Abstract
Background:
In China, Atractylodes macrocephala is mainly distributed in Zhejiang province. It is, therefore, desirable to determine a reliable and accurate methodology to differentiate the samples collected from Zhejiang province. Although some studies on the fingerprints of Atractylodes macrocephala using HPLC have been published, none of them compared the processed product of Atractylodes macrocephala from different areas of Zhejiang province.
Materials and Methods:
Reversed-phase high-performance liquid chromatography (RP-HPLC) coupled with hierarchical cluster analysis was employed in the fingerprint analysis of Atractylodes macrocephala from Zhejiang province, China. The LC assay was performed on a reversed-phase C18 column with linear gradient elution using water and acetonitrile. The LC data showed considerable variation of chemical constituents among Atractylodes macrocephala populations.
Results:
21 and 22 characteristic peaks in the 14 grants of Atractylodes macrocephala and its processed product were determined in samples from different habitats of Zhejiang province, respectively. Their chromatographic patterns were generally consistent although their contents of chemical compositions were greatly different. The results of hierarchical cluster analysis showed that the samples could be divided into four groups; it was able to select excellent resources from the groups.
Conclusion:
This was the first report of hierarchical cluster analysis of crude and processed Atractylodes macrocephala according to their chemical fingerprints and could be applied to the intrinsic quality control of crude and processed Atractylodes macrocephala. The processing technique of Atractylodes macrocephala through the pilot-scale experiment was first studied and is simple and suitable for prepared yin pian of Atractylodes macrocephala industrial manufacture.
Keywords: Atractylodes macrocephala, chemical fingerprinting, hierarchical cluster analysis, high-performance liquid chromatography, processed
INTRODUCTION
Atractylodes macrocephala (Baizhu in Chinese), derived from the rhizome of Atractylodes macrocephala Koidz, is one of the oldest and most frequently used Chinese herbs for oriental medicine in China.[1,2,3] Pharmacological studies and clinical practice have demonstrated that Atractylodes macrocephala possesses various bioactivities, including diarrhea, abdominal pain, and insufficiency of the stomach, intestine, liver, kidney, or insufficiency of the spleen with abundance of dampness.[4,5,6] In recent years, the study of Atractylodes macrocephala is more and more urgent with the emergence of the concept of modernization on traditional Chinese medicine.[7,8,9] Many components, such as volatile oils, sesquiterpenoids, polysaccharides, amino acids, vitamins, resins and other ingredients have been found in Atractylodes macrocephala up till recently.[10,11,12,13]
In China, Atractylodes macrocephala is mainly distributed in Zhejiang province. It is, therefore, desirable to determine a reliable and accurate methodology to differentiate the samples collected from Zhejiang province. Although some studies on the fingerprints of Atractylodes macrocephala using HPLC have been published,[14,15] none of them compared the processed product of Atractylodes macrocephala from different areas of Zhejiang province.
Atractylodes macrocephala is normally in great demand and sometimes in shortage due to its wide applications. As China's national essence, Atractylodes macrocephala has a long history of thousands of years. It is the precious wealth of the Chinese nation, and its efficacy has attracted worldwide attention. However, the qualities of Atractylodes macrocephala and its processed product are very labile, and its species are also confusable in the markets. Among a variety of quality control methods, chemical fingerprinting (CFP) has gained more and more attention recently.[16,17] CFP could provide an overall picture of the chemical composition of herbal medicines, and has been widely used for their quality control.[18] Recently, many reports on the fingerprinting analysis of Atractylodes macrocephala are available. Most of these reports constructed the chromatographic fingerprint of Atractylodes macrocephala by designating common peaks, which were usually the main constituents, and then evaluated the quality of Atractylodes macrocephala or differentiated Atractylodes macrocephala batches by comparing the peak areas of common peaks or by similarity evaluation using professional analytical software. However, the validity of chemical fingerprinting for quality control might be compromised by the instability of the medicinal materials and HPLC conditions, especially the pump precision.
In the present study, a new CFP methodology coupled with hierarchical cluster analysis were first used for evaluating the quality of Atractylodes macrocephala and its processed product grown in Zhejiang province. In addition, the identification of marker compounds for classification and evaluation of the quality of crude and processed extracts were performed by means of RP-HPLC analysis.
MATERIALS AND METHODS
Samples, chemicals and reagents
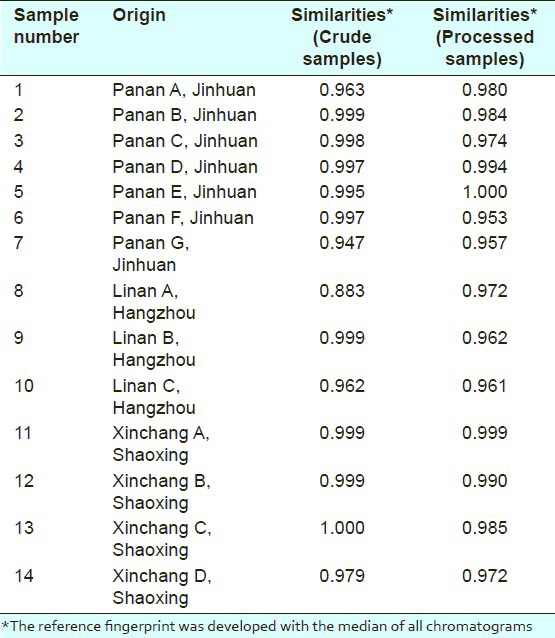
A total of 14 batches of crude Atractylodes macrocephala from different areas in Zhejiang province were investigated and collected [Table 1]. These herbal samples were authenticated by Professor Yun Zhang (Research Center of TCM Processing Technology, Zhejiang Chinese Medical University). Voucher specimens were stored at the Research Center of TCM Processing Technology. The reference standards Atractylenolide I and Atractylenolide III were purchased from the National Institute for the Control of Pharmaceutical and Biological Products. The chemical structures of these two compounds were confirmed by 1H, 13C nuclear magnetic resonance (NMR) spectroscopy and mass spectrum, and purities were over 98% by HPLC analysis.
Table 1.
Crude and processed materials used in this work and the similarity values of 14 chromatograms of Atractylodes macrocephala
The HPLC grade acetonitrile was purchased from Merck (Darmstadt, Germany), phosphoric acid was purchased from Tedia (Ohio, USA), and ultrapure water was prepared by a Milli-Q50 SP Reagent Water System (Millipore, Massachusetts, USA) for the preparation of samples and buffer solutions. Other reagents were of analytical grade.
Preparation of processed samples
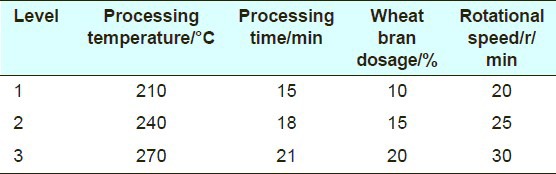
Atractylodes macrocephala was processed according to the Chinese Pharmacopoeia, edited in 2005 through pilot-scale experiment. In the processing procedure, the four relevant factors-processing temperature, processing time, wheat bran dosage, and rotational speed of stir-frying machines were investigated by the L9(34) orthogonal design [Table 2]. According to the scheme in the orthogonal list, 9 samples were obtained. Crude Atractylodes macrocephala is lurid, but the processed one is brown. The coarse powder of 9 processed samples was pretreated in the same way as mentioned above.
Table 2.
Orthogonal factor level test
Instrumentation and high-performance liquid chromatographic conditions
Analyses were performed using HPLC system Agilent 1200 (Agilent Technologies, Palo Alto, CA, USA) with diode array detector. Detection wavelength was set at 220 nm. An Agilent Zorbax Eclipse XDB- C18 column (250 mm × 4.6 mm, 5 μm) was used with a flow rate of 1.0 mL/min. The injection volume was 10 μL and the column temperature was maintained at 25°C. Mobile phase was composed of (A) acetonitrile and (B) water using a gradient elution of 30–60% A at 0–10 min, 60–65% A at 10–20 min, 65–100% A at 20–25 min, 100% A at 25–37 min.
Preparation of standard solutions
The primary stock standard solutions of two reference standards (Atractylenolide I and Atractylenolide III) were prepared by dissolving them with methanol, respectively, to get a concentration of 0.03 mg/mL and were stored at 4°C. The working mixed standard solutions were prepared daily by mixing and diluting the primary stock standard solutions with methanol. The RP-HPLC chromatogram of mixed standards is shown in Figure 1.
Figure 1.
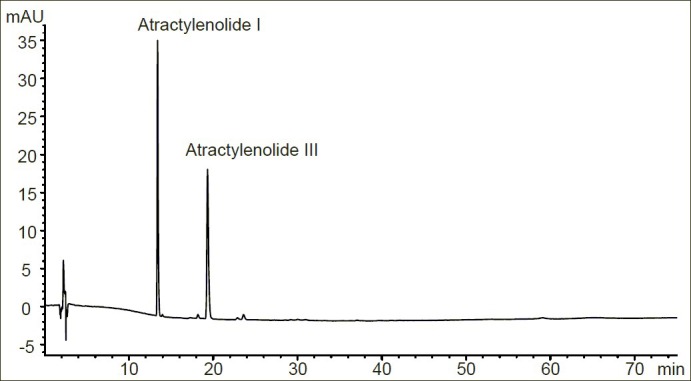
High-performance liquid chromatographic chromatogram of mixed standards
Preparation of sample solutions
The powders of crude and processed Atractylodes macrocephala samples were precisely weighed (0.300 g), and transferred into the dark brown calibrated flasks. They were extracted with 25 mL of methanol in an ultrasonic bath for 30 min. Additional methanol was added to make up the lost. The supernatants were filtered through a 0.45 μm membrane prior to injection.
Precision, repeatability and stability
To evaluate validation of the method, the crude Atractylodes macrocephala samples were extracted. The precision test was determined by repetitive injection of the same crude sample solution for six times in one day. The repeatability test was analyzed by injecting six independently prepared samples. The stability test was determined by six injections with one sample solution during 24 h. The relative standard deviations (RSDs) of relative retention times (RRTs) and relative peak areas (RPAs) of each test were calculated.
Data analysis and acquisition of characteristic information
After the analysis, recognition and comparison of chromatographic samples provide the characteristic information. One preferred index of composition is chosen as the reference standard, which is structurally accurate, stable and easy to obtain. Rational integral conditions are chosen and the characteristic peaks are identified. The nature of the RRT of the internal reference standard with regard to each characteristic peak is determined and the ratio of each chromatographic peak area to its weight is calculated by the following formula:
Xij = Aij / Wi (i = 1…m, j = 1…k)
where Xij is the ratio of the peak area j of the sample i to its weight, Aij is the peak area j of the sample i and Wi is the weight of the sample i. If there are no peaks for a certain fingerprint graph in a certain RRT and the RPA is zero, the relative series number is still given to guarantee that every chromatographic fingerprint has been obtained using the same number of chromatographic peaks.
The similarities, a commonly employed approach based on calculating the correlation coefficient and cosine value of the vector angle of the original data, are calculated based on the information obtained from the entire chromatographic profiles. The professional computer software based on chemometrics and recommended by SFDA, which is entitled the Similarity Evaluation System for Chromatographic Fingerprint of Traditional Chinese Medicine, is used in the similarity analysis of chromatographic and spectral patterns and was employed to calculate the similarities of fingerprint profiles. The similarity evaluation system for chromatographic fingerprint of TCM was developed by the Chinese Pharmacopoeia Commission in 2004 in Beijing; it could reflect the similarity of the distribution ratio of the chemical composition accurately, rather than as a function of the quantitative evaluation. The professional software used to evaluate the similarity of traditional Chinese medicine fingerprint profiles was in specific accordance with the state regulations of China and was purchased from the Chinese Pharmacopoeia Commission.
Hierarchical cluster analysis is a general approach to cluster analysis, in which the object is to group together objects or records that are “close” to one another. The hierarchical cluster analysis of samples was performed using Matlab. A method called average linkage between groups was applied and Pearson correlation was selected as a measurement. The common characteristic peaks, which were calculated by the Similarity Evaluation System, were selected for the hierarchical cluster analysis.
RESULTS AND DISCUSSION
Optimization of chromatographic conditions
In order to obtain better chromatograms, different mobile phase compositions, including water-acetonitrile; water-methanol; aqueous phosphoric acid (0.1%, v/v)-acetonitrile; aqueous phosphoric acid (0.2%, v/v)-acetonitrile were tested. As a result, the combination of water-acetonitrile for mobile phase was the best for separation. Furthermore, other chromatographic variables were also optimized, including analytical columns (Hanbon Lichrospher C18 and Agilent Zorbax Extend C18), the column temperatures (20°C, 25°C and 30°C) and the flow rates (0.5 mL/min, 0.8 mL/min and 1.0 mL/min). Eventually, the optimal separation was achieved on an Agilent Zorbax Extend C18 column (250 mm × 4.6 mm, 5 μm) at a column temperature of 25°C with a flow rate of 1.0 mL/min.
To obtain the optimal extraction efficiency, a previous study had chosen ethanol as extraction solvent. Simultaneously, in the present study, extraction methods (ultrasonic water bath), extraction time (30 min) and concentration of ethanol solution (80%, v/v) had also been selected. These findings were in accordance with the results previously reported.
Validation of analytical method
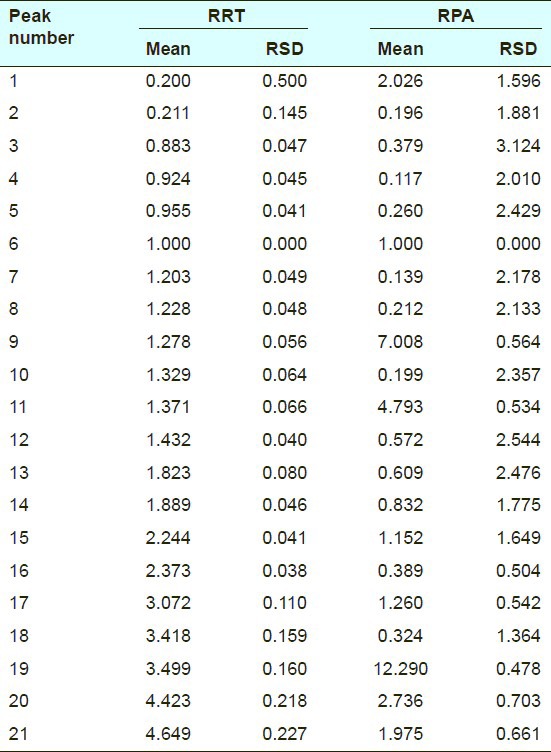
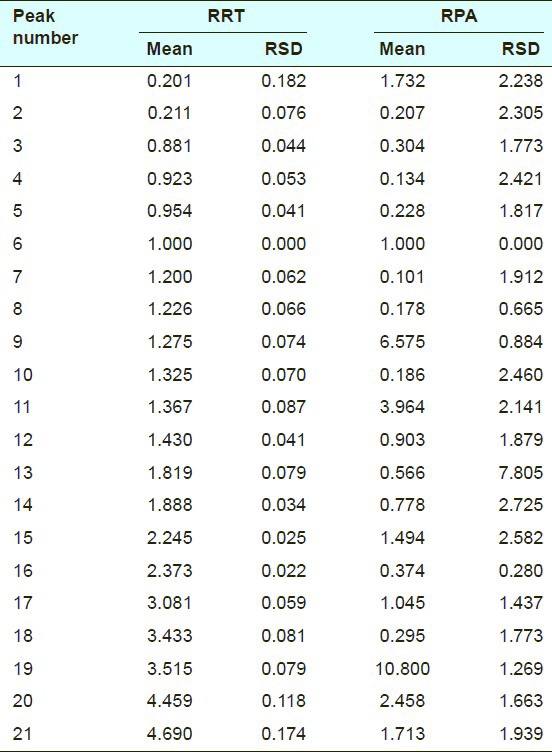
Injection precision was assessed by repetitive injection of the same crude Atractylodes macrocephala sample solution six times in one day. The results were shown in Table 3. The RSDs of RRT and RPA did not exceed 0.5% and 3.2%, respectively.
Table 3.
Results of precision test on highperformance liquid chromatographic fingerprint of crude Atractylodes macrocephala (n = 6)
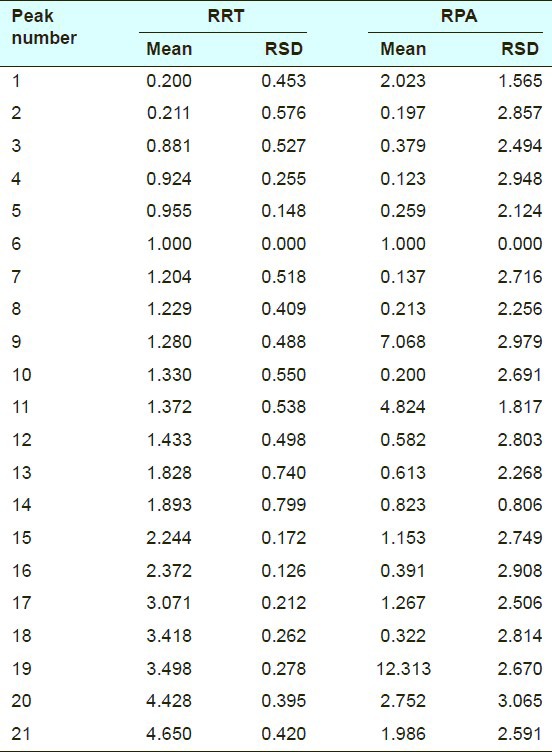
To further evaluate the repeatability of the developed assay, crude Atractylodes macrocephala were analyzed in six independently prepared samples as described above. The RSDs were taken as measurements of repeatability. The results were shown in Table 4. The RSDs of RRT and RPA were lower than 0.2% and 7.9%, respectively.
Table 4.
Results of repeatability test on highperformance liquid chromatographic fingerprint of crude Atractylodes macrocephala (n = 6)
Stability was tested with crude Atractylodes macrocephala at room temperature and analyzed at 0 h, 2 h, 4 h, 8 h, 12 h and 24 h, respectively. The results were shown in Table 5. The RSDs of RRT and RPA were below 0.8% and 3.1%, respectively.
Table 5.
Results of stability test on highperformance liquid chromatographic fingerprint of crude Atractylodes macrocephala (n = 6)
Analysis of chromatograms of crude and processed Atractylodes macrocephala
The chromatographic fingerprints showed abundant diversity of chemical constituents which were analyzed by authentication and quantification in crude and processed Atractylodes macrocephala from different populations [Figures 2 and 3]. A previous study showed that a LC method was mainly used for determining the contents of chemical constituents of Atractylodes macrocephala. In this paper, a LC chemical fingerprinting method was utilized and developed for quality control of crude and processed Atractylodes macrocephala and an evaluation system for crude and processed Atractylodes macrocephala with the sum of the contents of Atractylenolide I and Atractylenolide III (AI + AIII) was set up for the first time. Under the optimized conditions of extraction and chromatographic separation, a well-separated and reproducible chromatogram was achieved.
Figure 2.
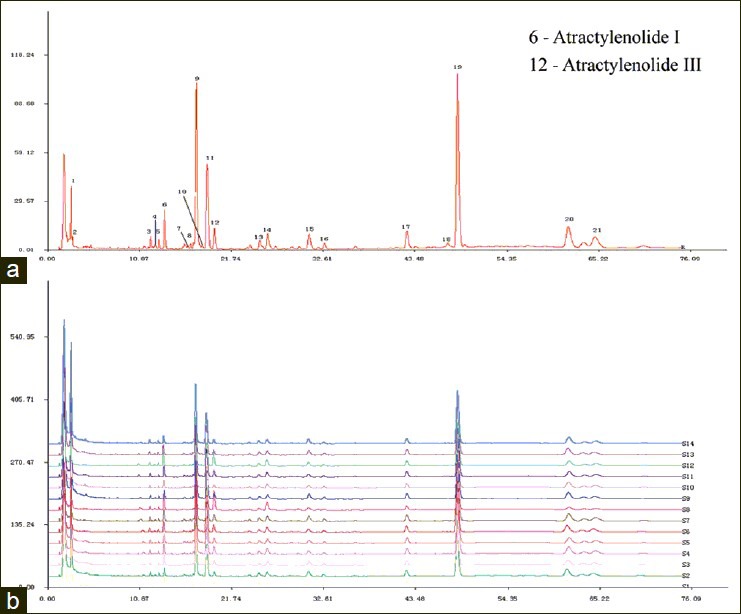
Original LC profiles of 21 common peaks (a) and the comparison of chromatographic fingerprints of crude Atractylodes macrocephala collected from 14 origins (b)
Figure 3.
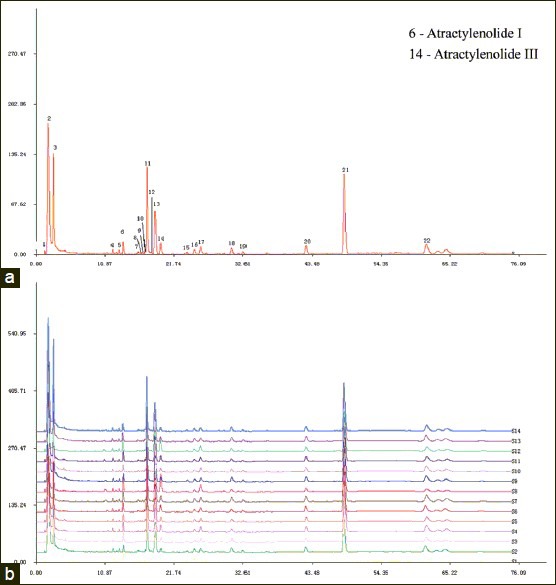
Original LC profiles of 22 common peaks (a) and the comparison of chromatographic fingerprints of processed Atractylodes macrocephala collected from 14 origins (b)
A total of 21 and 22 peaks were marked as the common peaks in the chromatograms in a total of 14 crude and processed Atractylodes macrocephala, respectively. 2 peaks were identified using standards. In the present study, according to the contents and pharmacological properties of major constituents in crude and its processed product, the peaks of number 6 and 12 in crude drug are Atractylenolide I (13.821 min) and Atractylenolide III (19.752 min). Peak 6 was used as a standard for the calculation of RRT and RPA, because it was a single peak in the chromatograms of all the 14 samples. Simultaneously, the peaks of number 6 and 14 in processed drug are Atractylenolide I (13.719 min) and Atractylenolide III (19.645 min) and the peak 6 was chosen as reference peak. The contents of above two compounds in crude and processed Atractylodes macrocephala samples analyzed were listed in Table 6. The results showed that the content of each compound in crude and processed drugs varied slightly. For instance, for Atractylodes macrocephala collected from Panan A, the contents of Atractylenolide I were 0.28 mg/g and 0.38 mg/g in crude and processed drugs, respectively, and the content of Atractylenolide III in crude sample was a little higher than that in its processed product. The variations might result from different processing procedures for Atractylodes macrocephala. It could also be seen that the total contents of two compounds varied significantly in the same type of samples from different suppliers, which might be due to the differences in soils and climates in each region. Thus it is necessary to control the main active components in Atractylodes macrocephala by good agricultural practice (GAP) and the norm of Chinese medicinal materials processing.
Table 6.
Contents of AI, AIII and AI + AIII in different crude and processed Atractylodes macrocephala (mg/g)
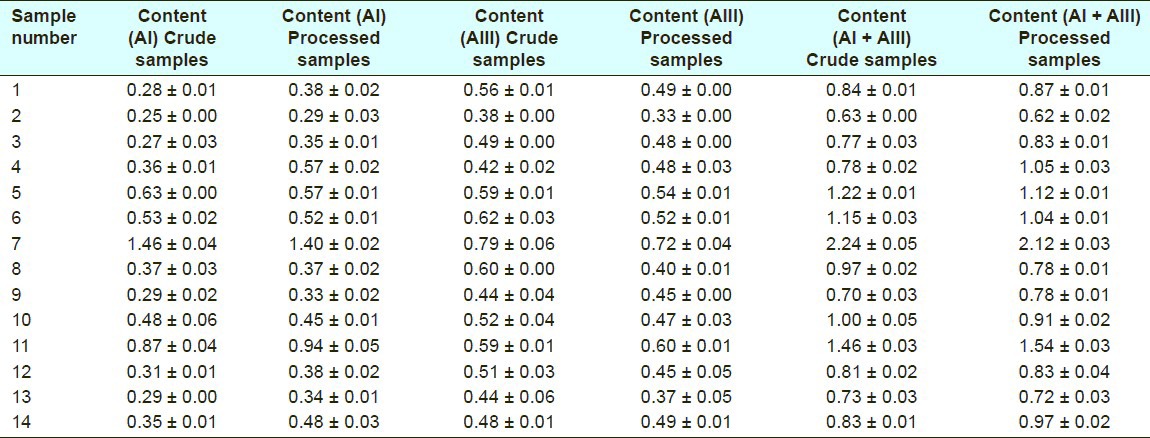
Altogether, 14 samples of crude or processed Atractylodes macrocephala were analyzed with the developed procedure, respectively. These samples were collected from a variety of geographical locations. Similarities among the 14 samples of crude or processed Atractylodes macrocephala were calculated using the similarity evaluation system recommended by SFDA. The results indicated that their chromatographic patterns were generally consistent, although the absorption intensities of some peaks were different. Authentication and identification of a herbal medicine can be accurately carried out using chromatographic fingerprints, even if batches or concentrations are not the same in analyzed samples.
Hierarchical cluster analysis
The areas of the 21 common peaks of crude samples were calculated; the areas of the 21 constituents of crude samples 1–14 formed a matrix of 21 × 14. The clustering analysis was operated in a Matlab software, and the results were shown in Figure 4. The result of hierarchical cluster analysis showed that the samples could be divided into four groups. Among them, the samples of S7 and S10 were included as separate categories, respectively; the samples of S3 and S5 were clustered into one group; and the remaining 10 samples were clustered into another group. Simultaneously, the result of hierarchical cluster analysis of processed Atractylodes macrocephala showed that the samples could also be divided into four groups [Figure 5]. The sample of Z13 was included as a separate category; the samples of Z8 and Z12 were clustered into one group; the samples of Z2, Z9, Z14 and Z10 were clustered into one group; and the remaining 7 samples were clustered into another group. In comparison to crude Atractylodes macrocephala and its processed product from different habitats, their contents of AI + AIII differ greatly, especially in samples No. 4, No. 8, and No. 14. As a result, we could choose excellent sources from these wild resources to breed new and better quality cultivars. This result agreed well with visual comparisons of their chromatograms [Figures 2 and 3]. The HCA provided a quantitative comparison.
Figure 4.
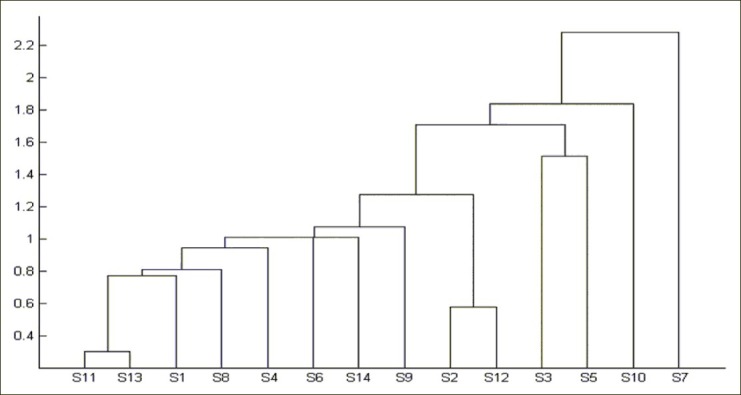
Results of hierarchical clustering analysis of crude Atractylodes macrocephala
Figure 5.
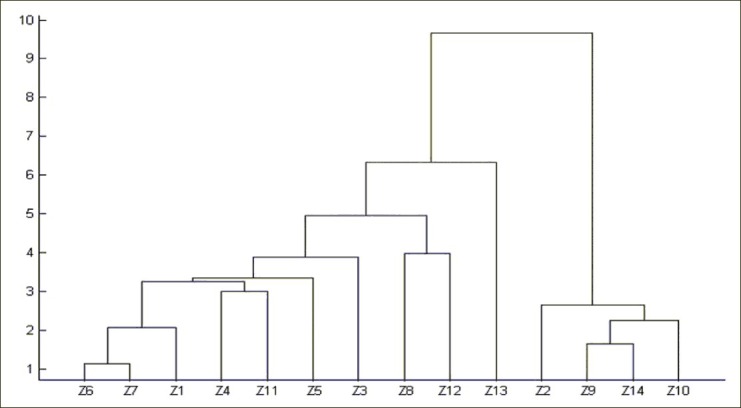
Results of hierarchical clustering analysis of processed Atractylodes macrocephala
CONCLUSION
In this study, a chemical fingerprinting combining similarity hierarchical cluster analysis method of characteristic bioactive compounds of Atractylodes macrocephala was developed, and 14 batches of crude and processed Atractylodes macrocephala from different regions in Zhejiang province were analyzed using this method. The 21 and 22 common fingerprint peaks in crude and processed samples were specified, respectively. The fingerprint analysis coupled with hierarchical cluster analysis method developed is reliable, reproducible, and valid and might be used as a more convenient approach for the species identification and quality assessment of crude and processed Atractylodes macrocephala from different regions in Zhejiang province. This result also provides a solid basis to establish good agriculture practice for Atractylodes macrocephala. In addition, selection of a suitable chromatographic system, screening of the optimal extraction condition, analysis of the chromatograms and validation of the analytical method were also included in this study. It was concluded that the established method could be useful for the quality control issues, which are critical to the production and processing industry of crude and processed Atractylodes macrocephala.
ACKNOWLEDGEMENTS
The authors are grateful to the financial support of the Medical Science Research Fund of the Ministry of Health of the People's Republic of China (No. WKJ2010-2-019), the National Natural Science Foundation of China (No.81202918, 81274056, 30873438 and 81173546), the Natural Science Foundation of Jiangsu Province, China (No. BK2009495), the International Science and Technology Cooperation Project of Jiangsu Province, China (No. BZ2011053), the Open Project of National First-Class Key Discipline for Science of Chinese Materia Medica, Nanjing University of Chinese Medicine (No. 2011ZYX2-006, No. 2011ZYX2-001), the Fund of Zhejiang Modernization of Traditional Chinese Medicine Item ([2008]436), and the Chinese Medicine Research Program of Zhejiang Province, China (2008ZA002).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Chen ZL. The acetylenes from Atractylodes macrocephala. Planta Med. 1987;53:493–4. doi: 10.1055/s-2006-962780. [DOI] [PubMed] [Google Scholar]
- 2.Endo K, Taguchi T, Taguchi F, Hikino H, Yamahara J, Fujimura H. Antiinflammatory principles of Atractylodes rhizomes. Chem Pharm Bull (Tokyo) 1979;27:2954–8. doi: 10.1248/cpb.27.2954. [DOI] [PubMed] [Google Scholar]
- 3.Li CQ, He LC, Jin JQ. Atractylenolide I and atractylenolide III inhibit lipopolysaccharide-induced TNF-α and NO production in macrophages. Phytother Res. 2007;21:347–53. doi: 10.1002/ptr.2040. [DOI] [PubMed] [Google Scholar]
- 4.He ST, He FZ, Wu CR, Li SX, Liu WX, Yang YF, et al. Treatment of rotaviral gastroenteritis with Qiwei Baizhu powder. World J Gastroenterol. 2001;7:735–40. doi: 10.3748/wjg.v7.i5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang YQ, Xu SB, Lin YC, Li Q, Zhang X, Lai YR. Antagonistic effect of 3 sesquiterpene lactones from Atractylodes macrocephala Koizd on rat uterine contraction in vitro. Acta Pharmacol Sin. 2000;21:91–6. [PubMed] [Google Scholar]
- 6.Huang HL, Chen CC, Yeh CY, Huang RL. Reactive oxygen species mediation of Baizhu-induced apoptosis in human leukemia cells. J Ethnopharmacol. 2005;97:21–9. doi: 10.1016/j.jep.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 7.Huang BS, Sun JS, Chen ZL. Isolation and identification of atractylenolide IV from Atractylodes macrocephala Koidz. Acta Botanica Sinica. 1992;34:614–7. [Google Scholar]
- 8.Resch M, Steigel A, Chen ZL, Bauer R. 5-lipoxygenase and cyclooxygenase-1 inhibitory active compounds from Atractylodes lancea. J Nat Prod. 1998;61:347–50. doi: 10.1021/np970430b. [DOI] [PubMed] [Google Scholar]
- 9.Dong HY, He LC, Huang M, Dong YL. Anti-inflammatory components isolated from Atractylodes macrocephala Koidz. Nat Prod Res. 2008;22:1418–27. doi: 10.1080/14786410801931629. [DOI] [PubMed] [Google Scholar]
- 10.Yu LL, Jian TZ, Cai Q. Research on the chemical references of Atractylodes macrocephala Koidz. Asia-Pacific Tradit Med. 2010;3:36–9. [Google Scholar]
- 11.Li W, Wen HM, Cui XB, Zhang FC, Dong J. Chemical constituents in rhizome of Atractylodes macrocephala. Chin Tradit Herbal Drugs. 2007;38:1460–2. [Google Scholar]
- 12.National Pharmacopoeia Committee. l. Beijing: Chemical Industry Press; 2005. China Pharmacopoeia; p. 68. [Google Scholar]
- 13.Li W, Wen HM, Cui XB, Li X, Hong M. Study on the effective components of rhizoma Atractylodis macrocephalae for invigorating the spleen. J Nanjing TCM Univ. 2006;22:366–7. [Google Scholar]
- 14.Shou D, Yu ZM, Zhang JM. Fingerprints establishment of rhizoma Atractylodis macrocephalae by high performance liquid chromatography and analysis of plant resource. China J Tradit Chin Med Pharm. 2010;25:466–9. [Google Scholar]
- 15.Yu C, Yang Y, Liu SY, Bai ZC, Zhang XM, Wang TT. Fingerprint of rhizoma Atractylodis macrocephalae by HPLC. Chin Tradit Herbal Drugs. 2006;37:766–9. [Google Scholar]
- 16.Tian L, Bi KS, Sun WJ, Zhao SC, Wu GF, Lv Y. Chemical pattern recognition in the rhizoma of Atractylodes macrocephala. China J Chin Materia Med. 2003;28:143–6. [PubMed] [Google Scholar]
- 17.Liu J, Chen XF, Yang WY, Zhang SP, Wang F, Tang ZX. Chemical fingerprinting of wild germplasm resource of Ophiopogon japonicus from sichuan basin, China by RP-HPLC coupled with hierarchical cluster analysis. Anal let. 2010;43:2411–23. [Google Scholar]
- 18.Wang Y, Han T, Zhang XG, Zheng CJ, Rahman K, Qin LP. LC Fingerprint and hierarchical cluster analysis of Crocus sativus L. from different locations in China. Chromatographia. 2009;70:143–9. [Google Scholar]


