Clinical summary
Childhood obesity has important consequences for health and wellbeing both during childhood and also in later adult life. The rising prevalence of childhood obesity poses a major public health challenge in both developed and developing countries by increasing the burden of chronic non-communicable diseases. Despite the urgent need for effective preventative strategies, there remains disagreement over its definition due to a lack of evidence on the optimal cut-offs linking childhood BMI to disease risks, and limited evidence on the most effective components of interventions to prevent childhood obesity. This article reviews the trends in childhood obesity, its genetic, nutritional and other risk factors, and preventative and treatment strategies. Particular emphasis is given to early-onset obesity in pre-school children, which, as a precursor to later childhood and adult obesity, provides insights into the developmental and genetic origins of obesity and also offers the potential for early preventative approaches with long-lasting benefits.
Prevalence and trends
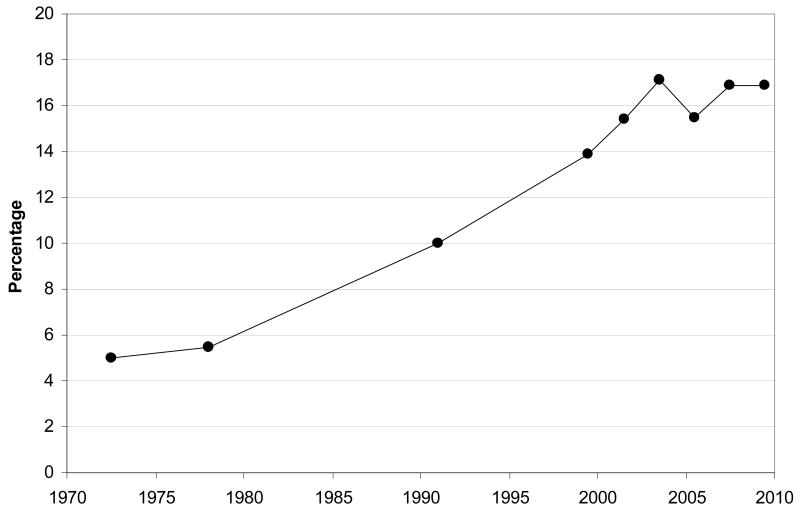
The prevalence of childhood obesity has almost tripled in US children and adolescents since 1980 1, suggesting that it may be increasing at a faster rate than adult obesity with major implications for the population’s future health. The proportion of children in the highest percentiles of BMI has seen the most rapid increase. In some western settings such as the US, Western Europe, Australia and Japan, recent data suggest that levels of childhood obesity may have reached a plateau in the last decade (Figures 1 and 2) 1-4. Even if this were the case, the prevalence of both childhood and adult obesity remains very high and understanding and managing the burden of childhood obesity presents a resilient challenge. Furthermore, the trends may be continuing among children from more disadvantaged social conditions 5 and among those from certain ethnic backgrounds. In the US, between 1971-1974 and 1999-2002 the prevalence of 6- to 11-year olds with a BMI > 95th percentile increased by 5-fold (4% to 20%) among black children compared to 3-fold (4% to 13%) among white children, with intermediate rates of increase seen in Mexican-American children.
Figure 1.
Prevalence of obesity (BMI > 95th centile) among children and adolescents aged 2-19 in the United States between 1971-74 to 2009-10 shows a recent plateau from 2003-04 onwards.
Source: Based on data from the National Health and Nutrition Examination Survey (NHANES) published in Ogden et al., Health E-Stat 2010 1 and Ogden et al., Journal of the American Medical Association 2012 4.
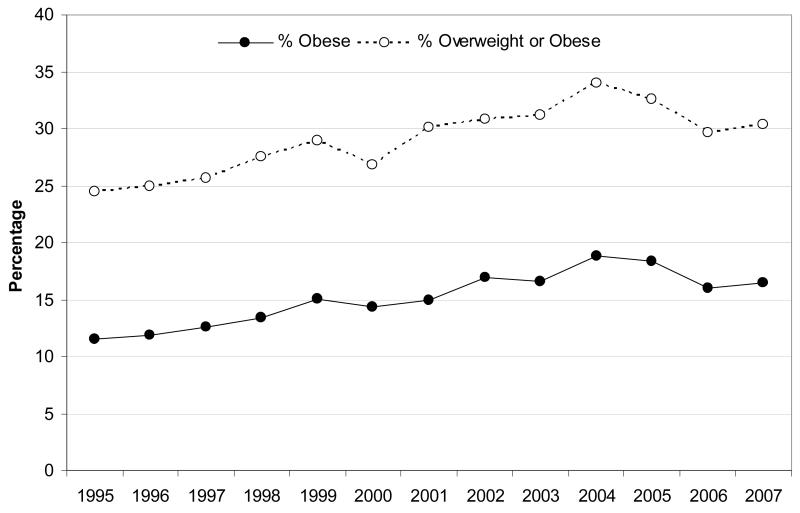
Figure 2.
Recent plateau in the prevalences of obesity (BMI > 95th centile) and ‘overweight or obesity’ (BMI > 85th centile) among children aged 2-15 in England, 1995-2007
Source: Based on data published by the Association of Public Health Observatories 2
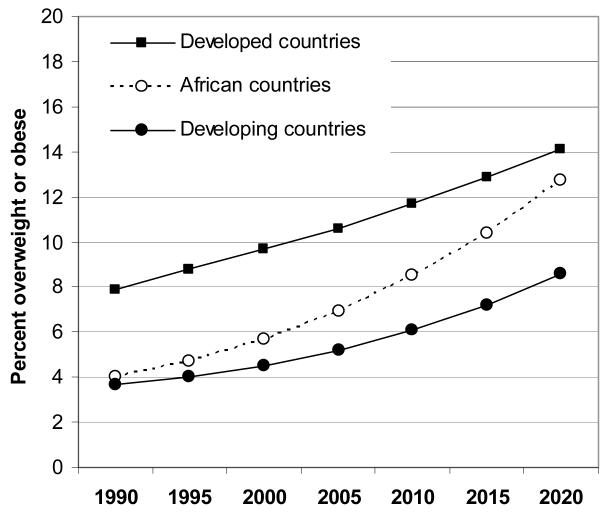
Increases in obesity levels have been observed even in very young preschool children and are predicted to continue. Based on an analysis of 450 nationally representative surveys from 144 countries, the World Health Organisation (WHO) estimated that the prevalence of children below age 5 years old with a BMI > +2 SD (equivalent to the 98th percentile) increased from 4.2% in 1990 to 6.7% in 2010, and is expected to reach 9.1% in 2020 6. Estimates in 2010 were higher in developed (11.7%) rather than developing countries (6.1%), although the relative changes in prevalence have been higher in developing countries, and particularly in African countries (Figure 3) 6. Nationally, there was wide variation in the prevalence of BMI > 98th centile in preschool children; the highest rates were seen in countries such as Albania, Bosnia and Herzegovina, and Ukraine, with prevalences >25% in the most recent surveys. In the US, based on the National Health and Nutrition Examination Survey 1999-2004, 8.3% of under-5 year-olds had a BMI > 98th centile using the same WHO reference 7. These worldwide trends in very young children are dramatic and have raised debate as to whether we can and should diagnose obesity even during infancy 8.
Figure 3.
Trends between 1990-2010 and predicted ongoing rise 2010-2020 in the prevalence of BMI > +2 SD (equivalent to the 98th centile) in preschool children in developed and developing countries.
Source: Based on data published in de Onis et al., Public Health Nutrition 2010 6, 74
Health consequences
Adult obesity & disease
There is strong evidence that childhood obesity leads to adult obesity and its related comorbidities 9. Furthermore, many obese children continue to progress in their severity of obesity. In the US National Longitudinal Study of Adolescent Health, nearly 40% of obese adolescents (BMI > 95th percentile) became severely obese (BMI > 40 kg/m2) by age 30 years, compared to less than 5% of normal weight teenagers 10. In a recent study of Pima Indians in Arizona US, those with the highest quartile of childhood BMI had double the incidence of death from endogenous causes in adult life compared to those in the lowest childhood BMI quartile (incidence-rate ratio 2.3 [1.46-3.62]) 11. In the US Bogalusa Heart Study, overweight during adolescence was associated with an 8.5-fold increase in hypertension, a 2.4-fold increase in the prevalence of high total serum cholesterol levels, a 3-fold increase in high LDL cholesterol levels and an 8-fold increase in low HDL cholesterol levels as adults aged 27–31 years 12. In a study of 276,835 Danish schoolchildren, childhood BMI at ages 7-13 years was positively associated with fatal and non-fatal CHD events during adulthood 13. The associations were linear at each age indicating that the risk of CHD increases across the entire BMI distribution. Furthermore, the effect of BMI increased with increasing age of the child, and adjustment for birth weight strengthened the results, suggesting that postnatal gains in weight or adiposity may explain these links 13. There is also evidence that childhood obesity may increase adult morbidity and mortality independently of adult BMI and other confounding factors such as family history of cardiovascular diseases or cancer and smoking 14.
Childhood comorbidities
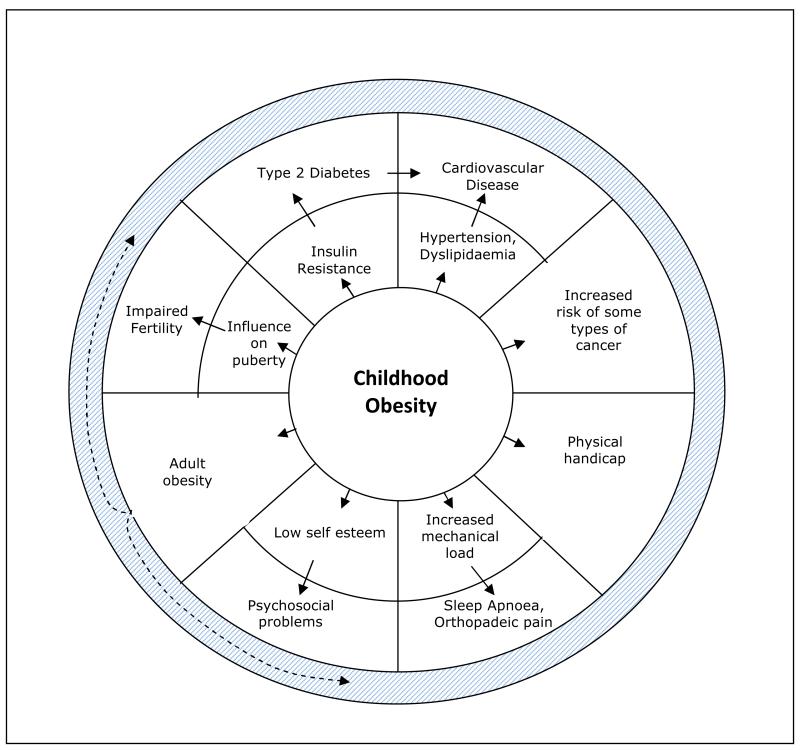
The need to tackle childhood obesity lies not only in the avoidance of poor adult health. Childhood obesity leads to many acute health problems and much suffering during childhood (Figure 4). Therefore parents, healthcare providers and policy-makers should be clearly impressed upon that prevention of childhood obesity is an important outcome in its own right. These BMI-related childhood and adolescent outcomes include type 2 diabetes, hypertension, early puberty, menstrual irregularities and polycystic ovary syndrome, steatohepatitis, sleep apnoea, asthma, benign intracranial hypertension, musculoskeletal disorders and psychological problems 15. A recent systematic review also found strong evidence for type 1 diabetes as a consequence of childhood obesity; that review identified 9 studies, comprising 2658 type 1 diabetes cases, where the assessment of childhood obesity preceded the diagnosis of diabetes 16. Some of the consequences of obesity, such as type 2 diabetes, hypertension and hyperlipidemia, were previously only seen in adults, but are now frequently observed in obese children in some populations. For type 2 diabetes the younger age at onset of disease not only prolongs the duration of the disease, but is associated with a more advanced rate of progression to beta cell failure 17 and is likely to lead to earlier presentation of adult life complications such as cardiovascular disease 18, kidney failure, visual impairment and limb amputations 19.
Figure 4.
Schematic summary of the complications of childhood obesity. Comorbidities of childhood obesity are depicted in the outer ring with their intermediate processes in the inner ring. Childhood obesity also increases the risk of adult obesity, which in turn also increases the likelihood of those comorbidities.
Furthermore, childhood obesity can severely influence quality of life via its impact on social and psychological functioning, having been linked with low self-esteem and depression 20, as well as educational attainment and interpersonal relationships 21. It is plausible that the causal relationships between many of these obesity-related complications are bi-directional; adverse health, stress or poor psychosocial functioning could lead to sedentary lifestyles and subsequent greater BMI.
Definition
Obesity is defined as a condition of ‘excess body fat which creates increased risk for morbidity and/or premature mortality’ and the adult BMI thresholds 25 and 30 kg/m2 for overweight and obesity respectively are based on prospective associations between BMI in middle- to late-aged adults in relation to their subsequent mortality 22. In contrast, there is little consensus as to the best way to operationalize this definition in children.
In many countries, including in the US since 2010 23, childhood obesity is defined as a BMI above the 95th percentile for age and sex (and >85th percentile for overweight), however there is a wide range of reference BMI charts available. In contrast, the WHO and UK use statistical based cut-offs corresponding to number of standard deviations above the median. The WHO uses a BMI > +2 SD, which is equivalent to the more extreme 98th percentile, to define overweight, while in the UK this same threshold is used to define obesity in clinical practice settings, because 91st and 98th percentile lines are typically displayed on growth charts, rather than the 85th and 95th percentiles 24. A third approach was proposed by the International Obesity Task Force (IOTF) based on identifying the childhood BMI thresholds that correspond with adult definitions. Reference charts were created from international data on 97,876 boys and 94,851 girls from Brazil, America, the UK, Hong Kong, Singapore and the Netherlands. BMI percentile lines were drawn from 2-18 years that pass through the adult overweight and obesity BMI cut-off points of 25 and 30 kg/m2 at age 18 years 25. These IOTF criteria are the most stringent of all the current definitions of childhood obesity because relatively few 18 year olds have such high BMI levels, and the IOTF threshold for childhood obesity is roughly equivalent to the 99th percentile…
Some may consider that stringent BMI thresholds for childhood obesity are more appropriate in order to better identify those children at highest risk of co-morbidities. However, while the use of higher BMI thresholds will increase specificity and positive predictive values, this should be balanced against the inevitable increase in the likelihood of false negatives (i.e. children with BMI below the 99th percentile but who are still at increased risk for obesity-related comorbidities). Currently there are little data on the true shape of the association between childhood BMI and any important child health outcomes to inform the optimal prediction thresholds.
Childhood risk factors
While the core of the problem of obesity can be simply stated as an imbalance between energy intake and energy expenditure over a prolonged period, the factors behind this are complex. The UK Foresight report described obesity as a ‘complex web of societal and biological factors that have, in recent decades, exposed our inherent vulnerability to weight gain’. That report presented an obesity system map with energy balance at its centre being influenced by over 100 variables acting at the individual, household, community or wider societal levels 26.
A description of these wide-ranging childhood risk factors is well beyond the scope of this article, and we refer the reader to recent systematic reviews that describe the evidence for a number of individual-level lifestyle factors that affect the energy intake-energy expenditure balance have been shown to behave been associated with childhood weight gain and obesity in school-aged children, including the intakes of sugar-sweetened beverages 27, dietary fat 28, dietary energy density 29, physical activity 30, sedentary behaviours 31,32, and short sleep duration 31.. In a review of prospective studies, the authors concluded that these classical individual-level lifestyle risk factors do seem to play a role in the development of obesity in school-age children and adolescents, and that the most consistent evidence was for sugar-sweetened beverages 28. They also commented on the value of studying new risk factors such as short sleep duration, chronic inflammation, anxiety, depression, and behavioural problems as potential targets for future interventions in this age group 28.
Early life and inter-generational factors
Considering the rapid rise in prevalence of early-onset obesity in pre-school children, and its links to later childhood and adult obesity, particular attention should be paid to identifying the early life risk factors for obesity with a view to developing strategies for the early prediction and prevention of obesity.
Birth weight and antenatal factors
There is consistent evidence from large cohort studies of a linear and positive association between birth weight and later life BMI, and this may be equally attributable to correlations with adiposity and lean mass 33. Furthermore, maternal obesity, gestational weight gain and glycaemia during pregnancy are positively associated with offspring obesity and metabolic disorders 34, 35. Such intergenerational associations may be explained by genetic transmission and shared postnatal environment. In addition, animal models show long-term ‘programming’ effects of antenatal exposure to maternal diet, and indirect support for this in humans comes from a study of pre-pregnancy bariatric surgery which was associated with a 52% lower risk of offspring obesity 36.
In contrast to the positive birth weight associations with obesity, studies of the major co-morbidities of obesity generally report inverse birth weight associations. For example, each 1 kg higher birth weight was associated with a 10-20% lower risk of ischaemic heart disease 37, and with a 1.5 mmHg lower systolic blood pressure in men and 2.8 mmHg lower in women 38. A meta-analysis of the association between birth weight and type 2 diabetes identified increased risks associated with both low (<2.5 Kg, odds ratio 47% higher) and high birth weights (>4 kg, odds ratio 36% higher) compared to the reference group (birth weight 2.5-4 kg) 39.
These contrasting associations with both low and high birth weight might be explained by the co-existence of two separate early life pathways to obesity and later metabolic disease.
The ‘thrifty’ or ‘mismatch’ pathway
Barker and Hales proposed that the relationship between low birth weight and later disease susceptibility was a result of fetal adaptations, such as insulin resistance, to survive antenatal undernutrition, but which were then inappropriate in the face of a subsequent affluent postnatal environment 40. To better explain why they should persist into postnatal life, Gluckman and colleagues proposed that these adaptations were ‘predictive adaptive responses’; i.e. that the fetus exposed to poor nutrition anticipates a similar harsh postnatal environment 41. These responses include: a preference for a high-fat diet, hyperphagia, less investment in muscle mass and greater deposition of visceral adipose stores. The term ‘thin-fat baby’ has been coined to describe this phenotype in some South Asian babies, and which is exacerbated by an obesogenic childhood environment 42. Thus later life obesity and metabolic risk may be determined by the mismatch between the intrauterine and subsequent postnatal environment 41, 43.
The ‘early life hyper-nutrition’ pathway
The second proposed developmental pathway to obesity follows the effects of hypernutrition during fetal and/or early postnatal life. Maternal hyperglycemia leads to increased glucose transport across the placenta, and in turn leads to increased insulin secretion by the fetal pancreas. Insulin is adipogenic in late fetal and infant life, and probably increases both fat cell number and content 44. Increased fetal adipogenesis is believed to underlie the macrosomia observed in infants of diabetic mothers 45. The relevance of this pathway may be of increasing importance in settings of rising maternal obesity, gestational weight gain and hyperglycemia.
Weight gain during infancy
Infancy is the period of life with the highest rates of weight gain, both in absolute terms (approximately 6 kg in the first year) and also relative to body size (the average infant triples in size during the first year). Faster infancy weight gain is consistently associated with increased risk of childhood and adult obesity, and there is increasing evidence from randomised controlled trials to support the existence of long-term ‘programming’ effects of infant nutrition and weight gain on later obesity and obesity-related diseases 46.
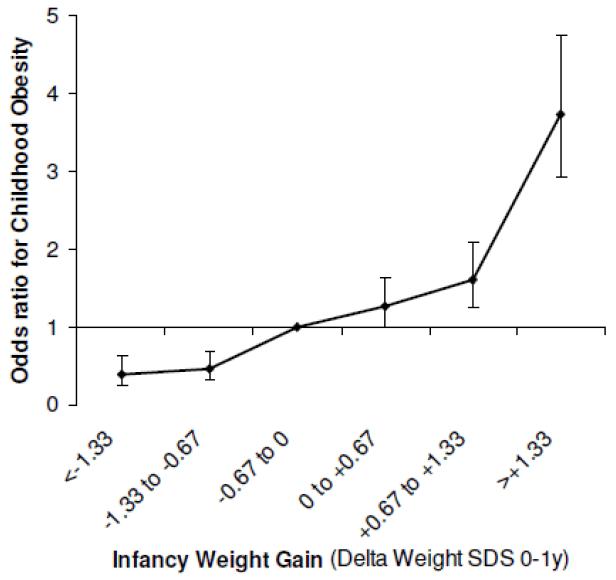
Overall, there appears to be a two- to three-fold increase in childhood obesity risk in those infants who cross upwards through at least one major weight centile band on their growth chart (e.g. 2nd to 9th or 9th to 25th centile etc.), which is equivalent to a gain in weight z-score > 0.67, between birth to age 1 year (Figure 5) 47. In a meta analysis of 7 studies, this rapid early growth pattern also predicted adult obesity up to age 66 years 47. In addition to higher BMI, rapid infancy weight gain is associated with greater total adiposity, central adiposity, and higher metabolic syndrome risk markers in young adults 48-50.
Figure 5.
Odds ratio for childhood obesity by infant weight gain between 0-1 year, adjusted for sex, age and birth weight.
Source: Figure reproduced from Druet et al, Paediatric and Perinatal Epidemiology, 2012 47
In historical cohorts, being thin at birth and thin until around age 2 years, but then showing rapid childhood weight gain after this period, was associated with increased risk for later cardiovascular disease 51 and impaired glucose tolerance 52. In contrast, in a contemporary cohort, rapid weight gain (> 0.67 z-score) within the first 3 months of life had an even greater influence on markers of cardiovascular disease and type 2 diabetes in early adulthood compared to a similar rate of rapid weight gain spread over the first 12 months of life 50. Hence both the degree and timing of infant and childhood weight gain may be important in conferring later disease risk.
Infant nutrition
In most developed settings, formula milk fed infants are heavier and grow faster than breastfed infants. Indeed, these early growth differences form the rationale for the WHO 2006 International Growth Standard for 0-5 year old children, which is based on predominantly breastfed children 53. Accordingly most observational studies report that obesity risk at school age is 15-20% lower in breastfed compared to formula milk-fed infants 54. Systematic reviews have also shown that breastfeeding is associated with a lower risk of type 2 diabetes 55, lower cholesterol levels 56 and blood pressure 57 in adulthood. However, breastfeeding is strongly socially patterned and is associated with other healthy behaviours and therefore it is possible that observational links between breastfeeding and better health outcomes are confounded by these and other unmeasured factors 58. A randomised controlled trial of breastfeeding promotion found no effect on childhood obesity 59, however in that setting breastfed infants were heavier than formula milk fed infants, which could explain the lack of protection against childhood obesity.
Among formula milk fed infants, higher intakes of milk and other sources of energy are positively associated with infancy weight gain and childhood BMI 60. Indeed, as a percentage of total energy intake, the energy demands for growth are substantially higher during infancy than during later childhood. Energy deposition as a percentage of total energy requirements decreases from: 40% at 1 month, to 17.5% at 3-6 months, 6% at 6 to 12 months, 3% at 12 months, 1-2% from 12 months until mid-adolescence, and gradually disappears by 20 years of age 61. Therefore infancy weight gain is more closely related to energy intake, than is weight gain in childhood or in later life.
A few studies have investigated the role of age at introduction of complementary foods (weaning) in childhood obesity. The evidence suggests that complementary foods displace milk intakes, with no clear effect on energy intake or obesity risk 62. Furthermore reverse causality cannot be ruled out because infants who gain weight rapidly are more likely to be weaned earlier 63.
Genetic factors
Monogenic obesity
Over the last 15 years, profound insights into the biological regulation of appetite, food intake and weight gain have been gained by identifying and characterising the rare genetic mutations in individuals and families with extreme obesity. Deleterious mutations have been detected in genes encoding leptin, the leptin receptor, pro-opiomelanocortin, melanocortin-4 receptor, and brain derived neurotropic factor 64. Other causes of severe monogenic obesity associated with hyperphagia and disorders or learning and behaviour arise due to large chromosomal deletions 65. It is noteworthy that many of these advances in the understanding of the genetics of obesity have been achieved through studies of extremely obese children with early-onset obesity, and this possibly reflects stronger effects of these rare mutations on BMI gains in children than in adults 66, 67.
Common genetic variants
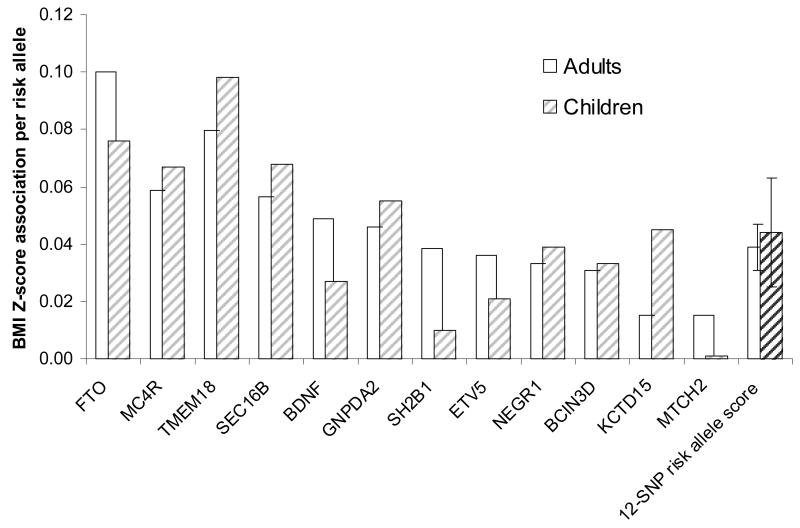
Advances in understanding the polygenic basis of common obesity and normal variations in BMI have only occurred much more recently. Genome-wide association studies (GWAS) in large-scale population-based studies have identified several common genetic variants that are robustly associated with adult BMI and obesity. The first BMI locus was found in the FTO locus in 2007, followed by MC4R in 2008, and loci in or near to TMEM18, SH2B1, KCTD15, MTCH2, NEGR1, BDNF, SEC16B, GNPDA2 and ETV5 in 2009 and 18 further loci in 2010 68. While the focus of those studies was on BMI or obesity in adult populations, those reports also showed that several of these variants showed significant associations with childhood BMI 68, 69. Indeed, recent follow-up studies have reported that most of these variants have comparable effect sizes in children and adolescents compared to adults, in terms of age standardised BMI Z-scores (Figure 6) 70.
Figure 6.
Comparison of the effect sizes on BMI Z-score in adults versus in children of various obesity-related genetic variants.
The 12 single nucleotide polymorphism (SNP) risk allele score represents the effect of each additional obesity risk allele averaged across all 12 variants (error bars represent 95% CI).
Source: Based on data published in den Hoed et al., Diabetes 2010 70
Other studies have explored longitudinal BMI and growth associations with these BMI loci. In the prospective 1946 British Birth Cohort, associations between the FTO and MC4R variants and BMI and body weight loci strengthened during childhood and adolescence, peaked at age 20, and then weakened into adulthood 71. In larger studies, the FTO variant has a surprising inverse association with infancy BMI; rather it confers an earlier adiposity rebound in early childhood and subsequent higher childhood BMI 72, but then appears to have no further positive effect on weight gain during adult life 73. In combination, the GWAS obesity variants appear to have little influence on birth weight, but promote more rapid weight gain and growth from even the earliest weeks of postnatal life, and prevent against risks of underweight and failure to thrive, which may point to a potential evolutionary advantage of genetic obesity susceptibility 74. These findings of the childhood timing of action of genetic mutations and common variants are supported by the observation that the heritability of BMI changes with age, increasing with age in childhood and decreasing with age in adults, and in general is higher in children than in adults 75.
With regard to its mechanism of action, the obesity susceptibility variant at FTO has been associated with increased dietary energy intake in children 76 and associated traits, such as lower levels of satiety 77 and a greater intake of dietary fat 78. However there is some discordance with the results of animal models which a highlight a role of FTO in the regulation of energy expenditure 79. While the use of further genomic approaches hope to explain substantially more of the heritability of BMI, the current findings are already helping to direct new avenues of research into the biological regulation of body weight 79.
Prevention
In 2010, Michelle Obama launched the ‘Let’s Move’ childhood obesity prevention campaign in the US, with the aim to ‘solve the childhood obesity problem within a generation’. In 2009, the UK government set a target to reduce the proportion of overweight and obese children to 2000 levels by 2020 80. Such ambitious targets require health and education policies that are based on well-evidenced intervention components.
Interventions in school-age children
Schools have been a popular setting for obesity intervention, as they offer continuous and intensive contact with children. School infrastructure and physical environment, policies, curricula and staff all have the potential to positively influence knowledge and lifestyle. A recent systematic review found strong evidence for beneficial effects of childhood obesity prevention programmes based on 27,946 children and adolescents in 37 studies 81. A large majority of the identified studies were based in school settings among children aged 6-12 years old and these appeared to be more effective in reducing BMI (mean change in BMI z-score: −0.17; 95% CI: −0.25 to −0.09) than studies that took place in non-educational or mixed settings (−0.07; 95% CI: −0.24 to 0.10). The most promising school-based intervention strategies were: inclusion of teaching on healthy eating, physical activity and body image within the school curriculum; more school-based sessions on physical activity and movement skills; better nutritional quality of schools food; and better support for teachers and staff to implement health promotion. However, the studies usually used complex interventions and there was wide heterogeneity in the results, which made it very difficult to identify the most effective intervention components, but suggest that combined diet and physical activity school-based interventions may help prevent children becoming overweight and there was no evidence for any adverse effects 81.
Only a few studies have tested obesity prevention strategies in older school-age children aged 13-18 years old, and together they provide only weak evidence for effectiveness as assessed by reduction in BMI (mean change in BMI z-score: −0.09; 95% CI: −0.20 to 0.03) 81. Furthermore, in this age group who are potentially more sensitive to issues related to body image, such as stigmatisation, low self-esteem and unhealthy dieting patterns, none of the studies explicitly reported on the potential harms of intervention 81.
Interventions in infants and pre-school children
There have been far fewer intervention studies in pre-school compared to school-age children, although the limited evidence suggests that larger treatment effects may be achieved in this age group 81. In particular, very early intervention during infancy could present a ‘window of opportunity’ for obesity prevention as it is a period of developmental plasticity, rapid weight gain and habit formation. Interventions in early life could potentially influence feeding patterns before they have been established and become more difficult to modify 82..
Long-term follow-up of earlier RCTs in preterm and small for gestational age infants have reported that standard versus nutrient-enriched formula milks prevent faster infancy weight gain and lead to lower adiposity and cardiovascular risk factors in adolescence 83, 84. The STRIP trial (the Special Turku Coronary Risk factor Intervention Project) showed that reduced saturated fat intake from age 7 months onwards had beneficial effects on serum cholesterol levels and lowered blood pressure during adolescence, although in that study the active intervention was continued well beyond infancy and pre-school ages 85.
Other trials of ‘childhood obesity prevention’ during infancy are in progress. Two RCTs are testing modified compositions of formula milk, while eight are testing behavioural interventions to promote breastfeeding, longer sleep duration, physical activity or healthy eating. For example, ERNEST (the multi-centre European Childhood Obesity Project) randomised 1000 infants to high- versus low-protein formula milks. Infants on the low-protein formula had lower weight gain, similar to the reference breastfed group 86. The US SLIMTIME pilot trial taught parents soothing strategies to reduce feeding for ‘non-hunger related fussiness’, to prolong sleep duration, to delay introduction of solid food and to increase acceptance of healthy foods through repeated exposure and has reported promising results 87.
In the slightly older pre-school age group, recent systematic reviews 88, 89 found an absence of effective interventions to prevent obesity. The first review 88 included only three studies that reported BMI as an outcome, while the second review included 23 studies that reported BMI, diet or physical activity as an outcome 89. It is interesting to note that the second review found that parental involvement was important, that parents were receptive to intervention programs and in some cases made positive changes to diet and physical activity in young children.
Treatment
The US Preventive Services Task Force (USPSTF) recently positively reviewed the evidence for lifestyle interventions in obese children 90. In 13 trials comprising 1258 overweight or obese children and adolescents they found adequate evidence for short-term (up to 12 months) improvements in BMI from moderate- to high-intensity (but not low intensity) comprehensive interventions that include dietary, physical activity, and behavioural counselling components in obese children and adolescents aged 6 years and older, with only small risks of harm. It is notable that no studies were identified that targeted those younger than 4 years. Based on these findings, and the adequacy of BMI as an acceptable measure for identifying children and adolescents with excess weight, the USPSTF were bold enough to recommend routine screening for obesity in children aged 6 years and older 90. Harms of screening were judged to be minimal and therefore, the net benefit of screening was judged to be at least moderate. However, how widely these recommendations are taken up will also depend on much needed health economics analyses to demonstrate the cost-effectiveness of such strategies.
The disappointing side-effect profiles and withdrawal of appetite suppressant medications, such as fluoxetine, rimonabant and sibutramine 91, have left limited pharmacological options for weight management in obese adults, let alone in obese children in whom the longer treatment durations needed to avoid disease end-points may lead to less favourable risk:benefit calculations. Orlistat, a gastric and pancreatic lipase inhibitor, modestly reduces BMI (by approximately −0.24 z-scores) in children and adolescents, but has an unacceptably high prevalence of gastrointestinal adverse effects leading to frequent premature discontinuation in the absence of adequate dietetic support 92.
Finally, there is limited evidence that the insulin sensitizing agent metformin has moderate efficacy in obese in children, reducing BMI by on average 1.42 kg/m2 93. In small randomised trials of girls presenting with precocious pubarche and history of low birth weight, who tend to have high central adiposity and insulin resistance, low-dose metformin during the peri-pubertal years has been reported to have remarkable benefits both on reducing short-term symptoms of oligomenorrhoea and hirsutism and also on long-term increases in lean body mass and height and reductions in adiposity and other markers of metabolic disease 94. Similar trials of metformin in other groups of insulin-resistant children are warranted to confirm these potential re-programming effects of insulin sensitisation therapy during puberty.
Conclusions
The promising signs that the rates of increase in obesity in children and adolescents are starting to slow have been attributed to wider awareness of its adverse health effects 26, 95. However, in order to turn the corner from plateau to steady decline in obesity rates will require informed and decisive actions, which, considering its complex causes and contributing factors 26, will need to involve multi-component and multi-sector policy interventions. Increasing understanding of the early developmental origins of obesity has led to a growing interest in the development and trials of interventions starting in early life, which have the potential for larger and longer-lasting benefits.
Footnotes
Disclosures
No conflicts to disclose
Reference List
- (1).Ogden CL, Carroll MD. Prevalence of Obesity Among Children and Adolescents: United States, Trends 1963-1965 Through 2007-2008. Health E-Stat. 2010 Available at: URL: http://www.cdc.gov/nchs/data/hestat/obesity_child_07_08/obesity_child_07_08.htm. [Google Scholar]
- (2).Association of Public Health Observatories International comparisons of obesity prevalence: National Obesity Observatory. Health Survey for England 1995-2007. 2009 Available at: URL: http://www.noo.org.uk/publications.php5?rid=718. [Google Scholar]
- (3).Rolland-Cachera MF, Peneau S. Stabilization in the prevalence of childhood obesity: a role for early nutrition. Int J Obes. 34:1524–5. doi: 10.1038/ijo.2010.64. 2010 online. [DOI] [PubMed] [Google Scholar]
- (4).Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Babey SH, Hastert TA, Wolstein J, Diamant AL. Income disparities in obesity trends among California adolescents. Am J Public Health. 2010;100:2149–55. doi: 10.2105/AJPH.2010.192641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).de Onis M, Blossner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. 2010;92:1257–64. doi: 10.3945/ajcn.2010.29786. [DOI] [PubMed] [Google Scholar]
- (7).Mei Z, Ogden CL, Flegal KM, Grummer-Strawn LM. Comparison of the prevalence of shortness, underweight, and overweight among US children aged 0 to 59 months by using the CDC 2000 and the WHO 2006 growth charts. J Pediatr. 2008;153:622–8. doi: 10.1016/j.jpeds.2008.05.048. [DOI] [PubMed] [Google Scholar]
- (8).McCormick DP, Sarpong K, Jordan L, Ray LA, Jain S. Infant obesity: are we ready to make this diagnosis? J Pediatr. 2010;157:15–9. doi: 10.1016/j.jpeds.2010.01.028. [DOI] [PubMed] [Google Scholar]
- (9).Serdula MK, Ivery D, Coates RJ, Freedman DS, Williamson DF, Byers T. Do obese children become obese adults? A review of the literature. Prev Med. 1993;22:167–77. doi: 10.1006/pmed.1993.1014. [DOI] [PubMed] [Google Scholar]
- (10).The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304:2042–7. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood Obesity, Other Cardiovascular Risk Factors, and Premature Death. N Engl J Med. 2010;362:485–93. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Srinivasan SR, Bao W, Wattigney WA, Berenson GS. Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism. 1996;45:235–40. doi: 10.1016/s0026-0495(96)90060-8. [DOI] [PubMed] [Google Scholar]
- (13).Baker JL, Olsen LW, Sorensen TIA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–37. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Must A, Jacques PF, Dallal GE, Bajema CJ, Dietz WH. Long-term morbidity and mortality of overweight adolescents. A follow-up of the Harvard Growth Study of 1922 to 1935. N Engl J Med. 1992;327:1350–5. doi: 10.1056/NEJM199211053271904. [DOI] [PubMed] [Google Scholar]
- (15).Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJH. Health consequences of obesity. Arch Dis Child. 2003;88:748–52. doi: 10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Verbeeten KC, Elks CE, Daneman D, Ong KK. Association between childhood obesity and subsequent Type 1 diabetes: a systematic review and meta-analysis. Diabet Med. 2011;28:10–8. doi: 10.1111/j.1464-5491.2010.03160.x. [DOI] [PubMed] [Google Scholar]
- (17).Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care. 2005;28:638–44. doi: 10.2337/diacare.28.3.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Gungor N, Thompson T, Sutton-Tyrrell K, Janosky J, Arslanian S. Early signs of cardiovascular disease in youth with obesity and type 2 diabetes. Diabetes Care. 2005;28:1219–21. doi: 10.2337/diacare.28.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Dean HJ, Sellers EA. Comorbidities and microvascular complications of type 2 diabetes in children and adolescents. Pediatr Diabetes. 2007;8(Suppl 9):35–41. doi: 10.1111/j.1399-5448.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- (20).Griffiths LJ, Parsons TJ, Hill AJ. Self-esteem and quality of life in obese children and adolescents: A systematic review. International Journal of Pediatric Obesity. 2010;5:282–304. doi: 10.3109/17477160903473697. [DOI] [PubMed] [Google Scholar]
- (21).Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329:1008–12. doi: 10.1056/NEJM199309303291406. [DOI] [PubMed] [Google Scholar]
- (22).World Health Organisation . Report of a Joint WHO/FAO Expert Consultation. Diet Nutrition and the Prevention of Chronic Diseases. WHO; Geneva: 2002. (WHO Technical Report Series no. 916). [PubMed] [Google Scholar]
- (23).Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. Natl Health Stat Report. 2010:1–5. [PubMed] [Google Scholar]
- (24).Cole TJ, Freeman JV, Preece MA. Body mass index reference curves for the UK, 1990. Arch Dis Child. 1995;73:25–9. doi: 10.1136/adc.73.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: an international survey. BMJ. 2000;320:1–6. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Butland B, Jebb S, Kopelman P, McPherson K, Thomas S, Mardell J. Tackling Obesities: Future Choices (Foresight Project Report) 2008. Available at: URL: http://www.bis.gov.uk/assets/bispartners/foresight/docs/obesity/17.pdf. [Google Scholar]
- (27).Malik VS, Schulze MB, Hu FB. Intake of sugar-sweetened beverages and weight gain: a systematic review. Am J Clin Nutr. 2006;84:274–88. doi: 10.1093/ajcn/84.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Must A, Barish EE, Bandini LG. Modifiable risk factors in relation to changes in BMI and fatness: what have we learned from prospective studies of school-aged children? Int J Obes (Lond) 2009;33:705–15. doi: 10.1038/ijo.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Johnson L, Wilks DC, Lindroos AK, Jebb SA. Reflections from a systematic review of dietary energy density and weight gain: is the inclusion of drinks valid? Obes Rev. 2009;10:681–92. doi: 10.1111/j.1467-789X.2009.00580.x. [DOI] [PubMed] [Google Scholar]
- (30).Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12:e119–e129. doi: 10.1111/j.1467-789X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- (31).Must A, Parisi SM. Sedentary behavior and sleep: paradoxical effects in association with childhood obesity. Int J Obes. 2009;33:S82–S86. doi: 10.1038/ijo.2009.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Tremblay MS, Colley RC, Saunders TJ, Healy GN, Owen N. Physiological and health implications of a sedentary lifestyle. Appl Physiol Nutr Metab. 2010;35:725–40. doi: 10.1139/H10-079. [DOI] [PubMed] [Google Scholar]
- (33).Rogers IS, Ness AR, Steer CD, Wells JC, Emmett PM, Reilly JR, Tobias J, Smith GD. Associations of size at birth and dual-energy X-ray absorptiometry measures of lean and fat mass at 9 to 10 y of age. Am J Clin Nutr. 2006;84:739–47. doi: 10.1093/ajcn/84.4.739. [DOI] [PubMed] [Google Scholar]
- (34).Catalano PM, Ehrenberg HM. The short- and long-term implications of maternal obesity on the mother and her offspring. BJOG. 2006;113:1126–33. doi: 10.1111/j.1471-0528.2006.00989.x. [DOI] [PubMed] [Google Scholar]
- (35).Hillier TA, Pedula KL, Schmidt MM, Mullen JA, Charles MA, Pettitt DJ. Childhood Obesity and Metabolic Imprinting: The ongoing effects of maternal hyperglycemia. Diabetes Care. 2007;30:2287–92. doi: 10.2337/dc06-2361. [DOI] [PubMed] [Google Scholar]
- (36).Kral JG, Biron S, Simard S, Hould F-S, Lebel S, Marceau M. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2-18 years. Pediatrics. 2006;118:e1644–e1649. doi: 10.1542/peds.2006-1379. [DOI] [PubMed] [Google Scholar]
- (37).Huxley R, Owen CG, Whincup PH, Cook DG, Rich-Edwards J, Smith GD, Collins R. Is birth weight a risk factor for ischemic heart disease in later life? Am J Clin Nutr. 2007;85:1244–50. doi: 10.1093/ajcn/85.5.1244. [DOI] [PubMed] [Google Scholar]
- (38).Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, Canoy D, Droyvold W, Eriksson JG, Forsen T, Gunnarsdottir I, Jarvelin MR, Koupil I, Lapidus L, Nilsen TI, Olsen SF, Schack-Nielsen L, Thorsdottir I, Tuomainen TP, Sorensen TIA, NordNet SG. Birth weight and Systolic blood pressure in adolescence and adulthood: Meta-regression analysis of sex- and age-specific results from 20 Nordic studies. Am J Epidemiol. 2007;166:634–45. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- (39).Harder T, Rodekamp E, Schellong K, Dudenhausen JW, Plagemann A. Birth weight and subsequent risk of type 2 diabetes: A meta-analysis. Am J Epidemiol. 2007;165:849–57. doi: 10.1093/aje/kwk071. [DOI] [PubMed] [Google Scholar]
- (40).Hales CN, Barker DJ. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- (41).Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008;359:61–73. doi: 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Yajnik CS, Fall CH, Coyaji KJ, Hirve SS, Rao S, Barker DJ, Joglekar C, Kellingray S. Neonatal anthropometry: the thin-fat Indian baby. The Pune Maternal Nutrition Study. Int J Obes Relat Metab Disord. 2003;27:173–80. doi: 10.1038/sj.ijo.802219. [DOI] [PubMed] [Google Scholar]
- (43).Yajnik C. Interactions of perturbations in intrauterine growth and growth during childhood on the risk of adult-onset disease. Proceedings of the Nutrition Society. 2000;59:257–65. doi: 10.1017/s0029665100000288. [DOI] [PubMed] [Google Scholar]
- (44).Wu Z, Puigserver P, Spiegelman BM. Transcriptional activation of adipogenesis. Curr Opin Cell Biol. 1999;11:689–94. doi: 10.1016/s0955-0674(99)00037-x. [DOI] [PubMed] [Google Scholar]
- (45).Catalano PM, Thomas A, Huston-Presley L, Amini SB. Phenotype of infants of mothers with gestational diabetes. Diabetes Care. 2007;30:S156–S160. doi: 10.2337/dc07-s209. [DOI] [PubMed] [Google Scholar]
- (46).Singhal A. Does early growth affect long-term risk factors for cardiovascular disease? Nestle Nutr Workshop Ser Pediatr Program. 2010;65:55–64. doi: 10.1159/000281145. [DOI] [PubMed] [Google Scholar]
- (47).Druet C, Stettler N, Sharp S, Simmons RK, Cooper C, Davey Smith G, Ekelund U, Levy-Marchal C, Jarvelin M-R, Kuh D, Ong KK. Prediction of childhood obesity by infancy weight gain: an individual-level meta-analysis. Paediatr Perinat Epidemiol. 2012;26:19–26. doi: 10.1111/j.1365-3016.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- (48).Victora CG, Sibbritt D, Horta BL, Lima RC, Cole T, Wells J. Weight gain in childhood and body composition at 18 years of age in Brazilian males. Acta Paediatr. 2007;96:296–300. doi: 10.1111/j.1651-2227.2007.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ekelund U, Ong KK, Linne Y, Neovius M, Brage S, Dunger DB, Wareham NJ, Rossner S. Association of Weight Gain in Infancy and Early Childhood with Metabolic Risk in Young Adults. J Clin Endocrinol Metab. 2007;92:98–103. doi: 10.1210/jc.2006-1071. [DOI] [PubMed] [Google Scholar]
- (50).Leunissen RWJ, Kerkhof GF, Stijnen T, Hokken-Koelega A. Timing and Tempo of First-Year Rapid Growth in Relation to Cardiovascular and Metabolic Risk Profile in Early Adulthood. JAMA. 2009;301:2234–42. doi: 10.1001/jama.2009.761. [DOI] [PubMed] [Google Scholar]
- (51).Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–9. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- (52).Bhargava SK, Sachdev HS, Fall CHD, Osmond C, Lakshmy R, Barker DJ. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350:865–75. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).de Onis M, Garza C, Onyango AW, Borghi E. Comparison of the WHO child growth standards and the CDC 2000 growth charts. J Nutr. 2007;137:144–8. doi: 10.1093/jn/137.1.144. [DOI] [PubMed] [Google Scholar]
- (54).Owen CG, Martin RM, Whincup PH, Davey SG, Cook DG. Effect of infant feeding on the risk of obesity across the life course: a quantitative review of published evidence. Pediatrics. 2005;115:1367–77. doi: 10.1542/peds.2004-1176. [DOI] [PubMed] [Google Scholar]
- (55).Owen CG, Martin RM, Whincup PH, Smith GD, Cook DG. Does breastfeeding influence risk of type 2 diabetes in later life? A quantitative analysis of published evidence. Am J Clin Nutr. 2006;84:1043–54. doi: 10.1093/ajcn/84.5.1043. [DOI] [PubMed] [Google Scholar]
- (56).Owen CG, Whincup PH, Kaye SJ, Martin RM, Smith GD, Cook DG, Bergstrom E, Black S, Wadsworth MEJ, Fall CH, Freudenheim JL, Nie J, Huxley RR, Kolacek S, Leeson CP, Pearce MS, Raitakari OT, Lisinen I, Viikari JS, Ravelli AC, Rudnicka AR, Strachan DP, Williams SM. Does initial breastfeeding lead to lower blood cholesterol in adult life? A quantitative review of the evidence. Am J Clin Nutr. 2008;88:305–14. doi: 10.1093/ajcn/88.2.305. [DOI] [PubMed] [Google Scholar]
- (57).Martin RM, Gunnell D, Smith GD. Breastfeeding in infancy and blood pressure in later life: Systematic review and meta-analysis. Am J Epidemiol. 2005;161:15–26. doi: 10.1093/aje/kwh338. [DOI] [PubMed] [Google Scholar]
- (58).Brion MJ. Commentary: Assessing the impact of breastfeeding on child health: where conventional methods alone fall short for reliably establishing causal inference. Int J Epidemiol. 2010;39:306–7. doi: 10.1093/ije/dyp318. [DOI] [PubMed] [Google Scholar]
- (59).Kramer MS, Matush L, Vanilovich I, Platt RW, Bogdanovich N, Sevkovskaya Z, Dzikovich I, Shishko G, Collet JP, Martin RM, vey Smith G, Gillman MW, Chalmers B, Hodnett E, Shapiro S, Promotion of Breastfeeding Intervention Trial (PROBIT) Study Group Effects of prolonged and exclusive breastfeeding on child height, weight, adiposity, and blood pressure at age 6.5 y: evidence from a large randomized trial. Am J Clin Nutr. 2007;86:1717–21. doi: 10.1093/ajcn/86.5.1717. [DOI] [PubMed] [Google Scholar]
- (60).Ong KK, Emmett PM, Noble S, Ness A, Dunger DB. Dietary energy intake at the age of 4 months predicts postnatal weight gain and childhood body mass index. Pediatrics. 2006;117:E503–E508. doi: 10.1542/peds.2005-1668. [DOI] [PubMed] [Google Scholar]
- (61).FAO. WHO. UNU . Human Energy Requirements. (Food and Nutrition Technical Report Series 1). 20074. [Google Scholar]
- (62).Kramer MS. Breastfeeding, complementary (solid) foods, and long-term risk of obesity. Am J Clin Nutr. 2010;91:500–1. doi: 10.3945/ajcn.2010.29199. [DOI] [PubMed] [Google Scholar]
- (63).Wright CM, Parkinson KN, Drewett RF. Why are babies weaned early? Data from a prospective population based cohort study. Arch Dis Child. 2004;89:813–6. doi: 10.1136/adc.2003.038448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Ramachandrappa S, Farooqi IS. Genetic approaches to understanding human obesity. J Clin Invest. 2011;121:2080–6. doi: 10.1172/JCI46044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Bochukova EG, Huang N, Keogh J, Henning E, Purmann C, Blaszczyk K, Saeed S, Hamilton-Shield J, Clayton-Smith J, O’Rahilly S, Hurles ME, Farooqi IS. Large, rare chromosomal deletions associated with severe early-onset obesity. Nature. 2010;463:666–70. doi: 10.1038/nature08689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Stutzmann F, Tan K, Vatin V, Dina C, Jouret B, Tichet J, Balkau B, Potoczna N, Horber F, O’Rahilly S, Farooqi IS, Froguel P, Meyre D. Prevalence of melanocortin-4 receptor deficiency in Europeans and their age-dependent penetrance in multigenerational pedigrees. Diabetes. 2008;57:2511–8. doi: 10.2337/db08-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Thearle MS, Muller YL, Hanson RL, Mullins M, Abdussamad M, Tran J, Knowler WC, Bogardus C, Krakoff J, Baier LJ. Greater impact of melanocortin-4 receptor deficiency on rates of growth and risk of type 2 diabetes during childhood compared with adulthood in Pima Indians. Diabetes. 2012;61:250–7. doi: 10.2337/db11-0708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Speliotes EK, Willer CJ, Berndt SI, Monda KL, Thorleifsson G, Jackson AU, Allen HL, Lindgren CM, Luan J, Magi R, Randall JC, Vedantam S, Winkler TW, Qi L, Workalemahu T, Heid IM, Steinthorsdottir V, Stringham HM, Weedon MN, Wheeler E, Wood AR, Ferreira T, Weyant RJ, Segre AV, Estrada K, Liang L, Nemesh J, Park JH, Gustafsson S, Kilpelainen TO, Yang J, Bouatia-Naji N, Esko T, Feitosa MF, Kutalik Z, Mangino M, Raychaudhuri S, Scherag A, Smith AV, Welch R, Zhao JH, Aben KK, Absher DM, Amin N, Dixon AL, Fisher E, Glazer NL, Goddard ME, Heard-Costa NL, Hoesel V, Hottenga JJ, Johansson A, Johnson T, Ketkar S, Lamina C, Li S, Moffatt MF, Myers RH, Narisu N, Perry JR, Peters MJ, Preuss M, Ripatti S, Rivadeneira F, Sandholt C, Scott LJ, Timpson NJ, Tyrer JP, van WS, Watanabe RM, White CC, Wiklund F, Barlassina C, Chasman DI, Cooper MN, Jansson JO, Lawrence RW, Pellikka N, Prokopenko I, Shi J, Thiering E, Alavere H, Alibrandi MT, Almgren P, Arnold AM, Aspelund T, Atwood LD, Balkau B, Balmforth AJ, Bennett AJ, Ben-Shlomo Y, Bergman RN, Bergmann S, Biebermann H, Blakemore AI, Boes T, Bonnycastle LL, Bornstein SR, Brown MJ, Buchanan TA, Busonero F, Campbell H, Cappuccio FP, Cavalcanti-Proenca C, Chen YD, Chen CM, Chines PS, Clarke R, Coin L, Connell J, Day IN, Heijer M, Duan J, Ebrahim S, Elliott P, Elosua R, Eiriksdottir G, Erdos MR, Eriksson JG, Facheris MF, Felix SB, Fischer-Posovszky P, Folsom AR, Friedrich N, Freimer NB, Fu M, Gaget S, Gejman PV, Geus EJ, Gieger C, Gjesing AP, Goel A, Goyette P, Grallert H, Grassler J, Greenawalt DM, Groves CJ, Gudnason V, Guiducci C, Hartikainen AL, Hassanali N, Hall AS, Havulinna AS, Hayward C, Heath AC, Hengstenberg C, Hicks AA, Hinney A, Hofman A, Homuth G, Hui J, Igl W, Iribarren C, Isomaa B, Jacobs KB, Jarick I, Jewell E, John U, Jorgensen T, Jousilahti P, Jula A, Kaakinen M, Kajantie E, Kaplan LM, Kathiresan S, Kettunen J, Kinnunen L, Knowles JW, Kolcic I, Konig IR, Koskinen S, Kovacs P, Kuusisto J, Kraft P, Kvaloy K, Laitinen J, Lantieri O, Lanzani C, Launer LJ, Lecoeur C, Lehtimaki T, Lettre G, Liu J, Lokki ML, Lorentzon M, Luben RN, Ludwig B, Manunta P, Marek D, Marre M, Martin NG, McArdle WL, McCarthy A, McKnight B, Meitinger T, Melander O, Meyre D, Midthjell K, Montgomery GW, Morken MA, Morris AP, Mulic R, Ngwa JS, Nelis M, Neville MJ, Nyholt DR, O’Donnell CJ, O’Rahilly S, Ong KK, Oostra B, Pare G, Parker AN, Perola M, Pichler I, Pietilainen KH, Platou CG, Polasek O, Pouta A, Rafelt S, Raitakari O, Rayner NW, Ridderstrale M, Rief W, Ruokonen A, Robertson NR, Rzehak P, Salomaa V, Sanders AR, Sandhu MS, Sanna S, Saramies J, Savolainen MJ, Scherag S, Schipf S, Schreiber S, Schunkert H, Silander K, Sinisalo J, Siscovick DS, Smit JH, Soranzo N, Sovio U, Stephens J, Surakka I, Swift AJ, Tammesoo ML, Tardif JC, Teder-Laving M, Teslovich TM, Thompson JR, Thomson B, Tonjes A, Tuomi T, van Meurs JB, van Ommen GJ. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat Genet. 2010;42:937–48. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Willer CJ, Speliotes EK, Loos RJ, Li S, Lindgren CM, Heid IM, Berndt SI, Elliott AL, Jackson AU, Lamina C, Lettre G, Lim N, Lyon HN, McCarroll SA, Papadakis K, Qi L, Randall JC, Roccasecca RM, Sanna S, Scheet P, Weedon MN, Wheeler E, Zhao JH, Jacobs LC, Prokopenko I, Soranzo N, Tanaka T, Timpson NJ, Almgren P, Bennett A, Bergman RN, Bingham SA, Bonnycastle LL, Brown M, Burtt NP, Chines P, Coin L, Collins FS, Connell JM, Cooper C, Smith GD, Dennison EM, Deodhar P, Elliott P, Erdos MR, Estrada K, Evans DM, Gianniny L, Gieger C, Gillson CJ, Guiducci C, Hackett R, Hadley D, Hall AS, Havulinna AS, Hebebrand J, Hofman A, Isomaa B, Jacobs KB, Johnson T, Jousilahti P, Jovanovic Z, Khaw KT, Kraft P, Kuokkanen M, Kuusisto J, Laitinen J, Lakatta EG, Luan J, Luben RN, Mangino M, McArdle WL, Meitinger T, Mulas A, Munroe PB, Narisu N, Ness AR, Northstone K, O’Rahilly S, Purmann C, Rees MG, Ridderstrale M, Ring SM, Rivadeneira F, Ruokonen A, Sandhu MS, Saramies J, Scott LJ, Scuteri A, Silander K, Sims MA, Song K, Stephens J, Stevens S, Stringham HM, Tung YC, Valle TT, Van Duijn CM, Vimaleswaran KS, Vollenweider P, Waeber G, Wallace C, Watanabe RM, Waterworth DM, Watkins N, Witteman JC, Zeggini E, Zhai G, Zillikens MC, Altshuler D, Caulfield MJ, Chanock SJ, Farooqi IS, Ferrucci L, Guralnik JM, Hattersley AT, Hu FB, Jarvelin MR, Laakso M, Mooser V, Ong KK, Ouwehand WH, Salomaa V, Samani NJ, Spector TD, Tuomi T, Tuomilehto J, Uda M, Uitterlinden AG, Wareham NJ, Deloukas P, Frayling TM, Groop LC, Hayes RB, Hunter DJ, Mohlke KL, Peltonen L, Schlessinger D, Strachan DP, Wichmann HE, McCarthy MI, Boehnke M, Barroso I, Abecasis GR, Hirschhorn JN. Six new loci associated with body mass index highlight a neuronal influence on body weight regulation. Nat Genet. 2009;41:25–34. doi: 10.1038/ng.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).den HM, Ekelund U, Brage S, Grontved A, Zhao JH, Sharp SJ, Ong KK, Wareham NJ, Loos RJ. Genetic susceptibility to obesity and related traits in childhood and adolescence: influence of loci identified by genome-wide association studies. Diabetes. 2010;59:2980–8. doi: 10.2337/db10-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Hardy R, Wills AK, Wong A, Elks CE, Wareham NJ, Loos RJ, Kuh D, Ong KK. Life course variations in the associations between FTO and MC4R gene variants and body size. Hum Mol Genet. 2010;19:545–52. doi: 10.1093/hmg/ddp504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Sovio U, Mook-Kanamori DO, Warrington NM, Lawrence R, Briollais L, Palmer CN, Cecil J, Sandling JK, Syvanen AC, Kaakinen M, Beilin LJ, Millwood IY, Bennett AJ, Laitinen J, Pouta A, Molitor J, Davey SG, Ben-Shlomo Y, Jaddoe VW, Palmer LJ, Pennell CE, Cole TJ, McCarthy MI, Jarvelin MR, Timpson NJ. Association between common variation at the FTO locus and changes in body mass index from infancy to late childhood: the complex nature of genetic association through growth and development. PLoS Genet. 2011;7:e1001307. doi: 10.1371/journal.pgen.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (73).Hertel JK, Johansson S, Sonestedt E, Jonsson A, Lie RT, Platou CG, Nilsson PM, Rukh G, Midthjell K, Hveem K, Melander O, Groop L, Lyssenko V, Molven A, Orho-Melander M, Njolstad PR. FTO, type 2 diabetes, and weight gain throughout adult life: a meta-analysis of 41,504 subjects from the Scandinavian HUNT, MDC, and MPP studies. Diabetes. 2011;60:1637–44. doi: 10.2337/db10-1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Elks CE, Loos RJ, Sharp SJ, Langenberg C, Ring SM, Timpson NJ, Ness AR, Davey SG, Dunger DB, Wareham NJ, Ong KK. Genetic markers of adult obesity risk are associated with greater early infancy weight gain and growth. PLoS Med. 2010;7:e1000284. doi: 10.1371/journal.pmed.1000284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Elks CE, den HM, Zhao JH, Sharp S, Wareham NJ, Loos RJ, Ong KK. Variability in the heritability of body mass index: a systematic review and meta-regression. Frontiers in Endocrinology. 2012;3:29. doi: 10.3389/fendo.2012.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Cecil JE, Tavendale R, Watt P, Hetherington MM, Palmer CN. An obesity-associated FTO gene variant and increased energy intake in children. N Engl J Med. 2008;359:2558–66. doi: 10.1056/NEJMoa0803839. [DOI] [PubMed] [Google Scholar]
- (77).Wardle J, Llewellyn C, Sanderson S, Plomin R. The FTO gene and measured food intake in children. Int J Obes (Lond) 2009;33:42–5. doi: 10.1038/ijo.2008.174. [DOI] [PubMed] [Google Scholar]
- (78).Timpson NJ, Emmett PM, Frayling TM, Rogers I, Hattersley AT, McCarthy MI, Davey SG. The fat mass- and obesity-associated locus and dietary intake in children. Am J Clin Nutr. 2008;88:971–8. doi: 10.1093/ajcn/88.4.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Tung YC, Yeo GS. From GWAS to biology: lessons from FTO. Ann N Y Acad Sci. 2011;1220:162–71. doi: 10.1111/j.1749-6632.2010.05903.x. [DOI] [PubMed] [Google Scholar]
- (80).Department of Health . Healthy Weight, Healthy Lives: A Cross Government Strategy for England. 2009. http://wwwdhgovuk/en/Publicationsandstatistics/Publications/PublicationsPolicyAndGuidance/DH_082378. [Google Scholar]
- (81).Waters E, de Silva-Sanigorski A, Brown T, Campbell KJ, Goa Y, Armstrong R, Prosser L, Summerbell CD. Interventions for preventing obesity in children. Cochrane Database Syst Rev. 2011:CD001871. doi: 10.1002/14651858.CD001871.pub3. [DOI] [PubMed] [Google Scholar]
- (82).Askie L, Baur L, Campbell K, Daniels L, Hesketh K, Magarey A, Mihrshahi S, Rissel C, Simes J, Taylor B, Taylor R, Voysey M, Wen LM, TEPoOiCC Study protocol: The Early Prevention of Obesity in CHildren (EPOCH) Collaboration - an Individual Patient Data Prospective Meta-Analysis. BMC Public Health. 2010;10:728. doi: 10.1186/1471-2458-10-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Singhal A, Kennedy K, Lanigan J, Fewtrell M, Cole TJ, Stephenson T, Elias-Jones A, Weaver LT, Ibhanesebhor S, MacDonald PD, Bindels J, Lucas A. Nutrition in infancy and long-term risk of obesity: evidence from 2 randomized controlled trials. Am J Clin Nutr. 2010;92:1133–44. doi: 10.3945/ajcn.2010.29302. [DOI] [PubMed] [Google Scholar]
- (84).Singhal A, Cole TJ, Fewtrell M, Deanfield J, Lucas A. Is slower early growth beneficial for long-term cardiovascular health? Circulation. 2004;109:1108–13. doi: 10.1161/01.CIR.0000118500.23649.DF. [DOI] [PubMed] [Google Scholar]
- (85).Niinikoski H, Jula A, Viikari J, Ronnemaa T, Heino P, Lagstrom H, Jokinen E, Simell O. Blood Pressure Is Lower in Children and Adolescents With a Low-Saturated-Fat Diet Since Infancy The Special Turku Coronary Risk Factor Intervention Project. Hypertension. 2009;53:918–24. doi: 10.1161/HYPERTENSIONAHA.109.130146. [DOI] [PubMed] [Google Scholar]
- (86).Koletzko B, von Kries R, Closa R, Escribano J, Scaglioni S, Giovannini M, Beyer J, Demmelmair H, Gruszfeld D, Dobrzanska A, Sengier A, Langhendries JP, Rolland Cachera MF, Grote V. Lower protein in infant formula is associated with lower weight up to age 2 y: a randomized clinical trial. Am J Clin Nutr. 2009;89:1836–45. doi: 10.3945/ajcn.2008.27091. [DOI] [PubMed] [Google Scholar]
- (87).Paul IM, Savage JS, Anzman SL, Beiler JS, Marini ME, Stokes JL, Birch LL. Preventing Obesity during Infancy: A Pilot Study. Obesity (Silver Spring) 2011;19:353–61. doi: 10.1038/oby.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Bond M, Wyatt K, Lloyd J, Welch K, Taylor R. Systematic review of the effectiveness and cost-effectiveness of weight management schemes for the under fives: a short report. Health Technology Assessment. 2009;13:1–75. doi: 10.3310/hta13610. [DOI] [PubMed] [Google Scholar]
- (89).Hesketh KD, Campbell KJ. Interventions to Prevent Obesity in 0-5 Year Olds: An Updated Systematic Review of the Literature. Obesity. 2010;18:S27–S35. doi: 10.1038/oby.2009.429. [DOI] [PubMed] [Google Scholar]
- (90).Barton M. Screening for obesity in children and adolescents: US Preventive Services Task Force recommendation statement. Pediatrics. 2010;125:361–7. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- (91).Downey M, Still C, Sharma AM. Is there a path for approval of an antiobesity drug: what did the Sibutramine Cardiovascular Outcomes Trial find? Curr Opin Endocrinol Diabetes Obes. 2011;18:321–7. doi: 10.1097/MED.0b013e32834a8726. [DOI] [PubMed] [Google Scholar]
- (92).Viner RM, Hsia Y, Tomsic T, Wong IC. Efficacy and safety of anti-obesity drugs in children and adolescents: systematic review and meta-analysis. Obes Rev. 2010;11:593–602. doi: 10.1111/j.1467-789X.2009.00651.x. [DOI] [PubMed] [Google Scholar]
- (93).Park MH, Kinra S, Ward KJ, White B, Viner RM. Metformin for obesity in children and adolescents: a systematic review. Diabetes Care. 2009;32:1743–5. doi: 10.2337/dc09-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Ibanez L, Lopez-Bermejo A, Diaz M, Marcos MV, de ZF. Early metformin therapy (age 8-12 years) in girls with precocious pubarche to reduce hirsutism, androgen excess, and oligomenorrhea in adolescence. J Clin Endocrinol Metab. 2011;96:E1262–E1267. doi: 10.1210/jc.2011-0555. [DOI] [PubMed] [Google Scholar]
- (95).Dietz WH. Reversing the tide of obesity. Lancet. 2011;378:744–6. doi: 10.1016/S0140-6736(11)61218-X. [DOI] [PubMed] [Google Scholar]