate Plasmodium sporozoite apical regulated exocytosis and hepatocyte infection. PLoS Pathog. 2008;4:e1000008. doi: 10.1371/journal.ppat.1000008.• This study shows that adenylyl cyclase α is required for regulated exocytosis in Plasmodium sporozoites, and its deletion results in 50% decrease in parasite infectivity.
- 113.Kebaier C, Vanderberg JP. Initiation of Plasmodium sporozoite motility by albumin is associated with induction of intracellular signalling. Int J Parasitol. 2010;40:25–33. doi: 10.1016/j.ijpara.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Beraldo FH, Almeida FM, da Silva AM, Garcia CR. Cyclic AMP and calcium interplay as second messengers in melatonin-dependent regulation of Plasmodium falciparum cell cycle. J Cell Biol. 2005;170:551–557. doi: 10.1083/jcb.200505117.• This study shows that the synchronization of Plasmodium blood stage cell cycle by melatonin is mediated through the interplay of cAMP and calcium signaling in the parasite.
- 115.Gazarini ML, et al. Melatonin triggers PKA activation in the rodent malaria parasite Plasmodium chabaudi . J Pineal Res. 2011;50:64–70. doi: 10.1111/j.1600-079X.2010.00810.x. [DOI] [PubMed] [Google Scholar]
- 116. Merckx A, et al. Plasmodium falciparum regulatory subunit of cAMP-dependent PKA and anion channel conductance. PLoS Pathog. 2008;4:e19. doi: 10.1371/journal.ppat.0040019.• This study demonstrates that in P. falciparum-infected red blood cells, anion conductance of the erythrocyte membrane is modulated by cAMP through the regulation of an anion channel.
- 117.Leykauf K, et al. Protein kinase a dependent phosphorylation of apical membrane antigen 1 plays an important role in erythrocyte invasion by the malaria parasite. PLoS Pathog. 2010;6:e1000941. doi: 10.1371/journal.ppat.1000941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dyer M, Day K. Expression of Plasmodium falciparum trimeric G proteins and their involvement in switching to sexual development. Mol Biochem Parasitol. 2000;110:437–448. doi: 10.1016/s0166-6851(00)00288-7. [DOI] [PubMed] [Google Scholar]
- 119.Kaushal DC, Carter R, Miller LH, Krishna G. Gametocytogenesis by malaria parasites in continuous culture. Nature. 1980;286:490–492. doi: 10.1038/286490a0. [DOI] [PubMed] [Google Scholar]
- 120.Read LK, Mikkelsen RB. Comparison of adenylate cyclase and cAMP-dependent protein kinase in gametocytogenic and nongametocytogenic clones of Plasmodium falciparum . J Parasitol. 1991;77:346–352. [PubMed] [Google Scholar]
- 121. Eaton MS, Weiss LM, Kim K. Cyclic nucleotide kinases and tachyzoite-bradyzoite transition in Toxoplasma gondii . Int J Parasitol. 2006;36:107–114. doi: 10.1016/j.ijpara.2005.08.014.• This study implicates both PKA and the cGMP-dependent kinase (PKG) in the cycling between the tachyzoite and bradyzoite life stages in T. gondii
- 122.Bao Y, Weiss LM, Braunstein VL, Huang H. Role of protein kinase A in Trypanosoma cruzi . Infect Immun. 2008;76:4757–4763. doi: 10.1128/IAI.00527-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Santos DO, Oliveira MM. Effect of cAMP on macromolecule synthesis in the pathogenic protozoa Trypanosoma cruzi . Mem Inst Oswaldo Cruz. 1988;83:287–292. doi: 10.1590/s0074-02761988000300004. [DOI] [PubMed] [Google Scholar]
- 124.Gonzales-Perdomo M, Romero P, Goldenberg S. Cyclic AMP and adenylate cyclase activators stimulate Trypanosoma cruzi differentiation. Exp Parasitol. 1988;66:205–212. doi: 10.1016/0014-4894(88)90092-6. [DOI] [PubMed] [Google Scholar]
- 125.Rangel-Aldao R, et al. Cyclic AMP as an inducer of the cell differentiation of Trypanosoma cruzi . Biochem Int. 1988;17:337–344. [PubMed] [Google Scholar]
- 126.Schoijet AC, et al. Defining the role of a FYVE domain in the localization and activity of a cAMP phosphodiesterase implicated in osmoregulation in Trypanosoma cruzi . Mol Microbiol. 2011;79:50–62. doi: 10.1111/j.1365-2958.2010.07429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oberholzer M, et al. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J. 2007;21:720–731. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- 128. Vassella E, Reuner B, Yutzy B, Boshart M. Differentiation of African trypanosomes is controlled by a density sensing mechanism which signals cell cycle arrest via the cAMP pathway. J Cell Sci. 1997;110(Pt 21):2661–2671. doi: 10.1242/jcs.110.21.2661.• This study shows that cell cycle regulation of bloodstream forms of African trypanosomes is regulated by cAMP signalling.
- 129.Bee A, et al. Transformation of Leishmania mexicana metacyclic promastigotes to amastigote-like forms mediated by binding of human C-reactive protein. Parasitology. 2001;122:521–529. doi: 10.1017/s0031182001007612. [DOI] [PubMed] [Google Scholar]
- 130.Rodriguez M, Kim B, Lee NS, Veeranki S, Kim L. MPL1, a novel phosphatase with leucine-rich repeats, is essential for proper ERK2 phosphorylation and cell motility. Eukaryot Cell. 2008;7:958–966. doi: 10.1128/EC.00442-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Frederick J, Eichinger D. Entamoeba invadens contains the components of a classical adrenergic signaling system. Mol Biochem Parasitol. 2004;137:339–343. doi: 10.1016/j.molbiopara.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 132.Swierczewski BE, Davies SJ. A schistosome cAMP-dependent protein kinase catalytic subunit is essential for parasite viability. PLoS Negl Trop Dis. 2009;3:e505. doi: 10.1371/journal.pntd.0000505. [DOI] [PMC free article] [PubMed] [Google Scholar]