Abstract
Although some primates, including chimpanzees, throw objects occasionally1,2, only humans regularly throw projectiles with high speed and great accuracy. Darwin noted that humans’ unique throwing abilities, made possible when bipedalism emancipated the arms, enabled foragers to effectively hunt using projectiles3. However, there has been little consideration of the evolution of throwing in the years since Darwin made his observations, in part because of a lack of evidence on when, how, and why hominins evolved the ability to generate high-speed throws4-8. Here, we show using experimental studies of throwers that human throwing capabilities largely result from several derived anatomical features that enable elastic energy storage and release at the shoulder. These features first appear together approximately two million years ago in the species Homo erectus. Given archaeological evidence that suggests hunting activity intensified around this time9, we conclude that selection for throwing in order to hunt likely played an important role in the evolution of the human genus.
Compared with other carnivores, hominins are slow, weak, and lack natural weapons such as fangs and claws. Yet, hominins were eating meat by at least 2.6 Ma, and likely hunting large prey by 1.9 Ma (Supplementary Note 1). Although contemporary hunter-gatherers rarely rely on throwing to kill prey, earlier hominins probably needed to throw projectiles frequently in order to acquire and defend carcasses before the relatively recent inventions of the atlatl and bow10. We can therefore surmise that the ability to throw well would confer a strong selective benefit to early hunters. However, in order to test when and how hominins evolved the ability to throw projectiles effectively, it is necessary to understand both throwing biomechanics and how changes in hominin anatomy affect throwing performance.
Throws are powered by rapid, sequential activation of many muscles, starting in the legs and progressing through the hips, torso, shoulder, elbow and wrist11-14. Torques generated at each joint accelerate segmental masses, creating rapid angular movements that accumulate kinetic energy in the projectile until its release. It has been shown that internal (medial) rotation around the long axis of the humerus is the largest contributor to projectile velocity15. This rotation, which occurs in a few milliseconds and can exceed 9,000°/sec13, is the fastest motion the human body produces. Although previous research has focused on the internal rotator muscles of the shoulder11,16,17, these muscles alone cannot explain how humans generate so much internal rotational power. For one, calculations of the maximum power production capacity of all the shoulder’s internal rotator muscles indicate that these muscles can contribute, at most, half of the shoulder rotation power generated during the throwing motion (Supplementary Notes 2,3). Peak internal rotation torque also occurs well before the humerus starts to rotate internally12. Furthermore, variation in muscle fiber orientation in these muscles produce actions other than internal humeral rotation that reduce power output for this action.
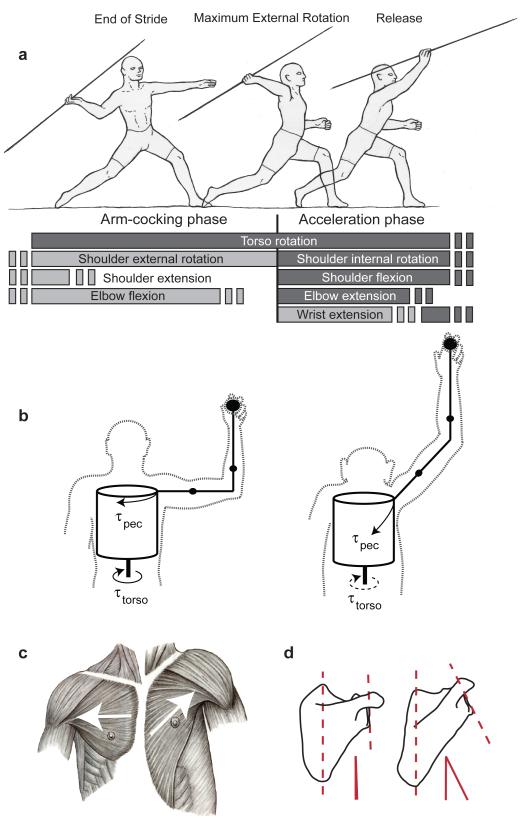
Elastic energy storage has been shown to be an important source of power amplification for many high-powered movements18,19. We propose that several evolutionarily novel features in the human shoulder help store and release elastic energy to generate much of the power needed for rapid humeral rotation during human throwing. According to this model, energy storage occurs during the arm-cocking phase (Fig. 1A), which begins with completion of a large step towards the target. As the foot hits the ground, the arm is already externally rotated, horizontally extended, and abducted nearly 90° at the shoulder, with forearm flexion approaching 90° at the elbow13. As the cocking phase begins, large torques are generated by rapid rotation of the torso towards the target and by the activation of the major shoulder horizontal flexor, Pectoralis major11,16. The positioning of the shoulder and elbow at this time increase the mass moment of inertia around the long axis of the humerus, causing the forearm and hand to lag behind the accelerating torso. Further, a flexed elbow during the cocking phase allows passive inertial forces to externally counter rotate the arm, stretching the short, parallel tendons, ligaments, and elastic components of muscles that cross the shoulder, potentially storing elastic energy in the large aggregate cross-sectional area of these structures (Supplementary Note 4). Then, when the biceps deactivate and elbow extension begins, the arm’s moment of inertia is reduced, allowing these stretched elements to recoil, releasing energy, and helping to power the extremely rapid internal rotation of the humerus (Supplementary Note 5).
Figure 1. Model of elastic energy storage.
Arm-cocking and acceleration phases of the overhand throw (A). Humans (left) and chimpanzees (right) differ in arm abduction and elbow flexion during throwing (B) because of differences in shoulder orientation, which alters the major line of action of the Pectoralis major (C). Aligning the long axis of the humerus with the major axis of P. major and flexing the elbow maximizes inertia to shoulder flexion torque and loads the elastic ligaments in the shoulder. However, chimpanzee morphology is compromised between maximizing humeral rotation or elbow extension. Signatures of shoulder orientation found in the scapula (D) can be used to reconstruct hominin shoulder orientation.
Three derived morphological features of humans not present in chimpanzees, our closest extant relatives, play a major role in storing and releasing elastic energy during throwing (Supplementary Note 6). First, humans’ tall, mobile waists decouple the hips and thorax, permitting more torso rotation20, in turn enabling high torque production over a large range-of-motion (ROM), needed to load the shoulder’s elastic elements. Second, humeral torsion, the angle between humeral head orientation and the axis of the elbow, is 10-20° lower in human throwers’ dominant arms compared to chimpanzee humeri5. Decreased torsion extends the rotational ROM at the shoulder externally21,22, potentially enabling more elastic energy storage during the cocking phase. Finally, humans have a more laterally oriented glenohumeral joint, which aligns the P. major flexion moment around the same axis as the torso rotation moment. This orientation allows humans to increase the arm’s moment of inertia by abducting the humerus in line with the torso rotation and shoulder flexion torques, maximizing resistance to both (Fig. 1B/C/D). In contrast, chimpanzees’ more cranially oriented glenohumeral joint and limited ability to produce torso rotation torque requires them to maximize inertial loading by abducting their humeri more than humans to bring their arm in line with the P. major flexion moment. This increased abduction, however, would force chimpanzees to position their elbow in a more extended posture to maximize the arm’s moment of inertia, resulting in a costly reduction in elbow extension during the throw.
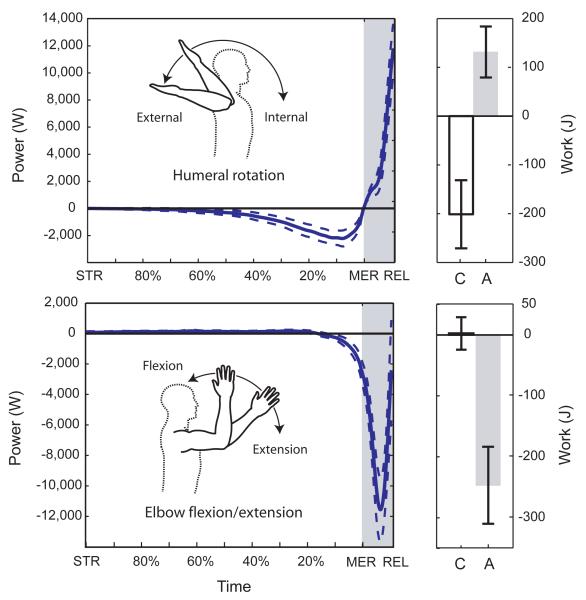
We tested the effects of these derived features on throwing performance using high-speed, 3D kinematic and kinetic data from 20 experienced human throwers to quantify power production at the shoulder during overhand baseball throwing (Supplementary Note 7). During the arm-cocking phase, the throwers’ humeri externally rotate 57±15 (mean±s.d.) past the active ROM limit achieved using their own muscular power, indicating passive stretching of the ligaments, tendons, and muscles crossing the shoulder. Inverse dynamics analysis shows that during this period, the shoulder produces an opposing internal rotation torque, causing a sustained period of power absorption (Fig. 2). During arm-cocking, the negative work of shoulder rotation averages −201±70J, with an average power of −631±337W. In contrast, the total rotational work of the subsequent internal rotation motion is 346±116J, with power during acceleration averaging 3,847±1,697W. If 90% of the negative work during arm-cocking is stored and returned elastically23, this energy can account for 54±15% of the internal humeral rotation work done during a typical throw.
Figure 2. Shoulder rotation and elbow flexion/extension power.
Mean shoulder rotational power (with 95% confidence intervals) shows a sustained period of negative power and work during arm-cocking, between stride (STR) and maximum external rotation (MER) - white. This negative work is recovered during acceleration, between MER and release (REL) - gray. Recovered work powers both internal rotation at the shoulder and extension of the elbow.
Elastic energy storage at the shoulder also augments the generation of joint velocity and power at the elbow. During acceleration, the elbow extends at very high angular velocities (2,434±552°/sec) despite large amounts of negative power and work (−246±63J), indicating that the triceps alone are not powering this rapid extension (Fig. 2). As previous studies have shown, elbow extension is powered primarily by more proximal segments15,24, especially the shoulder.
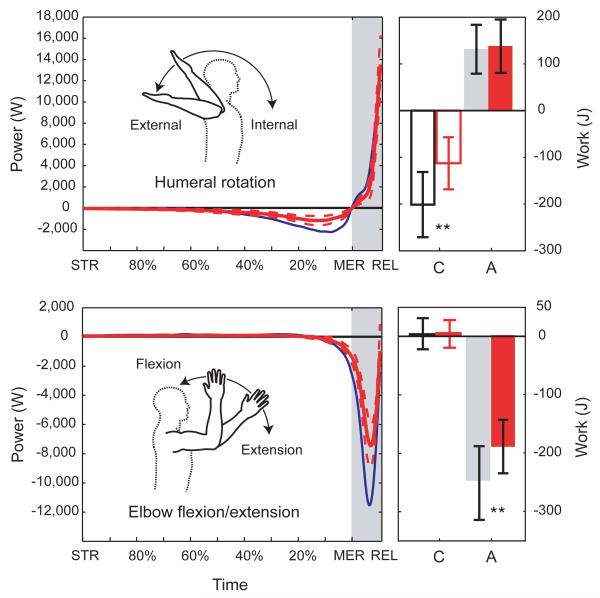
An additional line of evidence for the importance of elastic energy storage comes from experimentally limiting shoulder rotational ROM with therapeutic braces (Supplementary Notes 8-11), which restricted external rotation by 24±9°. During brace trials, shoulder rotation beyond the active ROM decreased by 50±36% and shoulder work during arm-cocking decreased by 39±16% (rmANOVA p < 0.001) (Fig. 3). Shoulder rotation work during the subsequent acceleration phase was not significantly different between conditions, but average shoulder rotation power during acceleration decreased significantly (−16±35%, rmANOVA p = 0.036). Wearing a shoulder brace also decreased elbow negative work during acceleration by 20±21% (rmANOVA p < 0.001). Overall, these work and power reductions from less elastic energy exchange significantly reduced humeral rotation angular acceleration (−24±29%, rmANOVA p < 0.001) and elbow extension angular velocity (−21±10%, rmANOVA p < 0.001), reducing ball speed by 8±6% (MANOVA, p < 0.001).
Figure 3. Shoulder brace restriction condition.
Brace restricted mean power (with 95% confidence intervals) for shoulder rotation and elbow flexion/extension are plotted in red alongside normal values in blue. Significant reductions (p < 0.05) in shoulder rotation work occur during arm-cocking and in elbow flexion/extension work during acceleration.
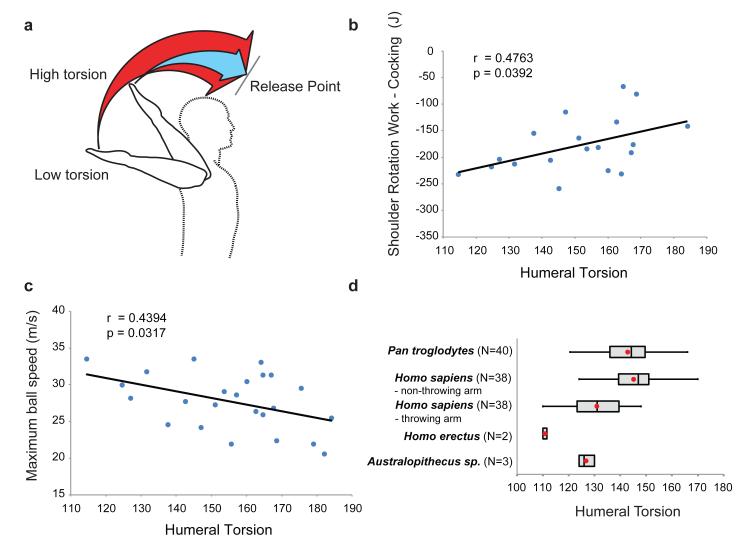
Natural variation in humeral torsion (Supplementary Note 12) yields similar performance effects. It has long been known that athletes such as pitchers have lower degrees of humeral torsion, by 10-15°, in their throwing versus non-throwing arms21,22,25. By maintaining relatively lower, juvenile levels of torsion into adulthood26, throwing athletes increase power generation by shifting the humerus’ rotational ROM externally21,22. This shift allows further external rotation during arm-cocking and increases internal rotation during acceleration (Fig. 4A), permitting more elastic energy storage and release (Fig. 4B/C). It is unknown whether the plasticity of humeral torsion is greater in humans than in other taxa, but we speculate that plasticity in humans may be advantageous, enabling low torsion to persist in the throwing arm, while higher torsion (useful for manipulative tasks) develops in the non-throwing arm5,25.
Figure 4. Humeral torsion and throwing performance.
Low humeral torsion shifts the shoulder rotational ROM externally (A), allowing increased negative work during arm-cocking to be stored as elastic energy (B), resulting in faster projectile speed (C). Humans and chimpanzees show comparable degrees of torsion5, although throwing athletes have reduced dominant arm torsion25 consistent with low torsion in Australopithecus and Homo erectus (D)5.
When high-speed throwing first evolved is difficult to test because the first projectiles were probably rocks and untipped wooden spears (Supplementary Notes 7,13). However, many of the derived morphological features that help human throwers store elastic energy can be assessed in the fossil record (Supplementary Note 14). These features evolved in a mosaic fashion, some predating the evolution of Homo. Tall, decoupled waists first appear in Australopithecus as adaptations for locomotion20. Low humeral torsion also appears in Australopithecus, likely resulting from the release of the forelimbs from weight-bearing during quadrupedal locomotion, and is present in early Homo5 (Fig. 4D). Although variation in glenoid orientation exists within Australopithecus27, a fully lateral glenoid position is first definitively present in Homo erectus28 (Supplementary Notes 15,16). Such laterally oriented shoulders probably decreased the mechanical advantage of the scapular rotator muscles during climbing, and probably had little or no effect on stone tool production. Throwing performance would likely also have benefited from low, wide shoulders, long legs, and hyperextendable wrists, which are present in H. erectus20,29. Although some of these features were probably selected for functions other than throwing, their combined configuration, first present in H. erectus, would have benefited throwing performance by enabling elastic energy storage in the shoulder, providing a selective advantage during hunting (Supplementary Note 1). Furthermore, high-speed throwing was likely a critical component of a suite of hunting behaviors that allowed early members of the genus Homo to thrive in new and varied habitats both in and out of Africa.
Today, technological advances such as the bow and arrow, nets, and firearms have reduced contemporary hunter-gatherers’ reliance on thrown projectiles, but the human ability and proclivity to throw persists in many sports, where athletes rely on the same mechanics (Supplementary Note 7). In this modern context, the evolution of adaptations for elastic energy storage during human throwing has implications for the high prevalence of injuries in throwing athletes. Paleolithic hunters almost certainly threw less frequently than modern athletes, who often deliver more than 100 high-speed throws in the span of a couple of hours. Unfortunately, the ligaments and tendons in the human shoulder and elbow are not well adapted to withstanding such repeated stretching from the high torques generated by throwing, and frequently suffer from laxity and tearing12,30. While humans’ unique ability to power high-speed throws using elastic energy may have been critical in enabling early hunting, repeated overuse of this motion can result in serious injuries in modern throwers.
METHODS
Subjects
Data were collected from 20 male subjects (ages 19-23). Nineteen of the subjects were collegiate athletes (16 baseball players, 3 non-throwing athletes). Prior to enrollment in the study, all participants were required to pass a throwing performance task (Supplementary Note 17) in order to exclude poor throwers. For all subjects, we collected weight, information on relevant injury/medical history, and basic anthropometric data (height, segment lengths and circumferences, joint range of motion). Humeral torsion was estimated using range of motion measures31. All subjects provided informed written consent in accordance with the Harvard Committee on the Use of Human Subjects.
Kinematics
Kinematic data were collected at 1000 Hz using an eight-camera Vicon T10s 3D infrared motion capture system (Vicon Inc, Centennial CO, USA). Each subject had twenty-one passive reflective markers taped on the throwing arm and torso (Supplementary Note 18). Subjects were given approximately 5 minutes to stretch and warm up before recording. After the warm up period, subjects were tasked to throw a 144g baseball at a 1m-radius target from 10m away. The subject then threw 8-10 normal pitches and 8-20 pitches using a Donjoy Shoulder Stabilizer (Donjoy Inc, Vista, CA) brace that restricts external rotational range of motion at the shoulder (Supplementary Note 19). As a sham, data from an intermediate condition in which the brace was applied but not tightened were also collected (Supplementary Note 8). Ball speed was measured using a Sports Radar Model 3600 radar gun. Ball release was timed using a synched FlexiForce A201 force sensor (Tekscan Inc, Boston MA, USA) collected at 1000 Hz taped to the palmar side of the distal phalanx of the third digit and synched with a 30 Hz Canon Vixia HV30 digital video camera (Canon Inc, Tokyo, Japan). In order to filter the kinematic data, a residual analysis32 of the entire throwing trial and the critical period during the humeral internal rotation motion was calculated in MATLAB (version R2010b) (Supplementary Note 20). A Butterworth 2nd order low-pass filter (cutoff 25Hz) was applied and marker gaps up to 100 frames were interpolated using C-Motion Visual3D software (v4). For analysis, each motion was then subdivided into five standard phases of the throw: windup/stride, arm cocking, arm acceleration, arm deceleration, and follow-through14.
Kinetics
Joint Euler angles were calculated and inverse dynamics analyses were performed using mass distribution data from Dempster33 in Visual3D. Joint angular velocities, moments, and power were calculated using each joint’s instantaneous axis of rotation (Supplementary Note 23). The sequence of rotations at each joint is described in Supplementary Note 22. Joint work was calculated in MATLAB using the trapz function.
Statistics
Kinetic data were standardized to phase length, interpolated, and resampled using custom MATLAB code to yield comparable data across all trials and subjects (Supplementary Note 21). Individual subject means were compared across experimental conditions using repeated measures ANOVA or MANOVA where appropriate. All statistical analyses were conducted using JMP software (v5). Differences were considered to be significant at alpha < 0.05.
Supplementary Material
Acknowledgments
We would like to thank the Wyss Institute, Leia Stirling, Andy Biewener, Richard Wrangham, Susan Larson, Brian Roach, Laszlo Meszoly, Amanda Lobell, and many undergraduate research assistants for their feedback, help, and support.
Funding was provided by the National Science Foundation (BCS-0961943 to NTR/DEL), the American School for Prehistoric Research (to NTR/DEL), and the Wellcome Trust/DBT India Alliance (500158/Z/09/Z to MV).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Information Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing or financial interests.
References
- 1.Goodall J. The chimpanzees of Gombe: Patterns of behavior. Harvard University Press; 1986. [Google Scholar]
- 2.Westergaard GC, Liv C, Haynie MK, Suomi SJ. A comparative study of aimed throwing by monkeys and humans. Neuropsychologia. 2000;38:1511–1517. doi: 10.1016/s0028-3932(00)00056-7. [DOI] [PubMed] [Google Scholar]
- 3.Darwin C. The descent of man, and selection in relation to sex. John Murray; 1871. [Google Scholar]
- 4.Churchill SE, Rhodes JA. In: The evolution of hominin diets: Integrating approaches to the study of Paleolithic subsistence. Hublin JJ, Richards MP, editors. Springer; 2009. [Google Scholar]
- 5.Larson SG. Evolutionary transformation of the hominin shoulder. Evol Anthropol. 2007;16:172–187. [Google Scholar]
- 6.Calvin WH. The throwing madonna: Essays on the brain. Bantam; 1983. [Google Scholar]
- 7.Fifer F. The adoption of bipedalism by the hominids: A new hypothesis. Hum Evol. 1987;2:135–147. [Google Scholar]
- 8.Bingham PM. Human evolution and human history: A complete theory. Evol Anthropol. 2000;9:248–257. [Google Scholar]
- 9.Stanford CB, Bunn HT. In: Human Evolution Series. Ciochon RL, Wood B, editors. Oxford University Press; New York: 2001. [Google Scholar]
- 10.Shea JJ. The origins of lithic projectile point technology: evidence from Africa, the Levant, and Europe. J Archaeol Sci. 2006;33:823–846. [Google Scholar]
- 11.Hirashima M, Kadota H, Sakurai S, Kudo K, Ohtsuki T. Sequential muscle activity and its functional role in the upper extremity and trunk during overarm throwing. J Sports Sci. 2002;20:301–310. doi: 10.1080/026404102753576071. [DOI] [PubMed] [Google Scholar]
- 12.Fleisig GS, Andrews JR, Dillman CJ, Escamilla RF. Kinetics of baseball pitching with implications about injury mechanisms. Am J Sports Med. 1995;23:233–239. doi: 10.1177/036354659502300218. [DOI] [PubMed] [Google Scholar]
- 13.Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13:216–222. doi: 10.1177/036354658501300402. [DOI] [PubMed] [Google Scholar]
- 14.Dillman CJ, Fleisig GS, Andrews JR. Biomechanics of pitching with emphasis upon shoulder kinematics. J Orthop Sports Phys Ther. 1993;18:402–408. doi: 10.2519/jospt.1993.18.2.402. [DOI] [PubMed] [Google Scholar]
- 15.Hirashima M, Kudo K, Watarai K, Ohtsuki T. Control of 3D limb dynamics in unconstrained overarm throws of different speeds performed by skilled baseball players. J Neurophysiol. 2007;97:680–691. doi: 10.1152/jn.00348.2006. [DOI] [PubMed] [Google Scholar]
- 16.DiGiovine NM, Jobe FW, Pink M, Perry J. An electromyography analysis of the upper extremity in pitching. J Shoulder Elbow Surg. 1992;1:15–25. doi: 10.1016/S1058-2746(09)80011-6. [DOI] [PubMed] [Google Scholar]
- 17.Gowan ID, Jobe FW, Tibone JE, Perry J, Moynes DR. A comparative electromyographic analysis of the shoulder during pitching: Professional versus amateur pitchers. Am J Sports Med. 1987;15:586–590. doi: 10.1177/036354658701500611. [DOI] [PubMed] [Google Scholar]
- 18.Astley HC, Roberts TJ. Evidence for a vertebrate catapult: elastic energy storage in the plantaris tendon during frog jumping. Biol Lett. 2012;8:386–389. doi: 10.1098/rsbl.2011.0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patek SN, Nowroozi BN, Baio JE, Caldwell RL, Summers AP. Linkage mechanics and power amplification of the mantis shrimp’s strike. J Exp Biol. 2007;210:3677–3688. doi: 10.1242/jeb.006486. [DOI] [PubMed] [Google Scholar]
- 20.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 21.Osbahr DC, Cannon DL, Speer KP. Retroversion of the humerus in the throwing shoulder of college baseball pitchers. Am J Sports Med. 2002;30:347–353. doi: 10.1177/03635465020300030801. [DOI] [PubMed] [Google Scholar]
- 22.Reagan KM, et al. Humeral retroversion and its relationship to glenohumeral rotation in the shoulder of college baseball players. Am J Sports Med. 2002;30:354–360. doi: 10.1177/03635465020300030901. [DOI] [PubMed] [Google Scholar]
- 23.Bennett MB, Ker RF, Dimery NJ, Alexander RM. Mechanical properties of various mammalian tendons. Zool Lond (A) 1986;209:537–548. [Google Scholar]
- 24.Werner SL, Fleisig GS, Dillman CJ, Andrews JR. Biomechanics of the elbow during baseball pitching. J Orthop Sports Phys Ther. 1993;17:274–278. doi: 10.2519/jospt.1993.17.6.274. [DOI] [PubMed] [Google Scholar]
- 25.Pieper HG. Humeral torsion in the throwing arm of handball players. Am J Sports Med. 1998;26:247–253. doi: 10.1177/03635465980260021501. [DOI] [PubMed] [Google Scholar]
- 26.Cowgill LW. Humeral torsion revisited: a functional and ontogenetic model for populational variation. Am J Phys Anthropol. 2007;134:472–480. doi: 10.1002/ajpa.20689. [DOI] [PubMed] [Google Scholar]
- 27.Haile-Selassie Y, et al. An early Australopithecus afarensis postcranium from Woranso-Mille, Ethiopia. Proc Natl Acad Sci U S A. 2010;107:12121–12126. doi: 10.1073/pnas.1004527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker A, Leakey RE. The Nariokotome Homo erectus skeleton. Harvard University Press; 1993. [Google Scholar]
- 29.Richmond BG, Begun DR, Strait DS. Origin of human bipedalism: The knuckle-walking hypothesis revisited. Yearb Phys Anthropol. 2001;44:70–105. doi: 10.1002/ajpa.10019.abs. [DOI] [PubMed] [Google Scholar]
- 30.Snyder SJ, Karzel RP, Del Pizzo W, Ferkel RD, Friedman MJ. SLAP lesions of the shoulder. Arthroscopy. 1990;6:274–279. doi: 10.1016/0749-8063(90)90056-j. [DOI] [PubMed] [Google Scholar]
- 31.Roach NT, Lieberman DE, Gill TJ, IV, Palmer WE, Gill TJ., III The effect of humeral torsion on rotational range of motion in the shoulder and throwing performance. J Anat. 2012;220:293–301. doi: 10.1111/j.1469-7580.2011.01464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Winter DA. Biomechanics and motor control of human movement. 2nd edn Wiley-Interscience; 1990. [Google Scholar]
- 33.Dempster WT. Space requirements for the seated operator. Wright Air Development Center; 1955. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.