Abstract
Background:
Arthropod venoms have attracted interest because they represent a source of neuroactive compounds that can be useful tools in neuroscience and pharmacological investigations.
Objective:
The purpose of the present work was to evaluate the anticonvulsant, anxiolytic, and behavioral effects of the peptide fraction separated from venom of the social wasp.
Materials and Methods:
The low- molecular-weight compounds of the venom were separated by ultrafiltration and the bioassays were performed to test anticonvulsant and anxiolytic effects, as well as alterations in the spontaneous behavior of the animals.
Results:
Intracerebroventricular injections of the compounds induced dose-dependent anticonvulsant effects and a potent anxiolytic activity. Regarding behavioral effects, no significant differences were observed in relation to the saline control group.
Conclusion:
The low-molecular-weight compounds of the venom of Polybia paulista include neuroactive peptides that can be used as pharmacological resources for anticonvulsant and anxiolytic drug research.
Keywords: Anticonvulsant, anxiolytic, behavioral effects, neuroactive compounds, peptides, wasp venom
INTRODUCTION
Neurological, mental, and behavioral disorders represent a huge burden to society, affecting more than 450 million people globally.[1] Furthermore, the data suggest that psychiatric and neurological disorders are a growing and important cause of morbidity.[2,3] Among the most common neurological disorders are depression, schizophrenia, Alzheimer's disease, anxiety disorders, and epilepsy.
Epilepsy is the second most common neurological disease in the world, affecting approximately 1% of the population.[4] It is defined as a chronic disorder of the brain characterized by recurrent and spontaneous unpredictable seizure activity, which is triggered by an abnormal discharge of neurons,[5,6] and it has psychological, cognitive, neurobiological, and social consequences for the patient.[7] Treatment for this disorder is based on the chronic administration of antiepileptic drugs.[5] These drugs often have strong side effects, which vary in frequency and severity after chronic treatment and may include chronic toxicity, cognitive impairment, sedation, teratogenesis, and hepatic failure.[8,9,10,11,12,13]
A significant proportion of patients with epilepsy (30%) suffer from intractable, that is, drug-resistant types of seizures.[6] Interestingly, most of the newer drugs available are more efficient than conventional anticonvulsants but still induce strong side effects at therapeutic doses.[6,13] As a result, the search continues for new drugs to treat the patients whose seizures are inadequately controlled by existing drugs, including adults with complex partial seizures and children with a variety of syndromes.[14,15]
Anxiety disorders, as a group, are the most prevalent mental health conditions in both developing and developed regions around the world.[16] To distinguish pathological anxiety from normal anxiety, it is necessary to establish whether the anxiety reaction is of short, self-limiting duration and linked to the moment. The pathological anxiety disorders affect about 400 million people worldwide, and an estimated 20% of the population will be subject to these disorders later in life. In Brazil, anxiety disorders are considered to represent a major public health problem, affecting more than 10 million people.[16]
Anxiety disorders are also enormously costly in terms of individual suffering and direct medical and indirect social cost.[17,18] Moreover, when first-line treatments are not effective, treatment-resistant anxiety disorders may be another serious problem.[19]
In this context, venoms of arthropods such as spiders, scorpions, and wasps, due to their high specificity and affinity for neuroactive substrates, may be useful in the design of novel drugs for the treatment of neurological disorders.[20,21,22]
Wasp venoms are complex mixtures of biochemically and pharmacologically active substances, which include amines, small peptides, and high-molecular-weight proteins such as enzymes, allergens, and toxins.[23,24,25,26] The venom of social wasps contains mainly low-molecular-weight compounds comprising peptides,[27,28] which are the most common compounds (about 70%) in this venom[28] and many of these have antimicrobial, myotoxic, hemolytic, and neural activity.[26,29,30,31] In relation to the latter, some peptides exert potent anticonvulsant, antinociceptive, and antipanic activity when applied directly to the central nervous system of rats.[30,31,33]
Very few studies have focused on the venoms of social wasps as sources of excitatory and inhibitory neuronal modulators. The purpose of this study was the investigation of the anticonvulsant and anxiolytic activity of the low-molecular-weight compounds isolated from the venom of the neotropical social wasp Polybia paulista, as well as an analysis of the side effects of these compounds on spontaneous behavior in Wistar rats.
MATERIALS AND METHODS
Animals
This study was approved by the Committee for Ethics in Animal Use of the Institute of Biological Sciences, University of Brasília, under license number 45.810/2009. Animals were maintained in accordance with the Brazilian Society for Neuroscience and Behavior ethical statements, which follow the guidelines for animal care prepared by the Committee on Care and Use of Laboratory Animal Resources, National Research Council, USA. Likewise, every effort was made to avoid unnecessary stress and pain to the experimental animals. Moreover, the collection of specimens of the wasps was authorized by the Chico Mendes Institute for Biodiversity Conservation in Brazil (license number 21723-1, date of issue 27/10/2009).
Wasps
P. paulista specimens were collected in Distrito Federal, Brazil, and they were identified by entomologist Prof. Dr. Fernando Barbosa Noll (UNESP, Brazil). The wasps’ nest was collected and immediately submitted to low temperature in a cooler with ice. In the laboratory, the nest was stored at -20°C for 5 hours to euthanize the wasps. A total of 1,500 venom sacs were dissected and macerated in a 1:1 acetonitrile/deionized water solution. The homogenate of the macerated material was centrifuged using an ultra filter (Millipore) with a 3kDa cut-off for 30 minutes at 10,000 x g. The resultant ultrafiltrate, characterized as low-molecular-weight compounds of the P. paulista venom (LMWC-Pp), was quantified and diluted in deionized water at concentrations of 70, 210, and 350 μg/μl.
Rats
Male Wistar rats (220-250 g) from the animal house of the University of Brasília, Distrito Federal, Brazil, were used in the assays. They were kept in wire-mesh cages in a room with a dark/light cycle of 12 hours (lights on at 7:00 a.m.), with water and food ad libitum. Conditions of luminosity and temperature (22°C) were kept constant in the housing and experiment rooms. Efforts were made to minimize the number and potential suffering of experimental subjects.
For surgical procedures, all animals were anesthetized with ketamine (60 mg/kg, Syntec, Brazil) and xylazine (10 mg/ kg, Syntec, Brazil) and fixed in a stereotactic frame (Insight, Brazil). A local injection of lidocaine was given, and the cranium was exposed for implantation of a stainless steel guide cannula (10 mm) in the right lateral ventricle. The coordinates used were 0.8-mm posterior to bregma, 1.6 mm lateral from the midline, and 3.4 mm ventral from the surface of the skull according to the atlas of Paxinos and Watson.[34] The cannula was fixed to the skull with dental acrylate. Before performing the assays, all animals were allowed 5-7 days to recover from surgery.
Bioassays
Anticonvulsant activity
Groups of rats (n=5-8) were injected intracerebroventricular (i.c.v.) with LMWC-Pp at 70, 210, 350 μg/animal and saline, with the aid of a Hamilton syringe driven by an infusion pump (Harvard Apparatus) injecting a volume of 1μl over 1 minute. Ten minutes after the injection, the animals received a single dose of the convulsant pentylenetetrazole (PTZ) (105mg/kg, s.c., Sigma) and were placed in the arena and filmed for 30 minutes. The dose of the convulsant was based on previous experiments to find the dose that produced convulsions in 97% of the animals (CD97) (data not shown). A positive control was also employed, in which PTZ was injected 30 minutes after Diazepam (DZP) (anticonvulsant, 2 mg/kg, i.p.). Seizures were evaluated using the score for seizure severity proposed by Lüttjohann et al.[35] The number of animals that presented a score 6 seizure (maximum score) and the latency to onset of seizures were recorded.
Anxiolytic activity
Groups of rats (n=5-8) were injected i.c.v. with LMWC- Pp (70, 210, and 350 μg/animal) and physiological saline, with the aid of a Hamilton syringe as described above. Ten minutes after treatment, the animals were placed in the center of an elevated plus maze (EPM), made of wood and consisting of four arms, each 50 cm in length and 10 cm in width. Two opposing arms were enclosed by walls 40 cm in height, while the two remaining (open) arms were surrounded by a raised lip to prevent falls from the apparatus. The maze was elevated 50 cm above the floor. The maze was cleaned with alcohol-soaked and dry cloths between animals. In addition to the saline group, two other control groups were employed: negative, in which the anxiogenic substance PTZ (30 mg/kg, s.c.) was injected and the rats were placed in the EPM 15 minutes later, and positive, in which the standard anxiolytic DZP (2 mg/kg, i.p.) was injected and the rats were placed in the EPM 30 minutes later. The behaviors of the experimental animals were recorded for 5 minutes by a video camera interfaced with a computer in an adjacent room. The following behavioral responses were analyzed: number of entries (1) in the open arms and (2) in the closed arms; time spent (3) in the open arms and (4) in the closed arms; and (5) number of head dips.
Behavioral effects
Adverse effects on the spontaneous behavior of animals were assessed using the open field test. Rats were placed in an acrylic arena (open field) 60 cm in diameter and 50 cm high, the floor of which was divided in 12 sections, after being injected as described before with LMWC-Pp at 70, 210, and 350 μg/animal, saline, and DZP. The animals were filmed for 30 minutes. The time spent in each behavior and the numbers of lines crossed were recorded. Behavioral categories were grouped according to Speller and Westby[36] with modifications, as follows: exploratory and immobility, and lines crossed.
Histological analysis
To verify the correct positioning of the cannula, after the assays, the animals received an overdose of sodium thiopental (Cristália, Brazil). Following euthanasia, the animals were injected i.c.v. with toluidine blue to mark the exact site of injection. Then, the brains were removed and frozen. After freezing, incisions were made in the brain to visualize the ventricular system and confirm the injection site. Correctly located cannulas were considered to be those that would enable a complete blue coloration of the lateral ventricles. Data from rats with guide cannula tips located outside the lateral ventricle were not included in behavioral and statistical analyses.
Statistical analysis
All statistical analyses were performed using GraphPad Prism Software version 4.0 for Windows (GraphPad Software, San Diego, USA). Differences were considered significant at values of P<0.05. With regard to anticonvulsant activity, the numbers of animals protected from seizures were analyzed using the Chi-square test, and ED50 values were calculated by the Probit method. To analyze the latency to onset of seizures in the anticonvulsant assays, the data collected were subjected to one-way analysis of variance (ANOVA) followed by the Tukey test. Regarding anxiolytic activity, to analyze the time spent on and number of entries into the open and closed arms of the EPM, as well as head dipping behavior, the results were subjected to ANOVA, followed by the Tukey test. The data obtained by the observation of exploratory and immobility behaviors were subjected to ANOVA followed by the Tukey test. In addition, the number of lines crossed was subjected to two-way ANOVA followed by the Bonferroni post-test.
RESULTS
Anticonvulsant activity
The results for protection against score 6 PTZ-induced seizures after administration of the LMWC-Pp are shown in Figure 1. Data from these experiments demonstrated that the administration of LMWC-Pp blocked PTZ-induced seizures in a dose-dependent manner. In experimental groups, the lower dose (70 μg/animal) did not protect any of the animals, while the medium dose (210 μg/ animal) protected 12%. However, only the highest dose of LMWC-Pp, 350 μg/animal, gave significant protection against score 6 PTZ-induced seizures compared to the saline group and the lowest dose of LMWC-Pp, protecting 60% of the animals (χ2 =23.18, P<0.001). The ED50 value was calculated at 314.75±0.08 μg/animal. In the saline group, all animals presented seizures. By contrast, in the DZP group, no animals presented seizures.
Figure 1.
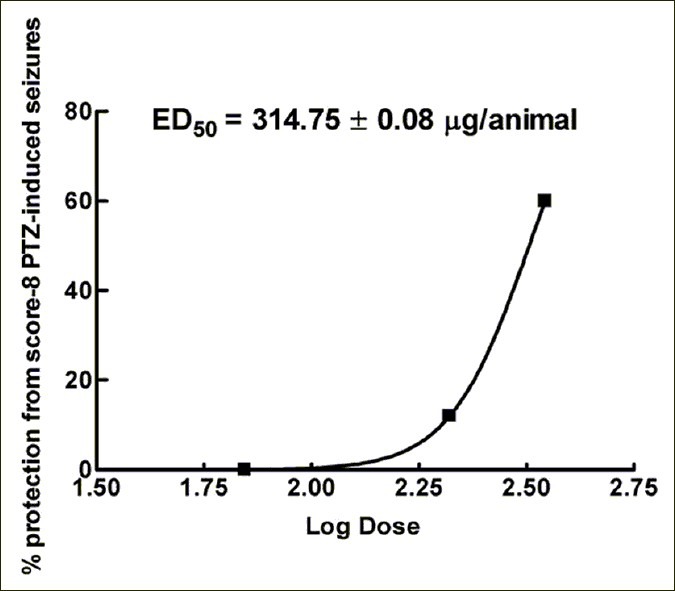
Anticonvulsant effect of low-molecular-weight compounds from the venom of Polybia paulista against score 6 PTZ-induced seizures. All data were subjected to Chi-square test. ED50 values were calculated by the Probit method
The latencies to the onset of PTZ-induced seizures in control and experimental groups can be seen in Figure 2. ANOVA revealed significant differences in the various experimental treatments [F(4,26) =21.23, P=0.0001]. Latencies in the saline and 70 and 210μg/animal LMWC- Pp groups were significantly shorter than that of the DZP group. Moreover, significant differences were also observed between the 210 μg/animal dose of LMWC-Pp and the saline and 70 μg/animal groups. The latency in the 350 μg/animal dose was significantly longer than those of the saline and 70 μg animal LMWC-Pp groups (P<0.001). Thus, a dose-dependent increase was observed in the latency to PTZ-induced seizure onset.
Figure 2.
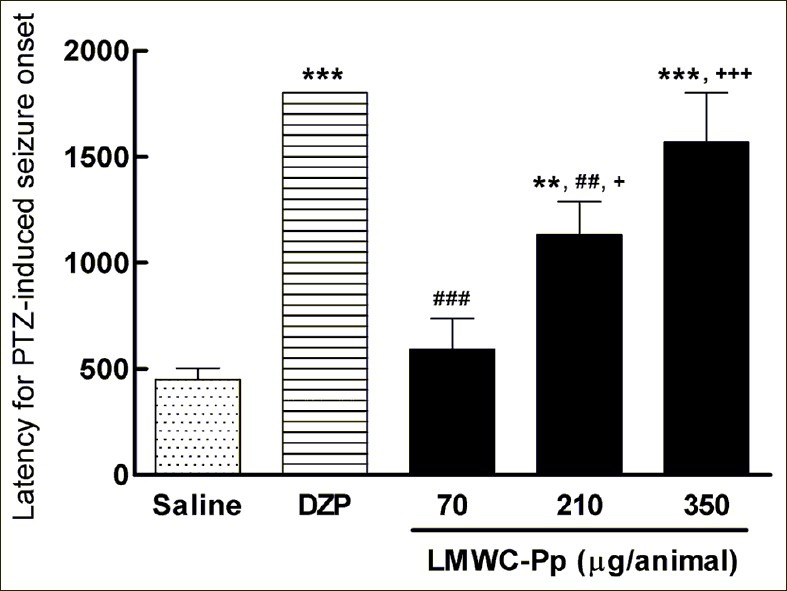
Latency to PTZ-induced seizure onset in control and experimental groups. Values represent means, with error bars denoting SEM. All data were subjected to one-way analysis of variance (ANOVA) followed by Tukey as a post-test. **P<0.01 and ***P<0.001 compared to saline group; ##P <0.01 and ###P <0.001 compared to DZP group; and +P <0.05 and +++P <0.001 compared to LMWC-Pp 70 μg/animal dose group
Anxiolytic activity
The time spent in the open arms of the EPM can be seen in Figure 3a. In relation to this parameter, ANOVA revealed significant differences in the various experimental treatments [F(5,28) =6.261, P=0.0008]. Animals treated with the highest dose of LMWC-Pp and DZP spent significantly more time in the open arms when compared with the groups treated with saline and PTZ. The number of entries in the open arms of the EPM was also significantly different [F(5,26) =5.939, P=0.0014] between the different groups. These data are shown in Figure 3c, where an increase in the number of entries can be seen in the group treated with the highest dose of LMWC-Pp.
Figure 3.
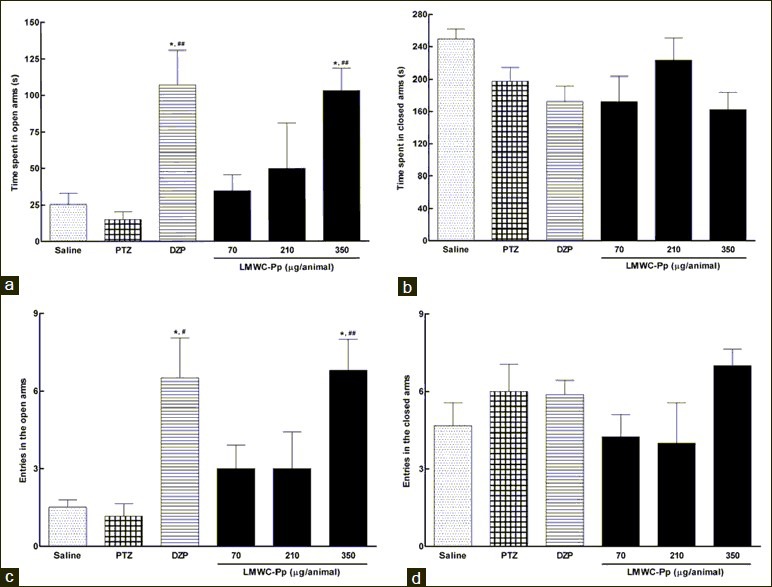
Effects of different doses of the LMWC-Pp on anxiety-related parameters in the Elevated Plus Maze (EPM). Time spent by animals in open arms (a) and in the closed arms (b) of the EPM. Number of entries in the open arms (c) and in the closed arms (d). Values represent means, with error bars denoting SEM. All data were subjected to one-way analysis of variance (ANOVA) followed by Tukey as a post-test. *P<0.05 compared to saline; #P <0.05 and ##P <0.01 compared to PTZ
Figure 3b illustrates the time spent in the closed arms of the EPM. ANOVA did not detect a significant difference between the groups [F(5,35) =2.530, P=0.0502]. Similar results were observed for the analysis of the number of entries in the closed arms Figure 3d, where there was no significant difference [F(5,35) =1.286, P=0.2958]. Also, there was a significant increase [F(5,30) =2.991, P=0.0299] in the time spent engaged in head dipping behavior when rats were given the highest dose of LMWC-Pp, compared with control groups. These observations are illustrated in Figure 4.
Figure 4.
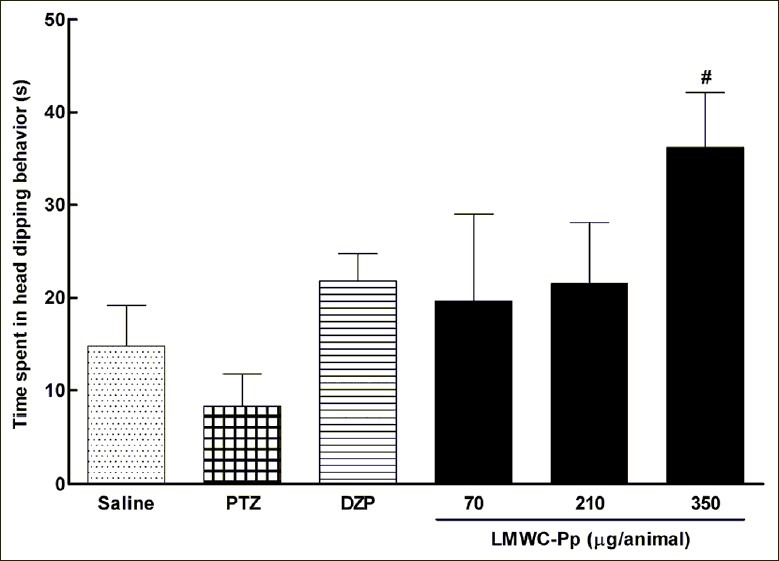
Head dipping behavior of the animals in the EPM. Values represent means, with error bars denoting SEM. All data were subjected to one-way analysis of variance (ANOVA) followed by Tukey as a post-test. #P <0.05 compared to PTZ
Behavioral effects
The effects of LMWC-Pp doses, saline, and DZP on exploratory and immobility behaviors are shown in Figures 5a and b. A significant difference between saline and DZP was observed (P<0.01), but not between saline and experimental groups, both for immobility [F(4,22) =4.135, P=0.012] and also exploratory behaviors [F(4,22) =3.70, P=0.0187].
Figure 5.
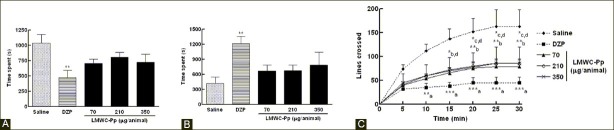
Time spent by animals in exploratory (A) and immobility (B) behaviors (C) Accumulated number of lines crossed by the animals in the arena in 30 minutes. values represent means, with error bars denoting SEM. Data for a and b were subjected to one-way analysis of variance (ANOVA) followed by Tukey as a post-test, while data for c were subjected to two-way analysis of variance (MANOVA) followed by Bonferroni as a post-test. *P<0.05, **P<0.01 and ***P<0.001 compared to saline. (a) DZP, (b) 70µg/animal, (c) 210µg/animal, and (d) 350μg/animal
Regarding the number of lines crossed by the animals in the arena, as shown in Figure 5c, there were significant effects of treatment [F(4,10) =22.94; P<0.0001] and time [F(5,102) =5.23; P<0.0003], but there was no significant difference in the treatment-vs-time interaction [F(20,102) =0.37; P=0.9936]. Post hoc analysis indicated that the number of lines crossed within 10 minutes by animals in the DZP group was significantly different from the saline group (P<0.01), and this difference became more significant after this period of time (P<0.001). At 15 minutes, there was a significant difference between the number of lines crossed in 70 and 350 μg/animal LMWC-Pp groups compared to the saline group (P<0.05); after this period, the number of lines crossed by animals in the saline group was significantly different from 70 (P<0.01), 210, and 350 μg/ animal LMWC-Pp groups (P<0.05).
DISCUSSION
Arthropod venoms have attracted interest from researchers because they represent a source of neuroactive compounds that can be useful tools in neuroscience and pharmacological investigations,[37] and, particularly, in this study, the low-molecular-weight compounds from the venom of P. paulista presented relevant anxiolytic and anticonvulsant activities.
In the present work, the low-molecular-weight compounds from the venom of the wasp P. paulista exerted an anticonvulsant effect in the bioassays with the 350 μg/ animal dose group, in which 60% of animals were protected from score 6 PTZ-induced seizures. This effect was also found in a study with crude denatured venom of Polybia occidentalis,[32] in which the authors observed protection from seizures induced by four distinct convulsants: bicuculline, picrotoxin, kainic acid, and PTZ, although protection against the latter was less effective. Another study[31] demonstrated similar effects of the denatured venom of Polybia ignobilis, with protection being observed from seizures induced by the same convulsants cited above, except PTZ. Compounds with anticonvulsant activity were found also in venom from other arthropods such as spiders.[38,39]
Also, with regard to anticonvulsant activity, the obtained ED50 was 314.75±0.08 μg/animal. Interestingly, in the study with crude denatured venom of P. occidentalis cited above,[32] the ED50 was 315 μg/animal. Finally, a dose-dependent increase was found in the latency to onset of PTZ-induced seizures, which was also observed in the work with P. occidentalis mentioned above.[32]
Interestingly, the PTZ test has been employed to evaluate compounds during initial screening to discover the potential of the test material against myoclonic seizures and absence of seizures,[40,41] in which active compounds may raise the seizure threshold for excitation in the neural tissues and thereby suppress seizure activity.[42] Thus, the LMWC-Pp probably contains compounds that are effective in the treatment of myoclonic seizures. Since PTZ-induced seizures can be blocked by either drugs that reduce the t-type calcium currents or drugs that enhance GABAA receptor-mediated inhibition,[43] it can be suggested that the ultrafiltrate compounds, consisting mainly of peptides, tested in the present work, may be acting on these mechanisms.
In this study, the EPM was also used to induce anxiety in animals, which are exposed to etiological aversive situations.[44] The EPM model is based on the innate fear of rodents for open spaces and heights, which is one of the factors that generate anxiety.[45] The most important parameters in evaluating the anxiolytic activity of the drug under study are the time spent on and the number of entries into the open arms.[44]
Benzodiazepines, such as DZP, have been used in the treatment of epilepsy and anxiety, but side effects, such as tolerance and sedation, are limitations that have restricted their use.[46] Meanwhile, in experimental models, PTZ is commonly administered as a convulsant or anxiogenic agent; its effect depends on the dose injected, and DZP can provide protection from seizures induced by PTZ.[47] In the bioassays of anxiolytic activity, only the highest dose of LMWC-Pp, 350 μg/animal, reduced the anxiety in the animals significantly compared to the saline group. Similar results were obtained in other studies, in which anxiolytic activity was demonstrated with the EPM model in compounds isolated from spider venom, although in this case the effect was dose-dependent.[38]
Another parameter used to assess anxiolytic activity is behavior which can be interpreted as assessing the potential risk involved in a situation: head dipping.[48] As can be seen in Figure 4, the highest dose of LMWC-Pp led to a significant increase in head dipping compared to PTZ given alone, with this increase being larger than that in the DZP group, suggesting the presence of a component with anxiolytic activity in this venom. Regarding the number of entries in the closed arms, there was no significant difference between the groups, which is quite interesting since some studies have shown that the frequency of closed arm entries may be an indicator of a change in overall motor activity.[38,48] Therefore, these results suggest that the anxiolytic compound present in the LMWC-Pp possesses minimal side effects.
The evaluation of behavioral effects demonstrated no significant differences between the doses of LMWC-Pp and saline groups in time spent in exploratory and immobility behaviors. However, in the study with crude denatured venom of P. occidentalis[32] cited above, the authors found a significant, dose-dependent increase in the time spent in immobility behavior and a decrease in the time spent in exploratory behavior. In the study with P. ignobilis[31] also mentioned above, the authors again found significant differences in immobility and exploratory behaviors, although these were not dose-dependent.
The number of lines crossed by the animals in the open-field arena provides evidence of changes in spontaneous locomotor activity. It was observed that the different doses of LMWC-Pp significantly reduced, in a manner independent of dose, the number of lines crossed by the animals, although this effect was moderate when compared to the DZP group. Other authors have also demonstrated this effect of wasp venom on spontaneous locomotor activity, with reports suggesting it to be dependent[32] or independent[31] of the dose.
The results of this study demonstrated that the low-molecular-weight compounds isolated from the venom of P. paulista exerted anticonvulsant and anxiolytic effects. The DZP treatment protected all animals from PTZ-induced seizures, but caused significant sedation. By contrast, the experimental groups presented only a trend toward sedation, although only the highest dose offered substantial protection from seizures. These activities have been observed in other Polybia species and reveal the great potential for the study of anticonvulsant and anxiolytic compounds isolated from social wasps’ venom.
Studies on the application of wasp venom in the treatment of anxiety and epilepsy are scarce. The results obtained indicate that wasp venom and its components are promising tools for experimental pharmacology, and further studies will be conducted in order to improve understanding of their effects and exploit their potential.
Footnotes
Source of Support: This work received financial support from the Brazilian Research Foundations; National Council for Scientific and Technological Development-CNPq, Universal Grant (No. 479873/2008) and Foundation for Research Support from the Distrito Federal-FAPDF, Induced Demand Grant (No. 193.000.539/2009)
Conflict of Interest: None declared.
REFERENCES
- 1.Mathers CD, Lopez A, Stein C, Ma Fat D, Rao C, Inoue M, et al. Bethesda, Md: Disease Control Priorities Project; [Accessed 10 october 2011]. Deaths and Disease Burden by Cause: Global Burden of Disease Estimates for 2001 by World Bank Country Groups. Working Paper 18. serial on the Internet. Available from: http://www.dcp2.org/file/33/wp18.pdf . [Google Scholar]
- 2.Sperling MR, Harris A, Nei M, Liporace JD, O’Connor MJ. Mortality after epilepsy surgery. Epilepsia. 2005;46:49–53. doi: 10.1111/j.1528-1167.2005.00410.x. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. What are neurological disorders? Question and answer archives [serial on the Internet] 2007. [Accessed 10 october 2011]. Available from: http://www.who.int/features/qa/55/en/index.html .
- 4.Blum DE. New drugs for persons with epilepsy. Adv Neurol. 1998;76:57–87. [PubMed] [Google Scholar]
- 5.Tunnicliff G. Basis of the antiseizure action of phenytoin. Gen Pharmacol. 1996;27:1091–7. doi: 10.1016/s0306-3623(96)00062-6. [DOI] [PubMed] [Google Scholar]
- 6.Löscher W. New visions in the pharmacology of anticonvulsion. J Pharmacol. 1998;342:1–13. doi: 10.1016/s0014-2999(97)01514-8. [DOI] [PubMed] [Google Scholar]
- 7.Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- 8.Verity CM, Hosking G, Easter DJ. A multicentre comparative trial of sodium valproate and carbamazepine in paediatric epilepsy. The Paediatric EPITEG Collaborative Group. Dev Med Child Neurol. 1995;37:97–108. doi: 10.1111/j.1469-8749.1995.tb11978.x. [DOI] [PubMed] [Google Scholar]
- 9.de Silva M, MacArdle B, McGowan M, Hughes E, Stewart J, Neville BG, et al. Randomised comparative monotherapy trial of phenobarbitone, phenytoin, carbamazepine, or sodium valproate for newly diagnosed childhood epilepsy. Lancet. 1996;347:709–13. doi: 10.1016/s0140-6736(96)90074-4. [DOI] [PubMed] [Google Scholar]
- 10.Painter MJ, Scher MS, Stein AD, Armatti S, Wang Z, Gardiner JC, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–9. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 11.Raza M, Shaheen F, Choudhary MI, Sombati S, Rafiq A, Suria A, et al. Anticonvulsant activities of ethanolic extract and aqueous fraction isolated from Delphinium denudatum. J Ethnopharmacol. 2001;78:73–8. doi: 10.1016/s0378-8741(01)00327-0. [DOI] [PubMed] [Google Scholar]
- 12.Bergin AM, Connolly M. New antiepileptic drug therapies. Neurol Clin. 2002;20:1163–82. doi: 10.1016/s0733-8619(02)00017-8. [DOI] [PubMed] [Google Scholar]
- 13.Suzette M, LaRoche SM, Helmers SL. The new antiepileptic drugs: clinical applications. JAMA. 2004;291:615–20. doi: 10.1001/jama.291.5.615. [DOI] [PubMed] [Google Scholar]
- 14.Meldrum BS. Identification and preclinical testing of novel antiepileptic compounds. Epilepsia. 1997;38:7–15. doi: 10.1111/j.1528-1157.1997.tb05204.x. [DOI] [PubMed] [Google Scholar]
- 15.Villetti G, Bregola G, Bassani F, Bergamaschi M, Rondelli I, Pietra C, et al. Preclinical evaluation of CHF3381 as a novel antiepileptic agent. Neuropharmacology. 2001;40:866–78. doi: 10.1016/s0028-3908(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 16.Demyttenaere K, Bruffaerts R, Posada-Villa J, Gasquet I, Kovess V, Lepine JP, et al. WHO World Mental Health Survey Consortium. Prevalence, severity, and unmet need for treatment of mental disorders in the World Health Organization World Mental Health Surveys. JAMA. 2004;291:2581–90. doi: 10.1001/jama.291.21.2581. [DOI] [PubMed] [Google Scholar]
- 17.Greenberg PE, Sisitsky T, Kessler RC, Finkelstein SN, Berndt ER, Davidson JR, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60:427–35. doi: 10.4088/jcp.v60n0702. [DOI] [PubMed] [Google Scholar]
- 18.Mogotsi M, Kaminer D, Stein DJ. Quality of life in the anxiety disorders. Harv Rev Psychiatry. 2000;8:273–82. [PubMed] [Google Scholar]
- 19.Stein DJ, Seedat S. Unresolved questions about treatment-resistant anxiety disorders. CNS Spectr. 2004;9:715. doi: 10.1017/s1092852900022355. [DOI] [PubMed] [Google Scholar]
- 20.Osborne NN, Chidlow G, Layton CJ, Wood JP, Casson RJ, Melena J. Optic nerve and neuroprotection strategies. Eye (Lond) 2004;18:1075–84. doi: 10.1038/sj.eye.6701588. [DOI] [PubMed] [Google Scholar]
- 21.Wang CZ, Chin CW. Conus peptides--a rich pharmaceutical treasure. Acta Biochim Biophys Sin. 2004;36:713–23. doi: 10.1093/abbs/36.11.713. [DOI] [PubMed] [Google Scholar]
- 22.Mortari MR, Cunha AO, Ferreira LB, dos Santos WF. Neurotoxins from invertebrates as anticonvulsants: from basic research to therapeutic application. Pharmacol Ther. 2007;114:171–83. doi: 10.1016/j.pharmthera.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Habermann E. Bee and wasp venom: the biochemistry and pharmacology of their peptides and enzymes are reviewed. Science. 1972;177:314–22. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- 24.Piek T. Delta-Philanthotoxin, a semi-irreversible blocker of ion-channels. Comp Biochem Physiol C. 1982;72:311–5. doi: 10.1016/0306-4492(82)90098-3. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima T. Biochemistry of vespid venoms. In: Tu AT, editor. Handbook of natural toxins. New York: Marcel Dekker; 1984. pp. 109–33. [Google Scholar]
- 26.Monteiro MC, Romão PR, Soares AM. Pharmacological perspectives of wasp venom. Protein Pept Lett. 2009;16:944–52. doi: 10.2174/092986609788923275. [DOI] [PubMed] [Google Scholar]
- 27.Ho CL, Shih YP, Wang KT, Yu HM. Enhancing the hypotensive effect and diminishing the cytolytic activity of hornet mastoparan B by D-amino acid substitution. Toxicon. 2001;39:1561–6. doi: 10.1016/s0041-0101(01)00128-3. [DOI] [PubMed] [Google Scholar]
- 28.Baptista-Saidemberg NB, Saidemberg DM, Palma MS. Profiling the peptidome of the venom from the social wasp Agelaia pallipes pallipes. J Proteomics. 2011;74:2123–37. doi: 10.1016/j.jprot.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Piek T. Neurotoxic kinins from wasp and ant venoms. Toxicon. 1991;29:139–49. doi: 10.1016/0041-0101(91)90098-c. [DOI] [PubMed] [Google Scholar]
- 30.Oliveira L, Cunha AO, Mortari MR, Pizzo AB, Miranda A, Coimbra NC, et al. Effects of microinjections of neurotoxin AvTx8, isolated from the social wasp Agelaia vicina (Hymenoptera, Vespidae) venom, on GABAergic nigrotectal pathways. Brain Res. 2005;1031:74–81. doi: 10.1016/j.brainres.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 31.Cunha AO, Mortari MR, Oliveira L, Carolino RO, Coutinho-Netto J, dos Santos WF. Anticonvulsant effects of the wasp Polybia ignobilis venom on chemically induced seizures and action on GABA and glutamate receptors. Comp Biochem Physiol C. 2005;141:50–57. doi: 10.1016/j.cca.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Mortari MR, Cunha AO, de Oliveira L, Vieira EB, Gelfuso EA, Coutinho-Netto J, et al. Anticonvulsant and behavioural effects of the denatured venom of the social wasp Polybia occidentalis (Polistinae, Vespidae) Basic Clin Pharmacol Toxicol. 2005;97:289–95. doi: 10.1111/j.1742-7843.2005.pto_137.x. [DOI] [PubMed] [Google Scholar]
- 33.Mortari MR, Cunha AO, Carolino RO, Coutinho-Netto J, Tomaz JC, Lopes NP, et al. Inhibition of acute nociceptive responses in rats after i.c.v. injection of Thr6-bradykinin, isolated from the venom of the social wasp Polybia occidentalis. Br J Pharmacol. 2007;151:860–69. doi: 10.1038/sj.bjp.0707275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paxinos G, Watson C. Sidney: Academic Press; 1986. The rat brain in stereotaxic coordinates. [DOI] [PubMed] [Google Scholar]
- 35.Lüttjohann A, Fabene PF, Luijtelaar GV. A revised Racine's scale for PTZ-induced seizures in rats. Physiol Behav. 2009;98:579–86. doi: 10.1016/j.physbeh.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Speller JM, Westby GW. Bicuculline-induced circling from the rat superior colliculus is blocked by GABA microinjection into deep cerebelar nuclei. Exp Brain Res. 1996;110:425–34. doi: 10.1007/BF00229142. [DOI] [PubMed] [Google Scholar]
- 37.Beleboni RO, Pizzo AB, Fontana ACK, Carolino ROG, Coutinho-Netto J, dos Santos WF. Spider and wasp neurotoxins: pharmacological and biochemical aspects. Eur J Pharmacol. 2004;493:1–17. doi: 10.1016/j.ejphar.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 38.Liberato JL, Cunha AO, Mortari MR, Gelfuso EA, Beleboni RO, Coutinho-Netto J, et al. Anticonvulsant and anxiolytic activity of FrPbAII, a novel GABA uptake inhibitor isolated from the venom of the social spider Parawixia bistriata (Araneidae: Araneae) Brain Res. 2006;1124:19–27. doi: 10.1016/j.brainres.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 39.Gelfuso EA, Cunha AO, Mortari MR, Liberato JL, Paraventi KH, Beleboni RO, et al. Neuropharmacological profile of FrPbAII, purified from the venom of the social spider Parawixia bistriata (Araneae, Araneidae), in Wistar rats. Life Sci. 2007;80:566–72. doi: 10.1016/j.lfs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 40.White HS, Woodhead JH, Wilcox KS, Stables JP, Kupferberg HJ, Wolf HH. Discovery and preclinical development of antiepileptic drugs. In: Levy RH, Mattson RH, Meldrum BS, Perucca E, editors. Antiepileptic drugs. Philadelphia: Lippincott; 2002. pp. 36–48. [Google Scholar]
- 41.White HS. Clinical significance of animal seizure models and mechanism of action studies of potential antiepileptic drugs. Epilepsia. 1997;38:S9–17. doi: 10.1111/j.1528-1157.1997.tb04523.x. [DOI] [PubMed] [Google Scholar]
- 42.Löscher W, Schmidt D. Which animal model should be used in the search for new antiepileptic drugs? A proposal based on experimental and clinical considerations. Epilepsy Res. 1988;2:145–81. doi: 10.1016/0920-1211(88)90054-x. [DOI] [PubMed] [Google Scholar]
- 43.Kwan P, Sills GJ, Brodie MJ. The mechanisms of action of commonly used antiepileptic drugs. Pharmacol Ther. 2001;90:21–34. doi: 10.1016/s0163-7258(01)00122-x. [DOI] [PubMed] [Google Scholar]
- 44.Dawson GR, Tricklebank MD. Use of the elevated plus maze in the search for novel anxiolytic agents. Trends Pharmacol Sci. 1995;16:33–6. doi: 10.1016/s0165-6147(00)88973-7. [DOI] [PubMed] [Google Scholar]
- 45.Montgomery KC. The relation between fear induced by novel stimulation and exploratory behavior. J Comp Physiol Psychol. 1955;48:254–60. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 46.Graeff FG, Brandão ML. São Paulo: Quinta Edição, Lemos Editora; 1999. Neurobiologia das Doenças Mentais; p. 254. [Google Scholar]
- 47.De Deyn PP, D’Hooge R, Marescau B, Pei YQ. Chemical models of epilepsy and their applicability in the development of anticonvulsants. Epilepsy Res. 1992;12:87–110. doi: 10.1016/0920-1211(92)90030-w. [DOI] [PubMed] [Google Scholar]
- 48.File SE. Behavioral detection of anxiolytic action. In: Elliott JM, Heal DJ, Marsden CA, editors. Experimental approaches to anxiety and depression. New York: John Wiley and Sons; 1992. pp. 25–44. [Google Scholar]


