Abstract
Background:
Drosera peltata Smith var. glabrata Y.Z.Ruan, a kind of wild carnivorous plants in the family Droseraceae, has been used for the treatment of rheumatism and bruises in Chinese folk. None of compounds in this herb has been quantified in the previous studies.
Objective:
To develop a validated and reliable HPLC method for the simultaneous determination of two bioactive constituents – quercetin and plumbagin, and establish a simple UV spectrophotometry method for the analysis of total flavonoids content.
Materials and Methods:
Chromatographic separation was performed by using a HPLC system consisting of an Agilent Eclipse XDB C18 column and a gradient elution system of acetonitrile and water (containing 0.1% phosphoric acid, V/V) within 20 minutes. Comparing with quercetin complex with Al(NO3)3, the total flavonoids were determined by UV spectrophotometry at 269 nm.
Results:
Both methods were validated for linearity (r2≥0.9994 for quercetin and plumbagin in the HPLC method, r2 = 0.9994 for quercetin in the UV spectrophotometry method), precision (The within-day and between-day variability was less than 0.738% and 1.64% for quercetin and plumbagin in the HPLC method, and was less than 1.67% for quercetin in the UV spectrophotometry method.) and recovery (The recoveries of the HPLC method were 96.7-100.4% and 97.4-100.4% for quercetin and plumbagin, respectively, and the recovery of the UV spectrophotometry method was 96.7-99.6% for quercetin.)
Conclusion:
The proposed methods are simple and accurate, and could be practiced to rapidly determine quercetin, plumbagin and total flavonoids in the herbal drug, which provide effective approaches for quality control.
Keywords: Drosera peltata Smith var. glabrata Y.Z.Ruan, high-performance liquid chromatography, plumbagin, quercetin, total flavonoids, UV spectrophotometry
INTRODUCTION
Drosera peltata Smith var. glabrata Y.Z.Ruan (Mao Gao Cai in Chinese), which occurs mainly in the Yangtze River and the southeastern provinces of China, belongs to insectivorous plant of the family Droseraceae.[1] It's a traditional folk medicinal herb for treating rheumatism, malaria, bruise, scabies and tumor.[2] The whole herb contains the quinones such as plumbagin, droserone, hydroxydroserone and the flavonoids such as quercetin, gossypetin, isoquercitrin, quercetin-3-O-(6″-O-galloyl)-β-D-glucoside, quercetin-3-digalactoside.[3,4] Although the pharmacological effects of Drosera peltata Smith var. glabrata Y.Z.Ruan are noteworthy, none of compounds in this drug has been quantified in the previous studies.
It has been demonstrated that quercetin can inhabit tumor and platelet aggregation,[5] while plumbagin has significant antibacterial, antiviral and anticancer effect.[6] Since some of the therapeutic properties of Drosera peltata Smith var. glabrata Y.Z.Ruan are mainly due to the presence of these two compounds [Figure 1], their contents were measured simultaneously by a validated and rapid HPLC method in the present paper. At the same time, comparing with quercetin complex with Al(NO3)3, the total flavonoids as the representative chemical effective parts in Drosera peltata Smith var. glabrata Y.Z.Ruan, were determined by an UV spectrophotometry method.
Figure 1.
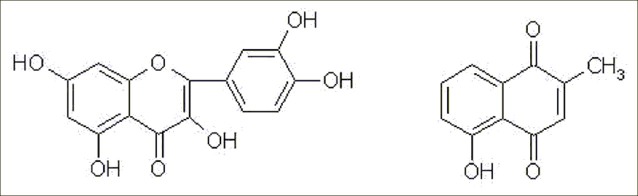
The chemical structures of quercetin and plumbagin (1) quercetin (2) plumbagin
MATERIALS AND METHODS
Plant material
The herbal drug Drosera peltata Smith var. glabrata Y.Z.Ruan used for this research was from HaoZhou LaoYiTang Chinese Herbal Drugs Co. Ltd. (Anhui province, China, batch number: 20110815), and was identified by Shuili Zhang, the associate professor from Zhejiang Chinese Medical University.
Reagents
Quercetin was obtained from National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China, batch number: 100081-200907). Plumbagin was made from Drosera peltata Smith var. glabrata Y.Z.Ruan in our laboratory. Methanol and phosphoric acid of analytical grade were supplied by Yongda Chemical Reagents Company (Tianjin,China) and aluminum nitrate of analytical grade was purchased from Zhenxin Chemical Reagents Company (Shanghai, China). HPLC-grade acetonitrile was supplied by TEDIA (Fairfleld, OH, USA), and water was purified by using a Milli-Q ultra-water system (Billerica, MA, USA).
Preparation of plumbagin standard
The powdered plant material Drosera peltata Smith var. glabrata Y.Z.Ruan was extracted by steam distillation, then the distillate was partitioned with petroleum ether (30~60°C). The extract solution was vaccum evaporated at 45°C. The residue was dissolved and recrystallized with anhydrous ethanol. The structure of the yellow-brown crystal was confirmed by comparison of the physical and spectral data with the reported data as plumbagin: molecular formula C11 H8 O3, molecular weight 188, mp 76~77°C; UVλmax (EtOH) 265nm (logε4.10), 415nm (logε3.58); IR (KBr) 3448 cm-1 (-OH), 2964 cm-1, 1644 cm-1 (-C=O), 1455 cm-1, 1365 cm-1, 753 cm-1 ;1 H-NMR (CDCl3)δppm 2.20 (3H, s, 2-CH3), 6.80 (1H, s, H-3), 7.25 (1H, d, H-6), 7.60 (2H, t, J=5.7, H-7,8), 11.96 (1H, s, -OH); EI-MS (m/z): 188 (M+, 100), 173 (M-CH3), 160 (M-CO), 145, 131, 120, 114, 103,[7] while its purity was further appraised by HPLC that has been achieved 98.9% in Area Percentage method.
HPLC: An Agilent Technologies 1200 series equipped with a G1322A on-line degasser, a G1311A quarternary pump, a G1316A column oven, a G1329A autosampler, a G1314B variable wavelength detector and Agilent Rev. B.04.01 chemstation for data analysis was used. An Agilent Eclipse XDB C18 column (4.6 × 150 mm, 5 μm) was eluted in gradient mode with mobile phase A as acetonitrile and mobile phase B consisting of water and phosphoric acid (100:0.1 v/v). The linear gradients between the time points were as follows: 0-5 min, 35-40%A; 5-8min, 40-50%A; 8-20 min, 50%A. The UV detection wavelength was set at 370 nm (the maximum absorption wavelength of quercetin) in the first 10 min, then was transferred to 268 nm (the maximum absorption wavelength of plumbagin) after 10 min. The flow rate was 0.8 ml/min and the column temperature was maintained at 25°C.
UV: The determination was carried out on an UV-2450 UV-Vis spectrophotometer (Shimadzu, Suzhou, China).
High performance liquid chromatographic quantification of quercetin and plumbagin
Preparation of mixed standard solution
Stock mixed standard solution with a concentration of 0.28mg/ml for quercetin and a concentration of 0.30mg/ml for plumbagin was prepared by methanol. From the stock solution, five calibration standard solutions were prepared by dilution with methanol.
Sample Preparation for high-performance liquid chromatography
For the preparation of sample, 5.0g powdered drug was accurately weighed and extracted by 50ml methanol for 60min two times. Then the extract was filtered and transferred quantitatively to a 100-ml measuring flask. After diluted to the volume with methanol, the solution was through 0.45μm syringe filter and injected 10μl.
Validation of the analytical method
The method was validated through calibration curve range, precision, stability and recovery.
The calibration curves were generated to evaluate the linear relationship between the peak areas and the concentrations of plumbagin and quercetin. Analysis of mixed standard solutions at each concentration was performed in duplicate. Slope, intercept, and coefficient of determination (r2) were calculated as regression parameters by weighted linear regression.
To determine the within-day precision of the method, the mixed standard solution with a concentration of 0.042 mg/ml for quercetin and a concentration of 0.045 mg/ml for plumbagin was analyzed five times on the same day. To determine the between-day precision, this mixed standard solution was run on each of five consecutive days.
In short-term stability, sample was analyzed after 0h, 2h, 4h, 8h, 12h, 24h at room temperature.
Recovery experiment was performed to evaluate the accuracy of the method. The recovery was investigated by analyzing six individual samples, and was determined by comparing the peak area of the analytes in the spiked samples.
UV spectrophotometric quantification of total flavonoids[8]
Preparation of standard solution
A stock quercetin standard solution of 0.038mg/ml was prepared by dissolving quercetin reference material in methanol.
Sample Preparation for UV spectrophotometry
The herbal drug methanol extract prepared for HPLC was diluted ten times by methanol, which was used as the sample solution for UV spectrophotometry.
Procedures for the determination of total flavonoids
A total of 3.0ml sample solution was pipetted into a 25- ml volumetric flask, and was treated with 4.0 ml of the 10% Al(NO3)3 solution and evenly mixed. The mixture was diluted to the volume with methanol, and allowed to stand for 10min before analyzing against the blank solution.
Selection of the detection wavelength
The 200-400nm absorption spectra of the standard solution and sample extraction complexed with Al(NO3)3 were obtained. Both of them had maximum absorption at 269nm which was chosen as the detection wavelength.
Validation of the analytical method
The validation procedure of the UV spectrophotometry method was quite similar with the HPLC method except the absorbance value substituting for the peak area.
RESULTS AND DISCUSSION
Chromatographic separation and conditions
Using the HPLC condition, plumbagin and quercetin were well separated from other compounds in sample, and their peaks displayed excellent peak resolution and symmetry. The retention time was about 5.1min and 15.1min for two compounds [Figures 2 and 3].
Figure 2.
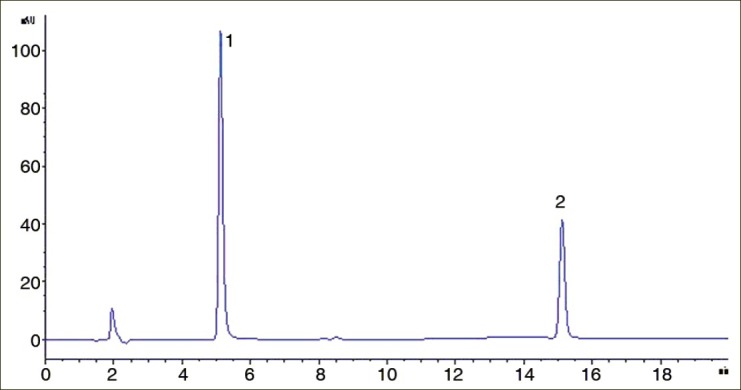
HPLC chromatogram of quercetin and plumbagin in standard solution (1) quercetin (2) plumbagin
Figure 3.
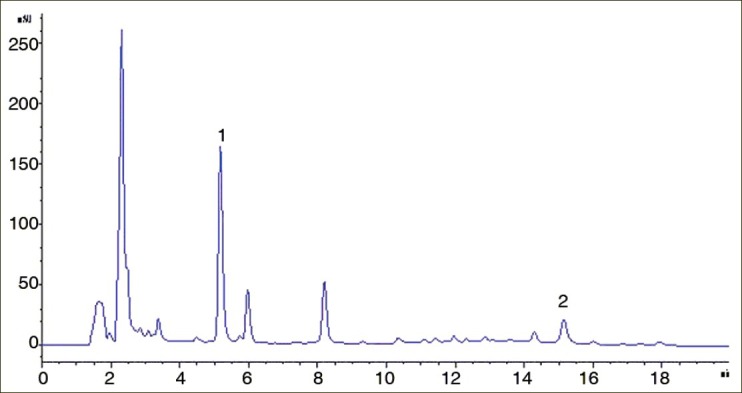
HPLC chromatogram of quercetin and plumbagin in sample solution
Calibration curves
HPLC: The standard curves for quercetin and plumbagin were linear over the range 0.014-0.070mg/ml and 0.015-0.075mg/ml, which could be described by the regression equation: Y=32425.21X+7.376 (r2=0.9996) for quercetin and Y=16104.82X-25.7863 (r2=0.9994) for plumbagin, where Y was the peak area of the analyte, and X was the concentration in mg/ml.
UV: The linearity of the method was tested by analyzing different amounts of the standard solution coupled with complexometric reagent according to the procedures for the determination of total flavonids. A good linear response was shown (r2=0.9994) over the range of 3.040-7.600 μg/ml, with an equation Y=0.0676X-0.0055, in which Y meant the absorbance value while X represented the concentration of the quercetin solution in μg/ml.
Precision
The within-day and between-day assay precision (expressed as RSD%) was less than 0.738 and 1.64% for quercetin and plumbagin respectively in the HPLC method [Table 1], and were less than 1.67% for quercetin in the UV spectrophotometry method [Table 2]. The results demonstrated that the corresponding assay methods are reliable and reproducible.
Table 1.
The within-day and between-day precision and stabilty for quercetin and plumbagin in highperformance liquid chromatography method
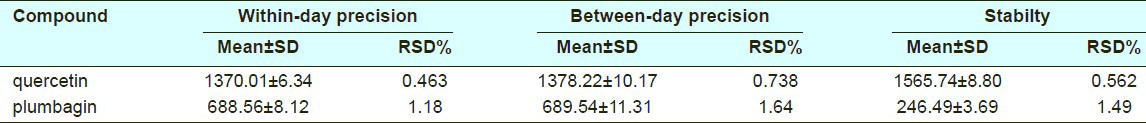
Table 2.
The within-day and between-day precision and stabilty for quercetin in UV spectrophotometry method

Stability
Analytes in samples were stable for HPLC determination at room temperature for 24h (RSD% were within 1.49% deviation) [Table 1]. But the complexed sample solution for UV analysis was stable at room temperature for 12h (RSD% was 2.31% deviation) [Table 2].
Recovery
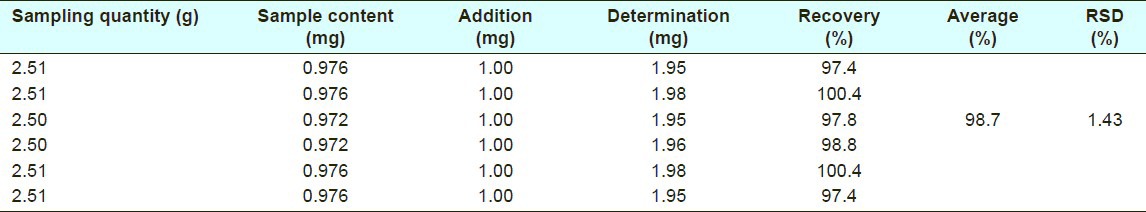
The recoveries of the HPLC method were 96.7-100.4% and 97.4-100.4% (n=6) for quercetin and plumbagin, respectively, and the average values were 98.1% (RSD%=1.32%) and 98.7% (RSD%= 1.43%) [Tables 3 and 4].
Table 3.
Recovery of quercetin in high-performance liquid chromatography method
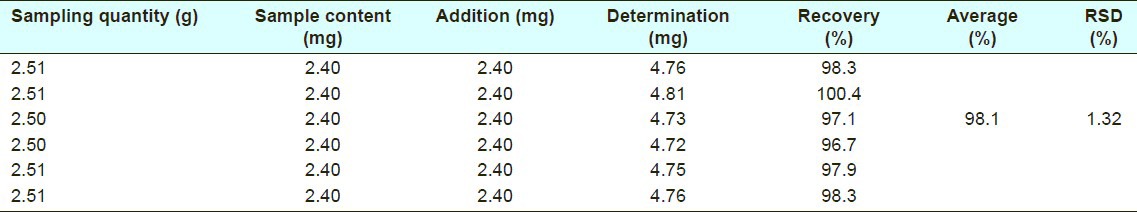
Table 4.
Recovery of plumbagin in high-performance liquid chromatography method
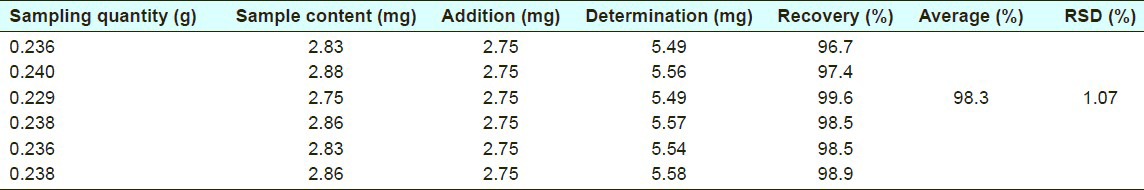
The recovery of the UV spectrophotometry method was 96.7-99.6% for quercetin, and the average value was 98.3% (RSD%=1.07%) [Table 5].
Table 5.
Recovery of quercetin in UV spectrophotometry method
Sample analysis
The powdered Drosera peltata Smith var. glabrata Y.Z.Ruan samples were weighed and treated according to sample preparation and the present HPLC or UV assay methods.
The results showed that the average contents of quercetin and plumbagin in Drosera peltata Smith var. glabrata Y.Z.Ruan are 0.0958% and 0.0389% (n=3), respectively, while the total flavonoids content is 1.20% (n=3).
CONCLUSION
Droserae herbal drugs are remarkable for their anticarcinogenic constituents such as quercetin and plumbagin. In the present research, quercetin and plumbagin of Drosera peltata Smith var. glabrata Y.Z.Ruan were determined simultaneously by a fast and sensitive HPLC method, and the total flavonoids were also analyzed by a simple UV method for the first time, whereby the chemical composition or the herbal quanlity could be evaluated. The developed methods herein would prove to be applied to intrinsic quality control of this drug or other medicinal preparations containing quercetin and plumbagin.
ACKNOWLEDGEMENTS
This research was sponsored by National Nature Science Foundation of China (No. 81102734), Zhejiang Province Key Science and Technology Innovation Team (2012R10044-06), Scientific Research Fund of Zhejiang Provincial Education Department (No. Y201120445) and Zhejiang Provincial Natural Science Foundation of China (No. Z2101201).
Footnotes
Source of Support: National Nature Science Foundation of China (No. 81102734), Zhejiang Province Key Science and Technology Innovation Team (2012R10044-06), Scientific Research Fund of Zhejiang Provincial Education Department (No. Y201120445) and Zhejiang Provincial Natural Science Foundation of China (No. Z2101201)
Conflict of Interest: None declared.
REFERENCES
- 1.Juniper R, Joel DM. New York: Academic Press; 1989. The Carnivorous Plants. [Google Scholar]
- 2.Editorial Committee of Flora of China, Chinese Academy of Sciences. Beijing: Science Press; 1984. Flora of China; pp. 15–28. [Google Scholar]
- 3.Wang QA, Su JY, Ceng LM. Study on chemical constituents in Lunate Peltate Sundew Herb from Tibet. China J Chinese Mat Med. 1998;23:683–5. [Google Scholar]
- 4.Hu XB, Yang PQ, Liu WJ. Study on flavonoids in Lunate Peltate Sundew Herb from Tibet. Chinese Trad Herbal Drugs. 1994;25:49–50. [Google Scholar]
- 5.Murakami A, Ashida H, Terao J. Multitargeted cancer prevention by quercetin. Cancer Lett. 2008;269:315–25. doi: 10.1016/j.canlet.2008.03.046. [DOI] [PubMed] [Google Scholar]
- 6.Hsu YL, Cho CY, Kuo PL, Huang YT, Lin CC. Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2 -terminal kinase-mediated phosphorylation at serine15 in vitro and in vivo. J Pharmacol Exp Ther. 2006;318:484–94. doi: 10.1124/jpet.105.098863. [DOI] [PubMed] [Google Scholar]
- 7.Du ZX. Determination on plumbagin in fresh and dry stem of Plumbago zeyanica L. J Anhui Agri Sci. 2009;37:16363–4. [Google Scholar]
- 8.Chen YJ, Wang J, Wan DR. Determination of total flavonoids in three Sedum crude drugs by UV–Vis spectrophotometry. Pharmacog Mag. 2010;6:259–63. doi: 10.4103/0973-1296.71784. [DOI] [PMC free article] [PubMed] [Google Scholar]


