Abstract
Padhy, Gayatri, Niroj Kumar Sethy, Lilly Ganju, and Kalpana Bhargava. Abundance of plasma antioxidant proteins confers tolerance to acute hypobaric hypoxia exposure. High Alt Med Biol 14:289–297, 2013—Systematic identification of molecular signatures for hypobaric hypoxia can aid in better understanding of human adaptation to high altitude. In an attempt to identify proteins promoting hypoxia tolerance during acute exposure to high altitude, we screened and identified hypoxia tolerant and susceptible rats based on hyperventilation time to a simulated altitude of 32,000 ft (9754 m). The hypoxia tolerance was further validated by estimating 8-isoprotane levels and protein carbonyls, which revealed that hypoxia tolerant rats possessed significant lower plasma levels as compared to susceptible rats. We used a comparative plasma proteome profiling approach using 2-dimensional gel electrophoresis (2-DGE) combined with MALDI TOF/TOF for both groups, along with an hypoxic control group. This resulted in the identification of 19 differentially expressed proteins. Seven proteins (TTR, GPx-3, PON1, Rab-3D, CLC11, CRP, and Hp) were upregulated in hypoxia tolerant rats, while apolipoprotein A-I (APOA1) was upregulated in hypoxia susceptible rats. We further confirmed the consistent higher expression levels of three antioxidant proteins (PON1, TTR, and GPx-3) in hypoxia-tolerant animals using ELISA and immunoblotting. Collectively, these proteomics-based results highlight the role of antioxidant enzymes in conferring hypoxia tolerance during acute hypobaric hypoxia. The expression of these antioxidant enzymes could be used as putative biomarkers for screening altitude adaptation as well as aiding in better management of altered oxygen pathophysiologies.
Introduction
Exponential decrease in partial pressure of inspired oxygen with increase in altitude results in hypobaric hypoxia. This condition of reduced tissue oxygen availability causes a number of life-threatening clinical manifestations, including shortness of breath, tachycardia, headache, dizziness, acute mountain sickness (AMS), pulmonary and cerebral edema (HAPE and HACE), mental confusion and memory deficit, ophthalmological disturbances, cerebral hemorrhages, and sleep disturbances (Hackett et al., 2004; Sharp et al., 2004; Weil 2004; Wilson et al., 2009). However, the major determinants of these high altitude maladies include altitude, the rate of ascent, and individual susceptibility.
Hypobaric hypoxia limits the availability of oxygen for reduction to H2O at cytochrome oxidase. This results in accumulation of reductive reducing equivalents within the mitochondrial respiratory sequence, also known as reductive stress. This stress leads to further ROS formation by auto-oxidation of one or more mitochondrial complexes, such as the ubiquinone–ubiquinol redox couple. Moreover xanthine dehydrogenase/oxidase system and nitric oxide synthase uncoupling also contribute to enhanced RNOS production during hypoxia. High altitude exposure also compromises the abundance and activity of antioxidant enzymes like SOD1, SOD2, and GPX (Moraga et al., 2007). Several human and animal studies have reported that increased levels of ROS production at high altitude, along with decreased antioxidant capacity, causes oxidative damage to cellular macromolecules and causative factor for altitude-induced maladies. However, long-term acclimatization and/or genetic adaptation attenuate or eliminate the high altitude-induced oxidative stress (Jefferson et al., 2004; Vij et al., 2005).
Two-dimensional gel electrophoresis (2-DGE) coupled with mass spectrometry (MS) for identification of proteins is a powerful approach to understand global protein dynamics in response to different stimuli. Plasma being circulative, representative of all the tissues for the pathological and physiological states, in addition to its ease of collection, monitoring, safe and noninvasive, has an immense diagnostic potential (Jacobs et al., 2005). Despite being a powerful approach for protein identification, proteomics studies have rarely been extended for high altitude adaptation. Comparing plasma proteome profiles of HAPE patients with normobaric controls, Ahmad et al. (2011) have identified increased expression of haptoglobin and apolipoprotein AI in HAPE patients. Similarly, muscle proteomics of high altitude Tibetans revealed higher expression pattern of GST-P1-1, enoyl CoA hydratase, and myoglobin whilst GST-P1-1 was also present in higher expression levels in low altitude Tibetans (Gelfi et al., 2004). Although much research has been focused on genetics and physiology of adaptation to hypobaric hypoxia, proteins responsible for this response has not been studied yet. Hence, identification of hypobaric hypoxia regulated proteins can provide a better insight into high altitude-induced proteomic adaptation that cannot be inferred from genomic studies.
In this study, we report the differential protein expression of hypoxia tolerant and susceptible rodents after an acute exposure of hypobaric hypoxia. Using 2-DGE combined with MALDI-TOF/TOF, we have identified 19 differentially expressed proteins in response to hypoxia. We have further verified the expression levels of identified proteins by ELISA and immunoblotting. These results indicate that hypobaric hypoxia differentially regulates expression of circulatory antioxidant proteins.
Materials and Methods
Animals
Male Sprague-Dawley (SD) rats of weight 150±10 g were used in the study. The animals were maintained at 12 h light/dark cycle period and were given food and water ad libitum. The experimental protocol was approved by the Institutional Animal Ethics Committee, and care of the animals was carried out as per the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forest, Government of India.
Hypoxia exposure procedure and sample collection
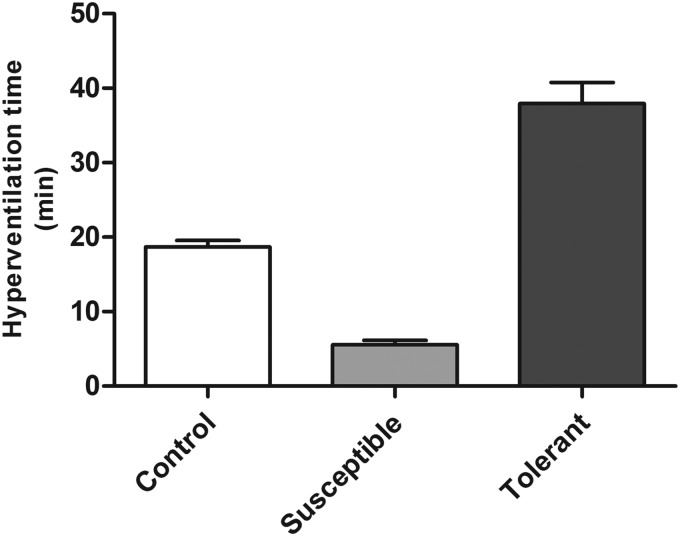
On simulation to acute hypobaric hypoxia, animals increase their oxygen supply by hyperventilation. Using hyperventilation time for hypoxia tolerance (Purushothaman et al., 2008), a total of 100 rats were screened for their tolerance to hypobaric hypoxia and grouped accordingly. Briefly, animals were simulated to hypobaric hypoxia with the barometric pressure of 9754 m (205 mmHg) at 32°C±1°C with air flow and humidity at 2 L/min and 65±5 %, respectively, and the hyperventilation time was determined. Based on the hyperventilation time, the rats were accordingly grouped into hypoxia tolerant (hyperventilation time ≥25 min), hypoxia susceptible (hyperventilation time ≤10 min), and control group (hyperventilation time 12–18 min, Fig. 1). Three successive hyperventilation exposures were performed with a washout period of 7 days each to identify the true hypoxia tolerant and susceptible rats. After the third exposure, blood from the ocular vein was collected in sodium heparinized vacutainers, and the plasma was collected by centrifuging at 1000 g for 15 min at room temperature. The isolated plasma samples were stored at −80°C till further use.
FIG. 1.
Hyperventilation time of Sprague Dawley rats on exposure to hypobaric hypoxia at 9754 m, 32°C. The hyperventilation time is expressed in Mean±SEM for each group of animals.
Estimation of plasma 8-isoprostane
Plasma 8-isoprostanes levels were measured using an 8-isoprostane EIA kit (Cayman Chemical Company, Ann Arbor, MI, USA) according to the manufacturer's instructions. Briefly, plasma samples were hydrolyzed with one volume of 15% (w/v) potassium hydroxide and incubated for 1 h at 40°C. The samples were neutralized with three volumes of 1 M potassium phosphate buffer (pH 7.2). Plasma 8-isoprotane was quantified using competitive binding with mouse anti-rabbit IgG monoclonal antibody. The enzymatic product was determined spectrophotometrically at 412 nm. Samples were assayed in duplicates and results were expressed in pg/mL.
Determination of protein carbonylation
The carbonylation of proteins results either from oxidative cleavage of the polypeptide backbone or by direct oxidation of the amino acid side chains. Carbonyl content in the plasma was determined using a protein carbonyl assay kit (Cayman Chemical Company) according to the manufacturer's instructions. In brief, protein carbonyls present in the plasma sample were derivatized to DNP hydrazone and was quantified spectrophotometrically at 370 nm with molar absorption coefficient 22,000 M−1cm−1 and results were expressed in nmol/mL.
Sample preparation for 2D gel electrophoresis
The blood plasma samples were depleted of the high abundant proteins using Proteoprep® Blue albumin & IgG depletion kit (Sigma Aldrich, St. Louis, MO) according to the manufacturer's instructions. The depleted plasma samples were precipitated using 10% TCA in acetone. The precipitated protein pellet was dissolved in lysis buffer (8 M urea, 2 M thiourea, 40 mM Tris-HCl, 4% (w/v) CHAPS, and 0.5% Igepal CA-630) and the concentration were determined using Bradford reagent. The depletion pattern was quantitatively and qualitatively determined on 10% SDS PAGE.
Isoelectric focusing
Precipitated protein samples (500 μg) were mixed with 350 μL of rehydration buffer (7M urea, 2 M thiourea, 1.2% (w/v) CHAPS, 20 mM DTT, 0.4% (w/v) ASB-14, 0.25% (v/v) pH 4–7 ampholyte, and 0.005% (w/v) bromophenol blue). The IPG strips (Immobiline DryStrip, pH 4–7, 18 cm, GE Healthcare, Sweden) were subjected to passive rehydration for 16 h. Following passive rehydration, the IPG strips were focused according to the following program; conditioning step of 500 V for 7 h to remove salts present in the sample, followed by constant voltage of 1000 V for 1 h, linear ramping of 8000 V for 3 h, linear ramping of 10,000 V for 3 h, and constant voltage of 10,000 V for 1 h for a total of 60,000 Vh (50 μA/strip) at 20°C using IPGphor system (Amersham Pharmacia Biotech). The focused IPG strips were equilibrated first with equilibration buffer (6 M urea, 30% v/v glycerol, 2% (w/v) SDS, 50 mM Tris pH 8.8) containing 1% (w/v) DTT for 15 min, and then with equilibration buffer containing 2.5% (w/v) iodoacetamide (IAA) for 15 min. The equilibrated IPG strips were subjected to second-dimension separation on 12% SDS PAGE. The gels were manually silver stained, as described by Baik et al. (2004), and image was scanned using Investigator™ ProPic II (Genomic Solutions). Differential spot pattern was analyzed by Progenesis Same spot analysis software (Nonlinear Dynamics, version 4.0). Spot assignment, statistical calculations, and background correction were performed using the default parameters.
In-gel digestion
The silver stained differentially expressed gel spots were excised manually and were subjected to destain with 30 mM potassium ferricyanide and 100 mM sodium thiosulfate (1:1). The destained gel spots were washed thrice with the milli Q water and subsequently with 10 mM ammonium bicarbonate and acetonitrile (1:1). The gel spots were rehydrated with 10 mM ammonium bicarbonate, dehydrated again with acetonitrile, and dried down by speed Vac (Heto MAXI dry plus, UK). Freshly prepared trypsin (20 ng/μL) (sequencing grade, Promega, Madison, WI) was added to the dried gel spots and incubated for 30 min at 4°C and overnight at 37°C. The tryptic digested peptides were extracted by sonicating with 50% acetonitrile and 0.1% TFA and were dried in Speed Vac.
Identification of differentially expressed proteins
The extracted peptides were resuspended in 5 μL of 50% v/v acetonitrile: 0.1% v/v TFA. The resolubilized peptides were mixed with saturated solution of cyano-4-hydroxy-cinnamic acid matrix (1:1) (CHCA, Bruker Daltonics), were spotted onto the MALDI target plate and allowed to air dry. The spectra were acquired by MALDI TOF/TOF (Ultraflex III, Bruker Daltonics, Bremen, Germany) in the positive ion mode at the accelerating voltage of 25 kV under the Flex Control software (Version 3.0, Bruker Daltonics). Before the analysis, the instrument was calibrated with mass standards: bradykinin (m/z 757.39), angiotensin II (m/z 1046.54), angiotensin I (m/z 1296.68), substance P (m/z 1347.73), bombysin (m/z 1619.82), ACTH fragment 1–17 (m/z 2093.08), ACTH fragment 18–39 (m/z 2465.39), and somatostatin (m/z 3147.47). For peptide mass fingerprinting (PMF), the generated peptide mass list was exported to Biotools (Version 3.1, Bruker Daltonics) using Flex Analysis 3.0 and submitted to in-house licensed version of the MASCOT database search engine (Matrix Science, Boston, MA). The SWISS-PROT database was used with the following search parameters; Rattus norvegicus as taxonomy with±100 ppm peptide mass tolerance, 1 maximum missed cleavage sites permitted, and carbamidomethyl (cysteine) as fixed modification. Proteins that returned MOWSE scores over a threshold 56 were considered significant. For each identified protein, at least one peptide was selected for confirmation by MS/MS analysis. The MS/MS spectra were acquired in LIFT mode using the same spot on the target and fragment mass tolerance of±0.8 Da was applied. All the peptide mass values are monoisotopic.
Expression analysis of serum paraoxonase 1 (PON1) by ELISA
ELISA-based validation of PON1 was carried out using plasma samples (n=6) from each group along with standards and negative control. PON1 activity in plasma was determined using EnzChek® Paraoxonase Assay kit (Molecular Probes, Invitrogen, USA) according to the manufacturer's protocol. In brief, 50 μL/well of the paraoxonase substrate was added to 10 μL of diluted plasma (1:250) and in the positive control wells, followed by 30 min incubation in dark at room temperature. The reaction was stopped by adding 10 μL of stop solution (12X DMSO). The excitation and emission was measured at 360 nm and 450 nm, respectively. Concentrations of PON1 were calculated as U/μL of plasma.
Validation of protein expression by immunoblot analysis
To validate the results of proteomics data, immunoblot of three candidate proteins, namely TTR, PON1, and GPx-3, in the plasma samples were performed. Plasma containing 30 μg of total protein were separated on 10% SDS-PAGE gels and transferred onto nitrocellulose membranes. The membranes were blocked in 5% non-fat milk in PBS-0.1% Tween-20 (PBST) overnight at room temperature. Followed by washing with PBST (5 min x 3), the membranes were incubated with mouse monoclonal to PON 1 (Abcam, U.K.), rabbit polyclonal to prealbumin (Transthyretin) (Abcam, U.K.), and rabbit polyclonal to glutathione peroxidase 3 (GPx-3) (Enzo Lifesciences, U.K.) at room temperature for 2 h. Subsequently, the membranes were washed with PBST thrice for 5 min each and were then incubated with their respective secondary antibodies for 2 h at room temperature. After washing thrice with PBST (5 min each), the membranes were developed by adding Supersignal chemiluminiscent substrate (Thermo Scientific, Germany). The autoradiograms were scanned and densitometry analysis was performed with Image J software (http://rsbweb.nih.gov/ij/).
Measurement of NOx (nitrate and nitrite) from plasma
The NOx level of plasma was measured using enzymatic conversion of nitrate to nitrite by nitrate reductase. The reaction was followed by colorimetric detection of nitrite as an azo dye product of the Griess reagent at 540 nm. The concentration of NOx was calculated as μmol/L.
Statistical analysis
All quantitative data were represented as mean±SEM. The results are representations of three independent experiments. Statistical analysis were done using one-way ANOVA with pair-wise multiple comparison procedures (Student-Newman-Keuls method), and a p value of<0.05 was considered significant.
Results
Identification of hypobaric hypoxia tolerant and susceptible rats
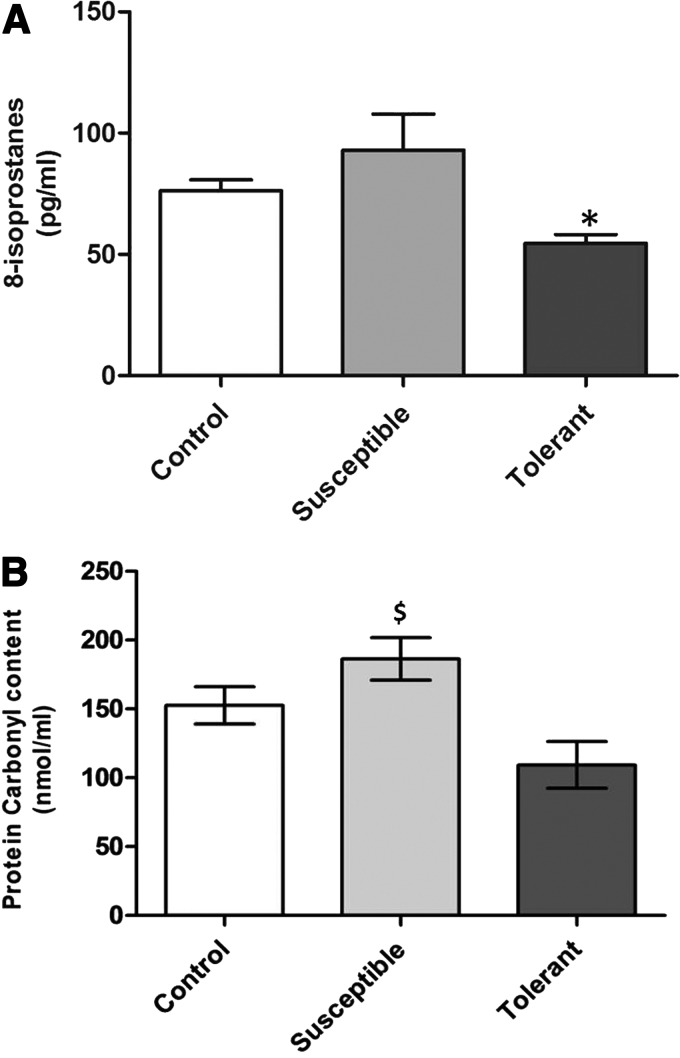
Animals hyperventilate on exposure to hypobaric hypoxia so as to improve their oxygen supply (Teppema et al., 2010). Based on three hyperventilation time recordings during acute hypobaric hypoxia exposure (Fig. 1), 17 tolerant and 21 susceptible rats were identified. The behavior, food, and water intake of these rats were similar to control (hyperventilation time 12–18 min) as well as normoxic rats. To further substantiate the hypoxia tolerance and susceptibility of rats, oxidative stress parameters such as 8-isoprostanes and protein carbonyl content were measured from plasma (Ali et al., 2012). The tolerant animals have significantly lower levels of plasma 8-isoprostane (54.53±3.62 pg/mL, p<0.05) and carbonyl content (116.9±15.9 nmol/mL) as compared to control animals (76.16±4.57 pg/mL and 152.65±12.9 nmol/mL, respectively) and exposure control animals ( 70.27±3.94 pg/mL and 144.28±5.64 nmol/mL, respectively). Similarly the susceptible animals possessed significantly higher levels of plasma 8-isoprostane (92.97±14.79 pg/mL, p<0.05) and carbonyl content (186.3±14.8 nmol/mL, p<0.05) as compared to control animals (Fig. 2A, B). Comparison of 8-isoprostane and protein carbonyl levels in hypoxia tolerant and susceptible rats revealed that tolerant rats have significant lower levels of 8-isoprostane (50.47%, p<0.01) and carbonyl content (45.4%, p<0.007) as compared to susceptible animals (Fig. 2A, B).
FIG. 2.
Determination of 8-isoprostane and protein carbonyls in hypoxia tolerant and susceptible rats. (A) Plasma 8-isoprostane levels were lower in tolerant while susceptible have higher levels and were measured in pg/mL. (B) The amount of protein carbonyls were observed to be higher in susceptible animals and expressed as nmol/mL. All the results are expressed as Mean±SEM. (* represents p<0.05 vs. Control, and $ indicates p<0.05 vs. hypoxia tolerant according to Newman–Keuls multiple comparison tests).
Identification of differentially expressed proteins
The plasma proteomes of tolerant and susceptible rats were resolved by 2-DGE and compared with control animals to identify differentially expressed proteins. Comparative gel analysis resulted in the identification of 19 differentially expressed spots (Fig. 3), which were further excised from gels and identified using MALDI TOF/TOF and MS/MS analysis (Fig. 4). From the 19 differentially expressed spots, 14 proteins were successfully identified (Table 1). Proteins such as transthyretin (TTR), glutathione peroxidase 3 (GPx-3), serum paraoxonase 1 (PON1), GTP-binding protein Rab-3D (Rab-3D), C-type lectin domain family 11 member A (CLC11), C-reactive protein (CRP), and haptoglobin (Hp) were upregulated in hypoxia tolerant animals, while proteins such as apolipoprotein A-I (APOA1) upregulated in hypoxia susceptible animals. Similarly proteins such as complement C-4 (CO4) and complement C-3 (CO3) were downregulated in hypoxia-susceptible animals (Fig. 4, Table 1). We further validated the expression of three antioxidant proteins PON1, TTR, and GPx-3 by both ELISA and immunoblotting.
FIG. 3.
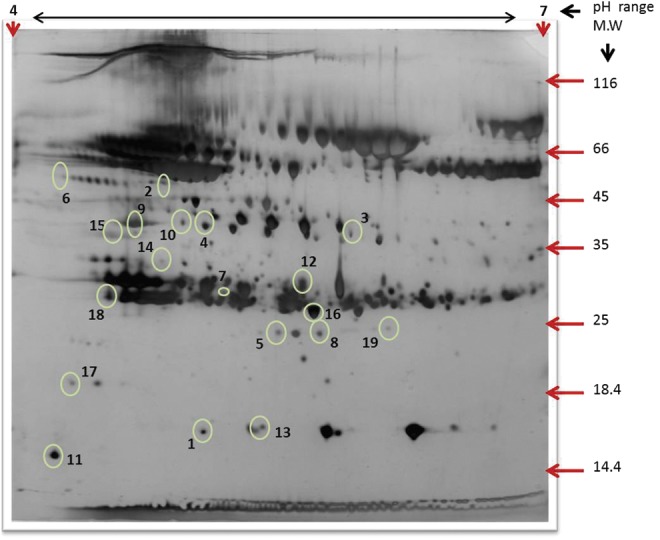
A representative 2-D gel image of hypobaric hypoxia exposed rat plasma following albumin and IgG depletion. Proteins (500 μg) were separated by IEF using pH 4–7, 18 cm IPG strips (GE Healthcare, Sweden) and 12% SDS PAGE. The gels were silver stained and analyzed by Progenesis Same spots 2D gel image analysis software (Nonlinear Dynamics). Spot numbers represent differentially expressed proteins (±1.5-fold, p<0.05) among tolerant and susceptible compared with control.
FIG. 4.
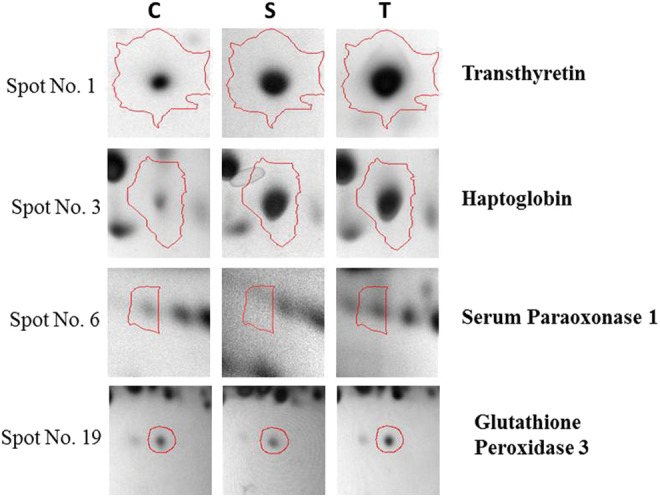
Zoom-in images of 2-DGE of differentially expressed spots in control, Susceptible, Tolerant rat plasma. Spots were identified by MS/MS. C, Control, S, Susceptible, T, Tolerant.
Table 1.
List of Differentially Expressed Plasma Proteins in Hypobaric Hypoxia Tolerant and Susceptible Rats Compared to Control Group
| |
|
|
|
|
|
Fold Change |
|
|
|---|---|---|---|---|---|---|---|---|
| Spot No. | Accession No. | Protein Name | pI/M.W | MASCOT Scorea | Sequence Coverage (%) | S | T | Protein Function |
| 6 | P55159 | Serum paraoxonase/arylesterase | 4.1/54 | 86 | 4 | 0.31 | 13.9 | Removes oxidised lipoprotein |
| 1 | P02767 | Transthyretin | 5.0/15 | 122 | 39 | 1.74 | 2.90 | Transports thyroxine and retinol |
| 14 | Q63942 | GTP-binding protein Rab-3D | 4.7/35 | 51 | 28 | 0.01 | 1.90 | Protein Transport |
| 5 | P23764 | Glutathione peroxidase 3 | 5.3/24 | 92 | 19 | 0.06 | 1.30 | Catalyses reduction of hydrogen peroxide |
| 19 | P23764 | Glutathione peroxidase 3 | 6.1/23 | 60 | 23 | 0.80 | 1.18 | |
| 17 | P08649 | Complement C4 | 4.2/18 | 197 | 1 | 1.09 | 1.92 | Activates classical pathway of the complement system |
| 9 | P01026 | Complement C3 | 4.6/41 | 51 | – | 0.85 | 1.09 | Positive regulator of angiogenesis |
| 15 | O88201 | C-type lectin domain family 11 member A | 4.4/39 | 59 | 28 | 0.12 | 0.55 | Stimulates the proliferation and differentiation of hematopoietic precursor cells |
| 18 | P48199 | C-reactive protein | 4.5/29 | 167 | 21 | 0.45 | 0.68 | Acute phase protein |
| 12 | P01026 | Complement C3 | 5.6/37 | 285 | 12 | 0.14 | 0.21 | Positive regulator of angiogenesis |
| 16 | P04639 | Apolipoprotein A-I | 5.6/26 | 63 | 3 | 1.46 | 1.33 | Component of HDL & promotes cholesterol efflux |
| 3 | P06866 | Haptoglobin | 5.8/38 | 190 | 10 | 4.34 | 4.42 | Prevents the toxic effects associated with release of cell free hemoglobin |
| 4 | P06866 | Haptoglobin | 5.0/40 | 95 | 7 | 1.45 | 1.45 | |
| 13 | P02767 | Transthyretin | 5.2/15 | 92 | 30 | 0.27 | 0.62 | Transports thyroxine and retinol |
Accession No., Swissprot database accession number; pI/MW, represent observed isoelectric point and molecular weight in kDa respectively of the matched protein; MASCOT probability-based MOWSE (molecular weight search) score calculated for PMF. Protein score is −10*Log (p), where p is the probability that the observed match is a random event and greater than 56 are significant (ap<0.05); Sequence coverage: percent of identified sequence to the complete sequence of the known protein.
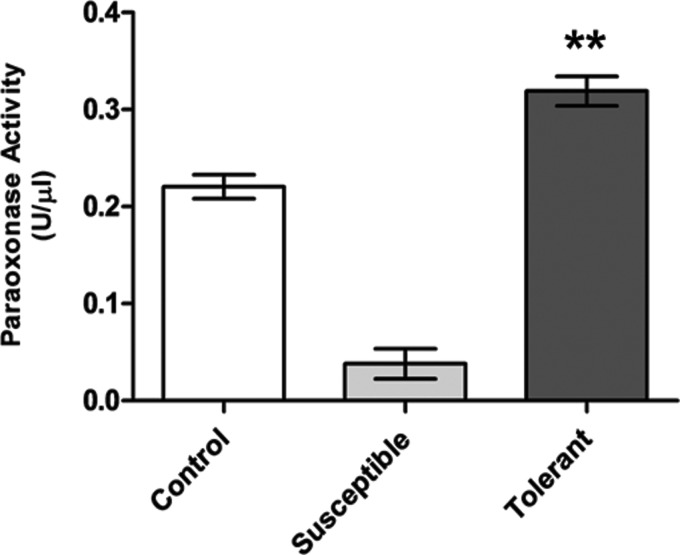
Validation of PON 1 enzyme activity by ELISA
The levels of PON1 enzyme were determined from plasma samples of the hypoxia susceptible, tolerant, and control animals. The hypoxia tolerant animals possess significant higher levels of PON1 (0.31±0.01 U/μL, p<0.001) as compared to the hypoxia susceptible (0.03±0.01 U/μL) and exposure control animals (0.09±0.02 U/μL). As compared to control rats, hypoxia susceptible rats exhibited significantly lower levels of PON1 (82.7%, p<0.001), further demonstrating relevance of PON1activity in hypoxia tolerance (Fig. 5).
FIG. 5.
Estimation of PON 1 activity in rat blood plasma. Results are given in Mean±SEM. (** indicates p<0.001 vs. Control). The concentration of plasma PON 1activity were higher in tolerant rats was 0.31±0.01 U/μL as compared to hypoxia susceptible animals (0.03±0.01 U/μL, p<0.001).
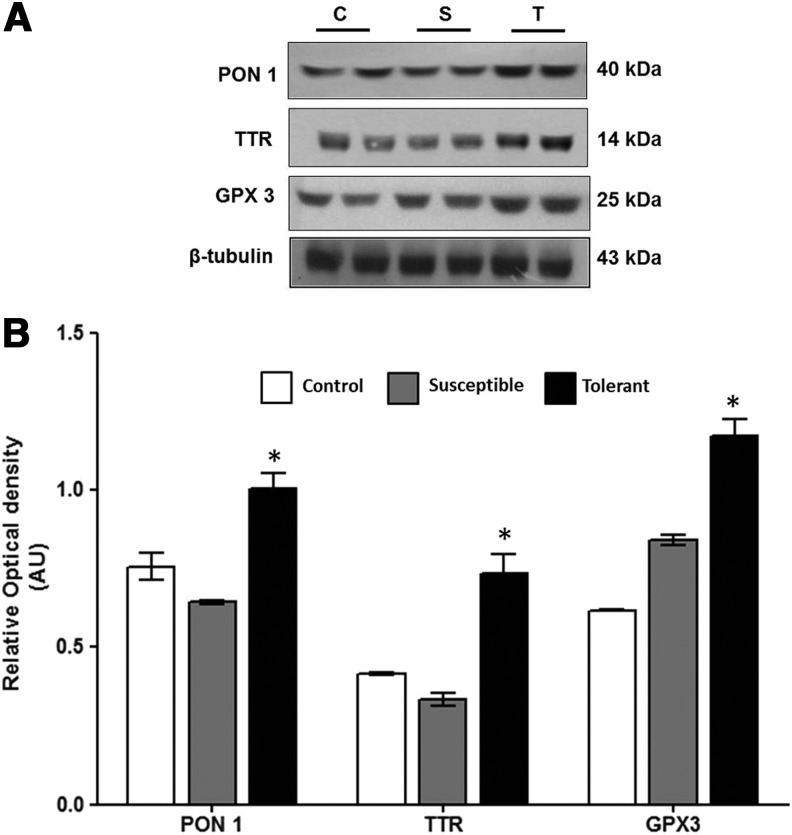
Validation of PON 1, GPx-3, and TTR expression level by immunoblot
Since higher levels of antioxidative proteins provide an advantage for hypoxia tolerance, the major antioxidant proteins identified in this study PON1, GPx-3, and TTR were validated by immunoblotting. Densitometry analysis revealed that hypoxia tolerant animals have higher expression levels for PON1 (1.56-fold, p<0.05), GPx-3 (1.39-fold, p<0.05), and TTR (2.19-fold, p<0.05) as compared with hypoxia susceptible animals (Fig. 6A, B).
FIG. 6.
(A) Validation of differentially expressed protein (PON 1, TTR, GPx-3) by immunoblot analysis. Total plasma protein (30 μg/lane) were separated by 10% SDS-PAGE and probed with primary antibody for PON 1, TTR, and GPx-3. (B) Graphical representation of the optical density of PON 1, TTR, GPx-3 normalized to β-tubulin indicating the expression levels. Data represent Mean±S.E.M of three independent experiments. (* represents p<0.05 vs. Control according to Newman–Keuls multiple comparison tests).
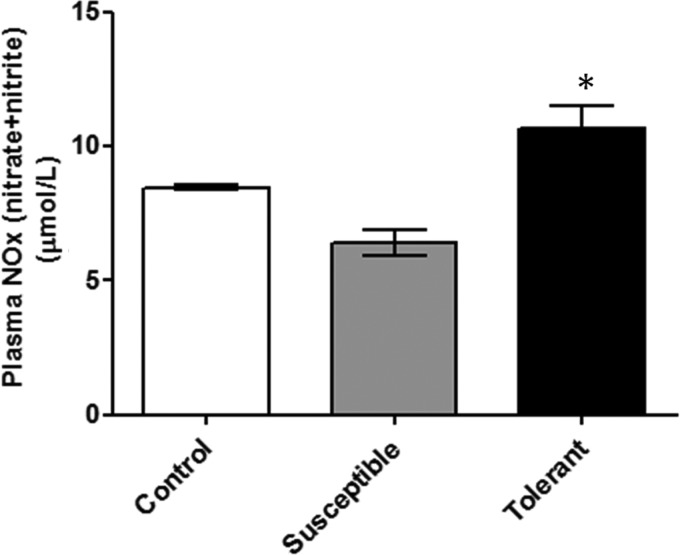
Estimation of NOx (nitrate and nitrite) levels of plasma
The level of NOx was determined in plasma sample of the hypoxia susceptible, tolerant, and control animals. The hypoxia tolerant animals possess significant higher levels of NOx (10.64±0.87 μmol/L, p<0.005), as compared to the hypoxia susceptible (6.4±0.48 μmol/L) and exposure control animals (7.6±0.35 μmol/L). As compared to control rats, hypoxia susceptible rats exhibited significantly lower levels of PON1 (24.7%, p<0.05), demonstrating relevance of NOx in hypoxia tolerance (Fig. 7).
FIG. 7.
Estimation of nitric oxide levels in rat blood plasma. Results are given in Mean±SEM. (* indicates p<0.05 vs. Control). The concentration of plasma nitric oxide was higher in tolerant rats (10.64±0.87 μmol/L, p<0.005) as compared to the hypoxia susceptible animals (6.4±0.48 μmol/L).
Discussion
Under acute hypobaric hypoxia, cellular energy production is dependent on anaerobic glycolysis, which is far less efficient than mitochondrial oxidative phosphorylation (TCA cycle). The lower mitochondrial consumption of protons also leads to cellular acidosis. Under these conditions, the leakage of electrons to oxygen to form superoxide becomes more prevalent. These conditions also activate a number of RONS generating systems such as mitochondrial electron transport chain, xanthine oxidase, and nitric oxide synthase. Furthermore, high altitude exposure decreases the activity and content of antioxidant enzymes that may have relevance to the nitric oxide (NO) availability and microcirculatory alterations associated with hypoxic exposure, including AMS, HAPE, and HACE (Bailey et al., 2009, 2010; Dosek et al., 2007; Guzy et al., 2006). Irrespective of severity of oxidative stress, variations in individual susceptibility is a major predisposing factor for high altitude illnesses. It has been advocated that systematic identification of molecular signatures for hypoxia tolerance can help in understanding of human adaptation to high altitude, as well as critical illnesses arising due to lack of tissue oxygenation.
Hyperventilation is the primary response under acute hypoxia to promote tissue oxygenation and is an indicator of hypoxia tolerance (Hohenhaus et al., 1995; Teppema et al., 2010). Thus monitoring hyperventilation time during acute exposure to 9754 m, we identified hypoxia tolerant and susceptible rats. To further confirm hypoxia tolerance, we also measured plasma levels of 8-isoprostane and protein carbonyls in both tolerant and susceptible animals. The hypoxia tolerant animal possessed significantly lower levels of 8-isoprostane and protein carbonyls than susceptible animals, indicating lower levels of oxidative damage to cellular macromolecules. Several human and animal studies have suggested that both intermittent and chronic hypoxia exposure-induced increase in ventilatory sensitivity is associated with increased oxidative stress (Peng et al., 2006; Pialoux et al., 2009). Our present results of higher oxidative damage in hypoxia-sensitive animals screened on the basis of hyperventilation time corroborate these findings. To understand the molecular basis of individual heterogeneity for hypoxia tolerance at proteome level, we compared the plasma proteomes of hypoxia tolerant and susceptible rats. This enabled us in the identification of profound upregulation of antioxidant proteins such as TTR, GPx-3, PON1, Rab-3D, CLC11, CRP, and Hp in hypoxia tolerant animals, and apolipoprotein A-I (APO-AI) in hypoxia susceptible animals (Table 1). Different classes of proteins such as transporters GTP-binding protein Rab-3D, thyroxine transporter, antioxidative GPx3, C-type lectin-stimulator to the hematopoietic precursor cells, haptoglobin, APO-AI, and complement factors, all play a pivotal role in mediating tolerance by working in orchestration.
The expression of serum paraoxonase 1 (PON1) was significantly upregulated (13.9-fold, p<0.05) in the tolerant rats and downregulated in susceptible rats as compared to control rats. PON 1 is a calcium-dependent 38–43 kDa glycoprotein synthesized in liver and circulates in plasma associated with the high density lipoprotein (HDL) fraction. This enzyme possesses both lactonase as well as esterase activity. The esterase activity is of importance in oxidative stress as it acts on oxidative stress mediators while lactonase work/act on other molecules, which could explain the antioxidative as well as anti-inflammatory role of PON1 (Rochu et al., 2007). The increased activity of PON 1 has been attributed to prevent both HDL and LDL from oxidation (Aviram et al., 1998) and PON1 knockout mice exhibits increased macrophage oxidative stress (Rozenberg et al., 2003). There is considerable evidence demonstrating beneficial effects of PON1 in atherosclerosis (Aviram et al., 1998; Fuhrman et al., 2010; Gaillard et al., 2011) and Apo E−/− mice overexpressing PON1 develops fewer atherosclerotic lesions (Tward et al. 2002). Moreover, PON1 gene polymorphism is also associated with a number of diseases attributed for oxidative stress such as type-2 diabetes, coronary heart disease, and Parkinson's disease (Precourt et al., 2011). These studies, in corroboration with our present finding, indicate higher protein levels of PON1 are prerequisite for hypoxia tolerance.
Transthyretin (TTR) is a 56 kDa homotetrameric protein that transports thyroxine and retinol produced by liver into plasma. It is a negative acute phase protein, and lower serum levels of TTR or misfolding in the tissues has been implicated in inflammation (Vascotto et al., 2007). It exists in both tetrameric as well as monomeric forms. The monomeric form is rapidly stabilized by modifications caused by oxidative stress. Thus, dissociation of transthyretin into its monomeric form could play a crucial role in determining the state of oxidative stress to the body (Takaoka et al., 2004; Zhang et al., 2003). Moreover, persistent low levels of serum TTR is a predictive of lethality whereas increased levels of TTR is associated with improved ventilatory responses (Schlossmacher et al., 2002). Corroborating these reports, our results also demonstrates higher levels of (2.19-fold, p<0.05) of TTR in hypoxia tolerant animals, possibly improving ventilatory functions during hypoxia exposure.
Plasma glutathione peroxidase (GPx-3) is a major antioxidant enzyme in plasma, synthesised in kidney and secreted into the blood. It is a member of the selenocysteine-containing GPx family and scavenges hydrogen peroxide as well as lipid hydroperoxides produced during normal metabolism or after oxidative insult, thereby maintaining the vasorelaxant and antithrombotic properties of the vascular endothelium (Bierl et al., 2004; Jin et al., 2011; Voetsch et al., 2007). GPx-3-mediated reduction in oxidative stress also protects against post-translational modifications of fibrinogen by ROS and NO-derived oxidants that increase its thrombogenicity (Bierl et al., 2004). In contrast, GPx-3 deficiency has been associated with platelet-dependent arterial thrombosis in human subjects (Voetsch et al., 2007). GPx-3 deficiency also impairs the reductive metabolism of ROS, leading in part to a decrease in NO bioavailability, thereby potentially impairing the inhibitory effect of extracellular (plasma-borne) NO on platelets (Jin et al., 2011). In the present study, we have observed 1.39-fold increase (p<0.05) in GPx-3 levels in hypoxia tolerant rats (Fig. 6B). Hypoxia promotes GPx-3 expression, probably through an HIF-1-dependent mechanism (Bierl et al., 2004), and the increased levels of GPx-3 in the tolerant rats along with decreased levels of lipid peroxides suggests lower oxidant production and oxidative damage.
A higher level of haptoglobin (Hp) was observed in hypoxia tolerant animals in comparison with susceptible animals. Haptoglobin possesses both anti-inflammatory and antioxidative properties by forming a complex with free hemoglobin released as a result of cell membrane damage by reactive oxygen species (Pimenova et al., 2010; Tseng et al., 2004). Free hemoglobin in circulation avidly binds and depletes nitric oxide (NO), the major vasodilator in the circulation (Boretti et al., 2009; Schaer et al., 2010; Watanabe et al., 2009). Furthermore, an antioxidative role of haptoglobin has been implicated in cardiovascular diseases, preventing formation of foam cells and free radical-induced damage to the cells (Salvatore et al., 2007). Besides having antioxidative functions, haptoglobin also acts as molecular chaperone and prevents misfolding and aggregation of protein. Haptoglobin also modulates the immune response by simulating monocyte and macrophages under a compromised oxygen state such as inflammation, injury, and respiratory burst (Levy 2003; Quaye et al., 2006). These results tend us to speculate that the increased levels of haptoglobin promotes better NO availability and limits oxidative stress there by conferring hypoxia tolerance.
NO is a critical regulator of vascular homeostasis and an integral part of the human physiological response to hypoxia. High altitude exposure reduces NO availability due to (i) decreased enzymatic production owing to limited activity of oxygen-dependent endothelial nitric oxide synthase (eNOS) and ii) paradoxical increase in the production of ROS leading to NO inactivation. Several lines of evidence suggest that multiple nitrogen oxides (including nitrite, nitrate, and S-nitrosothiols) contribute to improved hypoxia tolerance by improving NO availability (Erzurum et al., 2007; Levett et al., 2011; Lundberg, et al., 2008). In contrast, hypoxia exposure compromises eNOS-mediated NO production and HAPE susceptible subjects exhibit lower levels of exhaled NO as compared to HAPE-resistant subjects during acute hypoxia exposure (Berger et al., 2005; Brown et al., 2006; Busch et al., 2001). Studying the pulmonary artery systolic pressure (PASP) following active ascent to 4559 m, Bailey et al. (2010) reported that increase in PASP and vascular resistance are associated with free-radical mediated reduction in pulmonary NO availability. Similarly, increased levels of ROS and lipid peroxyl radicals decrease the NO availability in circulation, which in turn increases platelet-derived thrombosis (Loscalzo, 2001; Pidgeon et al., 2004). These results suggest that NO production, availability, and ROS-mediated quenching impart the final physiological outcome during hypoxia exposure. Owing to crucial roles of NO for the control of blood pressure, blood flow, and other vital bodily functions, it is imperative to protect the available NO from ROS-mediated depletion during hypoxia. Either reduced oxidant production or depletion of oxidants by antioxidants systems during hypoxia can confer hypoxia tolerance. Consistent with this evidence, we observed higher levels of NO in plasma of tolerant rats (50%, p<0.005) as compared to the susceptible rats (Fig. 7). In this regard, our present results reporting abundance of antioxidant enzymes like GPx-3 and haptoglobin, which in turn maintains vascular availability of NO during several clinical disorders, provides critical insight into hypoxia tolerance. Supporting our current results, Ali et al. (2012) reported that reduced superoxide leakage gives Drosophila melanogaster an advantage for survival in long-term hypoxia. These collective results highlight the crucial role of antioxidant proteins in conferring hypoxia tolerance, thereby increasing the availability of vasodilator nitric oxide into the circulation during acute altitude exposure.
Hyperventilation-based screening for hypoxia tolerance is one of the limitations of the present study. Though the hypoxia effect was similar between the studied groups, future studies measuring pulmonary arterial systolic pressure (PASP) along with hyperventilation time will aid in better screening. The other limitation in the present study is the gel-based identification of proteins. These methods are labor intensive, and have disadvantages of co-migration of proteins and resolution of limited number of proteins on the gel. In order to minimize both individual and gel to gel variations, we have resolved all the samples in triplicate with significant statistical stringency. However, future of use of gel-free proteomics methods will help in identification and quantification of larger number of proteins.
Conclusion
The present study reports the differential expression of plasma proteins in hypobaric hypoxia tolerant and susceptible rats. Comparing the expression profiles, we have identified higher expression of several antioxidant proteins like PON1, TTR, GPx-3, and haptoglobin, which were further validated by ELISA and Western blot. The increased expression of these antioxidant proteins with concomitant decrease in 8-isoprostane and protein carbonyls in hypoxia tolerant animals suggests significance of these proteins in conferring hypoxia tolerance, which in turn increase nitric oxide availability to the animal. The therapeutic potential of these proteins can be assayed as potential biomarkers for hypoxia tolerance.
Acknowledgments
This work is supported by grant number DIP-251/A-2.5 from Defence Research and Development Organization (DRDO), Ministry of Defence, Government of India. The authors acknowledge technical support provided by Kanika Jain, Shweta, Gaurav Kumar Keshri for screening of rats and Dr. Soma Sarkar for critically reading the manuscript. Gayatri Padhy is a recipient of senior research fellowship (SRF) from CSIR, Government of India.
Author Disclosure Statement
Authors declare no conflict of interest.
References
- Ahmad Y. Shukla D. Garg I. Sharma NK. Saxena S. Malhotra VK. Bhargava K. Identification of haptoglobin and apolipoprotein A-I as biomarkers for high altitude pulmonary edema. Funct Integr Genomics. 2011;11:407–417. doi: 10.1007/s10142-011-0234-3. [DOI] [PubMed] [Google Scholar]
- Ali SS. Hsiao M. Zhao HW. Dugan LL. Haddad GG. Zhou D. Hypoxia-adaptation involves mitochondrial metabolic depression and decreased ROS leakage. PLoS One. 2012;7:e36801. doi: 10.1371/journal.pone.0036801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviram M. Rosenblat M. Bisgaier CL. Newton RS. Primo-Parmo SL. La Du BN. Paraoxonase inhibits high-density lipoprotein oxidation and preserves its functions. A possible peroxidative role for paraoxonase. J Clin Invest. 1998;101:1581–1590. doi: 10.1172/JCI1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baik SC. Kim KM. Song SM, et al. Proteomic analysis of the sarcosine-insoluble outer membrane fraction of Helicobacter pylori strain 26695. J Bacteriol. 2004;186:949–955. doi: 10.1128/JB.186.4.949-955.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM. Dehnert C. Luks AM, et al. High-altitude pulmonary hypertension is associated with a free radical-mediated reduction in pulmonary nitric oxide bioavailability. J Physiol. 2010;588:4837–4847. doi: 10.1113/jphysiol.2010.194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey DM. Evans KA. James PE, et al. Altered free radical metabolism in acute mountain sickness: Implications for dynamic cerebral autoregulation and blood-brain barrier function. J Physiol. 2009;587:73–85. doi: 10.1113/jphysiol.2008.159855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger MM. Hesse C. Dehnert C, et al. Hypoxia impairs systemic endothelial function in individuals prone to high-altitude pulmonary edema. Am J Respir Crit Care Med. 2005;172:763–767. doi: 10.1164/rccm.200504-654OC. [DOI] [PubMed] [Google Scholar]
- Bierl C. Voetsch B. Jin RC. Handy DE. Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem. 2004;279:26839–26845. doi: 10.1074/jbc.M401907200. [DOI] [PubMed] [Google Scholar]
- Boretti FS. Buehler PW. D'Agnillo F, et al. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest. 2009;119:2271–2280. doi: 10.1172/JCI39115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE. Beall CM. Strohl KP. Mills PS. Exhaled nitric oxide decreases upon acute exposure to high-altitude hypoxia. Am J Hum Biol. 2006;18:196–202. doi: 10.1002/ajhb.20489. [DOI] [PubMed] [Google Scholar]
- Busch T. Bärtsch P. Pappert D, et al. Hypoxia decreases exhaled nitric oxide in mountaineers susceptible to high-altitude pulmonary edema. Am J Respir Crit Care Med. 2001;163:368–373. doi: 10.1164/ajrccm.163.2.2001134. [DOI] [PubMed] [Google Scholar]
- Dosek A. Ohno H. Acs Z. Taylor AW. Radak Z. High altitude and oxidative stress. Respir Physiol Neurobiol. 2007;158:128–131. doi: 10.1016/j.resp.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Erzurum SC. Ghosh S. Janocha AJ, et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA. 2007;104:17593–17598. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuhrman B. Gantman A. Aviram M. Paraoxonase 1 (PON1) deficiency in mice is associated with reduced expression of macrophage SR-BI and consequently the loss of HDL cytoprotection against apoptosis. Atherosclerosis. 2010;211:61–68. doi: 10.1016/j.atherosclerosis.2010.01.025. [DOI] [PubMed] [Google Scholar]
- Gaillard T. Parthasarathy S. Osei K. HDL dysfunctionality (Paraoxonase) is worse in nondiabetic, postmenopausal African American than in white women. Diabetes Care. 2011;34:e19. doi: 10.2337/dc10-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfi C. De Palma S. Ripamonti M, et al. New aspects of altitude adaptation in Tibetans: A proteomic approach. FASEB J. 2004;18:612–614. doi: 10.1096/fj.03-1077fje. [DOI] [PubMed] [Google Scholar]
- Guzy RD. Schumacker PT. Oxygen sensing by mitochondria at complex III: The paradox of increased reactive oxygen species during hypoxia. Exp Physiol. 2006;91:807–819. doi: 10.1113/expphysiol.2006.033506. [DOI] [PubMed] [Google Scholar]
- Hackett PH. Roach RC. High altitude cerebral edema. High Alt Med Biol. 2004;5:136–146. doi: 10.1089/1527029041352054. [DOI] [PubMed] [Google Scholar]
- Hohenhaus E. Paul A. McCullough RE. Kucherer H. Bartsch P. Ventilatory and pulmonary vascular response to hypoxia and susceptibility to high altitude pulmonary oedema. Eur Respir J. 1995;8:1825–1833. doi: 10.1183/09031936.95.08111825. [DOI] [PubMed] [Google Scholar]
- Jacobs JM. Adkins JN. Qian WJ. Liu T. Shen Y. Camp DG., 2nd Smith RD. Utilizing human blood plasma for proteomic biomarker discovery. J Proteome Res. 2005;4:1073–1085. doi: 10.1021/pr0500657. [DOI] [PubMed] [Google Scholar]
- Jefferson JA. Simoni J. Escudero E, et al. Increased oxidative stress following acute and chronic high altitude exposure. High Alt Med Biol. 2004;5:61–69. doi: 10.1089/152702904322963690. [DOI] [PubMed] [Google Scholar]
- Jin RC. Mahoney CE. Coleman Anderson L, et al. Glutathione peroxidase-3 deficiency promotes platelet-dependent thrombosis in vivo. Circulation. 2011;123:1963–1973. doi: 10.1161/CIRCULATIONAHA.110.000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levett DZ. Fernandez BO. Riley HL, et al. The role of nitrogen oxides in human adaptation to hypoxia. Sci Rep. 2011;1:109. doi: 10.1038/srep00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy AP. Genetics of diabetic cardiovascular disease: Identification of a major susceptibility gene. Acta Diabetol. 2003;40:S330–333. doi: 10.1007/s00592-003-0114-y. [DOI] [PubMed] [Google Scholar]
- Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ Res. 2001;88:756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- Lundberg JO. Weitzberg E. Gladwin MT. The nitrate–nitrite-nitric oxide pathway in physiology and therapeutics. Nat Rev Drug Discov. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- Moraga FA. Flores A. Serra J. Esnaola C. Barriento C. Ginkgo biloba decreases acute mountain sickness in people ascending to high altitude at Ollague (3696 m) in northern Chile. Wilderness Environ Med. 2007;18:251–257. doi: 10.1580/06-WEME-OR-062R2.1. [DOI] [PubMed] [Google Scholar]
- Peng YJ. Yuan G. Ramakrishnan D, et al. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–716. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pialoux V. Hanly PJ. Foster GE, et al. Effects of exposure to intermittent hypoxia on oxidative stress and acute hypoxic ventilatory response in humans. Am J Respir Crit Care Med. 2009;180:1002–1009. doi: 10.1164/rccm.200905-0671OC. [DOI] [PubMed] [Google Scholar]
- Pimenova T. Pereira CP. Gehrig P. Buehler PW. Schaer DJ. Zenobi R. Quantitative mass spectrometry defines an oxidative hotspot in hemoglobin that is specifically protected by haptoglobin. J Proteome Res. 2010;9:4061–4070. doi: 10.1021/pr100252e. [DOI] [PubMed] [Google Scholar]
- Pidgeon GP. Tamosiuniene R. Chen G. Leonard I. Belton O. Bradford A. Fitzgerald DJ. Intravascular thrombosis after hypoxia-induced pulmonary hypertension: Regulation by cyclooxygenase-2. Circulation. 2004;110:2701–2707. doi: 10.1161/01.CIR.0000145613.01188.0B. [DOI] [PubMed] [Google Scholar]
- Precourt LP. Amre D. Denis MC. Lavoie JC. Delvin E. Seidman E. Levy E. The three-gene paraoxonase family: Physiologic roles, actions and regulation. Atherosclerosis. 2011;214:20–36. doi: 10.1016/j.atherosclerosis.2010.08.076. [DOI] [PubMed] [Google Scholar]
- Purushothaman J. Suryakumar G. Shukla D, et al. Modulatory effects of seabuckthorn (Hippophae rhamnoides L.) in hypobaric hypoxia induced cerebral vascular injury. Brain Res Bull. 2008;77:246–252. doi: 10.1016/j.brainresbull.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Quaye IK. Ababio G. Amoah AG. Haptoglobin 2-2 phenotype is a risk factor for type 2 diabetes in Ghana. J Atheroscler Thromb. 2006;13:90–94. doi: 10.5551/jat.13.90. [DOI] [PubMed] [Google Scholar]
- Rochu D. Chabriere E. Masson P. Human paraoxonase: A promising approach for pre-treatment and therapy of organophosphorus poisoning. Toxicology. 2007;233:47–59. doi: 10.1016/j.tox.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Rozenberg O. Rosenblat M. Coleman R. Shih DM. Aviram M. Paraoxonase (PON1) deficiency is associated with increased macrophage oxidative stress: Studies in PON1-knockout mice. Free Radic Biol Med. 2003;34:774–784. doi: 10.1016/s0891-5849(02)01429-6. [DOI] [PubMed] [Google Scholar]
- Salvatore A. Cigliano L. Bucci EM, et al. Haptoglobin binding to apolipoprotein A-I prevents damage from hydroxyl radicals on its stimulatory activity of the enzyme lecithin-cholesterol acyl-transferase. Biochemistry. 2007;46:11158–11168. doi: 10.1021/bi7006349. [DOI] [PubMed] [Google Scholar]
- Schaer DJ. Alayash AI. Clearance and control mechanisms of hemoglobin from cradle to grave. Antioxid Redox Signal. 2010;12:181–184. doi: 10.1089/ars.2009.2923. [DOI] [PubMed] [Google Scholar]
- Schlossmacher P. Hasselmann M. Meyer N. Kara F. Delabranche X. Kummerlen C. Ingenbleek Y. The prognostic value of nutritional and inflammatory indices in critically ill patients with acute respiratory failure. Clin Chem Lab Med. 2002;40:1339–1343. doi: 10.1515/CCLM.2002.231. [DOI] [PubMed] [Google Scholar]
- Sharp FR. Bernaudin M. HIF1 and oxygen sensing in the brain. Nat Rev Neurosci. 2004;5:437–448. doi: 10.1038/nrn1408. [DOI] [PubMed] [Google Scholar]
- Takaoka Y. Ohta M. Miyakawa K. Nakamura O. Suzuki M. Takahashi K. Yamamura K. Sakaki Y. Cysteine 10 is a key residue in amyloidogenesis of human transthyretin Val30Met. Am J Pathol. 2004;164:337–345. doi: 10.1016/s0002-9440(10)63123-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teppema LJ. Dahan A. The ventilatory response to hypoxia in mammals: Mechanisms, measurement, and analysis. Physiol Rev. 2010;90:675–754. doi: 10.1152/physrev.00012.2009. [DOI] [PubMed] [Google Scholar]
- Tseng CF. Lin CC. Huang HY. Liu HC. Mao SJ. Antioxidant role of human haptoglobin. Proteomics. 2004;4:2221–2228. doi: 10.1002/pmic.200300787. [DOI] [PubMed] [Google Scholar]
- Tward A. Xia YR. Wang XP, et al. Decreased atherosclerotic lesion formation in human serum paraoxonase transgenic mice. Circulation. 2002;106:484–490. doi: 10.1161/01.cir.0000023623.87083.4f. [DOI] [PubMed] [Google Scholar]
- Vascotto C. Salzano AM. D'Ambrosio C, et al. Oxidized transthyretin in amniotic fluid as an early marker of preeclampsia. J Proteome Res. 2007;6:160–170. doi: 10.1021/pr060315z. [DOI] [PubMed] [Google Scholar]
- Voetsch B. Jin RC. Bierl C, et al. Promoter polymorphisms in the plasma glutathione peroxidase (GPx-3) gene: A novel risk factor for arterial ischemic stroke among young adults and children. Stroke. 2007;38:41–49. doi: 10.1161/01.STR.0000252027.53766.2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vij AG. Dutta R. Satija NK. Acclimatization to oxidative stress at high altitude. High Alt Med Biol. 2005;6:301–310. doi: 10.1089/ham.2005.6.301. [DOI] [PubMed] [Google Scholar]
- Watanabe J. Grijalva V. Hama S, et al. Hemoglobin and its scavenger protein haptoglobin associate with apoA-1-containing particles and influence the inflammatory properties and function of high density lipoprotein. J Biol Chem. 2009;284:18292–18301. doi: 10.1074/jbc.M109.017202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil JV. Sleep at high altitude. High Alt Med Biol. 2004;5:180–189. doi: 10.1089/1527029041352162. [DOI] [PubMed] [Google Scholar]
- Wilson MH. Newman S. Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8:175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- Zhang Q. Kelly JW. Cys10 mixed disulfides make transthyretin more amyloidogenic under mildly acidic conditions. Biochemistry. 2003;42:8756–8761. doi: 10.1021/bi030077a. [DOI] [PubMed] [Google Scholar]