Abstract
Background
Detailed associations between physical activity (PA) subcomponents, sedentary time, and body composition in preschoolers remain unclear.
Objective
We examined the magnitude of associations between objectively measured PA subcomponents and sedentary time with body composition in 4-y-old children.
Design
We conducted a cross-sectional study in 398 preschool children recruited from the Southampton Women’s Survey. PA was measured by using accelerometry, and body composition was measured by using dual-energy X-ray absorptiometry. Associations between light physical activity, moderate physical activity (MPA), vigorous physical activity (VPA), and moderate-to-vigorous physical activity (MVPA) intensity; sedentary time; and body composition were analyzed by using repeated-measures linear regression with adjustment for age, sex, birth weight, maternal education, maternal BMI, smoking during pregnancy, and sleep duration. Sedentary time and PA were also mutually adjusted for one another to determine whether they were independently related to adiposity.
Results
VPA was the only intensity of PA to exhibit strong inverse associations with both total adiposity [P < 0.001 for percentage of body fat and fat mass index (FMI)] and abdominal adiposity (P = 0.002 for trunk FMI). MVPA was inversely associated with total adiposity (P = 0.018 for percentage of body fat; P = 0.022 for FMI) but only because of the contribution of VPA, because MPA was unrelated to fatness (P ≥ 0.077). No associations were shown between the time spent sedentary and body composition (P ≥ 0.11).
Conclusions
In preschoolers, the time spent in VPA is strongly and independently associated with lower adiposity. In contrast, the time spent sedentary and in low-to-moderate–intensity PA was unrelated to adiposity. These results indicate that efforts to challenge pediatric obesity may benefit from prioritizing VPA.
INTRODUCTION
In England, more than one-fifth of children between 4 and 5 y of age are overweight or obese (1). It is expected that a considerable fraction of these over-fat children will become obese adults (2). Therefore, early childhood is an important time frame that should be targeted by preventive strategies intended to reduce body fat accumulation.
Increased physical activity (PA)4, which is the largest modifiable component of energy expenditure, is frequently prioritized alongside dietary restriction for the prevention or treatment of obesity (3, 4). It is logical that PA may assist the attainment of healthy weight by encouraging energy balance. However, there have been only a small number of investigations that have examined the association between preschool PA and body composition (5). At least in part, the small number of studies can be attributed to past difficulties in the measurement of the PA of young children. Uniquely, young children participate in low-intensity movement that is rapidly interspersed with brief, sporadic, and often unmemorable episodes of moderate-to-vigorous physical activity (MVPA) (6, 7). Children <10 y of age are unable to recall their own activity with accuracy and surrogate reports courtesy of caregivers have exhibited low validity (8). Alternative methodologies, such as accelerometry, are now available, and they can be feasibly used in large-scale studies to provide objective measurements of the free-living PA of young children (9). Accelerometers have been validated in preschoolers under a variety of conditions and by using different criterion methods. The vast majority of studies reported strong correlations between techniques (10, 11).
Accelerometers provide rich data about total activity and can further be used to measure subcomponent PA [eg, the time spent in light physical activity (LPA), moderate physical activity (MPA), and vigorous physical activity (VPA) intensity, and sedentary time (12)]. With the use of accelerometers, studies in young children have established inverse cross-sectional associations between PA and adiposity (5, 13-15). The studies have also indicated that high-intensity PA may be more strongly associated with adiposity than PA of a low-to-moderate intensity. However, it remains unknown if PA of greater intensity is associated with preschool adiposity independent of the time spent sedentary, which, itself, is a potential risk factor for obesity (16). Answering this is important for public health purposes, in terms of refining obesity interventions that are aimed at increasing PA, or reducing sedentary time, in young children.
The aim of the current study was to examine independent associations between a range of accelerometer-derived PA intensities and sedentary time (accomplished by mutually adjusting for one another) with body composition in a reasonably large, free-living population cohort of preschool children.
SUBJECTS AND METHODS
Subjects
The study was a cross-sectional investigation embedded within the Southampton Women’s Survey (SWS). Details of the SWS, which is a longitudinal birth cohort study, have been previously published (17). Briefly, between 1998 and 2002, all nonpregnant women aged 20–34 y who were registered with a general practitioner in Southampton, United Kingdom, were invited to participate in the SWS. Women who accepted and subsequently became pregnant (n = 3159) were studied during gestation, and their offspring were followed through childhood. At 4 y of age 1013 children underwent an assessment of body composition by using dual-energy X-ray absorptiometry (DXA); some children also underwent an assessment of habitual PA. This study constituted a complete-case analysis of participants with full data for body composition, PA, and important covariates at 4 y of age (n = 398). Approval for all components of the SWS was granted by the local research ethics committee, and parental written informed consent was obtained for all children.
Assessment of PA intensity and sedentary time
Free-living PA was measured by using the Actiheart combined heart rate and movement sensor (CambridgeNtech Ltd) attached to the chest (18). In young children, methods required for the interpretation of both heart rate and movement from the Actiheart sensor are still being developed, and thus for this study, accelerometer data were used in isolation.
Children were asked to wear the accelerometer continuously (including overnight wear) for 7 consecutive days including during swimming, bathing, and sleeping. Movement data were collected in 60-s epochs and, after upload to a computer, were reduced by means of a custom-designed program (MahUffe version 1.9.0.3; http://www.mrc-epid.cam.ac.uk). As part of data-reduction, periods of continuous zero counts ≥100 min in duration were used to indicate monitor removal from the torso and were erased. First and last days of observation were also discarded. We have previously observed that the SWS cohort (at 4 y of age) exhibited similar PA levels on both week and weekend days (KR Hesketh, AM McMinn, U Ekelund, et al, unpublished observations, May 2012). Therefore, weekend observations were deemed nonessential to providing reliable estimates of PA, and all children with at least one valid day of activity data were included in analyses. A valid day was defined as ≥600 min of nonsleeping data.
To derive the time (min/d) spent sedentary and in a range of PA intensities, age-specific cut-points commonly employed in the preschool age group were used (19-21). The cutoffs (sedentary: <37.5 counts; LPA: 38–419 counts; MPA: 420–841 counts; and VPA: ≥842 counts) were originally developed by using the Actigraph accelerometer (Manufacturing Technologies Inc) with 15-s epochs. To accommodate our data, we first reintegrated thresholds to 60 s (×4) and calculated Actiheart sensor equivalent cutoffs (÷5). The conversion factor was based on a comparison of counts in adolescents when both devices were worn simultaneously in the laboratory during treadmill walking and running and during free living (22, 23). It seems that accelerometer counts indicative of sedentary behavior and MVPA are largely independent of age and body size (24); thus, there is some reassurance that our conversion factor, although developed in adolescents, was suitable for application in preschoolers.
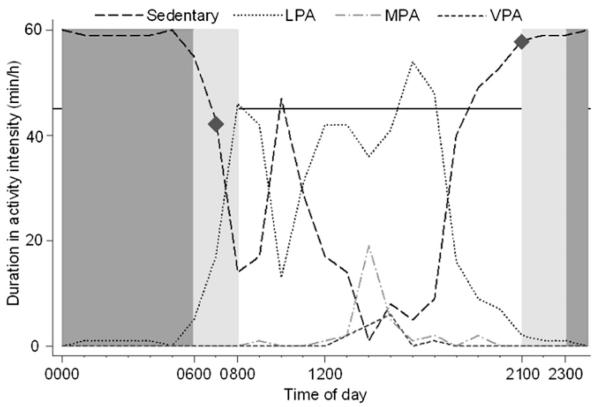
Daily sedentary time courtesy of the 24-h monitoring protocol was a combined record of true awake sedentary time and sleep. To eliminate sleep (a sedentary behavior albeit necessary for normal growth and functioning), distributions of registered sedentary time on an hourly basis were inspected. Overnight between 2300 and 0600, the median sedentary time was high (60 min/h; IQR: 3 min/h) and was presumed to reflect sleep; all data related to this 7-h period were removed. To account for interindividual and intraindividual variabilities in sleep patterns and durations, an additional exclusion rule was formulated. If a sedentary time >45 min was recorded within one of the hours between 0600–0800 and 2100–2300, the child was considered to have been sleeping, and the corresponding hour or hours were discarded from analyses (Figure 1). Napping has a low purported prevalence in this age group (25, 26), and hence, all periods of high sedentary time (>45 min/h) between the hours of 0800 and 2100 were considered true sedentariness and not daytime sleeping. With the use of our dismissal criteria, the average time of sleep onset for children was shown to be 2112, the sleep termination time was established as 0707, and the average (±SD) daily sleep duration was 9.9 ± 0.9 h. These times were reflective of objectively-measured sleep patterns and durations in 4-y-olds (25, 26) and indicated the credibility of our method. After the removal of sleep, daily averages for sedentary time and time spent in each of the PA intensities were constructed. Information concerning the number of bouts of MVPA (MPA + VPA) and VPA ≥5 min in duration was also extracted.
FIGURE 1.
Derivation of sedentary time. Sedentary time between 2300 and 0600 was assumed to reflect (in)activity during sleep; all corresponding data were removed (dark-gray shading). To account for interindividual and intraindividual variabilities in the sleep pattern, high sedentary times (>45 min/h; solid horizontal line) between 0600 and 0800 and between 2100 and 2300 were further assumed to reflect (in)activity during sleep, and all corresponding data were removed (light-gray shading). Remaining data provided an indication of sleep termination and onset times as well as sleep duration [0700 and 2100 (diamonds) and 10 h, respectively, in this scenario]. LPA, light physical activity; MPA, moderate physical activity; VPA, vigorous physical activity.
Assessment of body composition
Children were invited for an assessment of body composition by using DXA (Hologic Discovery; Hologic Inc) at Southampton General Hospital. To encourage child compliance, a sheet with appropriate colored cartoons was laid on the scanning bed before measurement, and suitable DVD cartoons were shown during scans. The DXA instrument was calibrated by using a spine phantom daily and a step phantom weekly. The total radiation dose for whole-body measurement by using the pediatric scan mode was 4.7 μSv.
Scans were analyzed by a trained technician with automated pediatric software (Vertec Scientific Ltd). Chosen body-composition variables for this study included 2 measures of total adiposity, namely the percentage of body fat and fat mass (FM), a single measure of lean mass (LM), and, as an indicator of truncal adiposity, trunk fat mass (TFM). TFM was estimated by using a region defined by a horizontal line below the chin, vertical borders lateral to the ribs, and oblique lines that passed through femoral necks. Subsequent to the measurement of child height (by using a Leicester height measurer), height-adjusted indexes of FM, LM, and TFM were computed (27). Height raised to the power of 2 was used to calculate fat mass index (FMI; kg/m2) and trunk fat mass index (TFMI; kg/m2). Height to the power of 2.5 was used to calculate LM index (LMI; kg/m2.5). Regression of these indexes against height at 4 y confirmed that they were no longer associated with stature (FMI: r2 = 0.0001, P = 0.88; TFMI: r2 = 0.0000, P = 0.95; LMI: r2 = 0.0008, P = 0.58). Weight was measured by using standard procedures with calibrated digital scales (Seca Ltd) and, together with height, was used to calculate BMI [in kg/m2 (weight divided by the square of height)]. For descriptive purposes, children were classified as normal weight, overweight, or obese according to International Obesity Task Force age- and sex-specific BMI growth charts (28).
Potential confounders
Data about several covariates known to be associated with PA or body composition were collected, including information about maternal education on enrollment to the SWS. These data were used to categorize mothers into 3 socioeconomic groups (low, moderate, and high) partitioned according to similarities in educational level [low: no qualifications and General Certificate of Secondary Education grades D or below (national school exams at age 16 y); moderate: General Certificate of Secondary Education grade C or above and A-level (national school exams at age 18 y); high: Higher National Diploma (higher education qualification considered equivalent to the first 2 y of a 3-y degree) and degree]. Maternal height and weight were measured with a stadiometer and calibrated digital scales, respectively, and were used to calculate maternal prepregnancy BMI. Infant sex and birth weight were abstracted from obstetric records, and maternal smoking was reported at 11 and 34 wk of gestation with responses being combined to indicate any smoking during pregnancy. Sleep duration was derived from the Actiheart sensor according to the method described in Figure 1. Finally, information about the duration of breastfeeding (weeks since birth) was maternally reported and available for a large subsample of participants (n = 383).
Statistical analysis
To determine the normality of continuous variables, histograms were viewed in conjunction with skewness and kurtosis statistics. The nonnormality of skewed dependent variables (FMI and TFMI) was rectified by using natural logarithmic transformations. Descriptive statistics for all variables were subsequently calculated for the total group and by sex after collapsing daily activity data to the average level (average data were used for the descriptive analysis only). To summarize normally distributed continuous variables, means and SDs were calculated, and sex differences were tested by using ANOVA. For skewed variables, median and IQRs were determined, and sex differences were examined by using Wilcoxon’s rank-sum test. For categorical variables, proportions were used, and sex differences were analyzed by using the chi-square test. Identical statistical tests were used to compare characteristics of children who contributed and did not contribute to the analysis (because of incomplete data).
To examine associations between PA intensity, sedentary time, and body-composition variables, Pearson’s correlation analysis was initially used followed by linear regressions with repeated measures. Repeated measures were used to account for clustered data that resulted from children who contributed >1 d of PA. All potential confounders including age, sex, birth weight, maternal education, maternal BMI, smoking during pregnancy, and sleep duration were initially added to regressions (model 1). Model 2 was further adjusted for sedentary time to see if the associations between PA intensity and body composition were independent of time spent sedentary. Similarly, when sedentary time was the exposure of interest, model 2 was further adjusted for MVPA. All finally adjusted models were examined for nonlinear variation by introducing quadratic terms and checking for an improved model fit by using likelihood ratio testing. The effect modification by sex was also inspected. Regression coefficients from models that included log-transformed dependent variables (FMI and TFMI) were converted for ease of interpretation. Coefficients were modified to represent the average percentage change in the outcome per unit increase in the independent variable by using the following formula:
| (1) |
Independent variables were also scaled according to guidelines for PA in children (ie, 60 min MVPA/d, which is the former guideline for young children) or according to durations that might be considered realistic ambitions for intervention studies (eg, 15 min VPA/d).
Subsequent to finalizing regression models 1 and 2, a number of sensitivity analyses were conducted to determine the robustness of coefficients. To enable adjustment for breastfeeding history, regression models were repeated in a marginally reduced sample size (n = 383; sensitivity analysis 1). Models were also rerun to allow for possible errors in the derivation of sedentary time (sensitivity analysis 2). This analysis was accomplished by replacing both sedentary time and sleep-duration variables with the one combined measure of awake sedentary time and sleep provided by the complete 24-h monitoring record. With appreciation that 1 d of observation may not accurately reflect the habitual PA pattern of children, we further used the intraclass correlation coefficient for accelerometer variables, in conjunction with the Spearman-Brown prophecy formula, to calculate the number of days needed to obtain 80% reliability in PA and sedentary time estimates. In close agreement with published recommendations (29), we showed that 6 d of accelerometry were needed to optimize reliability (data not shown). Models were thereby restricted to participants with at least this level of activity data and reanalyzed (sensitivity analysis 3). A significance level of P < 0.05 was chosen a priori, and all analyses were performed with Stata 12.0 software (StataCorp).
RESULTS
Descriptive characteristics of the sample are shown in Table 1. There was an equal sex contribution (51% of the sample were boys), but the sample was predominantly white (97% of mothers were white). Approximately 9% of children were born to mothers who smoked during pregnancy, and a similar proportion (7.8%) was born to mothers with no or few academic qualifications. At the time of the 4-y measurement, boys were marginally older than girls (P = 0.028). With reference to body composition, all indexes of adiposity (percentage of body fat, FM, FMI, TFM, and TFMI) were significantly higher in girls (P < 0.001), but there was no sex difference in the proportion of children categorized as normal weight, overweight, or obese (P = 0.34)
TABLE 1.
Descriptive characteristics of the study population1
| Characteristics | Total (n = 398) | Boys (n = 202) | Girls (n = 196) | P-sex difference |
|---|---|---|---|---|
| Age (y) | 4.10 (0.08)2 | 4.11 (0.08) | 4.10 (0.08) | 0.028 |
| Maternal ethnicity (% white) | 96.9 | 97.5 | 96.9 | 0.72 |
| Maternal education (%) | ||||
| Low | 7.83 | 6.9 | 8.6 | |
| Moderate | 59.6 | 57.9 | 61.2 | |
| High | 32.7 | 35.2 | 30.1 | 0.51 |
| Maternal BMI (kg/m2) | 24.3 (5.4) | 24.3 (4.9) | 24.0 (5.8) | 0.62 |
| Smoked during pregnancy (%) | 9.3 | 8.9 | 9.7 | 0.86 |
| Birth weight (kg) | 3.5 ± 0.54 | 3.6 ± 0.5 | 3.5 ± 0.5 | 0.054 |
| Breastfeeding (wk) | 13.0 (29.4) | 13.0 (25.9) | 13.0 (31.3) | 0.30 |
| Height (m) | 1.04 ± 0.04 | 1.05 ± 0.04 | 1.04 ± 0.04 | 0.21 |
| Weight (kg) | 17.8 (2.7) | 17.7 (2.8) | 17.8 (2.7) | 0.64 |
| BMI (kg/m2) | 16.3 (1.6) | 16.4 (1.6) | 16.3 (1.6) | 0.82 |
| Percentage of body fat | 26.8 ± 4.9 | 24.2 ± 3.7 | 29.5 ± 4.5 | <0.001 |
| Fat mass (kg) | 4.6 (1.4) | 4.2 (1.1) | 5.2 (1.5) | <0.001 |
| Fat mass index (kg/m2) | 4.2 (1.3) | 3.9 (0.9) | 4.7 (1.3) | <0.001 |
| Trunk fat mass (kg) | 1.6 (0.6) | 1.5 (0.5) | 1.8 (0.7) | <0.001 |
| Trunk fat mass index (kg/m2) | 1.5 (0.6) | 1.3 (0.4) | 1.7 (0.7) | <0.001 |
| Lean mass (kg) | 12.6 ± 1.5 | 13.1 ± 1.4 | 12.1 ± 1.4 | <0.001 |
| Lean mass index (kg/m2.5) | 11.2 ± 0.87 | 11.6 ± 0.81 | 10.8 ± 0.73 | <0.001 |
| Weight status (%)5 | ||||
| Normal | 79.9 | 82.7 | 77.0 | |
| Overweight | 15.6 | 13.9 | 17.4 | |
| Obese | 4.5 | 3.5 | 5.6 | 0.34 |
| LPA (min/d) | 423.6 ± 63.0 | 428.6 ± 64.8 | 418.5 ± 60.8 | 0.11 |
| MPA (min/d) | 58.6 (28.2) | 61.8 (33.3) | 56.8 (23.2) | 0.0073 |
| VPA (min/d) | 23.6 (21.3) | 24.8 (22.9) | 22.9 (19.7) | 0.48 |
| MVPA (min/d) | 84.7 (46.4) | 89.0 (53.8) | 82.3 (39.3) | 0.042 |
| Sedentary time (min/d) | 329.3 ± 72.7 | 327.1 ± 77.5 | 331.6 ± 67.5 | 0.54 |
| Sleep duration (min/d) | 598.2 ± 38.2 | 591.4 ± 39.9 | 605.2 ± 35.7 | 0.0003 |
LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate-to-vigorous physical activity; VPA, vigorous physical activity.
Median; IQR in parentheses (all such values). Median (IQR) and sex comparisons were made by using Wilcoxon’s rank sum tests.
Sex comparisons were made by using chi-square tests for categorical variables.
Mean ± SD (all such values). Mean ± SD and sex comparisons were made by using ANOVA.
On the basis of International Obesity Task Force age- and sex-specific weight-for-height growth charts.
There were no sex differences in the number of days of PA measurement (P = 0.47). Children were monitored for 1 (4%), 2 (4%), 3 (7%), 4 (7%), 5 (16%), 6 (60%), or 7 (2%) valid days (operationalized as ≥600 min nonsleeping data). Before removing sleep, the average registered observation time from the Actiheart sensor was 1425.6 ± 63.6 min/d, and >92% of days contained 1440 min (24 h) data. These values indicate a high adherence to the accelerometer protocol and low variability in wear time. An average of 5.2 d of accelerometry per child was collected and culminated in a total of 2045 d PA, which served as the unit of analysis in this investigation. As reported in Table 1, these activity data revealed that boys participated in more MPA and MVPA than girls did (P = 0.0073 and P = 0.042, respectively). Boys also conducted more bouts of MVPA that lasted ≥5 min in length [boys: 3.7 (IQR: 3); girls: 3.0 (IQR: 2.4); P = 0.0037]. Girls slept longer than boys (P = 0.0003), but there was no sex difference in LPA, VPA, or time spent sedentary (P ≥ 0.11; Table 1). The median number of VPA bouts that lasted ≥5 min in duration was comparably low across sexes [boys: 0.7 (IQR: 1.3); girls: 0.6 (IQR: 1.0); P = 0.076], which alluded to the sporadic nature of preschool PA.
Compared with children followed-up at 4 y of age with incomplete data, children who contributed to the analysis (n = 398) were, on average, 0.13 mo younger (P = 0.0085), 0.10 kg heavier at birth (P = 0.0054), and 0.9 cm taller at 4 y of age (P = 0.0019). In addition, the prevalence of maternal smoking in pregnancy was lower in children who contributed to the analysis than in children with incomplete data (9% compared with 16%, respectively; P = 0.005). In terms of body composition, 345 children underwent DXA at 4 y, but did not contribute to the analysis because data for PA or covariates were not collected. These children did not differ from the analysis sample in any aspect of body composition (P ≥ 0.43) or in the proportion classified as normal, overweight, or obese (P = 0.62). Similarly, 194 children had valid data for PA but did not contribute to the analysis because data for body composition or covariates were not available. Compared with these children, children who formed the analysis sample slept longer (589.5 compared with 598.2 min/d, respectively; P = 0.0063) and were less active because they performed fewer average minutes of LPA per day (434.9 compared with 423.6; P = 0.039), fewer average minutes of MPA per day (66.6 compared with 58.6; P = 0.0020), and fewer average minutes of MVPA per day (95.9 compared with 84.7; P = 0.032).
Bivariate correlations between independent and dependent variables are shown in Table 2. Moderate to strong correlations between most activity variables meant that, in an effort to avoid collinearity-induced errors, a series of individual linear regressions were performed for each PA intensity. Adiposity variables were also strongly correlated (P < 0.001 for all) and were all negatively correlated with LMI (P < 0.001). Correlations between activity and body-composition variables showed that all intensities of PA were negatively related to adiposity, but strengths of coefficients were higher for VPA (P < 0.001 for all) than the lower intensities of activity. In contrast, sedentary time was positively correlated with adiposity (P ≤ 0.01). Activity variables were positively correlated with LMI but unlike coefficients for adiposity, there was no discernible pattern toward stronger correlations for higher-intensity PA. Sedentary time was significantly negatively correlated with LMI (P < 0.001).
TABLE 2.
Bivariate correlations between PA intensity, sedentary time, and body composition variables1
| LPA | MPA | VPA | MVPA | Sedentary time | Percentage of body fat | FMI | TFMI | |
|---|---|---|---|---|---|---|---|---|
| MPA | 0.36 | |||||||
| VPA | 0.0182 | 0.61 | ||||||
| MVPA | 0.25 | 0.94 | 0.85 | |||||
| Sedentary time | −0.75 | −0.64 | −0.39 | −0.60 | ||||
| Percentage of body fat | −0.0463 | −0.11 | −0.13 | −0.13 | 0.080 | |||
| FMI | −0.0202 | −0.0734 | −0.12 | −0.10 | 0.0584 | 0.94 | ||
| TFMI | −0.0332 | −0.0594 | −0.10 | −0.084 | 0.0624 | 0.88 | 0.94 | |
| LMI | 0.095 | 0.12 | 0.0684 | 0.11 | −0.084 | −0.39 | −0.087 | −0.082 |
P < 0.001 unless otherwise indicated. Data were analyzed by using Pearson’s product-moment correlation. FMI, fat mass index; LMI, lean mass index; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; TFMI, trunk fat mass index; VPA, vigorous physical activity.
P ≥ 0.05.
P < 0.05.
P < 0.01.
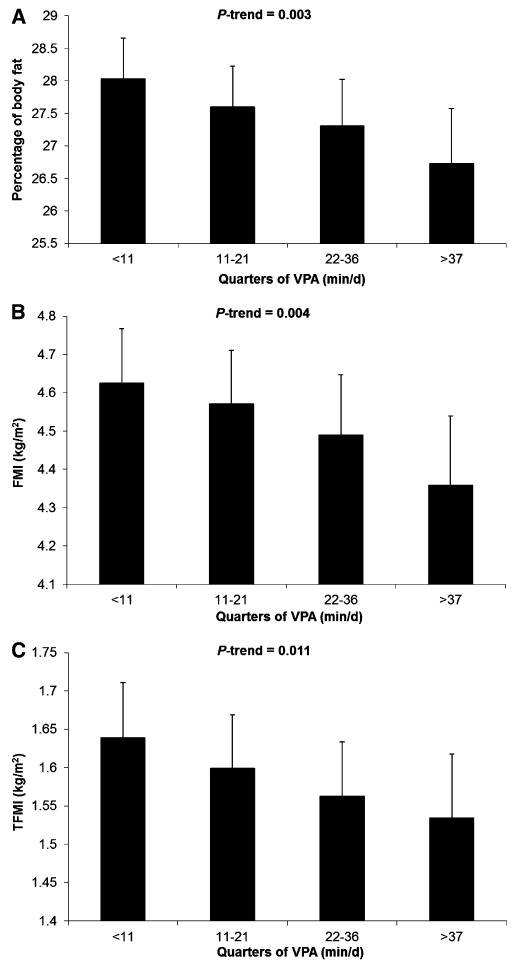
Adjusted associations for PA intensity and sedentary time with each adiposity measurement are shown in Table 3. Because there was no evidence of an effect modification by sex (P ≥ 0.14), data are related to the whole sample adjusted for sex. The most-consistent results were established for VPA, which was significantly inversely associated with all indicators of adiposity, independent of covariates including sedentary time (P < 0.001 for percentage of body fat and FMI; P = 0.002 for TFMI). A total of 15 min VPA/d was associated with a 0.36 lower percentage of body fat, 1.75% lower FMI, and 1.90% lower TFMI. Data were analyzed in continuous form, but for presentation purposes, adjusted means of the percentage of body fat, FMI, and TFMI stratified by quarters of VPA are shown in Figure 2. An inverse dose-response relation with lower adiposity as a function of increasing VPA is illustrated in Figure 2 (P-trend ≤ 0.011).
TABLE 3.
Associations between PA intensity, sedentary time, and adiposity1
| Percentage of body fat |
FMI (kg/m2)2 |
TFMI (kg/m2)2 |
||||
|---|---|---|---|---|---|---|
| Coefficient (95% CI) | P | Percentage difference (95% CI) | P | Percentage difference (95% CI) | P | |
| LPA (60 min/d) | ||||||
| Model 1 | −0.069 (−0.35, 0.22) | 0.64 | 0.09 (−1.26, 1.47) | 0.89 | −0.13 (−1.90, 1.66) | 0.88 |
| Model 2 | 0.53 (0.091, 0.98) | 0.018 | 2.60 (0.38, 5.00) | 0.022 | 2.50 (−0.40, 5.00) | 0.095 |
| MPA (30 min/d) | ||||||
| Model 1 | −0.27 (−0.57, 0.029) | 0.077 | −0.87 (−2.36, 0.64) | 0.26 | −0.86 (−2.80, 1.12) | 0.39 |
| Model 2 | −0.16 (−0.54, 0.22) | 0.42 | −0.69 (−2.62, 1.27) | 0.49 | −0.32 (−2.83, 2.25) | 0.80 |
| VPA (15 min/d) | ||||||
| Model 1 | −0.38 (−0.56, −0.20) | <0.001 | −1.68 (−2.52, 0.83) | <0.001 | −1.88 (−3.01, −0.73) | 0.001 |
| Model 2 | −0.36 (−0.55, −0.17) | <0.001 | −1.75 (−2.65, −0.85) | <0.001 | −1.90 (−3.10, −0.68) | 0.002 |
| MVPA (60 min/d) | ||||||
| Model 1 | −0.54 (−0.92, −0.0028) | 0.005 | −2.11 (−3.89, −0.29) | 0.023 | −2.26 (−4.59, 0.13) | 0.064 |
| Model 2 | −0.53 (−0.98, −0.091) | 0.018 | −2.60 (−5.00, −0.38) | 0.022 | −2.50 (−5.00, 0.40) | 0.095 |
| Sedentary time (120 min/d) | ||||||
| Model 1 | 0.37 (−0.087, 0.82) | 0.113 | 1.00 (−1.15, 3.19) | 0.36 | 1.36 (−1.46, 4.26) | 0.35 |
| Model 2 | 0.013 (−0.54, 0.57) | 0.96 | −0.70 (−3.30, 1.97) | 0.60 | −0.26 (−3.67, 3.26) | 0.88 |
Model 1 was adjusted for age, sex, birth weight, maternal education, maternal BMI, smoking during pregnancy, and sleep duration. For LPA, MPA, VPA, and MVPA, model 2 was adjusted as for model 1 and for sedentary time. For sedentary time, model 2 was adjusted as for model 1 and for MVPA. Data were analyzed by using repeated-measures linear regression. FMI, fat mass index; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; TFMI, trunk fat mass index; VPA, vigorous physical activity.
Coefficients were on the log scale and have been retransformed; estimates represent the percentage change in outcome per unit change in the independent variable and were calculated by using the following formula: [(exponentiated β − 1) × 100] × scaling factor.
FIGURE 2.
Adjusted means (95% CIs) of percentage body fat, FMI, and TFMI stratified by quarters of VPA (min/d). Data were adjusted for age, sex, birth weight, maternal education, maternal BMI, smoking during pregnancy, sleep duration, and sedentary time (P-trend ≤ 0.011 for all). FMI, fat mass index; TFMI, trunk fat mass index; VPA, vigorous physical activity.
Greater MVPA was associated both with a lower percentage of body fat and FMI (P = 0.018 and P = 0.022, respectively) but not lower TFMI (P = 0.095; Table 3). Because MPA was unrelated to all measures of adiposity (P ≥ 0.077), by process of elimination, the observed associations between MVPA, percentage of body fat, and FMI were exclusively explained by the contribution of VPA. LPA was positively associated with the percentage of body fat and FMI after adjustment for sedentary time (P = 0.018 and P = 0.022, respectively), but no associations were shown between sedentary time and adiposity (P ≥ 0.35). Variance inflation factors and tolerance statistics were within acceptable limits (variance inflation factors ≤10 and tolerances ≥0.1) for all regressions, but these tests can not verify the absence of collinearity within models. Indicators of collinearity include large shifts in coefficients and theoretically questionable changes in signs of the effect on the introduction of a correlated variable. LPA and sedentary time were strongly negatively correlated (r = −0.75), and after we compared models 1 and 2 for LPA, as shown in Table 3, it appeared that the positive associations between LPA and adiposity were probably spurious artifacts because LPA and sedentary time were heavily collinear.
Adjusted associations for PA intensity and sedentary time with LMI are shown in Table 4. There was no evidence for an effect modification by sex (P ≥ 0.094), and thus, data are presented for the whole sample with adjustment for sex. Initially, there was borderline evidence for a positive relation between MPA (P = 0.066) and MVPA (P = 0.063) with LMI, but adjustment for sedentary time weakened these associations (P ≥ 0.45). Similarly, there was some evidence to indicate that greater sedentary time was associated with lower LMI (P = 0.056), but after adjustment for MVPA, there was no longer evidence of an association (P = 0.25).
TABLE 4.
Associations between PA intensity, sedentary time, and LMI1
| LMI (95% CI) (kg/m2.5) | P | |
|---|---|---|
| LPA (60 min/d) | ||
| Model 1 | 0.036 (−0.014, 0.086) | 0.16 |
| Model 2 | −0.033 (−0.12, 0.053) | 0.45 |
| MPA (30 min/d) | ||
| Model 1 | 0.05 (−0.0035, 0.11) | 0.066 |
| Model 2 | 0.022 (−0.046, 0.089) | 0.53 |
| VPA (15 min/d) | ||
| Model 1 | 0.030 (−0.0067, 0.066) | 0.11 |
| Model 2 | 0.014 (−0.023, 0.052) | 0.45 |
| MVPA (60 min/d) | ||
| Model 1 | 0.070 (−0.0038, 0.14) | 0.063 |
| Model 2 | 0.033 (−0.053, 0.12) | 0.45 |
| Sedentary time (120 min/d) | ||
| Model 1 | −0.080 (−0.16, 0.0022) | 0.056 |
| Model 2 | −0.058 (−0.16, 0.040) | 0.25 |
Model 1 was adjusted for age, sex, birth weight, maternal education, maternal BMI, smoking during pregnancy, and sleep duration. For LPA, MPA, VPA, and MVPA, model 2 was adjusted as for model 1 and for sedentary time. For sedentary time, model 2 was adjusted as for model 1 and for MVPA. Data were analyzed by using repeated-measures linear regression. LMI, lean mass index; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; VPA, vigorous physical activity.
In sensitivity analyses 1 and 2, coefficients for all body-composition variables remained materially unchanged. When analyses were restricted to children with ≥6 d valid PA data, point estimates strengthened by a small amount, but changes in significance did not occur (data not shown). There was no evidence to indicate nonlinear associations in any models (data not shown).
DISCUSSION
The results from this study suggested that inverse associations exist between VPA and both total and abdominal adiposity. In contrast, sedentary time and low-to-moderate PA intensities (when the statistical artifact was accounted for) appeared to be unrelated to adiposity. Fifteen minutes of accumulated VPA per day was associated with 0.36 lower percentage of body fat, 1.75% lower FMI, and 1.90% lower TFMI. There are no established health-related reference values for adiposity in preschoolers, and thus, it is difficult to decipher the clinical importance of these data. However, overweight and obesity track over time, and adiposity increases with age, and thus, any reduction in adiposity at 4 y is unlikely to be trivial (30). Furthermore, truncal adipose tissue is strongly related to various cardiovascular and metabolic risk factors (31, 32). The inverse association between VPA and TFMI may point toward a role for high-intensity movement in the determination of the metabolic health of young children.
We are aware of only one other study that has examined the association between objectively measured sedentary time and adiposity in preschoolers (33). Similar to our adjusted associations, the investigation showed no correlation between sedentary behavior and weight status. Television viewing, which is a specific type of sedentary behavior, has been shown to exhibit positive associations with adiposity and overweight/obesity in preschoolers (34). However, dietary factors could mediate this relation because television viewing seems to be associated with a higher energy intake and poorer diet quality (35, 36). Observations in older children by using objective measures of sedentary time have offered the same inference as in the current study, that the total time spent sedentary may not be associated with adiposity (37-40).
Differences in accelerometer models and methodologies, the derivation of activity variables, and measures of body composition preclude a direct comparison of our PA data with other investigations. Nonetheless, our results are in agreement with other cross-sectional studies conducted in preschoolers. Janz et al (13) showed that VPA was negatively correlated with the percentage of body fat, FM, and TFM in 434 US children aged 4–6 y. Children in the lowest one-quarter for VPA had a higher percentage of body fat and 1 kg greater FM than did children in the topmost one-quarter. Similarly, in an ethnically and socially diverse group of 245 American preschoolers, overweight boys, compared with normal weight boys, were shown to participate in less MVPA and fewer hourly intervals of MVPA and VPA (14). In another diverse US cohort, it was further shown that odds of early childhood overweight were reduced by 6% and 32%, respectively, with each additional minute of VPA and very vigorous PA per day (5). Finally, in 281 Portuguese children aged 4–6 y, it was reported that children in the lowest one-third for VPA, relative to children in the highest third for VPA, had ~4-fold higher odds of being overweight (15). Our results contribute to the literature by showing that VPA is strongly inversely associated with both total and abdominal adiposity, irrespective of the time spent sedentary, and regardless of whether sedentary time is measured as awake-time only or total sedentary time including sleep. Together, the available data imply that weight interventions and PA guidelines may attain greater success in combating early childhood obesity if a paradigm shift toward VPA occurred. This recommendation concurs with the closing remarks of a recent systematic review that summarized the available evidence of weight-management schemes in young children (41). Currently, UK guidance states that children younger than 5 y should be active 180 min/d while simultaneously minimizing sedentary behaviors, but there is no recommendation regarding activity intensity (42).
Mechanistically, the inverse relation between VPA and adiposity may be due to maximal fat oxidation, which occurs during intense exercise (43). It has also been suggested that high-intensity PA may stimulate a preference for fat as an energy source, create greater elevations in postactivity energy expenditure (when the body returns to a condition of homeostasis), and transiently suppress (or elevate to a lesser extent compared with low-to-moderate–intensity PA) postactivity energy intake (44, 45). Because high-intensity activities often involve large ground-reaction forces and repetitive or forceful muscular contractions, it has further been hypothesized that vigorous movement may encourage stem cell differentiation into muscle and bone to the detriment of adipose tissue formation (46). However, we showed no association between VPA and LMI independently of sedentary time. Evidence for each of the aforementioned mechanisms that theoretically links VPA with reduced fatness remains limited. Whether VPA performed habitually by young children (ie, vigorous activity that is sporadic and unsustained) can stimulate these pathways remains to be determined.
In contrast to the results for adiposity, we showed no association between PA with LMI. Elsewhere, a positive association between MVPA and fat-free mass in girls was observed (13), but this study did not adjust for sedentary time. Furthermore, fat-free mass includes lean mass and the sum of bone mineral content. We have previously shown that MVPA is positively associated with hip-bone size and density (47). Thus, the results from the SWS indicated that, although objectively measured PA in youth may be influential in terms of bone density, it does not seem to be associated with more lean tissue independent of sedentary time. Similarly, we did not show an association between sedentary time and LMI. There are few comparable data, but Janz et al (13) failed to find an association between parental-reported television viewing and fat-free mass. Moreover, in children ≥10 y of age, self-reported sedentary behaviors appear to be unrelated to follow-up fat-free mass index (48).
Strengths of the current study included the (relatively) large and homogenous sample, objective measures of PA and body composition, and adjustment for several potential confounders. However, the sample signified a population with low minority representation, and thus, the generalizability of findings may be limited. We also incorporated a complete-case analysis in which children with missing data were excluded; this approach can yield biased variable estimates. Although we could not rule out bias as a result of missing data, we did compare associations that were both adjusted and unadjusted for predictors of missingness. Results were comparable (data not shown) and, thus, provided some indication against systematic error because of missing information. As regards the measurement of PA, accelerometers placed on the torso struggle to adequately capture static activities that involve movement of the limbs. Such activities include bicycling, lifting and pushing, climbing, swinging, and throwing. Many children spend time performing these types of activities, and, as recently indicated, they are more prevalent in high-active children (49). The failure to register these kinds of activities to a greater extent in high- than low-active children may have resulted in an attenuation of associations. Smaller epochs (<60 s) would have also been advantageous because they have been shown to provide greater distinctions in daily VPA between more- and less-active children by minimizing systematic measurement error (50). The likely outcome of the use of an extended epoch duration is analogous to the error incurred by missing certain types of activities; a dilution in effect estimates may have occurred, and thus, coefficients displayed for VPA and MVPA were plausibly underestimates of their true associations with body composition. However, residual confounding by unknown and unmeasured factors such as energy intake and diet composition could not be excluded. To finish, our cross-sectional study precluded us from making a strong inference about causality, let alone its direction; low participation in VPA may be a consequence and not a cause of higher adiposity because of physical limitations, psychological factors, and social barriers (51). To aid causality, prospective studies or randomized controlled trials are needed.
In conclusion, in young children aged 4 y, the time engaged in VPA appears to be strongly and independently associated with lower adiposity. In contrast, sedentary time and time spent in low-to-moderate intensity PA were unrelated to adiposity. Thus, the data suggest that, if the obesity epidemic is to be successfully curbed, interventions and PA guidelines alike may need to encourage VPA from an early age. Prospective study designs are needed to determine the direction of the association between PA intensity and adiposity in early childhood.
Acknowledgments
We are grateful to the women of Southampton and their children who took part in the study and the research nurses and other staff who collected and processed data. We also thank Stephen Sharp and Jian’an Luan for their statistical advice.
Footnotes
Supported by grants from the MRC, the British Heart Foundation, Arthritis Research UK, the National Osteoporosis Society, the International Osteoporosis Foundation, the Cohen Trust, The Food Standards Agency, the National Institute for Health Research (NIHR) Southampton Biomedical Research Centre, University of Southampton and University Hospitals Southampton National Health Service Foundation Trust, and NIHR Musculoskeletal Biomedical Research Unit, University of Oxford. Participants were drawn from a cohort study funded by the MRC and the Dunhill Medical Trust. KMG is supported by the NIHR through the NIHR Southampton Biomedical Research Centre.
Abbreviations used: DXA, dual-energy X-ray absorptiometry; FM, fat mass; FMI, fat mass index; LM, lean mass; LMI, lean mass index; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate-to-vigorous physical activity; PA, physical activity; SWS, Southampton Women’s Survey; TFM, trunk fat mass; TFMI, trunk fat mass index; VPA, vigorous physical activity.
REFERENCES
- 1.Department of Health . National child measurement programme: England, 2011/12 school year London. Department of Health; United Kingdom: 2012. Available from: http://www.ic.nhs.uk/ncmp (cited December 2012) [Google Scholar]
- 2.Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa Heart Study. Pediatrics. 2001;108:712–8. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- 3.Hesketh KD, Campbell KJ. Interventions to prevent obesity in 0-5 year olds: an updated systematic review of the literature. Obesity (Silver Spring) 2010;18(suppl 1):S27–35. doi: 10.1038/oby.2009.429. [DOI] [PubMed] [Google Scholar]
- 4.Graf C. Preventing and treating obesity in pediatrics through physical activity. European Association for Predictive. EPMA J. 2011;2:261–70. doi: 10.1007/s13167-011-0091-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metallinos-Katsaras ES, Freedson PS, Fulton JE, Sherry B. The association between an objective measure of physical activity and weight status in preschoolers. Obesity (Silver Spring) 2007;15:686–94. doi: 10.1038/oby.2007.571. [DOI] [PubMed] [Google Scholar]
- 6.Berman N, Bailey R, Barstow TJ, Cooper DM. Spectral and bout detection analysis of physical activity patterns in healthy, prepubertal boys and girls. Am J Hum Biol. 1998;10:289–97. doi: 10.1002/(SICI)1520-6300(1998)10:3<289::AID-AJHB4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Baquet G, Stratton G, Van Praagh E, Berthoin S. Improving physical activity assessment in prepubertal children with high-frequency accelerometry monitoring: a methodological issue. Prev Med. 2007;44:143–7. doi: 10.1016/j.ypmed.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Kohl HW, Fulton JE, Caspersen CJ. Assessment of physical activity among children and adolescents: a review and synthesis. Prev Med. 2000;31:S54–76. [Google Scholar]
- 9.Ekelund U, Tomkinson GR, Armstrong N. What proportion of youth are physically active? Measurement issues, levels and recent time trends. Br J Sports Med. 2011;45:859–65. doi: 10.1136/bjsports-2011-090190. [DOI] [PubMed] [Google Scholar]
- 10.Oliver M, Schofield GM, Kolt GS. Physical activity in preschoolers: understanding prevalence and measurement issues. Sports Med. 2007;37:1045–70. doi: 10.2165/00007256-200737120-00004. [DOI] [PubMed] [Google Scholar]
- 11.Adolph AL, Puyau MR, Vohra FA, Nicklas TA, Zakeri IF, Butte NF. Validation of uniaxial and triaxial accelerometers for the assessment of physical activity in preschool children. J Phys Act Health. 2012;9:944–53. doi: 10.1123/jpah.9.7.944. [DOI] [PubMed] [Google Scholar]
- 12.Corder K, Ekelund U, Steele RM, Wareham NJ, Brage S. Assessment of physical activity in youth. J Appl Physiol. 2008;105:977–87. doi: 10.1152/japplphysiol.00094.2008. [DOI] [PubMed] [Google Scholar]
- 13.Janz KF, Levy SM, Burns TL, Torner JC, Willing MC, Warren JJ. Fatness, physical activity, and television viewing in children during the adiposity rebound period: the Iowa Bone Development Study. Prev Med. 2002;35:563–71. doi: 10.1006/pmed.2002.1113. [DOI] [PubMed] [Google Scholar]
- 14.Trost SG, Sirard JR, Dowda M, Pfeiffer KA, Pate RR. Physical activity in overweight and nonoverweight preschool children. Int J Obes Relat Metab Disord. 2003;27:834–9. doi: 10.1038/sj.ijo.0802311. [DOI] [PubMed] [Google Scholar]
- 15.Vale SM, Santos RM, da Cruz Soares-Miranda LM, Moreira CM, Ruiz JR, Mota JA. Objectively measured physical activity and body mass index in preschool children. Int J Pediatr. 2010;2010:479439. doi: 10.1155/2010/479439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maffeis C. Aetiology of overweight and obesity in children and adolescents. Eur J Pediatr. 2000;159(suppl 1):S35–44. doi: 10.1007/pl00014361. [DOI] [PubMed] [Google Scholar]
- 17.Inskip HM, Godfrey KM, Robinson SM, Law CM, Barker DJ, Cooper C. Cohort profile: The Southampton Women’s Survey. Int J Epidemiol. 2006;35:42–8. doi: 10.1093/ije/dyi202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brage S, Brage N, Franks PW, Ekelund U, Wareham NJ. Reliability and validity of the combined heart rate and movement sensor Actiheart. Eur J Clin Nutr. 2005;59:561–70. doi: 10.1038/sj.ejcn.1602118. [DOI] [PubMed] [Google Scholar]
- 19.Pate RR, Almeida MJ, McIver KL, Pfeiffer KA, Dowda M. Validation and calibration of an accelerometer in preschool children. Obesity (Silver Spring) 2006;14:2000–6. doi: 10.1038/oby.2006.234. [DOI] [PubMed] [Google Scholar]
- 20.Williams HG, Pfeiffer KA, O’Neill JR, Dowda M, McIver KL, Brown WH, Pate RR. Motor skill performance and physical activity in preschool children. Obesity (Silver Spring) 2008;16:1421–6. doi: 10.1038/oby.2008.214. [DOI] [PubMed] [Google Scholar]
- 21.Pfeiffer KA, Dowda M, McIver KL, Pate RR. Factors related to objectively measured physical activity in preschool children. Pediatr Exerc Sci. 2009;21:196–208. doi: 10.1123/pes.21.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Corder K, Brage S, Wareham NJ, Ekelund U. Comparison of PAEE from combined and separate heart rate and movement models in children. Med Sci Sports Exerc. 2005;37:1761–7. doi: 10.1249/01.mss.0000176466.78408.cc. [DOI] [PubMed] [Google Scholar]
- 23.Ridgway CL, Brage S, Sharp SJ, Corder K, Westgate KL, van Sluijs EM, Goodyer IM, Hallal PC, Anderssen SA, Sardinha LB, et al. Does birth weight influence physical activity in youth? A combined analysis of four studies using objectively measured physical activity. PLoS ONE. 2011;6:e16125. doi: 10.1371/journal.pone.0016125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reilly JJ, Penpraze V, Hislop J, Davies G, Grant S, Paton JY. Objective measurement of physical activity and sedentary behaviour: review with new data. Arch Dis Child. 2008;93:614–9. doi: 10.1136/adc.2007.133272. [DOI] [PubMed] [Google Scholar]
- 25.Acebo C, Sadeh A, Seifer R, Tzischinsky O, Hafer A, Carskadon MA. Sleep/wake patterns derived from activity monitoring and maternal report for healthy 1- to 5-year-old children. Sleep. 2005;28:1568–77. doi: 10.1093/sleep/28.12.1568. [DOI] [PubMed] [Google Scholar]
- 26.Tikotzky L, Sadeh A. Sleep patterns and sleep disruptions in kinder-garten children. J Clin Child Psychol. 2001;30:581–91. doi: 10.1207/S15374424JCCP3004_13. [DOI] [PubMed] [Google Scholar]
- 27.Wells JC. A critique of the expression of paediatric body composition data. Arch Dis Child. 2001;85:67–72. doi: 10.1136/adc.85.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–3. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Penpraze V, Reilly JJ, MacLean CM, Montgomery C, Kelly LA, Paton JY, Aitchison T, Grant S. Monitoring of physical activity in young children: how much is enough? Pediatr Exerc Sci. 2006;18:483–91. [Google Scholar]
- 30.Riddoch CJ, Leary SD, Ness AR, Blair SN, Deere K, Mattocks C, Griffiths A, Smith GD, Tilling K. Prospective associations between objective measures of physical activity and fat mass in 12-14 year old children: the Avon Longitudinal Study of Parents and Children (ALSPAC) BMJ. 2009;339:b4544. doi: 10.1136/bmj.b4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goran MI, Gower BA. Relation between visceral fat and disease risk in children and adolescents. Am J Clin Nutr. 1999;70:149S–56S. doi: 10.1093/ajcn/70.1.149s. [DOI] [PubMed] [Google Scholar]
- 32.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99:541–5. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 33.Dolinsky DH, Brouwer RJ, Evenson KR, Siega-Riz AM, Ostbye T. Correlates of sedentary time and physical activity among preschool-aged children. Prev Chronic Dis. 2011;8:A131. [PMC free article] [PubMed] [Google Scholar]
- 34.te Velde SJ, van Nassau F, Uijtdewilligen L, van Stralen MM, Cardon G, De Craemer M, Manios Y, Brug J, Chinapaw MJ, ToyBox-study group Energy balance-related behaviours associated with overweight and obesity in preschool children: a systematic review of prospective studies. Obes Rev. 2012;13(suppl 1):56–74. doi: 10.1111/j.1467-789X.2011.00960.x. [DOI] [PubMed] [Google Scholar]
- 35.Miller SA, Taveras EM, Rifas-Shiman SL, Gillman MW. Association between television viewing and poor diet quality in young children. Int J Pediatr Obes. 2008;3:168–76. doi: 10.1080/17477160801915935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matheson DM, Killen JD, Wang Y, Varady A, Robinson TN. Children’s food consumption during television viewing. Am J Clin Nutr. 2004;79:1088–94. doi: 10.1093/ajcn/79.6.1088. [DOI] [PubMed] [Google Scholar]
- 37.Andersen LB, Harro M, Sardinha LB, Froberg K, Ekelund U, Brage S, Anderssen SA. Physical activity and clustered cardiovascular risk in children: a cross-sectional study (The European Youth Heart Study) Lancet. 2006;368:299–304. doi: 10.1016/S0140-6736(06)69075-2. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell JA, Mattocks C, Ness AR, Leary SD, Pate RR, Dowda M, Blair SN, Riddoch C. Sedentary behavior and obesity in a large cohort of children. Obesity (Silver Spring) 2009;17:1596–602. doi: 10.1038/oby.2009.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ekelund U, Luan J, Sherar LB, Esliger DW, Griew P, Cooper A. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307:704–12. doi: 10.1001/jama.2012.156. Published erratum appears in JAMA 2012;307:1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chaput JP, Lambert M, Mathieu ME, Tremblay MS, O’ Loughlin J, Tremblay A. Physical activity vs. sedentary time: independent associations with adiposity in children. Pediatr Obes. 2012;7:251–8. doi: 10.1111/j.2047-6310.2011.00028.x. [DOI] [PubMed] [Google Scholar]
- 41.Bond M, Wyatt K, Lloyd J, Taylor R. Systematic review of the effectiveness of weight management schemes for the under fives. Obes Rev. 2011;12:242–53. doi: 10.1111/j.1467-789X.2010.00713.x. [DOI] [PubMed] [Google Scholar]
- 42.Department of Health . Physical Activity, Health Improvement and Protection. Start active, stay active: a report on physical activity from the four home countries’ Chief Medical Officers. Department of Health; London: 2011. [Google Scholar]
- 43.Riddell MC. The endocrine response and substrate utilization during exercise in children and adolescents. J Appl Physiol. 2008;105:725–33. doi: 10.1152/japplphysiol.00031.2008. [DOI] [PubMed] [Google Scholar]
- 44.Hunter GR, Weinsier RL, Bamman MM, Larson DE. A role for high intensity exercise on energy balance and weight control. Int J Obes Relat Metab Disord. 1998;22:489–93. doi: 10.1038/sj.ijo.0800629. [DOI] [PubMed] [Google Scholar]
- 45.Boutcher SH. High-intensity intermittent exercise and fat loss. J Obes. 2011;2011:868305. doi: 10.1155/2011/868305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gutin B. Child obesity can be reduced with vigorous activity rather than restriction of energy intake. Obesity (Silver Spring) 2008;16:2193–6. doi: 10.1038/oby.2008.348. [DOI] [PubMed] [Google Scholar]
- 47.Harvey NC, Cole ZA, Crozier SR, Kim M, Ntani G, Goodfellow L, Robinson SM, Inskip HM, Godfrey KM, Dennison EM, et al. Physical activity, calcium intake and childhood bone mineral: a population-based cross-sectional study. Osteoporos Int. 2012;23:121–30. doi: 10.1007/s00198-011-1641-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fulton JE, Dai S, Steffen LM, Grunbaum JA, Shah SM, Labarthe DR. Physical activity, energy intake, sedentary behavior, and adiposity in youth. Am J Prev Med. 2009;37:S40–9. doi: 10.1016/j.amepre.2009.04.010. suppl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Howie EK, Brown WH, Dowda M, McIver KL, Pate RR. Physical activity behaviours of highly active preschoolers. Pediatric Obesity. doi: 10.1111/j.2047-6310.2012.00099.x. Epub ahead of print 8 October 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dorsey K, Herrin J, Krumholz H, Irwin M. The utility of shorter epochs in direct motion monitoring. Res Q Exerc Sport. 2009;80:460–8. doi: 10.1080/02701367.2009.10599584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Metcalf BS, Hosking J, Jeffery AN, Voss LD, Henley W, Wilkin TJ. Fatness leads to inactivity, but inactivity does not lead to fatness: a longitudinal study in children (EarlyBird 45) Arch Dis Child. 2011;96:942–7. doi: 10.1136/adc.2009.175927. [DOI] [PubMed] [Google Scholar]