Abstract
Engrailed-2 (En-2), a homeodomain transcription factor, is expressed in a caudal-to-rostral gradient in the developing mid-brain, where it has an instructive role in patterning the optic tectum—the target of topographic retinal input1,2. In addition to its well-known role in regulating gene expression through its DNA-binding domain, En-2 may also have a role in cell–cell communication, as suggested by the presence of other domains involved in nuclear export, secretion and internalization3. Consistent with this possibility, here we report that an external gradient of En-2 protein strongly repels growth cones of Xenopus axons originating from the temporal retina and, conversely, attracts nasal axons. Fluorescently tagged En-2 accumulates inside growth cones within minutes of exposure, and a mutant form of the protein that cannot enter cells fails to elicit axon turning. Once internalized, En-2 stimulates the rapid phosphorylation of proteins involved in translation initiation and triggers the local synthesis of new proteins. Furthermore, the turning responses of both nasal and temporal growth cones in the presence of En-2 are blocked by inhibitors of protein synthesis. The differential guidance of nasal and temporal axons reported here suggests that En-2 may participate directly in topographic map formation in the vertebrate visual system.
The first molecular insight into the mechanism of topographic mapping in the nervous system is usually credited to Sperry4, whose chemoaffinity hypothesis proposed matching gradients of receptors and ligands within the retina and tectum. The first candidate molecules fulfilling the requirements of this hypothesis, the EphrinA ligands, were identified in the tectum and found to be repulsive to retinal axons expressing EphA receptors5,6. Temporal axons expressing high levels of EphA receptors map to the rostral tectum and avoid the EphrinA-rich caudal tectum. En-2, which is also expressed in a caudal-to-rostral gradient in the developing tectum, has been shown to promote the expression of tectal EphrinA ligands7,8 and, through its transcriptional activity, is thought to have a major role in setting up the EphrinA gradient. However, work in knockout mice has shown that in the total absence of EphrinAs, a rough retinotectal map still forms9,10, implicating the existence of other potential guidance cues. In light of the evidence that En-2 can be secreted and transferred from one cell to another3, we decided to test whether En-2 could also act as a guidance factor.
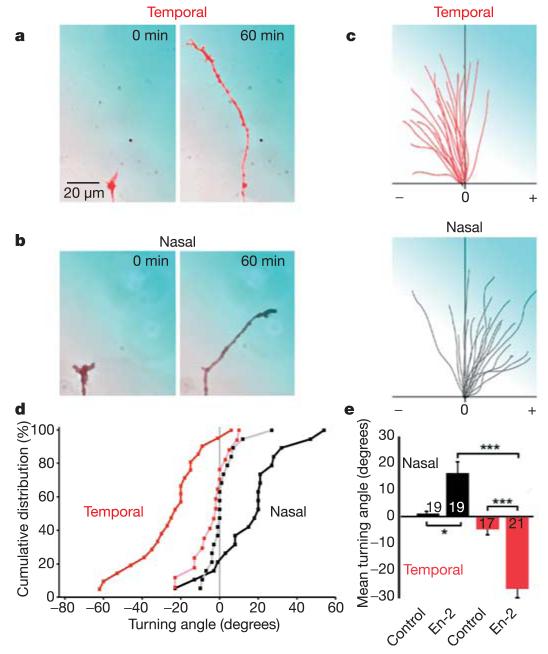
We first asked whether a source of pure En-2 protein could guide Xenopus retinal axons. Retinal growth cones from nasal and temporal parts of the retina were individually exposed to gradients of En-2 in chemotropic turning assays (see Methods)11,12. Notably, nasal axons were attracted by En-2, whereas temporal axons were repelled (Fig. 1a–e). These opposite turning responses correspond to the in vivo organization of the retinotectal map, where nasal axons terminate in the En-2-rich caudal tectum but temporal axons avoid it. Similar results were obtained when the experiment was performed ‘blind’ with respect to the nasal or temporal origin of the axons (Supplementary Fig. 1). The effect was not due to differential growth rates in the presence of En-2, as both nasal and temporal axons grew at approximately 30 μm h−1 in these assays (Supplementary Fig. 2). These data demonstrate that En-2 can act as a soluble factor that differentially guides axons from temporal and nasal parts of the retina.
Figure 1. En-2 gradient repels temporal and attracts nasal retinal axons.
a, b, Examples of temporal (red) and nasal (black) growth cones tested in turning assays with 10 μg ml−1 En-2 in the pipette (300 pM at the growth cone). The pipette is positioned in the top right, and the En-2 gradient is represented in blue (a–c). c, Trajectory plots of temporal (red) and nasal (black) neurites in En-2 gradients. Each line represents a single growth cone trajectory; the origin represents the centre of the growth cone at 0 min, and positive (+) and negative (−) turning angles are indicated. d, Cumulative distributions of turning angles of temporal (red) and nasal (black) growth cones in En-2 (bold) and control (light) gradients. e, Mean turning angles of growth cones in the experimental conditions shown in d, numbers on or beside the bars denote the number of growth cones tested. Significance was calculated using a Kolmogorov–Smirnov test, and indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001). Error bars indicate s.e.m.
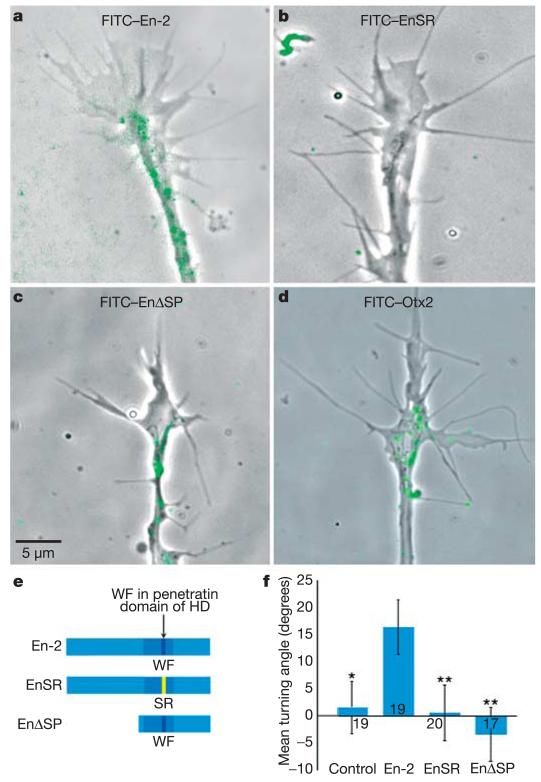
As the En-2 protein possesses an internalization sequence13, we asked whether En-2 was acting within the retinal growth cones to direct their growth. To visualize internalization, we exposed growth cones to media containing fluorescein isothiocyanate (FITC)-tagged En-2 for 5 min and then washed them in media free of FITC–En-2. Within 15 min, a fluorescent signal accumulated in living growth cones, occasionally residing in filopodia and lamellipodia, but mostly concentrated in puncta in the body of the growth cone and along the neurite shaft (Fig. 2a). Previous studies have shown that internalization of En-2 is compromised when there is a mutation in the coding sequence of the penetratin domain, changing the tryptophan (W) and phenylalanine (F) residues at positions 48 and 49 in the homeodomain to serine (S) and arginine (R) residues, respectively (W48S-F49R; see Fig. 2e)14. A FITC-tagged version of this mutant protein (FITC–EnSR) did not enter growth cones, even at high doses (Fig. 2b). Therefore, we used the EnSR mutant protein to test whether En-2 needs to be internalized to elicit axon turning. When nasal retinal growth cones were exposed to a gradient of the EnSR mutant protein they did not exhibit any directional turning bias (Fig. 2f), strongly suggesting that internalization is critical for axon guidance.
Figure 2. En-2 is internalized in the growth cone and guidance depends on internalization.
a–c, Live retinal growth cones following 5 min exposure to FITC-tagged proteins. Over 50% of growth cones showed internalized fluorescent puncta (green) following exposure to FITC-En-2 (a) or FITC–EnΔSP (c), but not with FITC–EnSR (b). d, FITC–Otx2 is internalized. e, En-2 constructs used in the turning assays: En-2 is the full-length wild-type protein; EnSR has a mutation in the penetratin domain (amino acids WF replaced by SR); and EnΔSP lacks the N-terminal part of the protein (where a putative eIF4E-binding site resides). HD, homeodomain. f, Mean turning angles of nasal growth cones in gradients of En-2, EnSR and EnΔSP. Nasal axons turn towards En-2 but not EnSR or EnΔSP. For Otx2 activity, see Supplementary Fig. 1d. For P values, see Fig. 1 legend, and comparisons are to En-2. Error bars indicate s.e.m.
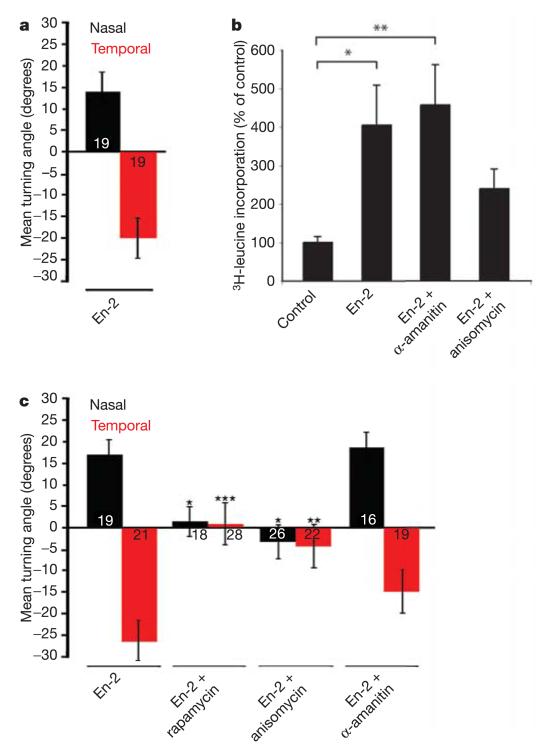
Turning behaviour was usually initiated in response to En-2 within 15–20 min of exposure—too rapidly for a transcriptional role of En-2 in axon guidance, especially as the growth cone is often >100 μm from the soma, or cell body. Recent studies have shown that the homeoprotein HoxA9, and a large number of other homeodomain proteins, contain a highly conserved binding site to eukaryotic initiation factor 4E (eIF4E) that is typically found in translational regulators15,16. Of particular note, HoxA9 has been found to positively regulate translation by directly competing with factors that bind and repress eIF4E (ref. 16). Extracellular guidance cues, such as Sema3A and Netrin-1, lead to local protein synthesis within retinal growth cones, and this rapidly activated translation is essential for chemotropic turning in gradients of these cues17. Therefore, we examined the possibility that En-2 stimulates turning responses in retinal growth cones by affecting local translation. First, a set of experiments was conducted to rule out a transcriptional mechanism of En-2 action. Nasal growth cones transected from their cell bodies still turned towards an En-2 gradient, and isolated temporal growth cones continued to turn away from this gradient (Fig. 3a and Supplementary Fig. 3). We then asked whether these transected and isolated growth cones synthesized new proteins in response to En-2 by measuring the incorporation of tritiated (3H)-leucine. Indeed, 10 min of stimulation with En-2 caused isolated growth cones to incorporate significantly higher amounts of 3H-leucine than controls, and this incorporation was severely reduced by the protein synthesis inhibitor anisomycin, but not the transcription inhibitor α-amanitin (Fig. 3b). En-2 binds eIF4E (ref. 18), but the EnΔSP mutant19 lacks the amino-terminal region of the protein (Fig. 2e) that contains a putative eIF4E-binding domain. The FITC-tagged EnΔSP protein is internalized normally (Fig. 2c), but in turning assays, gradients of EnΔSP do not attract nasal axons (Fig. 2f), consistent with the hypothesis that En-2-induced turning requires the N-terminal domain. Finally, another set of experiments was conducted to test whether pharmacological reagents that specifically interfere with transcription or translation affected growth cone turning in response to En-2. As shown in Fig. 3c, anisomycin abolished the ability of nasal axons to turn towards, and temporal axons to turn away, from a source of En-2. Similarly, rapamycin—an antibiotic that blocks the translation of 5′-capped messenger RNA by inhibiting target of rapamycin (TOR)—strongly inhibited axon turning responses. In contrast, α-amanitin—an inhibitor of transcription—had no effect on En-2-induced axon turning. Together, these results suggest that translation, rather than transcription, is essential for En-2-mediated retinal axon guidance in Xenopus.
Figure 3. Retinal ganglion cell growth cone guidance by En-2 is dependent on protein synthesis.
a, Temporal (red) and nasal (black) growth cones isolated from their somas (see Supplementary Fig. 1c) show opposite turning responses to an En-2 gradient, eliminating a role for transcription. b, 3H-leucine incorporation in growth cones isolated from their cell bodies. En-2 stimulates significant incorporation of 3H-leucine, which is abolished by anisomycin but not α-amanitin. c, Mean turning angles of temporal and nasal growth cones in a gradient of En-2 in the presence of inhibitors. En-2-induced turning is blocked by anisomycin and rapamycin, but is unaffected by α-amanitin. The number of growth cones tested in each case is indicated on the bars. b, Significance was calculated using a Kruskal–Wallis test, and indicated by asterisks (*P < 0.05; **P < 0.01). c, Significance was calculated using a Kolmogorov–Smirnov test, and indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001), and comparisons are to En-2. Error bars indicate s.e.m.
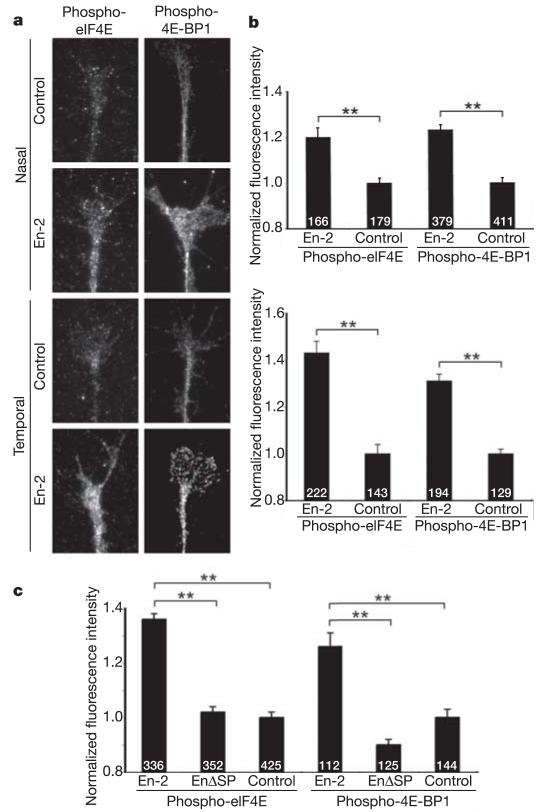
TOR-dependent translation can be regulated by eIF4E-binding protein 1 (4E-BP1), which silences translation by competitively binding eIF4E and sequestering it from the translation initiation complex20. Phosphorylation of 4E-BP1 by TOR releases eIF4E and allows it to initiate translation21. In growth cones exposed to Sema3A or Netrin-1, a rapid rise in the phosphorylation of both eIF4E and 4E-BP1 has been reported using antibodies that recognize the phosphorylated forms of the proteins17. Therefore, we postulated that if En-2 was acting through this pathway, then 4E-BP1 should also be phosphorylated in growth cones exposed to En-2. We compared the levels of phospho-4E-BP1 and phospho-eIF4E in control and En-2-exposed retinal growth cones using phospho-specific antibodies (Fig. 4). Digital quantification revealed that En-2 triggers a significant rise in both phospho-4E-BP1 and phospho-eIF4E immunofluorescence levels after 5 min in temporal and nasal growth cones (Fig. 4a, b). In contrast, EnΔSP—even though it is internalized (Fig. 2c)—does not stimulate the phosphorylation of either of these translation-associated proteins (Fig. 4c). These data are consistent with the possibility that En-2 activates translation in growth cones by interacting with components of the translational pathway.
Figure 4. En-2 stimulates the phosphorylation of translational regulatory proteins.
a, Fluorescent micrographs of unstimulated control and En-2-stimulated growth cones using phospho-eIF4E and phospho-eIF4E-BP1 antibodies. Exposure to En-2 for 5 min increases the level of phosphorylation of eIF4E and eIF4E-BP1 in nasal and temporal growth cones. b, Histograms showing the digital quantification of the fluorescence signal in growth cones. c, Exposure of growth cones to EnΔSP does not lead to phosphorylation of either eIF4E or eIF4E-BP1. The number of growth cones tested in each case is indicated on the bars. Significance was calculated using a two-tailed Mann–Whitney test, and indicated by asterisks (**P < 0.0001). Error bars indicate s.e.m.
A key question is that of specificity. Do all homeodomain proteins elicit the same response, or are these responses specific to En-2? To address this, we tested Otx2, another homeodomain transcription factor that shares the secretion, internalization and eIF4E-binding properties of En-2 (ref. 18). In vivo, Otx2 is distributed fairly uniformly in the developing Xenopus forebrain and midbrain22. FITC–Otx2 is internalized in retinal growth cones (Fig. 2d). However, when applied in a gradient, Otx2 weakly attracted both the nasal and temporal populations of retinal growth cones (Supplementary Fig. 4). Thus, unlike En-2, Otx2 does not elicit opposite turning responses in the two populations of retinal axons, indicating that En-2 acts specifically to induce these divergent behaviours.
Not only is the caudal-to-rostral gradient of En-2 in the tectum perfectly suited to having a function in topographic mapping, but the non-transcriptional function of En-2 can explain previously unresolved issues in retinotectal mapping, such as what attracts nasal axons to the caudal tectum or to patches of the tectum that retrovirally overexpress En-2 (ref. 23). Our findings may also help to explain why a fair degree of order remains along the rostrocaudal axis in the retinotectal maps of EphrinA2/EphrinA5 double knockout mice9, although they do not challenge the view that EphrinA ligands have a major role in retinotopic guidance. Rather, they suggest that En-2 may complement this role by eliciting different responses in nasal versus temporal axons. It is not clear how En-2 exerts its differential effect. One possibility is that En-2 triggers the translation of different proteins in the nasal and temporal axon populations. Alternatively, En-2 may activate translation of the same proteins, but in a spatially opposite manner. Providing answers to these questions will require further investigation.
Recent reports have demonstrated a role for morphogens like Wnt, Bone morphogenetic protein and Hedgehog in axonal guidance24, and so perhaps it is not unexpected that if a homeodomain protein is secreted, and its extracellular concentration reflects its pattern of expression in the brain, then such concentration gradients could be also used to guide growing axons. Notably, previous studies have shown that there is a high degree of correlation between the expression domains of homeodomain transcription factors and early axonal pathways25. In the context of the results presented here, the possibility that many of these richly patterned transcription factors have a direct role in axonal guidance should not be overlooked.
METHODS
Xenopus embryos
Embryos were obtained by in vitro fertilization of oocytes from Xenopus laevis females stimulated with human chorionic gonadotropin (Sigma). Embryos were raised in 0.1 × Modified Barth’s Saline, pH 7.4, and were staged according to the tables of Nieuwkoop and Faber.
Growth cone turning assays
The extreme nasal and temporal thirds of stage 32–33 retinas were explanted onto 50-mm glass-bottomed Petri dishes (MaTek) precoated with 10 μg ml−1 poly-l-lysine followed by 10 μg ml−1 Laminin-1 (Sigma), as this substrate promotes good outgrowth from retinal explants and is thought to be a substrate for retinal axon growth in vivo26. Cultures were grown at 20 °C for 12–26 h to generate good neurite outgrowth17. Stable gradients of En-2 protein were produced as described previously11,12 by pulsatile ejection of En-2 protein (10 μg ml−1). The pipette tip was positioned 100 μm from the centre of the growth cone at a 45° angle to the direction of growth. These standard conditions give rise to an estimated 1,000-fold dilution of the factor between the pipette and the growth cone, and a concentration gradient across the growth cone of 5–10% (ref. 11). The turning angle for each growth cone was defined as the angle between the original and final direction of growth after 60 min of culture. Only growth cones with a net extension >5 μm over the assay period were included in the analysis.
Pharmacological reagents
The following pharmacological reagents were bath-applied to cultures immediately before the application of En-2 protein in the turning assay: 40 μM anisomycin (Sigma), which inhibits the peptidyl transferase activity on the ribosome; 10 μg ml−1 α-amanitin (Calbiochem), which inhibits RNA polymerase II; and 10 nM rapamycin (Calbiochem), which inhibits mammalian TOR.
Protein production
The En-2, EnSR and EnΔSP proteins were produced in isopropyl-β-d-thiogalactoside (IPTG)-inducible bacteria using published methods27, and Otx2 was produced in insect cells from a recombinant baculovirus (a gift from G. Corte). Protein purity was analysed by SDS–polyacrylamide gel electrophoresis. After concentration measurement, the proteins were stored at −20 °C in culture medium supplemented with 10% glycerol. Before use, the glycerol was removed by dialysis against 1 × PBS. FITC was conjugated to purified En2 using standard procedures (FITC-Celite, Sigma).
Measurement of 3H-leucine incorporation
3H-leucine incorporation in isolated growth cones was assayed following 10 min of stimulation with En-2 according to methods described previously17. En-2 (0.3 μg ml−1) was added globally to the cultures and the experiments were done in six independent trials, each in duplicate.
FITC-protein internalization
FITC–tagged proteins (FITC–En-2, 2.3 μg ml−1; FITC–EnSR, 20.7 μg ml−1; FITC-EnΔSP, 6.5 μg ml−1; FITC–Otx2, 8.3 μg ml−1) were added to retinal cultures for 5 min after blocking for 30 min with 4% BSA. The cultures were then washed extensively in FITC–protein-free medium, and imaged. Fluorescence was visualized in living growth cones with a ×100 objective on a Nikon Eclipse 2000U microscope, and images were captured with a Hamamatsu digital CCD camera using Openlab software (Improvision). Approximately 30 cultures were examined for each condition, with approximately 40 growth cones per culture. More than half of the growth cones were labelled when exposed to FITC–En-2 or FITC–EnΔSP, but not a single growth cone was found labelled with FITC–EnSR.
Quantitative immunocytochemistry
Antibodies were used at the following dilutions: phospho-4E-BP1 primary antibody (Cell Signaling Technology) at 1:100; phospho-eIF4E primary antibody (Cell Signaling Technology) at 1:25; and goat anti-rabbit Cy3 secondary antibody (Chemicon) at 1:700. Xenopus temporal or nasal retinal explants were dissected at stage 32 and grown overnight at 20 °C on coverslips coated with poly-l-lysine (10 μg ml−1) and Laminin-1 (10 μg ml−1). Growth cones were stimulated with En-2 (1 μg ml−1) added globally to the medium for 5 min, fixed in 4% paraformaldehyde and 15% sucrose for 30 min, permeabilized in 0.1% Triton-X100, blocked with 10% goat serum, and incubated sequentially in primary and secondary antibodies for 1 h. Coverslips were mounted in FluorSave (Calbiochem) and growth cones were imaged at ×100 on a Nikon Optiphot. For digital quantification, growth cones were selected at random under phase optics and a phase image was captured, followed by a fluorescent image, using a Hamamatsu digital CCD camera and taking care to avoid pixel saturation. The growth cone outline was traced on the phase image, superimposed on the fluorescent image, and the fluorescence intensities were measured digitally using Openlab software yielding values of pixel intensity per unit area. The background fluorescence was measured by placing the outline of the growth cone in an adjacent area devoid of cellular material, and subtracted from the growth cone values to give a background-corrected intensity value. For presentation of the data, the fluorescence intensity values were normalized to the respective control experiments. Each experiment was performed four independent times and run in duplicate.
Statistical analyses
All data are shown as mean ± s.e.m. Significance was assessed using the two-sample Kolmogorov–Smirnov test, unless otherwise stated. Statistical analyses were carried out using the statistical package ‘R’ (www.r-project.org).
Supplementary Material
Acknowledgements
We thank all of our colleagues for discussions, and A. Dwivedy and E. Ipendey for technical assistance. This work was supported by the Wellcome Trust, UK (to C.H. and W.H.), the Human Frontier Sciences Program and the European Commission (to A.P.).
Footnotes
Supplementary Information is linked to the online version of the paper at www.nature.com/nature.
Author Information
Reprints and permissions information is available at npg.nature.com/reprintsandpermissions.
The authors declare no competing financial interests.
References
- 1.Retaux S, Harris WA. Engrailed and retinotectal topography. Trends Neurosci. 1996;19:542–546. doi: 10.1016/s0166-2236(96)10062-x. [DOI] [PubMed] [Google Scholar]
- 2.Itasaki N, Nakamura H. A role for gradient en expression in positional specification on the optic tectum. Neuron. 1996;16:55–62. doi: 10.1016/s0896-6273(00)80023-9. [DOI] [PubMed] [Google Scholar]
- 3.Prochiantz A, Joliot A. Can transcription factors function as cell-cell signalling molecules? Nature Rev. Mol. Cell Biol. 2003;4:814–819. doi: 10.1038/nrm1227. [DOI] [PubMed] [Google Scholar]
- 4.Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc. Natl Acad. Sci. USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng HJ, Nakamoto M, Bergemann AD, Flanagan JG. Complementary gradients in expression and binding of ELF-1 and Mek4 in development of the topographic retinotectal projection map. Cell. 1995;82:371–381. doi: 10.1016/0092-8674(95)90426-3. [DOI] [PubMed] [Google Scholar]
- 6.Drescher U, et al. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 7.Shigetani Y, Funahashi JI, Nakamura H. En-2 regulates the expression of the ligands for Eph type tyrosine kinases in chick embryonic tectum. Neurosci. Res. 1997;27:211–217. doi: 10.1016/s0168-0102(96)01151-0. [DOI] [PubMed] [Google Scholar]
- 8.Logan C, et al. Rostral optic tectum acquires caudal characteristics following ectopic engrailed expression. Curr. Biol. 1996;6:1006–1014. doi: 10.1016/s0960-9822(02)00645-0. [DOI] [PubMed] [Google Scholar]
- 9.Feldheim DA, et al. Genetic analysis of ephrin-A2 and ephrin-A5 shows their requirement in multiple aspects of retinocollicular mapping. Neuron. 2000;25:563–574. doi: 10.1016/s0896-6273(00)81060-0. [DOI] [PubMed] [Google Scholar]
- 10.Frisen J, Holmberg J, Barbacid M. Ephrins and their Eph receptors: multitalented directors of embryonic development. EMBO J. 1999;18:5159–5165. doi: 10.1093/emboj/18.19.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lohof AM, Quillan M, Dan Y, Poo MM. Asymmetric modulation of cytosolic cAMP activity induces growth cone turning. J. Neurosci. 1992;12:1253–1261. doi: 10.1523/JNEUROSCI.12-04-01253.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de la Torre JR, et al. Turning of retinal growth cones in a netrin-1 gradient mediated by the netrin receptor DCC. Neuron. 1997;19:1211–1224. doi: 10.1016/s0896-6273(00)80413-4. [DOI] [PubMed] [Google Scholar]
- 13.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 14.Joliot A, et al. Identification of a signal sequence necessary for the unconventional secretion of Engrailed homeoprotein. Curr. Biol. 1998;8:856–863. doi: 10.1016/s0960-9822(07)00346-6. [DOI] [PubMed] [Google Scholar]
- 15.Topisirovic I, et al. The proline-rich homeodomain protein, PRH, is a tissue- specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J. 2003;22:689–703. doi: 10.1093/emboj/cdg069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Topisirovic I, et al. Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels. Mol. Cell. Biol. 2005;25:1100–1112. doi: 10.1128/MCB.25.3.1100-1112.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campbell DS, Holt CE. Chemotropic responses of retinal growth cones mediated by rapid local protein synthesis and degradation. Neuron. 2001;32:1013–1026. doi: 10.1016/s0896-6273(01)00551-7. [DOI] [PubMed] [Google Scholar]
- 18.Nedelec S, et al. Emx2 homeodomain transcription factor interacts with eukaryotic translation initiation factor 4E (eIF4E) in the axons of olfactory sensory neurons. Proc. Natl Acad. Sci. USA. 2004;101:10815–10820. doi: 10.1073/pnas.0403824101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Foucher I, Montesinos ML, Volovitch M, Prochiantz A, Trembleau A. Joint regulation of the MAP1B promoter by HNF3β/Foxa2 and Engrailed is the result of a highly conserved mechanism for direct interaction of homeoproteins and Fox transcription factors. Development. 2003;130:1867–1876. doi: 10.1242/dev.00414. [DOI] [PubMed] [Google Scholar]
- 20.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 21.Gingras AC, Raught B, Sonenberg N. eIF4 initiation factors: effectors of mRNA recruitment to ribosomes and regulators of translation. Annu. Rev. Biochem. 1999;68:913–963. doi: 10.1146/annurev.biochem.68.1.913. [DOI] [PubMed] [Google Scholar]
- 22.Kablar B, et al. Xotx genes in the developing brain of Xenopus laevis. Mech. Dev. 1996;55:145–158. doi: 10.1016/0925-4773(96)00497-2. [DOI] [PubMed] [Google Scholar]
- 23.Friedman GC, Leary DD. Retroviral misexpression of engrailed genes in the chick optic tectum perturbs the topographic targeting of retinal axons. J. Neurosci. 1996;16:5498–5509. doi: 10.1523/JNEUROSCI.16-17-05498.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charron F, Tessier-Lavigne M. Novel brain wiring functions for classical morphogens: a role as graded positional cues in axon guidance. Development. 2005;132:2251–2262. doi: 10.1242/dev.01830. [DOI] [PubMed] [Google Scholar]
- 25.Macdonald R, et al. Regulatory gene expression boundaries demarcate sites of neuronal differentiation in the embryonic zebrafish forebrain. Neuron. 1994;13:1039–1053. doi: 10.1016/0896-6273(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J, Burne JF, McKinlay C, Winter J. The role of laminin and the laminin/fibronectin receptor complex in the outgrowth of retinal ganglion cell axons. Dev. Biol. 1987;122:407–418. doi: 10.1016/0012-1606(87)90305-8. [DOI] [PubMed] [Google Scholar]
- 27.Montesinos ML, et al. The neuronal microtubule-associated protein 1B is under homeoprotein transcriptional control. J. Neurosci. 2001;21:3350–3359. doi: 10.1523/JNEUROSCI.21-10-03350.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.