Abstract
A novel continuous subcritical n-butane extraction technique for Camellia seed oil was explored. The fatty acid composition, physicochemical properties, and benzo[a]pyrene content of Camellia seed oil extracted using this subcritical technique were analyzed. Orthogonal experiment design (L9(34)) was adopted to optimize extraction conditions. At a temperature of 45 °C, a pressure of 0.5 MPa, a time of 50 min and a bulk density of 0.7 kg/L, an extraction yield of 99.12 ± 0.20 % was obtained. The major components of Camellia seed oil are oleic acid (73.12 ± 0.40 %), palmitic acid (10.38 ± 0.05 %), and linoleic acid (9.15 ± 0.03 %). Unsaturated fatty acids represent 83.78 ± 0.03 % of the total fatty acids present. Eight physicochemical indexes were assayed, namely, iodine value (83.00 ± 0.21 g I/100 g), saponification value (154.81 ± 2.00 mg KOH/g), freezing-point (−8.00 ± 0.10 °C), unsaponifiable matter (5.00 ± 0.40 g/kg), smoke point (215.00 ± 1.00 °C), acid value (1.24 ± 0.03 mg KOH/g), refrigeration test (transparent, at 0 °C for 5.5 h), and refractive index (1.46 ± 0.06, at 25 °C). Benzo[a]pyrene was not detected in Camellia seed oil extracted by continuous subcritical n-butane extraction. In comparison, the benzo[a]pyrene levels of crude Camellia seed oil extracted by hot press extraction and refined Camellia seed oil were measured at 26.55 ± 0.70 and 5.69 ± 0.04 μg/kg respectively.
Keywords: Subcritical n-butane extraction, Camellia seed oil, Fatty acid composition, Physicochemical properties, Benzo[a]pyrene
Introduction
Camellia, which belongs to Camellia oleifera plants rich in oil, have been cultivated in China for more than 2000 years. C. oleifera plants are mainly distributed in south China (3.5 million hm2), and produce about 5.6 million tons of Camellia seeds each year [1]. Camellia seed oil, which is also called oriental olive oil, is recommended as a health-care plant oil by FAO because of its high content of unsaturated fatty acids, polyphenols, vitamin E and carotene.
Traditional processing methods such as hot press extraction and organic solvent extraction are generally applied to obtain the seed oil [2]. Supercritical fluid extraction is also attracting increasing attention because of its advantages of high yield and superior oil quality [3–5]. There are negative aspects to all three processing methods: traditional hot press extraction results in lower than desired yields, and organic solvent extraction is associated with environmental concerns; supercritical fluid extraction requires high pressures and is expensive, limiting its industrial application.
Recently, the presence of benzo[a]pyrene, a known carcinogen, in some commercially available Camellia seed oils has aroused widespread attention in society (http://finance.ifeng.com/news/special/jhcy/20100903/2586740.shtml), and the effect of different production processes on benzo[a]pyrene content in Camellia seed oil has become a focus of research. High temperature processing during both hot press and solvent extraction production techniques is considered to be a main cause of excessive benzo[a]pyrene formation [6]. Therefore, a more efficient, safer, and low-cost technique for extracting Camellia seed oil is needed.
Subcritical fluid extraction is performed at lower temperatures and pressures than those employed in supercritical fluid extraction. Subcritical fluid extraction is a continuous counter current process, After extraction, the solvent is removed using a vacuum at a low temperature. The subcritical fluid extraction process is safe and efficient, and does not damage the heat-sensitive components of the materials nor does it result in formation of benzo[a]pyrene. As there are few reports on the novel extraction of Camellia seed oil from Camellia seeds, the purpose of this study was to determine optimal continuous subcritical fluid extraction conditions for the production of Camellia seed oil. The study employed orthogonal experimental design and the parameters investigated in the extracted oil were fatty acid composition by gas chromatography–mass spectrometry (GC–MS), physicochemical properties, and benzo[a]pyrene content by high-performance liquid chromatography (HPLC).
Materials and Methods
Materials and Reagents
Camellia seeds, which were cultivated in Hunan Province, were supplied by Jing Hui Forestry Technology Development Center (Hengyang, China). After picking, drying and dehulling, the seed samples were stored at 4 °C in polythene bags until extraction. Crude Camellia seed oil and refined Camellia seed oil extracted by hot press extraction were supplied by Guangdong Taiyuan Nongke Co., Ltd. (Meizhou, China).
n-Butane solvent (99.99 %) was purchased from Shenzheng Shenyan Gas Co., Ltd. (Shenzhen, China). Benzo[a]pyrene was supplied by the Guangzhou Bureau of Quality and Technical Supervision (Guangzhou, China). Other chemicals were of analytical grade.
Preparation of Camellia Seed Oil by Continuous Subcritical n-Butane Fluid Extraction
The continuous subcritical fluid extractor used in the experiments was constructed by our laboratory. The extractions were conducted in a 5-L extraction vessel. Before extraction, the Camellia seeds were ground into a powder (10 mesh) and dried at 55 °C for 4–6 h to ensure moisture levels of <4 %. Based on single factor experiments, an orthogonal design L9(34) was conducted to optimize the extraction conditions (Table 1). The extraction yield was calculated with the following formula:
Table 1.
Primary variables and levels used in the orthogonal design
| Levels | Main variables | |||
|---|---|---|---|---|
| Pressure (A, MPa) | Time (B, h) | Temperature (C, °C) | Bulk density (D, kg/L) | |
| 1 | 0.4 | 30 | 35 | 0.6 |
| 2 | 0.5 | 50 | 40 | 0.7 |
| 3 | 0.6 | 70 | 45 | 0.8 |
Analysis of Fatty Acids by GC–MS
Before GC–MS analysis, the fatty acids of the experimental Camellia seed oil were derivatized into methyl esters [4, 7]. 50 mg of Camellia seed oil was weighed into a stoppered test tube and dissolved in 2 mL petroleum ether. Two (2) mL of 0.4 mol/L potassium hydroxide methanol solution was then added to the test tube, stirred, and held at room temperature for 30 min. Finally, distilled water was added and the organic phase was injected into a GC–MS system (Agilent Co., USA) for analysis. Qualitative analysis was performed using the standard peak retention times of fatty acids and the mass spectra library. Quantitative analysis of fatty acids was determined by measuring peak area.
The GC–MS conditions were set as follows: column oven initial temperature was 100 °C, programmed from 100 to 230 °C at 10 °C/min, and held for 40 min. The temperatures of the quadrupoles, the ion source chamber, and the split injector were held at 150, 230, and 250 °C, respectively. Helium was used as the carrier gas, and had a split ratio of 10:1.
Determination of the Physicochemical Properties
The physicochemical properties of the experimental Camellia seed oil were also measured, These included acid value, iodine value, saponification value, unsaponifiable matter, refractive index, freezing point, refrigeration test and smoke point, and were assayed according to the Chinese Pharmacopeia (2005) and GB 11765-2003 (a Chinese detection techniques and quality standard for the physicochemical properties detection of Camellia seed oil). All assays were carried out in triplicate.
Analysis of Benzo[a]pyrene Content by HPLC
The analysis of benzo[a]pyrene in Camellia seed oil was carried out according to the guidelines published by Wu et al. [6] and GB/T 5009.27-2003 (a Chinese detection techniques standard for benzo[a]pyrene detection in food). Before HPLC analysis, the Camellia seed oil was filtered through a chromatography column loaded with 22 g of alumina and 8.5 g of anhydrous sodium sulfate for purification. About 0.4 mg of the Camellia seed oil was weighed into a beaker and dissolved in 2 mL of petroleum ether. The sample solution was then added to the chromatography column and eluted with 80 mL of petroleum ether (flow rate of 1 mL/min). The eluent was dried with nitrogen. The residue was dissolved in 100 μL of acetone, mixed by vortex, and filtered through a 0.45-μm membrane filter before HPLC analysis. Qualitative analysis was performed using the retention time of standard benzo[a]pyrene, and quantitative calculation was performed using a standard curve. The peak areas of the sample in the chromatograms were correlated with the concentrations according to the calibration curve. The linearity was determined by using six standard solutions of benzo[a]pyrene with equidistant concentrations in the range of 0.05–30 μg/kg.
The HPLC chromatographic conditions were as follows: the benzo[a]pyrene was separated with a reversed-phase C-18 column (DiKMA, China) (250 × 4.6 mm, 5 μm). The mobile phase was a mixture of acetonitrile:water (88:12, v/v), and the flow rate was 1.0 mL/min. The benzo[a]pyrene was detected by a fluorescence detector at an excitation wavelength of 384 nm and an emission wavelength of 406 nm.
Statistical Analysis
One-way analysis of variance was used to show significant differences among the different treatments and the least significant differences were calculated at p = 0.05 level. All assays were carried out in triplicate and the results were expressed as mean values ± standard deviations.
Results and Discussion
Optimization of Continuous Subcritical Fluid Extraction
Based on the single factor experiments, an orthogonal design L9(34) was conducted to optimize the extraction pressure, time, temperature and bulk density (Table 2). Based on the R value, the effect of extraction variables on extraction yield decreased in the following order: bulk density (D) >time (B) >pressure (A) >temperature (C). The bulk density had a significant effect on the yield of the Camellia seed oil. Based on the K values of the variables, the potential highest extraction yield was expected to be obtained at a bulk density of 0.7 kg/L, time of 50 min, pressure of 0.5 MPa and temperature of 45 °C. Under these conditions, three confirmatory tests were carried out. The average extraction yield was 99.12 ± 0.20 %. Therefore, from a long-term perspective, continuous subcritical n-butane fluid extraction could be an alternative to the extraction of Camellia seed oil.
Table 2.
Results obtained under the experimental conditions using the L9(34) orthogonal design
| Trial | Pressure (A, MPa) | Time (B, h) | Temperature (C, °C) | Bulk density (D, kg/L) | Extraction yield (%) |
|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 97.71 ± 0.32 |
| 2 | 1 | 2 | 2 | 2 | 97.75 ± 0.39 |
| 3 | 1 | 3 | 3 | 3 | 98.1 ± 0.21 |
| 4 | 2 | 1 | 2 | 3 | 97.94 ± 0.93 |
| 5 | 2 | 2 | 3 | 1 | 96.76 ± 0.77 |
| 6 | 2 | 3 | 1 | 2 | 98.82 ± 0.86 |
| 7 | 3 | 1 | 3 | 2 | 98.23 ± 1.14 |
| 8 | 3 | 2 | 1 | 3 | 96.11 ± 0.91 |
| 9 | 3 | 3 | 2 | 1 | 96.34 ± 0.44 |
| k1 | 96.91 | 97.75 | 97.56 | 96.94 | |
| k2 | 97.84 | 97.96 | 97.34 | 98.27 | |
| k3 | 97.85 | 96.89 | 97.7 | 97.4 | |
| R | 0.94 | 1.07 | 0.35 | 1.33 |
Analysis of Fatty Acids
The fatty acid composition of Camellia seed oil produced by continuous subcritical n-butane fluid was analysed by GC–MS. The area normalization method was used to calculate the relative content of the fatty acids (Table 3). The major fatty acids of the Camellia seed oil were oleic acid, hexadecanoic acid, and linoleic acid. Oleic acid (73.12 ± 0.40 %) was the principal unsaturated fatty acid, followed by linoleic acid (9.15 ± 0.03 %). Palmitic acid (10.38 ± 0.05 %) was the predominant saturated fatty acid, followed by stearic acid (2.57 ± 0.40 %). The unsaturated fatty acids represented 83.78 ± 0.03 % of the total, including 73.98 ± 0.11 % monounsaturated fatty acids (MUFA) and 9.8 ± 0.04 % polyunsaturated fatty acids (PUFA). Studies have shown that MUFA and PUFA have many important physiological functions, such as reducing coronary heart disease risks [8], stabilizing the integrity of the cell membrane [9], and suppressing arthritis-associated inflammation [10]. The nutrient and healthcare functions of Camellia seed oil will become a research hotspot in the future because of superior fatty acid composition.
Table 3.
Fatty acid composition of Camellia seed oil extracted by continuous subcritical n-butane fluid extraction
| No. | Fatty acids | Retention time | Molecular formula | Chain | Relative content (%) |
|---|---|---|---|---|---|
| 1 | Myristic acid | 14.98 | C14H28O2 | C14:0 | 0.57 ± 0.06 |
| 2 | Hexadecenoic acid | 16.24 | C16H30O2 | C16:1 | 0.12 ± 0.01 |
| 3 | Palmitic acid | 17.9 | C16H32O2 | C16:0 | 10.38 ± 0.05 |
| 4 | Heptadecenoic acid | 19.29 | C17H32O2 | C17:1 | 0.24 ± 0.02 |
| 5 | Linoleic acid | 21.41 | C18H32O2 | C18:2 | 9.15 ± 0.03 |
| 6 | Oleic acid | 21.96 | C18H34O2 | C18:1 | 73.12 ± 0.40 |
| 7 | Octadecanoic acid | 22.33 | C18H36O2 | C18:0 | 2.57 ± 0.04 |
| 8 | Linolenic acid | 23.09 | C18H30O2 | C18:3 | 0.12 ± 0.00 |
| 9 | Arachidonic acid | 23.74 | C20H32O2 | C20:4 | 0.28 ± 0.05 |
| 10 | Eicosenoic acid | 25.78 | C20H38O2 | C20:1 | 0.5 ± 0.01 |
| 11 | Eicosanoic acid | 26.14 | C20H40O2 | C20:0 | 0.33 ± 0.00 |
| 12 | Others | 26.29 | C15H22O10 | C15:4 | 0.24 ± 0.07 |
| 14 | 26.41 | C18H36O4 | C18:0 | 0.02 ± 0.00 | |
| 15 | 27.51 | C27H42O4 | C27:6 | 0.01 ± 0.00 | |
| Saturated fatty acids | 13.89 ± 0.21 | ||||
| Unsaturated fatty acids | 83.78 ± 0.03 | ||||
| MUFA | 73.98 ± 0.11 | ||||
| PUFA | 9.8 ± 0.04 |
Physicochemical Properties
The physicochemical properties of an oil provide information regarding its structural stability and quality [11]. The physicochemical properties of Camellia seed oil obtained by continuous subcritical n-butane fluid are listed in Table 4. The iodine value (83.20 ± 0.21 gI/100 g), saponification value (154.81 ± 2.00 mg KOH/g), refractive index (1.46 ± 0.06, at 25 °C), freezing-point (−8.00 ± 0.10 °C), unsaponifiable matter (5.00 ± 0.40 g/kg), refrigeration test (transparent, at 0 °C for 5.5 h) and smoke point (215.00 ± 1.00 °C) met the Chinese national standards fora first-class solvent extracted oil based on GB 11765-2003. Although the acid value of 1.24 ± 0.03 mg KOH/g was higher than the value (≤0.25 mg KOH/g) in GB11765-2003, it met the requirement of GB 2716-2005 (4.0 mg KOH/g). Moreover, the higher acid value, probably caused by the long preservation time of Camellia seeds, could be reduced to 0.13 ± 0.02 mg KOH/g via molecular distillation. These physicochemical indexes show the possibility of obtaining a high-quality, high-grade, edible Camellia seed oil via continuous subcritical n-butane fluid extraction.
Table 4.
Physicochemical properties of Camellia seed oil extracted by continuous subcritical n-butane fluid extraction
| Physicochemical indexes | Camellia seed oil extracted by continuous subcritical n-butane fluid | National standard of a first-class solvent extraction oil (China) |
|---|---|---|
| Iodine value (gI/100 g) | 83.20 ± 0.21 | ≤85 |
| Saponification value (mg KOH/g) | 154.81 ± 2.00 | ≤185 |
| Refractive index (25 °C) | 1.46 ± 0.06 | 1.460–1.464 |
| Freezing point (°C) | −8.00 ± 0.10 | −5 to −10 |
| Unsaponifiable matter (g/kg) | 5.00 ± 0.40 | ≤15 |
| Refrigeration test (0 °C, 5.5 h) | Clear, transparent | Clear, transparent |
| Smoking point (°C) | 215.00 ± 1.00 | ≥215 |
| Acid value (KOH mg/g) | 1.24 ± 0.03 | ≤0.25 |
Benzo[a]pyrene Content
A number of polycyclic aromatic hydrocarbons (PAH) have been reported to be genotoxic carcinogens. Benzo[a]pyrene, a PAH, has become the focus of attention in Camellia seed oil, and researchers have found that different production processes had a great effect on benzo[a]pyrene content in Camellia seed oil [6]. The benzo[a]pyrene content in continuous subcritical n-butane fluid extraction and hot press extraction of Camellia seed oil (crude Camellia seed oil and refined Camellia seed oil) are shown in Fig. 1. No benzo[a]pyrene was present in Camellia seed oil extracted using the continuous subcritical n-butane fluid process, while a benzo[a]pyrene content of 26.55 ± 0.70 μg/kg was obtained in crude Camellia seed oil and 5.69 ± 0.04 μg/kg was obtained in refined Camellia seed oil extracted using a hot press process.
Fig. 1.
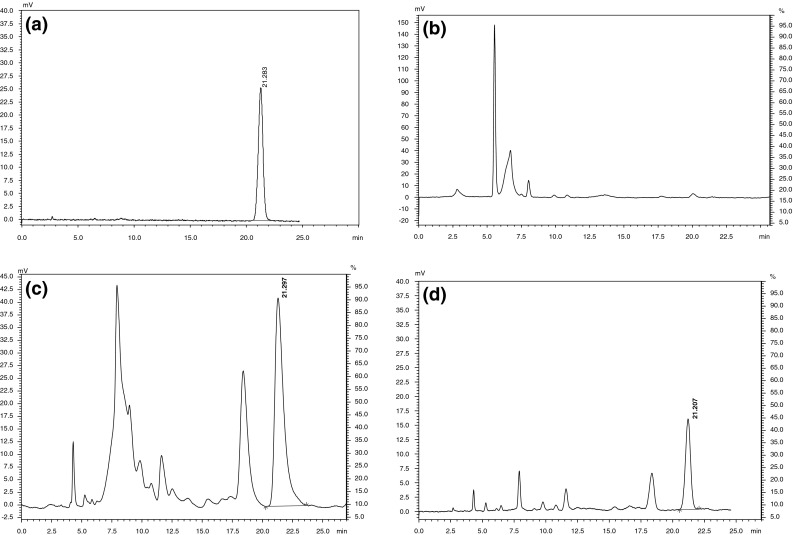
The analysis of benzo[a]pyrene content in different Camellia seed oils by HPLC. a HPLC chromatogram of a standard benzo[a]pyrene: the retention time was 21.28 min, b the analysis of benzo[a]pyrene content in Camellia seed oil extracted using continuous subcritical n-butane fluid: Benzo[a]pyrene was not found, c the analysis of benzo[a]pyrene content in crude Camellia seed oil extracted using hot pressing extraction: 26.55 μg/kg, d the analysis of benzo[a]pyrene content in refined Camellia seed oil extracted using hot pressing extraction 5.69 μg/kg
The major cause of excessive benzo[a]pyrene content in Camellia seed oil produced by the hot press method is the high temperature employed during extraction, whereas the subsequent refining process, bleaching and winterization, may lead to a reduction in the benzo[a]pyrene content. Because continuous subcritical n-butane extraction was carried out at the relatively mild temperature of 45 °C, there was no oil cracking, and therefore no benzo[a]pyrene formation. By using the simple processing method of continuous subcritical n-butane extraction, an oil of sufficiently high quality may be obtained that would obviate the need for further refining.
Conclusions
In this study, Camellia seed oil was produced by continuous subcritical n-butane fluid extraction. The composition of fatty acids, physicochemical properties, and benzo[a]pyrene content of Camellia seed oil were analyzed. Based on the results, the best conditions obtained for continuous subcritical n-butane fluid extraction were a bulk density of 0.7 kg/L, a time of 50 min, a pressure of 0.5 MPa, and a temperature of 45 °C. The major fatty acids of Camellia seed oil produced by continuous subcritical n-butane fluid included oleic acid (73.12 ± 0.40 %), palmitic acid (10.38 ± 0.05 %) and linoleic acid (9.15 ± 0.03 %); unsaturated fatty acids accounted for 83.78 ± 0.03 % of the total fatty acids. Eight main physicochemical properties reflecting the essential characteristics of Camellia seed oil produced by continuous subcritical n-butane fluid extraction were also analyzed. In particular, benzo[a]pyrene was not found in Camellia seed oil produced by continuous subcritical n-butane fluid extraction. The benzo[a]pyrene content in crude Camellia seed oil was 26.55 ± 0.70 and 5.69 ± 0.04 μg/kg in refined Camellia seed oil extracted using hot press extraction. The experimental results demonstrated that continuous subcritical n-butane fluid extraction is a simple process technology, by which a safe and high-quality Camellia seed oil could be produced.
Acknowledgments
The authors would like to express their sincere gratitude to the 948 project (2009-4-67) of the State Forestry Administration, P. R. China for the financial support of this research.
Footnotes
J. Miao and K. Che contributed equally to this article and should be considered co-first authors.
References
- 1.Zhang LL, Wang YM, Wu DM, Xu M, Chen JH. Microwave-assisted extraction of polyphenols from Camellia oleifera fruit hull. Molecules. 2011;16:4428–4437. doi: 10.3390/molecules16064428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jimenez P, Masson L, Barriga A, Chavez J, Robert P. Oxidative stability of oils containing olive leaf extracts obtained by pressure, supercritical and solvent-extraction. Eur J Lipid Sci Technol. 2011;113:497–505. doi: 10.1002/ejlt.201000445. [DOI] [Google Scholar]
- 3.Xu W, Chu KD, Li H, Chen LD, Zhang YQ, Tang YC. Extraction of Lepidium apetalum seed oil using supercritical carbon dioxide and anti-oxidant activity of the extracted oil. Molecules. 2011;16(12):10029–10045. doi: 10.3390/molecules161210029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Liu XS, Chen Y, Wang LH. Characterization of fatty oil of Zizyphi spinosi semen obtained by supercritical fluid extraction. J Am Oil Chem Soc. 2011;88:467–472. doi: 10.1007/s11746-010-1700-0. [DOI] [Google Scholar]
- 5.Xia L, You JM, Li GL, Sun ZW, Suo YR. Compositional and antioxidant activity analysis of Zanthoxylum bungeanum seed oil obtained by supercritical CO2 fluid extraction. J Am Oil Chem Soc. 2011;88:23–32. doi: 10.1007/s11746-010-1644-4. [DOI] [Google Scholar]
- 6.Wu SX, Zhang ZM, Liu RX, Huang SS (2012) Effect of production process on benzo[a]pyrene content in Camellia oil. Adv Mater doi:10.4028/www.scientific.net/AMR.554-556.1099
- 7.Kou XY, Yu GP. Researches on the methyl method of fat and fatty acid. Food Res Dev. 2006;26:46–47. [Google Scholar]
- 8.Stark AH, Madar Z. Olive oil as a functional food: epidemiology and nutritional approaches. Nutr Rev. 2002;66(6):170–176. doi: 10.1301/002966402320243250. [DOI] [PubMed] [Google Scholar]
- 9.López-Miranda J, Gómez P, Castro P, Marín C, Paz E, Bravo MD, Blanco J, Fuentes F, Pérez-Jiménez F. Mediterranean diet improves low density lipoprotein susceptibility to oxidative modifications. Med Clin. 2000;115(10):361–365. doi: 10.1016/S0025-7753(00)71561-2. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56(8):365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 11.Mabaleha MB, Yeboah SO. Characterization and compositional studies of the oils from some legume cultivars, Phaseolus vulgaris, grown in Southern Africa. J Am Oil Chem Soc. 2004;81(4):361–364. doi: 10.1007/s11746-004-0907-6. [DOI] [Google Scholar]


