Abstract
Objective The objective of the current study was to examine the effectiveness of a multidisciplinary weekly family-based behavioral group delivered via telemedicine to rural areas, compared with a standard physician visit intervention. Methods A randomized controlled trial was conducted with 58 rural children and their families comparing a family-based behavioral intervention delivered via telemedicine to a structured physician visit condition. Outcome measures included child body mass index z-score (BMIz), 24-hr dietary recalls, accelerometer data, Child Behavior Checklist, Behavioral Pediatrics Feeding Assessment Scale, and feasibility and fidelity. Results Child BMIz outcomes were not statistically different between the 2 groups (F = 0.023, p = .881). Improvements in BMIz, nutrition, and physical activity were seen for both groups. Conclusions Both telemedicine and structured physician visit may be feasible and acceptable methods of delivering pediatric obesity treatment to rural children.
Keywords: obesity, randomized controlled trial, weight management
Introduction
The prevalence of obesity among children in the United States has increased rapidly during the past several years (Strauss & Pollack, 2001) to the point that pediatric obesity is now termed a public health epidemic (Strauss, 2002). Many adult health concerns are linked to childhood obesity independent of adult weight status (Must & Strauss, 1999). Current data indicate that weight status by age 7 years is directly related to adult weight status and adult health concerns (Dietz, 1998). Thus, treating obesity in childhood may lead to improved adult weight status and improved adult health outcomes. In 2007, the Expert Committee Recommendations for the Prevention and Treatment of Pediatric Obesity were released (Barlow, 2007). These recommendations divide the treatment into four stages: Stage 1 (which occurs individually in the primary care office), Stage 2 (which includes a multidisciplinary obesity team), Stage 3 (family-based behavioral groups), and Stage 4 (which includes many intensive treatments, such as bariatric surgery).
Research indicates that children in rural areas are more likely to be obese than children in urban areas (Tai-Seale & Chandler, 2003). This may be because they are more likely to consume more “red” foods (foods with >7 g of fat and/or 12 g of sugar; Davis, James, Curtis, Felts, & Daley, 2008), to consume more fried foods and meat, and less likely to meet physical activity recommendations (Davis, Bennett, Befort, & Nollen, 2011). However, research indicates that these children are also less likely to have access to treatment (Tai-Seale & Chandler, 2003). Some studies have been published on Stage 1 clinical rural obesity services delivered by primary care physicians or other health care providers (Irby, Boles, Jordan, & Skelton, 2012; Shaikh, Nettiksimmons, & Romano, 2011), or on the perspectives of rural health care providers who are faced with the pediatric obesity epidemic (Steele et al., 2011). For example, Shaikh et al. (2011) published a report on the use of telemedicine for the clinical management of adolescents with obesity. Specifically, a weight management specialist or endocrinologist consulted via telemedicine with the primary care provider and patient to review the case and recommend additional testing or management options. Results indicate that consulting with the specialist increased the number of patient diagnoses significantly and changed/added treatment recommendations. Of the 50 patients seen more than once, 14 (22.6%) showed weight reduction.
However, when searching for more intense weekly programs that are recommended as Stage 3 programs by the Expert Committee, we could find only one such published study that targeted rural children (Janicke et al., 2008). Janicke et al. (2008) describe 71 rural children and families who were randomly assigned to two types of Stage 3 interventions (one with parent and child, and a second with parent alone) or a wait-list control group. All interventions were delivered by the investigative team at the cooperative extension service offices in the rural areas. Body mass index z-score (BMIz) data indicate that both intervention groups were superior to the wait-list group at the 10-month time point.
To extend this line of research, our team was interested in finding whether such a Stage 3 family-based behavioral group intervention could be delivered to rural families in their communities solely relying on interactive technology, including phone and televideo, also known as telemedicine. Relying solely on these modalities would greatly decrease the travel burden on providers and families, as well as extend the reach of providers to almost all rural sites—interactive televideo is extremely common for distance learning purposes in rural areas. Therefore, our team developed and piloted a rurally tailored pediatric obesity intervention (Davis, James, Boles, et al., 2011). This initial pilot study was promising, indicating high acceptability and feasibility, but it also had several weaknesses, including an intervention duration of only 8 weeks, lack of any changes in BMIz or other outcome measures, and a very small sample size (n = 17).
To extend this initial report, our team conducted a study to tailor this existing intervention to the needs of rural families. Focus groups were held with parents of rural children to learn more about the specific barriers they faced in helping their children in rural areas to live healthier lives. Findings indicated that parents wanted the intervention to have more information on eating at social gatherings and less information on eating out, for example (Davis, James, Curtis et al., 2008). We then modified the existing intervention to be more rurally tailored based on the focus group findings. Given the lack of change in BMIz in the initial study, our team also lengthened the intervention from 8 weeks to 8 months by adding monthly sessions after the initial 8-week burst. Our team then conducted a randomized controlled trial of this tailored intervention, and only the initial baseline data from this trial have been reported elsewhere (Gallagher, Davis, Malone, Landrum, & Black, 2011). The purpose of the current article is to describe the immediate posttreatment outcome of the families who participated in this randomized controlled trial. Therefore, the objective of the current study was to examine the effectiveness of a multidisciplinary weekly family-based behavioral group delivered via telemedicine to rural areas, compared with a standard physician visit intervention.
Methods
Recruitment
Recruitment occurred at the school level, and once a school completed the enrollment process, individuals within each school were recruited. Schools in rural Kansas were recruited during the 2007/2008 and 2008/2009 school years via a single flyer sent statewide to elementary school nurses and elementary school principals, relevant professional list serves, professional conferences, and word of mouth. Criteria for school participation included having rural designation (in a town or county with a population <20,000) and telemedicine capabilities (common in rural districts for distance learning). Interested schools were required to send in a letter from their principal or superintendent indicating their willingness to participate, and name a designated school representative (school nurse, gym teacher, principal, or computer teacher) who would be the liaison for the project. Ten elementary schools expressed interest, and the first seven to complete this process were enrolled in the study. Representatives from these schools then received the required institutional online training in Human Subjects Research, Conflict of Interest, and HIPAA, as well as training on study-specific procedures, which was conducted by the investigators, lasted 2 hr, and focused on properly measuring height and weight, reviewing the manual and supplies, and recruitment procedures of subjects at the school. All study procedures were approved by the relevant institutional review board.
Participants
The study was designed to target 3rd–5th grade children, but schools were allowed to invite any elementary grades they felt were pertinent. Recruitment letters were sent home with children by school personnel to determine which families were interested in participation, and interested families signed consent forms and completed baseline measures at home and returned these to school personnel. Research staff members were available via a toll-free number for questions, if needed. Inclusion criteria included living in a rural area and attending elementary school, BMI percentile ≥85th for age/gender, and parent’s ability to speak English. Exclusion criteria included having a developmental disability that would prevent the child from participating in the group, or being immobile, which would prevent them from increasing exercise. Parents and children who chose to participate gave informed consent and assent, respectively. Children were screened at school by the school representative, and children who met these criteria and whose parents completed baseline measures were enrolled in the study. Children within each school were ranked based on an obesity factor (child BMI percentile plus primary parent BMI) and stratified based on a household factor (single or dual parent household), and gender, according to previous research, which indicates these factors are closely linked to obesity and to treatment outcome (Cutting, Fisher, Grimm-Thomas, & Birch, 1999; Favaro & Santonastaso, 1995; Gordon-Larsen, Adair, & Popkin, 2003). One child from each stratification was then randomly assigned (via a random numbers table) to the telemedicine intervention (TM) with the other half of the pair being assigned to the physician visits (PV) intervention. Families were notified of their group assignment in a letter sent home from the school. Individuals assigned to the TM group were contacted by phone and a time for the group arranged by consensus (typically groups were offered on Tuesday or Thursday evenings). Individuals assigned to the PV group were contacted by phone to gather the name of their child’s primary care physician (PCP) and insurance status with the study planning to assign a PCP and pay for the visit, if necessary. However, every family had an existing PCP and the required insurance for the visit; therefore, coverage by the study was not necessary.
Intervention Groups
Telemedicine Intervention
Children and families randomized to the TM intervention participated in 8 weekly psychoeducational groups over telemedicine led by trained PhD-level psychologists or trained graduate students/postdoctoral fellows, followed by 6 monthly meetings. Telemedicine is a technology similar to other interactive televideo modalities (i.e., Skype©) except that it is point-to-point such that data are transferred directly from one site to another and do not move through an off-site server, thus increasing patient confidentiality. From a provider perspective, telemedicine is similar to face-to-face in that the provider can see and speak with the patient in real time.
Before the current study, preexisting pediatric obesity intervention treatment manuals for parents and children (Davis, James, Boles, et al., 2011) were tailored to better meet the needs of rural families based on published focus group findings (Davis, James, Curtis, et al., 2008). Specifically, the intervention was lengthened to 8 months, information was added on dressing for larger body sizes, modifying recipes, and eating at potluck gatherings, whereas information on eating out was condensed. In addition to being rurally tailored, the manual covered the existing topics of behavior, activity and nutrition, with a heavy focus on the stop-light diet (Epstein & Squires, 1988). Topics covered by week can be found in Table I.
Table I.
Topics Covered by Session
| Session number | Topics covered |
|---|---|
| 1 | Overview of program and expectations; goal setting |
| 2 | Use of goal charts; reinforcement and incentives |
| 3 | Stop-light diet and nutrition recommendations |
| 4 | Screen time and sedentary activity; the importance of tracking; activity monitor results: your child’s data |
| 5 | Praising and ignoring: role play and homework |
| 6 | Diet recall results: your child’s data; calorie counting; healthy substitutions |
| 7 | Portion sizes: lesson, demonstration, and quiz |
| 8 | Self-esteem; dressing for larger body sizes; adult modeling: what I like about myself |
| 9 | Reading food labels and vitamins/minerals |
| 10 | The concept of nutrient density |
| 11 | Potlucks, BBQs, and other events: how to be smart |
| 12 | Exercising as a family |
| 13 | The application of energy balance |
| 14 | The use of privileges and maintenance |
After an introduction and review of weekly progress, children participated as a group in an age-appropriate lesson with the school representative on site using a standardized treatment manual, whereas parents separately, but simultaneously, met as a group with the group leader over TM using a similar standardized treatment manual. Group leaders were either licensed psychologists experienced with weight management or graduate level students/postdoctoral fellows who observed at least one complete intervention cycle with a school and received training in weight management from the research team. Supervision was given to all students and postdoctoral fellows on a weekly basis during intervention delivery. Parents and children covered the same topics and were reunited at the end of the meeting for goal setting. After 8 weekly sessions were completed, groups met monthly for an additional 6 months during the follow-up period. Sessions during the summer break occurred individually with parents over the phone. All group meetings lasted ∼1 hr.
Physician Visit Group
Children and families randomized to the physician visit group agreed to meet with their primary care physician to discuss a standardized list of topics. If they did not have a primary care physician, staff agreed to provide one for them, but this was never necessary. Before the visit, research staff sent a list of pediatric obesity-related topics to both the family and the physician’s office, requesting that the physician discuss the topics with the family during the visit, sign the form, and return it to the research staff in a postage paid envelope. Topics that were covered included the causes of obesity and the relationship between diet, exercise, and body mass index; the importance of eating a balanced diet; and current exercise and sedentary behavior recommendations for children.
Measures
Measures were taken at two time points: baseline, before the start of the intervention; and posttreatment, following the intervention period, which was ∼8 months after baseline.
Demographics
At baseline, the target child’s birth date, gender, grade level, and ethnicity were gathered. Information pertaining to maternal and paternal age, height and weight, marital status, education, occupation, and income level were also collected.
Primary Outcome
BMIz
Height and weight were assessed by school nurses via a Harpenden Holtain stadiometer, Model 603 (Holtain, Crymych, UK) and a portable SECA digital scale (SECA, Hamburg, Germany). Height and weight were taken in triplicate and used to calculate BMIz, which was used for primary outcome based on previous similar research (Janicke et al., 2008) and BMI percentile for children (which was used for educational purposes) based on the Centers for Disease Control and Prevention’s growth charts (Centers for Disease Control and Prevention, 2000).
Secondary Outcomes
The 24-hr Dietary Recall
The 24-hr diet recall is a standardized three-pass method, developed by the U.S. Department of Agriculture for use in national dietary surveillance. This measure has been shown to be a valid and reliable representation of a child’s overall diet (Crawford, Obarzanek, Morrison, & Sabry, 1994). Dietary recall data were gathered by trained Master’s and PhD-level researchers over the phone and were found to be reliable in diet recall procedures by a registered dietician. Parents were asked to sit with their child and write down all food items consumed at home and away from home (i.e., at school) every day. Parents completed the phone recalls regarding their child’s diet for two weekdays and one weekend day at each time point using standardized procedures. All dietary data were analyzed using NDSR software version 2005 developed by the Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN, USA. Daily intake of calories, percent calories from fat, fruit and vegetable servings, sugar-sweetened beverage servings, and servings of “red” foods (foods with >12 g of sugar and/or 7 g of fat [Epstein & Squires, 1988]) were assessed.
Accelerometers
The ActiGraph (Actigraph LLC, Pensacola, FL, USA) is a small light-weight device worn on an adjustable belt over the nondominant hip that measures physical activity duration and intensity. The ActiGraph has been shown to provide valid assessments of physical activity for adults and children during daily living activities (Sirard, Melanson, Li, & Freedson, 2000). Participants were asked to wear the activity monitor for at least 6 hr a day for a minimum of 3 days during a 1-week period (Masse et al., 2005). All data were run through Santech MeterPlus software, which accounts for age and gender cut-offs when determining moderate or vigorous activity (for specific cut-offs, see Troiano et al., 2008). Data are reported as minutes of moderate to vigorous activity per day.
Child Behavior Checklist
Previous data have indicated that children with overweight/obesity problems are more likely to have psychological issues (Phillips et al., 2012). As the current study compared a behavioral intervention with a physician visit intervention, we were interested in comparing outcomes on a measure of global behavioral issues, such as the Child Behavior Checklist (CBCL). The CBCL (Achenbach, 1991) is a standardized measure that assesses parental report of child competencies and behavioral or emotional problems. Values for total score, internalizing behavior, and externalizing behavior were assessed.
Behavioral Pediatrics Feeding Assessment Scale
Previous data indicate that children with overweight/obesity problems have higher rates of mealtime behavior problems (Faith & Hittner, 2010), such as those measured by the Behavioral Pediatrics Feeding Assessment Scale (BPFAS). As the current study compared a behavioral intervention with a physician visit intervention, we were interested in comparing outcomes on a measure of mealtime behavior problems, such as the BPFAS. The measure is composed of 35 items: 25 describe the child’s feeding behavior and 10 describe parent’s feelings about or strategies for dealing with eating problems. Parents are also asked to rate on a scale from 1 to 5 how much they agree or disagree with each statement, as well as whether each of the 35 items are a problem. Thus, the measure results in a child frequency score, child problem score, parent frequency score, and parent problem score. Higher scores are suggestive of more problematic feeding behaviors. Previous research has shown the BPFAS to be a valid and reliable representation of a child’s and parent’s mealtime behavior (Crist, Dobbelsteyn, Brousseau, & Napier-Phillips, 2004).
Analysis Plan
Analyses were planned a priori. Analyses included all participants who completed pre- and posttime point measures, with no exclusions based on attendance. Analysis of covariance (ANCOVA), with corresponding baseline values entered as covariates, was used to assess the primary outcome of child BMIz, as well as all secondary variables (Huck & McLean, 1975). T-tests for each group from pre to post were also conducted only for the primary outcome variable of BMIz. Analyses were conducted using PASW 18.0 (IBM Inc., Chicago, IL, USA). Because this was a stratified design, a matched pair analysis plan was considered post hoc. However, this would have resulted in ∼25% of the participants being excluded because they did not have a true match (often in small rural schools, it was not possible to identify an appropriate match). Values are reported as M (SD). Effect sizes were calculated using Cohen’s d, which was calculated as the mean difference between the two scores divided by the standard deviation. Effect sizes were only calculated for BMIz, as this was our primary outcome.
Power Analysis
A priori power analyses were used to determine the number of subjects necessary to detect a change in the primary outcome from pre to post based on a published review of 27 studies (Epstein, Myers, Raynor, & Saelens, 1998). Effect sizes of 80% power and two-tailed .05 significance value were used. Analyses indicated at least 20 subjects per group were necessary to have a reasonable chance of detecting existing significance.
Results
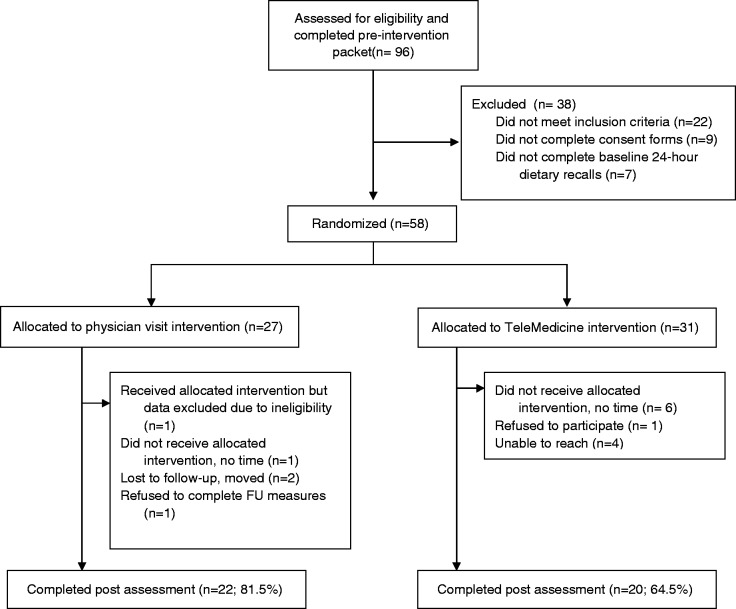
Baseline demographic data are given in Table II, and participant flowchart through the study is presented in Figure 1. A total of 96 elementary school children expressed interest in the study via the flyers sent home from school and completed the baseline packet. Of these, 58 families were randomized. Of these, 31 were randomly assigned to the TM group and 27 were randomly assigned to the PV group; there were no significant between-group differences at baseline. Children ranged in age from 5 to 11 years (M = 8.55, SD = 1.74). Fifty-two children were Caucasian (89.66%), three were Native American (5.17%), and three (5.17%) did not indicate their race/ethnicity; this ethnic breakdown is representative of the geographic area from which the children were drawn. Forty-one participants (70.69%) were male subjects. Approximately one-third (31.03%) of the children were eligible for free or reduced lunch, and the mean parent BMI was in the obese range (M = 30.83, SD = 9.30).
Table II.
Demographics of Randomized Sample
| Variable | Full sample (n = 58) | Telemedicine (n = 31) | Physician visit (n = 27) |
|---|---|---|---|
| Age in years M (SD) | 8.55 (1.74) | 8.48 (1.73) | 8.69 (1.78) |
| % Male (N) | 70.69 (41) | 70.97 (22) | 70.37 (19) |
| % Caucasian (N) | 89.66 (52) | 96.77 (30) | 81.48 (22) |
| Child BMI percentile M (SD) | 94.18 (4.27) | 94.69 (4.13) | 93.78 (4.35) |
| Maternal BMI M (SD) | 30.83 (9.30) | 30.47 (8.93) | 31.25 (9.88) |
| Annual household income M (SD) | 52,762.83 (29,030.70) | 56,603.10 (25,989.81) | 48,922.55 (31,990.65) |
| Free/reduced lunch (N) | 18 | 9 | 9 |
Figure 1.
CONSORT flow of participants through study.
Child BMI Change
ANCOVA results were not statistically significant by group (F = 0.023, p = .881). The R2 of .833 suggests that 83% of the variability in post-BMIz was explained by our model, with a significant amount of this variability coming from baseline BMIz (F = 186.2, p = .000) (Table III). Mean child BMIz score decreased from 1.88 (SD = 0.52) to 1.76 (SD = 0.52) in the TM group (t = 3.018, p = .007, d = .231) and from 1.70 (SD = 0.45) to 1.55 (SD = 0.59) in the PV group (t = 2.684, p = .014, d = .288) from pre- to posttreatment. This translates into a mean child BMI percentile drop from 94.69 (SD = 4.13) to 93.97 (SD = 5.48) in the TM group and from 93.78 (SD = 4.35) to 90.48 (SD = 8.79) in PV group.
Table III.
Changes From Pretreatment to Posttreatment in Child BMIz
| Child BMIz |
t (p-value) | |||
|---|---|---|---|---|
| Pretreatment M (SD) | Posttreatment M (SD) | Δ M | ||
| Telemedicine group | 1.88 (0.52) | 1.76 (0.52) | −0.12 | 3.018 (.007) |
| Physician visit group | 1.70 (0.45) | 1.55 (0.59) | −0.15 | 2.684 (.014) |
The 24-hr Diet Recall
ANCOVA results for mean child kilocalories were not statistically significant by group (F = 0.376, p = .544). Mean child kilocalories decreased from 1999.29 (SD = 531.29) to 1910.31 (SD = 499.79) in the TM group and from 2090.60 (SD = 630.88) to 1988.89 (SD = 462.52) in the PV group (Table IV). Percent calories from fat for the TM group dropped from 34.63 (SD = 5.98) to 33.59 (SD = 5.51) and from 36.14 (SD = 4.47) to 35.26 (SD = 5.79) in the PV group. Servings of fruits and vegetables increased for the TM group from 3.75 (SD = 1.60) to 4.43 (SD = 2.18) and decreased from 3.44 (SD = 2.14) to 3.31 (SD = 1.78) in the PV group. Servings of sugar-sweetened beverages decreased in the TM group from .99 (SD = 1.01) to .78 (SD = .97) and maintained at a level of .92 (SD = 1.07) to .92 (SD = .98) in the PV group. Finally, servings of “red” foods (Epstein & Squires, 1988) were assessed. The TM group decreased their servings of “red” foods from 7.25 (SD = 3.09) to 6.01 (SD = 2.98) and the PV from 7.76 (SD = 2.75) to 6.27 (SD = 2.68).
Table IV.
Health Behavior Changes From Pretreatment to Posttreatment Intervention by Group
| Telemedicine group |
Physician visit group |
|||
|---|---|---|---|---|
| Pretreatment M (SD) | Posttreatment M (SD) | Pretreatment M (SD) | Posttreatment M (SD) | |
| Dietary behaviors/day | ||||
| Kilocalories | 1,999.29 (531.29) | 1,910.31 (499.79) | 2,090.60 (630.88) | 1,988.89 (462.52) |
| % Kilocalories from fat | 34.63 (5.98) | 33.59 (5.51) | 36.14 (4.47) | 35.26 (5.79) |
| Fruit and vegetable servings | 3.75 (1.60) | 4.43 (2.18) | 3.44 (2.14) | 3.31 (1.78) |
| Sugar-sweetened beverage servings | 0.99 (1.01) | 0.78 (0.97) | 0.92 (1.07) | 0.92 (0.98) |
| Red fooda servings | 7.25 (3.09) | 6.01 (2.98) | 7.76 (2.75) | 6.27 (2.68) |
| Physical activity behaviors | ||||
| Minutes of M/V activity | 76.90 (36.27) | 104.31 (134.88) | 102.87 (53.62) | 76.69 (43.10) |
Note. aRed foods were defined as foods that have >7 g of fat and/or 12 g of sugar according to the well accepted Stop-Light Diet (Epstein & Squires, 1988).
M/V = moderate to vigorous.
Activity Monitors
ANCOVA results for number of minutes of moderate to vigorous physical activity were not statistically significant by group (F = 1.001, p = 0.327). Mean number of minutes of moderate to vigorous physical activity per day increased in the TM group from 76.90 (SD = 36.27) to 104.31 (SD = 134.88; Table IV). Mean minutes of moderate to vigorous physical activity per day decreased in the PV group from 102.87 (SD = 53.62) to 76.69 (SD = 43.10).
Child Behavior Checklist
The ANCOVA on total score on the CBCL by group was not significant (F = 0.698, p = .409). Mean child total score decreased from 54.84 (SD = 10.99) to 51.20 (SD = 11.12) in the TM group. Internalizing scores decreased from 53.37 (SD = 9.24) to 49.75 (SD = 11.26) and externalizing scores decreased from 53.68 (SD = 10.84) to 51.05 (SD = 9.48). In the PV group, mean child total problem score decreased from 53.58 (SD = 8.14) to 50.73 (SD = 6.28), internalizing scores decreased from 54.58 (SD = 9.34) to 50.95 (SD = 7.79), and externalizing scores decreased from 52.92 (SD = 7.02) to 51.00 (SD = 5.95).
Behavioral Pediatrics Feeding Assessment Scale
ANCOVAs for child problem score (F = 0.271, p = .606) and parent problem score (F = 0.545, p = .465) by group were not significant. Mean child frequency score decreased from 1.71 (SD = 0.31) to 1.67 (SD = 0.27) in the TM group and from 1.68 (SD = 0.33) to 1.64 (SD = 0.31) in the PV group. The total child problem score decreased from 2.25 (SD = 2.89) to 1.65 (SD = 1.02) in the TM group and from 3.08 (SD = 2.78) to 2.05 (SD = 1.79) in the PV group. Mean parent frequency score decreased from 1.56 (SD = .34) to 1.44 (SD = .33) in the TM group and remained the same at 1.46 (SD = .41) at pre and 1.46 (SD = .40) at post for the PV group. Finally, the parent problem score decreased in the TM group from 1.2 (SD = 1.5) to 0.95 (SD = 1.05) and from .70 (SD = 1.15) to .45 (SD = .51) in the PV group.
Intervention Feasibility and Fidelity
“Unanticipated problems” logs (our measure of feasibility) kept by group leaders indicated no significant problems in the groups delivered via TM. In general, group leaders reported that the groups were similar to other face-to-face clinical experiences. Fidelity coding was conducted on 20% of the TM sessions (Wickersham et al., 2011). A checklist of 8–10 topics was developed for each session, and independent trained graduate-level coders scored the videotapes for the presence/absence of the topics. Videotapes were selected for coding at random, but they were balanced across group leaders and session numbers. Analyses indicate that 82% of the topics from the manual were covered by group leaders. As for group session attendance, families attended an average of 12.7 sessions (SD = 1.78) of the 14 possible (90.7%). For the PV group, 100% of primary care physicians returned the study form, indicating that they had covered the complete list of visit topics 100% of the time.
Discussion
The objective of the current study was to examine the effectiveness of a multidisciplinary weekly family-based behavioral group delivered via telemedicine to rural areas, compared with a standardized physician visit intervention. Results indicate no statistically significant differences between the two groups on any of the outcome measures. This suggests that the telemedicine group was equally as effective as the physician visit group in changing health behaviors, which was a surprising finding to our team. The physician visit intervention was designed to serve as a “control” group; however, we gave the physicians a structured list of topics to discuss with each patient, and this seemed to be as effective as our 8-month behavioral intervention. Previous research has indicated that physicians are not effective at treating pediatric obesity, typically because they do not feel competent or comfortable in addressing it during visits (Jelalian, Boergers, Alday, & Frank, 2003), they do not identify it as a concern during patient visits (O’Brien, Holubkov, & Reis, 2004; Louthan et al., 2005), and when they do, they are unlikely to deliver specific obesity-related counseling (Vila et al., 2004). Published data from the patient perspective corroborate this finding, with only 36.7% of parents of children who are overweight or obese reporting they have been informed of their child’s weight issue by their child’s physician (Morbidity and Mortality Weekly Report, 2005). Therefore, prescribing a physician visit focused on pediatric obesity and providing the physicians a specific list of topics to cover may address some of the barriers noted earlier in the text. Overall, this type of physician visit seems to be as effective an intervention as a series of family-based behavior groups, at least with regard to rural elementary school children. Future research should examine this type of intervention versus usual care to further determine whether this type of “minimal intervention” would be effective in altering physician attitudes and practices and enhancing physician’s willingness and ability to address concerns regarding pediatric obesity during the child’s normal check-ups or other primary care visits.
Comparing our health behavior findings for the entire sample with the published literature or to national recommendations, children were consuming>30% of their calories from fat both before and after the interventions, with recommendations indicating children should consume <30% of their calories from fat. Children were also far below the recommendation of five fruits and vegetables a day both before and after the interventions. Also, a number of red food servings were extremely high, at over six per day. The traditional Stop Light Diet recommends no more than four red foods per week. Regarding physical activity, however, our data indicate children were active on average, meeting the national recommendation of 60 min of moderate to vigorous physical activity at all time points.
Results from the CBCL indicate that children were psychologically healthy at baseline in both groups, and this remained the case at follow-up, with no differences by group. CBCL scores were slightly lower than those obtained from a previous study, which included children who were more obese than our sample (Vila et al., 2004). A recent review (Vander Wal & Mitchell, 2011) indicates that children who are obese are significantly more likely to have problems with body dissatisfaction, symptoms of depression, impaired social relationships, obesity-related stigma, and decreased health-related quality of life. It would be wise to include measures of these specific concepts in future treatment outcome studies to determine whether a family-based behavioral group intervention delivered via telemedicine is any more or less effective at treating these issues than groups delivered face-to-face, or treatment from a primary care physician.
Results from the BPFAS, which is designed to assess mealtime behaviors, indicate that the children and parents in both groups did demonstrate some mealtime behavior difficulties at baseline, and that these problems decreased slightly in both intervention groups. Unfortunately, the BPFAS has not previously been used with children who are overweight or obese; therefore, it is difficult to compare our findings with those of existing research. However, previous research has found that caregiver report of “negative reaction to food presentation” was related to increased weight gain for girls in a large cohort study (Faith & Hittner, 2010), suggesting that at least some mealtime behavior problems may be related to pediatric obesity, and more specifically, to pediatric obesity treatment outcome. A great deal of future research is needed in this area. First, researchers need to modify the BPFAS or develop a new mealtime behavior scale that is pediatric obesity specific. Previous research (Zeller et al., 2007) has used a similar measure of mealtime behavioral difficulties (the About Your Child’s Eating—Revised; AYCE-R) with a sample of obese children, but this measure was also developed for a nonobese population (children with cancer). The development and validation of a measure of mealtime behavioral problems specific to pediatric obesity would be helpful. Second, there is a dearth of information about mealtime behavior problems in children who are obese. Clinically, it is easy to imagine that such a relationship exists for some children, despite the fact that we did not find one on the overall scale scores with the BPFAS. Future studies should examine mealtime behavior problems, as they differ between obese and nonobese children.
In terms of other outcome measures, attrition was not significantly different by group, but there was a trend for slightly higher attrition in the TM group compared with the PV group. Given the increased treatment demands associated with the TM intervention, this is not surprising. However, as the PV group was almost as effective as the TM group, consideration should be given to its low drop-out rate. Regarding intervention feasibility and fidelity, results indicate that telemedicine interventionists were adherent to the treatment manual, and that they found the telemedicine intervention similar to previous face-to-face clinical experiences. No significant technological problems were noted in the telemedicine group. This is significant, as all of the previous telemedicine intervention studies that have been published focus on the delivery of an individual clinic visit, not a group of 8–10 families as was conducted in this study. Before the start of the study, concerns were noted about the ability to facilitate a group this large over telemedicine. However, our results suggest that doing so is not difficult or problematic.
Equally surprisingly, our project found 100% adherence in the physician visit group, with every practitioner completing and returning the form as instructed. Of note, we also received several unsolicited positive written comments on the returned form, indicating that the physicians found the intervention materials extremely helpful and planned to use them with many of their patients. As noted earlier in the text, previous research suggests that physicians are not adequately delivering pediatric obesity services in their offices, specifically in terms of recognizing the problem and delivering specific counseling (O’Brien et al., 2004; Louthan et al., 2005). However, our results suggest that physicians are highly willing to be involved when a family desires obesity treatment, as well as to discuss specific health behaviors when conveniently provided via a one page handout. Future research will need to examine the effectiveness of this type of physician visit intervention on a larger scale, and possibly study its effects when delivered as an adjunct to other forms of pediatric obesity intervention services, such as family-based behavioral groups.
The clinical implications of this study are many. First, for rural families facing the issue of pediatric obesity, telemedicine or other methods of interactive televideo seem to be feasible for the delivery of empirically supported interventions. Families from rural areas who commit to this type of intervention are likely to show up for treatment and to encounter few technical difficulties. In terms of effects on health behavior, rural families are likely to benefit from interventions delivered via these modalities and demonstrate changes in health behavior in the expected directions. Feasibility is heightened in that family, and provider travel times are decreased, and access to highly trained specialists is increased. However, the results also suggest that referral back to the child’s primary care physician with a list of topics to discuss related to weight management may be equally effective. This finding is particularly noteworthy given recent research investigations examining factors that impact physician practices related to pediatric obesity in primary care settings and mechanisms to enhance weight management treatment in pediatric primary care. For example, given that time has been identified as a barrier for addressing pediatric obesity during primary care visits (Jelalian et al, 2003), the structure provided by the list of topics may facilitate discussion regarding weight management in a quick and efficient manner. The list of topics may also help increase physician-perceived competence and comfort in discussing weight-related issues with patients and their families, also known to be significant barriers to weight management in primary care.
Limitations
This study does have several limitations. First, our sample was relatively small, and it was from several rural areas in only one state; therefore, our findings may not generalize to rural areas in other states. Related to this, our sample was predominantly Caucasian, but this was reflective of the population from which the sample was drawn and, therefore, expected. Second, some of our measures were not obesity specific, such as the CBCL and the BPFAS. Future researchers would be wise to expand the availability of obesity-specific measures, as well as to include such measures as much as possible. Third, neither intervention was dramatically effective, and fourth, we did not conduct intent-to-treat analyses for this small study.
Conclusions
In summary, the current study was the first to examine the effectiveness of family-based behavioral groups delivered to rural children via telemedicine and to compare such an intervention to a standardized visit with a primary care physician. Results indicate no differences between groups but many positive health behavior changes. Future research will need to examine these findings with larger and more diverse samples and to determine specific methods to increase treatment effects.
Acknowledgments
The authors thank all of the rural families who participated, and the physicians and other rural professionals who participated as well. A.M.D. wishes to acknowledge the vast contributions of her mentors, Dr Michael A. Rapoff, Dr Joseph Donnelly, and Dr Edward Ellerbeck. The authors also acknowledge the contributions of our deceased colleague Cinnamon Smith, who was a joy to work with. Baseline information was published previously (Gallagher, Davis, Malone, Landrum & Black, 2011).
Funding
National Institutes of Health (DK068221 to A.M.D.).
Conflicts of interest: None declared.
References
- Achenbach T M. Manual for the child behavior checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry; 1991. [Google Scholar]
- Barlow S E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics. 2007;120(Suppl 4):S164–S192. doi: 10.1542/peds.2007-2329C. Retrieved from http://pediatrics.aappublications.org/content/120/Supplement_4/S164.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Health Statistics. 2000 CDC growth charts: United States. Retrieved from http://www.cdc.gov/growthcharts. [Google Scholar]
- Crawford P B, Obarzanek E, Morrison J, Sabry Z I. Comparative advantage of 3-day food records over 24-hour recall and 5-day food frequency validated by observation of 9- and 10-year-old girls. Journal of the American Dietetic Asociation. 1994;94:626–630. doi: 10.1016/0002-8223(94)90158-9. doi:10.1016/0002-8223(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Crist W, Dobbelsteyn C, Brousseau A M, Napier-Phillips A. Pediatric assessment scale for severe feeding problems: Validity and reliability of a new scale for tube-fed children. Nutrition in Clinical Practice. 2004;19:403–408. doi: 10.1177/0115426504019004403. doi:10.1177/0115426504019004403. [DOI] [PubMed] [Google Scholar]
- Cutting T M, Fisher J O, Grimm-Thomas K, Birch L L. Like mother, like daughter: Familial patterns of overweight are mediated by mothers' dietary disinhibition. American Journal of Clinical Nutrition. 1999;69:608–613. doi: 10.1093/ajcn/69.4.608. Retrieved from http://ajcn.nutrition.org/content/69/4/608.full.pdf+html. [DOI] [PubMed] [Google Scholar]
- Davis A M, Bennett K, Befort C, Nollen N. Obesity and related health behaviors among urban and rural children in the United States: Data from the National Health and Nutrition Examination Survey 2003–2004 and 2005–2006. Journal of Pediatric Psychology. 2011;36:669–676. doi: 10.1093/jpepsy/jsq117. doi:10.1093/jpepsy/jsq117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A M, Boles R E, James R L, Sullivan D K, Donnelly J E, Swirczynski D L, Goetz J. Health behaviors and weight status among urban and rural children. Rural and Remote Health. 2008;8 810. Retrieved from http://www.rrh.org.au/articles/subviewnew.asp?ArticleID=810. [PMC free article] [PubMed] [Google Scholar]
- Davis A M, James R L, Boles R E, Goetz J R, Belmont J, Malone B. The use of telemedicine in the treatment of paediatric obesity: Feasibility and acceptability. Maternal & Child Nutrition. 2011;7:71–79. doi: 10.1111/j.1740-8709.2010.00248.x. doi:10.1111/j.1740-8709.2010.00248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis A M, James R L, Curtis M, Felts S, Daley C M. Pediatric obesity attitudes, services, and information among rural parents: A qualitative study. Obesity. 2008;16:2133–2140. doi: 10.1038/oby.2008.312. doi:10.1038/oby.2008.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz W H. Health consequences of obesity in youth: Childhood predictors of adult disease. Pediatrics. 1998;101(3 Pt 2):518–525. Retrieved from http://pediatrics.aappublications.org/content/101/Supplement_2/518.full.pdf+html. [PubMed] [Google Scholar]
- Epstein L, Squires S. The stoplight diet for children: An eight-week program for parents and children. Canada: Little Brown & Co; 1988. [Google Scholar]
- Epstein L H, Myers M D, Raynor H A, Saelens B E. Treatment of pediatric obesity. Pediatrics. 1998;101(3 Pt 2):554–570. Retrieved from http://pediatrics.aappublications.org/content/101/Supplement_2/554.full.pdf+html. [PubMed] [Google Scholar]
- Faith M S, Hittner J B. Infant temperament and eating style predict change in standardized weight status and obesity risk at 6 years of age. International Journal of Obesity (London) 2010;34:1515–1523. doi: 10.1038/ijo.2010.156. doi:10.1038/ijo.2010.156. [DOI] [PubMed] [Google Scholar]
- Favaro A, Santonastaso P. Effects of parents' psychological characteristics and eating behaviour on childhood obesity and dietary compliance. Journal of Psychosomatic Research. 1995;39:145–151. doi: 10.1016/0022-3999(94)00097-o. Retrieved from http://www.sciencedirect.com/science/article/pii/002239999400097O. [DOI] [PubMed] [Google Scholar]
- Gallagher K S, Davis A M, Malone B, Landrum Y, Black W. Treating rural pediatric obesity through telemedicine: Baseline data from a randomized controlled trial. Journal of Pediatric Psychology. 2011;36:687–695. doi: 10.1093/jpepsy/jsr011. doi:10.1093/jpepsy/jsr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon-Larsen P, Adair L S, Popkin B M. The relationship of ethnicity, socioeconomic factors, and overweight in US adolescents. Obesity Research. 2003;11:121–129. doi: 10.1038/oby.2003.20. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12529494. [DOI] [PubMed] [Google Scholar]
- Huck S W, McLean R A. Using a repeated measures ANOVA to analyze the data from a pretest-posttest design: A potentially confusing task. Psychological Bulletin. 1975;82:511–518. doi:10.1037/h0076767. [Google Scholar]
- Irby M B, Boles K A, Jordan C, Skelton J A. Telefit: Adapting a multidisciplinary, tertiary-care pediatric obesity clinic to rural populations. Telemedicine and e-Health. 2012;18:247–249. doi: 10.1089/tmj.2011.0117. doi:10.1089/tmj.2011.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janicke D M, Sallinen B J, Perri M G, Lutes L D, Huerta M, Silverstein J H, Brumback B. Comparison of parent-only vs family-based interventions for overweight children in underserved rural settings: Outcomes from project STORY. Archives of Pediatric & Adolescent Medicine. 2008;162:1119–1125. doi: 10.1001/archpedi.162.12.1119. doi:10.1001/archpedi.162.12.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelalian E, Boergers J, Alday C S, Frank R. Survey of physician attitudes and practices relataed to pediatric obesity. Clinical Pediatrics. 2003;42:235–246. doi: 10.1177/000992280304200307. doi:10.1177/000992280304200307. [DOI] [PubMed] [Google Scholar]
- Louthan M V, Lafferty-Oza M J, Smith E R, Hornung C A, Franco S, Theriot J A. Diagnosis and treatment frequency for overweight children and adolescents at well child visits. Clinical Pediatrics. 2005;44:57–61. doi: 10.1177/000992280504400107. doi:10.1177/000992280504400107. [DOI] [PubMed] [Google Scholar]
- Masse L C, Fuemmeler B F, Anderson C B, Matthews C E, Trost S G, Catellier D J, Treuth M. Accelerometer data reduction: A comparison of four reduction algorithms on select outcome variables. Medicine and Science in Sports and Exercise. 2005;37(Suppl 11):S544–S554. doi: 10.1249/01.mss.0000185674.09066.8a. doi:10.1249/01.mss.0000185674.09066.8a. [DOI] [PubMed] [Google Scholar]
- Morbidity and Mortality Weekly Report, Centers for Disease Control and Prevention (CDC) Children and teens told by doctors that they were overweight—United States, 1999—2002. Morbidity & Mortality Weekly Report. 2005, September 2;54:848–849. Retrieved from http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5434a3.htm. [PubMed] [Google Scholar]
- Must A, Strauss R S. Risks and consequences of childhood and adolescent obesity. International Journal of Obesity and Related Metabolic Disorders. 1999;23(Suppl 2):S2–S11. doi: 10.1038/sj.ijo.0800852. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10340798. [DOI] [PubMed] [Google Scholar]
- O'Brien S H, Holubkov R, Reis E C. Identification, evaluation, and management of obesity in an academic primary care center. Pediatrics. 2004;114:e154–e159. doi: 10.1542/peds.114.2.e154. Retrieved from http://pediatrics.aappublications.org/content/114/2/e154.full.html. [DOI] [PubMed] [Google Scholar]
- Phillips B A, Gaudette S, McCracken A, Razzay S, Sutton K, Speed L, Thompson J, Ward W. Psychosocial functioning in children and adolescents with extreme obesity. Journal of Clinical Psychology in Medical Settings. 2012;199:277–284. doi: 10.1007/s10880-011-9293-9. doi:10.1007/s10880-011-9293-9. [DOI] [PubMed] [Google Scholar]
- Shaikh U, Nettiksimmons J, Romano P. Pediatric obesity management in rural clinics in California and the role of telehealth in distance education. Journal of Rural Health. 2011;27:263–269. doi: 10.1111/j.1748-0361.2010.00335.x. doi:10.1111/j.1748-0361.2010.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirard J R, Melanson E L, Li L, Freedson P S. Field evaluation of the Computer Science and Applications, Inc. physical activity monitor. Medicine and Science in Sports and Exercise. 2000;32:695–700. doi: 10.1097/00005768-200003000-00022. doi:0195-9131/00/3203-0695/0. [DOI] [PubMed] [Google Scholar]
- Steele R G, Wu Y P, Jensen C D, Pankey S, Davis A M, Aylward B S. School nurses' perceived barriers to discussing weight with children and their families: A qualitative approach. Journal of School Health. 2011;81:128–137. doi: 10.1111/j.1746-1561.2010.00571.x. doi:10.1111/j.1746-1561.2010.00571.x. [DOI] [PubMed] [Google Scholar]
- Strauss R S. Childhood obesity. Pediatric Clinics of North America. 2002;49:175–201. doi: 10.1016/s0031-3955(03)00114-7. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11826804. [DOI] [PubMed] [Google Scholar]
- Strauss R S, Pollack H A. Epidemic increase in childhood overweight, 1986–1998. Journal of the American Medical Association. 2001;286:2845–2848. doi: 10.1001/jama.286.22.2845. doi:10.1001/jama.286.22.2845. [DOI] [PubMed] [Google Scholar]
- Tai-Seale T, Chandler C. Rural Healthy People 2010: A companion document to Healthy People 2010 (Vol. 2) College Station, TX: The Texas AM University System Health Science Center, School of Rural Public Health, Southwest Rural Health Research Center; 2003. Nutrition and overweight concerns in rural areas: A literature review. Retrieved from http://www.srph.tamhsc.edu/centers/rhp2010/Vol2nutrition.htm. [Google Scholar]
- Troiano R P, Berrigan D, Dodd K W, Masse L C, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Medicine and Science in Sports and Exercise. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. doi:10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- Vander Wal J S, Mitchell E R. Psychological complications of pediatric obesity. Pediatric Clinics of North America. 2011;58:1393–1401. doi: 10.1016/j.pcl.2011.09.008. doi:10.1016/j.pcl.2011.09.008. [DOI] [PubMed] [Google Scholar]
- Vila G, Zipper E, Dabbas M, Bertrand C, Robert J J, Ricour C, Mouren-Siméoni M C. Mental disorders in obese children and adolescents. Psychosomatic Medicine. 2004;66:387–394. doi: 10.1097/01.psy.0000126201.12813.eb. doi:10.1097/01.psy.0000126201.12813.eb. [DOI] [PubMed] [Google Scholar]
- Wickersham K, Colbert A, Caruthers D, Tamres L, Martino A, Erlen J A. Assessing fidelity to an intervention in a randomized controlled trial to improve medication adherence. Nursing Research. 2011;60:264–269. doi: 10.1097/NNR.0b013e318221b6e6. doi:10.1097/NNR.0b013e318221b6e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller M H, Reiter-Purtil J, Modi A C, Gutzwiller J, Vanatta K, Davies W H. Controlled study of critical parent and family factors in the obesigenic environment. Obesity (Silver Spring) 2007;15:126–136. doi: 10.1038/oby.2007.517. doi:10.1038/oby.2007.517. [DOI] [PubMed] [Google Scholar]