Abstract
Major depressive disorder (MDD), cognitive symptoms, and mild cognitive deficits commonly occur in HIV-infected individuals, despite highly active antiretroviral therapies. In this study, we compared neuropsychological performance and cognitive symptoms of 191 HIV-infected participants. Results indicated that participants with a formal diagnosis of current MDD performed significantly worse than participants without MDD in all seven neuropsychological domains evaluated, with the largest effect sizes in information processing speed, learning, and memory. In addition, a brief assessment of cognitive symptoms, derived from a comprehensive neuromedical interview, correlated significantly with neurocognitive functioning. Participants with MDD reported more cognitive symptoms and showed greater neurocognitive deficits than participants without MDD. These findings indicate that HIV-infected adults with MDD have more cognitive symptoms and worse neuropsychological performance than HIV-infected individuals without MDD. The results of this study have important implications for the diagnosis of HIV-associated neurocognitive disorders (HAND).
Keywords: Depression, Cognition, Cognitive complaints, Neuropsychological tests, Acquired immunodeficiency syndrome, Mood disorders
INTRODUCTION
Major depressive disorder (MDD) is the most common psychiatric condition associated with human immunodeficiency virus (HIV) infection (Zanjani, Saboe, & Oslin, 2007) and occurs more frequently among HIV+ individuals than the general population (Bing et al., 2001; Ciesla and Roberts, 2001). Neurocognitive abnormalities continue to occur in HIV-infected individuals, despite the widespread use of combination antiretroviral therapies (Heaton et al., 2010; Robertson et al., 2007; Simioni et al., 2010). Similar to non-HIV studies, HIV studies examining the correlation between depressive symptoms and neuropsychological performance have produced mixed results.
While mild cognitive impairments commonly occur in the context of HIV infection, few studies have linked depression to these deficits. Evidence indicates that depression is associated with slowed information processing speed which may partially mediate other cognitive abilities such as episodic memory, executive functions, visuoconstruction, and fluency (Becker et al., 1997; Lee, Hermens, Porter, & Redoblado-Hodge, 2011). There is some evidence that increased depressive symptoms are associated with worse verbal memory and motor speed (Hinkin et al., 1992; Castellon et al., 2006), executive functioning (Castellon et al., 2006), procedural memory (Kalechstein, Hinkin, van Gorp, Castellon, & Satz, 1998), psychomotor speed (Shimizu et al., 2011), and global neurcognitive performance (Stern et al., 1991). However, many studies have not found a consistent link between depression and neuropsychological impairment in HIV infection (Bix et al., 1995; Carter, Rourke, Murji, Shore, & Rourke, 2003; Cysique et al., 2007; Grant et al., 1993; Mapou et al., 1993; von Giesen, Backer, Hefter, & Arendt, 2001). One study that used a comprehensive neuropsychological battery found that clinically diagnosed MDD was associated with significantly worse performance in domains of attention, learning and memory, but MDD did not confer a greater risk of impairment in any of the eight ability areas assessed (Goggin et al., 1997). The inconsistent findings across studies may be attributable to the variable methodology used to ascertain levels of depression.
Many studies have used self-report rating scales to evaluate the presence and severity of depressive symptoms, rather than a clinical diagnosis of MDD (e.g., Bassel, Rourke, Halman & Smith, 2002; Carter et al., 2003; Castellon et al., 2006; Kalechstein et al., 1998; Shimizu, 2011; Stern et al., 1991). Moreover, studies using rating scales have not reliably examined the independent impact of cognitive, somatic, and affective symptoms on cognitive abilities. One disadvantage of self-report measures, particularly when used with medically ill patients, is that mild depressive symptomatology may be inflated by somatic symptoms or medication side effects (i.e., fatigue, loss of energy, changes in appetite and/or sleep) rather than a mood disturbance. In contrast, some semi-structured interviews that yield a clinical diagnosis allow the clinician to dissociate symptoms induced by physical illness or medications from mood dysregulation after minimizing possible misclassification due to other medical illnesses. One study that evaluated the differential effect of clinically diagnosed MDD using a structured interview (i.e., Composite International Diagnostic Interview) versus a self-report rating scale (i.e., Center for Epidemiological Studies – Depression [CES-D]) on neuropsychological performance in a large sample of HIV+ African American men found that MDD was associated with worse verbal memory and cognitive flexibility, while participant report of depressive symptoms was only associated with poorer motor skills (Richardson et al., 1999). This discrepant finding suggests that, among individuals with chronic medical illness, self-report rating scales of depressive symptoms may be more likely to correlate with physical impairments (i.e., motor skills); whereas a clinical diagnosis of MDD may be more indicative of a disrupted CNS process. Importantly, the relatively small percentage (11.5%; 28/243) of participants that met criteria for MDD was split between three groups, which may have limited the ability to detect a main effect in other domains. This study also found that MDD was not significantly associated with the CES-D using a cut-off score (≥ 16; e.g., Richardson et al., 1999), which further indicates the importance of using clinician-based psychiatric interviews for participants with chronic medical illness.
Cognitive complaints are common among HIV+ individuals with depressive symptoms, but the association between these factors and cognition is less clear. Some studies have found that cognitive complaints are associated with depressive symptoms (Rourke, Halman, & Bassel, 1999; Thames et al., 2011), but not neuropsychological performance (van Gorp et al., 1991). Others have reported that cognitive complaints, but not depressive symptoms, are associated with neuropsychological performance (Carter et al., 2003). One study found that depressive symptoms and some neuropsychological tests were independently associated with cognitive complaints (Bassel et al., 2002). Furthermore, some self-report measures of cognitive complaints include non-cognitive symptoms such as sensory perception and physical impairments. For example, the Patient's Assessment of Own Functioning Inventory is a self-report measure of cognitive symptoms (e.g., “How often do you forget something that has been told to you within the last day or two?”), but also contains non-cognitive functions (e.g., “How often do you have difficulty feeling things with your right hand?”) which may inflate scores and distort correlations with objective neuropsychological impairments, particularly among HIV+ individuals that develop peripheral nerve damage (i.e., peripheral neuropathy). The accuracy of subjective cognitive complaint assessment is particularly important because these symptoms are used in the diagnosis of symptomatic HIV-associated neurocognitive disorders (Antinori et al., 2007; Woods et al., 2004). Using cognitive complaint measures that are less susceptible to inflated scores due to physical illness may improve associations with neuropsychological tests.
Evidence indicates a shifting pattern of neurocognitive impairment in HIV+ individuals from deficits in motor ability, information processing speed, and verbal fluency before the use combination antiretroviral therapies, to memory and executive functioning impairments after the widespread use of these medications (Heaton et al., 2011). However, there is a paucity of research examining the association between MDD and the changing neurocognitive profile of HIV+ individuals in the era of combination antiretroviral therapies. This is particularly important considering the possible contribution of these antiretroviral medications to neurocognitive ability (Robertson et al., 2010) and neuropathological abnormalities (Giunta et al., 2011).
The aims of the current study were to (1) Compare neuropsychological performance between ethnically diverse HIV+ participants with current MDD and those without MDD; (2) Identify the association between cognitive symptoms and global cognitive functioning, and (3) Compare the rate of cognitive symptoms by MDD status.
METHOD
Participants
The Manhattan HIV Brain Bank (MHBB) (U01MH083501), a member of the National NeuroAIDS Tissue Consortium, is an ongoing prospective cohort study designed to provide a resource of nervous system tissues from HIV-infected donors who undergo comprehensive medical, neuropsychological, and psychiatric evaluation. The MHBB study is approved by the Mount Sinai School of Medicine Institutional Review Board and all participants provided informed consent. Eligibility criteria for the Manhattan HIV Brain Bank have been described in a previously published study (Morgello et al., 2004). The primary enrollment criterion for the MHBB is willingness to participate in organ donation at the time of death. Other criteria were designed to target patients with advanced disease (e.g., CD4 count less than 50, opportunistic infections) or other intractable medical condition. Baseline data collected from 1999 to 2011 were used in the current study. Exclusion criteria for the current study included nonstandard assessment procedures, missing psychiatric or neuropsychological assessment, active psychosis, head injury with a loss of consciousness greater than 30 minutes, seizures, cerebral vascular accident, or other neurological condition considered to confound testing (e.g., neurosyphillis, blindness, cerebellar degeneration, CNS opportunistic infection). While there is evidence that peripheral neuropathy in HIV+ individuals is associated with specific neuropsychological deficits (Fellows et al., 2012), we chose not to exclude these participants as peripheral nerve disease may simultaneously occur with HIV-associated CNS pathology. A total of 191 participants met inclusion criteria for the current study.
Procedure
Neuropsychological assessment
The neuropsychological assessment consisted of eleven tests that comprised seven neurocognitive domains sensitive to HIV infection. Details of the neuropsychological battery along with normative sources are presented in the Appendix. The classification of these particular tests into specific domains was theoretically, rather than empirically derived (e.g., factor analysis), to maintain consistency with previous studies that have successfully used these categorizations to detect HIV-related deficits with acceptable sensitivity (Antinori et al., 2007; Byrd et al., 2011; Carey et al., 2004; Cherner et al., 2010; Devlin et al., 2012; Fellows et al., 2012; Heaton et al., 2010, 1995; Heaton, 2011; Moore et al., 2011; Rippeth et al., 2004; Ryan et al., 2005; Woods et al., 2004). All assessments were administered by trained staff and reviewed by a clinical neuropsychologist for quality assurance. Raw scores were converted into t scores which adjusted for the following demographic factors, as available: age, education, gender, and ethnicity using normative data for each test. Individual test t scores were summed and averaged to create domain t scores. A composite or “global” score was calculated by adding and averaging domain t scores.
Cognitive symptoms
Cognitive symptoms were assessed with questions derived from a comprehensive neuromedical interview, in which participants were asked to rate their current degree of difficulty in four areas: (1) attention and concentration, (2) comprehension (i.e., understanding of reading materials and TV), (3) memory (i.e., remembering and forgetfulness), and (4) speech and language (i.e., word finding and communication difficulty). In this clinician-administered assessment the clinician asks the participant if they are having difficulty in any of the four areas of interest, and then discusses with the participant the degree to which they are affected to determine severity. The interview based format of this questionnaire is particularly important in our cohort, as many of our participants have low literacy. The questionnaire was administered by the neuromedical staff, not neuropsychological testers, before the neuropsychological evaluation. Symptom severity was measured using a scale ranging from 0 (normal; as good as it has always been) to 4 (severe). These four questions showed good internal consistency (Cronbach's α=.802). A total symptom severity score is calculated by adding scores from all four areas (range=0–16). In addition, responses to each of the four questions were assigned a rating of either 0 (normal or minimal difficulty) or 1 (mild/moderate/severe) to indicate a change in perceived ability. A composite score was calculated by adding ratings from each of the four cognitive symptom areas, with a score of 0 indicating no self-reported cognitive deficits and a score of 4, indicating that the participant reported impairment in all four areas evaluated.
Psychiatric evaluation
The Psychiatric Research Interview for Substance and Mental Disorders (PRISM; version 1.9B) is a semi-structured interview that was used to assess psychiatric disorders in accordance with DSM-IV criteria (Hasin et al., 1996). From this interview, we used MDD data and substance use characteristics for all participants. Using this interview for current MDD diagnoses has shown acceptable test/re-test reliability (kappa=.81) and inter-rater reliability (kappa=.78) among ethnically diverse individuals with substance use disorders (Hasin et al., 1996; Morgello et al., 2006). Interviews were administered by trained staff and reviewed by a clinical neuropsychologist to maintain diagnostic accuracy.
Neuromedical assessment
Participants received a neuromedical evaluation that included a review of medications, medical history, current symptoms, and a complete neurological examination. Blood samples were taken to measure CD4 cell counts and HIV loads. These immunologic indicators were only available for a subset of the study sample (n=167). In addition, four participants did not have complete neurological exams. A diagnosis of peripheral neuropathy was assigned by a neurologist based on previously established two sign criteria (Morgello et al., 2004).
Group assignment
Participants were divided into two groups to examine the association between MDD and neuropsychological test performance. Individuals who met DSM-IV criteria for a current (within the past 2 months) major depressive episode, not attributable to substance use or known physical illness, were assigned to the MDD group. All other participants, including those with past-MDD only, were classified into the No MDD group. To meet criteria for MDD, at least five symptoms of depression must be present nearly every day for a minimum of two weeks (American Psychiatric Association, 2000, pg. 356). Importantly, an individual continues to carry a diagnosis of current MDD until symptoms have remitted for at least two months (APA, 2000, pg. 369).
Statistical Analyses
Independent t test and χ2 analyses were used to compare demographic, substance use, and medical factors between groups. A multivariate analysis of covariance (MANCOVA) with MDD status as the independent variable was conducted to examine the effect of MDD on neuropsychological performance. Spearman correlations were used to examine associations between global neuropsychological performance and cognitive symptoms. Chi-square analyses were used to compare the rate of cognitive symptoms between groups. A significance level of p<.05 was set for all analyses.
RESULTS
Participants
Participant characteristics are presented in Table 1. Overall, 30% (n=58) of the participants had current MDD, and 27% (n=49) met criteria for past, but not current MDD. Most demographic, medical, and substance use characteristics did not differ by MDD status, with two exceptions. The group without MDD (n=133) had a longer duration of known HIV infection, F(1,191)=4.402, p=.037 and were more likely to be on antiretroviral medication, (78.9% vs. 65.5%), χ2=3.87, p=.049, OR=.507, 95% CI [.256–1.00]. Therefore, duration of known HIV infection and antiretroviral status were entered as covariates in a MANCOVA.
Table 1.
Participant characteristics
| MDD (n = 58) | No MDD (n = 133) | Total sample (N = 191) | |
|---|---|---|---|
| Age | |||
| Years | 43.3 (6.5) | 45.0 (7.8) | 44.5 (7.5) |
| Sex (% male) | 58.6 | 66.9 | 64.4 |
| Ethnicity (%) | |||
| African American | 41.4 | 54.9 | 50.8 |
| Hispanic or Latino | 31.0 | 20.3 | 23.6 |
| Caucasian | 27.6 | 22.6 | 24.1 |
| Other | 0 | 2.3 | 1.6 |
| Education (years) | 11.8 (3.0) | 12.4 (2.8) | 12.2 (2.9) |
| WRAT-3 (SS) | 83.3 (18.9) | 86.9 (17.4) | 85.8 (17.9) |
| Length of HIV infection, years | 9.9 (4.9) | 11.6 (5.3)* | 11.1 (5.2) |
| Antiretroviral medication (%) | 65.5 | 78.9* | 74.9 |
| Detectable HIV RNA (%) | 68.8 | 76.1 | 73.9 |
| Median CD4, cells/μL | 149 | 168 | 165 |
| CD4 (cells/μL) (%) | |||
| ≥ 500 | 20.0 | 21.4 | 21.0 |
| 200–499 | 24.0 | 23.9 | 24.0 |
| ≤ 199 | 56.0 | 54.7 | 44.9 |
| Substance dependence, current (%) | |||
| Alcohol | 12.1 | 12.0 | 12.0 |
| Cannabis | 12.1 | 5.3 | 7.3 |
| Cocaine | 27.6 | 17.3 | 20.4 |
| Opiate | 12.1 | 5.3 | 7.3 |
| Peripheral neuropathy (%) | 46.4 | 47.3 | 47.1 |
| Antidepressant medication (%) | 36.2 | 27.1 | 29.8 |
Note. WRAT-3 (SS) is Wide Range Achievement Test – 3rd Edition reading standard score; means and standard deviations are presented unless otherwise indicated.
p < .05
MDD and Neuropsychological Performance
The omnibus MANCOVA used to compare neuropsychological performance in seven domains between the group with MDD and the group without was significant, F(1,191)=2.24, p=.033. Results from the post hoc analyses are presented in Table 2. The group with MDD performed significantly worse in all seven cognitive domains: information processing speed (p=.005), learning (p=.001), memory (p=.001), fluency (p=.012), motor ability (p=.005), working memory (p=.017), and executive functioning (p=.048). The domains with the largest effect sizes were information processing speed (ηp2=.042), learning (ηp2=.059), and memory (ηp2=.056). The pattern of deficits in the two groups was similar: the most significant deficits in each were seen in learning and memory, motor and executive functioning domains. Domain t score marginal means and standard errors are presented in Figure 1.
Table 2.
Neuropsychological performance by MDD status
| MDD (n = 58) |
No MDD (n = 133) |
||||||
|---|---|---|---|---|---|---|---|
| Domain | Mean | SE | Mean | SE | F | p | η p 2 |
| Info. Processing Speed | 38.8 | 1.15 | 42.7 | .751 | 8.20 | .005 | .042 |
| Learning | 28.3 | 1.53 | 34.6 | 1.00 | 11.76 | .001 | .059 |
| Memory | 28.6 | 1.67 | 35.3 | 1.09 | 11.00 | .001 | .056 |
| Fluency | 43.8 | 1.45 | 48.2 | .952 | 6.36 | .012 | .033 |
| Motor | 31.5 | 1.41 | 36.3 | .922 | 8.01 | .005 | .041 |
| Working Memory | 40.9 | 1.17 | 44.3 | .768 | 5.81 | .017 | .030 |
| Executive Functioning | 38.2 | 1.27 | 41.3 | .833 | 3.96 | .048 | .021 |
Note. Marginal means and standard errors are presented. Antiretroviral status and duration of HIV infection were entered as covariates.
Fig. 1.
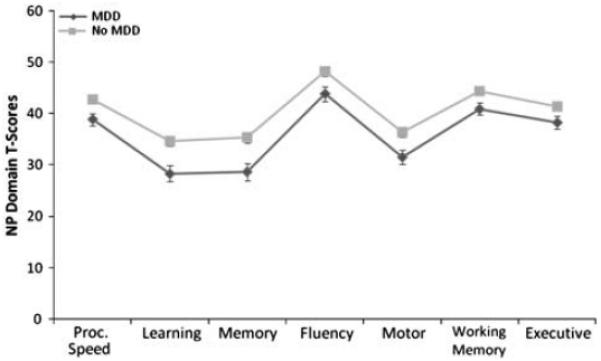
Neuropsychological marginal mean domain T-scores and standard errors. NP = neuropsychological; MDD = major depressive disorder.
Neuropsychological Performance and Cognitive Symptoms
Overall neuropsychological performance correlated with total number of cognitive symptoms, r=−.311, p<.001, and cognitive symptom severity, r=−.332, p<.001. Next, neuropsychological impairment, using a global neuropsychological t score cutoff of less than 40, and cognitive symptoms were evaluated using a χ2 analysis. Cognitively impaired participants were more likely to report one or more cognitive symptoms than those with intact neuropsychological performance, (51% vs. 24%), χ2=13.68, p<.001, OR=3.15, 95% CI [1.69–5.86].
MDD and Cognitive Symptoms
A χ2 analysis revealed that participants with MDD were significantly more likely to report one or more cognitive symptoms than participants without MDD (64% vs. 27%), χ2=23.07, p<.001, OR=4.75, 95% CI [2.46–9.17]. Next, χ2 analyses were computed to examine differences between groups in the rate cognitive symptoms in each of the four self-report areas. As shown in Figure 2, participants with MDD were significantly more likely to report a deficit in each of the four areas: attention/concentration, χ2=16.48, p<.001, OR=4.04, 95% CI [2.01–8.12], comprehension, χ2=15.29, p<.001, OR=4.49, 95% CI [2.04–9.93], memory, χ2=19.59, p<.001, OR=4.81, 95% CI [2.32–9.99] and speech/language, χ2=4.47, p=.034, OR=2.33, 95% CI [1.05–5.16].
Fig. 2.
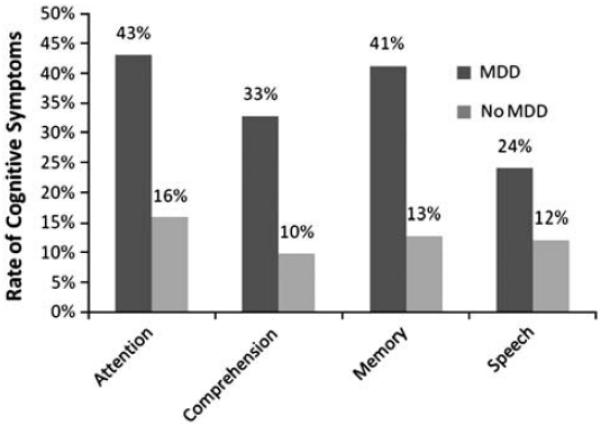
Rate of cognitive symptoms by major depressive disorder (MDD) status. Attention, comprehension, and memory, p<.001; speech, p<.05.
DISCUSSION
As the rate of affective disorders and mild neurocognitive impairment among HIV+ adults remains higher than the general population, more research is needed to characterize these co-occurring conditions. In this study, we compared the association between clinician-assessed MDD, cognitive symptoms, and neuropsychological performance in a diverse HIV+ cohort. Overall, 30% of participants had current MDD, which is consistent with previous studies that have reported the prevalence of depression among HIV+ men and women (e.g., Ickovics et al., 2001; Rabkin et al., 1997). The proportion of participants with MDD did not differ by mean age, gender, race/ethnicity, or current substance dependence. Moreover, the demographic diversity of this cohort is noteworthy considering that previous studies of depression and neurocognition in the context of HIV infection have consisted of primarily younger Caucasian male participants.
The primary finding of this study was that participants with MDD performed significantly worse than participants without MDD in all neuropsychological domains evaluated: including information processing speed, learning, memory, fluency, motor, working memory, and executive functioning. Also, the pattern of neuropsychological performance was similar between groups with notable deficits in learning, memory, motor, and executive functioning which are characteristic of HIV-associated neurocognitive impairment in the era of combined antiretroviral therapy (Heaton et al., 2011). This extends previous studies that have found an association between clinician-assessed MDD and worse attention, processing speed, fluency, learning, and memory among HIV+ individuals before the widespread use of highly active antiretroviral therapy (Becker et al., 1997; Goggin et al., 1997). Moreover, the diffuse pattern of deficits in the current study is similar to that of another study that examined the effects of clinician-assessed MDD on neuropsychological performance, but because of the Bonferroni corrections to post hoc analyses, only the domains of attention, learning, and memory were statistically significant at the corrected level (Goggin et al., 1997). However, the authors did note that participants with MDD tended to perform more poorly across all domains evaluated (Goggin et al., 1997). Indeed, the broad deficits among participants with MDD in the current study may be from processing speed impairments that have been shown mediate performance in other domains (Becker et al., 1997). The findings of the current study have important implications for the diagnosis and treatment of HIV-related neurocognitive disorders. If individuals with MDD perform more poorly on neuropsychological tests than those without MDD, as we found in this study, it will be important for clinicians to consider this condition in the differential diagnosis of HIV-related neurocognitive disorders, as MDD may produce or exacerbate these deficits. More research is needed to examine the mechanism of neuropsychological dysfunction associated with MDD in the context of HIV infection.
In contrast to previous research that have used only self-report rating scales to measure depressive symptoms, the results of the current study revealed a more diffuse pattern of neuropsychological dysfunction. This difference may be partially explained by the method of depressive symptom ascertainment. While self-report rating scales are useful as screening measures for depression, a clinical designation of MDD identifies an association between depressive symptoms and a significant change in social and/or functional impairment, unrelated to substance use or physical illness, which may be indicative of a more severe and/or organic CNS process. The results of the current study contrast with another study that reported no effect of incident MDD on neurocognitive functioning in a HIV+ sample evaluated before the widespread use of highly active antiretroviral therapies (Cysique et al., 2007). However, it is important to note that as that study was designed to assess incident MDD, individuals with a major depressive episode at baseline were not included in the analyses, which may have excluded individuals with more severe or chronic depression (Cysique et al., 2007). This may partially explain the discrepancy between the results of the current study and previous research. Future studies that track psychiatric and neuropsychological changes over time may be helpful in identifying depressive symptoms associated with physiological dysregulation endogenous to HIV disease progression (e.g., neuroinflammation), by examining differences between current, remitted, and incident MDD.
The accurate assessment of subjective cognitive symptoms is an important aspect of a comprehensive neuropsychological evaluation among HIV+ adults, because these symptoms are used in the determination of symptomatic HIV-associated neurocognitive disorders (HAND). In fact, according to the updated diagnostic criteria for HAND, an individual with neuropsychological impairment in two or more ability areas without self-reported functional decline or cognitive symptoms will be diagnosed with asymptomatic neurocognitive impairment (ANI) (Antinori et al., 2007), whereas functional decline or cognitive symptoms in the presence of neuropsychological impairment indicates a symptomatic diagnosis (e.g., mild cognitive motor disorder or HIV-associated dementia). Considering these diagnostic criteria, we examined the association between a brief assessment of cognitive symptoms and neuropsychological performance. The results revealed an association between cognitive symptoms and neuropsychological performance, which is consistent with previous research (e.g., Carter et al., 2003; Millikin, Rourke, Halman, & Power, 2003). However, the lack of association between neuropsychological performance and cognitive complaints in other studies has lead clinicians to be particularly cautious of using self-reported cognitive complaints in the diagnosis of HAND among individuals with increased depressive symptoms (Woods et al., 2004). In the current study, we found that participants with MDD were more likely to report cognitive symptoms and perform more poorly in all seven neuropsychological domains than participants without MDD. This suggests that subjective cognitive symptoms are related to neuropsychological ability, even among individuals with MDD. The correlation between objective performance and these cognitive symptoms provides evidence for the ecological validity of neuropsychological tests to detect the functional impact of HIV and MDD. These findings indicate that a brief assessment of cognitive symptoms, used in conjunction with a formal neuropsychological assessment, may be useful in the diagnosis of symptomatic HAND.
While we identified an association between MDD and neuropsychological performance in the current study, the mechanism of dysfunction is less clear. There is evidence that HIV infection causes cell-mediated immune activation and neuroinflammation that leads to serotonin depletion, induces neuronal apoptosis, and contributes to depressive symptoms and mild cognitive impairment (Leonard & Maes, 2012). This suggests that MDD and neurocognitive impairment may represent a unique CNS syndrome in the context of HIV infection. As research has shown that increased inflammatory cytokine activation may induce depressive symptoms, chronic low-level inflammation mediated by HIV infection may predispose patients to developing MDD. This is particularly important considering that HIV, depression, and neuropsychological impairment are independently associated with increased levels of neopterin, a proinflammatory immune marker (Dunbar, Pemberton, Perdices, & Brew, 1996; Schroecksnadel et al., 2008; Warriner et al., 2010). This may also partially explain the relatively high rate of MDD in HIV+ cohorts. As combined antiretroviral therapy effectively reduces viral replication, more research is needed to identify the association between depressive symptoms, neurocognitive impairment, and medication adherence. While research has indicated depression as a risk factor for suboptimal antiretroviral adherence (e.g., Gonzalez, Batchelder, Psaros, & Safren, 2011), conceptually, it is possible that poor adherence causes higher concentrations of virus in the brain and pro-inflammatory cytokines which induce depressive phenomena and neurocognitive impairment. This is particularly important considering HIV and MDD are associated with disturbed subcortical and frontostriatal neural circuitry (Tekin & Cummings, 2002). Therefore, future research may benefit from investigating the pathophysiologic mechanisms of MDD in the context of HIV infection.
This is the first study to examine the association between clinician-assessed MDD and neuropsychological performance among and ethnically diverse HIV+ men and women in the era of combined antiretroviral therapy. While the demographic heterogeneity and substance use characteristics of the sample is representative of the evolving HIV pandemic, these factors also complicate the clear interpretation of results. Even though substance dependence diagnoses did not differ by MDD status, the comparable rates of these disorders do not necessarily indicate an equal pattern of substance use. In addition, the lack of an HIV-negative comparison group limits the ability to clearly determine whether the deficits associated with MDD in this study are endogenous to HIV infection or processes specific to MDD. Moreover, as the primary aim of this study was to compare neuropsychological performance by MDD status, we did not examine the potential influence of antidepressant medications or specific antiretroviral therapies on neurocognition. It is particularly challenging to examine the interactions between MDD, antidepressants, and neurocognition among HIV+ participants, as some antidepressants are used to treat other HIV-related disorders, such as peripheral neuropathy (Verma, Estanislao, Mintz, & Simpson, 2004; for a review, see Saarto & Wiffen, 2007). Future studies of healthier HIV-infected individuals that can account for the effects of antidepressant medications would be helpful in evaluating the efficacy of depression treatment to improve neurocognitive deficits associated with MDD. There is evidence that protease inhibitors, often used as part of a combined antiretroviral regimen, reduce plasma levels of commonly prescribed antidepressants (i.e., paroxetine, buproprion), which may decrease the efficacy of these medications (Hesse, Greenblatt, von Miltke, & Court, 2006; Hogeland et al., 2007; van der Lee et al., 2007). As different medication classes may differentially impact MDD and neurocognition, more research is needed to characterize these neural pathways.
In summary, we identified an association between MDD and neurocognition, such that participants with MDD performed significantly worse than those without MDD in all seven neuropsychological domains assessed, with the largest effect sizes in information processing speed, learning, and memory. Additionally, participants with MDD were more likely to report cognitive symptoms than those without MDD. Finally, we found that current MDD and cognitive symptoms were independently associated with worse overall neurocognitive ability. More research is needed to examine the role of psychiatric illness in the pathogenesis of neurocognitive impairment in HIV-infected individuals.
ACKNOWLEDGMENTS
The authors thank the staff and participants of the Manhattan HIV Brain Bank. This research was supported by the National Institutes of Health (S.M., grant numbers U01MH083501, UL1RR029887).
APPENDIX.
NEUROPSYCHOLOGICAL TESTS AND NORMATIVE DATA SOURCES
| Neuropsychological domain/tests | Normative data source |
|---|---|
| Motor | |
| Grooved Pegboard—DH | Heaton et al. (1991)1,2,3 |
| Grooved Pegboard—NDH | Heaton et al. (1991)1,2,3 |
| Processing Speed | |
| Trail Making Test, Part A (TMT-A) | Heaton et al. (1991)1,2,3 |
| WAIS-III Digit Symbol | Wechsler (1997)1 |
| WAIS-III Symbol Search | Wechsler (1997)1 |
| Executive Functioning | |
| Trail Making Test, Part B (TMT-B) | Heaton et al. (1991)1,2,3 |
| Wisconsin Card Sorting Test (WCST) -Perseverative Responses | Kongs et al. (2000)1,2 |
| Learning | |
| Brief Visual Memory Test -Total Recall | Benedict et al. (1997)1 |
| Hopkins Verbal Learning Test -Total Recall | Benedict et al. (1998)1 |
| Memory | |
| Brief Visual Memory Test- Delayed Recall | Benedict et al. (1997)1 |
| Hopkins Verbal Learning Test- Delayed Recall | Benedict et al. (1998)1 |
| Working Memory | |
| WAIS-III Letter Number Sequencing | Wechsler (1997)1 |
| Paced Auditory Serial Addition Task | Diehr et al. (2003)1,2,3,4 |
| Verbal Fluency | |
| Controlled Oral Word Association Test | Gladsjo et al. (1999)1,2,4 |
| Reading Level | |
| Wide Range Achievement Test – Reading 3rd Edition | Wilkinson (1993)1 |
Note. Wechsler Adult Intelligence Scale (WAIS). Normative data provides adjustments for the following demographic characteristics, as indicated:
Age;
Education;
Gender
Ethnicity.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th ed., text revision American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, Wojna VE. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. doi:10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassel C, Rourke SB, Halman MH, Smith ML. Working memory performance predicts subjective cognitive complaints in HIV infection. Neuropsychology. 2002;16(3):400–410. doi: 10.1037//0894-4105.16.3.400. [DOI] [PubMed] [Google Scholar]
- Becker JT, Sanchez J, Dew MA, Lopez OL, Dorst SK, Banks G. Neuropsychological abnormalities among HIV-infected individuals in a community-based sample. Neuropsychology. 1997;11(4):592–601. doi: 10.1037//0894-4105.11.4.592. [DOI] [PubMed] [Google Scholar]
- Benedict RH. Brief Visuospatial Memory Test…Revised. Psychological Assessment Resources; Odessa, FL: 1997. [Google Scholar]
- Benedict RHB, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative data and analysis of inter-form and test-retest reliability. The Clinical Neuropsychologist. 1998;12(1):43–55. doi:10.1076/clin.12.1.43.1726. [Google Scholar]
- Bing EG, Burnam MA, Longshore D, Fleishman JA, Sherbourne CD, London AS, Shapiro M. Psychiatric disorders and drug use among human immunodeficiency virus-infected adults in the united states. Archives of General Psychiatry. 2001;58(8):721–728. doi: 10.1001/archpsyc.58.8.721. [DOI] [PubMed] [Google Scholar]
- Bix BC, Glosser G, Holmes W, Ballas C, Meritz M, Hutelmyer C, Turner J. Relationship between psychiatric disease and neuropsychological impairment in HIV seropositive individuals. Journal of the International Neuropsychological Society. 1995;1(6):581–588. doi: 10.1017/s1355617700000722. [DOI] [PubMed] [Google Scholar]
- Byrd DA, Fellows RP, Morgello S, Franklin D, Heaton RK, Deutsch R, CHARTER Group Neurocognitive impact of substance use in HIV infection. Journal of Acquired Immune Deficiency Syndromes. 2011;58(2):154–162. doi: 10.1097/QAI.0b013e318229ba41. doi:10.1097/QAI.0b013e318229ba41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey CL, Woods SP, Gonzalez R, Conover E, Marcotte TD, Grant I, HNRC Group Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. Journal of Clinical and Experimental Neuropsychology. 2004;26(3):307–319. doi: 10.1080/13803390490510031. doi:10.1080/13803390490510031. [DOI] [PubMed] [Google Scholar]
- Carter SL, Rourke SB, Murji S, Shore D, Rourke BP. Cognitive complaints, depression, medical symptoms, and their association with neuropsychological functioning in HIV infection: A structural equation model analysis. Neuropsychology. 2003;17(3):410–419. doi: 10.1037/0894-4105.17.3.410. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, van Gorp WG, Moore L. Components of depression in HIV-1 infection: Their differential relationship to neurocognitive performance. Journal of Clinical zand Experimental Neuropsychology. 2006;28(3):420–437. doi: 10.1080/13803390590935444. doi:10.1080/13803390590935444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherner M, Bousman C, Everall I, Barron D, Letendre S, Vaida F, HNRC Group Cytochrome P450-2D6 extensive metabolizers are more vulnerable to methamphetamine-associated neurocognitive impairment: Preliminary findings. Journal of the International Neuropsychological Society. 2010;16(5):890–901. doi: 10.1017/S1355617710000779. doi:10.1017/S1355617710000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciesla JA, Roberts JE. Meta-analysis of the relationship between HIV infection and risk for depressive disorders. The American Journal of Psychiatry. 2001;158(5):725–730. doi: 10.1176/appi.ajp.158.5.725. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Deutsch R, Atkinson JH, Young C, Marcotte TD, Dawson L, HNRC Group Incident major depression does not affect neuropsychological functioning in HIV-infected men. Journal of the International Neuropsychological Society. 2007;13(1):1–11. doi: 10.1017/S1355617707070026. doi:10.1017/S1355617707070026. [DOI] [PubMed] [Google Scholar]
- Devlin KN, Gongvatana A, Clark US, Chasman JD, Westbrook ML, Tashima KT, Cohen RA. Neurocognitive effects of HIV, hepatitis C, and substance use history. Journal of the International Neuropsychological Society: JINS. 2012;18(1):68–78. doi: 10.1017/S1355617711001408. doi:10.1017/S1355617711001408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehr MC, Cherner M, Wolfson TJ, Miller SW, Grant I, Heaton RK, HIV Neurobehavioral Research Center Group The 50 and 100-item short forms of the paced auditory serial addition task (PASAT): Demographically corrected norms and comparisons with the full PASAT in normal and clinical samples. Journal of Clinical and Experimental Neuropsychology. 2003;25(4):571–585. doi: 10.1076/jcen.25.4.571.13876. doi:10.1076/jcen.25.4.571.13876. [DOI] [PubMed] [Google Scholar]
- Dunbar N, Pemberton L, Perdices M, Brew BJ. Clinical markers of the presence of dementia and neuropsychological impairment in HIV infection. Journal of Neuro-AIDS. 1996;1(4):31–48. doi: 10.1300/j128v01n04_04. [DOI] [PubMed] [Google Scholar]
- Fellows RP, Byrd DA, Elliott K, Robinson-Papp J, Mindt MR, Morgello S. Distal sensory polyneuropathy is associated with neuropsychological test performance among persons with HIV. Journal of the International Neuropsychological Society. 2012;19:1–10. doi: 10.1017/S1355617712000707. doi:10.1017/S1355617712000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giunta B, Ehrhart J, Obregon DF, Lam L, Le L, Jin J, Shytle RD. Antiretroviral medications disrupt microglial phagocytosis of beta-amyloid and increase its production by neurons: Implications for HIV-associated neurocognitive disorders. Molecular Brain. 2011;4(1):23. doi: 10.1186/1756-6606-4-23. doi:10.1186/1756-6606-4-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladsjo JA, Schuman CC, Evans JD, Peavy GM, Miller SW, Heaton RK. Norms for letter and category fluency: Demographic corrections for age, education, and ethnicity. Assessment. 1999;6(2):147–178. doi: 10.1177/107319119900600204. [DOI] [PubMed] [Google Scholar]
- Goggin KJ, Zisook S, Heaton RK, Atkinson JH, Marshall S, McCutchan JA, Grant I. Neuropsychological performance of HIV-1 infected men with major depression. HNRC group. HIV neurobehavioral research center. Journal of the International Neuropsychological Society. 1997;3(5):457–464. [PubMed] [Google Scholar]
- Gonzalez JS, Batchelder AW, Psaros C, Safren SA. Depression and HIV/AIDS treatment nonadherence: A review and meta-analysis. Journal of Acquired Immune Deficiency Syndromes. 2011;58(2):181–187. doi: 10.1097/QAI.0b013e31822d490a. doi:10.1097/QAI.0b013e31822d490a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant I, Olshen RA, Atkinson JA, Heaton RK, Nelson J, McCutchan JA, Weinrich JD. Depressed mood does not explain neuropsychological deficits in HIV-infected persons. Neuropsychology. 1993;7(1):53–61. [Google Scholar]
- Hasin DS, Trautman KD, Miele GM, Samet S, Smith M, Endicott J. Psychiatric research interview for substance and mental disorders (PRISM): Reliability for substance abusers. The American Journal of Psychiatry. 1996;153(9):1195–1201. doi: 10.1176/ajp.153.9.1195. [DOI] [PubMed] [Google Scholar]
- Heaton R, Grant I, Matthews CG. Comprehensive norms for an expanded Halstead-Reitan Battery: Demographic corrections, research findings, and clinical applications. Psychological Assessment Resources; Odessa, FL: 1991. [Google Scholar]
- Heaton RK, Clifford DB, Franklin DR, Jr., Woods SP, Ake C, Vaida F, CHARTER Group HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. doi:10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, Leblanc S, HNRC Group HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: Differences in rates, nature, and predictors. Journal of Neurovirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. doi:10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RK, Grant I, Butters N, White DA, Kirson D, Atkinson JH, Ellis RJ. The HNRC 500–neuropsychology of HIV infection at different disease stages. HIV neurobehavioral research center. Journal of the International Neuropsychological Society. 1995;1(3):231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Hesse LM, Greenblatt DJ, von Moltke LL, Court MH. Ritonavir has minimal impact on the pharmacokinetic disposition of a single dose of bupropion administered to human volunteers. Journal of Clinical Pharmacology. 2006;46(5):567–576. doi: 10.1177/0091270006286981. doi:10.1177/0091270006286981. [DOI] [PubMed] [Google Scholar]
- Hinkin CH, van Gorp WG, Satz P, Weisman JD, Thommes J, Buckingham S. Depressed mood and its relationship to neuropsychological test performance in HIV-1 seropositive individuals. Journal of Clinical and Experimental Neuropsychology. 1992;14(2):289–297. doi: 10.1080/01688639208402829. doi:10.1080/01688639208402829. [DOI] [PubMed] [Google Scholar]
- Hogeland GW, Swindells S, McNabb JC, Kashuba AD, yee GC, Lindley CM. Lopinavir/ritonavir reduces bupropion plasma concentrations in healthy subjects. Clinical Pharmacology and Therapeutics. 2007;81(1):69–75. doi: 10.1038/sj.clpt.6100027. doi:10.1038/sj.clpt.6100027. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, HIV Epidemiology Research Study Group Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Long-itudinal analysis from the HIV epidemiology research study. JAMA. 2001;285(11):1466–1474. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, Hinkin CH, van Gorp WG, Castellon SA, Satz P. Depression predicts procedural but not episodic memory in HIV-1 infection. Journal of Clinical and Experimental Neuropsychology. 1998;20(4):529–535. doi: 10.1076/jcen.20.4.529.1473. doi:10.1076/jcen.20.4.529.1473. [DOI] [PubMed] [Google Scholar]
- Kongs SK, Thompson LL, Iverson GL, Heaton RK. WCST-64: Wisconsin Card Sorting Test-64 Card Version Professional Manual. Psychological Assessment Resources, Inc; Odessa, FL: 2000. [Google Scholar]
- Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. Journal of Affective Disorders. 2011 doi: 10.1016/j.jad.2011.10.023. doi:10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neuroscience and Biobehavioral Reviews. 2012;36(2):764–785. doi: 10.1016/j.neubiorev.2011.12.005. doi:10.1016/j.neubiorev.2011.12.005. [DOI] [PubMed] [Google Scholar]
- Mapou RL, Law WA, Martin A, Kampen D, Salazar AM, Rundell JR. Neuropsychological performance, mood, and complaints of cognitive and motor difficulties in individuals infected with the human immunodeficiency virus. The Journal of Neuropsychiatry and Clinical Neurosciences. 1993;5(1):86–93. doi: 10.1176/jnp.5.1.86. [DOI] [PubMed] [Google Scholar]
- Millikin CP, Rourke SB, Halman MH, Power C. Fatigue in HIV/AIDS is associated with depression and subjective neurocognitive complaints but not neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology. 2003;25(2):201–215. doi: 10.1076/jcen.25.2.201.13644. doi:10.1076/jcen.25.2.201.13644. [DOI] [PubMed] [Google Scholar]
- Moore DJ, Arce M, Moseley S, McCutchan JA, Marquie-Beck J, Franklin DR, Charter Group and HNRC Group Family history of dementia predicts worse neuropsycho-logical functioning among HIV-infected persons. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(3):316–323. doi: 10.1176/appi.neuropsych.23.3.316. doi:10.1176/appi.neuropsych.23.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgello S, Estanislao L, Simpson D, Geraci A, DiRocco A, Gerits P, Manhattan HIV Brain Bank HIV-associated distal sensory polyneuropathy in the era of highly active antiretroviral therapy: The manhattan HIV brain bank. Archives of Neurology. 2004;61(4):546–551. doi: 10.1001/archneur.61.4.546. doi:10.1001/archneur. 61.4.546. [DOI] [PubMed] [Google Scholar]
- Morgello S, Holzer CE, III, Ryan E, Young C, Naseer M, Castellon SA, Singer EJ. Interrater reliability of the psychiatric research interview for substance and mental disorders in an HIV-infected cohort: Experience of the national NeuroAIDS tissue consortium. International Journal of Methods in Psychiatric Research. 2006;15(3):131–138. doi: 10.1002/mpr.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabkin JG, Goetz RR, Remien RH, Williams JB, Todak G, Gorman JM. Stability of mood despite HIV illness progression in a group of homosexual men. The American Journal of Psychiatry. 1997;154(2):231–238. doi: 10.1176/ajp.154.2.231. [DOI] [PubMed] [Google Scholar]
- Richardson MA, Satz PF, Myers HF, Miller EN, Bing EG, Fawzy FI, Maj M. Effects of depressed mood versus clinical depression on neuropsychological test performance among African American men impacted by HIV/AIDS. Journal of Clinical and Experimental Neuropsychology. 1999;21(6):769–783. doi: 10.1076/jcen.21.6.769.860. doi:10.1076/jcen.21.6.769.860. [DOI] [PubMed] [Google Scholar]
- Rippeth JD, Heaton RK, Carey CL, Marcotte TD, Moore DJ, Gonzalez R, HNRC Group Methamphetamine dependence increases risk of neuropsychological impairment in HIV infected persons. Journal of the International Neuropsychological Society: JINS. 2004;10(1):1–14. doi: 10.1017/S1355617704101021. doi:10.1017/S1355617704101021. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS (London, England) 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. doi:10.1097/QAD. 0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Robertson KR, Su Z, Margolis DM, Krambrink A, Havlir DV, Evans S, A5170 Study Team Neurocognitive effects of treatment interruption in stable HIV-positive patients in an observational cohort. Neurology. 2010;74(16):1260–1266. doi: 10.1212/WNL.0b013e3181d9ed09. doi:10.1212/WNL.0b013e3181d9ed09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rourke SB, Halman MH, Bassel C. Neurocognitive complaints in HIV-infection and their relationship to depressive symptoms and neuropsychological functioning. Journal of Clinical and Experimental Neuropsychology. 1999;21(6):737–756. doi: 10.1076/jcen.21.6.737.863. doi:10.1076/jcen.21.6.737.863. [DOI] [PubMed] [Google Scholar]
- Ryan EL, Baird R, Mindt MR, Byrd D, Monzones J, Bank SM, Manhattan HIV Brain Bank Neuropsychological impairment in racial/ethnic minorities with HIV infection and low literacy levels: Effects of education and reading level in participant characterization. Journal of the International Neuropsychological Society. 2005;11(7):889–898. doi: 10.1017/S1355617705051040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saarto T, Wiffen PJ. Antidepressants for neuropathic pain. Cochrane Database Syst Rev. 2007;4(4):CD005454. doi: 10.1002/14651858.CD005454.pub2. doi:10.1002/14651858.CD005454.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu SM, Chow DC, Valcour V, Masaki K, Nakamoto B, Kallianpur KJ, Shikuma C. The impact of depressive symptoms on neuropsychological performance tests in HIV-infected individuals: A study of the hawaii aging with HIV cohort. World Journal of AIDS. 2011;1(4):139–145. doi: 10.4236/wja.2011.14020. doi: 10.4236/wja.2011.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroecksnadel K, Sarcletti M, Winkler C, Mumelter B, Weiss G, Fuchs D, Zangerle R. Quality of life and immune activation in patients with HIV-infection. Brain, Behavior, and Immunity. 2008;22(6):881–889. doi: 10.1016/j.bbi.2007.12.011. doi:10.1016/j.bbi.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, Du Pasquier RA. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS (London, England) 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. doi:10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- Stern Y, Marder K, Bell K, Chen J, Dooneief G, Goldstein S, Williams J. Multidisciplinary baseline assessment of homosexual men with and without human immunodeficiency virus infection. III. neurologic and neuropsychological findings. Archives of General Psychiatry. 1991;48(2):131–138. doi: 10.1001/archpsyc.1991.01810260039006. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thames AD, Becker BW, Marcotte TD, Hines LJ, Foley JM, Ramezani A, Hinkin CH. Depression, cognition, and self-appraisal of functional abilities in HIV: An examination of subjective appraisal versus objective performance. The Clinical Neuropsychologist. 2011;25(2):224–243. doi: 10.1080/13854046.2010.539577. doi:10.1080/13854046.2010.539577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Lee MJ, Blenke AA, Rongen GA, Verwey-van Wissen CP, Koopmans PP, Pharo C, Burger DM. Interaction study of the combined use of paroxetine and fosamprenavir-ritonavir in healthy subjects. Antimicrobial Agents and Chemotherapy. 2007;51(11):4098–4104. doi: 10.1128/AAC.01243-06. doi:10.1128/AAC.01243-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gorp WG, Satz P, Hinkin C, Selnes O, Miller EN, McArthur J, Paz D. Metacognition in HIV-1 seropositive asymptomatic individuals: Self-ratings versus objective neuropsychological performance. multicenter AIDS cohort study (MACS) Journal of Clinical and Experimental Neuropsychology. 1991;13(5):812–819. doi: 10.1080/01688639108401091. doi:10.1080/01688639108401091. [DOI] [PubMed] [Google Scholar]
- Verma S, Estanislao L, Mintz L, Simpson D. Controlling neuropathic pain in HIV. Current HIV/AIDS Reports. 2004;1(3):136–141. doi: 10.1007/s11904-004-0020-0. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Backer R, Hefter H, Arendt G. Depression does not influence basal ganglia-mediated psychomotor speed in HIV-1 infection. The Journal of Neuropsychiatry and Clinical Neurosciences. 2001;13(1):88–94. doi: 10.1176/jnp.13.1.88. [DOI] [PubMed] [Google Scholar]
- Warriner EM, Rourke SB, Rourke BP, Rubenstein S, Millikin C, Buchanan L, Gough K. Immune activation and neuropsychiatric symptoms in HIV infection. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(3):321–328. doi: 10.1176/jnp.2010.22.3.321. doi:10.1176/appi.neuropsych.22.3.321. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third edition: Administration and scoring manual. Harcourt Brace; San Antonio, TX: 1997. [Google Scholar]
- Wilkinson G. Wide Range Achievement Test (3rd ed.) administration manual. Wide Range Inc; Wilmington, DE: 1993. [Google Scholar]
- Woods SP, Rippeth JD, Frol AB, Levy JK, Ryan E, Soukup VM, Heaton RK. Interrater reliability of clinical ratings and neurocognitive diagnoses in HIV. Journal of Clinical and Experimental Neuropsychology. 2004;26(6):759–778. doi: 10.1080/13803390490509565. doi:10.1080/13803390490509565. [DOI] [PubMed] [Google Scholar]
- Zanjani F, Saboe K, Oslin D. Age difference in rates of mental health/substance abuse and behavioral care in HIV-positive adults. AIDS Patient Care and STDs. 2007;21(5):347–355. doi: 10.1089/apc.2006.0043. doi:10.1089/apc.2007.0043. [DOI] [PubMed] [Google Scholar]


