Abstract
It has been suggested that the concentrations of tamoxifen and its demethylated metabolites increase with age. We measured the serum concentrations of the active tamoxifen metabolites, 4OHtamoxifen (4OHtam), 4-hydroxy-N-desmethyltamoxifen (4OHNDtam, Endoxifen), tamoxifen and its demethylated metabolites. Their relations to age were examined. One hundred fifty-one estrogen receptor and/or progesterone receptor positive breast cancer patients were included. Their median (range) age was 57 (32–85) years. Due to the long half-life of tamoxifen, only patients treated with tamoxifen for at least 80 days were included in the study in order to insure that the patients had reached steady-state drug levels. Tamoxifen and its metabolites were measured by liquid chromatography-tandem mass spectrometry. Their serum concentrations were related to the age of the patients. To circumvent effects of cytochrome (CYP) 2D6 polymorphisms we also examined these correlations exclusively in homozygous extensive metabolizers. The concentrations of 4OHNDtam, tamoxifen, NDtam (N-desmethyltamoxifen), and NDDtam (N-desdimethyltamoxifen) were positively correlated to age (n = 151, p = 0.017, 0.045, 0.011, and 0.001 respectively). When exclusively studying the CYP2D6 homozygous extensive metabolizers (n = 86) the correlation between 4OHNDtam and age increased (p = 0.008). Up to tenfold inter-patient variation in the serum concentrations was observed. The median (inter-patient range) concentration of 4OHNDtam in the age groups 30–49, 50–69, and >69 years were 65 (24–89), 116 (25–141), and 159 (26–185) ng/ml, respectively. We conclude that the serum concentrations of 4OHNDtam (endoxifen), tamoxifen, and its demethylated metabolites increase with age during steady-state tamoxifen treatment. This may represent an additional explanation why studies on the effects of CYP2D6 polymorphisms on outcome in tamoxifen-treated breast cancer patients have been inconsistent. The observed high inter-patient range in serum concentrations of tamoxifen and its metabolites, especially in the highest age group, suggest that use of therapeutic monitoring of tamoxifen and its metabolites is warranted.
Keywords: Tamoxifen, Metabolites, Age, Endoxifen, CYP2D6
Introduction
The selective estrogen receptor modulator (SERM) tamoxifen is used in the treatment of breast cancer patients with estrogen receptor positive (ER+) tumors. It is the gold standard in adjuvant endocrine therapy for premenopausal women and may also be used as a preventive agent in premenopausal as well as postmenopausal women with a high risk of breast cancer.
Tamoxifen acts as an estrogen antagonist or agonist depending on tissue type [1] which may be due to variation of ER co-activators and inhibitors in tissues. The agonistic effects of tamoxifen are associated with favorable effects, such as prevention of bone fractures, but also some rare, serious detrimental effects may occur, including endometrial cancer and venous thromboembolic events. Furthermore, adverse effects like hot flushes and vaginal discharge are often observed [2, 3]. Used adjuvantly a compliance of only 50 % has been observed after 5 years treatment [4–6].
The anticancer effect of tamoxifen is believed to be due to the hydroxylated metabolites, 4-hydroxytamoxifen (4OHtam), and 4-hydroxy-N-desmethyltamoxifen (4OHNDtam/endoxifen), because of their high affinity for the ER [7, 8], whereas the demethylated metabolites of tamoxifen have been associated with its side effects [9]. As 4OHNDtam is present in blood in concentrations about ten times higher than 4OHtam it is supposed to be the most important metabolite [10]. Multiple enzymes are involved in activation and inactivation of tamoxifen. Some of these enzymes are polymorphic, including CYP2D6, CYP2C19, CYP3A4 sulfotransferase (SULT), Uridine 5′-diphospho-glucuronosyltransferase (UGT) [11–14], and other drugs may modulate their activity [15–18] .
Multiple factors including drug interactions [15–18], genetic polymorphisms of tamoxifen metabolizing enzymes [10, 18–20], and seasonal variation [21] influence the pharmacokinetics of tamoxifen. Effects of these factors may explain inconsistent results from clinical studies linking genetic polymorphisms of these enzymes to clinical outcome during tamoxifen treatment [22–25].
In the present study we analysed serum samples from breast cancer patients obtained during steady-state tamoxifen treatment and observed that the serum levels of tamoxifen and its metabolites increase with age. Furthermore, the inter-patient range of serum concentrations was up tenfold and highest in the oldest age group. The results suggest that tamoxifen treatment may be improved by means of therapeutic drug monitoring.
Materials and methods
Study population
The study protocol and the main results have been described in detail elsewhere [10]. From October 2002 to October 2003, Caucasian women with breast cancer were consecutively recruited from Haukeland University Hospital, St Olav University Hospital and Førde Central Hospital in Norway. All patients were ER and/or PR positive. Written informed consent was obtained from each patient. The protocol was approved by the National Committee for Medical and Health Research Ethics (NEM). Due to the long half-life of tamoxifen, only patients treated with 20 mg tamoxifen adjuvant per day for more than 80 days were included in the study in order to insure that patients had reached steady-state drug levels. Postmenopausal status was defined as FSH levels >20 IU/L and estradiol (E2) levels <70 pmol/L, using the automated analyzer Immulite 2000 (Diagnostic Products Corporation, Los Angeles, CA). When only one of these criteria was fulfilled, the patients were defined as perimenopausal.
Determination of tamoxifen and its metabolites concentrations in serum
We used a high-pressure liquid chromatography–tandem mass spectrometry system that was developed for the determination of tamoxifen and its metabolites in serum [10, 26]. The assay was modified to improve the sensitivity by changing the API 2000/Qtrap mass spectrometry system from Applied Biosystems (AB MDS Sciex, Concord, Canada) to the API 4000, equipped with TurboIonSpray.
CYPD6 genotype determination
Genomic leukocyte DNA was extracted from EDTA anti-coagulated whole blood using the QIAamp DNA Blood Mini Kit (Qiagen, Hilden, Germany). The CYP2D6 genotypes were tested previously and subdivided into four groups and ranked according to their predicted increasing enzymatic activity: poor metaboliser [subjects with any combination of two non-functional variant type (Vt) alleles *3, *4, *5, and *6], intermediate metabolizer [heterozygous; wild type/variant type], wild type/wild-type (Wt/Wt) extensive metabolizer (EM) CYP2D6, and ultra-rapid metabolizer [heterozygous; Vt allele *2 × 2/wild type] [10].
Statistical analysis
Two-tailed Spearman correlation rank tests were used to examine the correlations between tamoxifen and metabolite concentrations in serum and breast cancer tissues. p < 0.05 was considered statistically significant. The Kruskal–Wallis test was used to compare samples from different age groups. All p values were two-sided. Statistical analyses were performed with IBM SPSS Statistics software, version 19 (SPSS Inc, Chicago, Illinois).
Results
One hundred fifty-one breast cancer patients were included in the study. The median (range) age of the patients was 57 (32–85) years. Fifteen of the patients were premenopausal, 17 perimenopausal, and 119 postmenopausal. We observed a more than tenfold inter-patient variation in serum concentrations of tamoxifen and some of its metabolites (Table 1). The concentrations of tamoxifen, 4OHNDtam, and NDDtam were positively correlated to age (Table 1). The number of poor, intermediate, extensive, and ultra-rapid metabolizers were 11, 49, 86, and 5, respectively. The overall CYP2D6 genotype distribution was in accordance with an expected Hardy–Weinberg equilibrium.
Table 1.
Serum concentrations of tamoxifen and its metabolites correlate with age
| Tamoxifen | 4OHtama | 4OHNDtam | NDtam | NDDtam | TamNox | |
|---|---|---|---|---|---|---|
| Serum concentrations (ng/ml)b | ||||||
| All (n = 151) | 89.9 (27.1–302.1) | 5.7 (1.7–17.2) | 49.6 (24.3–184.8) | 225.7 (90.1–690.9) | 37.0 (11.4–93.4) | 9.4 (2.7–37.9) |
| Wt/Wt EM (n = 86) | 90.5 (33.9–291.1) | 5.8 (2.2–17.2) | 52.3 (24.3–184.8) | 216.7 (90.1–596.3) | 37.7 (11.4–93.4) | 9.0 (3.2–29.0) |
| Correlations with age (R(p))c | ||||||
| All (n = 151) | 0.163 (0.045) | 0.120 (0.141) | 0.195 (0.017)) | 0.207 (0.011) | 0.260 (0.001) | 0.112 (0.172) |
| Wt/Wt EM (n = 86) | 0.203 (0.060) | 0.190 (0.080) | 0.285 (0.008) | 0.250 (0.020) | 0.263 (0.014) | 0.191 (0.079) |
a 4OHtam 4-hydroxytamoxifen, 4OHNDtam 4-hydroxy-N-demethyltamoxifen, NDtam N-demethyltamoxifen, NDDtam N-dedimethyltamoxifen, tamNox tamoxifen-N-oxide, Wt/Wt EM wild type/wild type extensive metabolizer CYP2D6
b Median (range)
c R and p values are derived from two-tailed Spearman correlation rank test
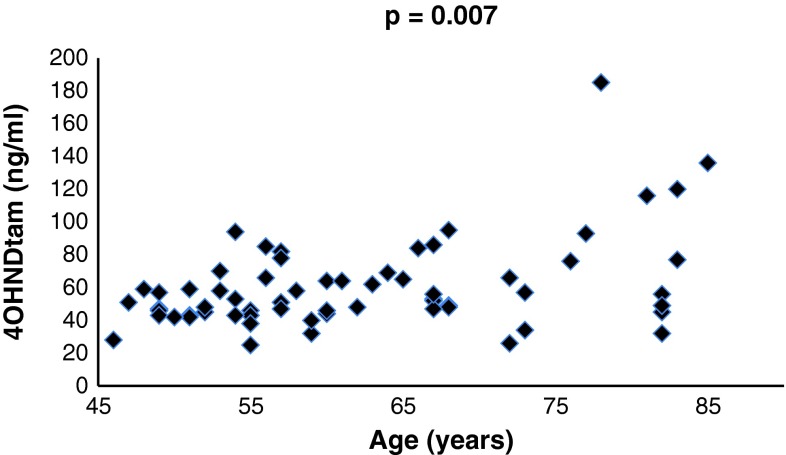
To circumvent effects of CYP2D6 polymorphisms we also studied these correlations exclusively in EMs. Although the number of patients included in this calculation was reduced from 151 to 86, the positive correlation between 4OHNDtam and age increased and the p value improved (Table 1). In the total subgroup of postmenopausal women (n = 119) the relationship between 4OHNDtam and age was not significant (p = 0.107, correlation coefficient 0.148). However, in the subgroup of CYP2D6 extensive metabolizers (n = 69), the correlation was highly significant (p = 0.007, correlation coefficient 0.322) (Fig. 1).
Fig. 1.
Serum levels of 4OHNDtam and age in postmenopausal patients with wt/wt CYP2D6 alleles (EM)
When analyzing the premenopausal subgroup separately, the serum concentrations of tamoxifen and its metabolites were not related to age (data not shown), possibly due to the low number of premenopausal patients (n = 15). However, a trend was observed for 4OHNDtam (p = 0.053). We also examined the median and range of the serum concentrations in the age groups 30–49 years, 50–69 years, and >69 years separately (Table 2). There was an up to tenfold inter-patient variation in the serum concentrations, and the inter-patient range increased with age. The Kruskal–Wallis test showed statistical differences in the serum levels of tamoxifen, 4OHtam, NDtam, and NDDtam between the age groups of 30–49, 50–69, and >69 years (data not shown).
Table 2.
Tamoxifen and metabolite concentrations in different age groups
| Age (years) | Tamoxifen | 4OHtam | 4OHNDtam | NDtam | NDDtam | TamNox |
|---|---|---|---|---|---|---|
| 30–49 (n = 33) | ||||||
| Median (ng/ml) | 75 | 5.5 | 44 | 187 | 26 | 7.8 |
| Range (min–max) | 176 (27–203) | 9.5 (1.7–11.3) | 65 (24–89) | 473 (90–563) | 77 (11–89) | 19 (2.7–21.2) |
| 50–69 (n = 83) | ||||||
| Median (ng/ml) | 95 | 5.8 | 51 | 229 | 38 | 9.5 |
| Range (min–max) | 271 (31–302) | 10 (2.8–13.0) | 116 (25–141) | 575 (116–691) | 78 (16–93) | 34 (3.5–38.0) |
| >69 (n = 35) | ||||||
| Median (ng/ml) | 96 | 5.5 | 54 | 255 | 42 | 10 |
| Range (min–max) | 188 (36–224) | 15 (2.2–17) | 159 (26–185) | 415 (97–512) | 72 (18–90) | 21 (3.9–25) |
Discussion
We observed in the present study that the serum concentrations of the active tamoxifen metabolite, 4OHNDtam (endoxifen), increase with age during steady-state tamoxifen treatment. Our findings are in line with a study published earlier this year by Teft et al. [21]. Peyrade et al. [27] and Sheth et al. [28] have previously observed that the serum concentrations of tamoxifen and its demethylated metabolites increased significantly with age.
Another important finding is that the high inter-patient variation in serum concentrations of 4OHNDtam was most pronounced in the oldest patient group, probably due to more concomitant diseases and increased use of additional drugs that may interact with tamoxifen metabolism. As creatinine clearance declines gradually with age and urinary elimination represents a major excretory route for 4OHNDtam [29], this may also cause higher 4OHNDtam levels in older patients [30]. The observation of higher 4OHNDtam levels and a high inter-patient variation of 4OHNDtam in the oldest patient group may in part explane the inconsistencies between studies linking CYP2D6 polymorphisms to outcome. This may further explain the finding of Margolin et al. [31] that the effect of CYP2D6 seems to derive mainly from premenopausal patients.
Tamoxifen dosage is based on the one-dose-fits-all approach. The up to tenfold variation in serum and tissue concentrations of tamoxifen and its metabolites [10, 32], which have been observed in multiple clinical studies, may influence the outcome and occurrence of adverse effects. It is still not sufficiently defined which concentrations are needed for optimal effects and at which concentrations side effects develop. Decensi et al. [33] observed in a preoperative study, using 1, 5, or 20 mg tamoxifen daily for 4 weeks, that the expression of the tumor proliferation marker Ki-67 decreased to the same extent irrespective of dose. In line with this, Moi et al. [34] observed in tumor tissues that an increase of coactivator mRNA levels seems to be an early response to tamoxifen without dose–response relationship in the 1- to 20-mg range.
Madlensky et al. [35] identified a threshold for the serum concentrations of 4OHNDtam suggesting that the women in the upper four quintiles of 4OHNDtam concentrations had a lower recurrence rate than women in the bottom quintile. Furthermore, Love et al. [36] suggested a J-shaped relationship between 4OHNDtam concentrations in individual patients and likelihood of recurrence. Quite recently, Gong et al. [37] using human MCF7 xenografts in athymic nu/nu mice observed a relationship between endoxifen concentrations and tumor growth inhibition.
Side effects of tamoxifen seem to be related to the serum levels of tamoxifen and its metabolites [38, 39]. Two studies report that the proportion of tamoxifen and its demethylated metabolites in serum was higher in patients with toxicity versus those not experiencing toxicity [9, 27]. In order to increase the serum level of 4OHNDtam in patients with decreased CYP2D6 activity, Kiyotani et al. [40] increased the daily doses of tamoxifen from 20 to 30 or 40 mg for at least 8 weeks, with no increase of side effects except from hyperhidrosis. Furthermore, some side effects like endometrial carcinoma and retina disease usually appear after long-term treatment and may be related to the cumulative dose [40]. Thus, we need more studies to better define the therapeutic range of 4OHNDtam serum levels. Are the high levels of tamoxifen and its metabolites in postmenopausal women of any clinical relevance after the advent of aromatase inhibitors?
Tamoxifen may still be a first line drug in the adjuvant treatment to some groups of postmenopausal women and could certainly be used in the last 3 years of the 5-year endocrine treatment period. In a recent study where 90 % of the patients included were postmenopausal, it was observed that prolonged tamoxifen treatment from 5 to 10 years halved breast cancer mortality during the second decade after diagnosis [41]. In the BIG 1-98 randomized trial, about half of the patients were node negative, i.e., at a low risk [42]. In this group the overall survival favored tamoxifen, although insignificantly, compared with treatment with the aromatase inhibitor letrozole. Moreover, osteoporosis and bone fractures were increased with letrozole compared to tamoxifen mono-therapy.
Tamoxifen treatment may be further improved by dose adjustment based on direct measurement of the active tamoxifen metabolites. Moreover, direct blood measurements may help to detect low compliance, which is a problem at least outside clinical studies [43]. Common criteria for therapeutic drug monitoring are fulfilled for tamoxifen, such as major inter-individual variation in metabolism, difficulties in observing toxicity clinically, and possibility of interactions and problems related to compliance.
In summary, we observed that the serum concentrations of 4OHNDtam (endoxifen) in breast cancer patients increase with age during steady-state tamoxifen treatment. The observed high inter-patient range in serum concentrations of tamoxifen and its metabolites, especially in the highest age group, suggest that use of therapeutic monitoring of tamoxifen and its metabolites is warranted inside as well as outside clinical studies.
Acknowledgments
This study has received support from The Western Norway Regional Health Authority, the Norwegian Cancer Society, the Research Council of Norway, the Grieg Foundation, the Frank Mohn Foundation, Torsteds Legat, and Odd Fellow Ordenen. We thank Elfrid Blomdal for technical assistance and Carol Cook for proof-reading the manuscript.
Conflict of interest
The authors declare they have no conflict of interest.
Abbreviations
- 4OHtam
4-Hydroxytamoxifen
- 4OHNDtam/endoxifen
4-Hydroxy-N-desmethyltamoxifen
- tamNox
Tamoxifen-N-oxide
- NDtam
N-desmethyltamoxifen
- NDDtam
N-desdimethyltamoxifen
- SERM
Selective estrogen receptor modulator
- ER
Estrogen receptor
References
- 1.Jordan VC. Metabolites of tamoxifen in animals and man: identification, pharmacology, and significance. Breast Cancer Res Treat. 1982;2:123–138. doi: 10.1007/BF01806449. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, Vogel V, Robidoux A, Dimitrov N, Atkins J, Daly M, Wieand S, Tan-Chiu E, Ford L, Wolmark N. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 3.Takei H, Ohsumi S, Shimozuma K, Takehara M, Suemasu K, Ohashi Y, Hozumi Y. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04) Breast Cancer Res Treat. 2012;133:227–236. doi: 10.1007/s10549-011-1943-y. [DOI] [PubMed] [Google Scholar]
- 4.Partridge AH, Wang PS, Winer EP, Avorn J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J Clin Oncol. 2003;21:602–606. doi: 10.1200/JCO.2003.07.071. [DOI] [PubMed] [Google Scholar]
- 5.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, Kwan M, Gomez SL, Neugut AI. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126:529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Owusu C, Buist DS, Field TS, Lash TL, Thwin SS, Geiger AM, Quinn VP, Frost F, Prout M, Yood MU, Wei F, Silliman RA. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26:549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 7.Lien EA, Solheim E, Kvinnsland S, Ueland PM. Identification of 4-hydroxy-N-desmethyltamoxifen as a metabolite of tamoxifen in human bile. Cancer Res. 1988;48:2304–2308. [PubMed] [Google Scholar]
- 8.Robertson DW, Katzenellenbogen JA, Long DJ, Rorke EA, Katzenellenbogen BS. Tamoxifen antiestrogens. A comparison of the activity, pharmacokinetics, and metabolic activation of the cis and trans isomers of tamoxifen. J Steroid Biochem. 1982;16:1–13. doi: 10.1016/0022-4731(82)90137-6. [DOI] [PubMed] [Google Scholar]
- 9.Gallicchio L, Lord G, Tkaczuk K, Danton M, Lewis LM, Lim CK, Flaws JA. Association of tamoxifen (TAM) and TAM metabolite concentrations with self-reported side effects of TAM in women with breast cancer. Breast Cancer Res Treat. 2004;85:89–97. doi: 10.1023/B:BREA.0000021050.92539.b0. [DOI] [PubMed] [Google Scholar]
- 10.Gjerde J, Hauglid M, Breilid H, Lundgren S, Varhaug JE, Kisanga ER, Mellgren G, Steen VM, Lien EA. Effects of CYP2D6 and SULT1A1 genotypes including SULT1A1 gene copy number on tamoxifen metabolism. Ann Oncol. 2008;19:56–61. doi: 10.1093/annonc/mdm434. [DOI] [PubMed] [Google Scholar]
- 11.Tamminga WJ, Wemer J, Oosterhuis B, de Zeeuw RA, de Leij LF, Jonkman JH. The prevalence of CYP2D6 and CYP2C19 genotypes in a population of healthy Dutch volunteers. Eur J Clin Pharmacol. 2001;57:717–722. doi: 10.1007/s002280100273. [DOI] [PubMed] [Google Scholar]
- 12.Nishiyama T, Ogura K, Nakano H, Ohnuma T, Kaku T, Hiratsuka A, Muro K, Watabe T. Reverse geometrical selectivity in glucuronidation and sulfation of cis- and trans-4-hydroxytamoxifens by human liver UDP-glucuronosyltransferases and sulfotransferases. Biochem Pharmacol. 2002;63:1817–1830. doi: 10.1016/S0006-2952(02)00994-2. [DOI] [PubMed] [Google Scholar]
- 13.Zanger UM, Raimundo S, Eichelbaum M. Cytochrome P450 2D6: overview and update on pharmacology, genetics, biochemistry. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:23–37. doi: 10.1007/s00210-003-0832-2. [DOI] [PubMed] [Google Scholar]
- 14.Raftogianis RB, Wood TC, Otterness DM, Van Loon JA, Weinshilboum RM. Phenol sulfotransferase pharmacogenetics in humans: association of common SULT1A1 alleles with TS PST phenotype. Biochem Biophys Res Commun. 1997;239:298–304. doi: 10.1006/bbrc.1997.7466. [DOI] [PubMed] [Google Scholar]
- 15.Kivisto KT, Villikka K, Nyman L, Anttila M, Neuvonen PJ. Tamoxifen and toremifene concentrations in plasma are greatly decreased by rifampin. Clin Pharmacol Ther. 1998;64:648–654. doi: 10.1016/S0009-9236(98)90055-8. [DOI] [PubMed] [Google Scholar]
- 16.Lien EA, Anker G, Lonning PE, Solheim E, Ueland PM. Decreased serum concentrations of tamoxifen and its metabolites induced by aminoglutethimide. Cancer Res. 1990;50:5851–5857. [PubMed] [Google Scholar]
- 17.Zhao XJ, Jones DR, Wang YH, Grimm SW, Hall SD. Reversible and irreversible inhibition of CYP3A enzymes by tamoxifen and metabolites. Xenobiotica. 2002;32:863–878. doi: 10.1080/00498250210158230. [DOI] [PubMed] [Google Scholar]
- 18.Stearns V, Johnson MD, Rae JM, Morocho A, Novielli A, Bhargava P, Hayes DF, Desta Z, Flockhart DA. Active tamoxifen metabolite plasma concentrations after coadministration of tamoxifen and the selective serotonin reuptake inhibitor paroxetine. J Natl Cancer Inst. 2003;95:1758–1764. doi: 10.1093/jnci/djg108. [DOI] [PubMed] [Google Scholar]
- 19.Nowell S, Sweeney C, Winters M, Stone A, Lang NP, Hutchins LF, Kadlubar FF, Ambrosone CB. Association between sulfotransferase 1A1 genotype and survival of breast cancer patients receiving tamoxifen therapy. J Natl Cancer Inst. 2002;94:1635–1640. doi: 10.1093/jnci/94.21.1635. [DOI] [PubMed] [Google Scholar]
- 20.Gjerde J, Geisler J, Lundgren S, Ekse D, Varhaug JE, Mellgren G, Steen VM, Lien EA. Associations between tamoxifen, estrogens, and FSH serum levels during steady state tamoxifen treatment of postmenopausal women with breast cancer. BMC Cancer. 2010;10:313–325. doi: 10.1186/1471-2407-10-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teft WA, Gong IY, Dingle B, Potvin K, Younus J, Vandenberg TA, Brackstone M, Perera FE, Choi YH, Zou G, Legan RM, Tirona RG, Kim RB. CYP3A4 and seasonal variation in vitamin D status in addition to CYP2D6 contribute to therapeutic endoxifen level during tamoxifen therapy. Breast Cancer Res Treat. 2013;139:95–105. doi: 10.1007/s10549-013-2511-4. [DOI] [PubMed] [Google Scholar]
- 22.Lash TL, Lien EA, Sorensen HT, Hamilton-Dutoit S. Genotype-guided tamoxifen therapy: time to pause for reflection? Lancet Oncol. 2009;10:825–833. doi: 10.1016/S1470-2045(09)70030-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goetz MP, Schaid DJ, Wickerham DL, Safgren S, Mushiroda T, Kubo M, Batzler A, Costantino JP, Vogel VG, Paik S, Carlson EE, Flockhart DA, Wolmark N, Nakamura Y, Weinshilboum RM, Ingle JN, Ames MM. Evaluation of CYP2D6 and efficacy of tamoxifen and raloxifene in women treated for breast cancer chemoprevention: results from the NSABP P1 and P2 clinical trials. Clin Cancer Res. 2011;17:6944–6951. doi: 10.1158/1078-0432.CCR-11-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moyer AM, Suman VJ, Weinshilboum RM, Avula R, Black JL, Safgren SL, Kuffel MJ, Ames MM, Ingle JN, Goetz MP. SULT1A1, CYP2C19 and disease-free survival in early breast cancer patients receiving tamoxifen. Pharmacogenomics. 2011;12:1535–1543. doi: 10.2217/pgs.11.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Regan MM, Leyland-Jones B, Bouzyk M, Pagani O, Tang W, Kammler R, Dell’orto P, Biasi MO, Thurlimann B, Lyng MB, Ditzel HJ, Neven P, Debled M, Maibach R, Price KN, Gelber RD, Coates AS, Goldhirsch A, Rae JM, Viale G, Breast International Group 1-98 Collaborative G CYP2D6 genotype and tamoxifen response in postmenopausal women with endocrine-responsive breast cancer: the breast international group 1-98 trial. J Natl Cancer Inst. 2012;104:441–451. doi: 10.1093/jnci/djs125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gjerde J, Kisanga ER, Hauglid M, Holm PI, Mellgren G, Lien EA. Identification and quantification of tamoxifen and four metabolites in serum by liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2005;1082:6–14. doi: 10.1016/j.chroma.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 27.Peyrade F, Frenay M, Etienne MC, Ruch F, Guillemare C, Francois E, Namer M, Ferrero JM, Milano G. Age-related difference in tamoxifen disposition. Clin Pharmacol Ther. 1996;59:401–410. doi: 10.1016/S0009-9236(96)90108-3. [DOI] [PubMed] [Google Scholar]
- 28.Sheth HR, Lord G, Tkaczuk K, Danton M, Lewis LM, Langenberg P, Lim CK, Flaws JA. Aging may be associated with concentrations of tamoxifen and its metabolites in breast cancer patients. J Womens Health (Larchmt) 2003;12:799–808. doi: 10.1089/154099903322447765. [DOI] [PubMed] [Google Scholar]
- 29.Kisanga ER, Mellgren G, Lien EA. Excretion of hydroxylated metabolites of tamoxifen in human bile and urine. Anticancer Res. 2005;25:4487–4492. [PubMed] [Google Scholar]
- 30.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 31.Margolin S, Lindh JD, Thoren L, Xie H, Koukel L, Dahl ML, Eliasson E. CYP2D6 and adjuvant tamoxifen: possible differences of outcome in pre- and post-menopausal patients. Pharmacogenomics. 2013;14:613–622. doi: 10.2217/pgs.13.47. [DOI] [PubMed] [Google Scholar]
- 32.Gjerde J, Gandini S, Guerrieri-Gonzaga A, Haugan Moi LL, Aristarco V, Mellgren G, Decensi A, Lien EA. Tissue distribution of 4-hydroxy-N-desmethyltamoxifen and tamoxifen-N-oxide. Breast Cancer Res Treat. 2012;134:693–700. doi: 10.1007/s10549-012-2074-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Decensi A, Robertson C, Viale G, Pigatto F, Johansson H, Kisanga ER, Veronesi P, Torrisi R, Cazzaniga M, Mora S, Sandri MT, Pelosi G, Luini A, Goldhirsch A, Lien EA, Veronesi U. A randomized trial of low-dose tamoxifen on breast cancer proliferation and blood estrogenic biomarkers. J Natl Cancer Inst. 2003;95:779–790. doi: 10.1093/jnci/95.11.779. [DOI] [PubMed] [Google Scholar]
- 34.Moi LL, Flageng MH, Gjerde J, Madsen A, Rost TH, Gudbrandsen OA, Lien EA, Mellgren G. Steroid receptor coactivators, HER-2 and HER-3 expression is stimulated by tamoxifen treatment in DMBA-induced breast cancer. BMC Cancer. 2012;12:247–258. doi: 10.1186/1471-2407-12-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madlensky L, Natarajan L, Tchu S, Pu M, Mortimer J, Flatt SW, Nikoloff DM, Hillman G, Fontecha MR, Lawrence HJ, Parker BA, Wu AH, Pierce JP. Tamoxifen metabolite concentrations, CYP2D6 genotype, and breast cancer outcomes. Clin Pharmacol Ther. 2011;89:718–725. doi: 10.1038/clpt.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Love RR, Desta Z, Flockhart D, Skaar T, Ogburn ET, Ramamoorthy A, Uy GB, Laudico AV, Van Dinh N, Quang LH, Van To T, Young GS, Hade E, Jarjoura D. CYP2D6 genotypes, endoxifen levels, and disease recurrence in 224 Filipino and Vietnamese women receiving adjuvant tamoxifen for operable breast cancer. SpringerPlus. 2013;2:52–56. doi: 10.1186/2193-1801-2-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong IY, Teft WA, Ly J, Chen YH, Alicke B, Kim RB, Choo EF. Determination of clinically therapeutic endoxifen concentrations based on efficacy from human MCF7 breast cancer xenografts. Breast Cancer Res Treat. 2013;139:61–69. doi: 10.1007/s10549-013-2530-1. [DOI] [PubMed] [Google Scholar]
- 38.Gualino V, Cohen SY, Delyfer MN, Sahel JA, Gaudric A. Optical coherence tomography findings in tamoxifen retinopathy. Am J Ophthalmol. 2005;140:757–758. doi: 10.1016/j.ajo.2005.04.042. [DOI] [PubMed] [Google Scholar]
- 39.Lindahl B, Andolf E, Ingvar C, Ranstam J, Willen R. Adjuvant tamoxifen in breast cancer patients affects the endometrium by time, an effect remaining years after end of treatment and results in an increased frequency of endometrial carcinoma. Anticancer Res. 2008;28:1259–1262. [PubMed] [Google Scholar]
- 40.Kiyotani K, Mushiroda T, Imamura CK, Tanigawara Y, Hosono N, Kubo M, Sasa M, Nakamura Y, Zembutsu H. Dose-adjustment study of tamoxifen based on CYP2D6 genotypes in Japanese breast cancer patients. Breast Cancer Res Treat. 2012;131:137–145. doi: 10.1007/s10549-011-1777-7. [DOI] [PubMed] [Google Scholar]
- 41.Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Muller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrutia G, Valentini M, Wang Y, Peto R, for the Adjuvant Tamoxifen: Longer Against Shorter Collaborative G Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: aTLAS, a randomised trial. Lancet. 2012;381:805–816. doi: 10.1016/S0140-6736(12)61963-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viale G, Regan MM, Dell’Orto P, Mastropasqua MG, Maiorano E, Rasmussen BB, MacGrogan G, Forbes JF, Paridaens RJ, Colleoni M, Lang I, Thurlimann B, Mouridsen H, Mauriac L, Gelber RD, Price KN, Goldhirsch A, Gusterson BA, Coates AS. Which patients benefit most from adjuvant aromatase inhibitors? Results using a composite measure of prognostic risk in the BIG 1-98 randomized trial. Ann Oncol. 2011;22:2201–2207. doi: 10.1093/annonc/mdq738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109:832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]