Abstract
Objective To examine the efficacy of an adjunct motivational and autonomy-enhancing intervention (self-directed) for behavioral family-based pediatric obesity relative to the standard prescription of uniform behavioral skills use and interventionist goal assignment (prescribed). Methods In this randomized clinical trial, 72 overweight/obese children and their parents/caregivers were assigned to either self-directed or prescribed intervention for 20 weeks, with approaches diverging after week 5. Anthropometric measurements from child and participating parent at baseline, posttreatment, and 3-month, 6-month, 1-year, and 2-year follow-ups were evaluated for change (n = 59 in follow-up analyses). Results The approaches demonstrated similar child body mass index (BMI) z-score and parent BMI change from baseline to posttreatment and throughout follow-up, with child and parent weight status lower than baseline at 2 years after treatment cessation. Conclusions An adjunct motivational and autonomy-enhancing approach to behavioral family-based pediatric obesity treatment is a viable alternative to the standard intervention approach.
Keywords: health promotion and prevention, obesity, weight management
Introduction
Family-based behavioral interventions are effective for pediatric obesity (Epstein, Paluch, Roemmich, & Beecher, 2007; Whitlock, O'Connor, Williams, Beil, & Lutz, 2010). The U.S. Preventive Services Task Force now recommends moderate–high-intensity behavioral treatment for obese children ≥6 years old (Barton, 2010). Effective interventions target nutrition and physical activity changes, emphasize lifelong behaviors, and target caregivers as agents of change (Spear et al., 2007). Often interventions train and encourage families to use specific behavioral skills (e.g., food and physical activity monitoring, contingency management) that purportedly begin and sustain behavior change. Interventionists hold families accountable for consistent and comprehensive skills use, as better skills use is related to better outcomes (Mockus et al., 2011; Saelens & McGrath, 2003). However, the intervention approach that will maximize families’ implementation of behavior skills is unknown.
Given widespread calls for patient-centered care (Institute of Medicine, 2001) and increasing use of collaborative approaches that support patient autonomy, behavioral obesity treatments stand out as more prescriptive and interventionist-directed than other clinical interventions. The growing health care trend toward patient-centered motivation-enhancing approaches to behavior change is exemplified by the National Institutes of Health obesity guidelines and reviews of best practices in illness self-management (Bodenheimer, Lorig, Holman, & Grumbach, 2002; National Institutes of Health, & National Heart Lung and Blood Institute, 1998; Tobacco Use and Dependence Guideline Panel, 2008). A more patient-centered model for weight management has emerged, rooted in motivational interviewing (MI) on the basis of its presumed greater acceptability by patients (Martins & McNeil, 2009). MI emphasizes patient autonomy, readiness to change, examination/resolution of ambivalence around behavior change and motivation enhancement (Miller & Rollnick, 2002). In contrast to the standard approach of prescribing behavioral skills use and behavioral targets, MI-based interventions are tailored to the specific patient or family’s situation and guided more by their input. MI and related counseling styles successfully promote change in various adult behaviors (e.g., alcohol, tobacco, medication adherence), and MI has been proposed as an obesity treatment approach (Martins & McNeil, 2009). MI is effective when used as an adjunct to standard behavioral treatment for adult obesity, improving outcomes by enhancing adherence (Armstrong et al., 2011; Spahn et al., 2010).
The American Academy of Pediatrics (American Academy of Pediatrics, 2012) and other proponents (Erickson, Gerstle, & Feldstein, 2005; Resnicow, Davis, & Rollnick, 2006) have suggested MI should be a mainstay of behavior change in the care of overweight and obese children, despite limited supporting evidence. The central role of specialized skills in evidence-based behavioral treatment raise some doubts as to whether a solely motivational approach would be sufficient to propel an individual or family toward lifestyle behavior change. In contrast to MI, effectiveness may require the interventionist to teach families key behavior change skills and to sustain high expectations for comprehensive and consistent skills use. In fact, pediatric studies to date that have evaluated MI for obesity have deployed it in the absence of a providing a foundation of behavioral skills training for parents and children, which may account for the lack of effectiveness (Macdonell, Brogan, Naar-King, Ellis, & Marshall, 2012; Schwartz et al., 2007; Taveras et al., 2010; Taylor et al., 2010).
To our knowledge, MI has not been evaluated, however, as an adjunct to the standard prescribed approach, with a transition during treatment from skills prescription to a more MI-based approach. Barriers to MI use in conjunction with this treatment may pose greater challenges in pediatric settings because of the dyadic (interventionist–one client) context. Family-based pediatric obesity treatment involves both parent and child, raising questions as to whose ambivalence is being explored, whose readiness-to-change is being enhanced, and whose autonomy is being supported. It remains unknown if incorporating MI and related components following initial skills training into pediatric obesity interventions sustains efficacy by better preparing parents to continue to facilitate child behavior change following treatment cessation. The present study examines the effect on child and parent weight status of a newly developed intervention model that initiates treatment with a standard approach of prescribing behavioral skills training and targets (“skills boot camp”), but then shifts to more patient-centered strategies, using MI techniques and emphasizing parental autonomy (i.e., a more self-directed approach) compared with the standard prescribed approach throughout treatment.
Methods
Study Design and Randomization
This randomized clinical trial included baseline (before treatment starting), posttreatment, 3-month, 6-month, 1-year, and 2-year follow-up assessment, with follow-up time referring to amount of time after posttreatment. Families were randomly assigned to receive either the prescribed or self-directed approach, with child gender and child level of overweight [< or >60% above median body mass index (BMI) for age and gender] as stratification variables. Randomization blocks were randomly selected to be either four or six participating families. Families were also randomly assigned to their interventionist. The randomization process was conducted for each of the three cohorts of families recruited and enrolled in this “Family Overweight: Comparing Use of Strategies” (FOCUS) study.
Participants
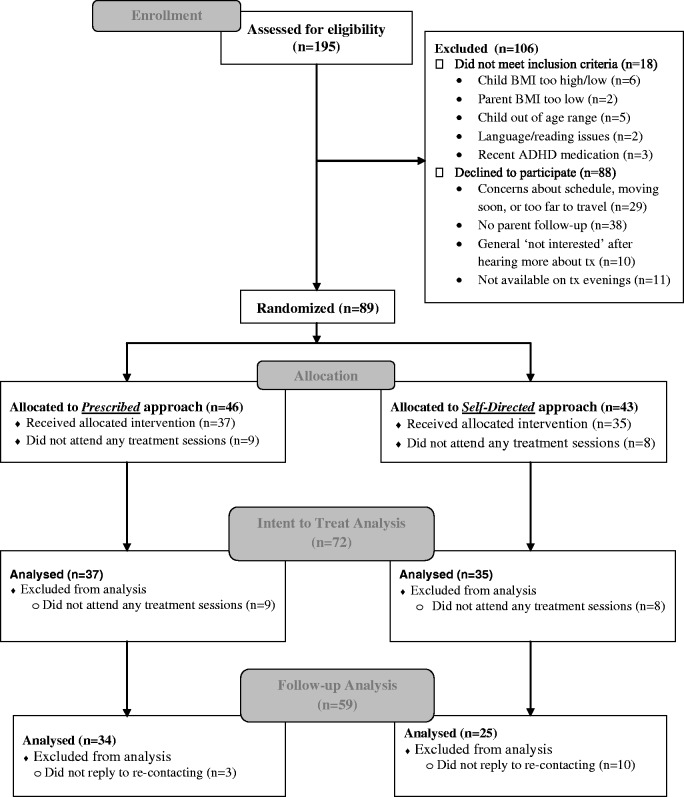
Families were recruited through pediatric offices, advertisements, and direct mailings. Only 7–11-year-old children at or above the 85th percentile for age- and gender-specific BMI (Kuczmarski et al., 2000), but no >175% above the median BMI for age and gender were eligible. Only children with at least one overweight parent (BMI ≥ 25.0) (National Institutes of Health & National Heart Lung and Blood Institute, 1998) were included to ensure targeting a higher risk group (Freedman, Khan, Dietz, Srinivasan, & Berenson, 2001; Whitaker, Wright, Pepe, Seidel, & Dietz, 1997). Children with conditions known to promote obesity were excluded (e.g., Prader-Willi), along with those in another weight control program or who had recently started taking weight-affecting medications (e.g., stimulants). Parents’ participation in other programs targeting their own weight change was not exclusionary (rare), if the behavioral changes recommended were consistent with FOCUS targets. Participating parents and children were required to (a) not have an existing thought disorder, suicidality, or substance abuse disorder, (b) not have disability or illness that would preclude them from engaging in at least moderate-intensity physical activity, (c) be English-speaking and at least at a second-grade reading level, (d) not have a current or prior diagnosed eating disturbance, and (e) live <50 miles from the treatment site. At least one parent/caregiver of the eligible child was required to consistently attend treatment sessions and engage in his/her own behavior change around eating and physical activity. Inclusion/exclusion criteria were evaluated based on parent report, with the exception of child and parent weight status, which were measured. Participant characteristics are provided in Table I, and Figure 1 indicates participant flow. Parents provided written consent and children provided assent. Families received incentives for completing posttreatment ($15) and follow-up assessments ($40–$50), but did not receive compensation for or pay for treatment. This study was approved by the institutional review board of the Seattle Children’s Research Institute.
Table I.
Sample Baseline Demographics
| Child, parent, our household characteristic | Total sample n = 72 M (SD) | Prescribed approach n = 37 M (SD) | Self-directed approach n=35 M (SD) |
|---|---|---|---|
| Child age | 9.8 (1.4) | 9.8 (1.4) | 9.7 (1.4) |
| Child weight (kg) | 54.6 (14.9) | 55.9 (15.7) | 53.1 (14.0) |
| Child height (cm) | 142.2 (11.4) | 142.6 (11.8) | 141.9 (11.0) |
| Child BMI | 26.5 (4.1) | 27.0 (4.2) | 25.9 (4.0) |
| Child BMI percentile for age-and-sex | 97.6 (2.0) | 97.9 (1.8) | 97.4 (2.2) |
| Child BMI z-score | 2.1 (0.3) | 2.0 (0.3) | 2.1 (0.3) |
| Parent BMI | 33.3 (7.7) | 33.6 (8.1) | 32.9 (7.4) |
| Percentage (%) | |||
| Male children | 33.3 | 32.4 | 34.3 |
| Children > 95th BMI percentile for age and sex | 88.9 | 91.9 | 85.7 |
| Child race | |||
| Caucasian | 84.7 | 83.8 | 85.7 |
| African American | 6.9 | 8.1 | 5.7 |
| Asian | 2.8 | 5.4 | 0 |
| Other or multiple races | 5.6 | 2.7 | 8.6 |
| Child ethnicity (Hispanic) | 12.5 | 8.1 | 17.1 |
| Parents married or living with partner | 75.0 | 73.0 | 77.1 |
| Mothers as participating parent | 87.5 | 81.1 | 94.3 |
| Parents with BMI > 30 | 63.9 | 62.1 | 65.7 |
| Annual Household income | |||
| $<30K | 15.3 | 13.5 | 17.1 |
| $30–69K | 23.6 | 27.0 | 20.0 |
| 70–99K | 29.2 | 29.7 | 28.6 |
| 100+K | 31.9 | 29.7 | 34.3 |
Note. There were no significant differences (all p > .05) for any prescribed versus self-directed comparisons.
Figure 1.
Participant flow.
Treatment Conditions
Common Features of Both Treatment Conditions
Both treatment conditions included 20 weekly sessions across 21 or 22 weeks (one intentional ‘skip’ week and one holiday skip week in two cohorts), consistent with U.S. Preventive Services Task Force recommendation for moderate- to high-intensity interventions (>25 contact hours) for childhood obesity treatment (Barton, 2010). For both treatment conditions, weekly treatment consisted of a 20–30-min individual family session where each parent–child dyad met with a family interventionist and 40–50-min separate child and parent group sessions immediately before or after individual family sessions. Interventionists were doctoral, masters’ level, or doctoral candidates with experience in providing behavioral interventions. Interventionists were provided 14–16 hr of training in intervention delivery for both approaches, including specific training for the self-directed approach focusing on MI patient-centered strategies. Interventionists received weekly supervision to ensure fidelity to the approach being delivered. All but one of the study interventionists provided both prescribed and self-directed interventions. During consenting, families were provided a brief description of each approach, but were otherwise blind to approach differences during treatment.
The eating plan was based on Epstein’s Stoplight Eating plan (Epstein & Squires, 1988), with a focus on calorie, dietary fat, and added sugar intake reduction, and increasing nutrient density. Total daily calorie ranges were based on the caloric intake needed for children to lose ∼½ pound per week, but no <1,000 kcal/day for children and no <1,200 kcal/day for parents. Foods are categorized into GREEN (Go! foods), YELLOW (Proceed with caution foods), and RED (Stop! eat sparingly foods). The eating plan helps children and parents reduce, but not eliminate, RED foods and increase GREEN foods. Increases in physical activity frequency, duration, and intensity were targeted (to 90 min/day for children and 60 min/day for parents), as were decreases in sedentary behavior (e.g., screen time), to <2 hr/day.
During the first five treatment weeks, families in both conditions were trained in the use of and encouraged to fully implement these behavioral skills:
Food monitoring, which included child (often with parents’ help) and parent recording of all foods/beverages consumed and amounts, calories, and categorization of food/beverages. Activity monitoring included duration and type of physical activity.
Contingency management, with behavioral (e.g., lower RED foods) and weight loss (i.e., ½ lb loss per week) goals for children and parents, parents encouraged to praise children’s behavior successes, and parents providing rewards for weekly behavioral and weight loss goal attainment.
Environmental control, with parents and children changing environments (e.g., in their home) to increase access to healthy foods and opportunities to be active and decrease unhealthy food access.
Similarities and Differences Between Treatment Conditions
Information provided about healthy eating and physical activity was the same between the treatment conditions throughout treatment. On entering treatment, it is hypothesized that most parents lack the knowledge or consistent use of the behavioral skills required to be successful in helping their child and themselves have success with weight management. So, in both treatment conditions, the first 5 weeks were devoted to bringing all parents and children to similar levels of knowledge and skills use for application to the healthy eating and physical activity plan, including food and physical activity monitoring, contingency management, and environmental control. After these first 5 weeks, the two conditions diverged, particularly regarding the accountability and autonomy for behavioral skills use and goal assignment. Families in the prescribed arm were encouraged to continue to adhere to treatment standards (i.e., consistent skills implementation). In contrast, families in the self-directed arm were given more autonomy in making choices about skills use (e.g., which skills to use, what goals to have).
Prescribed Treatment Condition
The prescribed approach purports that skills use leads to improved weight outcomes, which then leads to self-efficacy (child and parent), which then leads back to continued skills use, and so on. This approach takes the stance that after initial skills use, training needs to continue thereafter by the interventionist guiding, providing support for, highlighting the importance of, and helping families problem-solve to consistently and comprehensively use behavioral skills. The interventionist sets weekly treatment goals for parent and child, with little or no input from the family or tailoring of goals, and evaluates and holds accountable families for consistent adherence to the behavioral skills use. During weeks 17–20, the interventionist engages families in long-term planning for continued skills use.
Self-Directed Treatment Condition
The self-directed approach also involves interventionist focus on skills use training, feedback, and holding families accountable for consistent skills use during the first 5 weeks of treatment, the same as the prescribed approach. Thereafter, the interventionist shifts toward encouraging more autonomy and self-efficacy around skills use, by acknowledging parental (and child) ambivalence about behavior change and struggles with continued skills use (e.g., common for families to struggle with continuous daily monitoring of food and physical activity). The self-directed intervention assists the family in developing the ability to set tailored realistic and meaningful goals, guides and facilitates experimentation for families to determine for themselves which skills are feasible that will optimize their long-term behavior change, guided by the family’s readiness to change (see Table II). This includes the interventionist seeking families’ input regarding which behavioral goals and which behavioral skills (if any) they want to continue using after week 5, while providing feedback regarding any discrepancy between families’ stated goals and skills use (i.e., cannot have a daily calorie goal if not recording calories). The interventionist supports the parent’s autonomy and asks the parent (and child) weekly to consider what they are ready to undertake (e.g., changing the weekly weight loss goal, selecting which skills to use), their confidence in their ability to be successful in meeting goals, and their own behavioral expectations. Family autonomy from the interventionist, inherent to this approach and required for long-term implementation (i.e., after treatment ends), is further solidified by starting long-term planning in the self-directed approach in week 12, notably earlier than in the prescribed approach.
Table II.
Examples of Interventionist Responses to Common Situations and Rationale by Treatment Condition
| Situation in intervention visit | Prescribed approach | Self-directed approach |
|---|---|---|
| Goal-setting for the coming week | “This week, your goals will be ≤15 RED foods per week, 1000–1200 calories/day, 90 min of physical activity per day, and a ½ pound of weight loss.” | “What goals do you want to target this week … How confident are you that you and your child can meet this goal?” |
|
|
|
| Child did not meet any eating or physical activity goals this past week | To parent: “How will you help him meet his goals next week?” | To parent and child: “What got in the way? … What do you make of that? … What did you learn? … What might you do differently next week?” |
|
|
|
| Parent questions or rejects standard goal (e.g., “90 min of physical activity isn’t realistic.”) or use of a behavioral skill (e.g., “I don’t think I can keep up the monitoring.”). |
|
|
|
|
|
Treatment Condition Fidelity and Parent Self-Efficacy in Facilitating Child Lifestyle Change
A blinded coder rated 54 randomly selected audio recordings of randomly selected family sessions occurring between week 6 and week 19 on five interventionist prescribed approach and nine self-directed approach behaviors. Summed scores (item response ranges not at all = 0, somewhat = 1, definitely or a lot = 2) were derived separately for the different interventionist approach behaviors. A second coder rated 11 of these sessions, with high inter-rater reliability [intra-class correlation (ICC) = .89 for interventionist prescribed approach behaviors; ICC = .76 for interventionist self-directed approach behaviors].
At each assessment point, parents rated their self-efficacy or confidence to help their child make and maintain eating and physical activity lifestyle changes using two items (response ranges strongly disagree = 1 to strongly agree = 5; items were averaged). It was expected that parents-provided self-directed intervention would have more positive changes in self-efficacy over time than parents provided prescribed intervention.
Treatment Attendance
Twelve of the 72 families (16%) who attended any treatment session did not attend past session 5, half of which (n = 6) attended only the first treatment session. Of the 60 families attending past session 5 (when treatment conditions began diverging), the median number of sessions attended was 18 out of 20 total sessions in both the prescribed (n = 32) and self-directed (n = 28) approaches.
Anthropometric Measures
Children and participating parents were weighed three times in light clothing without shoes using a digital Scaletronix scale, with more measurements until agreement within 0.1 kg, and those values averaged. Height was measured with a Heightronic stadiometer at least in triplicate, until agreement within 0.5 cm, with those values averaged. In one instance at 2-year follow-up, child weight and height information was obtained only by parent-report of child measures at a recent pediatrician appointment. BMI was calculated as kg/m2. Children’s percent above median BMI and BMI z-scores were calculated using Centers for Disease Control and Prevention growth charts for age-specific median, standard deviation, and distribution skewness correction and the LMS method (Kuczmarski et al., 2000). Assessors were not interventionists and were blind to approach differences. Child change scores were also examined categorically, as a BMI z-score decrease of at least 0.25 improves blood pressure, lipids, and insulin sensitivity (Ford, Hunt, Cooper, & Shield, 2010), with greater improvements for decreases >0.5 (Reinehr, Kiess, Kapellen, & Andler, 2004; Sabin et al., 2007).
Sample Size and Data Analysis
Sample size estimates were based on examining whether the approaches resulted in different levels of behavioral skills use and whether this was related to child outcomes and on whether these approaches resulted in different child and parent outcomes. Using the observed average posttreatment effect size difference between active interventions and waitlist controls from a meta-analysis [Hedge’s g = .75 (Wilfley et al., 2007); with a standard deviation = 1.0], 29 participants per condition would have power >80% at p < .05.
There were 57 assessment completers at posttreatment, 58 at 3-month follow-up, 54 at 6-month follow-up, 52 at 1-year follow-up, and 46 at 2-year follow-up. There was no significant difference in posttreatment assessment completion by treatment condition [χ2(1) = 2.47, p = .12], although fewer self-directed than prescribed families completed any follow-up assessments [χ2(1) = 5.09, p = .024]. Separate intent-to-treat analyses were conducted for baseline to posttreatment (n = 72 for both children and parents), posttreatment through all follow-up assessments (n = 59 for children, n = 60 for parents), and baseline to 2-year follow-up only (n = 72 for both children and parents). Eleven families had no posttreatment or follow-up data, so baseline values were carried forward to posttreatment. Four families without posttreatment data had 3-month follow-up data, so their posttreatment data was interpolated. Only families with at least one follow-up assessment were included in the posttreatment through all follow-up analyses. Missing data at follow-ups was interpolated if measured values bounded the missing follow-up (e.g., missing 1-year follow-up was interpolated proportional with time for those with 6-month and 2-year follow-up values). Missing follow-up data without any subsequent follow-up data (e.g., data available at 3- and 6-month follow-up, but no 1- or 2-year follow-up) were replaced with the highest value ever measured.
T-tests and chi-squared tests examined treatment condition differences at baseline for continuous and categorical variables, respectively. Repeated measures analysis of variance were used for the child and parent weight status outcome and parental self-efficacy analyses, with corresponding baseline value used as a covariate in the all follow-up analyses. Partial eta squared values are reported as effect size estimates and significance level was set at p < .05 double-sided. All measures demonstrated acceptable skewness and kurtosis and thus no measures required transformation for analysis.
Results
There were no significant baseline differences in demographics or measures of child and parent anthropometrics between treatment conditions (Table I). Prescribed sessions scored higher than self-directed sessions on interventionist prescribed behaviors: Mean (M) = 3.9 [standard deviation (SD) = 2.0] versus M = 1.8 (SD = 1.5); p < .0001. Self-directed approach sessions scored higher than prescribed approach sessions on interventionist self-directed behaviors: M = 6.1 (SD = 3.2) versus M = 1.5 (SD = 1.9); p < .0001. The differential change from baseline to posttreatment in parent’s confidence between the prescribed [n = 33; mean 3.7 (SD = 0.9) to 3.6 (1.0)] versus self-directed families [n = 24; 3.9 (0.8) to 4.3 (0.7)] was not significant (p = .068) or from baseline to 2-year follow-up, prescribed [n = 25; 2.9 (1.0)] and self-directed approach [n = 19; 3.6 (1.0)] (p = .16).
Child Outcomes
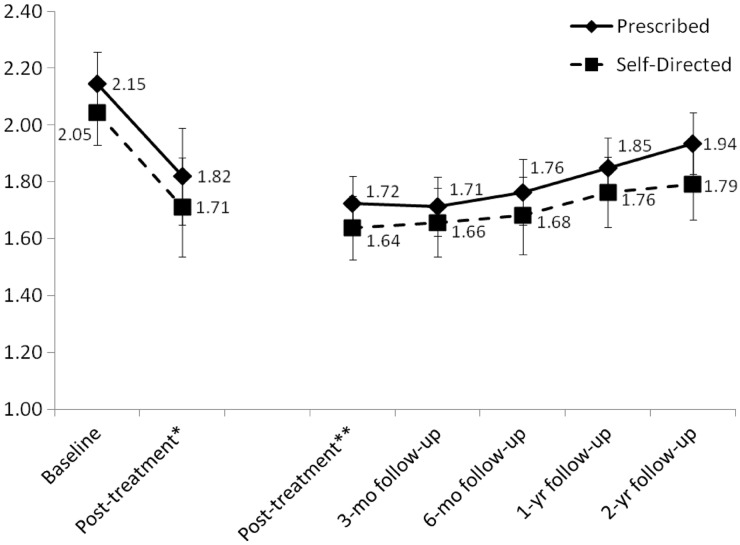
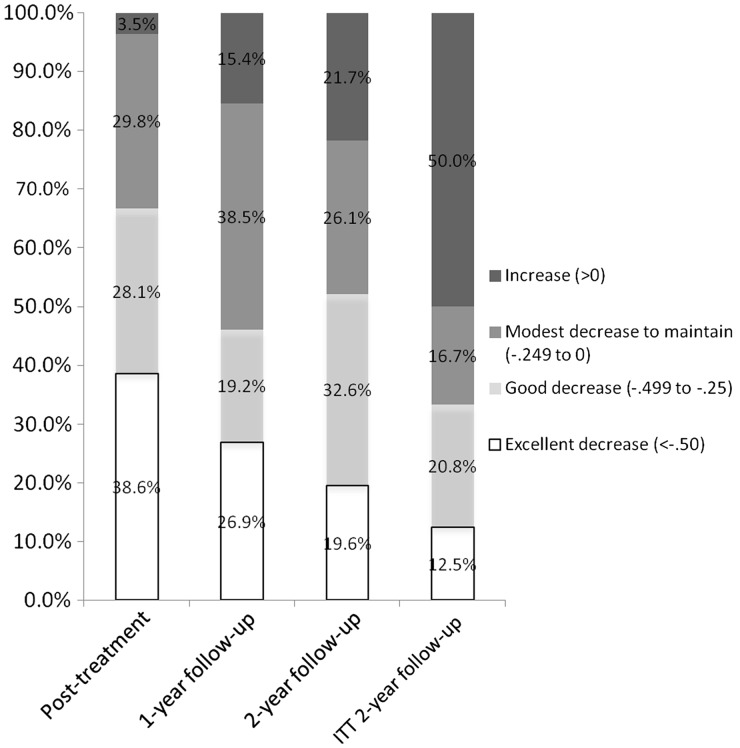
Baseline to posttreatment analyses (n = 72) revealed a significant effect of time, with a decrease in child BMI z-score [F(1,70) = 82.9, p < .001; partial eta squared = .542], but no significant time by treatment condition interaction [F(1,70) = 0.17, p = .90; partial eta squared < .001] (see Figure 2). Among the follow-up sample (n = 59) from posttreatment through all follow-ups, there was no significant effect of time [F(4,53) = 1.11, p = .36; partial eta squared = .077] or time by condition interaction [F(4,53) = 0.86, p = .55; partial eta squared = .061] for child BMI z-score (see Figure 2). Examining intent-to-treat changes directly from baseline to the 2-year follow-up among all children starting and receiving any treatment (n = 72), child BMI z-score decreased significantly [F(1,70) = 25.20, p < .001; partial eta squared = .265] in both the prescribed [M = 2.15 (standard error (SE) = .06) to M = 2.00 (SE = .08)] and self-directed [M = 2.05 (SE = .06) to M = 1.83 (SE = .08)] approach, but the time by condition interaction was not significant [F(1,70) = 1.37, p = .25; partial eta squared = .019]. Categorical changes in child BMI z-score changes are provided in Figure 3. At the most distal follow-up, ∼80% of children who completed 2-year follow-up assessment maintained or reduced their BMI z-score, although this was at 50% if assessment noncompleters are assumed to be in the increased BMI z-score category.
Figure 2.
Change in child BMI z-score for the prescribed and self-directed treatment approaches; *baseline to posttreatment among children randomized and attending first treatment session (n = 72); **posttreatment through all follow-ups among children with any follow-up data (n = 59); error bars indicate two standard errors above and below the mean for each approach at each time point.
Figure 3.
Categories of child BMI z-score change for assessment completers at each time point (n = 57 for posttreatment; n = 52 for 1-year follow-up; n = 46 for 2-year follow-up) and for ITT (intent-to-treat) sample of children (n = 72) assuming an increase in BMI z-score at 2-year follow-up for those with missing 2-year follow-up data (n = 26).
Parent Outcomes
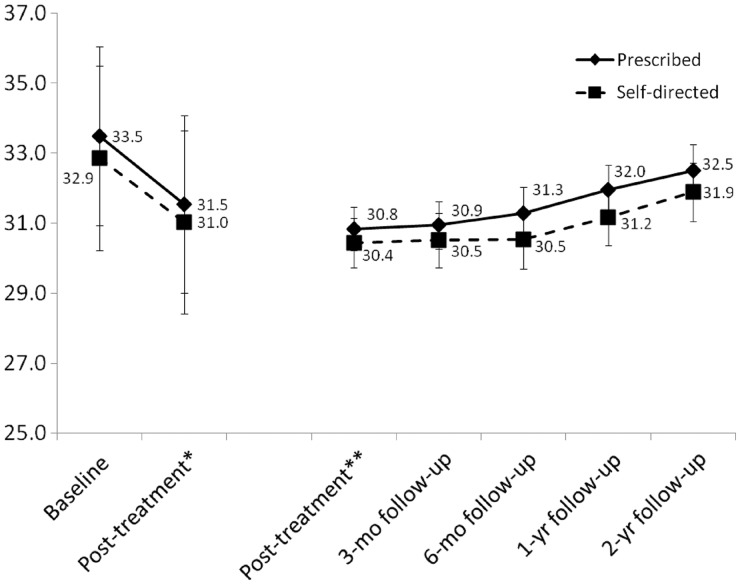
In baseline to posttreatment analyses (n = 72), parent BMI significantly decreased [F(1,70) = 73.5, p < .001; partial eta squared = .512], but there was no significant time by treatment condition interaction [F(1,70) = 0.064, p = .80; partial eta squared = .001] (see Figure 4). From posttreatment through all follow-ups (n = 60), there was no significant time effect [F(4,54) = 0.51, p = .73; partial eta squared = .036] and no significant time by treatment condition interaction [F(4,54) = 0.60, p = .67; partial eta squared = .042] for parent BMI (see Figure 4). Among parents starting treatment (n = 72), there was a significant decrease [F(1,70) = 6.11, p = .016; partial eta squared = .080] in parent BMI from baseline to 2-year follow-up in the prescribed [M = 33.5 (SE = 1.3) to M = 33.1 (SE = 1.3)] and self-directed approaches [M = 32.9 (SE = 1.3) to M = 32.1 (SE = 1.3)], but no significant time by condition interaction [F(1,70) = 0.60, p = .46; partial eta squared = .008].
Figure 4.
Change in participating parent BMI for the prescribed and self-directed treatment approaches; *baseline to posttreatment among parents randomized and attending first treatment session (n = 72); **posttreatment through all follow-ups among parents with any follow-up data (n = 60); error bars indicate two standard errors above and below the mean for each approach at each time point.
Discussion
There were no differences in child or parent weight outcomes between the prescribed versus self-directed approaches either at treatment end or through follow-up. Independent fidelity ratings suggested that interventionists delivered different interventions, but there was no significant difference between approaches in the expected differential change in parental self-efficacy for helping their child initiate and sustain healthy weight-related behaviors. The lack of differences finding is similar to other evidence from pediatric and adult obesity treatment studies that fail to find differences even among interventions that differ substantively in dietary or physical activity change methods (Epstein, Paluch, Gordy, & Dorn, 2000; Kirk et al., 2012). It is noteworthy that the intervention approaches evaluated in the present study both retained many components known to relate to outcome, among them moderate-to-high intensity (Whitlock, O'Connor, Williams, Beil, & Lutz, 2010) and a focus on both parent and child behavior change (Jelalian & Saelens, 1999). The average changes in child BMI z-scores herein are similar in magnitude to other efficacious interventions for pediatric obesity (Wilfley et al., 2007) and support the U.S. Preventive Task Force Services recommendations for overweight and obese children (Barton, 2010). More than two thirds of the children who were posttreatment assessment completers had achieved at least a decrease of .25 in BMI z-score by then. Even with conservative intent-to-treat, 50% of children provided any treatment had lower BMI z-scores at 2 years following treatment compared with baseline. There was some relapse following treatment cessation, although at the longest follow-up, children’s outcomes were better than at baseline and better than parental BMI change.
The standard prescribed approach continues to demonstrate efficacy and may be appealing to interventionists and families for whom consistent and clear directives within treatment are beneficial. This approach likely facilitates motivation by successful implementation of skills use and attaining expected outcomes. However, having an alternative approach provides an option for interventionists with a more patient-centered orientation. Translation of such an approach may be facilitated by incorporating explicit respect for patient/parent autonomy and acknowledgement of ambivalence regarding behavior change. The present study provided a test of only one way to incorporate a more self-directed and explicitly motivational approach into pediatric weight management. Other ways that perhaps vary timing or type of the self-directed intervention strategies used should be examined. Also, pending more evidence regarding which family or other characteristics would make a family more or less responsive to a standard prescribed versus a motivation enhancement approach, the best approach may be based on interventionist preference and experience with these approaches. Data regarding interventionists’ preference for treatment approach was not collected, although many health behavior change programs in use in health care systems use patient-centered approaches that share many characteristics with the self-directed approach.
Study limitations include the self-directed approach was not singularly motivational but rather a hybrid between the standard prescribed and a motivational approach. Families self-selected into the trial and thus already had high reported motivation for behavior change, perhaps unlike families approached through primary care providers (Resnicow et al., 2012). Both approaches retained a focus on the caregiver as the primary change agent and had the same format, dose, and information provided, so families may not have perceived approach differences. This study was not powered to detect small to moderate differences between approaches and the parental self-efficacy measures have not been validated. Median treatment attendance was high and similar between approaches beyond the fifth session (when approaches began to diverge), so it is not clear whether the differential dropout by approach was related to approach differences.
This study suggests that a family-based intervention approach for pediatric obesity that incorporates more motivational and autonomous components and a corresponding intervention stance after initial behavioral skills training performs similarly to the standard prescribed approach. Children and families having different characteristics may differentially benefit from one intervention approach, but more study is needed to examine this tailoring hypothesis.
Acknowledgments
The authors would like to thank the families and other interventionists for their time and dedication to this project.
Funding
Research reported in this publication work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health under award number R21HD054871 and the Seattle Children’s Hospital Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest: None declared.
References
- American Academy of Pediatrics. Motivational interviewing: Healthy active living for families implementation guide. 2012. Retrieved from http://www.aap.org/en-us/advocacy-and-policy/aap-health-initiatives/HALF-Implementation-Guide/communicating-with-families/Pages/Motivational-Interviewing.aspx. [Google Scholar]
- Armstrong M J, Mottershead T A, Ronksley P E, Sigal R J, Campbell T S, Hemmelgarn B R. Motivational interviewing to improve weight loss in overweight and/or obese patients: A systematic review and meta-analysis of randomized controlled trials. Obesity Reviews. 2011;12(9):709–723. doi: 10.1111/j.1467-789X.2011.00892.x. [DOI] [PubMed] [Google Scholar]
- Barton M. Screening for obesity in children and adolescents: US preventive services task force recommendation statement. Pediatrics. 2010;125(2):361–367. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. Journal of the American Medical Association. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Epstein L H, Paluch R A, Gordy C C, Dorn J. Decreasing sedentary behaviors in treating pediatric obesity. Archives of Pediatrics & Adolescent Medicine. 2000;154(3):220–226. doi: 10.1001/archpedi.154.3.220. [DOI] [PubMed] [Google Scholar]
- Epstein L H, Paluch R A, Roemmich J N, Beecher M D. Family-based obesity treatment, then and now: Twenty-five years of pediatric obesity treatment. Health Psychology. 2007;26:381–391. doi: 10.1037/0278-6133.26.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L H, Squires S. The stoplight diet for children. Boston, MA: Little, Brown and Co; 1988. [Google Scholar]
- Erickson S J, Gerstle M, Feldstein S W. Brief interventions and motivational interviewing with children, adolescents, and their parents in pediatric health care settings: A review. Archives of Pediatrics Adolescent Medicine. 2005;159(12):1173–1180. doi: 10.1001/archpedi.159.12.1173. [DOI] [PubMed] [Google Scholar]
- Ford A L, Hunt L P, Cooper A, Shield J P. What reduction in BMI SDS is required in obese adolescents to improve body composition and cardiometabolic health? Archives of Diseases in Childhood. 2010;95(4):256–261. doi: 10.1136/adc.2009.165340. [DOI] [PubMed] [Google Scholar]
- Freedman D S, Khan L K, Dietz W H, Srinivasan S R, Berenson G S. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: The Bogalusa heart study. Pediatrics. 2001;108(3):712–718. doi: 10.1542/peds.108.3.712. [DOI] [PubMed] [Google Scholar]
- Institute of Medicine. Committee on quality of health care in America: Crossing the quality chasm: A new health system for the 21st century. Washington, DC: National Academies Press; 2001. [Google Scholar]
- Jelalian E, Saelens B E. Empirically supported treatments in pediatric psychology: Pediatric obesity. Journal of Pediatric Psychology. 1999;24(3):223–248. doi: 10.1093/jpepsy/24.3.223. [DOI] [PubMed] [Google Scholar]
- Kirk S, Brehm B, Saelens B E, Woo J G, Kissel E, D'Alessio D, Bolling C, Daniels S R. Role of carbohydrate modification in weight management among obese children: A randomized clinical trial. Journal of Pediatrics. 2012;161(2):320–327. doi: 10.1016/j.jpeds.2012.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski R J, Ogden C L, Grummer-Strawn L M, Flegal K M, Guo S S, Wei R, Mei Z, Curtin L R, Roche A F, Johnson C L. Hyattsville, MD: National Center for Health Statistics; 2000. CDC growth charts: United States advance data from vital and health statistics; no. 314. [PubMed] [Google Scholar]
- Macdonell K, Brogan K, Naar-King S, Ellis D, Marshall S. A pilot study of motivational interviewing targeting weight-related behaviors in overweight or obese African American adolescents. Journal of Adolescent Health. 2012;50(2):201–203. doi: 10.1016/j.jadohealth.2011.04.018. [DOI] [PubMed] [Google Scholar]
- Martins R K, McNeil D W. Review of motivational interviewing in promoting health behaviors. Clinical Psychology Review. 2009;29(4):283–293. doi: 10.1016/j.cpr.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Miller W R, Rollnick S. Motivational interviewing, second edition: Preparing people for change. New York: Guilford Press; 2002. [Google Scholar]
- Mockus D S, Macera C A, Wingard D L, Peddecord M, Thomas R G, Wilfley D E. Dietary self-monitoring and its impact on weight loss in overweight children. International Journal of Pediatric Obesity. 2011;6(3–4):197–205. doi: 10.3109/17477166.2011.590196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institutes of Health, & National Heart Lung, Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. Obesity Research. 1998;6:51S–129S. [PubMed] [Google Scholar]
- Reinehr T, Kiess W, Kapellen T, Andler W. Insulin sensitivity among obese children and adolescents, according to degree of weight loss. Pediatrics. 2004;114(6):1569–1573. doi: 10.1542/peds.2003-0649-F. [DOI] [PubMed] [Google Scholar]
- Resnicow K, Davis R, Rollnick S. Motivational interviewing for pediatric obesity: Conceptual issues and evidence review. Journal of the American Dietetic Association. 2006;106(12):2024–2033. doi: 10.1016/j.jada.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Resnicow K, McMaster F, Woolford S, Slora E, Bocian A, Harris D, Drehmer J, Wasserman R, Schwartz R, Myers E, Foster J, Snetselaar L, Hollinger D, Smith K. Study design and baseline description of the BMI2 trial: Reducing paediatric obesity in primary care practices. Pediatric Obesity. 2012;7(1):3–15. doi: 10.1111/j.2047-6310.2011.00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin M A, Ford A, Hunt L, Jamal R, Crowne E C, Shield J P. Which factors are associated with a successful outcome in a weight management programme for obese children? Journal of the Evaluation of Clinical Practice. 2007;13(3):364–368. doi: 10.1111/j.1365-2753.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- Saelens B E, McGrath AM. Self-monitoring adherence and adolescent weight control efficacy. Children's Health Care. 2003;32(2):137–152. [Google Scholar]
- Schwartz R P, Hamre R, Dietz W H, Wasserman R C, Slora E J, Myers E F, Sullivan S, Rockett H, Thoma K A, Dumitru G, Resnicow K A. Office-based motivational interviewing to prevent childhood obesity: A feasibility study. Archives of Pediatrics and Adolescent Medicine. 2007;161(5):495–501. doi: 10.1001/archpedi.161.5.495. [DOI] [PubMed] [Google Scholar]
- Spahn J M, Reeves R S, Keim K S, Laquatra I, Kellogg M, Jortberg B, Clark N A. State of the evidence regarding behavior change theories and strategies in nutrition counseling to facilitate health and food behavior change. Journal of the American Dietetic Association. 2010;110(6):879–891. doi: 10.1016/j.jada.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Spear B A, Barlow S E, Ervin C, Ludwig D S, Saelens B E, Schetzina K E, Taveras E M. Recommendations for treatment of child and adolescent overweight and obesity. Pediatrics. 2007;120(Suppl 4):S254–S288. doi: 10.1542/peds.2007-2329F. [DOI] [PubMed] [Google Scholar]
- Taveras E M, Gortmaker S, Horan C, Kleinman K, Mitchell K, Price S, Prosser L, Rifas-Shiman S, Gillman MW. The high five for kids study: An intervention to improve primary care to prevent childhood obesity. 2010. Presented at the Pedaitrc Academic Societies Meeting, Vancouver. [Google Scholar]
- Taylor R W, Brown D, Dawson A M, Haszard J, Cox A, Rose E A, Taylor B J, Meredith-Jones K, Treacy L, Ross J, William S M. Motivational interviewing for screening and feedback and encouraging lifestyle changes to reduce relative weight in 4-8 year old children: Design of the MInT study. BMC Public Health. 2010;10:271. doi: 10.1186/1471-2458-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobacco Use and Dependence Guideline Panel. Treating tobacco use and dependence: 2008 update. Rockville, MD: US Dept of Health and Human Services, Public Health Service; 2008. [Google Scholar]
- Whitaker R C, Wright J A, Pepe M S, Seidel K D, Dietz W H. Predicting obesity in young adulthood from childhood and parental obesity. New England Journal of Medicine. 1997;337(13):869–873. doi: 10.1056/NEJM199709253371301. [DOI] [PubMed] [Google Scholar]
- Whitlock E P, O'Connor E A, Williams S B, Beil T L, Lutz K W. Effectiveness of weight management interventions in children: A targeted systematic review for the USPSTF. Pediatrics. 2010;125(2):e396–e418. doi: 10.1542/peds.2009-1955. [DOI] [PubMed] [Google Scholar]
- Wilfley D E, Tibbs T L, Van Buren D J, Reach K P, Walker M S, Epstein L H. Lifestyle interventions in the treatment of childhood overweight: A meta-analytic review of randomized controlled trials. Health Psychology. 2007;26:521–532. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]