Chair: Meeta
Co-Chairs: Leela Digumarti, Neelam Agarwal, Nirmala Vaze, Rashmi Shah, Sonia Malik.
Advisory Board: Asha Kapadia, Atul Munshi, Duru Shah, Rama Vaidya, Saroj Srivastava, Sonia Malik, Sunila Khandelwal, Urvashi Prasad Jha.
External Review Board: Anita Gadgil, Dayasagar, G R Sridhar, Hema Divakar, P K Shah, Rakesh Sahay, Sanjay Kalra.
Resource Faculty: Alka Kumar, Anil Mahajan, Anita Kant, Anita Shah, Anu Vij, Archana Tripathi, Asha Kapadia, Ashok Khurana, Atul Munshi, Bhavana Sheth, Bipasa Sen, C. H. Trivedi, Chellamma V.K, Choranur Ambuja, Duru Shah, G Nagamani, Gurava A Reddy, H. P. Pattanaik, Jaideep Malhotra, Jaishree Gajraj, Jignesh Shah, Jyothi Unni, Jyoti Hak, Jyoti M Shah, Kaushal Chundawat, Lakshm Ratna, Lakshmi Seshadri, Leela Digumarti, Madhukar Reddy, Mala Raj, Mandakini Parihar, Maninder Ahuja, Manjit Kaur Mohi, Meeta, Monica Chauhan, Muzammil S. Shaikh, Navneet Magon, Navneet Takkar, Neelam Agarwal, Nidhi Gupta, Nila A Mohile, Nirmala Vaze, Phagun N Shah, Preeti D Galvankar, Pushpa Sethi, Rajesh S Kumar, Rama Vaidya, Ranu Patni, Rashmi Shah, Rekha Sharma, Renu Makwana, Rita Shah, Rohit Shetty, Roza Olyai, Saroj Srivastava, Seema Sharma, Shailender Singh, Sharad Kumar, Sharmila Pimple, Sheela V. Mane, Shobhana Mohandas, Sonal Bathla, Sonia Malik, Sudha Sharma, Sudhakar Krishnamurti, Sunila Khandelwal, Surendra Shastri, Sushmita Dave, Suvarna Khadilkar, Tanvir, Tripti Nagaria, Urvashi Prasad Jha, Urvashi Yavalkar, Usha Rani Poli, Vandana Bansal, Yashodhara Pradeep.
INTRODUCTION
Guidelines are a method of translating the best available evidence into clinical, communicable, organizational, and policy making statements in the hope of improving health-care and/or policies. Unlike protocols, guidelines are meant to aid the clinician in decision making. Do we need country-specific guidelines? Yes, we do, given the fact that the model of health-care delivery system and the prevailing environment of one country may not be extrapolated to that of another.
“Working with what you have, where you are, and not with what you wish for” is the principle each one of us follow in clinical practice to give the best to our patients. This guideline hopes to bridge the gap between evidence-based practice, backed by scientific evidence and experience-based practice, based on the published and unpublished Indian data and expert opinions. The target readers of the guidelines are the adult women, members of the Indian Menopause Society (IMS), allied professionals, health-care providers and policy makers.
India is a land of rich and diverse cultural heritage. It is a land of diversity in terms of socio-economic, religion, culture, beliefs, education, and nutrition, urban, rural, and geographical regions. The dilemmas and challenges are unique to different regions, and solutions need to be planned accordingly. The specific issues pertaining to Indian women include an early age of natural menopause, genetic, and environmental influences, nutritional deficiencies and excesses resulting in physiological differences. These factors contribute significantly to an increased incidence of diabetes, cardiovascular disease, osteoporosis, and thyroid dysfunction. Genetic components are likely to play a prominent role in these disorders; for example, polymorphisms in estrogen receptors alpha and vitamin D receptor have been implicated in the pathogenesis of osteoporosis. Indians are known to be deficient in vitamin B12, folic acid, and vitamin D. In India, cancer cervix is the leading cause of genital cancers, and the peak incidence of breast cancer occurs at an earlier age than the Caucasians. India has the problem of urbanization bringing in new cultures and life-style leading to problems of obesity. There is a change from the traditional food to stored fast food. In the urban areas, there is breakdown of joint family system leading to nuclear families. The social support from the family during the transitional phase and ageing is dwindling on one side, and on the other side, life span has increased in the last two decades. The earlier age at menopause has several implications and challenges for health-care in India. There will be a large number of women who spend a substantial part of their life after menopause. Health-care providers will need to initiate programs and provide appropriate care for the large population of women living beyond menopause. In addition, attention needs to be directed toward implementing programs that will help to sensitize and increase awareness of menopause among women in India.
OBJECTIVES
To assist health-care practitioners in providing optimal and holistic care to the women in transition phase.
To aid primary care physicians to decide when to refer patients with difficult problems to the relevant specialists.
To sensitize the health-care professionals, policy makers toward the health of the ageing woman and thus promote the concept of menopausal clinics.
To stimulate interest in research on all aspects of menopausal medicine.
METHODS
The planning to publishing of the document took 24 months. The core committee was formed and a broad-based multi-disciplinary list of experts was invited to write on the topic of their expertise. Majority of the reviews and deliberations were by e-mail. A one day intensive contact program of the contributors was convened at Hyderabad on September 8, 2012, and each topic was presented and deliberated upon. Consensus was obtained by an automated response system. Finally, the document was validated by an external review board.
The guideline is based on three previous documents released by the IMS and other global guidelines on menopause management. Data were sourced from the electronic database PubMed, MEDLINE, Cochrane Data-base of Systematic Reviews and published guidelines on menopause management. The Appraisal of Guidelines Research and Evaluation,[1] instrument was used to appraise published guidelines. Abstracts from papers and posters presented at the National IMS meetings, published and unpublished studies, expert opinion was considered. Cost-effectiveness of diagnosis and treatment is based on the available market value.
System for grading: Evidence used in the document
The quality of evidence and the level of recommendation were done using the Grades of Recommendation, Assessment, Development, and Evaluation system.[2]
Recommendations are based on strong evidence and, suggestions on experience-based evidence. This method is adapted to unite the diverse conditions of India with the best available data and the rich experience-based evidence from the experts.
Grades of evidence
High quality Grade A: Further research is very unlikely to change our confidence in the estimate of effect.
Moderate quality Grade B: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low quality Grade C: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low quality Grade D: We are very uncertain about the estimate.
Strength of recommendation
In terms of the strength of the recommendation, strong recommendations use the phrase “recommend,” and weak recommendations use the phrase “suggest.”
Research questions are placed at the end of each chapter in the monogram.
Benefits of using the guidelines
Benefits of using this guidelines are (i) improved quality of care (ii) Early detection and management of non-communicable disease (iii) understanding the urgent need of conducting preventive health programs by all stakeholders related to women’s health, and (iv) additionally, in view of the great lacunae in Indian data, it is hoped that the guidelines will help stimulate interest in research in various aspects of menopause.
CONCLUSIONS
The onus of developing specialty menopause clinics akin to antenatal clinics in the private and public sectors besides developing management of menopause as a medical specialty within obstetrics and gynecology care lies with the government and non-government organizations. Meanwhile, the aim of the guideline is to provide a resource book to aid the busy clinician in extending optimal care to the aging woman. The guideline is no doubt limited by the paucity of robust research evidence in India due to various factors, but effort has been directed to tailor the recommendations to the diverse Indian scenario with the best available evidence.
This is one of the endeavors of the IMS to work toward the slogan “Fit @ Forty, Strong @ Sixty, Independent @ Eighty.”
ACKNOWLEDGMENT
We thank the experts who took time out of their busy family life, academics, and work to contribute to the document on management of menopause in India. A special thanks to Dr. Shaantanu Donde, Dr. Ganesh Uchit for sourcing the data.
DISSEMINATION OF THE GUIDELINE
Executive Summary and Recommendations is available on the IMS website www.indianmenopausesociety.org.com. indianmenopausesociety.org.com It is published in the Journal of midlife, official publication of the IMS. M/s Jaypee Brothers Medical Publishers are our partners in publishing the monogram on the clinical practice guidelines on menopause.
Revision of the guideline
It is recommended that the Guidelines are upgraded every 5 years.
Editorial independence
The views expressed are independent of any extraneous influences.
REFERENCES
Agree Next Steps Consortium. The Agree II Instrument [Electronic version], 2009. Available from: http://www.agreetrust.org. [Last accessed on 2012 Feb 10].
Atkins D, Best D, Briss PA, Eccles M, Falck.Ytter Y, Flottorp S. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490.
SECTION I
GENERAL CONSIDERATIONS
1. Menopause is a transition phase from the reproductive to the non-reproductive phase in a woman’s life.[1] It is nature’s protective phenomenon against reproductive morbidity and mortality in the ageing population. Today, we are aware that menopause has much wider implications than simply loss of fertility. It sets the stage for ageing and accelerates the process of non-communicable disorders.
2. Menopause is diagnosed retrospectively by history. Markers for diagnosis of menopause are preferably restricted for use in special situations and for fertility issues. Levels of (FSH) Folllicular Stimulating Hormone > 10 IU/L are indicative of declining ovarian function. FSH levels > 20 IU/L are diagnostic of ovarian failure in the peri-menopausal age group with vasomotor symptoms (VMS) even in the absence of cessation of menstruation. FSH levels > 40 IU/L done 2 months apart is diagnostic of menopause. Anti-mullerian hormone becomes undetectable, inhibin levels fall, and antral follicular count and ovarian volume decreases at menopause. Menstrual irregularity is the only objective marker to define and establish the menopause transition.[2]
TERMINOLOGY
3. Natural or spontaneous menopause: It is recognized to have occurred after 12 months of amenorrhea for which there are no obvious pathological and physiological causes. It is a retrospective diagnosis. It occurs due to depletion of ovarian follicles resulting in near complete, but natural diminution of ovarian hormone secretion. There is no independent biological marker for menopause.[3,4,5]
4. Pre-menopause: It is often used to refer the entire reproductive period, up to the final menstrual period.[4]
5. Peri-menopause: It is the period immediately prior to and up to 1 year after the final menstrual period. It may last for 3-5 years. The characteristics are increased blood levels of FSH, anovulatory cycles, significantly reduced fertility and erratic menstrual periods, and onset of symptoms. This term is used interchangeably with menopause transition.[4,5]
6. Menopause transition: It is the term coined by Stages of Reproductive Aging Workshop (STRAW) group, and during this period, disturbed menstrual cycle and endocrine changes are observed.[5]
7. Climacteric: Literally, it means the rungs of a ladder. It is interchangeable with peri-menopause and menopause transition. When associated with symptoms, it is termed as the climacteric syndrome. This term is preferably not to be used in scientific papers.[4]
8. Post-menopause: It is the span of time dating from the final menstrual period, regardless of whether the menopause was spontaneous or iatrogenic.[5]
9. Senescence: It is the period after the age of 60 years.[3]
10. Premature menopause: It is the spontaneous menopause occurring two standard deviations (SDs) below the mean estimated age for the reference population. Traditionally, it is considered to be below the age of 40 years.[4,5] We may consider it as occurring below 38 years*.
11. Induced menopause: Cessation of menstruation that follows bilateral oophorectomy or iatrogenic ablation of ovarian function.[4]
12. Temporary menopause: It is a term preferably not to be used, since definition of menopause is complete cessation of menstruation. Rarely, ovarian function is interrupted for a period of time and later resumes.[4]
13. Early menopause: It is the time span between the spontaneous or iatrogenic menopause occurring between the age of 40 years and the accepted typical age of menopause for a given population.
14. Delayed menopause: It is not defined but may be important in terms of the increased problems associated with the hyperestrogenism and is used in this guideline. It is two SDs above from the natural average age of menopause in a given population. We may consider it to be beyond 54 years*.
*We need population-based studies to derive at the cut off values.
15. Post-menopausal bleeding (PMB): It is the occurrence of vaginal bleeding following a woman’s final menstrual cycle and not on cyclical hormone therapy. However, vaginal bleeding that occurs 6 months after amenorrhea should be considered suspicious and warrants investigation.
16. Staging system: The staging system of a physiological event is to improve comparability of strategies and facilitate clinical decision making. In 1997, Behram Ankelesaria in India, published a simple method of staging of menopause to understand and deal with the problems of the transition phase and beyond.[6,7] STRAW (2001) aimed to classify the woman’s life in three phases: Reproductive, menopause transition, and post-menopause based on the menstrual cycle, endocrine parameters, and ovarian reserve markers. This was applicable only to healthy women.[5] 2012 STRAW + 10- provides a greater clarity for menstrual pattern and is applicable to most women, except for those with premature ovarian failure (POF).[8]
17. The life expectancy in India has taken a quantum jump from 30 years in 1940s to 61 years in 1990s. According to the world health organization’s (WHO’s) health statistics 2011, in India an average female life expectancy in 2011 is 68 years and is projected an increase to 73 years by 2021.
18. The estimated mean age of menopause is 46 years in India, and is lower than that of the Caucasians.[9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] From the available Indian data, it is hypothesized that an early age of menopause predisposes a woman to chronic health disorders a decade earlier than a Caucasian woman. It is reported that osteoporotic fractures occur 10-20 years earlier in Indians compared to Caucasians.[27,28] The first myocardial infarction attack occurs in 4.4% of Asian women at a younger age than in European women.[29] In India Type 2 Diabetes Mellitus (DM) occurs a decade earlier than the Caucasians.[30] Breast cancer incidence peaks before the age of 50 years.[31] Cervical cancer is leading cause of mortality due to cancers in women. The highest age specific incidence rate of 98.2/100,000 for cancer cervix was seen in the 60-64 year age group.[32]
19. The burden of cardiovascular disease (CVD) in India is projected to increase by 115% from 1990 to 2020,[33] and cerebrovascular incidence by 104%.[34] The migrant population from the Indian subcontinent in the UK is known to be at a significantly higher risk of developing diabetes and CVD.[35] The mean bone mineral density (BMD) in India is about two SDs lower than in women in the western population.[36,37,38,39,40,41,42,43,44,45] The prevalence of low bone mass is to the extent of 40% from the age of 40 years and increases to more than 62% by age 60 years and 80% by the age of 65 years.[46,46,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] The above facts indicate the need to have well planned cost effective systems in place to promote a healthy and an active ageing population.
INDIVIDUALIZED PLAN FOR MENOPAUSE
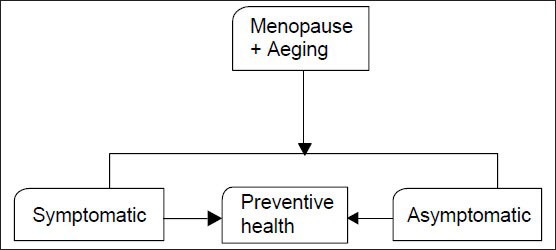
20. Each woman needs an individualized health plan management. It is most important to distinguish between a symptomatic and an asymptomatic menopausal woman. Women may present at the menopausal clinic with menstrual problems, menopausal symptoms or request for a general health check-up, or as an opportunistic contact to be picked up by the health professional [Flowchart 1 and 2].[67,68,69,70,71]
Flowchart 1.
The Physician’s role and approach
Flowchart 2.
Issues in symptomatic women
SECTION II
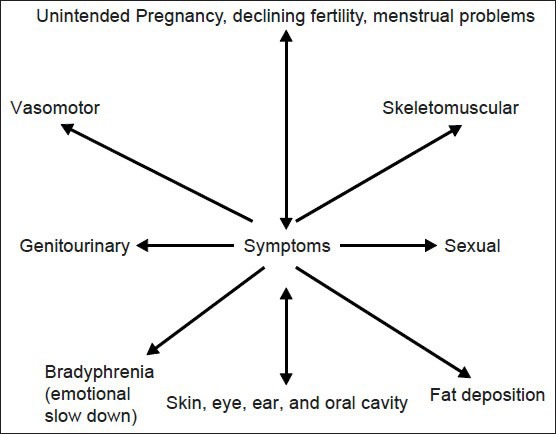
SYMPTOMS OF MENOPAUSE, ISSUES RELATED TO MENOPAUSE TRANSITION, AND AGEING
Fertility
21. After the age of 30 years, if a woman does not conceive naturally within 6 months, the couple should have an infertility work-up (Grade B).
22. In women with a single ovary, previous ovarian surgery, poor response to Gonadotropins, previous exposure to chemotherapy or radiation, or unexplained infertility should undergo ovarian reserve testing even before the age 30 years and in all women it is done beyond ≥ 30 years (R: Grade B).
23. In women > 40 years who do not conceive within 1 to 2 cycles of controlled ovarian hyperstimulation, (IVF) In vitro Fertilization should be considered (Grade B).
24. The only effective treatment for ovarian ageing is oocyte donation. A woman with decreased ovarian reserve should be offered oocyte donation as an option as pregnancy rates associated with this treatment are significantly higher than those associated with controlled ovarian hyperstimulation or In vitro fertilization with a woman’s own eggs (Grade B).
25. The risk of spontaneous pregnancy loss and chromosomal abnormalities increases with age, and the couple need to be counseled on this aspect (Grade B).
26. Preconception counseling with an emphasis on optimal general health, screening for medical conditions such as hypertension, diabetes, and pregnancy-related risks should be addressed for women of more than 40 years (Grade B).
Contraception
27. Pregnancies in elderly women are associated with higher maternal and perinatal morbidity and mortality. There is an increased risk of fetal malformations. This can also lead to psychological and potential domestic and social consequences.
28. Pattern of contraception use in the age group of 35-49 years in different countries Table 1.[72,73,74,75]
Table 1.
Percent pattern of contraception use in the age group of 35-49 years in different countries

29. The annual risk of deaths associated with using no method of contraception far exceeds that for use of any method among all age groups Table 2.[73]
Table 2.
Risk of deaths with contraception compared to no contraceptive method

30. Sterilization: It is highly effective, safe and a single act, case fatality rate with tubectomy is 1-2/100,000 procedures. However, it is a permanent method. Vasectomy is even safer except for minor complications (Grade A).
31. Oral contraceptives pill (OCPs): These are effective, easy to use, and reversible. Low-dose OCPs have non-contraceptives health benefits with an increased safety profile (Grade A).
32. For women, above the age of 35, careful personal and family history, and accurate measurement of blood pressure (BP), breast examination, screening for diabetes, and lipid profile should be performed (Grade A).
33. Healthy women of normal weight, non-users of tobacco, doing well on a combination contraceptive pill can continue this method until the age of menopause and up to a year or two later, after analyzing the risks and benefits (Grade B).
34. If oral contraceptives are continued before major surgery, heparin prophylaxis should be considered (Grade B).
35. Administration of OCPs in normal eumenorrheic women has no effect on BMD and bone metabolism. Conversely, depot medroxyprogesterone acetate (DMPA), is associated with bone loss, which returns to normal, after stopping DMPA. Yet, caution needs to be exercised in women at a high-risk of osteoporosis. Short- or long-term use of DMPA in healthy women should not be considered as an indication for dual X-ray energy absorptiometry (DXA) or other tests that assess BMD (Grade C).
36. Change over from oral contraceptive to Hormone Therapy (HT) is carried out at an arbitrary, age of 45-50 years or if serum FSH: (LH) Luetinising Hormone ratio of > 1, FSH > 30 IU/L (Grade B).
37. Progesterone only contraceptive is an ideal method in women with a past history of venous thromboembolism (VTE) and gallstones. Limitations are erratic and scanty periods. The levonorgesterol -Intra Uterine System (LNG-IUS) - this is correct apart from being used as a hormonal contraception is most effective hormonal therapy for heavy menstrual bleeding and for treating bleeding disturbances associated with endometrial hyperplasia (Grade B).
38. Intra-uterine contraceptive devices (IUCDs) are effective, but sometimes can cause menorrhagia and dysmenorrhea (Grade B).
39. Emergency contraception is an effective emergency method, but it is not as effective and consistent as the use of other contraceptive (Grade C).
Perimenopausal bleeding
40. It is suggested to incorporate the use of PALM-COEIN (polyp, adenomyosis, leiomyoma, malignancy and hyperplasia, coagulopathy, ovulatory dysfunction, endometrial, iatrogenic, and not yet classified) classification for abnormal uterine bleeding (Grade C).
41. Common cause are anovulatory bleeding, leiomyoma, endometrial, polyp, endometrial hyperplasia, and endometrial cancer (EC).[76]
42. Substantial evidence exists to indicate that sonohysterography is superior to transvaginal ultrasonography (TVS) in the detection of intra-cavitary lesions, such as polyps and submucosal leiomyomas (Grade A).
43. Endometrial tissue sampling should be performed in patients with (AUB) Abnormal Uterine Bleeding who are older than 40 years (Grade C).
44. TVS is the primary screening test for AUB, and Magnetic resonance imaging (MRI) should be considered when the diagnosis is inconclusive (Grade C).
45. Persistent bleeding with a previous benign pathology, such as proliferative endometrium, requires further testing to rule out focal endometrial pathology or a structural pathology, such as a polyp or leiomyoma (R: Grade B).
46. Management depends on the cause, cost benefit analysis of therapy and the patient’s choice (R: Grade C).
PMB
47. PMB is defined as uterine bleeding occurring after at least 1 year of amenorrhea. Its incidence is about 10-15%.
48. Women with PMB have a 10-15% chance of having EC. Conversely, 90% of the EC in the post-menopausal period present with PMB. Hence, immediate evaluation is required.
49. Common cause of PMB is due to atrophic changes in the vagina and the endometrium.
50. A detailed clinical and drug history is important as some over the counter drugs like “Ginseng” can cause PMB.
51. A through clinical examination is carried out to rule out cervical, vulval and vaginal cancer, atrophic vaginitis, urinary, and anal causes for bleeding.
52. Women with PMB may be assessed initially with TVS, an endometrial biopsy (Grade A).
53. Endometrial thickness is measured as the maximum anterior – posterior thickness of the endometrial echo on a long-axis transvaginal view of the uterus.
54. Women with PMB with an endometrial thickness of ≤ 4 mm in transvaginal scan do not require endometrial sampling unless they are at a high-risk for endometrial carcinoma or bleeding is episodic.
55. If endometrial thickness is > 4 mm in TVS, it is important to consider endometrial sampling. In women with homogeneous and normal morphology, women on HT and hypertensive medication, the acceptable combined thickness is 6 mm.
56. A focal increased echogenicity or a diffuse heterogenecity in the endometrium even in a thin endometrium warrants further investigations.
57. Out-patient endometrial sampling devices such as Pipelle and out-patient hysteroscopy can be carried out wherever possible.
58. If the endometrial biopsy tissue is reported as insufficient for diagnosis, and endometrial thickness on transvaginal ultrasonography is less than 4 mm, follow-up is sufficient. Recurrent episodes warrant’s further investigations.
59. Dilatation and curettage and fractional curettage are useful in low resource settings. Saline infusion sonography and 3D (USG) Ultrasonography play a limited role in PMB evaluation.
Quality of life (QOL)
60. The WHO defines QOL as an individual’s perception of their position in life in the context of the culture and value system in which they live and in relation to their goals, expectations, standards, and concerns. The two terms in common usage are global QOL and health-related Quality of life (HRQOL). WHO- Several questionnaires are used to assess HRQOL.
61. QOL as it relates to menopausal women is usually referring to health-related QOL, taking into account a woman’s symptoms.[77,78] Commonly used are Greene Climacteric Scale, Women’s Health Questionnaire, Menopause Rating Scale and Utian Quality of Life Scale.
62. When evaluating drug therapies, besides safety, and efficacy, it is important to know the effect of the drug on QOL.[79]
63. Some studies show that low dose horm replacement therapy (HRT) significantly improves overall measures of QOL in early menopause.
64. Some studies show that low dose HT significantly improves overall measures of QOL. HT had mixed effects on QOL among older women from the (HERS) Heart and Estrogen/Progesterone Replacement Study trial, whereas the Women’s Health Initiative (WHI) trial investigators found that estrogen plus progestin did not have a clinically meaningful effect on HRQOL.
65. An Indian study has shown an improvement in QOL in women receiving tibolone.[80]
VMS
66. In a multi-centric hospital, urban-based study conducted by the Indian Menopause Society (IMS), the incidence of VMS was found to be 75%.[50] There is a wide variation in prevalence of symptom reporting, ranging from 19% to 75% from various studies conducted in India.[81,82,83,84,85,86] The prevalence in UK Asians was reported as 71%,[87] and in Australian Indians as 33%.[26]
67. VMS present as hot flushes, cold sweats, and night sweats. VMS may be reported in the menopause transition, reach the maximum intensity during the first 2 years post-menopause and then declines over time. VMS generally last for 6 months to 2 years, although some may experience for 10 years or longer. We need to exclude other causes of flushing before planning treatment.
68. Grading of VMS is important to plan management, follow-up and for research. Grades of hot flashes are classified as: Mild – feeling of heat without sweating; moderate – feeling of heat with sweating; and severe – feeling of heat with sweating and palpitation that disrupts usual activity.
69. Life-style modifications may be recommended to reduce mild VMS (Grade A).
70. The most effective treatment for VMS is HT (Grade A) Ref Section V.
71. Low dose oral contraceptive pills can be used in the menopause transition phase for relief of symptoms (Grade A).
72. Non-hormonal prescription agents may relieve VMS, but have their own side-effects. These can be considered when HT is contraindicated or not desired (Level 1B).
73. Complementary and alternative treatments should be advised with caution as the data are still insufficient especially in moderate to severe VMS (Level 1B).
Urogenital symptoms
74. The prevalence of urogenital symptoms in the post-menopause in the IMS study was 15%.[50] It presents as vaginal dryness in 32%, pruritus vulvae 10-17%, dyspareunia, and urinary urgency 10%.[88,89] It is due to urogenital atrophy as a result of declining estrogen levels and may also present as recurrent urinary tract infections.[90] Though it effects the QOL, women in general do not complain about it; hence, suggestive questions need to be posed during history taking.
75. Physical signs of vulvovaginal atrophy are variable and include reduced vulval fat, reduced vaginal rugae, and blood flow leading to a pale appearance; a change from moderately acidic range (pH 3.5-5.0) to a neutral range (pH 6.0-8.0) in vaginal pH, there is a shift in the vaginal maturation index.
76. Vaginal lubricants can be recommended for subjective symptom improvement of dyspareunia (Grade C).
77. Vaginal moisturizers can be offered for vaginal dryness and dyspareunia (Grade A).
78. Estrogen therapy (ET) – Ref Section V 241-245.
79. Lifestyle modification, bladder drill, and pelvic floor exercises are recommended for urinary incontinence (Grade B).
Sexual problems
80. A woman’s sexual response to her partner is significantly related to her baseline feelings for the partner, their relationship qualities, and partner’s age and health.
81. Sexual dysfunction is multifactorial and needs to be addressed accordingly.
82. Vaginal atrophy with ageing leads to dyspareunia.[88] Dyspareunia leading to sexual dysfunction is corrected by local ET.
83. Acquired sexual desire disorder in some women responds to testosterone therapy. Formulations of testosterone for use in women are not available in India. Testosterone preparations meant for males should not be prescribed for women. Tibolone is a good option; since, it contains androgenic activity and can be used to treat libido problems.
Non-communicable diseases
CVD
84. The incidence of CVD in Indian women has been noted to have significantly risen. The projected death’s from CVDs by 2020 is estimated to be 42% of the total deaths. The prevalence rate of stroke is 545.1/100,000 persons. The case fatality rate is 41% in 30 days.[91,92] The prevalence of hypertension is 20.4-22% in the urban area and 12-17% in rural area.[93] From the Indian Million Death Study 2009, CVD emerges as the major cause of mortality, 16.8% in the rural and 28.6% in the Urban area. 79% of sudden cardiac deaths in rural South India occurred at home.[94]
85. Risk factors – Ref Section IV Clinical Evaluation - 212, 215.
86. Prevention and management
Life-style interventions (Grade A).
Encourage optimal BP < 120/80 through life-style approaches (Grade A).
Pharmacotherapy if BP ≥ 140/90 to avoid end-organ damage, more so in diabetes (Grade A).
Use thiazide diuretics unless there is an absolute contraindication. Optimal lipid targets (Grade A).
Low density lipoprotein (LDL) < 100 mg/dL, high density lipoprotein (HDL) > 50 mg/dL, triglycerides < l50 mg/dL, non-HDL cholesterol < 100 mg/dL (Grade A).
High-risk: Initiate statin if LDL > 100 mg/dL (Grade A).
Inter-mediate risk: Initiate statin if LDL > 130 mg/dL (Grade A).
Life-style approaches and pharmacotherapy to achieve near-normal HbAlc Glycosylated Haemoglobin (<7%) in women with diabetes (Grade A).
Aspirin in high-risk women (75-162 mg/day) (Grade A).
Routine use of aspirin in women < 65 years of age is not recommended for Ml prevention (Grade C).
HT is not indicated solely for primary or secondary cardio protection (Grade B).
Do not use antioxidant supplements for CVD prevention (Grade C).
Do not use folic acid, with or without B6 or B12 supplements for CVD prevention (Grade C).
The metabolic syndrome: Insulin resistance (IR)
87. The prevalence reported in the peri-menopause in India is 22.2% rising to 32.2% to 48% in the post-menopuse.[95,96] It is 1.5-2 times more common in women than in men.
88. The metabolic syndrome is also known as IR syndrome and syndrome X and an average of 40% of the Indian women are affected.
89. Clinical conditions associated with IR include type 2 diabetes, CVD, polycystic ovary syndrome (PCOS), non-alcoholic fatty liver, obstructive sleep apnoea, and certain cancers. It is also a prominent feature of the metabolic syndrome.
90. Diagnosis of metabolic syndrome: Abdominal obesity defined as > 35 inches in females; serum triglycerides > 150 mg/dL; BP > 130/85 mmHg; and fasting plasma glucose > 110 mg/dL.
91. Effect of HT: A meta-analysis of pooled data from 107 trials concluded that HT reduced IR, abdominal obesity, new-onset diabetes, lipids, BP, adhesion molecules, and procoagulant factors in women without diabetes and reduced fasting glucose and IR in women with diabetes. The effects were diminished by the addition of progestin (Grade A).
92. The basis of dietary recommendations is to reduce exposure to insulin both as a result of dietary stimulus and through decreased IR (Grade B).
93. We should advocate exercise as it improves insulin sensitivity, aiming for a minimum of 30 min of moderate physical activity/exercise per day.
94. Indications for intervention by Body mass index (BMI) category [Table 3].
Table 3.
BMI category

DM
95. India has 63 million people with diabetes and is second largest in numbers, the first being China. The prevalence rates of diabetes in the last 30 years has increased from 2.3% in urban and 1.2% in rural areas (1971) to 15-20% in urban and 10% in rural areas (2012).The prevalence in hospital based multi-centric study by the IMS in post-menopausal woman was 12%. In India, Type 2 DM occurs a decade earlier than the Caucasians. More than 50% of the subjects are undiagnosed.[97]
96. Risk factors: Ref Section IV - 213.
97. Screening: Opportunistic screening for all women above the age of 30 years, every 3 years for younger women with risk factors (Grade C). Diabetic women should be screened for hypertension, dyslipidemia, micro-albuminuria, and undergo yearly eye check.
98. The goal in management is to maintain the HbA1c around < 7% and control risk factors for CVD.
99. It may be indicated to evaluate the endometrium by transvaginal scan before starting HT.
Thyroid disease
100. The prevalence from hospital-based data in post-menopausal women for hypothyroid in India is 3-7%.[50,98]
101. Hypothyroidism is much more common in older than younger individuals. Symptoms and signs include lethargy, constipation, dry skin, alopecia, memory impairment, and depression. The individual is often obese and may have elevated cholesterol.
102. The prevalence of hypothyroidism is approximately 5% in otherwise healthy individuals. Thyroid-stimulating hormone (TSH) is a good screening test.
Anemia
103. Anemia is common in the elderly people in India. Prevalence of iron-deficiency anemia, vitamin B12 deficiency, and folate deficiency is common, and should be an integral part of management of menopause.
CENTRAL NERVOUS SYSTEM
Dementia
104. In 2010, there are 3.7 million Indians with dementia, 2.1 million women and 1.5 million men and the total societal costs is about 14,700 crore. While the numbers are expected to double by 2030, costs would increase 3 times. Prevalence of dementia is 0.6-3.5% in rural India and 0.9-4.8% in Urban India.[99]
105. The core mental functions are memory, communication and language, ability to focus and pay attention, reasoning and judgment, activities of daily living, and visual perception. Impairment of any two functions is suggestive of dementia (B).
106. Many dementias are progressive, early diagnosis allows a person to get the maximum benefit from available treatments and provides an opportunity to plan for the future (B).
107. Factors that increase the risk of dementia are family history, genetic factor apolipoprotein E (APOE), minimal cognitive impairment (MCI), CVD risk factors, physical inactivity, diabetes, hypertension, dyslipidemia, smoking, obesity, autoimmune diseases, depression and stress, social engagement and diet, head trauma and traumatic brain injury, and age (Grade B).
108. An objective marker is examination of (CSF) cerebrospinal fluid for amyloid beta or tau protein and phosphorylated tau protein concentration. They have the sensitivity of between 94% and 100% (A).
109. ET is not currently recommended for reducing risk of dementia developing in post-menopausal women or retarding the progress of diagnosed AD (A).
110. For best preservation of memory and cognition, women should be advised about the importance of good overall health, good cardiac and vascular health, exercise, maintenance of active mind, avoidance of excessive alcohol consumption, and measures to reduce risk of diabetes and hypertension. HT is not indicated for neuroprotection (A).
111. Introduction of accessible diagnostic and early stage dementia care services such as memory clinics is recommended (Grade C).
Sleep
In a study conducted in UK Asians, sleep problems were noted in 32%. A large study of over 9,000 older adults age of > 65 year found that 42% of participants reported difficulty initiating and maintaining sleep.[100] The estimate of prevalence of sleep disorders in India, by WHO extrapolated from US data is 156,628,027 in 1,065,070,607 population.
112. A detailed assessment of menopausal symptoms should always include questions about sleep pattern. Sleep questionnaires or sleep diaries can be useful to assess sleep in detail (Grade C).
113. Adverse life-style factors, social factors, and risk factors should be considered and treated accordingly (Grade C).
114. If insomnia is identified, medical or psychiatric causes of insomnia should be ruled out and if present, treated accordingly. If specific neurological or breathing disorders are suspected, further investigations and referrals to specialists should be initiated (Grade B).
115. Sleep hygiene measures and life-style modifications should be recommended as first line of treatment. Psychological treatments such as (CBT) Cognitive Behavioral Therapy should also be considered (Grade C).
116. If insomnia is resistant to life-style modifications, then hypnotics, benzodiazepines or melatonin agonists can be used in the short-term, but there is no definite or convincing evidence to suggest its efficacy. These should only be prescribed by supervision or after liaison with psychiatrists or sleep experts (Grade C).
117. No recommendations can be made about use of herbal remedies for insomnia as there is insufficient evidence. Mind body therapies such as yoga and tai chi have some evidence, but need further rigorous studies to prove its effectiveness (Grade D).
SKELETOMUSCULAR SYSTEM
Osteoporosis
Basic concepts
118. WHO defines osteoporosis as “a systemic skeletal disease characterized by low bone mass (measured as BMD) and microarchitectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture and involves the wrist, spine, hip, pelvis, ribs or humerus.”[101] The National Institute of Health definition is “a disease characterized by decreased bone strength and propensity to fall.”[102]
119. The diagnosis of an osteoporotic fracture, the clinical end-point of osteoporosis is by the presence of fragility fracture (clinical or by investigation) and or by BMD [Table 4].
Table 4.
WHO BMD (T-score) based diagnosis of osteoporosis for postmenopausal women
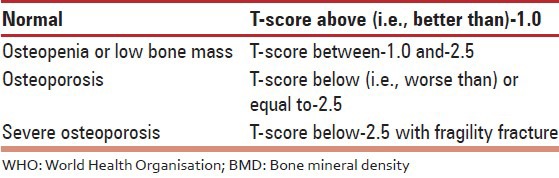
120. The “gold standard” method of BMD testing is by DXA. Its value is expressed in SD units from the population mean in young adults (T-score) or from the mean in an age-matched population (Z-score). The reference range recommended by the International Osteoporosis Foundation, International Society of Clinical Densitometry, WHO and National Osteoporosis Foundation for calculating the T-score in post-menopausal women is the National Health and Nutrition Examination Survey III reference database in Caucasian women aged 20-29 years for BMD (T-score) based diagnosis of osteoporosis for post-menopausal women WHO.[103,104]
121. The Z-score describes the number of SDs by which the BMD in an individual differs from the mean value expected for age and sex. It is mostly used in children adolescents and pre-menopausal women. A Z-score below - 2 is regarded as abnormal and should be referred to as “low for age.” A low Z-score in a post-menopausal woman indicates the need to evaluate for secondary osteoporosis.
122. Osteoporosis is classified as primary and secondary
Primary osteoporosis is seen in post-menopausal women in whom there is no specific pathogenetic mechanism other than age. There is an accelerated bone loss at the rate of 2-5% per year due to declining estrogens levels and is seen in the first 5-7 years after menopause. Later age-related bone loss occurs at a rate of 1% per year in both sexes and affects the cortical and trabecular bone.
Secondary osteoporosis is due to specific causes.
123. Bone is a dynamic tissue with a continuous remodeling leading to formation of new bone and absorption of old bone. A mismatch of this process forms the basis for osteoporosis while defective mineralization of the newly formed osteoid is called osteomalacia.
124. A fragility fracture has been defined by the WHO as “a fracture caused by injury that would be insufficient to fracture normal bone: The result of reduced compressive and/or torsional strength of bone.”
125. Clinically, a fragility fracture can be defined as one that occurs as a result of minimal trauma, such as a fall from a standing height or less or no identifiable trauma.
Screening and diagnosis
126. Osteoporosis is asymptomatic unless a fracture occurs. Early diagnosis in the asymptomatic period is and timely management of osteoporosis will prevent the associated morbidity and mortality. In the absence of a validated population screening tool for post-menopausal osteoporosis in India, a case finding strategy utilizing clinical risk factors with the addition of DXA as needed is suggested (Grade C).
127. Opportunistic screening for women above 40 years is suggested. Risk assessment factors for fractures are derived by history and clinical examination.
128. It is important to distinguish between those risk factors, which lead to reduced bone mass from those which predispose to osteoporotic fractures with a BMD not in the osteoporotic range.
129. Major risk factors defined by WHO are advancing age, prior fragility fracture, low BMI, family history of fracture, smoking, and more than three drinks of alcohol per day (Grade A).
130. Environmental factors include nutrition (calcium intake using the quick dietary calculator, protein) physical activity and sunlight exposure, which are important modifiable risk factors in India. Relevance of risk of falling increases with ageing (Grade B).
131. Case finding for secondary osteoporosis is practiced in high-risk disease subgroups, such as chronic glucocorticoid users and patients with rheumatoid arthritis, collagen vascular disease, or inflammatory bowel disease, hypogonadism, thyroid dysfunction, type 2 diabetes (Grade A).
132. Women presenting with fracture complain of severe pain, which is sudden in onset with minimal trauma, or chronic pain localized to the mid back, may radiate to the abdomen. Generalized bone pain indicates osteomalacia or metastasis.
133. Physical examination should include the height and weight annually, check for balance and gait, get up, and go test by asking the women to get up from the chair without using their arms. Kyphosis and dowgers hump is seen in the late stage of osteoporosis (Grade A).
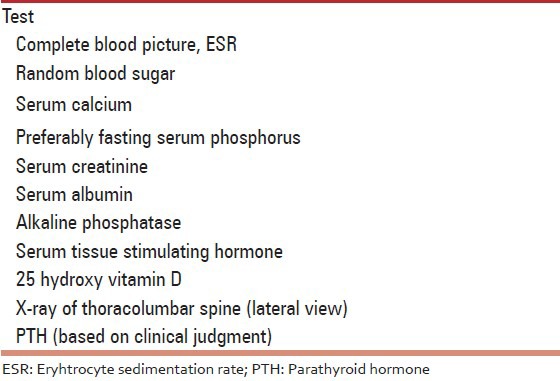
134. Laboratory studies [Table 5]
Table 5.
Essential R (Grade A)
135. The fracture risk assessment tool (WHO FRAX)
For online use is available for India (http: www.shef.ac.uk/FRAX). FRAX is a validated and widely accepted tool used world-wide to identify patients in the osteopenia group most likely to benefit from treatment. It predicts the 10 year absolute risk for a fracture in an individual and the cost-effective analysis determines the interventional threshold above which treatment is cost effective. All this is possible and valid when adequate data on the prevalence of osteoporotic fractures, mortality rates, and health economics data are available for the country. FRAX is country specific, and until more Indian data is available on the prevalence of osteoporotic fractures and mortality rates, the usage of FRAX in the Indian context for uniform guidance on intervention threshold is to be applied cautiously. Having said that, an enormous advantage of FRAX is that it can be used without BMD also to identify cases at risk for fractures. In view of the limited availability of (DXA) Dual Energy Xray Absorptiometry machines in India, it will be helpful to use FRAX without BMD in Indian context. Given the heterogenecity of Indian scenario, intervention thresholds and management may need to be individualized (Grade C).
136. Heterogeneity in different regions of the country and the prevalence of nutritional and other risk factors unique to the Indian population have not been considered in the calculation of FRAX (R: Grade B).
137. It is suggested to conduct central DXA of spine and hip in all women 5 years beyond the natural age of menopause and in women than 5 years since menopause with 1 high clinical risk or more than 2 clinical risk factors. This suggestion is based on the following. Early age of natural menopause that is 46.7 years in Indian women,[10] life expectancy of a woman is 68 years (WHO statistics 2011), accrual of low peak bone mass,[38] early age of presentation of fracture,[27,39] accelerated bone loss in the immediate 5 years of menopause and the trabecular bone is affected more.[43] Stratification by age shows that the prevalence of low bone mass is to the extent of 40% from the age of 40 years and increases to more than 80% by the age of 65 years (Grade C).
138. Indications for DXA (Grade B):
All post-menopausal women more than 5 years of menopause.
Women with fragility fractures.
Post-menopausal women less than 5 years of menopause with risk factors.
Women in menopause transition with secondary causes.
Radiological evidence of osteopenia and presence of vertebral compression fracture.
Before initiating pharmacotherapy for osteoporosis.
To monitor therapy - the interval to the next test should depend on the calculated individual risk and would mostly be scheduled between 1 years and 5 years later.
Emerging indications are to measure total body fat and lean tissue mass.
139. The diagnosis is based on central DXA of the spine, total hip, and neck of femur. If this is not feasible, lower one-third of the radius (33%) is measured. The Caucasian female normative database is used as a reference for T-scores (R: Grade A).
140. The lowest BMD score obtained from all sites is used for diagnosis (R: Grade A).
141. Screen post-menopausal women for secondary osteoporosis if history or examination show systemic disease or low Z-scores on DEXA (R: Grade A).
142. R peripheral DEXA (X-ray based) may be used as a mass screening tool because of its high negative predictive value (R: Grade C).
Management
143. Involves a population and a personalized-based approach. The target is primary prevention (population-based), intervention, and rehabilitation (individualized).
144. Fracture risk is obtained by BMD (both primary and secondary causes) and the presence of clinical risk factors for osteoporotic fracture. For treatment purpose, combining BMD with clinical risk factors provides a better estimate of fracture risk. We simply should not treat T-scores, but must take a patient’s full clinical status into account to make therapeutic decisions.
145. The term prevention and treatment in the context of osteoporosis has to be understood. The term prevention is used to denote the prevention of bone loss in post-menopausal women with osteopenia (T-score between 1 and 2.5) and increased fracture risk. Treatment is defined as a reduction in fracture risk in post-menopausal women with osteoporosis.
Universal recommendations
146. Life-style management: Balanced diet, adequate physical activity, exposure to sunlight, avoidance of bone depleting agents such as tobacco, alcohol, etc.
Nutrition
The recommended dietary allowance (RDA) of calcium intake for Indian population [Table 6].[105]
Assess the total calcium intake from dietary sources and if needed, supplements are used to correct the deficient balance. The intake should exceed > 800 mg/day (Grade B). The risk of cardiovascular events, calculi are not observed with the recommended doses of calcium.
The following tool depicted in Table 7 can be used for a quick calculation of daily calcium intake.
Calcium content of Indian foods [Table 8].
Low sodium intake: Daily salt intake should not exceed 5 g (1 tsp). Protein should be 1 g/kg body weight.[105]
Decrease caffeine intake (<3 cups/day), limit alcohol and avoid use of tobacco (Grade B). A cup (150 mL) of brewed coffee contains 80-120 mg of caffeine and instant coffee 50-65 mg while tea contains 30-65 mg of caffeine. Caffeine stimulates the central nervous system and induces physiological dependency. In general, low doses (20-200 mg) of caffeine produce mild positive effects such as a feeling of wellbeing, alertness, and energy. Higher doses (>200 mg) can produce negative effects such as nervousness and anxiety, especially in people who do not usually consume caffeine-containing beverages.[105]
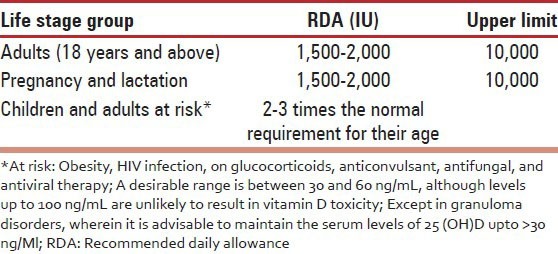
In the background of widespread vitamin D deficiency in all age groups, it is prudent to adopt the US Endocrine Society 2011 [Table 9] RDA.[106] There is an urgent need for an Indian update on RDA for different age groups.
Vitamin D: Dietary sources are limited, adequate sunlight exposure has limitations and presently, food fortified with adequate vitamin D is unavailable in India. Urgent and cost-effective measures need to be implemented. Hence, it is recommended to use vitamin D as supplements (Grade A).
-
Recommendations for management of vitamin D deficiency and maintenance are: (Grade B).
- Cholecalciferol (vitamin D3) is available in the form of oral tablets and oral spray of 1000 IU and 2,000 IU.
- It is also available in the form of granules and tablet of 60,000 IU.
- Intramuscular (IM) injections of vitamin D3 are available in doses of 300,000 IU and 60,000 IU per ampoule. Injections of cholecalciferol are cost-effective may be recommended in cases of malabsorption and to increase compliance. The disadvantage is being an oily injection, it is painful, and since it is administered intramuscularly and can produce an erratic blood levels.
- Cholecalciferol is the preferred therapy for correction of deficiency and maintenance.
Table 6.
Recommended dietary allowance of calcium in women

Table 7.
Quick dietary calcium assessment chart: A tool for a quick assessment of total dietary calcium intake
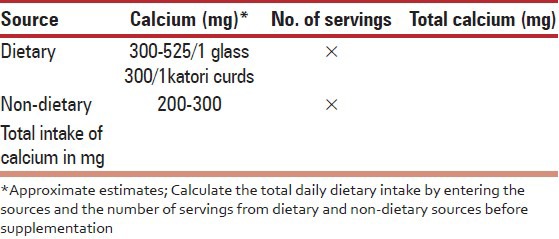
Table 8.
Calcium content of Indian foods
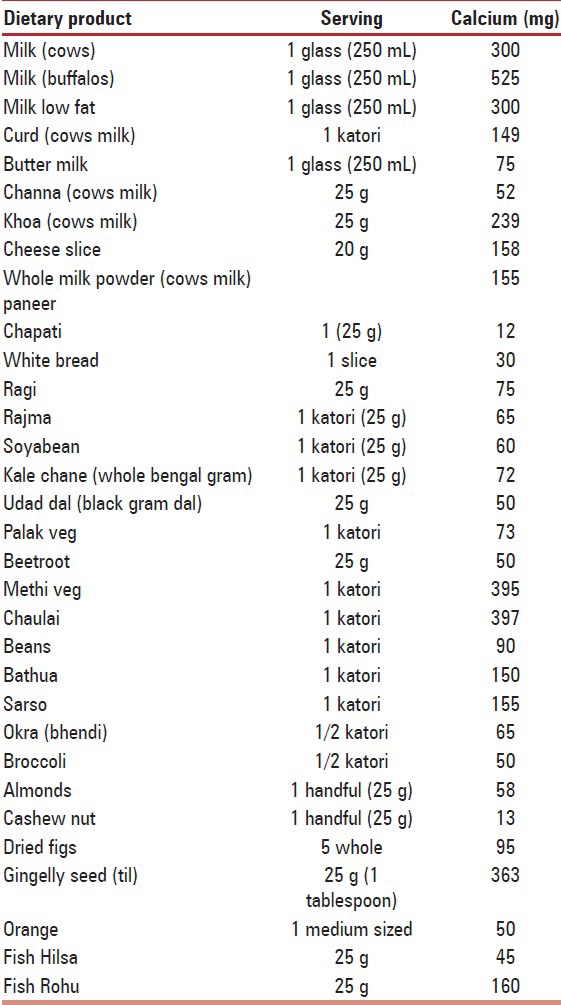
Table 9.
US endocrine society 2011 RDA
Management of deficiency
Cholecalciferol (vitamin D3) tablet or powder 60,000 IU/once a week for 8 weeks preferably with milk or.
One IM injection of 600,000 IU is given to correct the deficiency.(not to be repeated before 6 months and may be given after confirmation of persisting low levels of vitamin D).
Maintenance therapy (from natural sources or supplements) is advised after correction of the deficiency.
Maintenance therapy
Cholecalciferol tablet or powder 60,000 IU once a month in summer or twice a month in winter.
Vitamin D supplements by oral spray or oral tablets of 2,000 IU/day, or.
Injection of Cholecalciferol 300,000 IU IM, twice a year or 600,000 IU IM once a year.
Cholecalciferol, 1,000 IU daily, will raise blood levels, on average, by approximately 10 ng/mL.
Upper acceptable limit
The dose for treatment should not exceed 4000 IU/day and hypocalcaemia has been reported when the dose exceeds 10,000 IU/day.
-
x.
Vitamin D derivatives: Calcitriol, the active form of vitamin D is reserved only for patients with chronic renal and hepatic disease Alfacalcidol is a synthetic analog of the active vitamin D metabolite calcitriol (1,25-dihydroxyvitamin D3), and it is metabolized to calcitriol by its 25-hydroxylation in the liver. It is less potent than calcitriol. The use of vitamin D derivatives necessitates monitoring of serum and possibly urine calcium. There is the risk of hypercalcaemia and hypercalciuria. Adverse effects of prolonged hypercalcemia include impairment of renal function and nephrocalcinosis.
-
xi.
It is preferable to get vitamin D through sunlight by exposing 20% of body surface area (face, neck, and both arms and forearms) without sunscreen for at least 30 min between 10 am and 3 pm, depending on the season, latitude, altitude, pollution, and skin pigmentation. The sunlight between 11 am to 2 pm is preferably the best.
-
xii.
In post-menopausal women, the intake of vitamin D should be in addition to sunlight exposure. Vitamin D supplementation (≥500-2,000 IU/day) was favorable in the reduction of hip fracture and any non-vertebral fracture in persons 65 years of age or older.
-
xiii.
Vitamin K: For women of post-menopausal age, 180-350 μg/day of vitamin K2-7 may need to be supplemented along with the recommended intake of calcium, magnesium, vitamin D, and a balanced diet. The current RDA of vitamin K2-7 WHO/of 65-80 μg/day is too low and needs to be raised up to at least 100 μg/day throughout life, with larger doses when needed.[107] Both bone and cardiovascular health of women with osteoporosis would benefit from vitamin K2-7 intake (Grade C).
-
xiv.
Interestingly, exposure to complex nutrients and food constituents interact to affect bone mass, it is, however, left to individual clinician to decide on supplementing vitamin A, vitamin B12, and phytoestrogens (Grade B).
Prevention of falls
-
xv.
Patients should receive a multifactorial risk assessment and intervention because it is the most consistently effective strategy to prevent falls (Grade A).
16. Home hazard assessment and modification, exercise, and physical therapy are recommended to prevent falls and injuries from falls. Biomechanics of posture and safe movements are a vital component of counseling (Grade A).
Flowcharts 3 and 4 show an approach to management of asymptomatic postmenopausal woman and postmenopausal woman with fragility fracture, respectively.
Flowchart 3.
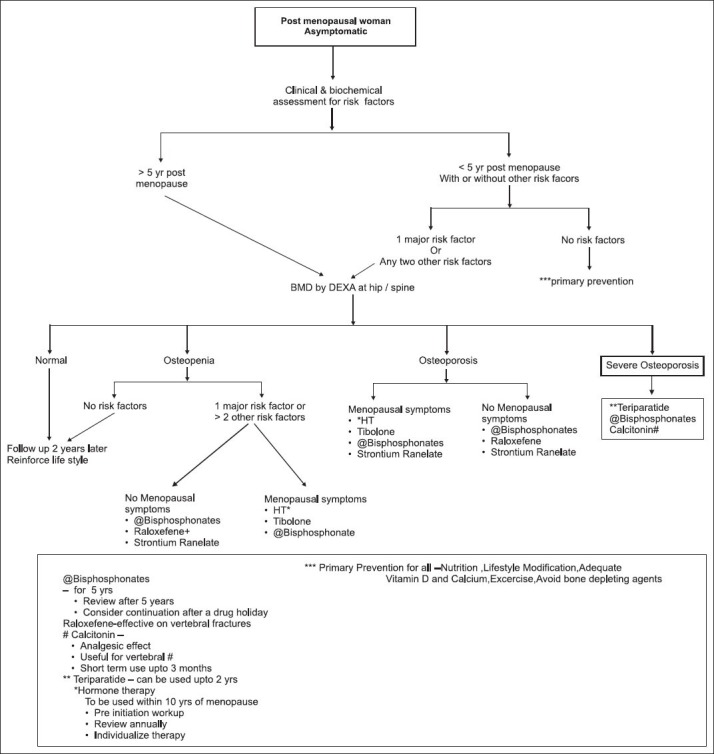
Treatment algorithm for postmenopausal women asymptomatic woman
Flowchart 4.
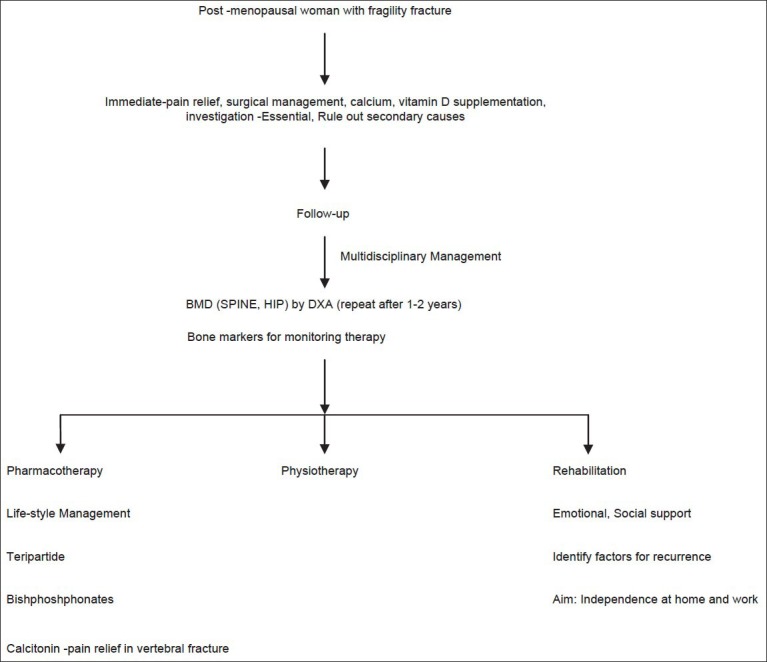
Treatment algorithm for postmenopausal women with fragility fracture
Frailty
147. Frailty: Fried et al. have standardized the definition as three or more of the following five criteria; unintentional weight loss, self-reported exhaustion, weakness (grip strength), slow motor performance (walking speed), and low physical activity.
Frailty-related falls and fractures have been reported with OR of 1.38-2.4 for falls and recurrent falls, 1.40 and 1.7 for hip fracture in old women. Women’s health-care programs targeting post-menopausal women’s comprehensive care can contribute a lot by educating women as to take care of their musculoskeletal health through life-long commitment to proper nutrition, exercise, and understanding about issues related to prevention of falls.
Osteoarthritis
148. The prevalence of osteoarthritis in India as reported from a community dwellers in a small study conducted in Delhi was 47.3% and in others it is reported to be between 22% and 39%.[108,109,110] Age, weight, female sex, quadriceps weakness, and overloading of the knee joint (climbing stairs, squatting posture, etc.,) are the main contributors than menopause per se in the incidence of osteoarthritis. Those contributing factors should be addressed on a priority basis.
149. Epidemiological studies of a potential role for estrogens in osteoarthritis showed two very different findings. First, estrogen deprivation at the menopause seems to be associated with increases in the frequency of knee, hip, and finger osteoarthritis, and in the severity of hip osteoarthritis. Second, HT for the menopause may decrease the incidence and progression of hip and knee osteoarthritis.
150. The identification of the alfa and beta estrogen receptors in normal and osteoarthritic cartilage and the effects of 17 beta estradiol on cartilage in vivo in animals and In vitro confirm that the cartilage responds to estrogens. Finally, this response is dose-dependent: Physiological doses (as with HT) are protective and higher dosages are deleterious.
151. Perimenopausal women can be advised about HT and they should be aware of the fact that only long-standing (>5 years) use of HT can be beneficial.
152. Once osteoarthritis sets in, there is no protection from HT and osteoarthritis takes its own course. In such cases, osteoarthritis should be treated on its own merits.
153. Age, weight, female sex, quadriceps weakness, and overloading of knee joint (climbing stairs, squatting posture, etc.,) are the main contributors than menopause per se in the incidence of osteoarthritis. Those contributing factors should be addressed on priority basis.
154. First two stages of osteoarthritis can be addressed by life-style modification, pharmacotherapy, and physical therapy (Grade A).
155. Third and fourth stages need surgical intervention for which total knee replacement is the gold standard (Grade B).
Eye
156. Blindness was more likely with increasing age and decreasing socio-economic status, and in female subjects and in rural areas. The causes of blindness were easily treatable in 60.3% (cataract, 44%; refractive error, 16.3%).[111] Preventable corneal disease, glaucoma, complications of cataract surgery, and amblyopia caused another 19% of the blindness [Table 10].
Table 10.
The causes of blindness

Glaucoma
157. Glaucoma is the most common cause of irreversible, but preventable blindness world-wide. There is level one evidence to show that prevalence and/or incidence of glaucoma increases with age, women are more pre-disposed to angle closure glaucoma. Established risk factors for glaucoma are age, family history, diabetes, shallow anterior chamber, refractive status, and race (Grade A).
158. Blindness due to primary angle-closure glaucoma is potentially avoidable if this condition is detected early and peripheral iridotomy or iridectomy is performed. This requires detection of occludable angles, which lead to primary angle-closure glaucoma, using slit-lamp examination and gonioscopy. Blindness due to primary open-angle glaucoma is more difficult to prevent and medication in open angle glaucoma could prevent the progression of the disease (Grade A).
Dry eye
159. There is increased risk of dry eye in both genders with age due to decreased tear production. The incidence is more in women than men. Menopause also contributes to the ocular surface impairment due to hormonal imbalance.
160. HRT after menopause, especially unopposed ET has been proven to cause the dry eye (Grade B).
161. Prevention of blindness:
Improvement in the quality of cataract surgery, and increase in the number of surgeries on persons blind in both eyes
Effective screening to detect refractive error blindness and provision of spectacles
Initiation of long-term strategies to prevent corneal and glaucoma blindness
Effective control of diabetes and yearly eye checkup to prevent diabetic retinopathy.
Cancers
162. A population-based study (Million Death Study cancer mortality in India: A nationally representative survey 2012) revealed that 1 in 22 men or women aged 30 years alive today in rural India is likely to die of cancer before 70 years of age based on the rates of actual deaths and in the absence of other disorders. In urban areas, the risks are 1 in 20 for men and 1 in 24 for women.[112]
Breast cancer
163. In India, breast cancer is the second most common cancer with an estimated 115,251 new diagnoses and the second most common cause of cancer-related deaths with 53,592 breast cancer deaths in 2008.[113,114] The age-standardized incidence rate for breast cancer in India is 22.9 per 100,000, one-third that of Western countries, and the mortality rates are disproportionately higher.[115,116]
164. The data from atlas project suggest that breast cancer in urban areas of India is 3 times higher than in rural parts of the country.[117,118,119] Indian women are more likely to develop breast cancer at earlier ages than their Western counterparts.[31]
165. Non-modifiable risk factors for breast cancer are age, family history, benign breast disease, BRCA – Breast Cancer) 1 or 2 carriers, early menarche (<12 years), late age at menopause (after age 55), increased breast density, and a chest irradiation between ages 25 years and 55 years.
166. Modifiable risk factors are age at first child, breast-feeding, parity, obesity, physical activity, and menopausal HT.
Screening in breast cancer
167. The debate about value of screening continues. There is no organized, systematic, government funded screening program for breast cancer in India. The screening in developing countries can be regarded as “opportunistic screening.” There are no evidence-based guidelines for breast cancer screening in India at present.
Methods
Breast cancer screening includes 3 methods of early detection (Grade C).
Breast self-examination (BSE) monthly starting in the 20 s.
Clinical breast exams (CBE) every 3 years starting in the 20 s till 39, and annually thereafter mammographic screening (annually) starting at the age of 40 years.
BSE
BSE is performed by the woman herself and involves examination of the breast, skin, and axillae based on palpations by her hands.
The woman should examine the look and feel of her breasts as well as any signs, symptoms or changes to the breasts.
BSE is recommended so that women understand their breasts for detecting any suspicious changes over time.
Initially, BSE should be performed very frequently and regularly so that a woman understands the physiological changes that occur during the different phases of menstrual cycle and then continue monthly around 7th or 8th day of cycle. They are encouraged to report any recent or persistent changes.
Nodular and lumpy feel of the breasts and increased pain and tenderness, which is a physiological finding prior to menstruation, needs to be explained to the patient.
Women can be taught to examine the breasts in any of the following ways in both supine as well as standing positions.
CBE
CBE and increasing awareness of breast cancer are viable alternative in view of limited health-care resources and advanced stage of disease distribution for Indian women in age group less than 50 years of age. Early results of trial by WHO in India (JNCI 2011) (Journal of the National Cancer Institute) and studies for cost effectiveness of screening in Indian women support that CBE is an effective way and survival can be improved by up to 16% at half the cost by use of CBE (JNCI 2008).
For women between 50 years and 70 years of age, annual CBE and selective use of mammography, once in 3 years, in high-risk groups, determined by the above mentioned criteria has been found to be equally effective (JNCI 2011).
CBE is performed by the clinician or other health professional and involves a systematic examination of the breast skin and tissue.
The health professional is looking for signs and symptoms or if any changes occur, including development of a lump or swelling, skin irritation or dimpling, nipple pain or retraction (turning inward), redness or scaliness of the nipple or breast skin, or a discharge other than breast milk.
CBE should include all the 4 quadrants of the breast and the central nipple areola complex followed by examination of axilla and supraclavicular fossae.
Fibroadenoma, a benign condition feels as a firm and freely mobile swelling, characteristically described as a “mouse in the breast” where as an irregular hard painless lump is characteristic of malignancy. These findings are generalized and all lumps may not classically fit into these descriptions.
Normal breasts may feel lumpy and tender prior to menstruation, especially if felt with the tips of the fingers; hence, use of a flat hand is recommended.
Mammogram
In India, breast cancer incidence peaks before the age of 50 years, and a recent review of the evidence in younger women (aged 39-49 years) based on 8 trials conducted between 2001 and 2008, suggests that mammographic screening is also beneficial in this younger age group.
An approximate 12-15% reduction in breast cancer mortality is associated with mammography screening for women aged 40-69 years.
Limitations of mammography in developing countries are economic constraints and quality assurance. Cost affectivity and false positives are the other limitations in the use of mammography in India.
The decision to perform mammography should be determined with shared decision making about risks and benefits and by individual patient values.
168. MRI Currently MRI screening in combination with mammography is targeted to high-risk patients, which includes:
BRCA 1 or 2 mutation carriers.
Untested women who have a first degree relative with a BRCA 1 or 2 mutation.
Lifetime risk of breast cancer of 20-25% or more.
Received radiation treatment to the chest between ages 10 and 30.
Genetic mutation in the TP53 Tumour Protein 53(Li-Fraumeni syndrome) or PTEN (Phosphatase and Tensin homolog) genes (Cowden syndrome).
169. Role of (PET) Positron Emission Tomography imaging: PET has currently a limited role in breast can cer, due to its low sensitivity and is not recommended in most of the cases, especially in early disease. The most useful application of PET/CT is monitoring the changes in 18F-FDG (Flu Deoxy Glucose) uptake during chemotherapy in order to detect an early response to treatment.
Breast cancer prevention
170. The risk of breast cancer may be lowered to some extent by lifestyle changes, working on modifiable risk factors, and diligent use of HRT.
171. The best way to protect one’s self is through early detection.
Prevention in high-risk population
172. Indications of risk reducing surgery, mastectomy, salpingo oophorectomy, and chemoprevention can be discussed with experts. The decision is individualized.
Cancer cervix
173. Cervical cancer is the leading cause of cancer death in women in both rural and urban areas. The cervical cancer death rate of 16/100,000 reported in the million woman death study 2012 suggests that a 30-year-old Indian woman has about 0.7% risk of dying from cervical cancer before 70 years of age in the absence of other diseases. By contrast, the risk of dying during the pregnancy for Indian women aged 15-49 years is about 0.6%.[112]
174. India contributes to over 25% of the disease burden and more than 26% of the deaths due to cervical cancer world-wide. More than 75% of the cases presenting in the late stage of the disease renders poor prospects for survival and cure. About 1, 34, 420 new case are being diagnosed every year.[120,121]
175. Risk factors: HPV-Human Papiloma Virus, sexual intercourse at an early age, multiple sexual partners, sexual partners who have had multiple partners, HIV positive status, and smoking.
176. In India, currently only 4.9% of urban women aged 18-69 years are screened every 3 years (WHS India -World Health Surveys. Geneva: WHO; 2003) and 2.3% of rural women aged 18-69 years are screened every 3 years (WHS India).
177. Screening tests available
Visual inspection.
Visual inspection with acetic acid (VIA).
Visual inspection with Lugol’s iodine.
PAP Papinacolou smear both conventional and liquid base cytology.
HPV DNA testing.
Cervicography.
Papnet.
Polar probe.
The first three are useful at community and low resource setting whereas, the last three are still in the experimental phase.
Primary care (Rural/Urban)
Cytology-based screening has made little impact in developing countries due to relatively high false negative rate and lack of organized screening program and referral pattern.
Several studies have shown the benefit of a single visit approach in the form of “see and treat,” which involves VIA followed by cryotherapy. This unique approach is based on the principle that the screening test should provide rapid and accurate results and the treatment modality should be appropriate, adequate, and effective. VIA and cryotherapy satisfied these criteria and yielded satisfying results. A randomized trial in South India done by Sankaranarayanan et al., in 2007 has shown 25% reduction in cervical cancer incidence and 35% reduction in mortality compared to control with VIA and cryotherapy.[122] This approach is useful in primary care level to make the screening program more cost-effective. This can be carried out both by physicians and trained nurses and mid wives.[120,121,122,123,124,125,126,127,128,129]
HPV testing also has been tried in a screen and treat approach. A few studies reported screening with HPV DNA testing followed by cryotherapy. However, it has two limitations - time and infrastructure required for current HPV testing and a lack of consensus about appropriate follow-up for test positives and also treatment strategy. Hence, in some other studies, HPV DNA positive women had VIA followed by cryotherapy if VIA was positive.
Some studies suggest that cryotherapy is protective against the future development of cervical disease among women with current HPV infection. Because of this, and due to the low morbidity of cryotherapy, the occasional treatment of screen-positive women without confirmed cervical disease is acceptable.
Secondary and tertiary Level
PAP smear,[130,131] and HPV DNA testing are being used commonly at secondary and tertiary care level.
Applicability of screening techniques at different settings both in rural and urban [Table 11].
HPV co-testing is to be performed only if the woman crosses 30 years of age as most of the HPV infection clears by then with natural immunity. If both PAP and HPV are negative, the screening interval can be increased, which again becomes cost-effective.
Colposcopy: For screen-positive women, post any primary screening method adopted, for diagnostic confirmation with guided biopsies. Because of hormonal changes, many post-menopausal women will have an unsatisfactory colposcopy. Estrogen treatment (estrogen cream application intra-vaginally each evening for 4 weeks and stopped 1 week before repeat cytology) will cause enough ectropion of the endocervical cells to result in a satisfactory examination.
-
Screening recommendations from different organizations [Table 12].
- Women with negative PAP and positive HPV testing can be either rescreened with contesting in 1 year or with a test specific for type of HPV (HPV 16 and 1).
- All these screening methods may be sometimes inconclusive in menopausal women whose transformation zone is inside the cervical canal or due to atrophic changes. Hence, choosing the appropriate test is important.
- High-risk (oncogenic) HPV DNA testing could be adopted for appropriate triage management of post-menopausal women with unequivocal cytology results.
- Post-colposcopy management of women of any age with initial cytologic result of atypical glandular cells or ASC-H -′Atypical squamous cells- cannot exclude-High grade squamous intraepithelial lesion" in initial work-up does not identify a high grade lesion).
- In the event of availability of low-cost and rapid HPV testing as primary screening test every 5 years up to the age of 65 is recommended. With HPV testing as the primary screening method, PAP or VIA testing can be used to triage to evaluate those with HPV-positive test results to plan for appropriate treatment options.
- Above recommendation holds true for women seeking opportunistic services in apex and secondary care levels in public and private sector health facilities where good quality PAP cytology services and molecular testing for HPV DNA are available
Table 11.
Screening at different levels for cancer cervix

Table 12.
Screening recommendations from different organisations
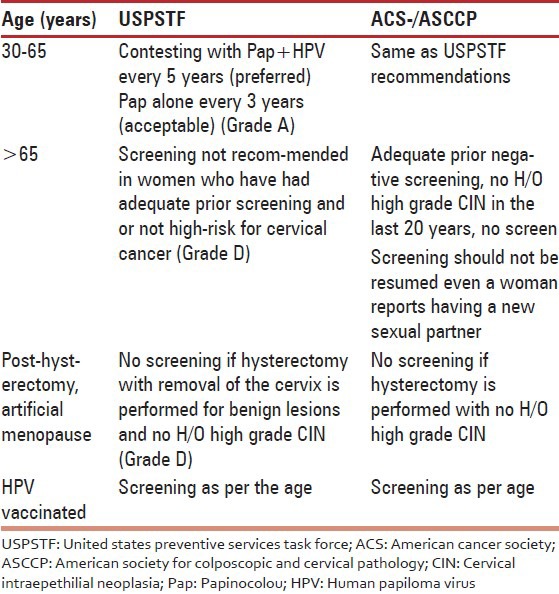
178. In the absence of organized cervix cancer screening for the vast women population in rural and urban areas, once in a life time screening by contesting by combined use of cervical cytology and high-risk HPV DNA testing would be appropriate.
Primary prevention
179. Women should be educated early on to think of cervical cancer as an extension of a sexually transmitted disease.
180. Behavioral changes to reduce the risk of cervical cancer include limiting the number of sexual partners, delaying initial age of sexual intercourse, and avoiding sexually transmitted disease. The association of cigarette smoking with cervical cancer should also be emphasized.
181. An HPV vaccine needs to be promoted especially in the age group of 9 years to the age of first sexual debut. Data from a large placebo-controlled trial showed that the vaccine reduced the incidence of both HPV-16 infection and HPV-16 related (CIN) Cervical intraepithelial neoplasia.
Cancer endometrium
182. Indian incidence of EC: 4.3/100,000 as per Delhi population based cancer registry.
EC is commonly occurs in post-menopausal women
Overall morbidity and mortality of EC is low because most patients present at an early stage because of abnormal bleeding or PMB.
A strong influence of modifiable risk factors such as increasing obesity, life expectancy, and adjuvant tamoxifen use for breast cancer has been attributed.
Adenomatous and atypical hyperplasia are the common precursors of endometrial carcinoma.
Factors that increase the risk of EC are those associated with increase in endogenous estrogens or HT with estrogens.
Unopposed ET in women with an intact uterus increases the risk of EC 2- to 10-fold, and risk increases with duration of use.
Cyclic or continuous progestin given along with estrogens reduces the risk of EC.
Relative risk of EC with obesity is 3.0 in women 21-50 lb overweight and 10 in women more than 50 lb overweight.
Women taking tamoxifen for more than 2 years have a 2.3- fold to 7.5-fold relative risk of EC.
The lifetime risk of EC for women with hereditary non-polyposis colorectal cancer (HNPCC) and for women who are at high-risk for HNPCC is as high as 60%.
There is no evidence that screening by ultrasonography (e.g. endovaginal ultrasound or transvaginal ultrasound) or endometrial biopsy reduces mortality from EC. Most cases of EC (85%) are diagnosed at low stage because of symptoms, and survival rates are high.
There is no indication that screening for EC is warranted for women who have no identified risk factors.
It is recommended that, at the time of menopause, women at average risk should be informed about risks and symptoms of EC, and strongly encouraged to report any unexpected bleeding or spotting.
For those with increased risk and special situations such as on HT, genetic risk, and on tamoxifen therapy should have a complete diagnostic evaluation for abnormal bleeding.
Regular screening for high-risk group for endometrial carcinoma has not been fully evaluated.
Women diagnosed with EC should have the benefit of multidisciplinary team approach.
Cancer ovary
183. The general or lifetime risk of ovarian cancer is 1.4%.
The most common sign of ovarian cancer is enlargement of the abdomen caused by accumulation of fluid or a large ovarian mass. However, many women have bloating or weight gain in the abdominal area, making this sign non-specific.
In women over 40, digestive disturbances that persist and cannot be explained by any other cause indicate the need for a thorough evaluation for ovarian cancer, including a carefully performed pelvic examination and ultrasound.
184. Risk factors
A first degree relative with ovarian cancer (mother, sister or daughter).
Personal H/o breast cancer < 40 years or age.
Personal H/o breast cancer < 50 and one or more close relative with breast or ovary cancer at any age.
2 or more close relative with breast cancer < 50 years of age or ovarian cancer at any age.
185. Screening: No screening guidelines are available for mass screening for ovarian cancer. Recommendation for screening is dependent on the risk status of women.
186. A heightened awareness of the symptoms of early ovarian cancers on the parts of the patients and practitioners may help to reduce the delay in diagnosis and hopefully result in an improvement in outcome of some progress.
187. For general population - annual pelvic examination, PAP smear, and transvaginal sonography are recom-mended as a part of post-menopausal surveillance.
188. Primary prevention: Limited data are available on the efficacy of prophylactic oophorectomy in decreasing the risk of ovarian cancer in mutation carriers. Still, it is recommended that prophylactic surgery be considered in BRCA mutation carriers who have completed childbearing.
Vulvar cancer
189. Epidemiology: Cancer of the vulva is a rare disease that accounts for approximately 5% of gynecological cancers. The median age of onset is approximately 65-70 years for invasive cancer and approximately 45-50 years for carcinoma in situ.
190. Risk factors for vulvar cancer include the following: HPV, previous genital warts, greater number of sexual partners, current smoking, abnormal PAP smear, diabetes, obesity, chronic vulvar pruritis, and poor personal hygiene have also been suggested as contributing to risk.
191. Protected intercourse, monogamy, and adequate hygiene of the external genitalia protect against vulvar cancer.
192. Prevention and detection: The prevention of vulvar cancer rests in the avoidance of risk factors and application of protective factors as summarized above. Annual examinations should be performed to check for vulvar cancer. High-risk patients should be examined every 6 months. White lesions and chronic ulcerative lesions should be biopsied for evaluation.
Stomach cancer
193. In women aged 30-69 years, the second most common fatal cancers were stomach (14.1%). Stomach cancer rates were higher in rural than in urban areas of India due to increased prevalence of chronic Helicobacter pylori infection.
Million death study cancer mortality in India: A nationally representative survey 2012. This may include stomach and primary liver cancer. Prevalence of hepatitis B virus (HBV) in India was less than 1.9% in 72,000 pregnant women aged 15-49 years who were tested in 2002.
194. Nearly, 37% of all female cancer deaths were from infection-related cervical, stomach, and liver cancers and 18.3% were from tobacco-related cancers. This underscores the importance of vaccination, control of infection.[112] Vaccination against HBV would reduce future liver cancer deaths and cirrhosis. Use of tobacco in pan and beedi should be strongly discouraged.
SECTION III
ABNORMAL MENOPAUSE
Premature menopause
195. The National Family Health Survey of 1998-99, collected information from a sample of more than 90,000 married women aged between 15 and 49 and covering 99% of India’s population living in 26 states. 3.1% of the women are already in menopause by the age of 30-34, and the incidence rises to 8% for the age bracket of 35-39. At age of 48-49 years 66% of the women are amenorrheic. This is probably an overestimate for the study did not differentiate between natural, surgical or secondary causes.[67]
196. Menopause occurring at an age less than 2 SD below the mean estimated age for the reference population is called as premature menopause.
197. Diagnosis is established by hormone analysis repeated 1 month apart. Serum FSH levels > 40 U/mL are diagnostic of POF.
198. Appropriate counseling, life-style modification and HRT form the mainstay of treatment. HRT should be started as early as possible in women with POF and continued till age of natural menopause. Androgen replacement should be considered for women with persistent fatigue, loss of libido in spite of estrogen replacement.
199. No evidence that HT increases risk of breast cancer, CVD or dementia, over and above that found in menstruating women with a normally timed menopause.
200. Women with untreated premature menopause are at increased risk of developing osteoporosis, cardiovascular disease dementia, cognitive decline, and Parkinson’s and all-cause mortality.
201. Women receiving chemotherapy/radiotherapy (pelvis) should be cautioned about iatrogenic premature menopause.
202. Hysterectomy alone can sometimes cause early menopause.
Induced menopause
203. The exact prevalence of surgical menopause is not known, but varies in the rural to urban areas and across states.
204. A significant number of hysterectomies along with bilateral oophorectomies are performed at a young age. This trend of unwarranted hysterectomies and surgical castration for fear of cancer by the professional and the women should be discouraged.
205. There is wide diversity in awareness, about public health problems and QOL among both physicians and population. There is a great need of awareness program about consequence of surgical menopause risk/benefit and in prevention of problems due to surgical menopause.
206. The exact prevalence of surgical menopause is not known, but varies in the rural to urban areas and across states.
206. The physicians should have appropriate knowledge to recognize menopausal symptoms and whenever in doubt should get the test (FSH > 40 IU/mL, E2 < 40 pg/mL.
207. Women who need oophorectomy before menopause should be counseled about the risk of surgical menopause.
208. Routine HT is not recommended for surgical menopause in post-menopausal women as primary prevention for chronic conditions.
209. HT should be considered in women less than 50 who have undergone surgical menopause.
SECTION IV
CLINICAL EVALUATION
General considerations
210. Clinical examination includes a holistic approach to health, rather than simply looking for features of menopause in isolation and this leads to diagnose the latent and overt NICD. Non communicable disease.[68,39,70,71,132] A thorough assessment of the health-related problems helps in formulating treatment plan. Examination can be broadly divided into three main categories:
General physical examination: Examination of respiratory, cardiovascular system, and bones may detect common age related problems
Breast examination: This should be carried out regularly due to an increased risk of breast cancer as women get older
Pelvic examination: This is performed to assess for complications of menopause, such as urogenital atrophy and must include PAP smear.
Assessment
Detailed history.
Evaluate women’s need.
Evaluation of women’s individual risk factor.
Assess general condition of patient.
-
Physical examination:
Pulse
BP
- Optimal BP (<130/85) to be rechecked every 2 years.
- Normal level (<140/90 mmHg) to be checked yearly.
- Greater than 140/90 mmHg need second measurement to confirm diagnosis of hypertension.
Auscultation of the heart and lungs
Height.
Weight.
Waist circumferences.
Calculate BMI.
Breast examination.
Pelvic examination.
211. Risk Factors for Osteoporosis: Major risk factors as defined by WHO are advancing age, prior fragility fracture, low BMI, family history of fracture, smoking and more than three drinks of alcohol per day (R: Grade A).
Environmental factors include nutrition (calcium intake using the quick dietary calculator, protein) physical activity and sunlight exposure, which are important modifiable risk factors in India. Relevance of risk of falling increases with ageing. R (Grade A).
212. Risk factors for coronary heart disease: Pre- maturemenopause, hypertension, dyslipidemia, homocystenemia, lipoprotein(a), high-risk CRP, DM, obesity, sedentary life-style, smoking, and metabolic syndrome.
213. Risk factors for DM: Advancing age, obesity, family history, hypertension, dyslipidemia, personal history of gestational DM or impaired glucose tolerance, PCOS, and physical inactivity.
214. Risk factor for deep vein thrombosis: Personal or family history of clot, if so, when and why? Prolonged immobilization-surgery or while pregnant or on the contraceptive pills. Any tests to confirm the clot history of the treatment with anticoagulants.
215. Risk factors for stroke: Hypertension, diabetes, smoking, obesity, atrial fibrillation, asymptomatic carotid stenosis, and hyperlipidemia.
216. Risk factors for Alzheimer’s disease: Age, family history, genetic factor APOE, MCI, CVD risk factors, physical inactivity, diabetes, hyper-tension, dyslipidemia, smoking, obesity, auto-immune diseases, depression and stress, social engagement and diet, and head trauma and traumatic brain injury.
Polypharmacy and thyroid disease are two examples of reversible causes of memory loss in older adults.
Investigations
217. These are necessary to establish the diagnosis, determine etiology, and screen for complication. Some investigations may be necessary to perform for diagnosis or to help in formulating a treatment plan.
218. Recommended laboratory tests:
Complete blood picture.
Urine test routine.
Fasting glucose level.
Lipid profile.
Serum TSH.
Stool for occult blood.
PAP smear.
Transvaginal ultrasound.
Mammogram/ultrasound.
Eye checkup – intraocular pressures, refractive index, and retina.
219. The following investigations are not mandatory and should be chosen judiciously depending on the women’s history and examination [Table 13].
Table 13.
Test performed solely on indication
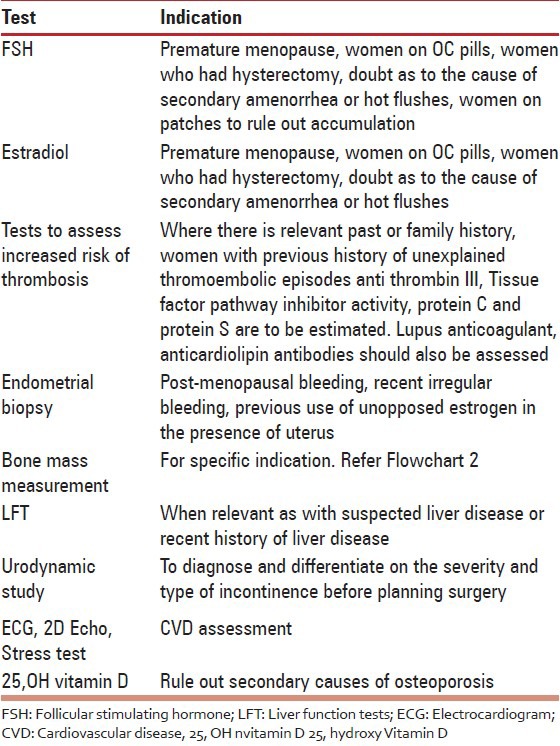
SECTION V
MANAGEMENT OPTIONS
A. Counseling
220. Today, the art of medical counseling and translating the statistics in simple language is an important part of the consultation.
221. The objectives of counseling include addressing women’s questions and concerns, providing patient education, and enhancing the patient’s confidence in the decision making. If a therapy is chosen, the patient and clinician should agree on the goals, risks, and benefits, whether they are short-term (menopause symptom relief), long-term (primary or secondary prevention of diseases associated with aging), or both.[133,134,135,136]
222. The clinician should review the decisions about menopause management with the patient at subsequent visits.
Dietary prescription
223. The National Institute of Nutrition plan for an adult sedentary woman is a good strategy for healthy living [Table 14].
Table 14.
Nutrition plan for an adult sedentary woman
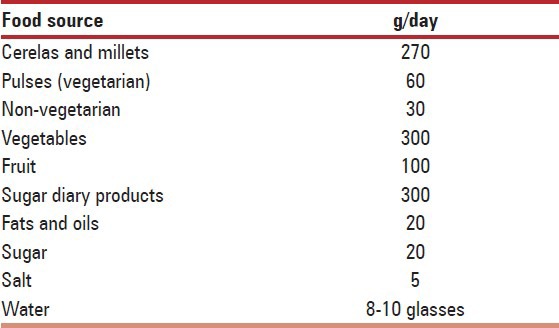
Exercise prescription
224. Physical exercise helps to maintain a healthy weight, improves bone density, coordination and balance, muscle strength and joint mobility, lipid profiles, genitourinary problems, relieves depression, and induces sleep.
225. Combination of exercises, diet, and yoga helps the post-menopausal women to increase her metabolic rate and maintain a healthy weight.
226. Social interactions either in an exercise program or otherwise, help the post-menopausal women to improve mood, relieve depression, and anxieties.
227. Euphoria created with activity promotes her QOL.
Immunization prescription
228. Hepatitis B vaccination is indicated for all unvaccinated adults at risk for HBV infection and all adults seeking protection from HBV infection including post-exposure prophylaxis. Pre-vaccination screening in general population has not been found to be cost-effective in India (Level B).
229. The expert group of Association of Physicians of India recommends vaccination of the entire community at risk during an outbreak situation (Grade B).
230. Two doses of varicella vaccine are strongly recommended in adults at increased risk for exposure of varicella (Grade B).
Pharmacotherapy
Complementary and alternative therapies
231. Non-hormonal prescription agents may relieve VMS, but have their own side effects. These can be considered when HRT is contraindicated or not desired.[137] (Grade A).
232. Complementary and alternative treatments should be advised with caution as the data is still insufficient, especially in moderate to severe VMS (Grade A).
233. Awareness should be created regarding the phytoestrogens and lycopene rich foods in the Indian diet.[138] (Grade C).
234. It is recommended to validate the effects of locally used herbs in the Indian context, according to modern medicine and prescribe them rationally using clinical research tools and well-designed and documented RCTs. Whilst prescribing or recommending herbs, it would be essential to fully inform the women that very little human data is available on the usefulness of these formulations and side-effects of the herbs have not been studied. It is important to read labels to determine isoflavone content and to warn them that in India, there are no regulations to ensure the content or quality of such products (Grade C).
HT
Terminology
235. HT covers therapies including estrogens, progestogens, combined therapies, androgens, and tibolone.
236. Terminology used in HT: HT, HT; ET; Estrogen progesterone therapy (EPT), EPT; and androgen therapy (AT).
237. Three indications for post-menopausal HT, which have constantly withstood the test of time, derived from the results of various clinical trials are the beneficial effect of estrogens on symptom relief, urogenital atrophy, and bone.
Patient characteristics that may be favorable for estrogen/androgen combination
238. Surgical menopause continued VMS despite estrogen replacement, decreased wellbeing despite estrogen replacement, and acquired sexual desire dysfunction.
Indications for HT
239. The most effective treatment for VMS is HT (Grade A).
240. Progesterones or Low dose oral contraceptive pills can be used in the menopause transition phase for relief of symptoms (Grade A).
241. Vaginal ET is most effective in the treatment of urogenital atrophy. Low dose vaginal preparations are as effective as systemic therapy. Some women on oral ET may require additional local therapy (Grade A).
242. Recurrent attacks of atrophic vaginitis require the use of the smallest effective dose over a period of time. After control of acute symptoms, the dose of local estrogen can be tapered for long-term maintenance therapy. Treatment may be continued indefinitely, although safety data from studies do not go beyond 1 year (Grade C).
243. Recurrent urinary tract in this age after ruling out other causes may benefit from the local application of ET (Grade A).
244. Progesterone supplement for endometrial protection is not needed along with the use of vaginal estrogen (Grade C).
245. Endometrial surveillance is not necessary in low risk asymptomatic woman. Unscheduled bleeding should be investigated by an ultrasound and endometrial biopsy (Grade A).
246. EPT/ET may be used for prevention and treatment of osteoporosis in the early post-menopause in symptomatic women unless there is a contra-indication. ET/EPT prevents all osteoporotic fractures even in low risk population, it increases lumbar spine BMD up to 7.6% and femoral neck BMD up to 4.5% over 3 years. It reduces the risk of spine, hip, and other osteoporotic fractures by 33-40% (Grade A).
247. HT should not be started solely for bone protection after 10 years of menopause. Extended use of HT in women with reduced bone mass is an option after considering the risk benefit analysis compared to the other available therapies for osteoporosis. The bone protective effect is lost after stopping HT (Grade B).
248. HT should be offered to women with POF or early menopause (and it can be recommended until the age of natural menopause (Grade C).
249. Estrogen can be prescribed to enhance mood in women with depressive symptoms. The effect appears to be greater for perimenopausal symptomatic women than for post-menopausal women (Grade A).
Possible benefits
250. HT (conjugated equine estrogens±medroxy- progesterone) was associated with a decrease in the risk for type 2 diabetes (Grade B).
251. HT decreases the abdominal obesity (Grade B).
252. Estrogens may have a protective effect on osteoarthritis (Grade B).
253. Estrogen benefits verbal memory over the short period when initiated soon after surgical menopause (Grade B).
254. HT reduces the neovascular macular lesions (Grade C).
255. HT in the early menopausal period improves QOL by its effects on vasomotor and urogenital symptoms, improvement on sleep, and mood (Grade B).
HT use in disease
256. All preparations including low dose, non-oral routes of estrogen are effective in symptom control and in preserving bone mass. In women with hypertriglyceridemia, obesity, glucose intolerance, history of deep vein thrombosis, and tobacco users, non-oral route should be the preferred (Grade B).
257. Women who have general risk of breast cancer can be prescribed HT according to their need after a detailed history, examination, and counseling. They should be provided information about breast cancer risk with HT as per evidence.
258. Women who are at high-risk of breast cancer also can be prescribed HT after risk benefit analysis.
259. HT does not appear to influence the clinical pattern of benign breast disease in a post-menopausal woman (Grade C).
260. Use of HT in breast cancer survivors is debatable. It is recommended to use non-hormonal therapies.
261. Women with cervix, ovary, and EC endometrial cancer can be given HT if needed.
262. (HT) Hormone Therapy given to women below the age of 60 or within 10 years of menopause, the risks are rare. The tables below two elaborate the benefits and risks in terms that can be easily communicated during counseling.
263. Classification of frequency of drug reactions according to WHO and CIOMS – The Council for International Organizations of Medical Sciences
Very common > 1/10; common (frequent) >1/100 and < 1/10; uncommon (infrequent) >1/1,000 and < 1/100; rare > 1/10,000 and < 1/1,000; and very rare < 1/10,000.
264. Harms
265. Based on WHI: Number of excess events on HT versus placebo per 10,000 women per year of HT Use between the age group of 50 years and 59 years (R: Grade A) [Table 15].
Table 15.
Based on WHI: number of excess events on HT vs. placebo per 10,000 women per year of HT use between the age group of 50–59 years (R: Grade A)
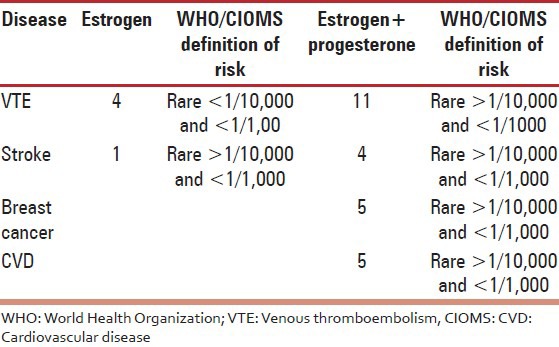
266. Benefits
Based on WHI: Number of less events on estrogen versus placebo per 10,000 women per year of HT use between the age group of 50 years and 59 years (R: Grade A) [Table 16].
Table 16.
Based on WHI: number of less events on estrogen vs. placebo per 10,000 women per year of HT use between the age group of 50-59 years (R: Grade A)
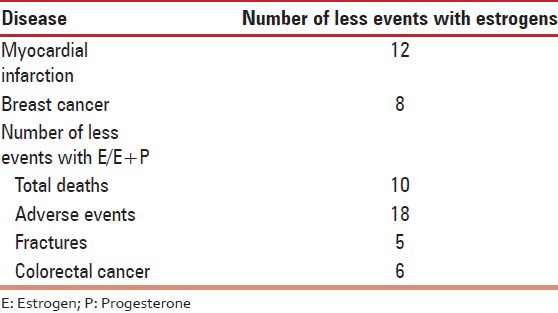
Absolute contraindications of HT
267. Active endometrial and gynecological hormone dependent cancers, active breast cancer, estrogen progestogen receptor positive cancers, known or suspected pregnancy, undiagnosed, abnormal vaginal bleeding, severe active liver disease with impaired/abnormal liver function, estrogen dependent venous thmbosis, and inherent increased risk of thromboembolism.
Precautions
268. Progesterone in adequate dose should be supplemented along with oral estrogens in women with uterus (Grade A).
269. Estrogen alone is given in hysterectomized women (Grade A).
270. Progesterone supplement for endometrial protection is not needed along with the use of vaginal estrogen (Grade C).
271. Endometrial surveillance is not necessary in low risk asymptomatic woman. Unscheduled bleeding should be investigated by an ultrasound and endometrial biopsy (Grade A).
272. Pre-HT work-up and an annual follow-up are essential when prescribing HT. The dose and duration of use of HT should be individualized and a risk-benefit assessment carried out annually. A full gynecological assessment is mandatory prior to starting HT and at regular intervals thereafter. Self-breast examination is advised monthly and CBE at least annually. Mammogram/US, where available should be carried out 1-3 yearly if the initial mammogram is normal (Grade C).
Duration of use
273. Premature menopause-HT can be prescribed up to the natural age of menopause; further continuation of therapy is a shared decision between the woman and the physician according to the indication and the need (Grade C).
274. Natural menopause: Safety data of EPT therapy with CEE+MPA is 3-5 years with ET safety data for use is 7 years of treatment with 4 years follow-up. Role of extended use of HT is a shared decision between the woman and the physician and may be considered in cases of recurrence of symptoms after stopping therapy, in cases of management of osteoporosis when other therapies are contraindicated (Grade A).
275. Stopping HT: May be abrupt or the dose and duration may be tapered off gradually (Grade C).
Potency and non-oral routes
276. Minimum effective dose is the principle to be followed while prescribing HT. The potency needed by the woman may change over time. After starting standard dose therapy, dose can be lowered and maintained accordingly. Low dose and ultralow dose therapy are effective in relieving symptoms and increasing bone mass.
277. Transdermal estrogen has a neutral effect on triglycerides, CRP, and sex hormone binding globulin and is preferable for use in women with hypertriglyceridemia, obesity, glucose intolerance, high-risk of deep vein thrombosis, and tobacco users.
HT and CVD
278. HT should not be prescribed for primary or secondary prevention of CVD. However, healthy women within 10 years of menopause tend to have a lower risk.
279. HT increases VTE risk by 2-fold (Grade A).
280. Standard dose oral HT increased stroke risk by about one third in generally healthy post-menopausal women (Grade B). Low dose ET may not increase the risk of stroke (Grade C).
HT and breast cancer
Estrogen alone
281. Estrogen alone increases percentage mammographic density, not as much as estrogen and progesterone together (Level A).
282. Estrogen increases the risk of breast cancer after more than 5 years of use, particularly in recently post-menopausal women (Level B).
283. The precise duration of exposure needed to exert this effect is not clear, but a linear model suggests a 3% relative increase per year of exposure in thin women and a lesser risk in obese women (Level C).
284. The attributable or excess risk for 5 years usage is 0/1000 to 2.59/1000 (Level C). It falls under the rare category.
285. Increased risk dissipates within 5 years of discontinuing the HT (Level B).
286. Use of estrogen for less than 5 years may reduce the risk especially in women who start HT many years after menopause (Level B).
287. Tumors in HT used women are usually ER positive and lobular type (Level C).
Estrogen+progesterone (E+P)
288. E+P increase percentage mammographic density significantly (Level A).
289. E+P particularly with synthetic progesterones increase the risk of invasive breast cancer within 3-5 years of initiation and increases progressively beyond that time (Level B).
290. Emerging data from 2 independent studies report that progesterone (micronized progesterone/dydrogesterone) with estrogen does not increase the risk if given for less than 5 years (Level C).
291. The risk returns to approximately that of non-users within 3 years of cessation (Level B).
Androgens
292. Available data is of low quality and conflicting regarding the risk of breast cancer relating to use of androgens (Level D).
293. Prospective randomized double-blind trials are needed (Level D).
Tibolone
294. It reduces the risk of breast cancer in post-menopausal women (Grade B).
295. It increases the risk of breast cancer recurrences.
Raloxefene
296. It decreases the risk of development of breast cancer.
Maximum benefits, minimum side-effects of HT can be achieved by judicious use
297. Age of initiation: Ideally therapy begins within 10 years of menopause or below 60 years of age – “window of opportunity.”
298. Low dose: Use of low-dose estrogen with low-dose progestin when appropriate.
299. Route of administration: Transdermal administration has reduced risk of blood clotting (VTE risk) compared to oral administration.
300. Progestin: Side-effect profile of various progestins may play a clinical role in selecting the optimum treatment regimen. Natural progesterone is a choice.
301. Tissue selective estrogen complex (TSEC): Newer formulations of combination therapy of estrogen and selective estrogen receptor modulators are soon to be available.
Tibolone
302. Tibolone is a selective tissue estrogenic activity regulator. It is a synthetic steroid compound, which has estrogenic, progestogenic, and androgenic properties. It has an estrogenic effect on bone, inhibiting bone resorption by reducing osteoclastic activity.
303. Tibolone is approved in 90 countries to treat menopausal symptoms and in 45 countries to prevent osteoporosis.
Tibolone is effective in treating VMS and improves urogenital atrophy (Grade A).
304. It improves mood and libido (Grade B).
305. Tibolone is prescribed in a single daily dose of 2.5 mg orally. A lower dose of 1.25 mg has been found to be equally effective for most indications, including osteoporosis. It should be prescribed 1 year after amenorrhea (Grade A).
306. Tibolone reduces the risk of vertebral and non-vertebral fracture in older osteoporotic women. Tibolone prevents bone loss and is as effective as standard doses of conventional post-menopausal HT. Tibolone increases lumbar spine and total hip BMD to a statistically significantly greater extent than raloxifene (Grade A).
307. It does not increase the risk of VTE and CVD events (Grade B).
308. It does not induce endometrial hyperplasia or carcinoma in post-menopausal women (Grade A).
309. Tibolone may be preferable to HRT in symptomatic menopausal women with mammographically dense breast tissue (Grade A).
310. Tibolone may be used as add back therapy with GnRH analogs for VMS and to maintain BMD (Grade B).
311. Tibolone may be used in women with myomas and endometriosis.
312. Tibolone should not be used in breast cancer survivors as it increases the recurrence risk (Grade A).
313. It reduces the risk of breast cancer in post-menopausal women (Level B).
314. Tibolone should be used with caution in women over 60 years and should not be used in those who have strong risk factors for stroke (Grade A).
Selective estrogen receptor modulators
315. Selective estrogen receptor modulators, e.g. raloxifene at 60 mg daily improve and preserve bone density at the spine (2.6%) and hip (2.1%) after 4 years with a simultaneous reduction by 76% in the risk of invasive breast cancer.
Antifracture efficacy on the hip is lacking (Grade A).
316. Raloxifene has been shown to be beneficial in reducing new vertebral fracture risk by 69% in post-menopausal women with osteoporosis and 47% in postmenopausal women with osteopenia over 3 years (Grade A).
317. Raloxifene can be used as therapy for the prevention and treatment of osteoporosis especially for women with an increased risk of breast cancer (Grade A).
318. Raloxifene and estrogen are associated with a similar increased risk of VTE (Grade A). Other side-effects include hot flushes, which are more likely in the perimenopausal period, and leg cramps.
SECTION VI
Economics of menopause management
319. Indian health-care system is one of the most privatized systems where government spends much less and individual has to pay for health insurance.
320. Insurance may be described as a social device to reduce or eliminate the risk of life and property. Under the plan of insurance, many people associate themselves by sharing risk, attached to individual insurance plan that covers only health-care costs and is called health insurance.
321. It is indeed very important to enroll in any of the good health insurance schemes for a secure future. Health-care insurance provides a cushion against medical emergencies. Most companies stop enrolment after 65-70 years of age.[139]
322. Menopause management is associated with significant direct and indirect costs.
323. Direct costs include physician’s visits, specialist’s visit and traditional pharmacotherapy or alternative and complimentary medicines modality.
324. Indirect costs include laboratory testing, management of adverse events, loss of productivity at home and at work, and treatment of associated medical disorders.
325. Rates prevailing in different regions of India are compared and the preliminary cost (without medication) is found to a range between Rs. 5,800 and Rs. 8,400.
326. Various oral estrogen and tibolone preparations are available in Indian market, cost of which ranges from Rs. 40 to Rs. 990.
327. Local and transdermal estrogen preparations are scarce in Indian market, cost of which ranges from Rs. 134 to Rs. 658.
328. Various oral and non-oral progesterone preparations are available in Indian market, cost of which ranges from Rs. 10 to Rs. 820.
329. Various groups of molecules are available for prevention and treatment of osteopenia and osteoporosis. Cost of therapy varies according to the indication whether they are prescribed for prevention or treatment of osteopenia or osteoporosis.
330. Alternative and complimentary medications are usually not considered to be part of mainstream medicine, but are popularly available in Indian market, cost of which ranges from Rs. 42 to Rs. 473.
331. Menopause is a time of significant changes, which often have a negative impact on QOL. However, it is possible to live well with menopause. Adopting a healthy life-style is cost-effective. 140
REFERENCES
- 1.Milsom I. Menopause-related symptoms and their treatment. In: Erkkola R, editor. The Menopause. Philadelphia: Elsevier; 2006. p. 16. [Google Scholar]
- 2.Fritz MA, Speroff L. 8th ed. Ch. 17. Philadelphia: Lippincot Williams and Wolters Kluwer business; 2011. Clinical Gynaecological Endocrinological and Infertility; p. 681. [Google Scholar]
- 3.Research on the Menopause in the 1990s Report of a WHO Scientific Group. World Health Organisation, WHO Technical Report Series No. 866. 1996 [PubMed] [Google Scholar]
- 4.Utian WH. The International Menopause Society menopause-related terminology definitions. Climacteric. 1999;2:284–6. doi: 10.3109/13697139909038088. [DOI] [PubMed] [Google Scholar]
- 5.HRT for “Mature” Women using a Clinical Staging System: Dr. B.S. Anklesaria Gujarat. Med J. 1997:54. [Google Scholar]
- 6.Anklesaria BS. The Staging of Menopause in Jeffcoate's Principles of Gynecology. In: Kumar P, Malhotra N, editors. 7th ed. India: Japyee Brothers Medical Publishers; 2008. pp. 862–4. [Google Scholar]
- 7.Soules MR, Sherman S, Parrott E, Rebar R, Santoro N, Utian W, et al. Executive summary: Stages of Reproductive Aging Workshop (STRAW) Park City, Utah, July, 2001. Menopause. 2001;8:402–7. doi: 10.1097/00042192-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Harlow SD, Gass M, Hall JE, Lobo R, Maki P, Rebar RW, et al. Executive summary of the Stages of Reproductive Aging Workshop+10: Addressing the unfinished agenda of staging reproductive aging. Fertil Steril. 2012;97:843–51. doi: 10.1016/j.fertnstert.2012.01.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma S, Tandon VR, Mahajan A. Menopausal Symptoms in Urban Women. JK Sci J Med Educ Res. 2007;9 Vol. 13 No. 1, Jan-March 2011. [Google Scholar]
- 10.Singh M. Early age of natural menopause in India, a biological marker for early preventive health programs. Climacteric. 2012;15:581–6. doi: 10.3109/13697137.2011.643514. [DOI] [PubMed] [Google Scholar]
- 11.Singh L, Ahuja S. Trend of menopause among the women of Punjab. Anthropol Anz. 1980;38:297–300. [PubMed] [Google Scholar]
- 12.Sengupta S, Gogoi G. Menarche and menopause among the Kaibarta women of Dibrugarh, Assam. J Assam Science Society. 1993;35:113–9. [PubMed] [Google Scholar]
- 13.Kulkarni VS, Joshi M. Reproductive life of two endogamous groups of Maharashtra. Man India. 1979;59:71–90. [PubMed] [Google Scholar]
- 14.Kar RK, Mahanta S. Menarche and menopause among the women of Arunachal Pradesh. Ind J Phys Anthro Human Genet. 1975;1:51–57. [Google Scholar]
- 15.Balgir RS. Age at menarche and menopause among the Sikligars of Punjab. J Indian Med Assoc. 1985;83:195–7. [PubMed] [Google Scholar]
- 16.Sharma N, Singh R. Patiala India: 1980. Age at menarche and menopause of Brahmins and Choudhary females of kangra valley. Proceedings of international symposium on human growth. [Google Scholar]
- 17.Gosh AK, Kumari S. Effect of menarcheal age on fertility. J Indian Anthropol Soc. 1973;8:165–72. [Google Scholar]
- 18.Singh A, Arora AK. Profile of menopausal women in rural north India. Climacteric. 2005;8:177–84. doi: 10.1080/13697130500117920. [DOI] [PubMed] [Google Scholar]
- 19.Shah R, Kalgutkar S, Savardekar L, Chitlang S, Iddya U, Balaiah D. Menopausal symptoms in urban Indian women. Obstet Gynaecol Today. 2004;11:667–70. [Google Scholar]
- 20.Bagga A. Age and symptomatology of menopause: A case study. Obstet Gynaecol Today. 2004;11:660–6. [Google Scholar]
- 21.Rakshit S. Reproductive life of maharashtrian Brahmin women. Man India. 1962;56:65–70. [Google Scholar]
- 22.Sengupta S, Rjkhowa M. Menarche and menopause in Ahom women of Dibrogarh in Assam. J Hum Ecol. 1996;7:211–13. [Google Scholar]
- 23.Kaw D, Khanna B, Vasishtha K. Factors that influence the age at natural menopause. J Obstet Gynaecol India. 1994;44:273–7. [Google Scholar]
- 24.Mastana SS. Age at menopause among the Lobanas of North West India. J Hum Ecol. 1996;7:151–3. [Google Scholar]
- 25.Kiplani A, Banerjee K. An overview of age of menopause in Northern India. Maturitas. 2005;52:199–204. doi: 10.1016/j.maturitas.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Shatrugna V, Kulkarni B, Kumar PA, Rani KU, Balakrishna N. Bone status of Indian women from a low-income group and its relationship to the nutritional status. Osteoporos Int. 2005;16:1827–35. doi: 10.1007/s00198-005-1933-1. [DOI] [PubMed] [Google Scholar]
- 27.Gupta A. Osteoporosis in India the nutritional hypothesis. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic Bone Disorders. Lucknow: Hindustani Book Depot; 1998. p. 115. [Google Scholar]
- 28.Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet. 2004;364:937–52. doi: 10.1016/S0140-6736(04)17018-9. [DOI] [PubMed] [Google Scholar]
- 29.Ramachandran A, Ma RC, Snehalatha C. Diabetes in Asia. Lancet. 2010;375:408–18. doi: 10.1016/S0140-6736(09)60937-5. [DOI] [PubMed] [Google Scholar]
- 30.Nandakumar A, Ramnath T, Chaturvedi M. The magnitude of cancer cervix in India. Indian J Med Res. 2009;130:219–21. [PubMed] [Google Scholar]
- 31.Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases: Part I: General considerations, the epidemiologic transition, risk factors, and impact of urbanization. Circulation. 2001;104:2746–53. doi: 10.1161/hc4601.099487. [DOI] [PubMed] [Google Scholar]
- 32.Das SK, Banerjee TK, Biswas A, Roy T, Raut DK, Mukherjee CS, et al. A prospective community-based study of stroke in Kolkata, India. Stroke. 2007;38:906–10. doi: 10.1161/01.STR.0000258111.00319.58. [DOI] [PubMed] [Google Scholar]
- 33.London: Department of Health; 1998. Acheson Report: The Independent Inquiry into Inequalities in Health. [Google Scholar]
- 34.Nangia S, Arya V, Gujral Ratni B, Mithal A. Lucknow: Presented at 27th Annual Meeting of the Endocrine Society of India; 1997. Spinal bone mineral density in normal Indian females. [Google Scholar]
- 35.Arya V, Nangia S, Gujral RB, Mithal A. Lucknow: Presented at 27th Annual Meeting of the Endocrine Society of India; Femoral bone mineral density in normal Indian females. [Google Scholar]
- 36.ICMR Taskforce Study. New Delhi: Indian Council of Medical Research; 2010. Population based reference standards of peak bone mineral density of Indian males and female. An ICMR multicentre task force study. [Google Scholar]
- 37.Gupta A. Osteoporosis in India the nutritional hypothesis. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic Bone Disorders. Lucknow: Hindustani Book Depot; 1998. p. 115. [Google Scholar]
- 38.Teotia SP, Teotia M. Nutritional bone disease: The continuing challenge to neonatal bone health. Postgrad Med. 2009;23:30–8. [Google Scholar]
- 39.Nangia S, Arya V, Gujral RB, Mithal A. Spinal bone mineral density in normal Indian females. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic Bone Disorders. Lucknow: Hindustani Book Depot; 1998. p. 213. [Google Scholar]
- 40.Arya V, Nangia S, Gujral RB, Mithal A. Femoral bone mineral density in normal Indian females. In: Mithal A, Rao DS, Zaidi M, editors. Metabolic Bone Disorders. Indian Society for Bone and Mineral Research; 1998. p. 215. [Google Scholar]
- 41.Singh M, Magon N, Singh T. Major and minor discordance in the diagnosis of postmenopausal osteoporosis among Indian women using hip and spine dual-energy X-ray absorptiometry. J Midlife Health. 2012;3:76–80. doi: 10.4103/0976-7800.104457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki TT, Grecu EO, Srinivas PR, Prescott P, Benbarka M, Arcangeli MM. Prevalence of osteoporosis in women: Variation with skeletal site of measurement of bone mineral density. Endocr Pract. 2000;6:127–31. doi: 10.4158/EP.6.2.127. [DOI] [PubMed] [Google Scholar]
- 43.Reddy PG, Mithal A, Rao DS. Bone mineral density in healthy Asian Indian women: Development of a reference database and implications for diagnosis of osteoporosis in Indian women living in the United States [abstract] J Bone Miner Res. 2002;17(Suppl 1):SA270. [Google Scholar]
- 44.Joshi VR, Mangat G, Balakrishnan C, Mittal G. Osteoporosis – Approach in Indian scenario. J Assoc Physicians India. 1998;46:965–7. [PubMed] [Google Scholar]
- 45.Makkar A, Mishra G, Singh BP. Peak bone density at multiple skeletal sites in Indian subjects. Arch Osteoporos. 2008;3:25–3. [Google Scholar]
- 46.Chibber G, Roy R, Eunice M, Srivastava M, Ammini AC. Prevalence of osteoporosis among elderly women living in Delhi and rural Haryana. Indian J Endocrinol Metab. 2007;1:11–4. [Google Scholar]
- 47.Nangia S, Arya V, Gujral Ratni B, Mithal A. Lucknow: Presented at 27th Annual Meeting of the Endocrine Society of India; 1997. Spinal bone mineral density in normal Indian females. [Google Scholar]
- 48.Gandhi A, Shukla AR. Evaluation of BMD of women above 40 years of age. J Obstet Gynecol India. 2005;55:265. [Google Scholar]
- 49.Meeta . Spain: Poster Presented At International Menopause Meeting; 2008. Evaluation of Risk Factors for Dexa Referral in Indian Women. [Google Scholar]
- 50.Krishna U, Mehta RU. Osteoporosis – Incidence and implications. J Ob Gyn of India. 2000;50:150–6. [Google Scholar]
- 51.Babu AS, Ikbal FM, Noone MS, Joseph AN, Samuel P. Osteoporosis and osteopenia in India: A few more observations. Indian J Med Sci. 2009;63:76–7. [PubMed] [Google Scholar]
- 52.Sharma S, Tandon VR, Mahajan A, Kour A, Kumar D. Preliminary screening of osteoporosis and osteopenia in urban women from Jammu using calcaneal QUS. Indian J Med Sci. 2006;60:183–9. [PubMed] [Google Scholar]
- 53.Pande KC. Prevalence of low bone mass in healthy Indian population. J Indian Med Assoc. 2002;100:598–600. 602. [PubMed] [Google Scholar]
- 54.Meeta Osteoporosis Revisited. Osteoporosis Alert. (1):1. [Google Scholar]
- 55.Paul TV, Thomas N, Seshadri MS, Oommen R, Jose A, Mahendri NV. Prevalence of osteoporosis in ambulatory postmenopausal women from a semiurban region in Southern India: Relationship to calcium nutrition and vitamin D status. Endocr Pract. 2008;14:665–71. doi: 10.4158/EP.14.6.665. [DOI] [PubMed] [Google Scholar]
- 56.Aggrawal N, Bathla S, Juneja S. Measurement of bone mineral density by dexa scan in postmenopausal women. Obs abd Gynae Today. 2004;9:768–71. [Google Scholar]
- 57.Meeta . Chandigarh: Poster at the Annual Indian Menopause Society meeting; 2006. Meeta Two hundred and six reasons to be informed about osteoporosis. [Google Scholar]
- 58.Unni J, Garg R, Pawar R. Bone mineral density in women above 40 years. J Midlife Health. 2010;1:19–22. doi: 10.4103/0976-7800.66989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Savardekar LS, Shah RS, Iddya U, Balaiah D, Parihar A, Jaknkaria B. Bone density in normal Indian women: Assessment by USG and DEXA. Obs abd Gynae Today. 2004;9:772–6. [Google Scholar]
- 60.Meeta, Shaantanu, Tanvir Assessment of Risk Factors for Osteoporosis in Indian Women submitted for publication [Google Scholar]
- 61.Dhanwal DK, Dixit V, Jameson K, Mithal A, Cooper C. Hip fracture incidence in Rohtak, North India. Osteoporosis Int Supplement. 2011 doi: 10.1007/s11657-013-0135-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.International Osteoporosis Foundation. The Asian Audit: Epidemiology, costs and burden of osteoporosis in Asia. 2009. [Last accessed on 2012 Oct 01]. Available from: http://www.iofbonehealth.org .
- 63.Sankaran B. Clinical studies: Incidence of fracture neck of femur and intertrochanteric fractures in three Delhi hospitals. In: Sankaran B, editor. Osteoporosis. New Delhi: South East Asia Regional Office, World Health Organisation; 2000. pp. 9–18. [Google Scholar]
- 64.Jha RM, Mithal A, Malhotra N, Brown EM. Pilot case-control investigation of risk factors for hip fractures in the urban Indian population. BMC Musculoskelet Disord. 2010;11:49. doi: 10.1186/1471-2474-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marwaha RK, Tandon N, Gupta Y, Bhadra K, Narang A, Mani K, et al. The prevalence of and risk factors for radiographic vertebral fractures in older Indian women and men: Delhi Vertebral Osteoporosis Study (DeVOS) Arch Osteoporos. 2012;7:201–7. doi: 10.1007/s11657-012-0098-8. [DOI] [PubMed] [Google Scholar]
- 66.Kulkarni S. India: 1998-99. fred Arnold-Family Planning; National Family Health Survey (NFSH-2) p. 85. 87. [Google Scholar]
- 67.Herbert B peterson. JAMA. Vol. 279. Contraception Information Center; 1998. May 27, MD Discussant-A 40 year-old Women considering Contraception; pp. 1651–8. p. 1. [DOI] [PubMed] [Google Scholar]
- 68.Welling K, Field J, Johnson AM, Wadsworth J. Contraception in the Over-40s. Menopause Digest. 2001;13:5. [Google Scholar]
- 69.Haines CJ, Ludicke F. Geneva: Proceedings of the First Consensus Meeting on Menopause in the East Asian Region; 1997. Contraception in the late premenopause; p. 55. [Google Scholar]
- 70.Lakshmi RM, Kusumalatha K, Shraddha S. Rajkot: 12th National Indian Menopause Society Meeting; 2007. Analysis of 200 Perimenopausal Women: A Prospective Study; p. 25. [Google Scholar]
- 71.Bhavna S. Souvenir, Surat, Souvenir: 9th National Indian Menopause Society Meeting; 2009. Quallity of Life During Menopause. [Google Scholar]
- 72.Bansal S, Thaker R. Ahmedabad, Souvenir: Abstract 10th National Indian Menopause Society Meeting; 2005. Age, Symptoms and Perception of Menoapuse amongst Underprivilaged Women of Ahmedabad. (QOL) p. 61. [Google Scholar]
- 73.Patni R. Ahmedabad Souvenir, Souvenir: 10th National Indian Menopause Society Meeting; 2005. Managing Induced Menopause In Treated Cancer Patients Introduction; p. 53. [Google Scholar]
- 74.Bhattacharya SM. Effects of tibolone on health-related quality of life in menopausal women. Int J Gynaecol Obstet. 2007;99:43–5. doi: 10.1016/j.ijgo.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 75.Sharma S, Tandon VR, Mahajan A. Menopausal Symptoms in urban Women. JK Science. 2007;9:13. [Google Scholar]
- 76.Singh A, Arora AK. Profile of menopausal women in rural north India. Climacteric. 2005;8:177–84. doi: 10.1080/13697130500117920. [DOI] [PubMed] [Google Scholar]
- 77.Shah R, Kalgutkar S, Savardekar L, Chitlang S, Iddya U, Balaiah D. Menopausal symptoms in urban Indian women. Obstet Gynaecol Today. 2004;11:667–70. [Google Scholar]
- 78.Bagga A. Age and symptomatology of menopause: A case study. Obs Gynae Today. 2004;11:660–6. [Google Scholar]
- 79.Kaur S, Walia I, Singh A. How menopause affects the lives of women in suburban Chandigarh, India. Climacteric. 2004;7:175–80. doi: 10.1080/13697130410001713779. [DOI] [PubMed] [Google Scholar]
- 80.Bipasa S. Chandigarh, Souvenir: 11th National Indian Menopause Society Meeting; 2003. Diversity of Menopausal Symptoms in Urban Women of West Bengal; p. 203. [Google Scholar]
- 81.Gupta P, Sturdee DW, Hunter MS. Mid-age health in women from the Indian subcontinent (MAHWIS): General health and the experience of menopause in women. Climacteric. 2006;9:13–22. doi: 10.1080/13697130500515776. abstract. [DOI] [PubMed] [Google Scholar]
- 82.Hafiz I, Liu J, Eden J. A quantitative analysis of the menopause experience of Indian women living in Sydney. Aust N Z J Obstet Gynaecol. 2007;47:329–34. doi: 10.1111/j.1479-828X.2007.00746.x. [DOI] [PubMed] [Google Scholar]
- 83.Meeta, Majumdar S, Shah JM, Srivastava S, Joshi SA, Sharma S, et al. Attitude towards sexuality in the perimenopausal and postmenopausal women in India. In Press. [Google Scholar]
- 84.Agarwal M, Sinha R, Gupta N, Poonam . Faridabad, Souvenir: 17th National Indian Menopause Society; 2012. Study of urogenital complaints in postmenopausalnwomen over 45 yrs in Safdarjung Hospital; p. 48. [Google Scholar]
- 85.Garjie SB, Bharucha KE. UTIS Recurrent the estriol option. Natl Indian Menopause Soci News lett. 1998;3:10. [Google Scholar]
- 86.Jha UP, Gajraj J, Unni J, Meeta, Malik S. The IMS International Congress; 2008. Indian Menopause Society consensus summary statement. Abstract book. [Google Scholar]
- 87.Unni J. Third consensus meeting of Indian Menopause Society (2008): A summary. J Midlife Health. 2010;1:43–7. doi: 10.4103/0976-7800.66987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta R, al-Odat NA, Gupta VP. Hypertension epidemiology in India: Meta-analysis of 50 year prevalence rates and blood pressure trends. J Hum Hypertens. 1996;10:465–72. [PubMed] [Google Scholar]
- 89.Jha P, Laxminarayan R. Geneva: CTSU, Oxford, and 21 Medical Colleges and Research Institutions in India.--correct; CGHR, University of Toronto, Toronto and New Delhi, 2009. Causes of Death in India: Registrar General of India. SRS, India; CGHR, University of Toronto, Canada; ICMR, Delhi; SJRI, Bangalore; PGI, Chandigarh; WHO. [Google Scholar]
- 90.Shah D. The annual conference of the British menopause society-June 2010. J Midlife Health. 2010;1:48–50. doi: 10.4103/0976-7800.66983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pandey S, Srinivas M, Agashe S, Joshi J, Galvankar P, Prakasam CP, et al. Menopause and metabolic syndrome: A study of 498 urban women from western India. J Midlife Health. 2010;1:63–9. doi: 10.4103/0976-7800.76214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Evidence Based Diabetes Management. New Delhi, Chennai: Public Health Foundation of India, Dr Mohan's educational Academy; 2010. p. 31. 2010. [Google Scholar]
- 93.Shringi MS, Vaidya RA, Joshi JV. Subclinical hypothyroidism in perimenopausal-a study of 648 women in Maitreyi's healthcare programme. Obstet Gynecol Today. 2004;9:671. [Google Scholar]
- 94.Shaji KS, Jotheeswaran AT, Girish N, Bharath S, Dias A, Pattabiraman M, et al. Alzheimer's and Related Disorders Society of India. The Dementia India Report: Prevalence, impact, costs and services for Dementia. 2010 [Google Scholar]
- 95.Roepke SK, Ancoli-Israel S. Sleep disorders in the elderly. Indian J Med Res. 2010;131:302–10. [PubMed] [Google Scholar]
- 96.Consensus Development Conference: Prophylaxis and treatment of osteoporosis. Am J Med. 1991;90:107–10. doi: 10.1016/0002-9343(91)90512-v. [DOI] [PubMed] [Google Scholar]
- 97.NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7-29, 2000: Highlights of the conference. South Med J. 2001;94:569–73. [PubMed] [Google Scholar]
- 98.Kanis JA. Shiffield: World Health Organisation Scientific Group, WHO Collaboration Centre for Metabolic Bone Diseases University of Shiffield; 2008. Assessment of osteoporosis at the primary health care level, 2008. [Google Scholar]
- 99.Dawson-Hughes B National Osteoporosis Foundation Guide Committee. A revised clinician's guide to the prevention and treatment of osteoporosis. J Clin Endocrinol Metab. 2008;93:2463–5. doi: 10.1210/jc.2008-0926. [DOI] [PubMed] [Google Scholar]
- 100.Delhi: 2011. National Institute of Nutrition. ICMR, Dietary Guidelines for Indians: A Manual; p. 89. [Google Scholar]
- 101.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, et al. Guidelines for preventing and treating vitamin D deficiency and insufficiency revisited. J Clin Endocrinol Metab. 2012;97:1153–8. doi: 10.1210/jc.2011-2601. [DOI] [PubMed] [Google Scholar]
- 102.Recommended Dietary Allowances. 10th ed. Washington, DC: National Academy Press; 1989. Subcommittee on the Tenth Edition of the Recommended Dietary Allowances, Food and Nutrition Board. [Google Scholar]
- 103.Chopra A, Patil J, Bilampelly V. The Bhigwan (India) COPCORD: Methodology and first information report, APLAR. J Rheumatol. 1997;1:145–54. [Google Scholar]
- 104.Chopra A, Patil J, Billempelly V, Relwani J, Tandle HS WHO-ILAR COPCORD Study. WHO International League of Associations from Rheumatology Community Oriented Program from Control of Rheumatic Diseases. Prevalence of rheumatic diseases in a rural population in western India: A WHO-ILAR COPCORD Study. J Assoc Physicians India. 2001;49:240–6. [PubMed] [Google Scholar]
- 105.Mahajan A, Jasrotia DS, Manhas AS, Jamwal SS. Prevalence of major rheumatic disorders in Jammu. JK Science. 2003;5:63–6. [Google Scholar]
- 106.Dandona L, Dandona R, Srinivas M, Giridhar P, Koxvai V. Blindness in the Indian State of Andhra Pradesh. Invest Ophthalmol Vis Sci. 2001;42:908. [PubMed] [Google Scholar]
- 107.Dikshit R, Gupta PC, Ramasundarahettige C, Gajalakshmi V, Aleksandrowicz L, Badwe R, et al. Cancer mortality in India: A nationally representative survey. Lancet. 2012;379:1807–16. doi: 10.1016/S0140-6736(12)60358-4. [DOI] [PubMed] [Google Scholar]
- 108.Ferlay JB, Pisani P, Parkin DM. Globocan 2000: Cancer Incidence, Mortality and Prevalence Worldwide. Version 1.0. 2001 [Google Scholar]
- 109.Ferlay JS, Bray F, Forman D, Mathers C, Parkin DM. Globocan 2008 v1.2. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 10. 2010. [Last cited on 2011 Oct 25]. Available from: http://www.globocan.iarc.fr .
- 110.Agarwal G, Ramakant P. Breast Cancer Care in India: The Current Scenario and the Challenges for the Future. Breast Care (Basel) 2008;3:21–7. doi: 10.1159/000115288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Agarwal G, Ramakant P, Forgach ER, Rendón JC, Chaparro JM, Basurto CS, et al. Breast cancer care in developing countries. World J Surg. 2009;33:2069–76. doi: 10.1007/s00268-009-0150-z. [DOI] [PubMed] [Google Scholar]
- 112.Two-year report of the population based cancer registries 2004-2005. Incidence and Distribution of Cancer. 2008 [Google Scholar]
- 113.Yeole BB, Jayant K, Jussawalla DJ. Trends in breast cancer incidence in greater Bombay: An epidemiological assessment. Bull World Health Organ. 1990;68:245–9. [PMC free article] [PubMed] [Google Scholar]
- 114.Programme NCR. Bangalore, India: Indian Council of Medical Research; 2009. Time Trends in Cancer Incidence Rates 1982-2005. [Google Scholar]
- 115.Kakarala M, Rozek L, Cote M, Liyanage S, Brenner DE. Breast cancer histology and receptor status characterization in Asian Indian and Pakistani women in the U.S. – A SEER analysis. BMC Cancer. 2010;10:191. doi: 10.1186/1471-2407-10-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Lyon, France: IARC Cancer Base No. 10; International Agency for Research on Cancer; 2010. [Last accessed on 2012 Nov 01]. GLOBOCAN 2008. Cancer Incidence and Mortality Worldwide. Available from: http://www.globocan.iarc.frGlobocan 2008 . [Google Scholar]
- 117.Vallikad E. “Cervical Cancer: The Indian Perspective”. International J Gynaecol Obstet. 2006;95:S215–33. doi: 10.1016/S0020-7292(06)60037-4. [DOI] [PubMed] [Google Scholar]
- 118.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu India: A cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 119.Shastri SS. PI-Mumbai trial for Breast and Cervical Cancer Screening, NIH RO1 awarded study Unpublished personal communication dated Oct 1. 2011 [Google Scholar]
- 120.Sankaranarayanan R, Basu P, Wesley RS, Mahe C, Keita N, Mbalawa CC, et al. Accuracy of visual screening for cervical neoplasia: Results from an IARC multicentre study in India and Africa. Int J Cancer. 2004;110:907–13. doi: 10.1002/ijc.20190. [DOI] [PubMed] [Google Scholar]
- 121.Sankaranarayanan R, Wesley R, Thara S, Dhakad N, Chandralekha B, Sebastian P, et al. Test characteristics of visual inspection with 4% acetic acid (VIA) and Lugol's iodine (VILI) in cervical cancer screening in Kerala, India. Int J Cancer. 2003;106:404–8. doi: 10.1002/ijc.11245. [DOI] [PubMed] [Google Scholar]
- 122.Sankaranarayanan R, Shastri SS, Basu P, Mahé C, Mandal R, Amin G, et al. The role of low-level magnification in visual inspection with acetic acid for the early detection of cervical neoplasia. Cancer Detect Prev. 2004;28:345–51. doi: 10.1016/j.cdp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 123.Sankaranarayanan R, Gaffikin L, Jacob M, Sellors J, Robles S. A critical assessment of screening methods for cervical neoplasia. Int J Gynaecol Obstet. 2005;89:S4–S12. doi: 10.1016/j.ijgo.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 124.Arbyn M, Sankaranarayanan R, Muwonge R, Keita N, Dolo A, Mbalawa CG, et al. Pooled analysis of the accuracy of five cervical cancer screening tests assessed in eleven studies in Africa and India. Int J Cancer. 2008;123:153–60. doi: 10.1002/ijc.23489. [DOI] [PubMed] [Google Scholar]
- 125.Goldie SJ, Gaffikin L, Goldhaber-Fiebert JD, Gordillo-Tobar A, Levin C, Mahé C, et al. Cost-effectiveness of cervical-cancer screening in five developing countries. N Engl J Med. 2005;353:2158–68. doi: 10.1056/NEJMsa044278. [DOI] [PubMed] [Google Scholar]
- 126.Legood R, Gray AM, Mahé C, Wolstenholme J, Jayant K, Nene BM, et al. Screening for cervical cancer in India: How much will it cost? A trial based analysis of the cost per case detected. Int J Cancer. 2005;117:981–7. doi: 10.1002/ijc.21220. [DOI] [PubMed] [Google Scholar]
- 127.Usha Rani P, Meeta, Batool L, Anjaneyulu V, Murthy Ramana Reddy CH. Kolkotta, Souvenir: 9th National Indian Menopause Society Meeting; 2008. Evaluation of cervical smears by the Betesda System for cervical screening in postmenopausal women. abstract. [Google Scholar]
- 128.Mumbai: IIPS; 2000. International Institute for Population Sciences and ORC Macro. National Family Health Survey (NFHS-2), 1998-99. [Google Scholar]
- 129.Bipasa S. Chennai: 15th National Indian Menopause Society Meeting; 2010. First Time Detection of Medical Disorders in Forty-Plus Women in Health Camps; p. 68. Abstract. [Google Scholar]
- 130.Santhakumar K, Nivedita Bharati K, Jaishree Gajraj A, Samundeswari V. Souvenir, Chennai: 15th National Indian Menopause Society Meeting; 2010. Routine Investigations in Women 40 Years and Above; p. 69. [Google Scholar]
- 131.Savithri P. Hyderabad: 7th National Indian Menopause Society Meeting; 2002. Screening Before and During the Use HRT-Cost Effectiveness; p. 77. [Google Scholar]
- 132.Ranade S. Is Treatment Seeking Behaviour Specific to Socioeconomic Status in the Middle Aged Women? Obstet Gynecol Today. 2004;9:9. [Google Scholar]
- 133.Hina K, Manju T, Mehandale SS, Wagh GN. Faridabad, Souvenir: 17th National Indian Menopause Society; 2012. Mid life management clinic for screening of metabolic disorders and osteoporsis in women aged above 35 yrs; p. 45. [Google Scholar]
- 134.Kakkar V, Arshdeep K, Darshanjot K, Kanwaljit C, Indu Pal K. Chandigarh: 11th National Indian Menopause Society Meeting; 2003. Combined Effect of Drug Therapy and Counselling in the Relief of Menopause Symptoms; p. 209. [Google Scholar]
- 135.Bharati A. Chennai, Souvenir: 15th National Indian Menopause Society Meeting; 2010. Awareness of Health Check up in Peri-menopausal Women; p. 67. [Google Scholar]
- 136.Nimala V. Rajkot, Souvenir: National Indian Menopause Society Meeting; 2007. Counselling 12th women: Is it worth the time; p. 34. [Google Scholar]
- 137.Asha D, Shitole . Faridabad, Souvenir: 17th National Indian Menopause Society Meeting; 2012. Counselling-A basic management of perimenopaue and menopause period; p. 35. [Google Scholar]
- 138.Laxmi S. Rajkot, Souvenir: 12th National Indian Menopause Society Meeting; 2007. Cinical effects of soya flour on menopausal symptoms; p. 24. [Google Scholar]
- 139.Meeta . Faridabad, Souvenir: 17th National Indian Menopause Society; 2012. Risk analysis of cardiovascular disease and osteoporosis in Indian menopausal women and its relationship with blood lycopene levels; p. 39. [Google Scholar]
- 140.Pinaki C, Sanhati . 1st ed. Developed and Published by: National Coordination Committee, Jan Swasthya Abhiyan; 2006. Oct, Health care in India. September 1, 2009, Health System in India: Crisis and Alternatives. [Google Scholar]


