Abstract
Background
Patient navigation (PN) has been suggested as a way to reduce cancer health disparities; however, many models of PN exist and most have not been carefully evaluated. The goal of this study was to test the Ohio American Cancer Society model of PN as it relates to reducing time to diagnostic resolution among persons with abnormal breast, cervical, or colorectal cancer screening tests or symptoms.
Methods
862 patients from 18 clinics participated in this group-randomized trial. Chart review documented the date of the abnormality and the date of resolution. The primary analysis used shared frailty models to test for the effect of PN on time to resolution. Crude Hazard Ratios (HR)were used since there was no evidence of confounding. Costs were tracked with a 52-item instrument that recorded fixed costs of running a PN program and operational costs of navigating patients.
Results
HRs became significant at 6 months; conditional on the random clinic effect, the resolution rate at 15 months was 65% higher in the PN arm (p=0.012 for difference in risk across arms; p=0.009) for an increase in relative risk over time. The cost of navigating to diagnostic resolution averaged $150 per participant.
Conclusions
Participants with abnormal cancer screening tests or symptoms resolved faster if assigned to PN compared to those not assigned to PN. The effect of PN became apparent beginning six months after detection of the abnormality.
Impact
PN may help address health disparities by reducing time to resolution after an abnormal cancer screening test.
Keywords: cancer, patient navigation, abnormal screening tests, disparities
INTRODUCTION
Despite significant advances in prevention, screening, diagnosis, and treatment of cancer, reductions in mortality have not reached all populations equally (1). For example, mortality from breast cancer in blacks increased from 31.8% in 1980 to 35.6% in 2005 (p<0.001), while decreasing in whites from 32.6% to 25.8% (p<0.001) over the same period (2). Death rates for colorectal cancer (CRC) decreased 2.4 to 4.8% annually during 1993-2001 for those with at least 16 years of education, but there was no significant decrease for those with less than 12 years of education (3). Barriers, such as access, attitudes, insurance coverage and cost, permeate all points of the cancer continuum, ranging from individual to system and policy levels (4). These barriers, as well as differences in risk factors, screening rates, environmental and biological factors, all contribute to cancer disparities (5).
Freeman introduced patient navigation (PN) as a potential strategy to reduce disparities among African American patients in a Harlem, New York public hospital. Among this medically underserved population, PN significantly increased diagnostic follow-up rates and reduced time to treatment (6). Often ambiguously defined in the literature, Dohan et al. describe patient navigators as care coordinators who take a flexible problem-solving approach to helping patients overcome barriers to all points of care on the cancer continuum: prevention, screening, treatment, and survivorship(7). Patient navigators attempt to improve timeliness of diagnosis and treatment, as well as reduce loss to follow-up, after an abnormal screening finding until cancer-related treatment services have been completed (8).
To date, PN lacks a supportive body of evidence for its effectiveness as well consistent definitions and training for patient navigators (7). Its success has been measured in increases in cancer screening rates (9), down shifts in stage of diagnosis (10), improvement in follow-up rates for an abnormality (11), and reduced time to diagnosis (11) and treatment (10). However, very few rigorous evaluations of PN interventions (i.e. randomized studies) have been reported and of those conducted, most relate to increasing uptake of screening tests (9).
Few randomized controlled trials of PN have been conducted using diagnostic resolution as an outcome. Partly due to its potential to reduce disparities, the majority of PN studies have been conducted among medically underserved, high risk, or specific ethnic or racial populations. Moreover, previous trials focused on breast and cervical cancers only (12-16), limiting the generalizability of findings to other populations and other cancer sites. Overall, these randomized controlled trials demonstrated strong evidence for the effectiveness of PN in reducing loss to follow-up and diagnostic delay, although most suffered from small to moderate sample sizes and were limited to specific populations (8, 17). Thus, there is a need for large-scale randomized controlled trials of PN in a broad population, measuring diagnostic resolution and treatment as outcomes (7).
The primary goal of the Ohio Patient Navigator Research Program (OPNRP) was to test the Ohio American Cancer Society (ACS) model of PN as it relates to reducing time to diagnostic resolution among persons with abnormal breast, cervical, or CRC screening tests or symptoms in a heterogeneous patient population from primary care clinics and Federally-Qualified Health Centers (FQHC) located in central Ohio. Secondary objectives included evaluating the costs of the PN program and reporting on program process measures. This research program was one of nine funded grants conducted in 10 sites included in the National Cancer Institute-sponsored Patient Navigation Research Program (PNRP) (18).
METHODS
Study Design
The OPNRP employed a group-randomized trial (GRT) design, in particular, a nested cohort design (19). In this design, identifiable groups are randomized to study conditions and members of those groups are followed over time to assess the effect of an intervention. In the OPRNP, medical clinics were randomized to conditions (PN or comparison), and individual patients were followed over time to assess the effect of the PN intervention.
Setting
Clinics
Initially, patients from 12 primary care clinics from the Ohio State University (OSU) Primary Care Network (PCN) and Columbus Neighborhood Health Centers (CNHC) in Columbus, Ohio participated in the formal evaluation of the OPNRP. These clinics provide primary comprehensive health care to a culturally and economically diverse group of patients, including underserved populations (minority, poor, and elderly). At the time the OPNRP was implemented, the OSU PCN consisted of 15 primary care clinic locations providing care in family practice, internal medicine, occupational health, women’s health and pediatric clinics. Eight of these clinics participated in the OPNRP. The CNHC was a not-for-profit organization comprised of five FQHCs located in the city of Columbus, Ohio, with a preponderance of patients from underserved (i.e., racial/ethnic minorities, people of lower socioeconomic status, and elderly) populations. Four CNHC clinics participated in OPNRP. Thus, initially 8 PCN and 4 CNHC clinics participated in OPNRP, and clinics were paired for the GRT design within either the PCN or the CNHC groups.
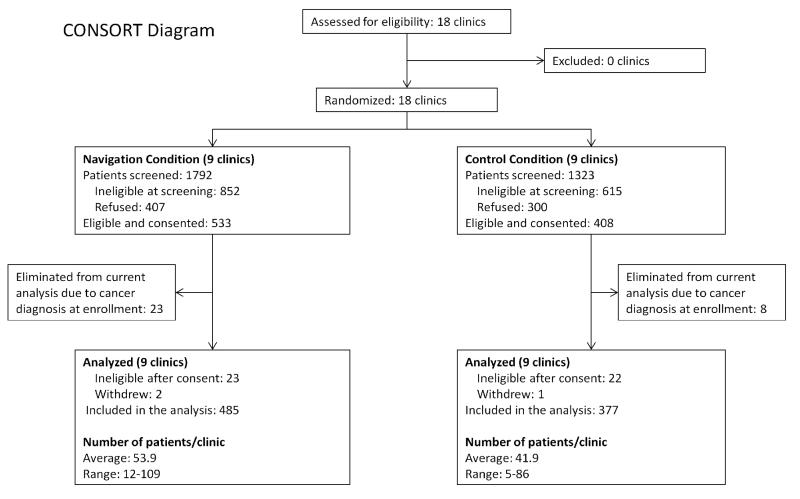
Recruitment began in the initial 12 clinics in groups of two pairs. The first group of clinics (2 comparison and 2 intervention) started patient recruitment in November 2006. The second group of four clinics began recruitment in February 2007, and the last group began recruitment in April 2007. Due to slow recruitment, two Ohio State University Medical Center gynecology clinics (OBGYN), two gastroenterology (GI) specialty clinic sites, and two smaller general internal medicine (GIM) clinics were added. Recruitment began in these 6 additional clinics in December 2007. Thus, in total, there were 18 clinics participating, 9 in each treatment arm. A CONSORT diagram (Figure 1) outlines the numbers of clinics and patients participating at various stages throughout the study.
Figure 1.
Participants
To be eligible for participation, patients must have been: 1) over 18 years old; 2) a regular patient of the primary care practice (i.e. not just coming for a second opinion or consult); 3) not cognitively impaired; 4) able to give informed consent; 5) identified as having either an abnormal screening test, an abnormal diagnostic test, or an abnormal clinical finding leading to diagnostic testing for cervical, breast; 6) without a prior history of cancer, except for non-melanoma cancer of the skin; 7) living outside a nursing home or institutional setting; 8) without prior history of medical navigation; and 9) able to speak and understand English or Spanish.
Several different mechanisms were developed to recruit participants. Some clinics agreed to use “passive consent” as a means of referring patients to the study, whereby research staff screened cytology reports, mammography reports, and charts to identify potential participants, and, if a potential participant was identified through the screening process, the research staff forwarded the name to the physician asking permission to contact the patient. Providers replied only if they did not want the research staff to contact the potential participant. Other clinics required active physician consent. Once consent was obtained from the physician, a letter introducing the study was sent to the patient prior to any contact by the research staff. Potential participants were then called, the study was explained, and they were asked if they would like to participate in the program. If they agreed to participate, they had the option of providing verbal consent and completing baseline activities via telephone or a meeting with the study staff. Meetings to further discuss the study and complete the informed consent process took place at either the clinic or a location that was mutually convenient for the patient and study staff.
Randomization
The initial 12 clinics were matched on clinic type (PCN vs. CNHC) and proportion of African American patients, then randomized from within pairs to study conditions, providing six clinics in each condition. The additional 6 clinics were paired on clinic type (OBGYN vs. GI vs. GIM) and randomized from within pairs to study conditions, providing three additional clinics in each condition. Thus, clinic randomization resulted in 9 intervention clinics and 9 control clinics.
PN Intervention
Behavioral Theory
The Chronic Care Model guided the overall development and implementation of the OPNRP intervention (20) by focusing on eliminating problems that exist within the healthcare system because of breakdowns in communication and coordination of care as patients navigate across different settings and among various providers. The PN intervention was also guided by social support theory (21) and addressed specific constructs of the Health Belief Model (e.g. perceived severity, perceived barriers, self-efficacy) (22).
Navigators first identified specific barriers to care and then assisted participants by taking actions tailored to the specific needs of the individual. Support was expressed as concern for the participant’s health, and actions taken by the navigators included supportive listening, providing educational materials, and providing referrals for psychological assistance, if needed. Navigators provided instrumental assistance by helping participants with making appointments, resolving child care problems, and helping with transportation issues, etc. At the participant-level, navigators addressed important HBM constructs by counseling them to take important health-related actions. For example, navigators addressed barriers to care or improved their confidence (self-efficacy) by providing support or encouragement as they progressed through diagnostic testing and/or treatment.
Patient Navigators
The three patient navigators for the OPNRP were female, over 30 years of age, and college graduates. Each had worked within the health care system prior to starting with the OPNRP. One navigator was bilingual and fluent in Spanish. If needed, a translation service was also available to assist with questionnaires and/or PN issues in Spanish.
Patient navigators completed on-site training and also attended numerous Ohio (OSU and ACS) and national (PNRP) training sessions (23). Each patient navigator developed their own resource book to assist participants in addressing barriers to care. The navigators were not embedded in the clinic; rather, they completed most activities by telephone, following the Ohio ACS model of PN.
Participants from intervention practices were assigned to one of the three patient navigators. Those who only spoke and understood Spanish were assigned to the navigator who was fluent in Spanish. The assigned patient navigator contacted the participants by phone (or in person, if no phone number was available) within 5 days following the assignment. The navigator then assessed participants’ needs, connected participants to community and social support services, facilitated interaction and communication with health care staff and providers, and provided health education to individuals.
Comparison Condition
Participants from comparison clinics were mailed educational materials focusing on their specific cancer test and/or abnormality/cancer within one month of completing the baseline questionnaire. Where possible for those who did not speak English, materials in Spanish were made available.
Measures
Participants Completed a baseline questionnaire when their abnormality was resolved or the end of their follow-up period (ie, censored at 365 days). The end-of-study questionnaire contained additional questionnaires for those participants who were enrolled from the PN clinics to assess process measures related to receiving the intervention. Outcome data (e.g. resolution of abnormality, and time to resolution) were obtained from the participants as well as their medical records. The surveys collected information on demographics (e.g. age, gender, race, insurance, etc. (see Table 1), as well as information contained in the Quality of Life Index (24), the Trust in Physician Scale (25), the Beck Anxiety Inventory(26), the CES-D (27), and the Perceived Social Support-Friends and Perceived Social Support-Family (28). Information from measures will be presented in future papers.
Table 1. Patient Characteristics.
Missing values have been omitted from the totals.
| Variable | Level | Control (n=377) N (%) |
Intervention (n=485) N (%) |
Total (n=862) N (%) |
||
|---|---|---|---|---|---|---|
| Cancer site | Breast | 199 (52.8%) | 282 (58.1%) | 481 (55.8%) | ||
| Cervix | 144 (38.2%) | 175 (36.3%) | 320 (37.1%) | |||
| Colorectal | 34 (9.0% | 27 (5.6%) | 61 (7.1%) | |||
| Gender | Female | 363 (96.3%) | 473 (97.5%) | 836 (97.0%) | ||
| Male | 14 (3.7%) | 12 (2.5%) | 26 (3.0%) | |||
| Race | White | 258 (69.5%) | 347 (71.5%) | 605 (70.7%) | ||
| Black | 88 (23.7%) | 98 (20.2%) | 186 (21.7%) | |||
| Other | 25 (6.7%) | 40 (8.2%) | 65 (7.6%) | |||
| Primary language English |
No | 23 (6.1%) | 18 (3.7%) | 41 (4.8%) | ||
| Yes | 351 (93.9%) | 467 (96.3%) | 818 (95.2%) | |||
| Marital status | Single | 119 (31.8%) | 137 (28.2%) | 256 (29.8%) | ||
| Married | 165 (44.1%) | 241 (49.7%) | 406 (47.3%) | |||
| Divorced/Widowed | 90 (24.1%) | 107 (22.1%) | 197 (22.9%) | |||
| Education level | Less than High School | 19 (5.1%) | 28 (5.8%) | 47 (5.5%) | ||
| High School | 38 (10.2%) | 79 (16.3%) | 117 (13.6%) | |||
| Some college/associate’s degree | 138 (37.0%) | 158 (32.6%) | 296 (34.5%) | |||
| College graduate/Graduate school | 178 (47.7%) | 220 (45.4%) | 398 (46.4%) | |||
| Housing status | Rent | 141 (37.7%) | 161 (33.3%) | 302 (35.2%) | ||
| Own | 206 (55.1%) | 294 (60.7%) | 500 (58.3%) | |||
| Live with Family, Friends/Other | 27 (7.2%) | 29 (6.0%) | 56 (6.5%) | |||
| Country of birth US | No | 34 (9.1%) | 45 (9.3%) | 79 (9.2%) | ||
| Yes | 340 (90.9%) | 440 (90.7%) | 780 (90.8%) | |||
| Number of dependents |
None | 210 (56.3%) | 262 (54.1%) | 472 (55.1%) | ||
| One | 79 (21.2%) | 102 (21.1%) | 181 (21.1%) | |||
| Two | 54 (14.5%) | 76 (15.7%) | 130 (15.2%) | |||
| Three or more | 30 (8.0%) | 44 (9.1%) | 74 (8.6%) | |||
| Household size including self |
One | 83 (22.2%) | 102 (21.0%) | 185 (21.5%) | ||
| Two | 143 (38.2%) | 173 (35.7%) | 316 (36.8%) | |||
| Three | 62 (16.6%) | 104 (21.4%) | 166 (19.3%) | |||
| Four | 49 (13.1%) | 63 (13.0%) | 112 (13.0%) | |||
| Five or more | 37 (9.9%) | 43 (8.9%) | 80 (9.3%) | |||
| Employment status | Full-time | 217 (58.3%) | 235 (48.7%) | 452 (52.9%) | ||
| Part-time | 53 (14.2%) | 66 (13.7%) | 119 (13.9%) | |||
| Retired | 30 (8.1%) | 58 (12.0%) | 88 (10.3%) | |||
| Disabled | 27 (7.3%) | 45 (9.3%) | 72 (8.4%) | |||
| Unemployed | 45 (12.1%) | 79 (16.4%) | 124 (14.5%) | |||
| Annual household income in last year |
Less than $10k | 49 (13.1%) | 50 (10.3%) | 99 (11.5%) | ||
| $10K-$29,999 | 72 (19.2%) | 83 (17.1%) | 155 (18.0%) | |||
| $30K-$49,999 | 60 (16.0%) | 81 (16.7%) | 141 (16.4%) | |||
| $50K+ | 170 (45.3%) | 237 (48.9%) | 407 (47.3%) | |||
| Don’t know | 24 (6.4%) | 34 (7.0%) | 58 (6.7%) | |||
| Public, private or uninsured |
Uninsured | 11 (3.0%) | 28 (5.9%) | 39 (4.7%) | ||
| Private | 257 (70.8%) | 325 (68.4%) | 582 (69.5%) | |||
| Public | 95 (26.2%) | 122 (25.7%) | 217 (25.9%) | |||
| Control | Intervention | Total | ||||
| Mean (std) | Range | Mean (std) | Range | Mean (std) | Range | |
| Age at consent | 43.4 (15.0) | 19 - 89 | 45.9 (14.3) | 18 - 86 | 44.8 (14.7) | 18 - 89 |
| Years in home | 7.3 (8.6) | 0.1 - 45 | 8.0 (9.1) | 0.1 - 60 | 7.7 (8.9) | 0.1 - 60 |
Approval to conduct the project was obtained from The Ohio State University Institutional Review Board.
Data Analysis
Primary Outcome
Baseline characteristics of intervention and control participants were compared descriptively using means for continuous variables and percentages for categorical variables (Table 1). A shared frailty model (29, 30) was used to test for differences in time to case resolution between the control and PN arms. The shared frailty model is an extension of the commonly used Cox proportional hazard model for survival data, which includes a gamma distributed random effect accounting for correlation among the responses from participants attending the same clinic. Our primary model contained a fixed effect for intervention arm (PN/control) and a random effect for clinic. Data from all sites (breast, cervical, and colorectal) were combined in our primary analysis; differences in the intervention effect by site were explored in a secondary analysis. The primary model was extended to adjust for participant-level characteristics collected in each PNRP study center (race, medical insurance, country of origin, primary language, marital status, and age) and exclusively in OPNRP (household income, education, household size, number of dependents, housing status, employment status, and number of years in current residence). The proportional hazard assumption of treatment arm and each covariate was evaluated using Schoenfeld residuals (31, 32) and a test of an interaction with the natural log of time; if violated, we included the interaction term in the model. These analyses were performed using Stata Intercooled 11 (33).
Cost Analyses
The 2009 costs of PN was estimated using navigator time logs and a 52-item survey that gathered information on the resources used to establish and maintain the PN intervention coordinated by each of the PNRP sites. This included information on staff time to recruit, train, supervise and support patient navigators; the cost of travel; office supplies; telecommunications; and the area of office space allocated to navigation staff. The total cost of providing PN was estimated as the cost of PN time, as recorded in the tracking logs, plus the associated cost to establish and maintain PN. To account for the uncertainty surrounding the final estimated cost, a probabilistic uncertainty analysis was completed using distributions fitted to each of the underlying parameters. A complete description of the costing methodology can be found in Bensink et al 2011 (34).
Navigation Process Measures
Patient navigators recorded information about their encounters with participants to obtain data regarding the PN process itself. A form enumerating the number and types of barriers a participant experienced, as well as action steps to be taken by the participant and/or patient navigator, and the time spent was collected at each encounter.
RESULTS
Participants
A total of 862 patients from 18 clinics participated in the study. Participant characteristics are summarized in Table 1. The average participant age was approximately 43 years, and the vast majority (97%) were female. A majority (70.7%) of the participants were white, 21.7% were black, and 7.6% identified as a race other than white or black. Nearly half of the participants were married, with roughly 30% single and 23% divorced/widowed. Almost half (46.4%) of the participants completed college or graduate school, while 34.5% had some college experience or an associate’s degree. Nearly 60% of the participants lived in a home they owned, and 47.3% reported annual household incomes in excess of $50,000. One quarter (25.9%) of the participants had public insurance, 69.5% had private insurance, and 4.7% of participants were uninsured. A total of 707 patients refused to participate in the study. Their gender distribution was the same as for participants (96.5% female), while on average their age at referral was 2.4 years less.
Primary Outcome: Time to Resolution
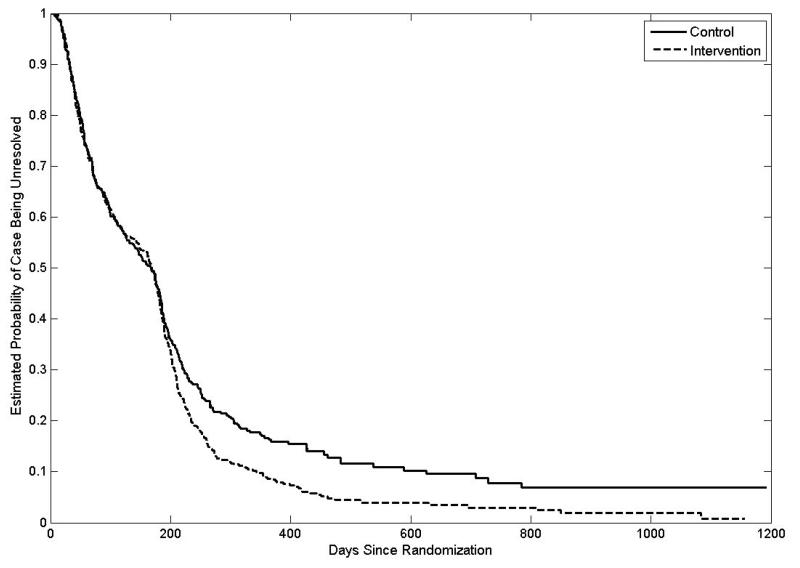
Survival curves for time to resolution by treatment arm are provided in Figure 2. The estimated probability that a participants was resolved in the first six months was approximately the same across the two study arms, after which the estimated probability of resolution was greater among navigated participants. Our shared frailty model confirmed that PN became more effective over time (p = 0.009). Table 2 provides hazard ratios comparing those in the PN group to those in the control group at 3, 6, 9, 12, and 15 months of follow-up. Conditional on the random clinic effect, the resolution rate at six months was 36% higher in the PN arm compared to the control arm, and the difference between arms increased with time. Similar results were found after adjusting for participant-level characteristics.
Figure 2. Estimated Survival Curves for Time to Resolution.
Curves estimated using shared frailty models fit separately to the intervention and control data. Curves assume mean frailty value (frailty = 1).
Table 2. Hazard Ratios (95% Confidence Intervals) of Case Resolution for Intervention versus Control.
| Month | Crude1 | Adjusted for Variables2VariablesVariablesVariables2Variables Collected in Each PNRP2 |
Adjusted for OPNRP Variables3 | |||
|---|---|---|---|---|---|---|
| 3 | 1.17 | (0.90, 1.52) | 1.15 | (0.95, 1.40) | 1.12 | (0.93, 1.36) |
| 6 | 1.36 | (1.03, 1.78) | 1.35 | (1.09, 1.67) | 1.32 | (1.07, 1.63) |
| 9 | 1.48 | (1.09, 1.99) | 1.48 | (1.15, 1.89) | 1.45 | (1.14, 1.85) |
| 12 | 1.57 | (1.13, 2.17) | 1.57 | (1.19, 2.08) | 1.55 | (1.18, 2.04) |
| 15 | 1.65 | (1.16, 2.33) | 1.66 | (1.22, 2.25) | 1.64 | (1.21, 2.21) |
n = 862
n = 835; Adjusted for race (black, white, other), medical insurance (private, public, none), country of birth (US, other), primary language (English, other), marital status (single, married, divorced/widowed), and age (in years).
n=851; Adjusted for household income (< $10k; $10k-$29,999; $30k-$49,999; $50k+; don’t know/refused), education (< high school, high school, some college, college grad/grad degree), household size (1, 2, 3, 4, 5+), number of dependents (none, 1, 2, 3+), housing status (rent, own, other), employment status (full-time, part-time, retired, disabled, unemployed), and number of years in current residence.
As an exploratory analysis, we tested for effect modifiers of the PN intervention. We found that the effect of PN on the resolution rate did not differ with race (black vs. white; p=0.43), insurance (public vs. private; p = 0.27), education (college grad vs. lower; p = 0.15), or cancer site (breast vs. cervical; p = 0.57), but there was a marginally significant difference in the effect with income (p = 0.07). For the first six months, the effect of the PN intervention on the resolution rate was greater among participants whose annual household income was less than $50,000; however, as time progressed, the effect became greater among participants whose household income was $50,000 or higher (Table 3).
Table 3. Monthly Hazard Ratios (95% Confidence Intervals) of Case Resolution by Annual Household Income.
| Months Post Randomization | < 50k | 50k + |
|---|---|---|
| 3 | 1.36 (1.04, 1.76) | 1.01 (0.77, 1.32) |
| 6 | 1.45 (1.08, 1.93) | 1.30 (0.96, 1.76) |
| 9 | 1.50 (1.07, 2.11) | 1.51 (1.06, 2.15) |
| 12 | 1.54 (1.05, 2.27) | 1.68 (1.13, 2.50) |
| 15 | 1.58 (1.03, 2.41) | 1.82 (1.18, 2.83) |
Secondary Outcomes
Cost
Navigation costs averaged $150 per participant (95% CI: $130 to $170) for the OPNRP model of PN using lay navigators. These cost estimates are based on the full OPNRP rather than individual PN cost estimates for each disease site (e.g., breast vs. colorectal). While time to resolution (and the associated navigator’s time) varied across disease sites, estimating site-specific PN costs would exclude economies of scale and scope, producing erroneously high r site-specific estimates.
Navigation Process
Fifty-nine participants (out of the 485 in the PN arm) refused PN after consent or were not successfully contacted by the navigator prior to diagnostic resolution or censoring. Over half (n=228; 53.5%) of the 426 contacted participants reported no barriers. Among those reporting barriers, 99 (50.0%) reported only one barrier, 53 (26.8%) reported two, and 46 (23.2%) reported three or more. The three most frequently reported barriers among those reporting barriers were misperception/beliefs about a test or treatment (16.4%), communication concerns with healthcare providers (15.0%), and problems with scheduling (11.5%).
Patient navigators reported 1,152 encounters with the participants in the PN arm. The majority (n=1,034; 89.8%) of encounters lasted less than 15 minutes. The mean number of encounters was 2.7 with a range of 1-38.
DISCUSSION
PN has emerged as a possible strategy to eliminate disparities in outcomes experienced by many underserved and minority populations (6). However, little evidence from well-designed studies exists on the efficacy or effectiveness of PN (17). The goal of this study was to assess the efficacy of a PN intervention—modeled after the Ohio ACS PN program—on improving rates of follow-up after an abnormal breast, cervical or CRC test/finding in a diverse population from academic and FQHC clinics in Columbus, Ohio. The results of this GRT indicate that this model of PN, mainly via telephone from a non-clinic location, improved resolution rates among participants, with equivalent effects among black and white participants, among those with and without a college education, and across cancer sites. Moreover, there was a suggestion that participants from lower incomes benefited earlier from PN.
Previous studies have demonstrated similar results (17). For example, PN reduced time to diagnostic resolution in two low-income, minority populations after an abnormal mammogram (13, 15) and increased follow-up in those two populations, as well as in another study which included Asian women (16). Additionally, two cohort studies and two pre/post policy analyses found that PN reduced diagnostic delay and improved follow-up in women after abnormal mammograms in high-risk, urban, safety-net populations (10, 11, 35, 36). Not all previous studies, however, have found a beneficial effect for PN. For example, PN did not improve follow-up when delivered by telephone in a low income, minority population with breast abnormalities (12) or among Hispanic women with breast or gynecological cancer (14).
Of interest was our finding that almost half of the participants from the intervention clinics (47.6%) reported no barriers. To our knowledge, no other studies examining PN have reported this result. This suggests that not all patients need PN or that many patients might already be receiving sufficient attention from their health care providers and other support systems. However, this result also points to the fact that almost half of patients do identify barriers and need assistance to address those barriers to complete recommended follow-up.
The fact that PN was effective in the first 6 months among individuals with lower incomes suggests that those individuals had immediate needs the PN could effectively address, whereas after 6 months those with higher incomes who had not resolved had barriers that at that time were more easily resolved with assistance from a patient navigator. Participants who experienced barriers reported primarily patient-focused barriers, such as mis-perception/beliefs about the test or treatment, communication concerns, and scheduling problems. These results provide clues as to where the health care system is breaking down for many patients and exactly how PN can help. Findings also indicate that a tailored PN program, i.e. one that focuses on the individual barriers each patient has, may be beneficial.
The cost of PN in the OPNRP was $150 per navigated participant. While there is a paucity of research on the cost of PN, making comparisons to our cost findings difficult, as it seems the overall cost of this intervention is modest. Factors contributing to the modest costs of this PN intervention appear to center on our use of lay navigators (as opposed to nurses, social workers, etc.), a phone-based intervention (vs. embedded in the clinics), and a high percentage of non-cancer patients (few cancer patients were enrolled).
This study possesses several strengths, including a group-randomized design, a large and diverse population of participants (both demographically and by cancer site), and a mix of clinic types. Moreover, an easy-to-replicate PN intervention was tested which used well-trained lay navigators representing the communities from which the participants were drawn. A cost analysis, with a well-defined methodology, was used, and process information was collected to help describe intervention delivery. Study limitations included recruitment of few cancer patients, thus limiting our ability to generalize results to that population. Approximately 40% of potential participants did not participate in the study, also limiting generalizability, however, non-participants were not very different from those who participated. In addition, more participants with breast and cervical abnormalities were recruited, again limiting our findings to women with these two cancer types.
In summary, the Ohio ACS model of PN appears to be effective in improving resolution rates among participants with abnormal breast, cervical and CRC tests. These findings have important implications for hospitals and clinics wishing to establish PN programs in order to help reduce disparities among underserved populations and reduce disease burden. More research, however, is needed to determine why some individuals use PN while others do not and if this PN model is effective in all populations, including cancer patients and men.
Acknowledgements
Ohio Patient Navigation Research Program: Mike Burgin, MD, Kelly Fleming, MD, Gail Grever, MD, Lisa Kedar,MD, Steve Koesters, MD, Mike Langan, MD, John McConaghy, MD, Fred Miser,MD, Rupal Oza,MD, Hosi Padamadan, MD, Benita Petri-Pickstone, MD, Bushra Siddiqi, MD, Neeraj Tayal,MD.
Financial support: Supported by Special Initiative Research Scholar Grant 112190-SIRSG-05-253-01 from the American Cancer Society and a supplement from the National Cancer Institute Center to Reduce Health Disparities to Award Number P30CA016058. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American Cancer Society, National Cancer Institute, or the National Center for Research Resources, Grant UL1RR025755, KL2RR025754, and TL1RR025753, and is now at the National Center for Advancing Translational Sciences, Grant 8KL2TR000112-05, 8UL1TR000090-05, 8TL1TR000091-05.
Footnotes
Disclosures: The authors report no conflicts of interest
References
- 1.Byers T. Two decades of declining cancer mortality: progress with disparity. Annu Rev Public Health. 2010;31:121–32. doi: 10.1146/annurev.publhealth.121208.131047. [DOI] [PubMed] [Google Scholar]
- 2.Whitman S, Ansell D, Orsi J, Francois T. The racial disparity in breast cancer mortality. J Community Health. 2011;36:588–96. doi: 10.1007/s10900-010-9346-2. [DOI] [PubMed] [Google Scholar]
- 3.Kinsey T, Jemal A, Liff J, Ward E, Thun M. Secular trends in mortality from common cancers in the United States by educational attainment, 1993-2001. J Natl Cancer Inst. 2008;100:1003–12. doi: 10.1093/jnci/djn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Warnecke RB, Oh A, Breen N, Gehlert S, Paskett E, Tucker KL, et al. Approaching health disparities from a population perspective: the National Institutes of Health Centers for Population Health and Health Disparities. Am J Public Health. 2008;98:1608–15. doi: 10.2105/AJPH.2006.102525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artinian NT, Warnecke RB, Kelly KM, Weiner J, Lurie N, Flack JM, et al. Advancing the science of health disparities research. Ethn Dis. 2007;17:427–33. [PMC free article] [PubMed] [Google Scholar]
- 6.Freeman HP, Muth BJ, Kerner JF. Expanding access to cancer screening and clinical follow-up among the medically underserved. Cancer Pract. 1995;3:19–30. [PubMed] [Google Scholar]
- 7.Dohan D, Schrag D. Using navigators to improve care of underserved patients: current practices and approaches. Cancer. 2005;104:848–55. doi: 10.1002/cncr.21214. [DOI] [PubMed] [Google Scholar]
- 8.Wells KJ, Battaglia TA, Dudley DJ, Garcia R, Greene A, Calhoun E, et al. Patient navigation: state of the art or is it science? Cancer. 2008;113:1999–2010. doi: 10.1002/cncr.23815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Percac-Lima S, Milosavljevic B, Oo SA, Marable D, Bond B. Patient Navigation to Improve Breast Cancer Screening in Bosnian Refugees and Immigrants. J Immigr Minor Health. 2011 doi: 10.1007/s10903-011-9539-5. [DOI] [PubMed] [Google Scholar]
- 10.Haideri NA, Moormeier JA. Impact of patient navigation from diagnosis to treatment in an urban safety net breast cancer population. J Cancer. 2011;2:467–73. doi: 10.7150/jca.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglia TA, Roloff K, Posner MA, Freund KM. Improving follow-up to abnormal breast cancer screening in an urban population. A patient navigation intervention. Cancer. 2007;109:359–67. doi: 10.1002/cncr.22354. [DOI] [PubMed] [Google Scholar]
- 12.Bastani R, Mojica CM, Berman BA, Ganz PA. Low-income women with abnormal breast findings: results of a randomized trial to increase rates of diagnostic resolution. Cancer Epidemiol Biomarkers Prev. 2010;19:1927–36. doi: 10.1158/1055-9965.EPI-09-0481. [DOI] [PubMed] [Google Scholar]
- 13.Ell K, Vourlekis B, Lee PJ, Xie B. Patient navigation and case management following an abnormal mammogram: a randomized clinical trial. Prev Med. 2007;44:26–33. doi: 10.1016/j.ypmed.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Ell K, Vourlekis B, Xie B, Nedjat-Haiem FR, Lee PJ, Muderspach L, et al. Cancer treatment adherence among low-income women with breast or gynecologic cancer: a randomized controlled trial of patient navigation. Cancer. 2009;115:4606–15. doi: 10.1002/cncr.24500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrante JM, Chen PH, Kim S. The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. J Urban Health. 2008;85:114–24. doi: 10.1007/s11524-007-9228-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maxwell AE, Jo AM, Crespi CM, Sudan M, Bastani R. Peer navigation improves diagnostic follow-up after breast cancer screening among Korean American women: results of a randomized trial. Cancer Causes Control. 2010;21:1931–40. doi: 10.1007/s10552-010-9621-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paskett ED, Harrop JP, Wells KJ. Patient navigation: an update on the state of the science. CA Cancer J Clin. 2011;61:237–49. doi: 10.3322/caac.20111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freund KM, Battaglia TA, Calhoun E, Dudley DJ, Fiscella K, Paskett E, et al. National Cancer Institute Patient Navigation Research Program: methods, protocol, and measures. Cancer. 2008;113:3391–9. doi: 10.1002/cncr.23960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murray D. Design and Analysis of Group-Randomized Trials. Oxford University Press; New York, NY: 1998. [Google Scholar]
- 20.Wagner EH, Bennett SM, Austin BT, Greene SM, Schaefer JK, Vonkorff M. Finding common ground: patient-centeredness and evidence-based chronic illness care. J Altern Complement Med. 2005;11(Suppl 1):S7–15. doi: 10.1089/acm.2005.11.s-7. [DOI] [PubMed] [Google Scholar]
- 21.Heaney CAIB. Social networks and social support. In: Glanz KRB, Viswanath K, editors. Health Behavior and Health Education Theory, Research, and Practice. 4 ed. Jossey-Bass; San Francisco, CA: 2008. pp. 189–207. [Google Scholar]
- 22.Rosenstock I. The health belief model and preventive health behavior. Health Education Monographs. 1974:354–86. doi: 10.1177/109019817800600406. [DOI] [PubMed] [Google Scholar]
- 23.Calhoun EA, Whitley EM, Esparza A, Ness E, Greene A, Garcia R, et al. A national patient navigator training program. Health Promotion Practice. 2010;11:205. doi: 10.1177/1524839908323521. [DOI] [PubMed] [Google Scholar]
- 24.Ferrans CE. Development of a quality of life index for patients with cancer. 1990;1990:15. [PubMed] [Google Scholar]
- 25.Lynda AA, Robert FD. Development of the Trust in Physician Scale: A measure to assess interpersonal trust in patient-physician relationships. Psychological Reports. 1990;67:1091–100. doi: 10.2466/pr0.1990.67.3f.1091. [DOI] [PubMed] [Google Scholar]
- 26.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and clinical Psychology. 1988;56:893. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 27.Hann D, Winter K, Jacobsen P. Measurement of depressive symptoms in cancer patients:: Evaluation of the center for epidemiological studies depression scale (Ces-d) Journal of Psychosomatic Research. 1999;46:437–43. doi: 10.1016/s0022-3999(99)00004-5. [DOI] [PubMed] [Google Scholar]
- 28.Procidano ME, Heller K. Measures of perceived social support from friends and from family: Three validation studies. American journal of community psychology. 1983;11:1–24. doi: 10.1007/BF00898416. [DOI] [PubMed] [Google Scholar]
- 29.Clayton D, Cuzick J. Multivariate Generalizations of the Proportional Hazards Model. J Roy Stat Soc a Sta. 1985;148:82–117. [Google Scholar]
- 30.Vaupel JW, Manton KG, Stallard E. Impact of Heterogeneity in Individual Frailty on the Dynamics of Mortality. Demography. 1979;16:439–54. [PubMed] [Google Scholar]
- 31.Schoenfeld D. Partial Residuals for the Proportional Hazards Regression-Model. Biometrika. 1982;69:239–41. [Google Scholar]
- 32.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika. 1994;81:515–26. [Google Scholar]
- 33.StataCorp . Stata Statistical Software: Release 11. StataCorp LP; College Station, TX: 2009. [Google Scholar]
- 34.Bensink MERS, Battaglia T, Everhart RM, Fiscella K, Hurd TC, McKoy JM, Patierno SR, Raich PC, Seiber E, Mears VW, Whitley E, Mandelblatt JS. Economic cost of providing a navigation program for underserved patients with abnormal findings on breast, colorectal, cervical and prostate cancer screening. 2011. Unpublished manuscript. [Google Scholar]
- 35.Psooy BJ, Schreuer D, Borgaonkar J, Caines JS. Patient navigation: improving timeliness in the diagnosis of breast abnormalities. Can Assoc Radiol J. 2004;55:145–50. [PubMed] [Google Scholar]
- 36.Clark CR, Baril N, Kunicki M, Johnson N, Soukup J, Ferguson K, et al. Addressing social determinants of health to improve access to early breast cancer detection: results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women’s Health Demonstration Project. J Womens Health (Larchmt) 2009;18:677–90. doi: 10.1089/jwh.2008.0972. [DOI] [PubMed] [Google Scholar]