Abstract
Background:
Hyperlipidemia is implicated as the cause for coronary heart diseases. Though varieties of synthetic drugs are used in the treatment, still the searches are on for better medicaments especially from the plant kingdom. Many medicinal plants have been studied in this context but most of them are seasonal or have restricted availability. One such weed, available throughout the year is Salvadora oleoides (decne.).
Materials and Methods:
Column chromatographic fractionation of the butanol fraction of leaves of Salvadora oleoides (decne.) yielded four fractions (fraction A-D). All sub-fractions were tested for their anti-hyperlipidemic activity. Fractions were administered at a dose of 65 mg/kg (oral) to the Triton WR-1339 induced hyperlipidemic rats.
Results:
Sub-fraction D showed maximum significant reduction (P<0.05) among four sub-fractions in comparison with standard drug fenofibrate.
Conclusion:
Further studies on the isolated fractions and constituents are needed to isolate compound responsible for activity and elucidate the mechanism by which Salvadora oleoides (decne.) exerts protective effects against hyperlipidemia.
Keywords: Antihyperlipidemic, Atherosclerosis, fenofibrate, Salvadora oleoides (decne.), Triton WR-1339
INTRODUCTION
Coronary heart diseases (CHD) are the main cause of death in western countries and Asia. Among CHDs, ischemic heart disease (IHD) leads to the highest mortality rate. The number of heart patients suffering from IHD worldwide is gradually increasing.[1]
In recent years much prominence has been given to the association of abnormal levels or values of lipid profile (e.g., total cholesterol, low density lipoprotein (LDL), very low density lipoprotein (VLDL) and triglycerides) with atherosclerosis and ischemic heart disease (IHD). Treatment of hyperlipidemia is preferably by the dietary factors accompanied by other natural regimes. Drug therapy is reserved for more intractable conditions. Thus, foremost in the development and management of atherosclerosis is the reduction of serum cholesterol levels. Individuals (men or women) with 33 to 44 years of age, the total cholesterol levels of 256mg/dL or over have a 5 times greater risks of developing coronary artery disease than those whose levels are below 220mg/dL.[2]
Herbal medicines are being used by about 80% of the world population primarily in the developing countries for primary health care. They have stood the test of time for their safety, efficacy, cultural acceptability and lesser side effects. The chemical constituents present in them are a part of the physiological functions of living flora and hence they are believed to have better compatibility with the human body.[3]
There are many ayurvedic and other herbs which is medicinal active against hyperlipidemia such as Terminalia arjuna,[4] Zingiber officinale,[4] Phyllanthus niruri,[4] Ginkgo biloba,[4] Allium Sativum,[4] Commiphora mukul,[4] Curcuma longa,[4] Erythrina variegate,[5] Murraya koenigii (curry leaf),[6] Camellia sinensis,[7] Solanum nigrum fruit,[8] Sida rhomboidea. Roxb[9] etc.
Salvadora oleoides (Decne.), belonging to family Salvadoraceae commonly found in Western region of India is an evergreen plant or shrub or a small tree. Bark is grey or whitish grey, Leaves linear-lanceolate, coriaceous, Flower sessile, greenish white minute in paniculate spikes, drupes globose, usually yellow when ripe; seeds are greenish yellow 3 m in diameter.[10] Leaves are used as purgative and as cure of cough. Leaves and stem are used for diabetes and hypercholestremia.[11]
EXPERIMENTAL
Drug and chemicals
Triton WR-1339 was purchased from Fisher Scientific, Belgium. Total cholesterol, Triglyceride, HDL estimations were done using the Seimen diagnostic kit. Silica gel 100-200 mesh size and solvents were purchased from Rankem ltd.
Plant material
Leaves of Salvadora oleoides (Decne.) were collected from Delhi (India). The plant material was deposited and authenticated by the Botanical Survey of India, Dehradun. Authenticated specimen number is Acc. No. 113246 and also authenticated by Dr. H. B. Singh, NISCAIR, New Delhi, reference no. NISCAIR/RHMD/Consult/2010-11/1675/273 and voucher specimen sample is preserved in Dept. of Pharmaceutical Sciences, S. B. S. P. G. I., Balawala, Dehradun for further reference.
The plant material was dried under shade and powdered. The 500g powdered material was extracted with methanol by cold percolation for 1 week. The extract was evaporated to dryness to obtain a residue.[12]
Fractionation by column chromatography
From total methanol extract, different fractions were prepared by cold percolation method using increasing polarity of solvents by separation technique i.e. Petroleum ether (Pet. ether), Chloroform, Ethyl acetate and Butanol. The Active butanol fraction was re-fractionated by column chromatography. Silica gel 100-200 mesh size was used for fractionation by column chromatography. Sufficient quantity of a column grade silica gel (100 - 200 mesh size) was wet-packed using chloroform solvent system. The active butanol fraction was first dissolved in methanol, and then mixed with sufficient amount of the silica gel to form slurry. The slurry was loaded into the wet packed column and continuously eluted with the mobile phase. From the column four sub-fractions A, B, C and D were obtained. The separation process was monitored by TLC (thin layer chromatography), using chloroform and methanol as mobile phase. The column was eluted with chloroform, 5% methanol in chloroform, 10% methanol in chloroform and 15% methanol in chloroform respectively. All four sub-fractions had different constituents as Sub-fraction ‘A’ showed three spots on TLC using mobile phase 5% methanol in Chloroform. The sub-fraction ‘B’ showed three spots on TLC using mobile phase 10% methanol in chloroform. The sub-fraction ‘C’ showed two spots on TLC using mobile phase 15% methanol, 10% ethyl acetate in chloroform. The sub-fraction ‘D’ showed two spots on TLC using mobile phase 30% methanol in chloroform.
Animals
Adult albino rats of both sexes weighing 180-300gm were procured from disease free CPCSEA approved animal house (Reg. no. 273/CPCSEA) of S. B. S. P. G. I. Dehradun. The animals were fed with standard pellet diet. The Institutional Animal Ethics committee (IAEC) of SBSPGI, Balawala, Dehradun approved the study.
Antihyperlipidemic study
Antihyperlipidemic studies were carried out and total cholesterol, triglycerides, HDL, LDL and VLDL level in the blood were checked.
Induction of hyperlipidemia
A single dose (350mg/kg body weight i.p) of Triton WR-1339 dissolved in 0.15 N NaCl solution was used for induction of hyperlipidemia in the rats. Hyperlipidemia was confirmed 48 hrs after triton injection by determining the blood cholesterol concentration.[13]
The quantities of individual drug (fraction) to be administered were calculated at a dose of 65 mg/kg b.w (Body weight). The drug was administered continuously for 7 days orally using infant feeding tube. The results were compared with that of the standard drug fenofibrate which was also given continuously for 7 days at a dose of 65 mg/kg b.w.[7]
Collection of blood and experimental setup
The rats were anaesthetized with diethyl ether and blood samples were drawn from the retro orbital plexus of eye. The rats were divided into seven groups having six animals in each group as follows:
Normal Group I - normal diet only
Control Group II
Group III (fraction A): received fraction ‘A’ at a dose of 65mg/kg b.w.
Group IV (fraction B): received fraction ‘B’ at a dose of 65mg/kg b.w.
Group V (fraction C): received fraction ‘C’ at a dose of 65mg/kg b.w.
Group VI (fraction D): received fraction ‘D’ at a dose of 65mg/kg b.w.
Group VII (Standard Drug): received fenofibrate at a dose of 65mg/kg b.w.
Blood cholesterol, triglycerides, LDL, HDL and VLDL profile were estimated before starting the treatment and end of the treatment period i.e. seven days.
Estimation of blood cholesterol and lipid profile
Total cholesterol estimation was done by using the seimen cholesterol diagnostic kit. Serum triglycerides were estimated by seimen triglycerides diagnostic kit. HDL cholesterol was estimated by seimen HDL diagnostic kit.
Cholesterol, triglycerides and HDL profile were estimated using standard monograph.
LDL cholesterol was calculated as[14]
LDL = Total Cholesterol - HDL - Triglycerides/5
VLDL was calculated using the formula[14]
VLDL = Triglycerides/5
Statistical analysis
All results are expressed as the mean±SEM. The results were analysed for statistical significance by Dunnett test of one-way ANOVA test.
RESULT AND DISCUSSION
Earlier we have preliminary reported that total methanol extract at a dose of 100mg/kg body weight shows reduction in cholesterol and triglycerides level in blood plasma.[15] After fractionation of methanol extract, we reported that the butanol fraction shows reduction in cholesterol, triglyceride, HDL, LDL and VLDL level in blood plasma.[16]
From this study we fractionated the butanol fraction by using column chromatography technique. Butanol fraction yielded four sub-fractions A-D. All four sub-fractions were tested against Triton WR-1339 induced hyperlipidemic rats. Results are shown in Tables 1-2 and Figures 1-5.
Table 1.
Effect of different sub-fractions of Salvadora oleoides (decne.) on cholesterol, triglycerides, HDL level in plasma of control and experimental rats
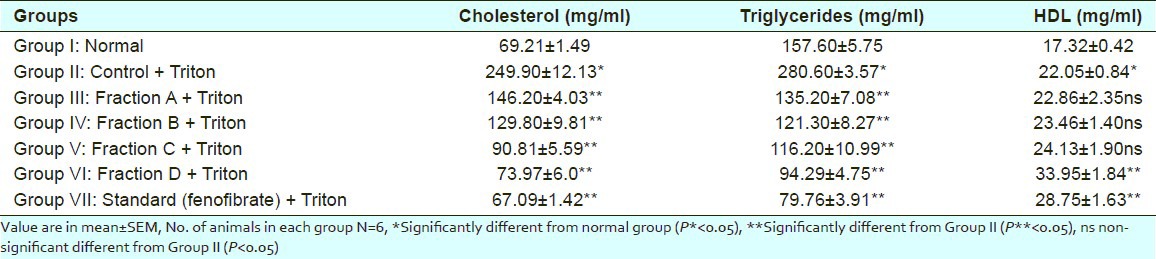
Table 2.
Effect of different sub-fractions of Salvadora oleoides (decne.) on LDL and VLDL level in plasma of control and experimental rats
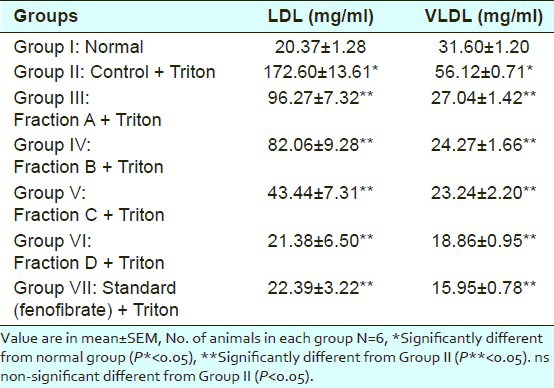
Figure 1.
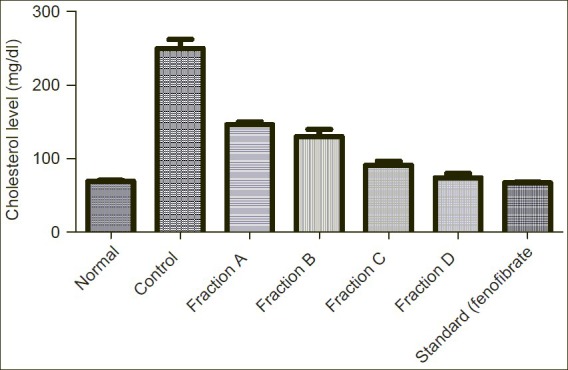
Showing effect of Sub-fractions on plasma cholesterol level on Triton induced hyperlipidemic rats
Figure 5.
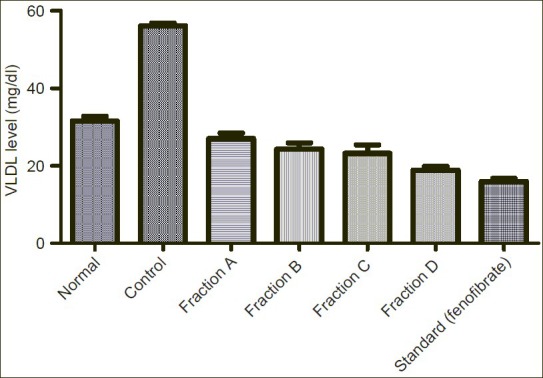
Showing effect of Sub-fractions on plasma VLDL level on Triton induced hyperlipidemic rats
Figure 2.
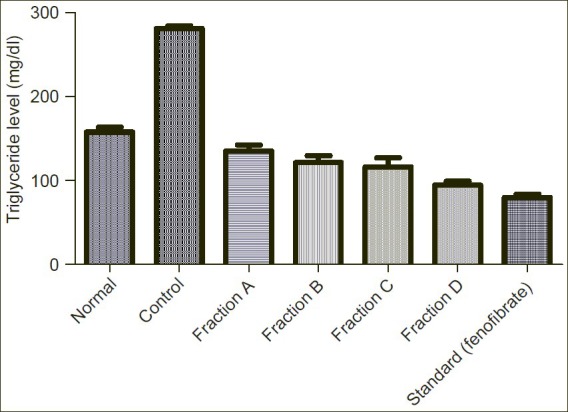
Showing effect of Sub-fractions on plasma triglycerides level on Triton induced hyperlipidemic rats
Figure 3.
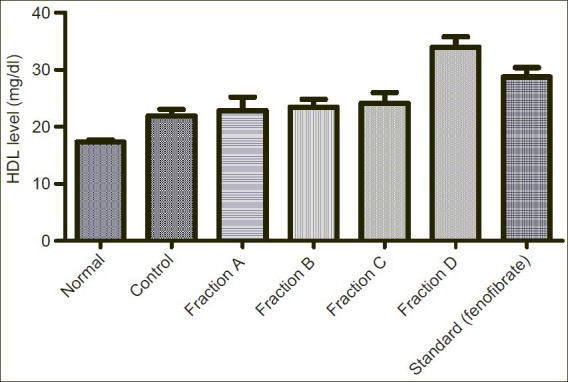
Showing effect of Sub-fractions on plasma HDL level on Triton induced hyperlipidemic rats
Figure 4.
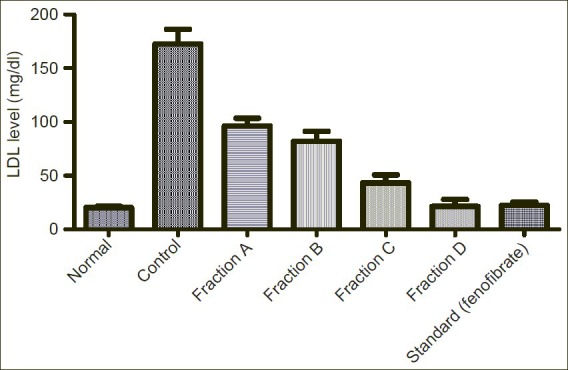
Showing effect of Sub-fractions on plasma LDL level on Triton induced hyperlipidemic rats
There was an elevation in plasma cholesterol, Triglycerides, HDL, LDL and VLDL-C levels in response to induction of hyperlipidemia by Triton WR- 1339 as compared to normal and control group. A significant increase was observed in cholesterol level from normal level 69.21mg/dl to 249.90mg/dl by Triton induced hyperlipidemic rats. On the treatment with all the sub-fractions A, B, C, D reduced the elevated cholesterol level to 146.20mg/dl, 129.80mg/dl, 90.81mg/dl and 73.97mg/dl respectively in comparison to standard drug (fenofibrate) 67.09mg/dl.
Triglyceride level was increased from normal level 157.60mg/dl to 280.60mg/dl. Sub-fraction A, B, C and D reduced the elevated triglyceride level to 135.20mg/dl, 121.30mg/dl, 116.20mg/dl and 94.29mg/dl respectively in comparison to standard drug (fenofibrate) 79.76mg/dl.
Elevated HDL level is good for health. After induction of Triton, HDL level increased from normal level 17.32mg/dl to 22.05mg/dl. Fraction A, B, C reduced the elevated HDL level reduced to 22.86mg/dl, 23.46mg/dl, 24.13mg/dl respectively but butanol fraction shows increase in HDL level to 33.95mg/dl in comparison to standard drug (fenofibrate) 28.75mg/dl.
LDL level was increased from normal level 20.37mg/dl to 172.60mg/dl by induction of Triton. Sub-fraction A, B, C and D reduced the elevated LDL level to 96.27mg/dl, 82.06mg/dl, 43.44mg/dl and 21.38mg/dl respectively in comparison to standard drug (fenofibrate) 22.39mg/dl.
VLDL level was increased from normal level 31.60mg/dl to 56.12mg/dl by induction of Triton. Sub-fraction A, B, C and D reduced the elevated VLDL level to 27.04mg/dl, 24.27mg/dl, 23.24mg/dl and 18.86mg/dl respectively in comparison to standard drug (fenofibrate) 15.95mg/dl.
All the results were statistically significant (P<0.05) and compared with normal and control group.
Thus among all fractions sub-fraction D showed significant reduction in plasma cholesterol (73.97mg/dl), triglyceride (94.29mg/dl), LDL (21.38mg/dl), VLDL (18.86mg/dl) and increase in HDL level (33.95mg/dl) as we know that HDL is good for health.
HDL is synthesized mainly in intestine and liver. HDL is considered to be a beneficial lipoprotein as it has an inhibitory effect in the pathogenesis of atherosclerosis. Low level of HDL is associated with high risk of coronary artery disease.[17]
CONCLUSION
From the above study it could be concluded that butanol sub-fraction D of Salvadora oleoides (decne.) not only have resulted in significant reduction in cholesterol, triglyceride, LDL, VLDL level but also increases the HDL level at a reduced dose level.
Further studies on the isolated fractions and constituents are needed to isolate compound responsible for activity and elucidate the mechanism by which Salvadora oleoides (decne.) exerts protective effects against hyperlipidemia.
ACKNOWLEDGEMENTS
Author's are thankful to Director and Management S. B. S. P. G. I., Balawala, Dehradun, India for providing necessary facilities and one of author is thankful to National Medicinal Plant Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India for financial support.
Footnotes
Source of Support: National Medicinal Plant Board, Department of AYUSH, Ministry of Health and Family Welfare, Government of India for financial support
Conflict of Interest: None declared.
REFERENCES
- 1.Patil RH, Prakash K, Maheshwari VL. Hypolipidemic Effect of Celastrus paniculatus in Experimentally Induced Hypercholesterolemic Wistar Rats. Indian J Clin Biochem. 2010;25:405–10. doi: 10.1007/s12291-010-0050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahmood ZA, Sualeh M, Mahmood SB, Karim MA. Herbal Treatment for Cardiovascular disease the evidence based therapy. Pak J Pharm Sci. 2010;23:119–24. [PubMed] [Google Scholar]
- 3.Kambooj V. Herbal medicine. Curr Sci. 2000;78:35–9. [Google Scholar]
- 4.Phadke SA. A review on lipid lowering activities of Ayurvedic and other herbs. Nat Prod Radiance. 2007;6:81–9. [Google Scholar]
- 5.Mangathayaru K, Balakrishna K, Kuruvilla S, Reddy C, Maheshwara U. Modulatory Effect of Erythrina vareigata on Experimental Hyperlipidaemia in Male Wistar Rats. Pharmacog Res. 2009;1:202–7. [Google Scholar]
- 6.Vinuthan MK, Girish Kumar V, Narayanaswamy N, Veena T. Lipid lowering effect of aqueous leaves extract of Murraya koenigii (curry leaf) on alloxan-induced male diabetic rats. Pharmacog Mag. 2007;3:112–5. [Google Scholar]
- 7.Saravana KA, Mazumder A, Saravanan VS. Antihyperlipidemic activity of Camellia sinensis leaves in Triton WR-1339 induced albino rats. Pharmacog Mag. 2008;4:60–4. [Google Scholar]
- 8.Arulmozhi V, Krishnaveni M, Karthishwaran K, Dhamodharan G, Mirunalini S. Antioxidant and antihyperlipidemic effect of Solanum nigrum fruit extract on the experimental model against chronic ethanol toxicity. Pharmacog Mag. 2010;6:42–50. doi: 10.4103/0973-1296.59965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devkar RV, Ramachandran AV, Patel DK. Assessment of lipid lowering effect of Sida rhomboidea Roxb. Methanolic extract in experimentally induced hyperlipidemia. J Young Pharma. 2009;1:233. [Google Scholar]
- 10.IX. New Delhi: Wealth of India, National Institute of Science and Communication, CSIR; 1999. pp. 193–4. [Google Scholar]
- 11.Yadav JP, Saini S, Kalia AN, Dangi AS. Hypoglycemic and hypolipidemic activity of ethanolic extract of Salvadora oloeides in normal and alloxan-induced diabetic rats. Indian J Pharmacol. 2008;40:23–7. doi: 10.4103/0253-7613.40485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Agarwal SS, Paridhavi M. Herbal Drug Technology. 1st ed, reprint. Universities Press (India) Private Ltd; 2009. pp. 323–4. [Google Scholar]
- 13.Schurr PE, Schultz JR, Parkinson TM. Triton-induced hyperlipidemia in rats as an animal model for screening hypolipidemic drugs. Lipids. 1972;7:68–74. doi: 10.1007/BF02531272. [DOI] [PubMed] [Google Scholar]
- 14.William TF, Robert IL, Donald SF. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 15.Kumar D, Parcha V, Dhulia I, Maithani A. Evaluation of Anti-Hyperlipidemic Activity of Methanol Extract of Salvadora oleoides (Linn.) leaves in Triton WR-1339 (Tyloxapol) induced hyperlipidemic rats. J Pharma Res. 2011;4:512–3. doi: 10.4103/0973-1296.103663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar D, Parcha V, Dhulia I, Maithani A. Effect and Evaluation of Antihyperlipidemic activity of fractions of total methanol extract of Salvadora oleoides (decne.) leaves on Triton WR-1339 (Tyloxapol) induced hyperlipidemic rats. Int J Res Pharma Sci. 2011;2:507–10. doi: 10.4103/0973-1296.103663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israni DA, Patel KV, Gandhi TR. Anti-Hyperlipidemic Activity of aqueous extract of Terminalia Chebula and Gaumutra in high cholesterol diet fed rats. Pharma Sci Monit. 2010;1:48–59. [Google Scholar]


