Abstract
Although lentiviruses such as human, feline and simian immunodeficiency viruses (HIV, FIV, SIV) rapidly gain access to cerebrospinal fluid (CSF), the mechanisms that control this entry are not well understood. One possibility is that the virus may be carried into the brain by immune cells that traffic across the blood–CSF barrier in the choroid plexus. Since few studies have directly examined macrophage trafficking across the blood–CSF barrier, we established transwell and explant cultures of feline choroid plexus epithelium and measured trafficking in the presence or absence of FIV. Macrophages in co-culture with the epithelium showed significant proliferation and robust trafficking that was dependent on the presence of epithelium. Macrophage migration to the apical surface of the epithelium was particularly robust in the choroid plexus explants where 3-fold increases were seen over the first 24 h. Addition of FIV to the cultures greatly increased the number of surface macrophages without influencing replication. The epithelium in the transwell cultures was also permissive to PBMC trafficking, which increased from 17 to 26% of total cells after exposure to FIV. Thus, the choroid plexus epithelium supports trafficking of both macrophages and PBMCs. FIV significantly enhanced translocation of macrophages and T cells indicating that the choroid plexus epithelium is likely to be an active site of immune cell trafficking in response to infection.
Keywords: HIV, FIV, Monocytes, Tcells, Cerebrospinal fluid, Brain, Blood–brain barrier, Trafficking
Introduction
Following systemic infection, lentiviruses rapidly penetrate into cerebrospinal fluid (CSF) (Bragg et al. 2002b; Lane et al. 1996; Ryan et al. 2003; Sasseville and Lackner 1997; Zink et al. 1999; Liu et al. 2006). The rapid penetration of virus occurs in spite of the presence of tight junctions at the choroid plexus epithelium that form the blood–CSF barrier. While numerous studies support the idea that CSF virus is closely related to virus circulating in the plasma (Bragg et al. 2002b; Eggers et al. 1999; Haas et al. 2003; Ritola et al. 2004; Ryan et al. 2003; Staprans et al. 1999), the precise relationship between these two compartments is still not well understood. Correlations between temporally matched plasma and CSF viral burdens have typically been low or absent (Bossi et al. 1998; Cinque et al. 1998; Ellis et al. 1997; Sei et al. 1996) indicating that the entry of virus into the CSF is not due to a simple passive transfer of virus from the plasma. While much of the virus in CSF is genotypically similar to virus in plasma (Ritola et al. 2004), the identification of a small population of HIV and FIV variants unique to the CSF (Ellis et al. 2000; Ritola et al. 2004; Strain et al. 2005) or brain (Chang et al. 1998; Epstein et al. 1991; Monken et al. 1995; Morris et al. 1999; Smit et al. 2001; Liu et al. 2006) in some people and animals is consistent with a local source of virus production. In addition, it has been suggested that the choroid plexus environment may encourage the evolution of unique HIV quasispecies (Burkala et al. 2005). Thus, evidence favors the idea that trafficking of virus from blood through the choroid plexus to CSF contributes in complex ways to the CSF viral burden. Our understanding of the significance of this pathway is hindered by a lack of knowledge regarding the mechanisms of lentivirus penetration of the blood–CSF barrier.
Mechanisms of virus penetration may include trafficking of macrophages or peripheral blood mononuclear cells (PBMC) across the epithelium, breakdown of the epithelial tight junction barrier, infection of the epithelium itself, or epithelial transport of virus.
Most observations support the idea that the virus is carried into the CSF by trafficking immune cells. The ability of lentiviruses to stimulate T cell trafficking into the CSF (Neuenburg et al. 2004; Shacklett et al. 2004) and to infect monocytes, macrophages and dendritic cells within the choroid plexus (Bragg et al. 2002a; Chen et al. 2000; Hanly and Petito 1998) lend support to the immune cell trafficking hypothesis. Adhesion molecule expression by the choroid plexus epithelium but not the vascular endothelium (Steffen et al. 1996) suggests that the epithelium participates heavily in the regulation of cellular trafficking. Macrophages that normally reside within the choroid plexus stroma could play a particularly important role in the local response to virus and transfer into the CNS due to their strategic location at the blood–CSF interface. Macrophage trafficking across the choroid plexus epithelium has been inferred by the presence of transepithelial and ventricular epiplexus cells (Ling 1981; Ling et al. 1998; Matyszak et al. 1992). However, few studies have directly assessed the extent to which choroid plexus macrophages traffic across the epithelium. To better understand the potential role of trafficking macrophages in normal tissue and in response to infection with feline immunodeficiency virus (FIV), we measured trafficking across feline choroid plexus epithelial cells grown on transwell membranes and macrophage translocation within ex vivo choroid plexus explants.
Materials and methods
Choroid plexus epithelial culture
Choroid plexus was harvested from the brains of cat fetuses ranging in age from approximately E30 to E60. The fetuses were removed from the uteri of cats undergoing spaying through a non-profit spay/neuter program. All procedures were conducted under strict protocols in accordance with NIH guidelines and the North Carolina State University Animal Care and Use Committee. After removal, each uterus was placed on ice. Each fetus was removed from the uterus and rinsed in 70% ethanol. A transection was made through the caudal brainstem, the brain was removed from the skull and the choroid plexus of each lateral ventricle was collected from the cerebral hemispheres and placed in HEPES-buffered Hank’s balanced salt solution (HBSS, pH 7.4) on ice. After washing three times in HBSS and twice in Dulbeccos Modified Eagle Medium (DMEM)+10% fetal bovine serum+20 μg/ml gentamicin (complete medium), the choroid plexus was placed in calcium-magnesium-free HBSS containing 2.4 U/ml dispase and 0.1% collagenase plus 2 U/ml DNase. The tissue was incubated at 37°C for 30–40 min and then triturated by 10 passages through the tip of a 5-ml pipette. Tissue pieces were allowed to settle for 2 min and the cell suspension was transferred to a 50-ml tube containing 20 ml of complete medium. Calcium-magnesium free HBSS (3 ml) was added back to the tissue pieces and the trituration/harvest procedure repeated until all the tissue was dispersed. The dispersed epithelium is unique from any other cell type in the nervous system, characterized by a very large, round cell body approximately 15–20 μm in diameter. Cells were negative for Factor VIII using an antibody that stains feline endothelial cells (1:500; DAKO) and positive for the epithelial marker cytokeratin (PCK-26, 1:300; Novus Biologicals). In initial studies, the resulting cell suspension was centrifuged for 5 min at 140g. Any remaining red blood cells were gently removed from the surface of the pellet leaving a pellet enriched with epithelium. Cells were seeded onto Matrigel®-coated Transwell® membranes (pore size, 8 μm) at a density of 8×105 cells/cm2. After the first day in culture, the epithelium was found to contain 1.2–2.1% associated macrophages. These cultures were used to assess intrinsic macrophage trafficking.
To provide purified epithelial cells free from the intrinsic macrophages, the dispersed cells from the enriched epithelium were centrifuged at 80g for 5 min, re-suspended in 20 ml HBSS and centrifuged again. Epithelial cells were recovered in the pellet and care was taken to avoid any red blood cells that had accumulated adjacent to the epithelium. Cells were then seeded into uncoated flasks in complete medium and incubated for 1 h to allow attachment of the macrophages. The non-adherent epithelial cells were collected and seeded into the transwell inserts. Epithelial cells from this preparation had negligible numbers of macrophages and no significant macrophage trafficking when grown on membranes. Cells were fed three times a week with EpiCM medium from Sciencell, which contained MEM supplemented with 2% fetal bovine serum, epithelial growth supplement and 100 U/ml each of penicillin G and streptomycin. Because these cells did not replicate well in the cultures, they were seeded at a density of 8×105 cells/cm2 to immediately provide a confluent layer. Choroid plexus epithelial cells typically attached loosely to the Matrigel-coated membrane surface within 24 h and it often took 2–3 days for full attachment and the development of a confluent monolayer of epithelium with a cobblestone appearance. Extra epithelial cells generally did not adhere and were washed free from the insert, although occasional clumps of cells were noted. Experiments were performed between 8 and 16 days in culture. Cultures were carefully monitored over time and only cultures with the cobblestone epithelial appearance were used and reported here. While this preparation allowed an analysis of the ability of the epithelium to support trafficking, the direction would represent movement from the basal to apical surface due to the polarization of the cells. Directional trafficking from the basal (in contact with the membrane) to the apical surface was much more difficult to assess. Additional measures were taken to evaluate migration from the basal to the apical surface (see below).
Half the cultures were inoculated with 105 TCID50/ml FIV NCSU1. This dose is roughly equivalent to 5.8×105 virus particles/ml, a value similar to what would be considered a moderate peak plasma viremia in vivo. The insert was then placed in a new well containing the same medium for an additional 24 h.
The density of macrophages associated with the epithelium was measured after the epithelial cells had settled and attached (designated Day 0). On subsequent days, macrophages appeared in both the lower well and on the upper surface of the epithelium. Trafficking of macrophages into the lower well was measured at 0, 1, 8, 9, 15 and 16 days. Macrophages were identified by morphology and confirmed by staining with 2 μg/ml Alexa488-acetylated LDL (green fluorescent marker; Molecular Probes/Invitrogen) or DiI-acetylated LDL (red fluorescent marker; Biomedical Technologies). Macrophages showed robust phagocytic uptake of each dye and, at this concentration, no staining of the epithelium was seen.
Cell counts taken from the bottom well at 8 and 15 days represented the total trafficking of macrophages over sequential 1-week periods. The transwell inserts were moved to new wells on days 8 and 15 and macrophages were counted in the bottom chamber 24 h later, on days 9 and 16, respectively. Measurement of the number migrating over each 24-h period was used for comparison with the migration seen on day 1.
Macrophages also appeared on the surface of the epithelium in increasing numbers and were quantified at days 1, 6 and 7. The upper surface of the epithelium would normally constitute the ventricle side of the choroid plexus (Thomas et al. 1992) and macrophages on this surface should better reflect potential trafficking from the stroma into the ventricle. However, large numbers of macrophages were seen floating in the medium in clusters above the epithelium that could not be quantified. A modified “sandwich” culture and ex vivo explants (see below) were used to obtain better estimates of the number of macrophages migrating from the stroma to the surface of the epithelium.
Choroid plexus “sandwich” cultures
The polarized growth of the epithelium on the Matrigel-coated membranes made it difficult to assess directional trafficking from the basal to the apical surface. To estimate the relative extent of this trafficking, we used an arrangement that sandwiched the macrophages between the epithelium and the membrane, a configuration that places them in a location similar to the stroma of the choroid plexus. The layers from the bottom of the well were: membrane, matrigel, macrophages, matrigel and epithelium. This forces the macrophages to migrate through matrigel and epithelium to appear on the upper surface of the culture. Trafficking toward the epithelium could then be compared to trafficking into the bottom chamber through the matrigel and membrane. Choroid plexus macrophages were seeded onto the Matrigel-coated insert membrane in a 24-well plate format. After 1 h, the macrophage layer was covered with an additional thin layer of matrigel (10 μl/well, approximately 1:1 with complete DMEM). The Matrigel was incubated for 30 min in a humidified tissue culture incubator to allow gelling. Purified choroid plexus epithelium was then seeded on top of the macrophage/matrigel layer (2×105 cells/cm2) and half the cultures were inoculated with 105 TCID50/ml FIV. DiI-acetylated LDL was added to the medium at 2 μg/ml to label the underlying macrophages. The culture was incubated for 24 h to allow epithelial attachment. The epithelium formed large clusters on the surface of all cultures but failed to form a complete monolayer under these conditions. However, labeled macrophages could be seen beneath the epithelium as unfocused red fluorescent spots. Since it was unlikely that the macrophages would move laterally to reach the epithelial surface, we proceeded to measure the appearance of DiI-labeled macrophages on the surface of the epithelium at days 1, 3 and 6 post-inoculation.
Choroid plexus explant cultures
We also reasoned that the movement of macrophages could be tracked in ex vivo choroid plexus. To accomplish this, we harvested lateral ventricle choroid plexus from the fetal brain as described above. The choroid plexus were washed vigorously in HBSS to remove blood, cut into three smaller pieces and washed again twice. The explants were placed onto poly-D-lysine-coated glass bottom 35-mm dishes (Biocoat®; BD) and covered with a thin layer of complete DMEM containing 2 μg/ml DiI-acetylated LDL to label the macrophages. The small explants attached loosely to the coverslip within 24 h and could then be fed with additional medium. To count the macrophages, the surface of the choroid plexus was scanned under phase contrast and three fields were imaged for DiI-labeled cells. Images were taken as close as possible to the surface of the epithelium based on the calcein stain and the areas in the focal plane were traced. The DiI+ macrophages in the traced regions and in the same focal plane were counted. The number of macrophages was divided by the area of the imaged field to determine the relative density of surface macrophages (DiI+ cells/mm2). Pre-inoculation measurements were collected (Day 0) and the explants were infected with FIV as described above. Measurements were taken from the same explants at 1, 3 and 6 days in culture.
Macrophage culture
Macrophages were initially isolated by culturing explants of the choroid plexus on poly-D-lysine coated coverslips in DMEM+10% fetal bovine serum+20 μg/ml gentamicin until sufficient numbers of macrophages migrated from the explants. In subsequent studies, we determined that an enriched preparation of macrophages could be obtained more easily by directly culturing the choroid plexus explants on low adhesion plastic (Costar, Ultra Low Cluster Plate; Corning). Under these circumstances, only the macrophages adhered to the plate and yielded macrophage cultures of high purity after approximately 1–2 weeks. Macrophages used for seeding transwell inserts were harvested from the plate by incubating in calcium-magnesium-free HBSS at 4°C for 20–30 min. The surface of the plate was washed with CMF-HBSS and the cells collected in 15- or 50-ml centrifuge tubes. The suspension was centrifuged at 80g for 5 min. The final pellet was re-suspended at a density of 0.5–1×106 cells/ml and seeded into the transwell insert (upper membrane surface) at the indicated densities.
Isolation and culture of PBMCs
Blood was collected from the external jugular vein of anesthetized specific pathogen free (SPF) cats into tubes containing citrate or EDTA anticoagulant. The blood was centrifuged at 1,500 rpm for 10 min. The plasma was removed and frozen in aliquots at −80°C. Two volumes of phosphate buffered saline were added to the blood cells and 4 ml of the cells were layered on top of a discontinuous Percoll gradient containing 4 ml of 62% Percoll and 4 ml of 45% Percoll in saline. The tube was centrifuged at 400g for 5 min followed by an additional 20 min at 800g. PBMCs were collected from the Percoll interface and diluted 3 times with HBSS. These gradients typically yielded PBMCs with an average of 5.8% monocytes, 30.8% B cells, 30.4% CD4+ T cells and 20.6% CD8+ T cells.
The isolated PBMCs were resuspended at 106 cells/ml and maintained in culture at 37°C, 5% CO2 in RPMI 1640 supplemented with 10% fetal bovine serum, 2 μl 2-β mercaptoethanol, 2 mM L-glutamine, 10 mM Hepes buffer (pH 7.2), 8.93 mM sodium bicarbonate and 20 μg/ml gentamicin. Fresh IL-2 (100 U/ml) was added to the medium at the time of culture and for each subsequent feeding (every 2–3 days).
Adherence and trafficking of PBMCs was assessed in separate experiments. In each case, 105 feline PBMCs freshly isolated from uninfected SPF cats were seeded into the transwell chamber onto the surface of the epithelial cells. The PBMCs were labeled with 5 μM Cell Tracker Orange (Molecular Probes) for 1 h at 37°C. The cells were washed twice in a 10× volume of HBSS and resuspended at a density of 5×105 cells/ml in culture medium composed of one part PBMC medium and one part DMEM supplemented with 10% fetal calf serum. A volume of 200 μl was added to both upper and lower chambers. Analysis of the final PBMC addition indicated that 7×104 cells were actually added to the epithelium. PBMCs were added to the epithelial culture 1 day after inoculation with 105 TCID50/ml FIV. The culture insert was washed and freshly isolated PBMCs were added in a 1:1 mix of the epithelial medium with PBMC medium. After 24 h, the inset chamber was washed twice with HBSS and the number of fluorescent cells (Cell Tracker Orange) adhering to the epithelial cell surface was counted with the aid of Metamorph® Imaging Software (Molecular Devices). Trafficking of PBMCs was assessed using the same protocol except that the number of fluorescent PBMCs in the lower chamber was counted. For subsequent immunostaining, the PBMCs in the lower well were transferred to coated glass slides.
Immunostaining for ZO-1 and occludin
The staining procedure for ZO-1 and Occludin was modified from Hakvoort et al. (1998). Confluent epithelium samples on matrigel inserts were fixed with 97% methanol/3% acetic acid (vol/vol) for 10 min at −20°C. Samples were washed three times with 0.1 M PBS (pH 7.4) and incubated in 3% bovine serum albumin/PBS solution for 1 h at room temperature. Primary antibody (10 μg/ml rat monoclonal anti-ZO-1, Chem-icon International/Millipore and/or 4 μg/ml goat anti-occludin, Santa Cruz) in 0.5% BSA/PBS was applied to the cells and incubated at 4°C for 24–72 h. Cells were washed three times in 0.1 M PBS and incubated with 4 μg/ml anti-rat IgG-FITC (Santa Cruz Biotechnology) or biotinylated anti-goat IgG followed by 2 μg/ml streptavidin Alexa568 (Molecular Probes) in PBS for 1 h at room temperature. Cells were washed three times and membranes were cut from the wells and mounted onto glass slides under a coverslip using Fluoromount-G (Southern Biotech). A similar protocol was used to stain for Factor VIII, which we have previously used to identify cultured feline endothelial cells (Hudson et al. 2005). The epithelium was negative for Factor VIII indicating little contamination from endothelial cells.
Albumin permeability assay
Confluent choroid plexus epithelium grown in a transwell insert was placed in serum-free MEM for 3 days prior to permeability analysis to eliminate serum proteins. On the third day, medium was removed and replaced with 250 μl serum-free MEM containing 1% bovine serum albumin (BSA). A volume of 250 μl serum-free MEM (not containing BSA) was also placed in the bottom well, below the membrane. Inserts containing a monolayer of non-epithelial cells (ZO-1 and occludin negative, fibroblast-like cells) and inserts with no cells were run to determine BSA permeability across cells without tight junctions and through the Matrigel-coated membrane, respectively. A 10-μl sample was taken from the bottom of each well at 1, 2, 4, 6 and 24 h after initial application of BSA, added to 90 μl sterile ultrapure H2O and frozen to −80°C for subsequent BCA assay (Pierce Biotechnology). Protein concentration in the lower well was determined, corrected for endogenous protein/medium background and plotted versus time to provide an indication of the rate of BSA penetration across each insert preparation.
Identification of CD3+ T cells and Mac387+ monocytes
PBMCs in the lower well of the transwell setup were harvested in a volume of 250 μl and 125 μl transferred to each of two coated slides. The cells on one slide were stained with an anti-feline CD3 antibody and the other slide was stained with an antibody that recognized feline monocytes (Mac387). Briefly, cells on the slide were re-hydrated for 60 min and washed once with 0.01 M PBS, pH 7.4. After incubating 60 min in blocking solution (3% normal goat serum in 0.01 M PBS), Mac387 antibody (1:100; DAKO) or anti-CD3 antibody (1:50; DAKO) was added in the blocking solution and incubated overnight at 4°C. Cells were washed 3×5 min in PBS and incubated in Biotinylated Universal Antibody (Vectastain ABC kit) at room temperature for 1 h. Cells were washed 2×5 min in PBS, once in 0.05 M Tris buffered saline and incubated in enhanced diaminobenzidine (DAB)/H2O2 according to the manufacturer’s recommendations (Pierce). The slides were rinsed in PBS, dehydrated and coverslipped. Stained cells were identified by a very dark brown/black stain. Unstained cells were clear.
Statistics
Comparisons were made by t-test or one-way analysis of variance using Graphpad Prism® statistical software. In one case, results from different runs were not normally distributed and a Kruskal–Wallis analysis of variance was used with Dunn’s multiple comparison test. A p value of ≤0.05 was considered to be significant.
Results
Macrophage trafficking across the choroid plexus epithelium
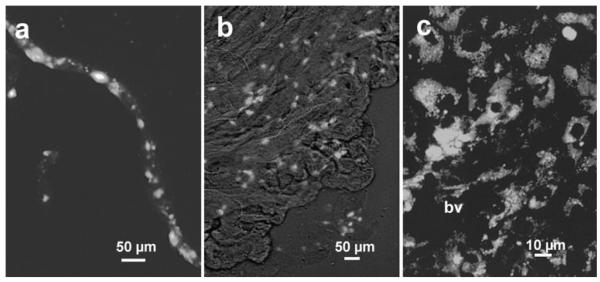
In situ, the choroid plexus contains numerous macrophages within the stroma between the vasculature and the cuboidal epithelium. After staining, a choroid plexus explant with 2 μM DiI acyl-LDL, phagocytic macrophages could be identified lining the vessels penetrating the choroid plexus (Fig. 1a) and throughout the stroma (Fig. 1b). At high magnification (Fig. 1c), dense collections of macrophages are often seen adjacent to blood vessels (bv) within the stroma. During the purification of the choroid plexus epithelium (Fig. 2a), these macrophages were either isolated by differential adhesion to uncoated culture plastic or were allowed to remain with the epithelium to follow the trafficking of the native macrophage population. In each case, the final preparation of epithelial cells was, on average, purified to >98%. Figure 2b shows the epithelial monolayer in cultures of purified epithelium that contained negligible macrophages. Partially purified epithelium contained a small population of macrophages (1.2–2.1% of the epithelium cell numbers). Epithelial cells expressed tight junctions as indicated by staining with an anti-occludin antibody (Fig. 2c). The same cells were also positive for ZO-1 (not shown). The purified epithelial monolayers completely inhibited the passage of bovine serum albumin from 0 to 6 h and after 24 h, the passage of the albumin was restricted to 5.5% of the diffusion seen in blank wells and 7.2% of that seen in cultures with nonepithelial cells (Fig. 3).
Fig. 1.
Macrophages within the choroid plexus. Fresh cultured explants of feline choroid plexus were incubated in 2 μg/ml DiI-acetylated LDL to label macrophages. a Fluorescent macrophages were abundant along the vessels penetrating into the choroid plexus. An isolated vessel is shown with associated macrophages. b Low magnification view of the choroid plexus in culture stretched flat to illustrate the widespread localization of macrophages. Fluorescent macrophages appear as bright objects against the tissue imaged with Hoffmann modulation contrast. c High magnification image illustrating the dense accumulation of macrophages in the choroid plexus stroma surrounding a blood vessel (bv) penetrating from the lower left
Fig. 2.

Isolation of choroid plexus epithelium. a Following enzymatic treatment and trituration, the epithelial cells were distinguished as large round cells approximately 20 μm in diameter (arrow). The figure illustates the dissociated epithelium just prior to the final purification. A few contaminating red blood cells and small unidentified cells are present but are largely eliminated in the final preparation. Inset Isolated epithelial cell after attachment to substrate stained for the epithelial marker cytokeratin (anti-cytokearatin with Alexa594 secondary antibody). b After purification of the epithelial cells and culture on Matrigel-coated membrane inserts, the epithelium forms a monolayer of cells with a distinctive “cobblestone” appearance under Hoffmann modulation contrast. Pores within the underlying membrane that allow trafficking of immune cells can be seen as unfocused bright spots. c Epithelium stained with an antibody to occludin and Alexa568 illustrating the presence of tight junctions between the epithelial cells. Inset Cells grown on a Matrigel-coated insert showed bright fluorescent immunostaining (Alexa594) for the epithelial marker cytokeratin
Fig. 3.

Penetration of BSA from the upper chamber of a transwell insert across the epithelium into the lower chamber. Total BSA content within the lower chamber is expressed as the percentage of the BSA in the upper chamber at various intervals from 1 to 24 h. Thus, a value of 100% represents total equilibration between the upper and lower chambers. BSA rapidly equilibrated across the Matrigel-coated membrane (No cells, n=4), slowly equilibrated across a layer of fibroblast-like non-epithelial cells (Non-epithelium, n=6) and showed negligible (<5%) penetration across the epithelial barrier (Epithelium n=6). The fibroblast-like cells were derived from cultures where the epithelium failed to attach and grow. Values fluctuated around zero, often with values less than matched control medium. Values represent the mean ± SEM
Macrophages associated with the epithelium showed a robust migration through the transwell insert as well as to the surface of the epithelium. The number of macrophages transmigrating into the lower chamber within a 24-h period at 1, 9 and 16 days in culture is illustrated in Fig. 4a. Significant increases in the number of macrophages entering the lower chamber over a 24-h period were seen at 9 and 16 days in culture relative to day 1. Macrophages that collected in the bottom well over the intervening intervals were also counted to give an indication of the total trafficking over time. By 16 days, the mean cumulative number of macrophages transmigrating into the bottom chamber was 20,461. This amount was 6.9 times the number initially present on the membrane (2,950±269 macrophages stained with DiI-acetylated-LDL). The number of macrophages on the surface of the epithelium also increased significantly by 2.8-fold at day 7 (Fig. 4b) and 5-fold (14,706±1,582 macrophages) by day 16 (not shown). An estimate of the rate of accumulation from the total epithelial + cumulative transmigration relative to day 1 was an average increase of 74% per day.
Fig. 4.
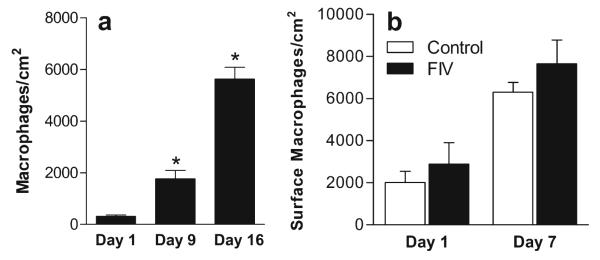
a Macrophage trafficking across the epithelial layer and the insert membrane into the lower well at day 1 (n=18), day 9 (n=9) and day 16 (n=18) in culture. Each bar represents the mean number of macrophages (± SEM) that accumulated in the lower well over a period of 24 h at the indicated day in culture. Significant increases in macrophage trafficking were seen over time. *p<0.0001 relative to trafficking on day 1. b Macrophages that accumulated on the surface of the epithelium on the transwell membrane at day 1 and day 7. On each day, a small increase was seen in the presence of FIV although the increase did not reach significance. Bars illustrate the mean ± SEM
The specific ability of the epithelial cells to support trans-migration was determined by counting the number of isolated choroid plexus macrophages that migrated across the trans-well chamber in the absence of epithelium. The percentage of macrophages migrating across the transwell membrane over a 24-h period was calculated from day 0 to day 1, day 8 to day 9 and day 15 to day 16 in culture and compared to migration in the presence of epithelium (Fig. 5). In the absence of epithelium, the migration of macrophages across the membrane at 1, 9 and 16 days in culture was 0.35, 0.57 and 1.91% of the total macrophages versus 3.93, 4.56 and 14.15%, respectively, in the presence of epithelium. Thus, the presence of the epithelium enhanced the macrophage transmigration by 7.4- to 11.3-fold. Since the percentages were based on the number of macrophages present at each time point, these estimates were not biased by any accumulation of macrophages over time.
Fig. 5.
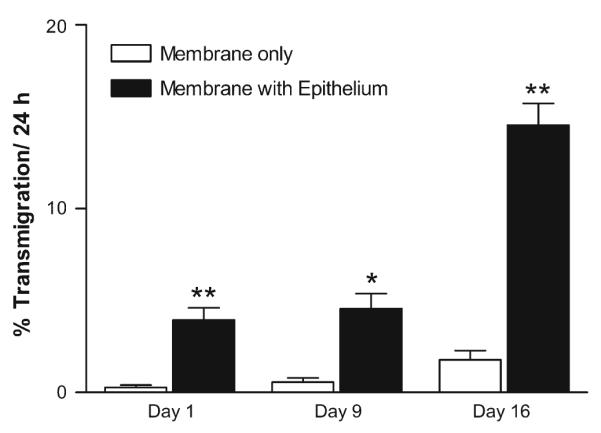
Choroid plexus epithelium facilitates trafficking of macrophages. The transmigration of macrophages across an epithelial monolayer on the insert membrane (membrane with epithelium) was contrasted with the transmigration of choroid plexus macrophages across the cell-free membrane (membrane only). Macrophages that migrated into the lower well over a 24-h period at the designated times in culture were normalized to the total number of macrophages seeded into the upper well to provide an indication of the relative rate of trafficking. Macrophages in the lower well are, therefore, expressed as the percentage of macrophages in the upper well. Significantly greater trafficking was consistently seen in the presence of epithelium (n=5, each condition). Bars mean ± SEM. *p=0.002, **p<0.0001 epithelium versus cell-free membrane
Macrophage and PBMC trafficking in the presence of FIV
Inoculation of the epithelial culture with FIV at 16 days in culture failed to induce an increase in trafficking of the macrophages into the lower chamber with an average of 4,603±912 macrophages/cm2 counted in the lower well versus 4,971±1,060 macrophages/cm2 in the absence of FIV. A small increase (43%) in the number of macrophages was seen on the surface of the epithelium, which did not reach significance. However, clusters of macrophages were noted floating in the medium above the epithelium that could not be quantified. This observation suggested that the macrophages may move to the epithelial surface and detach.
To better appreciate the directional trafficking of macrophages from the basal to apical surface, cultures were prepared in which the macrophages were sandwiched in a Matrigel layer between the membrane and the epithelium. To reach the surface of the epithelium, the macrophages must migrate through the Matrigel and epithelium. Macrophages on the upper (apical) surface of epithelial cell clusters were measured pre-inoculation (Day 0) and 1, 3 and 6 days after inoculation with 105 TCID50 FIV (Fig. 6a). A large 16.8–20.0-fold increase in the number of surface macrophages was seen on Day 1 (p<0.001) The relative rate of accumulation of surface macrophages decreased on days 3 (not shown) and 6 but remained 6.8–7.5-fold greater than the macrophages at day 0 (all p≤0.01). In these cultures, inoculation with FIV induced a significant increase in the migration of macrophages to the surface of the epithelium (p=0.045). The acceleration of trafficking by FIV was restricted to the first day post-inoculation as subsequent rates of accumulation were similar between FIV and control explants. A negligible number of macrophages appeared within the bottom well on day 1 indicating that the macrophage migration was highly directional. By day 6, numerous macrophages were seen in the bottom well with greater numbers in the control wells. A comparison of the relative number of macrophages on the epithelial surface versus the bottom well, shown in Fig. 6b, verified that 19.3±6.7% of the total macrophages were on the epithelium surface in the control cultures versus 44.1±8.0% in the FIV-inoculated cultures (p=0.044), again indicating preferred trafficking to the epithelial surface in the presence of FIV.
Fig. 6.

Effect of feline immunodeficiency virus (FIV) on the trafficking of macrophages that were “sandwiched” in an artificial stroma between the membrane and the basal surface of the epithelium. a. Macrophages began translocating to the surface of the epithelium where they could be visualized over the first day. Inoculation of the culture with FIV significantly increased the number of macrophages on the epithelial surface at Day 1 (p=0.0452) post-inoculation. b. Comparison of the relative number of macrophages on the epithelial surface (basal to apical migration) versus the bottom well at day 6 indicated a selective migration toward the apical surface of the epithelium in the presence of FIV (44.1% FIV vs. 19.3% Control, p=0.0445). Bars mean±SEM (n=5 controls, 6 FIV). *p<0.05 for FIV relative to temporal control
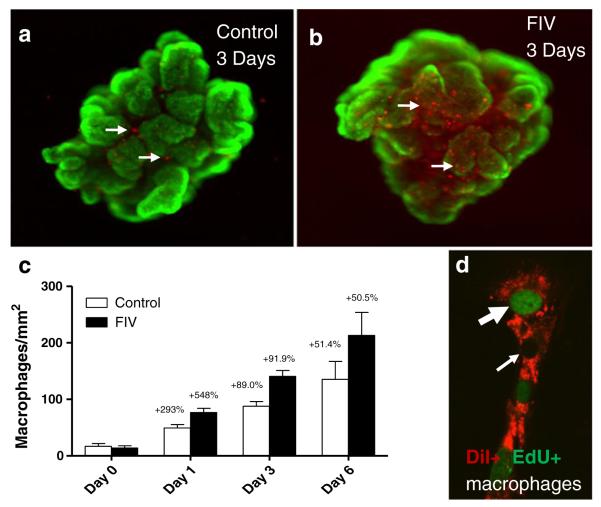
To further evaluate the natural movement of macrophages, choroid plexus explants were cultured and labeled with DiI-acetylated LDL. DiI was rapidly taken up by the macrophages and an initial measurement of the density on the epithelial surface was taken (Day 0). After 24 h, half the cultures were inoculated with 105 TCID50/ml FIV. The robust increase in macrophages on the surface of the epithelium over time is illustrated in Fig. 7. DiI-labeled macrophages (red) on the surface of the epithelium are illustrated in Fig. 7a, b at 3 days post-inoculation. The epithelium is stained with the live cell cytosolic marker calcein to demonstrate the viability of these cells and to facilitate visualization of the epithelial cells. The FIV-induced increase in macrophages (Fig. 7b) was typically quite large relative to controls (Fig. 7a) and often occurred in clusters. On day 1 post-inoculation, an average 2.9-fold increase in the number of macrophages was seen on the surface of the epithelium in the control cultures (Fig. 7c). A significantly greater increase of 5.5-fold (p=0.0106 vs. day 1 control) was seen in the FIV-inoculated cultures (Fig. 7c). The number of surface macrophages continued to increase at day 3 and day 6. However, the relative rate of increase (% per day) remained relatively constant at 50–61% per day for both conditions indicating that the effect of FIV on macrophage migration was transient. Since the large increase in macrophages also indicated active replication, we added 5-ethynyl-2′-deoxyuridine (EdU) to cultures from day 0 to day 1 and stained with the Click-iT® kit (Invitrogen) to determine how much of the FIV-induced increase in surface macrophages might be due to replicating cells. Newly replicated macrophages (Figure 7d), double labeled for DiI-acetylated LDL (red) and EdU (green nucleus, large arrow), were counted and compared to the total DiI+ macrophage population. An average of 5.98±2.37 EdU+ macrophages per mm2 was seen in control explants and 5.54±2.74 in the FIV-treated explants. This accounted for 24.7±9.8 and 14.0±7.0% of the DiI+ macrophages on the surface of the epithelium in the control and FIV cultures, respectively. Therefore, differential cell replication could not account for the large FIV-induced increases in macrophages on the surface of the epithelium.
Fig. 7.
Translocation of macrophages to the surface of ex vivo choroid plexus explants in the presence or absence of FIV. Fetal choroid plexus explants were placed into culture and inoculated with FIV. Macrophages were labed with DiI acylLDL (red) and viability of the epithelium was verified with calcein AM (green). a Macrophages visible on the apical (CSF) surface of the epithelium were low on the first day of culture and gradually accumulated. At low magnification, a low density of red fluorescent macrophages (arrows) is seen on an untreated control explant at 3 days in culture. b Three days after inoculation with FIV, a large accumulation of macrophages was often seen on the surface (arrows). c Quantification of the average macrophage density (macrophages/mm2) at days 0, 1, 3 and 6 in culture shows the progressive accumulation in both control and FIV cultures. At day 1, the increase was significantly greater in the FIV-inoculated explants (p=0.0106, n=12–13 explants from 3 runs). After day 1, the rate of increase did not differ in the control and FIV explants. Numbers above each bar represent the percent daily increase for each interval. d Three cultures were incubated with EdU from day 0 to day 1 post-inoculation to label dividing cells (fluorescent green nucleus). Three adjacent macrophages are illustrated. Most DiI-labeled macrophages (red fluorescence) were negative for EdU (small arrow, unstained nucleus) and 9–18% were positive (large arrow, green nucleus)
Trafficking of PBMCs across the epithelium was also examined, although these studies were restricted to an examination of apical to basal trafficking across the epithelial monolayer. The trafficking of PBMCs was measured 1 day after inoculation of purified epithelial cultures with FIV. PBMCs rapidly penetrated the epithelial and transwell barriers with an average of 12,111±2,104 cells counted in the lower well 24 h after seeding. This accounted for 17.3% of the total number of PBMCs added. The number of PBMCs migrating across the epithelium after exposure to FIV (26,051±2,433) was significantly enhanced (37% of total added) compared to uninoculated controls (p<0.05). PBMCs in the lower well were stained with antibodies that recognized CD3+ T cells or Mac387+ monocytes. Although more CD3+ T cells crossed versus monocytes, a comparison of the relative trafficking after accounting for the estimated numbers of T cells and monocytes in the PBMC mix indicated that 14.9% of the available monocytes crossed the barrier versus 3.5% of the T cells. However, in spite of this preference, the increases in the number of cells trafficking seen after inoculation with FIV largely reflected an increase in T cells. On average, CD3+ T cells accounted for 93.0±6.0% of the total increase in PBMC trafficking induced by FIV (t=15.59, p<0.001).
Discussion
Macrophage trafficking through the choroid plexus epithelium
Little is currently known about the function of the macrophage population within the stroma of the choroid plexus. One possibility is that these cells may represent a systemic immune “barrier” to prevent pathogens from entering the brain ventricular system. Choroid plexus cells have been shown to express a variety of monocytic and dendritic cell markers (MHC-II, CD11b, CD14, CD68, CD32, IL-10) (Serot et al. 2000) and can present foreign antigen to T cells (Nathanson and Chun 1989). Other studies have suggested that macrophages cross the epithelium in vivo and reside within the cerebral ventricles on the surface of the choroid plexus and ependyma (Ling 1981; Ling et al. 1998; Matyszak et al. 1992). While these latter observations suggested that the macrophages traffic from the choroid plexus into the ventricular system, the extent of macrophage trafficking via this route could not be assessed. To begin to assess trafficking across the choroid plexus epithelium, we established cultures of feline choroid plexus epithelium on Matrigel-coated transwell membranes based, in part, on previous studies (Hakvoort et al. 1998; Zheng et al. 1998; Thomas et al. 1992). The use of feline cells allowed the opportunity to examine the effects of FIV on trafficking across this barrier. Data generated in these experiments pave the way for more detailed studies of the effects of FIV on the epithelial barrier. However, these studies were difficult and limited by the availability of antibodies that cross-react with feline antigens. Thus, model systems in other species using a similar paradigm may be more suitable for studies of the epithelial barrier function where feline tissue is not a requirement (Hakvoort et al. 1998; Zheng et al. 1998; Thomas et al. 1992).
The current results indicate that the choroid plexus epithelium supports significant trafficking of macrophages as well as of T cells and monocytes. The results also indicate that the epithelium may differentially regulate trafficking of different cell subsets. Although the results indicated that macrophages could move in either direction across the epithelium, the presence of FIV shifted the direction of macrophage trafficking toward the apical surface (CSF side) of the epithelium.
The importance of the epithelium was demonstrated by showing that macrophage trafficking was much more dynamic in the presence of epithelium relative to the Matrigel-coated membrane alone. In the absence of epithelium, macrophages migrated across the transwell membrane in relatively small numbers, representing 0.35–1.91% of the total macrophages in the upper chamber after 24 h. In the presence of epithelium, macrophage trafficking into the lower chamber was increased by 7–11-fold. While these initial experiments demonstrated that the epithelium was highly permissive to macrophage trafficking, it was difficult to assess directional trafficking from the basal (stroma) to the apical (CSF space) surface, which would reflect the natural route across the blood–CSF barrier. Increased macrophages were seen at the surface of the epithelium in the transwell chambers but clusters of macrophages were noted in the medium above the epithelium suggesting that many had migrated into the medium. Two different models were used to better assess the translocation of macrophages to the apical surface of the epithelium. In the sandwich cultures, choroid plexus macrophages were grown, harvested and seeded onto the Matrigel-coated membrane. They were covered with additional Matrigel and then with the purified epithelial cells. These macrophages readily trafficked to the epithelial surface, although numbers were low in this paradigm. Increased migration in response to FIV in the “sandwich” explant cultures indicated that FIV increased the extent of trafficking. The disproportionate numbers of macrophages on the surface of the epithelium versus the lower well or plate also suggested that the trafficking was preferrentially directed to the apical surface in spite of the additional barriers.
The movement of macrophages to the surface of the epithelium was confirmed in ex vivo explant cultures where a dramatic accumulation of macropahages on the surface of the choroid plexus was seen. Enhancement of transmigration by FIV was again observed but limited to the first day post-inoculation. The 8-fold increase in surface macrophages over 6 days in the control explants also reinforces the idea that trafficking is normally very dynamic. The absence of differential EdU labeling of the FIV-treated versus control macrophages also indicated that the FIV-induced increase was not due to accelerated replication. Together, these observations illustrate the potential for robust movement of macrophages into the cerebral ventricles in response to the virus.
Trafficking of the macrophages may be enhanced significantly by replication in the setting of the choroid plexus. In each experiment, a large increase in total numbers of macrophages was seen. The number of macrophages associated with the surface of the epithelium increased 5-fold (2,950 to 14,706) over a period of 15 days in culture. The estimated rate of expansion of 74% in the epithelial cultures and 50–61% in the explants greatly exceeded replication rates of 3.4% per day previously measured in enriched cultures of feline choroid plexus macrophages in the absence of epithelium (Bragg et al. 2002b). While this may enhance the numbers of macrophages available, it did not account for the FIV-induced increase in trafficking. Large relative increases in macrophage trafficking were seen in response to FIV even when normalized to the total numbers in the upper chamber. Combined, these results indicate that the choroid plexus is poised to provide large numbers of macrophages for migration into the ventricular system.
Efficient macrophage trafficking across the choroid plexus in vivo is supported by studies showing the rapid appearance of systemically administered fluorescent dye in ventricular epiplexus cells (Lu et al. 1993) and the constitutive expression of adhesion molecules on the choroid plexus epithelium (Steffen et al. 1996). In addition, the ability to up-regulate the adhesion molecules ICAM-1, VCAM-1 and MadCAM-1 in the choroid plexus in response to cytokines or after induction of experimental autoimmune encephalitis suggests that trafficking across the epithelium is subject to positive regulation in vivo (Steffen et al. 1996; Wolburg et al. 1999).
Because of their location in advance of reaching the blood–CSF barrier, the choroid plexus macrophages also represent excellent targets for lentiviral infection. Previous studies have documented infection of the choroid plexus in HIV-infected humans (Falangola et al. 1995; Petito et al. 1999), SIV-infected macaques (Czub et al. 1996; Lackner et al. 1991) and FIV-infected cats (Bragg et al. 2002a). In vitro studies of the kinetics of FIV infection of choroid plexus macrophages have shown that, although these cells are infected, they produce very low or undetectable levels of FIV RNA (Bragg et al. 2002a). However, addition of a feline CD4+ T cell line resulted in a robust infection of the T cells indicating that infectious virus could easily be transferred to permissive cells (Bragg et al. 2002a). Transmigration of Tcells into the choroid plexus is an early event following FIV infection of cats. Ryan et al. (2005) showed prominent T cell infiltration of the choroid plexus at the earliest time point examined at 4 weeks post-infection. Thus, conditions are favorable for the efficient transfer of infectious virus within the choroid plexus. The possibility of virus transfer and trafficking at the choroid plexus is consistent with the relationship between CSF HIV load and accumulation of leukocytes in the CSF (Bossi et al. 1998; Morris et al. 1998), the observation that CSF contains a higher proportion of infected T cells than blood (Neuenburg et al. 2004) and T cell tropic activity of CSF from HIV-infected patients (Kolb et al. 1999; Shacklett et al. 2004). The potential of the choroid plexus pathway is particularly significant since previous studies have shown that the choroid plexus is a rich source of HIV variants (Burkala et al. 2005; Chen et al. 2000) that could seed the brain with unique strains. Once macrophages enter the ventricular compartment, their fate and functions are not well understood. They may interact with periventricular cells to promote CNS infection, traffic into the parenchyma, or rapidly exit the brain compartment carrying virus or viral antigens from the CSF into the lymph nodes (Boulton et al. 1999; Gordon et al. 1992; Stevenson et al. 1997). The latter possibility is reinforced by the demonstration of rapid systemic infection after injection of FIV into the CSF (Liu et al. 2006). Studies specifically designed to assess the functions of ventricular macrophages should provide greater insight into the potential role of these cells in pathogenesis.
In our studies, PBMCs seeded onto purified epithelial cultures transmigrated across the epithelial barrier with relative ease. This demonstrated that the epithelium is highly permissive to trafficking, although the interpretation is limited since trafficking from the basal to the apical surface could not be assessed. It is notable, however, that trafficking across the epithelium may be bi-directional, allowing potential communication of circulating ventricular macrophages or T cells with immune cells of the stroma. Penetration of Tcells into the CSF in humans is well documented. The potential role of the choroid plexus is reinforced by the demonstration of early T cell trafficking into the choroid plexus of FIV-infected cats (Ryan et al. 2005). Efficient trafficking of lymphocytes across an in vitro feline blood–brain barrier has also been demonstrated (Fletcher et al. 2009; Hudson et al. 2005) but the contribution to CSF T cells is not known. T cells harvested from the brains of HIV-infected individuals tend to express the chemokine receptors CXCR3 and CCR5. Since these co-receptors are more typically expressed on monocytic cells (Shacklett et al. 2004), the T cells that enter the brain compartment may somehow facilitate infectious interactions between T cells and macrophages/microglia. Regulation of T cell trafficking may precede detectable changes in the peripheral T cell populations and tends to favor penetration of CD8+ T cells (Elovaara and Muller 1993). Indeed, in FIV-infected cats, CD8+ T cells are prominent within the choroid plexus by 10 weeks (Ryan et al. 2005). The kinetics of HIV clearance from the CSF is consistent with the possibility that Tcells are a significant source of virus (Eggers et al. 1999; Haas et al. 2003). The choroid plexus provides an environment where T cells and macrophages can interact, setting the stage for many of the observed changes in the CSF virus. Cellular trafficking through the choroid plexus has numerous implications for HIV neuropathogenesis and more studies are needed to clarify the functional impact of cells at this interface.
Acknowledgement
This work was supported by NIH Grant MH 063646.
Contributor Information
Rick B. Meeker, Department of Neurology and Curriculum in Neurobiology, University of North Carolina, CB #7025, 6109F Neuroscience Research Building 103 Mason Farm Road, Chapel Hill, NC 27599, USA
D. C. Bragg, Department of Neurology and Curriculum in Neurobiology, University of North Carolina, CB #7025, 6109F Neuroscience Research Building 103 Mason Farm Road, Chapel Hill, NC 27599, USA
Winona Poulton, Department of Neurology and Curriculum in Neurobiology, University of North Carolina, CB #7025, 6109F Neuroscience Research Building 103 Mason Farm Road, Chapel Hill, NC 27599, USA.
Lola Hudson, Department of Molecular Biomedical Sciences, College of Veterinary Medicine, North Carolina State University, Raleigh, NC 27607, USA.
References
- Bossi P, Dupin N, Coutellier A, Bricaire F, Lubetzki C, Katlama C, Calvez V. The level of human immunodeficiency virus (HIV) type 1 RNA in cerebrospinal fluid as a marker of HIV encephalitis. Clin Infect Dis. 1998;26:1072–1073. doi: 10.1086/520301. [DOI] [PubMed] [Google Scholar]
- Boulton M, Flessner M, Armstrong D, Mohamed R, Hay J, Johnston M. Contribution of extracranial lymphatics and arachnoid villi to the clearance of a CSF tracer in the rat. Am J Physiol. 1999;276:R818–R823. doi: 10.1152/ajpregu.1999.276.3.R818. [DOI] [PubMed] [Google Scholar]
- Bragg D, Childers T, Tompkins M, Tompkins W, Meeker R. Infection of the choroid plexus by feline immunodeficiency virus. J Neurovirol. 2002a;8:211–224. doi: 10.1080/13550280290049688. [DOI] [PubMed] [Google Scholar]
- Bragg D, Hudson L, Liang Y, Tompkins M, Fernandes A, Meeker R. Choroid plexus macrophages proliferate and release toxic factors in response to feline immunodeficiency virus. J Neurovirol. 2002b;8:225–239. doi: 10.1080/13550280290049679. [DOI] [PubMed] [Google Scholar]
- Burkala EJ, He J, West JT, Wood C, Petito CK. Compartmentalization of HIV-1 in the central nervous system: role of the choroid plexus. AIDS. 2005;19:675–684. doi: 10.1097/01.aids.0000166090.31693.aa. [DOI] [PubMed] [Google Scholar]
- Chang J, Jozwiak R, Wang B, Ng T, Ge YC, Bolton W, Dwyer DE, Randle C, Osborn R, Cunningham AL, Saksena NK. Unique HIV type 1 V3 region sequences derived from six different regions of brain: region-specific evolution within host-determined quasispecies. AIDS Res Hum Retroviruses. 1998;14:25–30. doi: 10.1089/aid.1998.14.25. [DOI] [PubMed] [Google Scholar]
- Chen H, Wood C, Petito CK. Comparisons of HIV-1 viral sequences in brain, choroid plexus and spleen: Potential role of choroid plexus in the pathogenesis of HIVencephalitis. J Neurovirol. 2000;6:498–506. doi: 10.3109/13550280009091950. [DOI] [PubMed] [Google Scholar]
- Cinque P, Vago L, Ceresa D, Mainini F, Terreni MR, Vagani A, Torri W, Bossolasco S, Lazzarin A. Cerebrospinal fluid HIV-1 RNA levels: correlation with HIV encephalitis. AIDS. 1998;12:389–394. doi: 10.1097/00002030-199804000-00007. [DOI] [PubMed] [Google Scholar]
- Czub S, Muller JG, Czub M, Muller-Hermelink HK. Impact of various simian immunodeficiency virus variants on induction and nature of neuropathology in macaques. Res Virol. 1996;147:165–170. doi: 10.1016/0923-2516(96)80231-7. [DOI] [PubMed] [Google Scholar]
- Eggers CC, van Lunzen J, Buhk T, Stellbrink HJ. HIV infection of the central nervous system is characterized by rapid turnover of viral RNA in cerebrospinal fluid. J Acquir Immune Defic Syndr Hum Retrovirol. 1999;20:259–264. doi: 10.1097/00042560-199903010-00007. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Hsia K, Spector SA, Nelson JA, Heaton RK, Wallace MR, Abramson I, Atkinson JH, Grant I, McCutchan JA. Cerebrospinal fluid human immunodeficiency virus type 1 RNA levels are elevated in neurocognitively impaired individuals with acquired immunodeficiency syndrome. HIV Neurobehavioral Research Center Group. Ann Neurol. 1997;42:679–688. doi: 10.1002/ana.410420503. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Gamst AC, Capparelli E, Spector SA, Hsia K, Wolfson T, Abramson I, Grant I, McCutchan JA. Cerebrospinal fluid HIV RNA originates from both local CNS and systemic sources. Neurology. 2000;54:927–936. doi: 10.1212/wnl.54.4.927. [DOI] [PubMed] [Google Scholar]
- Elovaara I, Muller KM. Cytoimmunological abnormalities in cerebrospinal fluid in early stages of HIV-1 infection often precede changes in blood. J Neuroimmunol. 1993;44:199–204. doi: 10.1016/0165-5728(93)90043-x. [DOI] [PubMed] [Google Scholar]
- Epstein LG, Kuiken C, Blumberg BM, Hartman S, Sharer LR, Clement M, Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissuespecific evolution within host-determined quasispecies. Virology. 1991;180:583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Falangola MF, Hanly A, Galvao-Castro B, Petito CK. HIV infection of human choroid plexus: a possible mechanism of viral entry into the CNS. J Neuropathol Exp Sci. 1995;54:497–503. doi: 10.1097/00005072-199507000-00003. [DOI] [PubMed] [Google Scholar]
- Fletcher NF, Bexiga MG, Brayden DJ, Brankin B, Willett BJ, Hosie MJ, Jacque JM, Callanan JJ. Lymphocyte migration through the blood-brain barrier (BBB) in feline immunodeficiency virus infection is significantly influenced by the pre-existence of virus and tumour necrosis factor (TNF)-alpha within the central nervous system (CNS): studies using an in vitro feline BBB model. Neuropathol Appl Neurobiol. 2009;35:592–602. doi: 10.1111/j.1365-2990.2009.01031.x. [DOI] [PubMed] [Google Scholar]
- Gordon LB, Knopf PM, Cserr HF. Ovalbumin is more immunogenic when introduced into brain or cerebrospinal fluid than into extracerebral sites. J Neuroimmunol. 1992;40:81–87. doi: 10.1016/0165-5728(92)90215-7. [DOI] [PubMed] [Google Scholar]
- Haas DW, Johnson BW, Spearman P, Raffanti S, Nicotera J, Schmidt D, Hulgan T, Shepard R, Fiscus SA. Two phases of HIV RNA decay in CSF during initial days of multidrug therapy. Neurology. 2003;61:1391–1396. doi: 10.1212/wnl.61.10.1391. [DOI] [PubMed] [Google Scholar]
- Hakvoort A, Haselbach M, Wegener J, Hoheisel D, Galla H-J. The polarity of choroid plexus epithelial cells in vitro is improved in serum-free medium. J Neurochem. 1998;71:1141–1150. doi: 10.1046/j.1471-4159.1998.71031141.x. [DOI] [PubMed] [Google Scholar]
- Hanly A, Petito CK. HLA-DR-positive dendritic cells of the normal human choroid plexus. A potential reservoir of HIV in the central nervous system. Hum Pathol. 1998;29:88–93. doi: 10.1016/s0046-8177(98)90395-1. [DOI] [PubMed] [Google Scholar]
- Hudson LC, Bragg DC, Tompkins MB, Meeker RB. Astrocytes and microglia differentially regulate trafficking of lymphocyte subsets across brain endothelial cells. Brain Res. 2005;1058:148–160. doi: 10.1016/j.brainres.2005.07.071. [DOI] [PubMed] [Google Scholar]
- Kolb SA, Sporer B, Lahrtz F, Koedel U, Pfister HW, Fontana A. Identification of a T cell chemotactic factor in the cerebrospinal fluid of HIV-1-infected individuals as interferon-gamma inducible protein 10. J Neuroimmunol. 1999;93:172–181. doi: 10.1016/s0165-5728(98)00223-9. [DOI] [PubMed] [Google Scholar]
- Lackner AA, Smith MO, Munn RJ, Martfeld DJ, Gardner MB, Marx PA, Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991;139:609–621. [PMC free article] [PubMed] [Google Scholar]
- Lane JH, Sasseville VG, Smith MO, Vogel P, Pauley DR, Heyes MP, Lackner AA. Neuroinvasion by simian immunodeficiency virus coincides with increased numbers of perivascular macrophages/microglia and intrathecal immune activation. J Neurovirol. 1996;2:423–432. doi: 10.3109/13550289609146909. [DOI] [PubMed] [Google Scholar]
- Ling EA. Ultrastructure and mode of formation of epiplexus cells in the choroid plexus in the lateral ventricles of the monkey (Macaca fascicularis) J Anat. 1981;133:555–569. [PMC free article] [PubMed] [Google Scholar]
- Ling EA, Kaur C, Lu J. Origin, nature, and some functional considerations of intraventricular macrophages, with special reference to the epiplexus cells. Microsc Res Tech. 1998;41:43–56. doi: 10.1002/(SICI)1097-0029(19980401)41:1<43::AID-JEMT5>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Liu P, Hudson LC, Tompkins MB, Vahlenkamp TW, Colby B, Rundle C, Meeker RB. Cerebrospinal fluid is an efficient route for establishing brain infection with feline immunodeficiency virus and transfering infectious virus to the periphery. J Neurovirol. 2006;12:294–306. doi: 10.1080/13550280600889567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Kaur C, Ling EA. Intraventricular macrophages in the lateral ventricles with special reference to epiplexus cells: a quantitative analysis and their uptake of fluorescent tracer injected intraperitoneally in rats of different ages. J Anat. 1993;183:405–414. [PMC free article] [PubMed] [Google Scholar]
- Matyszak MK, Lawson LJ, Perry VH, Gordon S. Stromal macrophages of the choroid plexus situated at an interface between the brain and peripheral immune system constitutively express major histocompatibility class II antigens. J Neuroimmunol. 1992;40:173–182. doi: 10.1016/0165-5728(92)90131-4. [DOI] [PubMed] [Google Scholar]
- Monken CE, Wu B, Srinivasan A. High resolution analysis of HIV-1 quasispecies in the brain. AIDS. 1995;9:345–349. [PubMed] [Google Scholar]
- Morris L, Silber E, Sonnenberg P, Eintracht S, Nyoka S, Lyons SF, Saffer D, Koornhof H, Martin DJ. High human immunodeficiency virus type 1 RNA load in the cerebrospinal fluid from patients with lymphocytic meningitis. J Infect Dis. 1998;177:473–476. doi: 10.1086/517379. [DOI] [PubMed] [Google Scholar]
- Morris A, Marsden M, Halcrow K, Hughes ES, Brettle RP, Bell JE, Simmonds P. Mosaic structure of the human immunodeficiency virus type 1 genome infecting lymphoid cells and the brain: evidence for frequent in vivo recombination events in the evolution of regional populations. J Virol. 1999;73:8720–8731. doi: 10.1128/jvi.73.10.8720-8731.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson JA, Chun LL. Immunological function of the blood-cerebrospinal fluid barrier. Proc Natl Acad Sci USA. 1989;86:1684–1688. doi: 10.1073/pnas.86.5.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuenburg JK, Sinclair E, Nilsson A, Kreis C, Bacchetti P, Price RW, Grant RM. HIV-Producing T Cells in Cerebrospinal Fluid. J Acquir Immune Defic Syndr. 2004;37:1237–1244. doi: 10.1097/01.qai.0000136733.09275.fa. [DOI] [PubMed] [Google Scholar]
- Petito CK, Chen H, Mastri AR, Torres-Munoz J, Roberts B, Wood C. HIV infection of choroid plexus in AIDS and asymptomatic HIV-infected patients suggests that the choroid plexus may be a reservoir of productive infection. J Neurovirol. 1999;5:670–677. doi: 10.3109/13550289909021295. [DOI] [PubMed] [Google Scholar]
- Ritola K, Pilcher CD, Fiscus SA, Hoffman NG, Nelson JA, Kitrinos KM, Hicks CB, Eron JJ, Jr, Swanstrom R. Multiple V1/V2 env variants are frequently present during primary infection with human immunodeficiency virus type 1. J Virol. 2004;78:11208–11218. doi: 10.1128/JVI.78.20.11208-11218.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G, Klein D, Knapp E, Hosie MJ, Grimes T, Mabruk MJ, Jarrett O, Callanan JJ. Dynamics of viral and proviral loads of feline immunodeficiency virus within the feline central nervous system during the acute phase following intravenous infection. J Virol. 2003;77:7477–7485. doi: 10.1128/JVI.77.13.7477-7485.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan G, Grimes T, Brankin B, Mabruk MJ, Hosie MJ, Jarrett O, Callanan JJ. Neuropathology associated with feline immunodeficiency virus infection highlights prominent lymphocyte trafficking through both the blood-brain and blood-choroid plexus barriers. J Neurovirol. 2005;11:337–345. doi: 10.1080/13550280500186445. [DOI] [PubMed] [Google Scholar]
- Sasseville VG, Lackner AA. Neuropathogenesis of simian immunodeficiency virus infection in macaque monkeys. J Neurovirol. 1997;3:1–9. doi: 10.3109/13550289709015787. [DOI] [PubMed] [Google Scholar]
- Sei S, Stewart SK, Farley M, Mueller BU, Lane JR, Robb ML, Brouwers P, Pizzo PA. Evaluation of human immunodeficiency virus (HIV) type 1 RNA levels in cerebrospinal fluid and viral resistance to zidovudine in children with HIV encephalopathy. J Infect Dis. 1996;174:1200–1206. doi: 10.1093/infdis/174.6.1200. [DOI] [PubMed] [Google Scholar]
- Serot JM, Bene MC, Foliguet B, Faure GC. Monocyte-derived IL-10-secreting dendritic cells in choroid plexus epithelium. J Neuroimmunol. 2000;105:115–119. doi: 10.1016/s0165-5728(99)00240-4. [DOI] [PubMed] [Google Scholar]
- Shacklett BL, Cox CA, Wilkens DT, Karl KR, Nilsson A, Nixon DF, Price RW. Increased adhesion molecule and chemokine receptor expression on CD8+ T cells trafficking to cerebrospinal fluid in HIV-1 infection. J Infect Dis. 2004;189:2202–2212. doi: 10.1086/421244. [DOI] [PubMed] [Google Scholar]
- Smit TK, Wang B, Ng T, Osborne R, Brew B, Saksena NK. Varied tropism of HIV-1 isolates derived from different regions of adult brain cortex discriminate between patients with and without AIDS dementia complex (ADC): evidence for neurotropic HIV variants. Virology. 2001;279:509–526. doi: 10.1006/viro.2000.0681. [DOI] [PubMed] [Google Scholar]
- Staprans S, Marlowe N, Glidden D, Novakovic-Agopian T, Grant RM, Heyes M, Aweeka F, Deeks S, Price RW. Time course of cerebrospinal fluid responses to antiretroviral therapy: evidence for variable compartmentalization of infection. AIDS. 1999;13:1051–1061. doi: 10.1097/00002030-199906180-00008. [DOI] [PubMed] [Google Scholar]
- Steffen BJ, Breier G, Butcher EC, Schulz M, Engelhardt B. ICAM-1, VCAM-1, and MAdCAM-1 are expressed on choroid plexus epithelium but not endothelium and mediate binding of lymphocytes in vitro. Am J Pathol. 1996;148:1819–1838. [PMC free article] [PubMed] [Google Scholar]
- Stevenson PG, Hawke S, Sloan DJ, Bangham CR. The immunogenicity of intracerebral virus infection depends on anatomical site. J Virol. 1997;71:145–151. doi: 10.1128/jvi.71.1.145-151.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain MC, Letendre S, Pillai SK, Russell T, Ignacio CC, Gunthard HF, Good B, Smith DM, Wolinsky SM, Furtado M, Marquie-Beck J, Durelle J, Grant I, Richman DD, Marcotte T, McCutchan JA, Ellis RJ, Wong JK. Genetic composition of human immunodeficiency virus type 1 in cerebrospinal fluid and blood without treatment and during failing antiretroviral therapy. J Virol. 2005;79:1772–1788. doi: 10.1128/JVI.79.3.1772-1788.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T, Stadler E, Dziadek M. Effects of the extracellular matrix on fetal choroid plexus epithelial cells: changes in morphology and multicellular organization do not affect gene expression. Exp Cell Res. 1992;203:198–213. doi: 10.1016/0014-4827(92)90056-e. [DOI] [PubMed] [Google Scholar]
- Wolburg K, Gerhardt H, Schulz M, Wolburg H, Engelhardt B. Ultrastructural localization of adhesion molecules in the healthy and inflamed choroid plexus of the mouse. Cell Tissue Res. 1999;296:259–269. doi: 10.1007/s004410051287. [DOI] [PubMed] [Google Scholar]
- Zheng W, Zhao Q, Graziano JH. Primary culture of choroidal epithelial cells: characterization of an in vitro model of blood-CSF barrier. In Vitro Cell Dev Biol. 1998;34:40–45. doi: 10.1007/s11626-998-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–10488. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]