Abstract
Hepatic steatosis is a frequent complication in nonobese patients with breast cancer treated with tamoxifen, a potent antagonist of estrogen. In addition, hepatic steatosis became evident spontaneously in the aromatase-deficient (ArKO) mouse, which lacks intrinsic estrogen production. These clinical and laboratory observations suggest that estrogen helps to maintain constitutive lipid metabolism. To clarify this hypothesis, we characterized the expression and activity in ArKO mouse liver of enzymes involved in peroxisomal and mitochondrial fatty acid β-oxidation. Northern analysis showed reduced expression of mRNAs for very long fatty acyl-CoA synthetase, peroxisomal fatty acyl-CoA oxidase, and medium-chain acyl-CoA dehydrogenase, enzymes required in fatty acid β-oxidation. In vitro assays of fatty acid β-oxidation activity using very long (C24:0), long (C16:0), or medium (C12:0) chain fatty acids as the substrates confirmed that the corresponding activities are also diminished. Impaired gene expression and enzyme activities of fatty acid β-oxidation were restored to the wild-type levels, and hepatic steatosis was substantially diminished in animals treated with 17β-estradiol. Wild-type and ArKO mice showed no difference in the binding activities of the hepatic nuclear extracts to a peroxisome proliferator response element. These findings demonstrate the pivotal role of estrogen in supporting constitutive hepatic expression of genes involved in lipid β-oxidation and in maintaining hepatic lipid homeostasis.
Introduction
The association of alcohol with liver damage has been well established. During the early stages, the liver is enlarged owing to severe fatty change. Inflammatory changes and hepatocyte necrosis with the alcoholic hyaline of Mallory and variable extents of liver fibrosis are characteristic pathological features in alcoholic liver disease (1). During the 1980s, liver diseases with very similar pathological findings, which latently progress to liver cirrhosis, were recognized in nonalcoholics. Typical examples were observed as an extremely frequent complication of jejunoileal bypass surgery for morbidly obese patients and as a rare adverse reaction to a few medicines (2, 3). Since this disease entity was defined pathologically irrespective of etiology, both fatty liver hepatitis and nonalcoholic steatohepatitis (NASH) have been used to describe “the pathological and clinical features of non-alcoholic disease of the liver associated with the pathological features most commonly seen in alcoholic liver disease itself” (4).
Recently, NASH has become the second or third most common liver disease in outpatient hepatology practice in North America (2). This has led to a debate as to whether hepatic steatosis, which is easily defined from the liver/spleen ratio of computed tomography values in Hounsfield units less than 0.9 (5), is an “innocent bystander or guilty party” in NASH (6). Because liver diseases have not been widely appreciated as life-threatening complications of obesity, hepatic steatosis has been regarded as an innocent bystander of NASH. However, evidence implying obesity is a risk for liver diseases has recently been accumulated. For example, liver cirrhosis is approximately sixfold more prevalent in obese individuals than in the general population, and obesity increases the risk of liver cirrhosis (7), and, in addition, gradual progression from hepatic steatosis to NASH and eventually to cirrhosis is supported by epidemiologic findings (8). Thus, a consensus about NASH was recently provided; namely, that hepatic steatosis is regarded as a risk of NASH and that a “second hit” capable of inducing necrosis, inflammation, and fibrosis in the liver is required for NASH, as most patients with hepatic steatosis do not develop liver cirrhosis (2, 9, 10). An exposure to endotoxin/bacterial lipopolysaccharides, iron overload, and accumulation of long chain and very long chain fatty acids (VLCFAs) were suggested as candidates for the “second hit” (2, 7, 9).
In the 1990s, adjuvant tamoxifen became a standard treatment for women with early breast cancer. A 5-year treatment of adjuvant tamoxifen reduced the recurrence risk of estrogen receptor–positive cancer by 50% (11). Moreover, the same adjuvant trials showed a 40% reduction in the risk of cancer recurrence in the opposite breast and in the ductal carcinoma in situ (12, 13). These studies may further promote adjuvant tamoxifen, as 5-year treatment of tamoxifen for breast cancer undoubtedly outweighs the risks of the adverse effects. However, it was reported that rapidly progressive hepatic steatosis among nonobese nondiabetic breast cancer patients treated with tamoxifen was known to induce NASH and liver cirrhosis on rare occasions (8, 14–17). The frequency of progressive hepatic steatosis had increased to 36% (18, 19), and more than ten patients in our clinic were shown by liver biopsy to have tamoxifen-induced NASH. A body mass index (BMI; kg/m2) greater than 23 was a significant risk factor for tamoxifen-induced hepatic steatosis (19). As hepatic steatosis is suggested as a risk factor of NASH, it is important to elucidate the mechanisms involved in the rapid progression of hepatic steatosis during adjuvant tamoxifen.
Because tamoxifen is a potent antagonist of estrogen, and estrogen is believed to be profoundly involved in hepatic lipid metabolism (20), this suggested that tamoxifen could suppress hepatic lipid β-oxidation and accelerate lipid accumulation in hepatocytes. To clarify this, we developed aromatase-deficient (ArKO) mice lacking intrinsic estrogen production, because aromatase is a key enzyme in estrogen synthesis (21, 22). We investigated constitutive expression of PPAR-α and fatty acid–metabolizing enzymes in peroxisome, mitochondria, and microsomes of ArKO mice. Here, we show that hepatocellular fatty acid β-oxidation is impaired in ArKO mice and that the impairment in mitochondrial and peroxisomal fatty acid β-oxidation is restored to a wild-type level with supplementation of 17β-estradiol. These findings clarify the fundamental role of estrogens in constitutive expression of fatty acid–metabolizing enzymes to maintain hepatic lipid homeostasis, and implicate estrogen receptor-signaling pathways in the control of constitutive hepatic lipid metabolism.
Methods
Mice.
The aromatase gene (cyp 19 gene) was disrupted by homologous recombination (23). In brief, an 87-bp fragment located within exon 9 of cyp 19 gene in E14-1 cells (embryonic stem cell; ES cell) was replaced with a neomycin resistance gene derived from pMC1-neo. Selected ES cells were microinjected into the C57BL/6J blastocytes to generate chimeric mice. Chimeric male mice were then mated with C57BL/6J female mice to generate mice heterozygous for the mutation. Heterozygous mice were mated to obtain aromatase null (ArKO) mice because of the infertility of homozygous males and females. Genotypes of mice were determined by PCR using genomic DNA isolated from tail tips. ArKO (Ar–/–) male mice aged 6 months and their wild-type male siblings (Ar+/+) fed with a CE-2 diet (Clea Japan, Tokyo, Japan) were used in the present study. ArKO mice were divided into two groups: the Ar–/– group was nontreated, and the Ar–/– + E2 group was subcutaneously supplemented with 7.5 μg/mouse of 17β-estradiol (E2) every 4 days for the first 3 weeks after birth and once a week with 0.75 μg/mouse thereafter. Animal care and experiments were carried out in accordance with institutional animal care regulations.
Light microscopic observations.
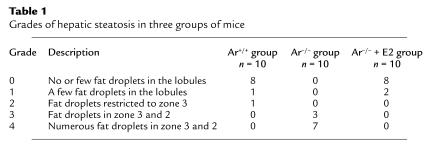
Liver tissues were routinely fixed in 10% phosphate-buffered formalin (pH 7.4), embedded in paraffin, and sectioned for hematoxylin-and-eosin staining. The degree of hepatic steatosis was classified into four grades according to the distribution pattern of the fat vesicles as follows: 0 = no or few fat droplets in the lobules; 1= a few fat droplets in the lobules; 2 = fat droplets restricted to zone 3; 3 = fat droplets in zones 3 and 2; 4 = numerous fat droplets in zones 3 and 2.
Analysis of mRNA expression for enzymes involved in fatty acid β-oxidation.
mRNA analysis was performed by Northern blotting. Total liver RNA was obtained from fresh liver using the acid guanidinium thiocyanate-phenol-chloroform extraction method. RNA was separated on 1% agarose gel and transferred to a nylon membrane. The membranes were incubated with 32P-labeled cDNA probes and analyzed on a Fuji system analyzer (Fuji Photo Film, Tokyo, Japan). The cDNA used for Northern blotting included catalase, very long fatty acyl-CoA synthetase (VLACS), peroxisomal acyl-CoA oxidase (AOX), medium-chain acyl-CoA dehydrogenase (MCAD), CYP2E1, CYP4A1 (20), GAPDH, and β-actin. Changes in mRNA levels were estimated by densitometric scanning of autoradiograms and analyzed by NIH Image software (version 1.52; National Institutes of Health, Bethesda, Maryland, USA) to show a relative ratio to the findings in the Ar+/+ group.
Fatty acid β-oxidation activity.
Fatty acid β-oxidation activity was measure as described previously (24). In brief, fresh liver was homogenized in four volumes of 0.25 M sucrose containing 1 mM EDTA in a Potter-Elvehjem homogenizer (Ikemoto Rika, Tokyo, Japan) using a tight-fitting Teflon pestle. Homogenate (1–10 mg) was incubated with the assay medium in 0.2 mL of 150 mM KCl, 10 mM HEPES (pH 7.2), 0.1 mM EDTA, 1 mM potassium phosphate buffer (pH 7.2), 5 mM malonate, 10 mM MgCl2, 1 mM carnitine, 0.15% BSA, 5 mM ATP, and 50 μM each fatty acid (105 cpm for radioactive substrates) (55 mCi/mmol; American Radiolabeled Chemicals, St. Louis, Missouri, USA): [1-14C]tetracosanoic acid (C24:0), [1-14C]palmitic acid (C16:0), or [1-14C]lauric acid (C12:0). The reaction was run for 30 minutes at 25°C and stopped by the addition of 0.2 mL of 0.6 N perchloric acid. The mixture was centrifuged at 2,000 g for 10 minutes, and the unreacted fatty acid in the supernatant was removed using 2 mL of n-hexane with three extractions. Radioactive degradation products in the water phase were counted. Fatty acid β-oxidation activity was expressed as nanomolars per minute per liver. In some experiments using [1-14C]palmitic acid, KCN, a potent inhibitor of the mitochondrial respiratory chain, was added to the assay medium to inhibit potent mitochondrial activity.
Lipid analysis.
Lipid composition of the livers (n = 3) and total plasma lipids (n = 5) were analyzed using gas chromatograph equipped with a glass capillary column. Three parameters of the VLCFAs in sphingomyelin from plasma, the ratio of hexacosanoic acid (C26:0) to docosanoic acid (C22:0), of pentacosanoic acid (C25:0) to C22:0, and of tetracosanoic acid (C24:0) to C22:0, were analyzed by chemical ionization mass spectrometry combined with capillary gas chromatography (25).
Preparation of nuclear extracts and electrophoretic mobility shift assay.
Nuclear extracts from the liver were prepared according to the method of Jiang and Eberhardt (26). The protein concentration of the nuclear extracts was determined by the method of Lowry et al. (27). The peroxisome proliferator response element (PPRE) of AOX (28) was synthesized (5′-CCAGGACAAAGGTCACG-3′) and subcloned at the SmaI site of a pUC118 plasmid. The EcoRI-BamHI fragments containing the PPRE were end labeled with [α-32P]dCTP using the Klenow fragment. The radiolabeled fragments were purified by spin column chromatography and used as a probe in the assay. The 32P-labeled probe of 65 pg (∼46 cpm/pg) was incubated at 20°C for 20 minutes in 20 μL of a mixture containing 10 mM HEPES-KOH (pH 7.9), 100 mM KCl, 1 mM EDTA, 1 mM dithiothreitol, 3 mM MgCl2, 5% (vol/vol) glycerol, 50 μg/mL poly(dI-dC), 20 μg of protein from the nuclear extracts, and various amounts of competitor DNAs. The unlabeled probe and C/EBP binding element were used as specific and nonspecific competitor DNAs, respectively. After incubation, the mixtures were subjected to electrophoresis in a 4% nondenaturing polyacrylamide gel in a Tris-glycine buffer (50 mM Tris base, 380 mM glycine, and 2 mM EDTA [pH 8.5]). The gel was then dried and autoradiographed. PPAR-α content was estimated by densitometric scanning and analyzed by NIH Image software (version 1.52).
Statistic analysis.
Data were analyzed using Student’s t test or Wilcoxon signed rank test.
Results
Light microscopic observation.
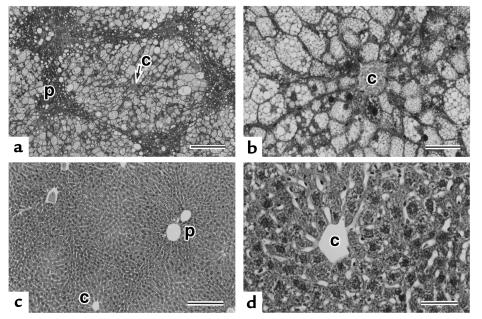
The growth of ArKO mice in the first 2 months after birth is similar to that of their wild-type siblings. Thereafter, they gradually accumulated abdominal fat in an estrogen-free condition, and hepatic steatosis became evident spontaneously, although there was a slight variation in the severity. As shown in Figure 1, liver cells in zones 3 and 2 (centrilobular and intermediate zones in the lobules) showed marked microvesicular steatosis in ArKO mice. Liver cells with features of microvesicular steatosis did not contain any large fat droplets, but the cytoplasm of some liver cells filled with numerous fat droplets had both small and large fat droplets. However, liver cells in zone 1 (periportal) remained intact. Such a zonal difference of steatosis within the liver lobule was very clear. Hepatic steatosis was not observed in their wild-type siblings and supplementation of 17β-estradiol retrieved ArKO mice from massive hepatic steatosis (Table 1). No necroinflammatory lesions were present in any group.
Figure 1.
Light micrographs of livers of ArKO mice (a and b) and ArKO mice supplemented with 17β-estradiol (c and d). (a) Zones 2 and 3 of liver lobules are composed of liver cells with marked fatty changes rimmed with narrower zone 1 areas of normal appearing liver cells. (b) Higher magnification of liver cells in zone 3. The enlarged cytoplasm is filled with numerous fat droplets, although microvesicular features are prominent. (c) In marked contrast to the findings in ArKO mice livers (a and b), liver tissues show the normal structure. (d) Higher magnification of a perivenular portion in c. All liver cells are normal in appearance. c, central vein; p, portal tract. Scale bar = 200 μm (a and c) and 50 μm (b and d). Hematoxylin-and-eosin staining.
Table 1.
Grades of hepatic steatosis in three groups of mice
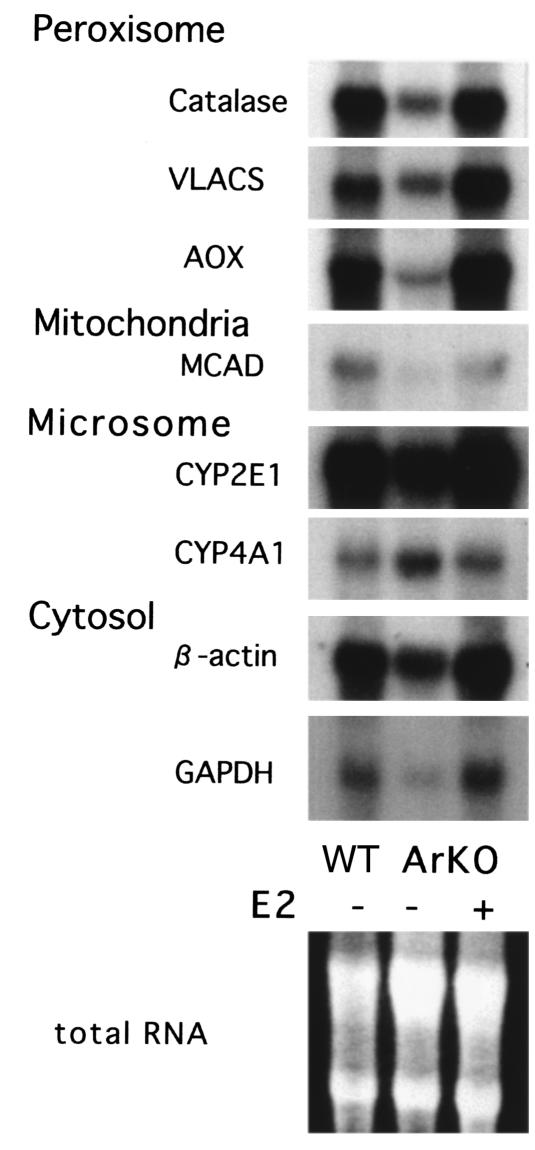
mRNA analysis.
To clarify whether estrogen is involved in hepatic lipid metabolism, constitutive mRNA expression of hepatic peroxisomal (catalase, VLACS, AOX), mitochondrial (MCAD), and microsomal (CYP2E1 and CYP4A1) enzymes were analyzed by Northern blots in the Ar+/+, Ar–/–, and Ar–/– + E2 groups as shown in Figure 2. Constitutive mRNA expression of mitochondrial MCAD (0.15 ± 0.05) and of three peroxisome-associated enzymes, VLACS (0.35 ± 0.05), AOX (0.19 ± 0.04), and catalase (0.17 ± 0.03), were significantly lower in the Ar–/– group than that in the Ar+/+ and Ar–/– + E2 group, whereas microsomal CYP4A1 mRNA expression (1.73 ± 0.09), which is well known to be induced by PPAR-α stimulation (29), was significantly higher in the Ar–/– group (P = 0.043 in Wilcoxon signed rank test).
Figure 2.
Northern blot analysis of gene expression in ArKO mice. Total cellular RNA was isolated from the liver samples of ArKO mice treated or not treated with 17β-estradiol (E2+, E2–) and their wild-type siblings (WT). Probes of peroxisomal and mitochondrial enzymes essential for β-oxidation were used in the assay. Equal amounts of total RNA were applied in the analysis.
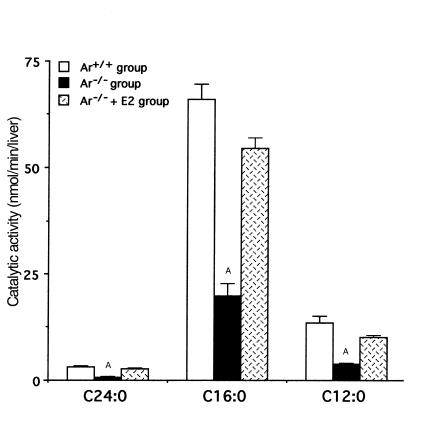
Fatty acid β-oxidation activity.
Compared with the Ar+/+ group, the basal levels of total fatty acid β-oxidation activity in the Ar–/– group were lower using [1-14C]C24:0 (3.26 ± 0.15 vs. 0.73 ± 0.08 nmol/min/liver; P < 0.001), [1-14C]C16:0 (66.0 ± 3.5 vs. 19.8 ± 2.9 nmol/min/liver; P < 0.001) or [1-14C]C12:0 (13.4 ± 1.7 vs. 3.9 ± 0.1 nmol/min/liver; P < 0.001) as the substrates (Figure 3). They reverted very close to the wild-type level by supplementation with 17β-estradiol (2.81 ± 0.14 nmol/min/liver [P < 0.001]; 54.4 ± 2.5 nmol/min/liver [P < 0.001]; and 10.0 ± 0.5 nmol/min/liver [P < 0.001], respectively).
Figure 3.
Impaired hepatic fatty acid β-oxidation activity in ArKO mice. Peroxisomal β-oxidation capacity was assessed using tetracosanoic acid (C24:0) as the substrate, and mitochondrial β-oxidation capacity, using palmitic acid (C16:0) and lauric acid (C12:0). The bars represent the mean ± SD from at least six samples in each group. AStatistically significant difference (P < 0.001) compared with the value obtained with the wild-type group (Ar+/+) and the ArKO mice supplemented with 17β-estradiol group (Ar–/– + E2).
Electrophoretic mobility shift assay.
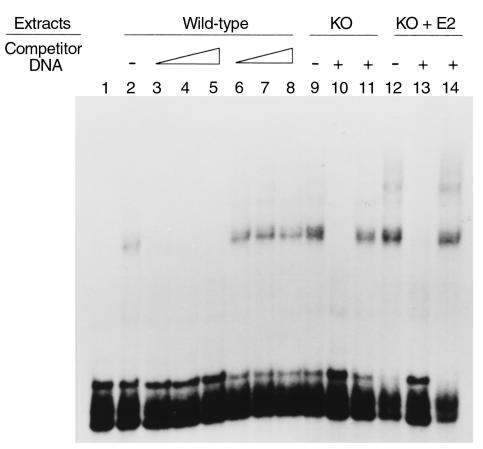
The nuclear extracts (20 μg protein) prepared from the liver of Ar+/+ (lanes 2–8), ArKO (lanes 9–11), or ArKO + E2 (lanes 12–14) mice were incubated with the 32P-labeled probe in the absence of the competitor DNA (lanes 2, 9, and 12) or in the presence of 25-fold molar excess (lanes 3 and 6), 50-fold molar excess (lanes 4 and 7), 100-fold molar excess (lanes 5, 8, 10, 11, 13, and 14) of the unlabeled probe (lanes 3–5, 10, and 13) or the C/EBP binding element (lanes 6–8, 12, and 14) (Figure 4). These findings show that PPAR-α expression was not impaired in ArKO mice and ArKO + E2 mice.
Figure 4.
Electrophoretic mobility shift assays. The nuclear extracts prepared from the liver of wild-type siblings (Ar+/+) (lanes 2–8), KO (Ar–/–) (lanes 9–11) or KO + E2 (Ar–/– supplemented with 17β-estradiol) (lanes 12–14) are incubated with the 32P-labeled PPRE in the absence of the competitor DNA (lanes 2, 9, and 12) or in the presence of 25-fold molar excess (lanes 3 and 6), 50-fold molar excess (lanes 4 and 7), 100-fold molar excess (lanes 5, 8, 10, 11, 13, and 14) of the unlabeled PPRE (lanes 3–5, 10, and 13) or of the C/EBP binding element (lanes 6–8, 12, and 14).
Lipid analysis.
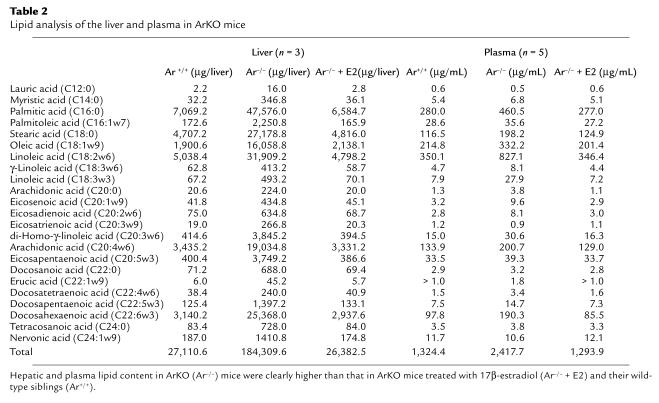
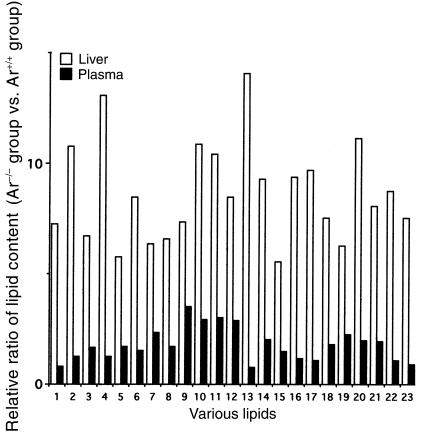
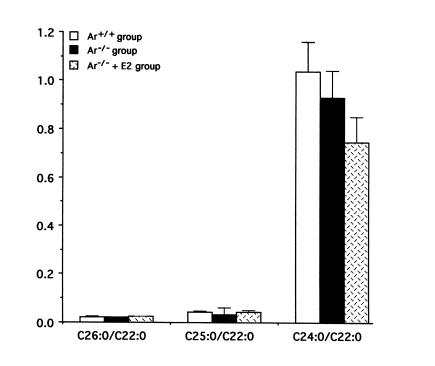
Hepatic lipid content in the Ar–/– group was significantly higher than that in the Ar+/+ and Ar–/– + E2 group (Table 2). Relative ratio of lipid content in Ar+/+ mice versus Ar–/– mice varied from 5.5 to 14.0 in the liver and from 0.75 to 3.5 in the plasma (Figure 5). Three parameters of VLCFAs in plasma sphingomyelin were also similar in these three groups. The C26:0/C22:0 ratio, C25:0/C22:0 ratio, and C24:0/C22:0 ratio were 0.022, 0.045, and 1.038, respectively, in the Ar+/+ group. They were 0.021, 0.033, and 0.930 in the Ar–/– group and 0.025, 0.045, and 0.746 in the Ar–/– + E2 group (Figure 6).
Table 2.
Lipid analysis of the liver and plasma in ArKO mice
Figure 5.
Increased lipid content of the liver and plasma in ArKO mice: a relative ratio of lipid content in Ar–/– mice versus in Ar+/+ mice. 1: C12:0, 2: C14:0, 3: C16:0, 4: C16:1w7, 5: C18:0, 6: C18:1w9, 7: C18:2w6, 8: C18:3w6, 9: C18:3w3, 10: C20:0, 11: C20:1w9, 12: C20:2w6, 13: C20:3w9, 14: C20:3w6, 15: C20:4w6, 16: C20:5w3, 17: C22:0, 18: C22:1w9, 19: C22:4w6, 20: C22:5w3, 21: C22:6w3, 22: C24:0, and 23: C24:1w9.
Figure 6.
Serum VLCFA content. Three parameters of VLCFAs in plasma sphingomyelin are similar in ArKO treated with 17β-estradiol (ArKO + E2) or in those not treated with 17β-estradiol (ArKO) and their wild-type siblings (Ar+/+).
Discussion
In the present study, we demonstrated that the disruption of the cyp 19 gene encoding aromatase resulted in massive hepatic steatosis (Figure 1) as observed in tamoxifen-induced hepatic steatosis in human (17, 18). Inflammatory changes were absent in ArKO mice with massive hepatic steatosis, although tamoxifen-induced hepatic steatosis in human occasionally developed NASH (8, 17–19). This strongly supports the idea that hepatic steatosis is not sufficient and that a “second hit” to hepatic steatosis is needed for the development of NASH (2, 9, 10). During adjuvant tamoxifen therapy, the plasma ferritin concentration was increased only in patients with hepatic steatosis (19). This suggests that oxidative stress and subsequent lipid peroxidation induced by excessive free iron could deteriorate mitochondrial function of hepatocytes and induce hepatocyte necrosis and inflammatory changes in tamoxifen-induced NASH.
Biochemical analysis of ArKO liver revealed that mRNA expression of enzymes involved in peroxisomal and mitochondrial β-oxidation were markedly suppressed in ArKO mice (Figure 2). AOX, VLACS, and MCAD have an essential role in fatty acid β-oxidation of hepatocytes; i.e., VLACS and AOX are enzymes involved in the first two steps of peroxisomal β-oxidation by which VLCFAs are exclusively metabolized, and MCAD is an enzyme catalyzing a rate-limiting step in the mitochondrial oxidation of medium-chain fatty acyl-thioesters produced by peroxisomal β-oxidation of long-chain fatty acids (20, 30). Such suppression of gene expression induced by the lack of estrogen, however, was not due to the generalized impairment of gene expression, because the expression of a gene such as cyp 4A1 was enhanced in the same liver.
Consistent with these findings, β-oxidation activities of C24:0, C16:0, and C12:0 were lower in ArKO mice than in wild-type mice (Figure 3). Supplementation with 17β-estradiol restored these defects and resulted in reverting the hepatic lipid content close to the levels of wild-type mice (Table 2). Furthermore, it was reported that tamoxifen, a potent antagonist of estrogens, frequently induced hepatic steatosis without significant changes in BMI among nonalcoholic, nondiabetic, and nonobese women with breast cancer (17–19). These clinical and laboratory observations in human and ArKO mice suggest that the estrogen receptor–mediated signaling pathway plays important roles in basal hepatic lipid homeostasis.
PPAR-α is another well-established nuclear molecule maintaining homeostasis in hepatocellular fatty acid uptake and utilization (20, 29–32). It binds to PPRE and regulates peroxisome proliferation. Although studies on PPAR-α–deficient mice have demonstrated that loss of the receptor did not affect constitutive gene expression of enzymes involved in peroxisomal β-oxidation (20), we assessed the binding activities of the hepatic nuclear extracts to a synthetic PPRE. The PPRE binding activity was similar in wild-type and ArKO mice (Figure 4), implying that estrogen is not essential in maintaining constitutive PPAR-α expression in hepatocyte nuclei. Therefore, this suggested that administration of peroxisome proliferator could improve hepatic lipid β-oxidation in estrogen deficiency. We administrated bezafibrate to ArKO and succeeded in treatment of severe hepatic steatosis (data not shown). With this finding, we administrated bezafibrate to treat tamoxifen-induced NASH, and, fortunately, severe hepatic steatosis was improved dramatically (18). These findings revealed an important finding that constitutive PPAR-α expression in hepatocyte nuclei is not sufficient enough to salvage defective constitutive lipid β-oxidation in estrogen deficiency, but administration of peroxisome proliferator can activate PPAR-α–mediated signaling pathways even in estrogen deficiency and can improve hepatic steatosis. Although the roles for maintaining constitutive lipid metabolism under normal physiological conditions are largely unknown, peroxisome proliferators are known to alter the expression of estrogen-metabolizing enzymes (33). This suggests that constitutive gene expression of the enzymes essential for fatty acid β-oxidation under normal physiological conditions might be orchestrated by an estrogen receptor–mediated signaling pathway in cooperation with a PPAR-α–mediated signaling pathway.
Long chain fatty acids and VLCFAs are assumed to be an important factor in the pathogenesis of NASH in AOX-deficient mice (2), and reduced mRNA expression of AOX and VLACS was observed in ArKO mice with normal levels of VLCFAs. We suggest that the ArKO mouse could be a useful animal model to investigate the mechanisms involved in tamoxifen-induced NASH among patients with breast cancer.
Acknowledgments
This work was partially supported by grants from the President Research Fund of Kochi Medical School Hospital (to T. Saibara, K. Toda, T. Okada, and H. Enzan), the Mitsui Life Social Welfare Foundation (to K. Toda, T. Okada, and T. Saibara), the Ono Medical Research Foundation, and the Toyota high-tech research grant program (to K. Toda), and by Grants-in-Aid for Scientific Research (C) 09670554 and 11670511 from the Ministry of Education, Science, Sports and Culture Japan (to T. Saibara).
References
- 1.Sherlock, S., and Dooley, J. 1997. Alcohol and the liver. In Diseases of the liver and biliary system. 10th edition. S. Sherlock and J. Dooley, editors. Blackwell Science Ltd. Oxford, United Kingdom. 385–404.
- 2.James O, Day C. Non-alcoholic steatohepatitis: another disease of affluence. Lancet. 1999;353:1634–1636. doi: 10.1016/S0140-6736(99)00163-4. [DOI] [PubMed] [Google Scholar]
- 3.Berson A, et al. Steatohepatitis-inducing drugs cause mitochondrial dysfunction and lipid peroxidation in rat hepatocytes. Gastroenterology. 1998;114:764–774. doi: 10.1016/s0016-5085(98)70590-6. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig J, Viggiano TR, McGill DB, Ott B. Nonalcoholic steatohepatitis: Mayo clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- 5.Kato K, et al. Evaluation of computed tomography in the diagnosis of liver diseases. Liver. 1980;21:1340–1351. [Google Scholar]
- 6.Day CP, James OF. Hepatic steatosis: innocent bystander or guilty party? Hepatology. 1998;27:1463–1466. doi: 10.1002/hep.510270601. [DOI] [PubMed] [Google Scholar]
- 7.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 8.Oien KA, et al. Cirrhosis with steatohepatitis after adjuvant tamoxifen. Lancet. 1999;353:36–37. doi: 10.1016/S0140-6736(05)74872-8. [DOI] [PubMed] [Google Scholar]
- 9.Yang SQ, Lin HZ, Lane MD, Clemens M, Diehl AM. Obesity increases sensitivity to endotoxin liver injury: implications for the pathogenesis of steatohepatitis. Proc Natl Acad Sci USA. 1997;94:2557–2562. doi: 10.1073/pnas.94.6.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day CP, James OFW. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114:842–845. doi: 10.1016/s0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 11.Wilcken NR. Tamoxifen hits the target in situ. Lancet. 1999;353:1986–1987. doi: 10.1016/S0140-6736(99)00194-4. [DOI] [PubMed] [Google Scholar]
- 12.Fisher B, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 13.Fisher B, et al. Tamoxifen in treatment of intraductal breast cancer: National Surgical Adjuvant Breast and Bowel Project B-24 randomised controlled trial. Lancet. 1999;353:1993–2000. doi: 10.1016/S0140-6736(99)05036-9. [DOI] [PubMed] [Google Scholar]
- 14.Pratt DS, Knox TA, Erban J. Tamoxifen-induced steatohepatitis. Ann Intern Med. 1995;123:236. doi: 10.7326/0003-4819-123-3-199508010-00018. [DOI] [PubMed] [Google Scholar]
- 15.Pinto HC, et al. Tamoxifen-associated steatohepatitis: report of three cases. J Hepatol. 1995;23:95–97. doi: 10.1016/0168-8278(95)80316-5. [DOI] [PubMed] [Google Scholar]
- 16.Van Hoof M, Rahier J, Horsmans Y. Tamoxifen-induced steatohepatitis. Ann Intern Med. 1996;124:855–856. doi: 10.7326/0003-4819-124-9-199605010-00015. [DOI] [PubMed] [Google Scholar]
- 17.Ogawa Y, Murata Y, Nishioka A, Inomata T, Yoshida S. Tamoxifen-induced fatty liver in patients with breast cancer. Lancet. 1998;351:725. doi: 10.1016/S0140-6736(05)78493-2. [DOI] [PubMed] [Google Scholar]
- 18.Saibara T, Onishi S, Ogawa Y, Yoshida S, Enzan H. Bezafibrate for tamoxifen-induced non-alcoholic steatohepatitis. Lancet. 1999;353:1802. doi: 10.1016/S0140-6736(05)75907-9. [DOI] [PubMed] [Google Scholar]
- 19.Saibara T, Onishi S, Ogawa Y, Yoshida S, Enzan H. Non-alcoholic steatohepatitis. Lancet. 1999;354:1299–1300. doi: 10.1016/S0140-6736(05)76071-2. [DOI] [PubMed] [Google Scholar]
- 20.Djouadi F, et al. A gender-related defect in lipid metabolism and glucose homeostasis in peroxisome proliferator-activated receptor a-deficient mice. J Clin Invest. 1998;102:1083–1091. doi: 10.1172/JCI3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Akhtar M, Calder MR, Corina DL, Wright JN. Mechanistic studies on C-19 demethylation in oestrogen biosynthesis. Biochem J. 1982;201:569–580. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fishman J, Goto J. Mechanism of estrogen biosynthesis. Participation of multiple enzyme sites in placental aromatase hydroxylations. J Biol Chem. 1981;256:4466–4471. [PubMed] [Google Scholar]
- 23.Toda, K., et al. 2000. Concentrations of monoamines and acetylcholine in the brains of mice lacking the aromatase cytochrome P450 gene. In Molecular steroidgenesis. Frontiers Science Series No. 29. M. Okamoto, Y. Ishimura, and H. Newada, editors. University Academic Press Inc. Tokyo, Japan. 141–143.
- 24.Shindo Y, Osumi T, Hashimoto T. Effects of administration of di-(2-ethylhexyl) phthalate on rat liver mitochondria. Biochem Pharmacol. 1978;27:2683–2688. doi: 10.1016/0006-2952(78)90042-4. [DOI] [PubMed] [Google Scholar]
- 25.Tsuji S, et al. Abnormality of long-chain fatty acids in erythrocyte membrane sphingomyelin from patients with adrenoleukodystrophy. J Neurochem. 1981;36:1046–1049. doi: 10.1111/j.1471-4159.1981.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 26.Jiang SW, Eberhardt NL. A micro-scale method to isolate DNA-binding proteins suitable for quantitative comparison of expression levels from transfected cells. Nucleic Acids Res. 1995;23:3607–3608. doi: 10.1093/nar/23.17.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Osumi T, Ishii N, Miyazawa S, Hashimoto T. Isolation and structural characterization of the rat acyl-CoA oxidase gene. J Biol Chem. 1987;262:8138–8143. [PubMed] [Google Scholar]
- 29.Yeldandi AV, Rao MS, Reddy JK. Hydrogen peroxide generation in peroxisome proliferator-induced oncogenesis. Mutat Res. 2000;448:159–177. doi: 10.1016/s0027-5107(99)00234-1. [DOI] [PubMed] [Google Scholar]
- 30.Aoyama T, et al. Altered constitutive expression of fatty acid-metabolizing enzymes in mice lacking the peroxisome proliferator-activated receptor α (PPARα) J Biol Chem. 1998;273:5678–5684. doi: 10.1074/jbc.273.10.5678. [DOI] [PubMed] [Google Scholar]
- 31.Hashimoto T. Peroxisomal beta-oxidation enzymes. Neurochem Res. 1999;24:551–563. doi: 10.1023/a:1022540030918. [DOI] [PubMed] [Google Scholar]
- 32.Ren B, Thelen AP, Peters JM, Gonzalez FJ, Jump DB. Polyunsaturated fatty acid suppression of hepatic fatty acid synthase and S14 gene expression does not require PPAR-alpha. J Biol Chem. 1997;272:26827–26832. doi: 10.1074/jbc.272.43.26827. [DOI] [PubMed] [Google Scholar]
- 33.Bocos CJC, Moreno ES, Merritt A, Cattley RC, Gustafsson JA. Peroxisome proliferators alter the expression of estrogen-metabolizing enzymes. Biochemie. 1997;79:151–162. doi: 10.1016/s0300-9084(97)81508-8. [DOI] [PubMed] [Google Scholar]