Abstract
Context:
As a traditional Uygur medicinal plant, Z. clinopodioides Lam has various uses in Xinjiang.
Aims:
A reversed-phase rapid resolution liquid chromatography (RP-RRLC) method with diode array detector (DAD) was developed for simultaneous determination of diosmin, linarin, and pulegone from Ziziphora clinopodioides Lam, a widely used in traditional Uygur medicine for treating heart disease, high blood pressure, and other diseases.
Settings and Design:
Compounds were separated on a XDB-C18 reversed-phase analytical column (50 mm × 4.6 mm, 1.8 μm) with gradient elution using methanol and 1% aqueous acetic acid (v/v) at 0.9 mL/min. he detection wavelength was set at 270 nm.
Materials and Methods:
Ziziphora clinopodioides Lam. were collected from ten different origins in Xinjiang, including the Ban fang ditch, Tuoli, the Altay mountains, Terks, Xiata Road, Zhaosu Highway, Guozigou, Fukang, Jimsar, Wulabo.
Statistical Analysis Used:
The intra-day and inter-day precisions of all three compounds were less than 0.89% and the average recoveries ranged from 97.4 to 104.1%. There were highly significant linear correlations between component concentrations and specific chromatographic peak areas (R2 > 0.999).
Results:
The proposed method was successfully applied to determine the levels of three active components in Z. clinopodioides Lam. samples from different locations in Xinjiang.
Conclusions:
The proposed method is simple, consistent, accurate, and could be utilized as a quality control method for Z. clinopodioides Lam.
Keywords: Diosmin, linarin, pulegone, reversed-phase-rapid resolution liquid chromatography-diode array detector, Ziziphora clinopodioides Lam
INTRODUCTION
Herbal medicines have been used over many centuries in Asia and have become more popular worldwide in recent decades. Medicinal herbs may contain hundreds of complex active components, and it is often impractical to identify all these substances by quantitative analysis[1] Therefore, we can only determine the composition is relatively high, and have bioactive ingredients in order to quality control. We decided to develop a Rapid Resolution LC method suitable for determination of different compounds in crude extracts of selected medicinal herbs, on a short C18 analytical column packed with 1.8 μm silica-based particles, using methanol as an organic solvent in a binary mobile phase system.
Traditional Uygur medicines are natural therapeutic agents used in accordance with the guiding theory of traditional Uygur medical science. They have been widely used in China since antiquity for prevention and treatment of diseases. Ziziphora clinopodioides Lam of the family Lamiaceae is indigenous to China, Mongolia, Turkey, Kazakhstan, and Kyrgyzstan. It is a semi-perennial shrub-like plant that grows on low hills, grasslands, and arid slopes.[2] As a traditional Uygur medicinal plant, Z. clinopodioides Lam has various uses in Xinjiang, including the treatment of heart disease, high blood pressure, asthma hyperhidrosis, palpitation insomnia, edema, cough, bronchitis, and lung abscess.[3] Several studies have revealed that it has a wide range of antimicrobial[4,5] and antioxidant[6] effects. Senejoux and others also explored the vasodilating effects of Z. clinopodioides Lam and the underlying mechanisms.[7] Till date, research on Z. clinopodioides Lam has focused mainly on the chemical constituents of its essential oils and their bioactive constituents, of which pulegone is considered the main ingredient.[8,9,10] In preliminary studies, our research group studied the stability of the plant's essential oils,[11] inhibit bacterial activity screening, and volatile oil chemical composition analysis of Z. clinopodioides Lam,[12] determined its oleanolic acid and ursolic acid content by HPLC[9] and simultaneous determination of caffeic acid and rosmarinic acid in Z. clinopodioides Lam from different sources in Xinjiang.[13] Moreover, we investigated the total polyphenolic and flavonoid content as well as the antioxidant activity of Z. clinopodioides Lam extracts of different polarity[6] and determination of ten metal elements in Z. clinopodioides Lam by microwave digestion-FAAS.[14] Other components in Z. clinopodioides Lam are caffeic acid, rosmarinic acid and oleanolic acid, ursolic acid, salylic acid, and flavonoids.[15] Flavonoids are known to possess potent anti-inflammatory activity in both humans and animals, and recently their topical application has met with considerable interest.[16,17] Diosmin and linarin are common naturally occurring flavonoids with a number of interesting biological activities. As a flavonoid, diosmin [Figure 1a] also exhibits anti-inflammatory, free-radical scavenging, and antimutagenic properties,[18] linarin [Figure 1b] is believed to possess parasiticide, anti-microbial, analgesic, anti-viral, anti-proliferative, anti-hypertensive, anti-oxidant, and anti-inflammatory properties.[19] These pharmacological effects are consistent with the herbs of Z. clinopodioides Lam. Pulegone [Figure 1c], a monoterpene hydrocarbon reported to be one of the major active components of Z. clinopodioides Lam, can act against Gram-positive and Gram-negative bacteria. It also has a wide range of antimicrobial and antioxidant effects.[20] We develop an RRLC method that allows for the simultaneous determination of at least three of these putative bioactive ingredients, diosmin, linarin, and pulegone. This method may form the basis for a more efficient analytic procedure to assess the medicinal quality of Z. clinopodioides Lam samples and as a preparative aid for future studies on therapeutic mechanisms.
Figure 1.
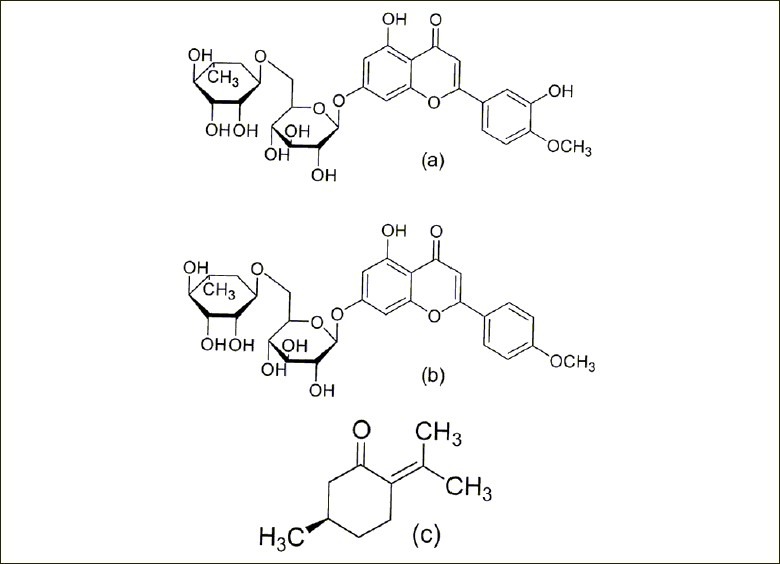
Chemical structures of the three bioactive components in Z. clinopodioides Lam
MATERIALS AND METHODS
Materials and reagents
Whole plant samples were collected from ten different origins in Xinjiang. Samples from the Ban fang ditch, Tuoli, the Altay mountains, Terks, Xiata Road, Zhaosu Highway, and Guozigou were obtained in July, 2010 (NO:ZYMZY20100701-07). Samples from Fukang and Jimsar were collected in August, 2009 (NO:ZYMZY20090801) and the samples from Wulabo were collected in July, 2008 (NO:ZYMZY20080701-02). All specimens were stored in the Traditional Chinese Medicine Ethnical Herbs Specimen Museum of Xinjiang Medical University. The plant materials were identified by Yonghe Li, a chief apothecary of the Chinese Medicine Hospital of Xinjiang. The standards of pulegone were purchased from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). The standards of diosmin and linarin ware purchased from Sigma (USA). Acetic acid of analytical grade was obtained from Tianjin, Fuyu Chemical Reagents Company. Reverse phase RRLC grade methanol was supplied by Fisher Scientific (USA) and water was obtained from a Millipore Q3 ultra-pure water system (Millipore, USA).
Rapid resolution liquid chromatography analysis
Analyses were carried out on an Agilent 1200 system with diode array detector (DAD). The detection wavelength was set at 270 nm. An Agilent XDB-C18 column (50 mm × 4.6 mm, 1.8 μm) was used with a flow rate of 0.9 mL/min. The injection volume was 5μl and the column temperature was maintained at 30°C. The mobile phase consisted of solvent A (1% acetic acid in water) and solvent B (methanol), using gradient elution as follows: 0–10 min, 62–52% A; 10–20 min, 52–40% A.
Preparation of standard solutions
Each standard of diosmin, linarin, and pulegone were accurately weighed, dissolved in methanol:DMSO (3:1 v/v) and diluted to the appropriate concentration for analysis. All stock solutions were stored at 4°C.
Preparation of samples
The herbal samples of Z. clinopodioides Lam. were first crushed into coarse powder. The pulverized powder (0.2000 g) was added to methanol-DMSO in a 30 ml Erlenmeyer flask. For chemical extraction, the mixture was first left at room temperature for 1 h and then ultrasonicated for 30 min. The solution was then filtered through the 0.22 μm membrane filter.
RESULTS
Method development and optimization
To obtain chromatograms with well-resolved peaks and minimal analysis time per run, chromatographic conditions were optimized, including the mobile phase, column temperature, and flow rate. Various mixing ratios of water to methanol as the mobile phase were used but no satisfactory separation was achieved. It was found that the presence of acids in the mobile phase enhanced the resolution. The results showed that 1% acetic acid buffer in the mobile phase significantly improved the retention behavior and peak shape of the different components in Z. clinopodioides Lam. However, using isocratic elution, the three compounds could not be separated effectively and so gradient elution was used throughout the study. Other chromatographic variables were also optimized, including column temperatures (25, 30, or 35°C) and flow rate (0.6, 0.8, 0.9, and 1.0 mL/min). Eventually, the optimal separation was achieved at a column temperature of 30°C and flow rate of 0.9 mL/min.
To determine the appropriate wavelength for simultaneous determination of diosmin, linarin, and pulegone solutions, standards were injected into the RRLC system and the UV spectra measured over the range 190 to 400 nm by DAD. The UV spectra of all three compounds showed the same absorption maximum at 270 nm.
Extraction efficiency of different solvents, including methanol-DMSO (75:25, v/v), methanol, methanol-water (50:50, v/v), ethanol, and ethanol-water (50:50, v/v), were compared. Diosmin and linarin were difficult to dissolve in methanol, but DMSO as a co-solvent helped in the separation.
A typical RRLC chromatogram is shown in Figure 2. Retention times for the three compounds were 3.66 min (diosmin), 7.98 min (linarin), and 14.23 min (pulegone).
Figure 2.
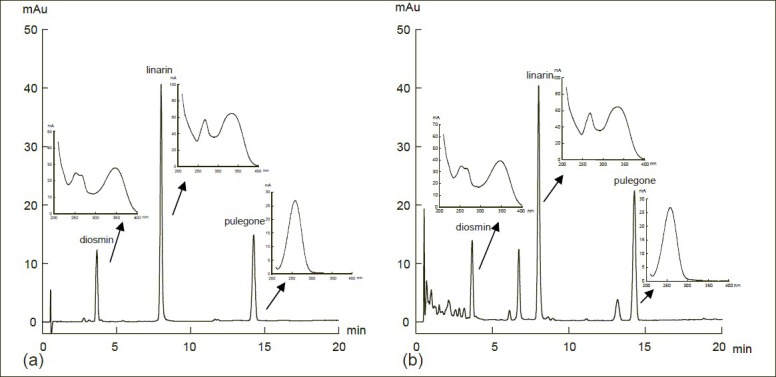
A typical rapid resolution liquid chromatography chromatogram and UV spectra of Z clinopodioides Lam. (a) Standard chromatogram; (b) sample chromatogram
System suitability
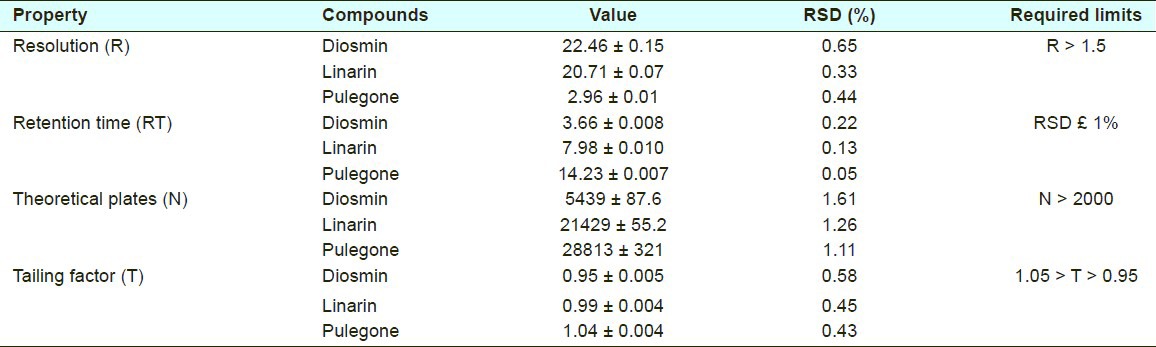
System suitability tests are an integral part of method development and are used to ensure adequate performance of the chromatographic system. Resolution (R), retention time (RT), number of theoretical plates (N), and tailing factor (T) were evaluated in five replicate injections of the drugs at 5μl. As shown in Table 1, all parameters were within acceptable limits.
Table 1.
System-suitability data. Mean ± SD, (n = 5)
Method validation
Linearity, limits of detection, and quantification
Regression analyses were performed using GraphPad Prism 4.00. The correlation coefficient r2 and linear regression equations were computed by the partial least square method and the quality of the curve fit obtained was assessed. The linear calibration curves were constructed from five concentration assays performed in triplicate. The linear regression equation was Y = aX + b, where Y and X are the values of peak area and concentration of the reference compound, respectively. The results of the regression analyses and the calculated correlation coefficients (r2) are listed in Table 2. The high correlation coefficient values (r2 > 0.999) indicated good linearity between peak areas (Y) and compound concentrations (X, mg/mL) over relatively wide concentration ranges.
Table 2.
Statistical results of linear regression equation analysis in the determination of the three investigated compounds

The limit of quantification (LOQ) was defined as the lowest concentration with peak height 10 times the baseline noise (S/N = 10). The minimum detectable concentration, as defined by a signal-to-noise ratio (S/N) of 3, was considered to be the limit of detection (LOD). The LOQ and LOD values for the three chemical components are also listed in Table 2.
Stability and precision
Intra-day and inter-day precision and accuracy were evaluated by analyzing quality-control samples. The intra-day variation was examined by analyzing five individual sample solutions from the same crude sample of Z. clinopodioides Lam on the same day. Inter-day precision and accuracy were determined by once daily trials for three consecutive days. Variations were expressed as relative standard deviation (RSD). The values of the intra-and inter-day variations were less than 2.0%. The instrumental precision was evaluated by five replicate injections of the ban fang ditch sample solution, and RSD value was below 0.89%.
Reproducibility
The reproducibility of extraction was also investigated for the three components by comparing six samples from six independent extractions. Six 0.2000 g samples of Z. clinopodioides Lam power from Ban fang ditch were accurately weighed, prepared, and analyzed by RRLC. The RSD values of the six replicates were less than 2.0% for all compounds, demonstrating the high reproducibility of the sample preparation procedure.
Recovery
Recovery tests were performed to further investigate the reproducibility and efficiency of the extraction and analysis method. Recoveries of the three compounds were determined by the method of standard addition. Three concentrations of the compound standard solutions were used to spike Z. clinopodioides Lam samples containing known amounts of each compound (namely 50% of the compound). The mixture was extracted and analyzed as described. The mean recoveries of the three compounds were 104.1% for diosmin, 102.3% for linarin, and 97.4% for pulegone, with RSD values of 1.6, 1.2, and 2.1%, respectively.
The obtained results indicate that the developed analytical method was reproducible with high accuracy. It is therefore satisfactory for quantitative analysis.
Application to the analysis of Ziziphora clinopodioides Lam samples
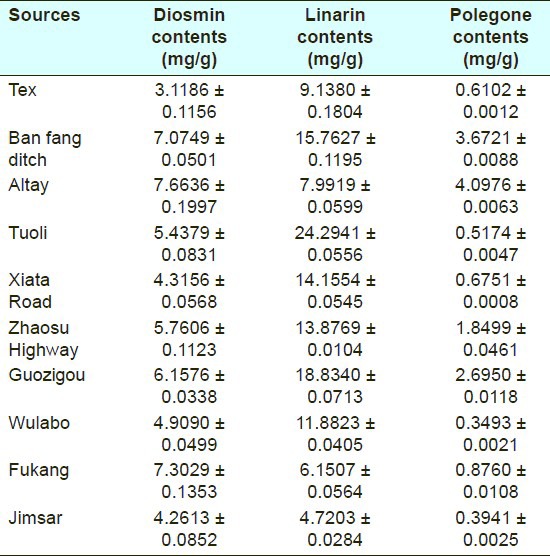
The developed analytical method was successfully applied for the simultaneous determination of the three components in ten different samples of Z. clinopodioides Lam. All three compounds were detected from every sample. Each sample was determined in triplicate and the peaks in the chromatograms were identified by comparing the retention times and UV spectra with the authentic standards. The three compounds were quantified in the ten samples [Table 3].
Table 3.
Contents of the three compounds in ten different origin samples of Ziziphora clinopodioides Lam. (n = 3)
DISCUSSION
We describe a new method of RRLC separation, using 1.8 μm particle size of stationary phase instead of the usual 5 μm columns, is proven to be efficient, precise, accurate, sensitive and time saving, and enabled determination of diosmin, linarin, and pulegone from Z. clinopodioides Lam using reversed-phase chromatography with gradient elution and a DAD detector. Simultaneous detection could allow for a large number of herbal samples to be analyzed in a relatively short period of time. Further studies are ongoing in our laboratory to further characterize activate compounds of Z. clinopodioides Lam. It is clear that there was a significant variation in the contents of the three compounds between the ten samples obtained from different regions. Similar variations have been found for other components and may be attributed to the different growing conditions at the sampling sites. These variations in the bioactive components influence the medicinal quality, so it was necessary to develop an effective qualitative and quantitative method to evaluate the overall quality of Z. clinopodioides Lam. The simultaneous analysis of several chemical components is a promising tool for the quality control of medicinal herbs.
ACKNOWLEDGEMENT
This work was supported by the National Natural Science Foundation of China (Grant No 81060365).
Footnotes
Source of Support: This work was financially supported by the National Natural Science Foundation of China (grant no. 81060365)
Conflict of Interest: None declared.
REFERENCES
- 1.Tang D, Yang D, Tang A, Gao Y, Jiang X, Mou J, et al. Simultaneous chemical fingerprint and quantitative analysis of Ginkgo biloba extract by HPLC–DAD. Anal Bioanal Chem. 2010;396:3087–95. doi: 10.1007/s00216-010-3536-8. [DOI] [PubMed] [Google Scholar]
- 2.Liu YM. Xinjiang: Science and Technology Health Publisher; 1999. Pharmacography of Uighur; p. 446. [Google Scholar]
- 3.Tian SG, Shi Y, Yu Q, Upur H. Determination content of oleanolic acid and ursolic acid in Ziziphora clinopodioides Lam by HPLC method. Pharmacogn Mag. 2010;6:116–9. doi: 10.4103/0973-1296.62898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Behravan J, Ramezani M, Hassanzadeh MK, Eskandari M, Kasaian J, Sabeti Z. Composition, antimycotic and antibacterial activity of Ziziphora clinopodioides Lam essential oil from Iran. J Essent Oil Bear Pl. 2007;10:339–45. [Google Scholar]
- 5.Ozturk S, Ercisli S. Antibacterial activity and chemical constitutions of Ziziphora clinopodioides. Food Control. 2007;18:535–40. [Google Scholar]
- 6.Tian SG, Shi Y, Zhou XY, Ge L, Upur H. Total polyphenolic (falconoid) content and antioxidant capacity of activity of different Ziziphora clinopodioides Lam. extracts. Pharmacogn Mag. 2011;7:65–8. doi: 10.4103/0973-1296.75904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Senejoux F, Girard C, Kerram P, Aisa HA, Berthelot A, Bévalot F, et al. Mechanisms of vasorelaxation induced by Ziziphora clinopodioides Lam. (Lamiaceae) extract in rat thoracic aorta. J Ethnopharmacol. 2010;132:268–73. doi: 10.1016/j.jep.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Sonboli A, Atri M, Shafiei S. Intraspecific variability of the essential oil of Ziziphora clinopodioides ssp. Rigida from Iran. Chem Biodivers. 2010;7:1784–9. doi: 10.1002/cbdv.200900336. [DOI] [PubMed] [Google Scholar]
- 9.Mohammadreza VR. Essential oil composition and biological activity of Ziziphora clinopodioides Lam from Iran. Research J Pharmacology. 2008;2:17–9. [Google Scholar]
- 10.Chitsaz M, Barton M, Bazargan M. Essential oil composition and antibacterial effects of Ziziphora clinopodioides. Int J Antimicrob Ag. 2007;2:203. [Google Scholar]
- 11.Shi Y, Bi K, Xu TH, Tian SG. The stability of the essential oil from Uygur medicine Ziziphora clinopodioides Lam. Chin J Ethnomed Ethnopharm. 2009;18:1–3. [Google Scholar]
- 12.Zhou XY, Shi Y, Ma XM, Gong HY, Tian SG. Inhibit bacterial activity screening and volatile oil chemical composition analysis of Ziziphora clinopodioides Lam. Chin Med J Res Prac. 2011;25:44–7. [Google Scholar]
- 13.Zhou XY, Yu Q, Gong HY, Zhang HK, Wang DD, Tian SG. Simultaneous determination of Caffeic acid and Rosmarinic acid in Ziziphora clinopodioides Lam. From different sources in Xinjiang, Lat. Am J Pharm. 2011;30:1651–5. [Google Scholar]
- 14.Tian SG, Zhou XY, Xu DH, Ding JB, Shan LJ, Shi Y. Determination of Ten Metal Elements in Ziziphora Clinopodioides Lam by Microwave Digestion-FAAS. Guang Pu Xue Yu Guang Pu Fen Xi. 2009;29:1993–6. [PubMed] [Google Scholar]
- 15.Yang XJ. Master thesis by Shenyang Pharmaceutical University; 2008. Isolation and identification of chem ical constituents from stem and root of Ziziphora clinopodioides Lam; p. 15. [Google Scholar]
- 16.Gul Akillioglu H, Karakaya S. Changes in total phenols, total flavonoids, and antioxidant activities of common beans and pinto beans after soaking, cooking, and in vitro digestion process. Food Sci Biotechnol. 2010;19:633–9. [Google Scholar]
- 17.Unal EL, Mavi A, Kara AA, Ahmet Cakir, Meryem Şengül. Ali Yildirim Antimicrobial and antioxidant activities of some plants used as remedies in Turkish traditional medicine. Pharm Biol. 2008;46:207–24. [Google Scholar]
- 18.Sanjay L. The Brooklyn Center: Long Island University; 2010. Protective effect of diosmin on lipopolysaccharide-induced PC12 cell death; p. 95. [Google Scholar]
- 19.Jia Y, Li YJ, Tan XJ, Li Q, Bi KS. RP-HPLC determination of linarin in beagle dog plasma after administration of Yejuhua injection. Chromatographia. 2006;64:303–5. [Google Scholar]
- 20.Tabotabaei-Anaraki M, Chalabian F, Masoudi S, Rustaiyan A. The chemical composition and in vitro antibacterial activities of the oil of Ziziphora clinopodioides Lam. from Iran. Planta Med. 2007;9:852–3. [Google Scholar]


