Abstract
Recently, alterations in the expression pattern of PPP2R5C associated with malignant transformation have been characterized, and PPP2R5C overexpression was demonstrated in leukemias. To confirm the role of PPP2R5C in proliferation and its molecular mechanism, three PPP2R5C-siRNAs and a scrambled nonsilencing siRNA control were used to treat Molt-4 and Jurkat T cells. After nucleofection, PPP2R5C expression and biological consequences based on a highly efficient and specific PPP2R5C-siRNA were demonstrated by qRT-PCR, CCK-8 assay, Annexin V/PI, and flow cytometry. The global gene expression profile of PPP2R5C-siRNA-treated Jurkat T cells was established. A significant reduction in the PPP2R5C mRNA level was observed at 24 to 72 h in Molt-4 and Jurkat T cells with all of the PPP2R5C-siRNAs. The proliferation rate of Molt-4 and Jurkat T cells transfected with different PPP2R5C-siRNAs was significantly decreased at 72 h compared with the control (p<0.05). However, the transfected cells did not show a significant increase in Annexin V/PI-positive cells (apoptosis). The highly efficient PPP2R5C-siRNA2 was used to treat Jurkat T cells for gene expression profile analysis. In total, 439 genes were upregulated, and 524 genes were downregulated at least twofold in PPP2R5C-siRNA-treated Jurkat T cells. Changes in signaling pathway genes closely related to the TCR, Wnt, calcium, MAPK, and p53 signaling pathways were observed. In conclusion, the suppression of PPP2R5C by RNA interference could effectively inhibit the proliferation of leukemic T cells, the PPP2R5C-siRNA treatment altered gene expression profiles, and the differential expression of the glycogen synthase kinase 3 beta (GSK-3β), ataxia telangiectasia mutated (ATM), and Mdm2 p53 binding protein homolog (MDM2) genes may play an important role in the effects of PPP2R5C knockdown in Jurkat T cells.
Knocking down PPP2R5C using siRNA altered downstream gene expression, and was associated with decreased proliferation of Jurkat cells. Among the differentially expressed genes are GSK-3β, ATM, and Mdm2 p53 binding protein homolog.
Introduction
T-cell acute lymphoblastic leukemia (T-ALL) accounts for 10–15% of newly diagnosed ALL cases in children and 20–25% of ALL cases in adults. Although treatment outcome for patients with T-ALL has improved in recent years, relapsed T-ALL remains a challenge (Aifantis et al., 2008). Monoclonal antibodies, gene inhibitors, and regulating microRNAs are promising tools for targeted cancer therapy (Peipp et al., 2002; Sato and Yamochi, 2005; Ishida et al., 2006; Ravandi et al., 2006; Rodig et al., 2006; Labbaye and Testa, 2012; Wang et al., 2012; Zinzani et al., 2012). However, most therapies have targeted B-cell malignancy and myeloid leukemia, and few targeted therapies are available for T-cell malignancies. For example, transforming Mer signals may contribute to T-cell leukemogenesis, and the regulation of Mer expression could be a novel therapeutic target for pediatric ALL therapy (Graham et al., 2006). The recent identification of Notch1-activating mutations in the majority of patients with T-ALL has stimulated interest in targeting the Notch signaling pathway for this disease (Palomero et al., 2009). Further advances in the treatment of T-cell malignancies require the development of novel agents that target specific malignancies without significant toxicity.
In 2001, synthetic small interfering RNAs (siRNAs) were demonstrated to be capable of inducing RNAi in mammalian cells by Thomas Tuschl (Elbashir et al., 2001). This discovery led to a surge in interest in harnessing RNAi for biomedical research and drug development. siRNA is a promising gene-targeting agent that has shown great potential, particularly in the field of cancer treatment (Devi, 2006; Oh et al., 2009; Whitehead et al., 2009). A combination of c-raf and bcl-2 siRNA induced apoptosis in HL-60, U937, and THP cell lines and increased chemosensitivity to etoposide and daunorubicin (Cioca et al., 2003). Based on the finding that B-cell chronic lymphocytic leukemia/lymphoma 11B (BCL11B) overexpression is detected in most T-cell malignancies (Oshiro et al., 2006; Liu et al., 2010; Huang et al., 2011), a previous study demonstrated that the inhibition of BCL11B expression by BCL11B-siRNA leads to apoptosis in Molt-4, Jurkat, and huT78 cell lines (Grabarczyk et al. 2007).
Recently, we characterized the novel chromosomal translocation t(14;14)(q11;q32), which causes rearrangement of the T-cell receptor (TCR) α gene J7 segment (TRAJ7) with the PPP2R5C gene (unpublished data) in a case of Sézary syndrome at the molecular level. PPP2R5C is a regulatory B subunit of protein phosphatase 2A (PP2A), which is a major cellular serine/threonine phosphatase that affects the phosphorylation status of many proteins (Muneer et al., 2002). The PPP2R5C gene encodes five differentially spliced variants: B56γ1, B56γ2, B56γ3, B56γ5, and B56γ6, whereas B56γ4 is identified only in mice. The locus for the functional PPP2R5C gene is at 14q32.2, whereas the nonfunctional B56γ1 pseudogene PPP2R5C is at 3p21.3 (Muneer et al., 2002; Lee et al., 2010). PPP2R5C plays a crucial role in cell proliferation, differentiation, and transformation based on its induction of the dephosphorylation of p53 at various residues (Shouse et al., 2008). It has been reported that the dynamic nuclear distribution of the B56γ3 regulatory subunit controls the nuclear PP2A activity and may be responsible for the tumor-suppressive function of PP2A (Lee et al., 2010). Recently, alterations in the expression pattern of PPP2R5C that are associated with malignant transformation have been characterized in lung cancer, and the PPP2R5C mutation F395C disrupts the B56γ–p53 interaction (Shouse et al., 2010).
We were also the first to find that PPP2R5C overexpression occurs in leukemias, including T-ALL (Zheng et al., 2011). To confirm the role of PPP2R5C in proliferation and its molecular mechanism, we screened highly efficient and specific PPP2R5C-siRNAs, which target different exons in the human T-ALL cell lines Molt-4 and Jurkat, and a highly efficient PPP2R5C-siRNA was used further to evaluate its molecular mechanism in the proliferation inhibition of leukemic T cells.
Methods
Cell culture
The human T-cell leukemia cell lines Molt-4 and Jurkat (Institutes for Biological Sciences Cell Resource Center, Chinese Academy of Sciences, Shanghai, China) were grown in the Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco-BRL) with 10% fetal calf serum (FCS) (Sijiqing Co.) and maintained in a humidified incubator at 37°C and 5% CO2. All experiments were performed using cells in the exponential growth phase.
siRNA design and synthesis
The siRNAs PPP2R5C-siRNA1, PPP2R5C-siRNA2 (Chinese patent number: ZL 2011 1 0340411.1), and PPP2R5C-siRNA3 (Chinese patent number: ZL 201110337837.1), which target domains in the fourth, sixth, and between the eighth and ninth exons in the PPP2R5C gene (ACCESSION NM_178587), respectively, and a scrambled nonsilencing siRNA control (SC) were designed with online software (www.invitrogen.com) and synthesized by Invitrogen (Table 1). An Alexa Red Oligo (Invitrogen) was used to measure transfection efficiency.
Table 1.
Sequences of PPP2R5C-siRNA and Scrambled Nonsilencing siRNA Control
| No. of siRNA | Target (nt) | Sense (5′-3′) | Antisense (5′-3′) | Chinese patent number |
|---|---|---|---|---|
| PPP2R5C-siRNA1 | 574–598 | UAGAGUCUCCAGAUUUCCAACCUAA | UUAGGUUGGAAAUCUGGAGACUCUA | |
| PPP2R5C-siRNA2 | 799–823 | GCAUAGCAGAGUUACUGGAAAUAUU | AAUAUUUCCAGUAACUCUGCUAUGC | 201110340411.1 |
| PPP2R5C-siRNA3 | 991–1015 | CAGUGGUGAUGGCACUUCUCAAAUA | UAUUUGAGAAGUGCCAUCACCACUG | 201110337837.1 |
| SC | UAGUCUACCUAGCUUAACCCAGUAA | UUACUGGGUUAAGCUAGGUAGACUA |
SC, scrambled nonsilencing siRNA control.
Nucleofection
Cells were collected by centrifugation and resuspended at 2.5×106 cells for Molt-4 and Jurkat cells per 100 μL of the appropriate Nucleofector™ kit solution (Amaxa Biosystems) (Huang et al., 2011; Chen et al., 2012; Zha et al., 2012). Malignant T cells were nucleofected with 3 μg of PPP2R5C-siRNAs or a control nonsilencing scrambled (SC) siRNA using the C-005 (Molt-4 cells) or X-001 (Jurkat cells) program of the Nucleofection Device II (Amaxa Biosystems). Mock-transfected cells nucleofected without siRNA were used as a negative control. After nucleofection, the cells were immediately mixed with 500 μL of prewarmed culture medium and transferred into culture plates. The treated cells were incubated at 37°C for 3 days for cell proliferation, apoptosis, and microarray analyses. Three independent experiments for Molt-4 or Jurkat T cells were preformed every 24 h.
RNA isolation, reverse transcription, real-time qRT-PCR, and microarray analysis
Total RNA was isolated from different samples (Molt-4 and Jurkat T cells) using TRIzol (Invitrogen). cDNA for qRT-PCR was synthesized using the Superscript II RNaseH reverse transcriptase kit (Invitrogen).
The expression level of PPP2R5C and the β2-MG reference gene was determined by SYBR Green I real-time PCR. PCR was performed as previously described (Zheng et al., 2011). The sequences for the primers used in qRT-PCR were as follows: PPP2R5C: 5′-GTAATAAAGCGGGCAGC AGG-3′ (forward) and 5′-CAAAGTCAAAGAGGACGCAACA-3′ (reverse) for PPP2R5C gene amplification and β2M: 5′-CAGCAAGGACTGGTCTTT CTAT-3′ (forward) and β2M 5′-GCGGCATCTTCAAAC CTC-3′ (reverse) for β2M gene amplification.
Total RNA (>3 μg) was sent for global gene expression profile analysis using the Affymetrix HG-U133 Plus 2.0 gene chip (Shanghai Biochip Co. Ltd). Affymetrix microarray analysis was performed using the Gene Spring GX11.0 software (Agilent Technologies) (Lai et al., 2011; Huang et al., 2011a; Chen et al., 2012). Probe sets displaying a signal log ratio indicating an increase or marginal increase [i.e., log ratio ≥1(n)] and the detection of an experimental group displaying a signal change with P represented upregulated genes. Conversely, probe sets displaying a signal log ratio indicating a decrease or marginal decrease [i.e., log ratio ≤−1(n)] and the detection of a control group displaying a signal change with P represented downregulated genes. The resulting data were analyzed using the SBC Analysis System. After normalization and correction, the log2 fluorescence intensity value for each gene was obtained (Huang et al., 2011b). The fold change for all genes was calculated by comparing PPP2R5C-siRNA2- and SC-treated Jurkat T cells. A minimum twofold difference was considered significant.
Cell proliferation assays
The proliferation of Molt-4 and Jurkat T cells was indirectly assayed using the CCK-8 kit (Dojindo), which stains living cells. After transfection, approximately 5×104 Molt-4 or Jurkat (100 μL) and control cells were incubated in triplicate in 96-well plates. At 24, 48, and 72 h, the CCK-8 reagent (10 μL) was added to each well and incubated at 37°C for 2 h. The optical density at 450 nm was measured using an automatic microplate reader (Synergy4; Bio-Tek).
Apoptosis analysis
Molt-4 and Jurkat T cells transfected with PPP2R5C-siRNAs or Alexa Red Oligo were monitored from 48 to 72 h post-transfection using a Delta Vision high-resolution imaging system (Applied Precision, Inc.). The Molt-4 and Jurkat T cells (5×105) in each group were collected 48 h after transfection and then prepared with FITC-labeled anti-Annexin-V (BD Pharmingen) and propidium iodide (Kaiji) according to the manufacturers' protocol and measured by flow cytometry (Beckman Coulter). The results were analyzed using Windows MDI 2.9 software.
Statistical analyses
Statistical analyses were performed with paired t-tests and one-way analysis of variance using SPSS 13.0 statistical software. Kruskal–Wallis analysis was used to analyze the PPP2R5C gene mRNA levels in different samples. Differences were considered statistically significant at p<0.05.
Results
PPP2R5C-specific siRNAs suppress PPP2R5C expression in Molt-4 and Jurkat T cells
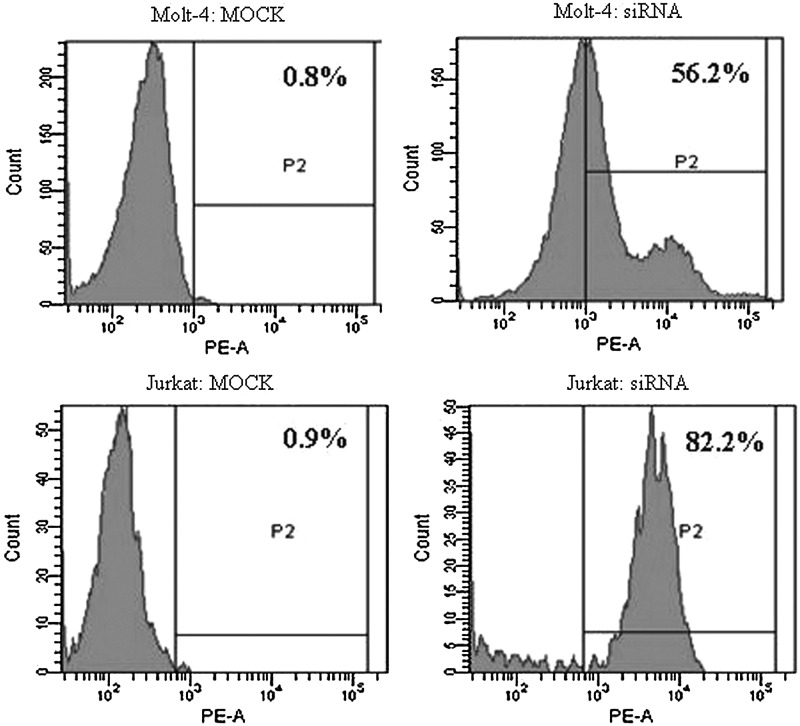
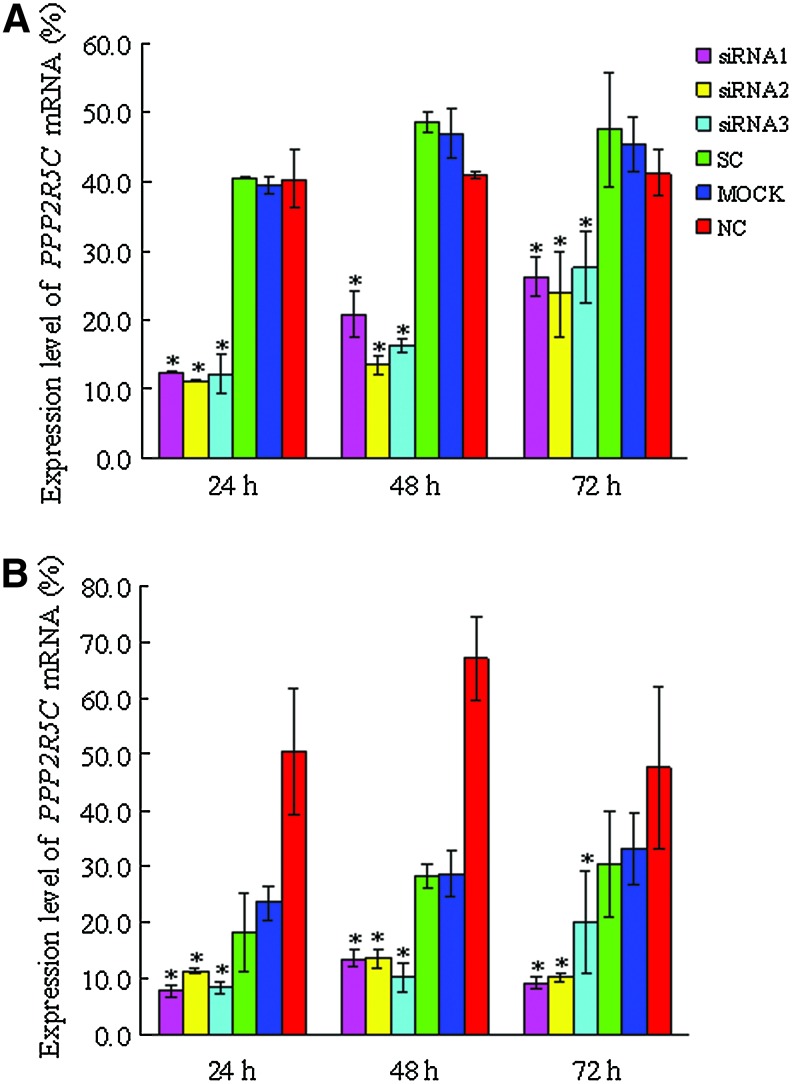
We first verified the transfection efficiency with Alexa Red Oligo-transfected Molt-4 and Jurkat T cells, which was found to be 58.12%±14.14% and 65.2%±10.3%, respectively (Fig. 1). To examine the knockdown of PPP2R5C expression in Molt-4 and Jurkat cells after siRNA treatment, PPP2R5C mRNA expression was analyzed by qRT–PCR 24, 48, and 72 h after nucleofection. The expression level of PPP2R5C was significantly decreased in Molt-4 and Jurkat T cells treated with all three PPP2R5C-siRNAs compared with the control (p<0.05) (Fig. 2) and the expression levels of PPP2R5C were obviously decreased (Figure S1; supplementary materials are available online at http://www.liebertpub.com/dna).
FIG. 1.
Alexa Red Oligo-transfected Molt-4 and Jurkat T cells and MOCK transfected control cells 10 h after transfection as measured by FCM (positive cells are shown in the P2 domain).
FIG. 2.
Inhibition of PPP2R5C expression in Molt-4 and Jurkat cells by RNA interference. The expression of PPP2R5C in Molt-4 (A) and Jurkat T cells (B) treated with different PPP2R5C-siRNAs and control. A significant decrease in the PPP2R5C expression level was observed in all siRNA treatment groups. *p<0.05. Color images available online at www.liebertpub.com/dna
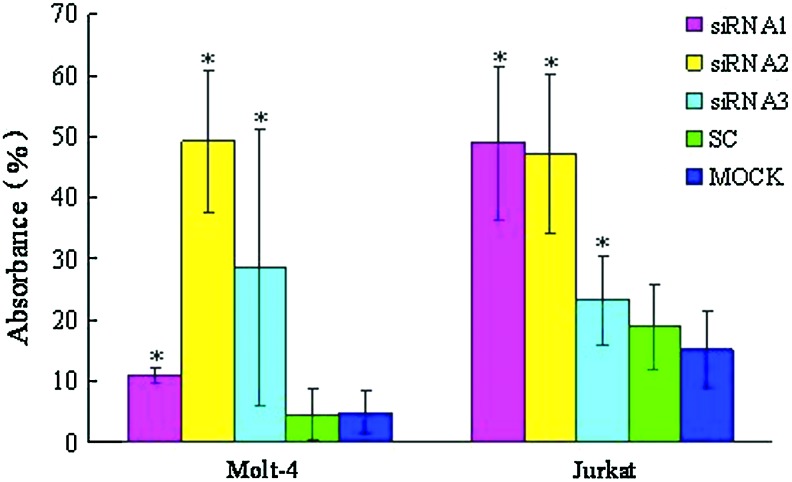
PPP2R5C suppression inhibits the proliferation of Molt-4 and Jurkat T cells
All three PPP2R5C-siRNAs were used to assess biological consequences. The proliferation rate of Molt-4 and Jurkat T cells transfected with different PPP2R5C-siRNAs was significantly decreased at 72 h compared with the control. In comparison with scrambled nonsilencing RNA-treated cells, Molt-4 and Jurkat T cells transfected with PPP2R5C-siRNAs had a significantly inhibited proliferation rate (p<0.05) (Fig. 3). The inhibition rate was 49.23%±2.12% for Molt-4 T cells treated with siRNA2 at 72 h and 48.87±0.69 and 47.12%±0.72% for Jurkat cells treated with siRNA1 and siRNA2, respectively, at 72 h. However, the transfected cells did not demonstrate a significant increase in Annexin V/PI-positive cells (apoptosis) at 48 or 72 h, and the apoptosis rate in cells from different groups was 12.80%±2.73% (siRNA1), 2.11%±2.75% (siRNA2), 13.35%±3.66% (siRNA3), 8.76%±2.19% (SC), 11.73%±2.11% (MOCK), and 6.60%±1.88% (NC) for Molt-4 T cells at 48 h, and 12.79%±1.39% (siRNA1), 22.41%±1.76% (siRNA2), 18.40%±6.36% (siRNA3), 11.48%±2.77% (SC), 16.60%±4.37% (MOCK), and 7.38%±2.45% (NC) for Jurkat T cells at 48 h. We have also checked the change of cell cycle of the siRNA-treated T cells by flow cytometry; there were no significant differences between different groups at 48 and 72 h after siRNA treatment and controls either in Jurkat or Molt-4 T cells (Figures S2 and S3).
FIG. 3.
The inhibition of the proliferation of Molt-4 and Jurkat T cells transfected with PPP2R5C-siRNAs at 72 h as measured by the CCK-8 method. *p<0.05 compared with scrambled, nonsilencing siRNA-treated cells. Color images available online at www.liebertpub.com/dna
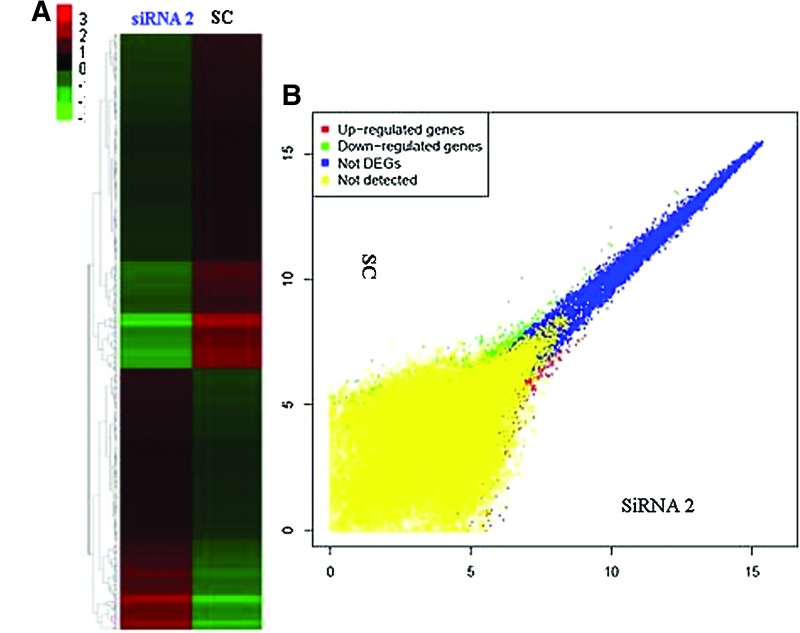
Global gene expression profile in PPP2R5C-siRNA2-treated Jurkat T cells
To elucidate the gene expression profile of Jurkat T cells after PPP2R5C suppression, global gene expression analysis was performed by comparing the transcriptome profiles of PPP2R5C-siRNA2- and SC-treated Jurkat cells using Affymetrix HG-U133 Plus 2.0 arrays. There was a clear dysregulation in global gene expression in Jurkat T cells with PPP2R5C knockdown as demonstrated by the degree of differential expression between the PPP2R5C-siRNA2 and SC groups (Fig. 4A). Subsequently, the dysregulated genes were clustered based on their differential expression (twofold up or down) and visualized using a heatmap (Fig. 4B). Gene Spring GX software analysis demonstrated that 439 genes (greater than twofold: 304 genes, greater than fourfold: 74 genes, greater than eightfold: 61 genes) were upregulated, and 524 genes (greater than twofold: 369 genes, greater than fourfold: 86 genes, greater than eightfold: 69 genes) were downregulated at least twofold in PPP2R5C-siRNA2-transfected Jurkat T cells compared with the SC control. Changes in signaling pathway genes closely related to cell proliferation, TCR signaling, Wnt signaling, calcium signaling, MAPK signaling, and p53 signaling were further analyzed by pathway analysis using the SBC Analysis System (Table 2).
FIG. 4.
Microarray analysis after PPP2R5C knockdown in Jurkat T cells. (A) The Affymetrix data were clustered, and the red and green colors represent the expression levels that are increased or decreased with respect to the average expression across samples. (B) Scatter plots comparing the gene expression profiles of siRNA2- and siRNA control (SC)-transfected cells. The yellow dots represent genes absent in both samples, blue dots represent genes present in both samples, red dots represent genes upregulated, and green dots represent genes downregulated. Color images available online at www.liebertpub.com/dna
Table 2.
The Detailed List of Genes with Expression Changes Related to Signaling Pathways in Jurkat T Cells After PPP2R5C Knockdown
| Gene symbol | NCBI accession | Fold change | Description | Pathway |
|---|---|---|---|---|
| MAP3K2 | NM_006609 | 2.67 | Mitogen-activated protein kinase kinase kinase 2 | MAPK signaling pathway |
| MAP3K6 | NM_004672 | 14.22 | Mitogen-activated protein kinase kinase kinase 6 | MAPK signaling pathway |
| MAP3K8 | NM_005204 | −2.08 | Mitogen-activated protein kinase kinase kinase 8 | MAPK signaling pathway/T-cell receptor signaling pathway |
| DDIT3 | NM_001195053 | −2.32 | DNA-damage-inducible transcript 3 | MAPK signaling pathway |
| FGF7 | NM_002009 | 3.68 | Fibroblast growth factor 7 | MAPK signaling pathway |
| CACNβ2 | NM_000724 | 6.34 | Calcium channel, voltage-dependent, beta 2 subunit | MAPK signaling pathway |
| RasGRP2 | NM_001098670 | 2.30 | RAS guanyl releasing protein 2 | MAPK signaling pathway |
| NF1 | NM_000267 | −2.09 | Neurofibromin 1 | MAPK signaling pathway |
| STMN1 | NM_001145454 | −9.63 | Stathmin 1 | MAPK signaling pathway |
| NF-κB2 | NM_001077493 | 2.24 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 2 | MAPK signaling pathway |
| PAK1 | NM_001128620 | −2.88 | p21 protein (Cdc42/Rac)-activated kinase 1 | MAPK signaling pathway/T-cell receptor signaling pathway |
| ATM | NM_000051 | −2.01 | Ataxia telangiectasia mutated | Cell cycle/p53 signaling pathway/Apoptosis |
| MDM2 | NM_002392 | 16.51 | Mdm2 p53 binding protein homolog | Cell cycle/p53 signaling pathway |
| GSK-3β | NM_001146156 | −2.19 | Glycogen synthase kinase 3 beta | Cell cycle/T-cell receptor signaling pathway/Wnt signaling pathway |
| PTEN | NM_000314 | 2.76 | Phosphatase and tensin homolog | p53 signaling pathway |
| ADRA1B | NM_000679 | −2.33 | Adrenergic, alpha-1B-, receptor | Calcium signaling pathway |
| ATP2A3 | NM_005173 | 9.84 | ATPase, Ca++ transporting, ubiquitous | Calcium signaling pathway |
| DRD5 | NM_000798 | −2.13 | Dopamine receptor D5 | Calcium signaling pathway |
| RYR3 | NM_001036 | 38.04 | Ryanodine receptor 3 | Calcium signaling pathway |
| HRH2 | NM_001131055 | 8.93 | Histamine receptor H2 | Calcium signaling pathway |
| HTR7 | NM_000872 | −4.56 | 5-hydroxytryptamine (serotonin) receptor 7 | Calcium signaling pathway |
| ITPR1 | NM_002222 | 2.00 | Inositol 1,4,5-triphosphate receptor, type 1 | Calcium signaling pathway |
| P2RX1 | NM_002558 | −2.31 | Purinergic receptor P2X, ligand-gated ion channel, 1 | Calcium signaling pathway |
| GRIN2C | NM_000835 | −2.12 | Glutamate receptor, ionotropic, N-methyl D-aspartate 2C | Calcium signaling pathway |
| CALM1 | NM_001166106 | 4.52 | Calmodulin 1 | Calcium signaling pathway |
| ADCY1 | NM_021116 | 2.35 | Adenylate cyclase 1 (brain) | Calcium signaling pathway |
| ADCY2 | NM_020546 | −5.51 | Adenylate cyclase 2 (brain) | Calcium signaling pathway |
| ADCY9 | NM_001116 | 2.41 | Adenylate cyclase 9 | Calcium signaling pathway |
| Dkk1 | NM_012242 | −2.16 | Dickkopf homolog 1 | Wnt signaling pathway |
| APC | NM_000038 | 2.37 | Adenomatous polyposis coli | Wnt signaling pathway |
| APC2 | NM_005883 | 3.13 | Adenomatosis polyposis coli 2 | Wnt signaling pathway |
| PPP2R5C | NM_178587 | −2.49 | Protein phosphatase 2, regulatory subunit B', gamma | Wnt signaling pathway |
| TCF7L1 | NM_031283 | −7.21 | Transcription factor 7-like 1 (T-cell specific, HMG-box) | Wnt signaling pathway |
| Daam1 | NM_014992 | −3.90 | Dishevelled associated activator of morphogenesis 1 | Wnt signaling pathway |
| CFLAR | NM_001127183 | 2.39 | CASP8 and FADD-like apoptosis regulator | Apoptosis |
| CSF2Rβ | NM_000395 | −31.26 | Colony stimulating factor 2 receptor, beta, low affinity | Apoptosis |
| PRKAR1A | NM_002734 | 2.50 | Protein kinase, cAMP-dependent, regulatory, type I, alpha | Apoptosis |
| XIAP | NM_001167 | 2.06 | X-linked inhibitor of apoptosis | Apoptosis |
| CDC14A | NM_003672 | −2.05 | Cell division cycle 14 homolog A | Cell cycle |
| STAG1 | NM_005862 | 2.32 | Stromal antigen 1 | Cell cycle |
| CDKN1A | NM_000389 | 2.42 | Cyclin-dependent kinase inhibitor 1A (p21, Cip1) | Cell cycle/p53 signaling pathway |
| CDKN1C | NM_000076 | 2.31 | Cyclin-dependent kinase inhibitor 1C (p57, Kip2) | Cell cycle |
| ORC5 | NM_002553 | −3.75 | Origin recognition complex, subunit 5 | Cell cycle |
Discussion
PPP2R5C plays a crucial role in cell proliferation, differentiation, and transformation based on its induction of the dephosphorylation of p53 at various residues (Shouse et al., 2008) and may be responsible for the tumor-suppressive function of PP2A (Lee et al., 2010). To confirm the role of PPP2R5C siRNA on the inhibition of leukemic T cells and its potential as a therapeutic agent, we compared different PPP2R5C-siRNAs that target different exon sequences and screened leukemic T cells with a highly efficient and specific PPP2R5C-siRNA.
Exogenous siRNA delivery always results in a transient RNAi effect. In general, RNAi effects are detected between 24 to 72 h after siRNA transfection. We demonstrated that the siRNAs effectively silenced PPP2R5C post-transcriptionally, and the control siRNA had no obvious influence 72 h after nucleofection. These results were not only confirmed by real-time PCR, but also by microarray analysis, which showed that PPP2R5C was downregulated 2.49- and 2.77-fold by two probe sets. siRNAs targeting different exon domains had different efficacies for PPP2R5C gene silencing and subsequent biological consequences. PPP2R5C-siRNA2, which targets exon six, demonstrated robust knockdown, whereas PPP2R5C-siRNA1 and PPP2R5C-siRNA3 had different effects on the different leukemic cell lines.
There are no reports regarding the effects of the suppression of PPP2R5C on changing cell biological functions. In this study, we demonstrated that the suppression of PPP2R5C by RNAi effectively inhibited the proliferation of the Molt-4 and Jurkat cell lines. However, unlike other reported siRNAs, such as BCL11B-siRNA and Notch1-siRNA (Guo et al., 2009; Huang et al., 2011), the suppression of PPP2R5C by RNAi could not significantly induce apoptosis, which was confirmed by gene expression profile analysis demonstrating that only a limited number of apoptotic genes are altered. Therefore, it is interesting to analyze the molecular mechanisms of the PPP2R5C siRNA-mediated suppression of cell proliferation.
To characterize the effects of PPP2R5C knockdown in Jurkat T cells, we analyzed gene expression profiles by gene chip. Data from global gene expression analysis demonstrated that approximately 1000 genes were differentially expressed; thus, we attempted to find genes involved in signaling pathways related to the downregulation of PPP2R5C.
Genes altered in the TCR signaling pathway
TCR engagement initiates intracellular signaling cascades that lead to T-cell proliferation, cytokine production, and differentiation into effector cells (Wilkinson et al., 2005). Therefore, the TCR signaling pathway is the most important pathway in T-cell activation and proliferation (Laky et al., 2006; Zha et al., 2013), and according to the KEGG (Kyoto Encyclopedia of Genes and Genomes) website, there are 108 genes involved in the TCR signaling pathway (Pathway: map04660) (Kanehisa, 2002; Zha et al., 2013). Our results demonstrated that only three genes, p21 protein (Cdc42/Rac)-activated kinase 1 (PAK1), mitogen-activated protein kinase kinase kinase 8 (MAP3K8), and glycogen synthase kinase 3 beta (GSK-3β), were downregulated in PPP2R5C-siRNA-treated Jurkat T cells (Table 1). All three genes are also involved in the MAPK signaling pathway, and GSK-3β is involved in the cell cycle pathway and is related to tumor proliferation (Varisli et al., 2011). The downregulation of GSK-3β is thought to decrease the phosphorylation of NFAT, while MAP3K8 downregulation effects the inhibition of activator protein-1 (AP-1), which is critically modulated by post-translational modifications, particularly phosphorylation, which mediates not only TCR, but also MAPK cascades (Torgersen et al., 2008). Therefore, our results indicate that the effect of the proliferation inhibition of Jurkat cells by PPP2R5C downregulation might be due to a common cell proliferation pathway, such as the MAPK pathway, rather than the TCR signaling pathway.
Genes altered in the p53 signaling pathway
p53 is an important tumor suppressor protein, in humans, whose main functions are involved in preventing genome mutations and regulating the cell cycle. In response to DNA damage and other stress signals, p53 is highly modified post-translationally, which increases its stability and promotes its activation and nuclear localization (Shouse et al., 2010; Yang and Phiel, 2010; Isin et al., 2012). B56γ-containing PP2A is an important p53 regulatory enzyme that directly regulates DNA damage-induced p53 stabilization. In response to DNA damage, B56γ-containing PP2A triggers Thr55 dephosphorylation on p53 and promotes its transcriptional activation (Li et al., 2007; Shouse et al., 2008). In addition, B56γ-containing PP2A could dephosphorylate Bcl2 and prevent the proteasome-dependent degradation of Bcl2, which inhibits p53 and resists apoptosis (Lin et al., 2006). Thus, B56γ-containing PP2As regulate the p53 network at multiple levels. In this study, the expression of p53 was not directly downregulated in Jurkat T cells by inhibiting PPP2R5C expression; however, the MDM2 gene, which is an important negative regulator of the p53 tumor suppressor, was upregulated 16.51-fold (Uhrinova et al., 2005), while the ataxia telangiectasia mutated (ATM) gene, a positive regulator of the p53 that triggers p53 Ser15 phosphorylation and promotes its transcriptional activation, was downregulated 2.1-fold (Ditch et al., 2012). Recently, it is proved that ATM directly phosphorylates and specifically regulates B56γ3, B56γ2, and B56δ, after DNA damage, the phosphorylation of B56γ3 at Ser510 leads to an increase in B56γ3-PP2A complexes, and direction of the PP2A phosphatase activity toward the substrate p53, activating its tumor-suppressive functions (Shouse et al., 2011). Both the MDM2 and ATM proteins act as an E3 ubiquitin ligase, which binds to the N-terminal domain of the p53 protein and inhibits p53 transcriptional activation (Vassilev et al., 2004). This result suggests that PPP2R5C knockdown in Jurkat T cells may lead to a decrease in the transcriptional activation of the p53 protein and p53 function by downregulating ATM and upregulating MDM2.
Genes altered in the canonical Wnt/β-catenin signaling pathway
The canonical Wnt/β-catenin signaling pathway plays an important role in the differentiation, polarization, and survival of mature T cells (Gattinoni et al., 2010). β-catenin is a pivotal signaling molecule in the canonical Wnt/β-catenin signaling pathway, and its function as a coactivator that cooperates with diverse DNA binding partners, such as the T-cell factor (TCF)/lymphoid enhancer binding factor (LEF) proteins, remodels chromatin and orchestrates transcriptional programs (Arce et al., 2006). In the presence of the Wnt signal, the disheveled Wnt/receptor complex is recruited to promote the phosphorylation of the β-catenin destruction complex, which consists of the scaffold proteins adenomatous polyposis coli (APC), axin, GSK-3β, and casein kinase 1. Phosphorylation leads the accumulation of β-catenin, and the accumulated β-catenin in its de- or underphosphorylated form is then translocated into the nucleus, where it interacts with TCF/LEF factors to regulate gene expression (MacDonald et al., 2009; Xue et al., 2012). It has been well established that B56s are upstream regulators of β-catenin in the canonical Wnt/β-catenin signaling pathway. B56s could interact with APC, promote the degradation of β-catenin, and inhibit the transcription of β-catenin target genes in mammalian cells (Seeling et al., 1999). Our data indicate that in addition to PPP2R5C, two genes upstream of β-catenin, GSK-3β and APC, were down- or upregulated, respectively, and one downstream gene TCF7L1 was downregulated. Our data suggest that the knockdown of PPP2R5C by siRNA did not directly effect the expression of β-catenin, but it might indirectly effect the reduction in the accumulation of β-catenin by the up- and downregulation of β-catenin regulatory factors and inhibit the expression of the TCF/LEF gene. Downregulation of the TCF/LEF gene could result in a decrease in T-cell development or activation. However, TCF1 was also described as a T-cell-specific tumor suppressor gene in addition to its established role as a Wnt-responsive transcription factor (Tiemessen et al., 2012), which appears to be in contrast with the present results. Whether there are different functions for different TCF family members requires further investigation.
Genes altered in the other signaling pathways
In this study, we also analyzed the differential regulation of genes involved in the MAPK, calcium, apoptosis and cell cycle signaling pathways. Many genes were upregulated or downregulated; however, it is hard to conclusively determine which are the direct results of PPP2R5C knockdown in Jurkat T cells. This finding is in agreement with results demonstrating insignificant differences in apoptosis and cell cycle genes in Jurkat T cells after PPP2R5C knockdown.
In conclusion, our findings provide evidence for the effect of proliferation inhibition in malignant T cells by PPP2R5C knockdown and characterize its relative molecular mechanism. PPP2R5C might be involved in the p53 and canonical Wnt/β-catenin signaling pathways. The differential expression of the GSK-3β, ATM, and MDM2 genes may play an important role in the effects of PPP2R5C downregulation. Moreover, GSK-3β plays a direct role in the stimulatory modification of the p65/RelA transcription factor, which targets NF-κB p50/52 in NF-κB signaling. Overall, PPP2R5C downregulation results in the inhibition of Jurkat T-cell proliferation, which involves numerous pathways, and decreased GSK-3β may be a critical factor in this process. However, further validation of the differentially regulated genes and their relative protein expression levels is needed.
Authors' Contributions
Y.L. contributed to the concept development and study design. Y.C., S.L., Q.S., X.Z., H.Z., L.Y., S.C., X.W., and B.L. performed the laboratory studies. Y.L. and Y.C. coordinated the study and helped draft the manuscript. All authors read and approved the final manuscript.
Supplementary Material
Acknowledgments
This work was supported by Grants from the National Natural Science Foundation of China (30871091 and 91129720), the Collaborated grant for HK-Macao-TW of the Ministry of Science and Technology (2012DFH30060), the Guangdong Science & Technology Project (2012B050600023), Science and Technology Innovation Key Project of Guangdong Higher Education Institutes (kjcxzd1013), and the Key Discipline Construction Foundation of Jinan University.
Disclosure Statement
The authors declare that they have no competing interests.
References
- Aifantis I. Raetz E. Buonamici S. Molecular pathogenesis of T-cell leukaemia and lymphoma. Nat Rev Immunol. 2008;8:380–390. doi: 10.1038/nri2304. [DOI] [PubMed] [Google Scholar]
- Arce L. Yokoyama N.N. Waterman M.L. Diversity of LEF/TCF action in development and disease. Oncogene. 2006;25:7492–7504. doi: 10.1038/sj.onc.1210056. [DOI] [PubMed] [Google Scholar]
- Chen S. Huang X. Chen S.H. Yang L. Shen Q. Zheng H. Li B. Grabarczyk P. Przybylski G.K. Schmidt C.A. Li Y. The role of BCL11B in regulating the proliferation of human naïve T cells. Hum Immunol. 2012;73:456–464. doi: 10.1016/j.humimm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- Cioca D.P. Aoki Y. Kijosawa K. RNA interference is a functional pathway with therapeutic potential in human myeloid leukemia cell lines. Cancer Gene Ther. 2003;10:125–133. doi: 10.1038/sj.cgt.7700544. [DOI] [PubMed] [Google Scholar]
- Devi R.S. siRNA-based approaches in cancer therapy. Cancer Gene Ther. 2006;13:819–829. doi: 10.1038/sj.cgt.7700931. [DOI] [PubMed] [Google Scholar]
- Ditch S. Paull T.T. The ATM protein kinase and cellular redox signaling: beyond the DNA damage response. Trends Biochem Sci. 2012;37:5–22. doi: 10.1016/j.tibs.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbashir S. Harborth J. Lendeckel W. Yalcin A. Weber K. Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–498. doi: 10.1038/35078107. [DOI] [PubMed] [Google Scholar]
- Gattinoni L. Ji Y. Restifo N.P. Wnt/beta-catenin signaling in T-cell immunity and cancer immunotherapy. Clin Cancer Res. 2010;16:4695–4701. doi: 10.1158/1078-0432.CCR-10-0356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabarczyk P. Przybylski G.K. Depke M. Völker U. Bahr J. Assmus K. Bröker B.M. Walther R. Schmidt C.A. Inhibition of BCL11B expression leads to apoptosis of malignant but not normal mature T cells. Oncogene. 2007;26:3797–3810. doi: 10.1038/sj.onc.1210152. [DOI] [PubMed] [Google Scholar]
- Graham D.K. Salzberg D.B. Kurtzberg J. Ectopic expression of the proto-oncogene Mer in pediatric T-cell acute lymphoblastic leukemia. Clin Cancer Res. 2006;12:2662–2669. doi: 10.1158/1078-0432.CCR-05-2208. [DOI] [PubMed] [Google Scholar]
- Guo D. Ye J. Dai J. Li L. Chen F. Ma D. Ji C. Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk Res. 2009;33:678–685. doi: 10.1016/j.leukres.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Huang X. Chen S. Shen Q. Chen S. Yang L. Grabarczyk P. Przybylski G.K. Schmidt C.A. Li Y. Down regulation of BCL11B expression inhibits proliferation and induces apoptosis in malignant T cells by BCL11B-935-siRNA. Hematology. 2011a;6:236–242. doi: 10.1179/102453311X13025568941961. [DOI] [PubMed] [Google Scholar]
- Huang X. Shen Q. Chen S. Chen S. Yang L. Weng J. Du X. Grabarczyk P. Przybylski G.K. Schmidt C.A. Li Y. Gene expression profiles in BCL11B-siRNA treated malignant T cells. J Hematol Oncol. 2011b;4:23. doi: 10.1186/1756-8722-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida T. Ueda R. CCR4 as a novel molecular target for immunotherapy of cancer. Cancer Sci. 2006;97:1139–1146. doi: 10.1111/j.1349-7006.2006.00307.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isin M. Yenerel M. Aktan M. Buyru N. Dalay N. Analysis of p53 tumor suppressor pathway genes in chronic lymphocytic leukemia. DNA Cell Biol. 2012;31:777–782. doi: 10.1089/dna.2011.1314. [DOI] [PubMed] [Google Scholar]
- Kanehisa M. The KEGG database. Novartis Found Symp. 2002;247:91–101. discussion 101–103, 119–128, 244–152. [PubMed] [Google Scholar]
- Labbaye C. Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J Hematol Oncol. 2012;5:13. doi: 10.1186/1756-8722-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laky K. Fleischacker C. Fowlkes B.J. TCR and Notch signaling in CD4 and CD8 T-cell development. Immunol Rev. 2006;209:274–283. doi: 10.1111/j.0105-2896.2006.00358.x. [DOI] [PubMed] [Google Scholar]
- Lai P. Weng J. Lu Z. Guo R. Luo C. Wu S. Ling W. Geng S. Du X. Gene expression profiling-based identification of CD28 and PI3K as new biomarkers for chronic graft-versus-host disease. DNA Cell Biol. 2011;30:1019–1025. doi: 10.1089/dna.2011.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T.Y. Lai T.Y. Lin S.C. Wu C.W. Ni I.F. Yang Y.S. Hung L.Y. Law B.K. Chiang C.W. The B56gamma3 regulatory subunit of protein phosphatase 2A (PP2A) regulates S phase-specific nuclear accumulation of PP2A and the G1 to S transition. J Biol Chem. 2010;285:21567–21580. doi: 10.1074/jbc.M109.094953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H.H. Cai X. Shouse G.P. Piluso L.G. Liu X. A specific PP2A regulatory subunit, B56gamma, mediates DNA damage-induced dephosphorylation of p53 at Thr55. EMBO J. 2007;26:402–411. doi: 10.1038/sj.emboj.7601519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.S. Bassik M.C. Suh H. Nishino M. Arroyo J.D. Hahn W.C. Korsmeyer S.J. Roberts T.M. PP2A regulates BCL-2 phosphorylation and proteasome-mediated degradation at the endoplasmic reticulum. J Biol Chem. 2006;281:23003–23012. doi: 10.1074/jbc.M602648200. [DOI] [PubMed] [Google Scholar]
- Liu P. Li P. Burke S. Critical roles of Bcl11b in T-cell development and maintenance of T-cell identity. Immunol Rev. 2010;238:138–149. doi: 10.1111/j.1600-065X.2010.00953.x. [DOI] [PubMed] [Google Scholar]
- MacDonald B.T. Tamai K. He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muneer S. Ramalingam V. Wyatt R. Schultz R.A. Minna J.D. Kamibayashi C. Genomic organization and mapping of the gene encoding the PP2A B56gamma regulatory subunit. Genomics. 2002;79:344–348. doi: 10.1006/geno.2002.6721. [DOI] [PubMed] [Google Scholar]
- Oh Y.K. Park T.G. siRNA delivery systems for cancer treatment. Adv Drug Deliv Rev. 2009;61:850–862. doi: 10.1016/j.addr.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Oshiro A. Tagawa H. Ohshima K. Karube K. Uike N. Tashiro Y. Utsunomiya A. Masuda M. Takasu N. Nakamura S. Morishima Y. Seto M. Identification of subtype-specific genomic alterations in aggressive adult T-cell leukemia/lymphoma. Blood. 2006;107:4500–4507. doi: 10.1182/blood-2005-09-3801. [DOI] [PubMed] [Google Scholar]
- Peipp M. Küpers H. Saul D. Schlierf B. Greil J. Zunino S.J. Gramatzki M. Fey G.H. A recombinant CD7-specific single-chain immunotoxin is a potent inducer of apoptosis in acute leukemic T cells. Cancer Res. 2002;62:2848–2855. [PubMed] [Google Scholar]
- Palomero T. Ferrando A. Therapeutic targeting of NOTCH1 signaling in T-ALL. Clin Lymphoma Myeloma. 2009;9(Suppl 3):S205. doi: 10.3816/CLM.2009.s.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravandi F. O'Brien S. Alemtuzumab in CLL and other lymphoid neoplasms. Cancer Invest. 2006;24:718–725. doi: 10.1080/07357900600981414. [DOI] [PubMed] [Google Scholar]
- Rodig S.J. Abramson J.S. Pinkus G.S. Treon S.P. Dorfman D.M. Dong H.Y. Shipp M.A. Kutok J.L. Heterogeneous CD52 expression among hematologic neoplasms: implications for the use of alemtuzumab (CAMPATH-1H)] Clin Cancer Res. 2006;12:7174–7179. doi: 10.1158/1078-0432.CCR-06-1275. [DOI] [PubMed] [Google Scholar]
- Sato T. Yamochi T. CD26 regulates p38 mitogen-activated protein kinase-dependent phosphorylation of integrin beta, adhesion to extracellular matrix, and tumorigenicity of T-anaplastic large cell lymphoma Karpas 299. Cancer Res. 2005;65:6950–6956. doi: 10.1158/0008-5472.CAN-05-0647. [DOI] [PubMed] [Google Scholar]
- Seeling J.M. Miller J.R. Gil R. Moon R.T. White R. Virshup D.M. Regulation of beta-catenin signaling by the B56 subunit of protein phosphatase 2A. Science. 1999;283:2089–2091. doi: 10.1126/science.283.5410.2089. [DOI] [PubMed] [Google Scholar]
- Shouse G.P. Cai X. Liu X. Serine 15 phosphorylation of p53 directs its interaction with B56gamma and the tumor suppressor activity of B56gamma-specific protein phosphatase 2A. Mol Cell Biol. 2008;28:448–456. doi: 10.1128/MCB.00983-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse G.P. Nobumori Y. Liu X. A B56gamma mutation in lung cancer disrupts the p53-dependent tumor-suppressor function of protein phosphatase 2A. Oncogene. 2010;29:3933–3941. doi: 10.1038/onc.2010.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shouse G.P. Nobumori Y. Panowicz M.J. Liu X. ATM-mediated phosphorylation activates the tumor-suppressive function of B56γ-PP2A. Oncogene. 2011;30:3755–3765. doi: 10.1038/onc.2011.95. [DOI] [PubMed] [Google Scholar]
- Tiemessen M.M. Baert M.R. Schonewille T. Brugman M.H. Famili F. Salvatori D.C. Meijerink J.P. Ozbek U. Clevers H. van Dongen J.J. Staal F.J. The nuclear effector of Wnt-signaling, Tcf1, functions as a T-cell-specific tumor suppressor for development of lymphomas. PLoS Biol. 2012;10:e1001430. doi: 10.1371/journal.pbio.1001430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torgersen K.M. Aandahl E.M. Tasken K. Molecular architecture of signal complexes regulating immune cell function. Handb Exp Pharmacol. 2008;2008:327–363. doi: 10.1007/978-3-540-72843-6_14. [DOI] [PubMed] [Google Scholar]
- Uhrinova S. Uhrin D. Powers H. Watt K. Zheleva D. Fischer P. McInnes C. Barlow P.N. Structure of free MDM2 N-terminal domain reveals conformational adjustments that accompany p53-binding. J Mol Biol. 2005;350:587–598. doi: 10.1016/j.jmb.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Varisli L. Gonen-Korkmaz C. Debelec-Butuner B. Erbaykent-Tepedelen B. Muhammed H.S. Bogurcu N. Saatcioglu F. Korkmaz K.S. Ubiquitously expressed hematological and neurological expressed 1 downregulates Akt-mediated GSK3β signaling, and its knockdown results in deregulated G2/M transition in prostate cells. DNA Cell Biol. 2011;30:419–429. doi: 10.1089/dna.2010.1128. [DOI] [PubMed] [Google Scholar]
- Vassilev L.T. Vu B.T. Graves B. Carvajal D. Podlaski F. Filipovic Z. Kong N. Kammlott U. Lukacs C. Klein C. Fotouhi N. Liu E.A. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- Wang K. Wei G. Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead K.A. Langer R. Anderson D.G. Knocking down barriers: Advances in siRNA delivery. Nat Rev Drug Discov. 2009;8:129–138. doi: 10.1038/nrd2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. Downey J.S. Rudd C.E. T-cell signalling and immune system disorders. Expert Rev Mol Med. 2005;7:1–29. doi: 10.1017/S1462399405010264. [DOI] [PubMed] [Google Scholar]
- Xue H.H. Zhao D.M. Regulation of mature T cell responses by the Wnt signaling pathway. Ann N Y Acad Sci. 2012;1247:16–33. doi: 10.1111/j.1749-6632.2011.06302.x. [DOI] [PubMed] [Google Scholar]
- Yang J. Phiel C. Functions of B56-containing PP2As in major developmental and cancer signaling pathways. Life Sci. 2010;87:659–666. doi: 10.1016/j.lfs.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X. Chen S. Yang L. Shi L. Li B. Wu X. Lu Y. Li Y. Up-regulated TCRζ enhances interleukin-2 production in T-cells from patients with CML. DNA Cell Biol. 2012;31:1628–1635. doi: 10.1089/dna.2012.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zha X. Yin Q. Tan H. Wang C. Chen S. Yang L. Li B. Wu X. Li Y. Alteration of the gene expression profile of TCRαβ-modified T-cells with DLBCL specificity. Hematology. 2013;18:138–143. doi: 10.1179/1607845412Y.0000000028. [DOI] [PubMed] [Google Scholar]
- Zheng H. Chen Y. Chen S. Niu Y. Yang L. Li B. Lu Y. Geng S. Du X. Li Y. Expression and distribution of the PPP2R5C gene in leukemia. J Hematol Oncol. 2011;4:21. doi: 10.1186/1756-8722-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinzani P.L. Khuageva N.K. Wang H. Garicochea B. Walewski J. Van Hoof A. Soubeyran P. Caballero D. Buckstein R. Esseltine D.L. Theocharous P. Enny C. Zhu E. Elsayed Y.A. Coiffier B. Bortezomib plus rituximab versus rituximab in patients with high-risk, relapsed, rituximab-naïve or rituximab-sensitive follicular lymphoma: subgroup analysis of a randomized phase 3 trial. J Hematol Oncol. 2012;5:67. doi: 10.1186/1756-8722-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.