Abstract
Background
The capacity of CD8+ T cells to control infections and mediate anti-tumor immunity requires the development and survival of effector and memory cells. IL-21 has emerged as a potent inducer of CD8+ T cell effector function and memory development in mouse models of infectious disease. However, the role of IL-21 and associated signaling pathways in protective CD8+ T cell immunity in humans is unknown.
Objective
To determine which signaling pathways mediate the effects of IL-21 on human CD8+ T cells and whether defects in these pathways contribute to disease pathogenesis in primary immunodeficiencies caused by mutations in components of the IL-21 signaling cascade.
Methods
Human primary immunodeficiencies resulting from monogenic mutations provide a unique opportunity to assess the requirement for particular molecules in regulating human lymphocyte function. Lymphocytes from patients with loss-of-function mutations in STAT1, STAT3 or IL21R were used to assess the respective roles of these genes in human CD8+ T cell differentiation in vivo and in vitro.
Results
Mutations in STAT3 and IL21R, but not STAT1, lead to a decrease in multiple memory CD8+ T cell subsets in vivo, indicating that STAT3 signaling – possibly downstream of IL-21R - regulates the memory cell pool. Furthermore, STAT3 was important for inducing the lytic machinery in IL-21-stimulated naïve CD8+ T cells. However, this defect was overcome by TCR engagement.
Conclusion
The IL-21R/STAT3 pathway is required for many aspects of human CD8+ T cell behavior but in some cases can be compensated by other signals. This helps explain the relatively mild susceptibility to viral disease observed in STAT3 and IL-21R-deficient individuals.
Keywords: autosomal dominant hyper-IgE syndrome, STAT3, STAT1, IL-21, human CD8+ T cells, memory, differentiation
INTRODUCTION
CD8+ T cell responses are essential for the control of viruses and protection against some tumors. Common γ-chain family cytokines are important regulators of CD8+ T cell behavior. Thus, IL-7 and IL-15 control lymphocyte homeostasis1-3, while IL-2 regulates differentiation of naïve cells into effector or memory populations4, 5. IL-21 has also been reported to control CD8+ T cell function. In vitro, IL-21 increases survival and proliferation of mouse6-9 and human CD8+ T cells10-12, induces effector molecules such as IFN-γ, granzyme B and perforin 6, 13-15, and transcription factors BCL6 and EOMES that control their differentiation into effector and memory populations16.
IL-21 is also implicated in controlling immune responses in vivo. Treatment of cancer patients with IL-21 resulted in upregulation of cytotoxic molecules such as granzyme B, perforin and IFN-γ in their CD8+ T cells and NK cells17. In mice, IL-21 enhanced memory CD8+ T cell responses during vaccinia infection9, 18 and was required for CD8+ T cell mediated control of chronic LCMV infection19-21. IL-21 alone, or together with IL-15, also increased the efficacy of anti-tumor responses by CD8+ T cells8, 9, 22-25. Thus, IL-21 is a potent inducer of CD8+ T cell effector function and memory with clinical relevance in both anti-viral and anti-tumor immunity.
IL-21 mediates its effects by activating JAK1 and JAK315, 26, 27 leading to phosphorylation of STAT1, STAT3 and STAT56, 15, 26. IL-21 can also activate MAPK and Akt6. It is not clear, however, which of these pathways mediates the stimulatory effects of IL-21 on human CD8+ T cells. Primary immunodeficiencies (PID) resulting from mutations in single genes provide a unique opportunity to address the role of individual molecules in regulating immune responses. Autosomal dominant hyper IgE syndrome (AD-HIES) is a PID characterized by chronic eczema, elevated serum IgE levels and recurrent infections of the skin, mucosa and lungs28, 29. Notably, some AD-HIES patients have impaired control of reactivation of infection with herpes viruses (HSV, VZV)29, 30, and are predisposed to developing non Hodgkin’s B-cell lymphoma29-32. The molecular lesion in AD-HIES is heterozygous mutations in STAT3, with mutant alleles working in a dominant negative manner33, 34. Mutations in STAT1 also result in infectious susceptibility to particular pathogens. Thus, mono- or bi-allelic loss-of-function STAT1 mutations severely compromise responses to IFN-γ. However, responses to IFN-α/β and IFN-λ are either intact (AD STAT1 deficiency; heterozygous mutations) or impaired (autosomal recessive [AR] STAT1 deficiency; bi-allelic mutations). Consequently, these mutations result in clinical disease caused by weakly virulent mycobacteria, and occasionally non-lethal viral infection35, 36. On the other hand, bi-allelic null mutations abolish STAT1-dependent cellular responses to IFN-γ, IFN-α/β and IFN-λ, thereby predisposing affected individuals to fatal infection with herpes viruses and mycobacteria35, 36. The importance of IL-21 signalling in humans was recently highlighted by the identification of four patients with IL21R mutations who develop recurrent respiratory and gastrointestinal infections, particularly with cryptosporidia resulting in chronic liver disease37. Two of these patients also exhibited ongoing infection with norovirus and rhinovirus, but immunity against herpes viruses and other pathogens that are commonly problematic for patients with combined immunodeficiencies (eg CMV, EBV) appeared to be intact37.
Here, we used STAT3 mutant (STAT3MUT), STAT1MUT and IL21RMUT patients to determine the requirement for STAT1 and STAT3 in regulating human CD8+ T cell responses. IL-21 in combination with IL-15 induced proliferation of, and granzyme expression in, naïve CD8+ T cells. Loss of STAT3 function impaired IL-21-induced granzyme B expression but did not affect its ability to induce proliferation. However, strong TCR/co-stimulatory signals could rescue granzyme expression in STAT3MUT T cells. Loss of STAT1 function did not affect controlled the formation/maintenance of effector and memory CD8+ T cell subsets in vivo, as proliferation or granzyme B production. We also found that STAT3, but not STAT1, evidenced by reduced frequencies of differentiated memory populations. We also observed some memory cell deficiencies in patients with IL21R mutations, implicating IL-21 as a potential STAT3-activating cytokine required for CD8+ memory T cell homeostasis. These findings provide insight into some of the clinical features of AD-HIES and IL-21R deficiency, including impaired control of viral infection and susceptibility to B-cell lymphoma.
Materials and Methods
Human Samples
Buffy coats from normal donors were purchased from the Australia Red Cross Blood Service. Peripheral blood (PB) was collected from patients with mutations in STAT3, STAT1 or IL21R (Refer to Table E1 in Online Repository for Patient details). All human experiments were approved by ethics committees in Canberra, Sydney, Melbourne, Brisbane and Perth, and institutional review boards of Necker Medical School, Rockefeller University and NIH.
T cell phenotyping & isolation
PB CD8+ T cells were stained with mAbs to CD4, CD8, CCR7 and CD45RA. Subsets were defined as naïve (CD8+CD4-CCR7+CD45RA+), central memory (TCM; CD8+CD4-CCR7+CD45RA-), effector memory (TEM; CD8+CD4-CCR7-CD45RA-) or TEMRA (revertant CD45RA effector memory; CD8+CD4-CCR7-CD45RA+). For experiments with STAT3MUT samples naïve cells were isolated from samples using a Positive Isolation Dynal Kit (Invitrogen) followed by sorting CD8+CCR7+CD45RA+ cells (FACSAria: BD). Due to limiting numbers of cells in STAT1MUT and IL-21RMUT samples, naïve CD8+T cells were isolated directly by sorting. For phenotyping cells were also stained for further cell surface markers (Table E2 in the Online Repository lists mAbs used)
Expression of phospho-STATs
Normal naïve CD8+ T cells were cultured for four days with TAE beads (Miltenyi Biotech), rested for 2 hours in OPTI-mem (LifeTech) plus Normicin (InVivogen), and then stimulated in the absence or presence of IL-2 (50 U/ml), IL-15 (50 ng/ml) and/or IL-21 (50 ng/ml) for 30 min. Cells were fixed with 2% paraformaldehyde, permeabilized with 90% methanol and stained with anti-phospho-STAT1, STAT3 and STAT5 mAbs.
In Vitro Stimulation of Naïve CD8+ T cells
Naïve CD8+ T cells were labeled with CellTrace™ Violet (CTV; Invitrogen) and then cultured (∼4 × 104 cells/200μl/well) with or without TAE beads (one bead/5 cells) for 4 or 10 d respectively; alone or together with 50 U/ml IL-2 (Millipore), 50 ng/ml IL-15 or 50 ng/ml IL-21 (PeproTech). Cells were thenharvested, permeabilized and stained with anti-perforin and anti-granzyme B mAb. Cell division and phenotype were determined using FlowJo software (Tree Star, Inc.).
Quantitative PCR analysis
RNA was isolated immediately after ex vivoisolation or after 4 or 10 days of culture using the QIAGEN RNeasy kit. For quantitative PCR, total RNA was reverse transcribed using oligo-dT. Expression of genes was determined by real-time PCR using the LightCycler 480 Probe Master Mix and System (Roche). All primers (Table E3 in the Online Repository) were from Integrated DNA Technologies. All reactions were standardized to GAPDH.
RESULTS
IL-21 activates STAT1, STAT3 and STAT5 in human CD8+ T cells
IL-21 activates numerous intracellular signaling pathways including STAT1, STAT3, STAT5, MAPK and Akt6, 15, 26. We assessed which pathways were activated by IL-21 in human naïve CD8+ T cells. IL-21 induced strong phosphorylation of STAT3, and a low level of STAT1 and STAT5 phosphorylation (Fig 1). We also analysed STAT activation induced by two other γc cytokines that are potent inducers of CD8+ T cell proliferation and differentiation, namely IL-2 and IL-15. In contrast to IL-21, IL-2 and IL-15 did not result in phosphorylation of STAT1 or STAT3, but did induce STAT5 phosphorylation (Fig 1). The combination of IL-15 and IL-21 did not alter the level of STAT phosphorylation above that observed with these cytokines alone (Fig 1A-F). Therefore, of these cytokines, IL-21 uniquely activates STAT1 and STAT3 in human CD8+ T cells.
Figure 1. IL-21 predominately activates STAT1, STAT3 and STAT5 in human CD8+T cells.
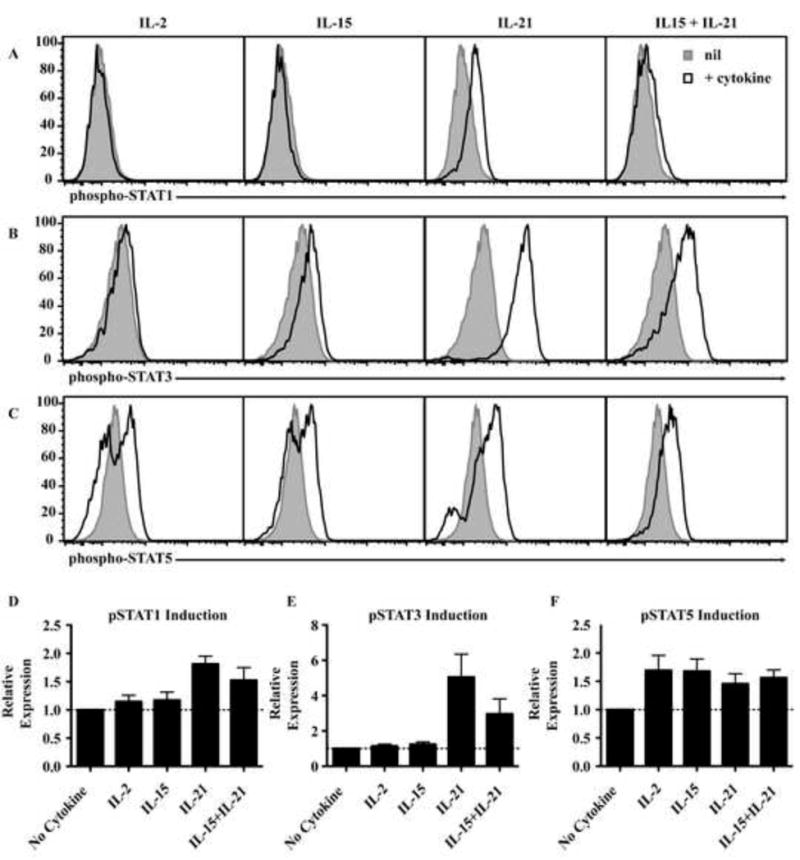
(A-C) Naïve CD8+T cells were activated for 4 days with TAE beads and recultured with cytokines for 30 min to determine phosphorylation of STAT1, 3 and 5. Histograms show nil or cytokine cultures and are representative of 4 experiments. (D-F) Graphs represent fold increase in MFI (mean ± SEM; n=4) of cells stimulated with cytokine over nil cultures. The dashed lines indicate a fold-change of 1 i.e. no change.
STAT1 and STAT3 mutations do not impair proliferation of naïve CD8+ T cells
IL-21 plays a pivotal role in inducing proliferation of CD8+ T cells10-12, 38. However, since IL-21 activates multiple signaling pathways, it is not clear which of these underlies this proliferative effect. To address this we utilized naïve CD8+ T cells from patients with mutations in STAT1 (n=8), STAT3 (n=15) or IL21R (n=3).
Homeostatic cytokines support survival of CD8+ T cells and can induce proliferation and differentiation in the absence of extrinsic TCR stimulation. Thus, IL-15 mediates homeostatic proliferation of memory cells and combined with IL-21 drives naïve cells to effector phenotypes9, 12, 38, 39. In vitro culture of naïve CD8+ T cells with IL-2 or IL-15 for 10 days significantly increased the recovery of viable cells (Fig 2A). In contrast, IL-21 alone did not increase survival above that seen with media alone. However, co-culture with IL-15 plus IL-21 induced significant proliferation as assessed by CTV dilution (Fig 2B). STAT3MUT or STAT1MUT CD8+ T cells stimulated with IL-15 and IL-21 showed comparable proliferation to controls (Fig 2B-D), however proliferation of IL-21RMUT CD8+ T cells was strongly reduced, with the residual proliferation likely being induced by IL-15 (Fig 2C, 2D). Thus, IL-21’s involvement in the homeostatic turnover requires a functional IL-21R but is unaffected by loss-of function mutations in STAT3 or STAT1.
Figure 2. Cytokine-induced proliferation is impaired in IL-21R-deficient, but not STAT1- or STAT3-deficient, naïve CD8+ T cells.
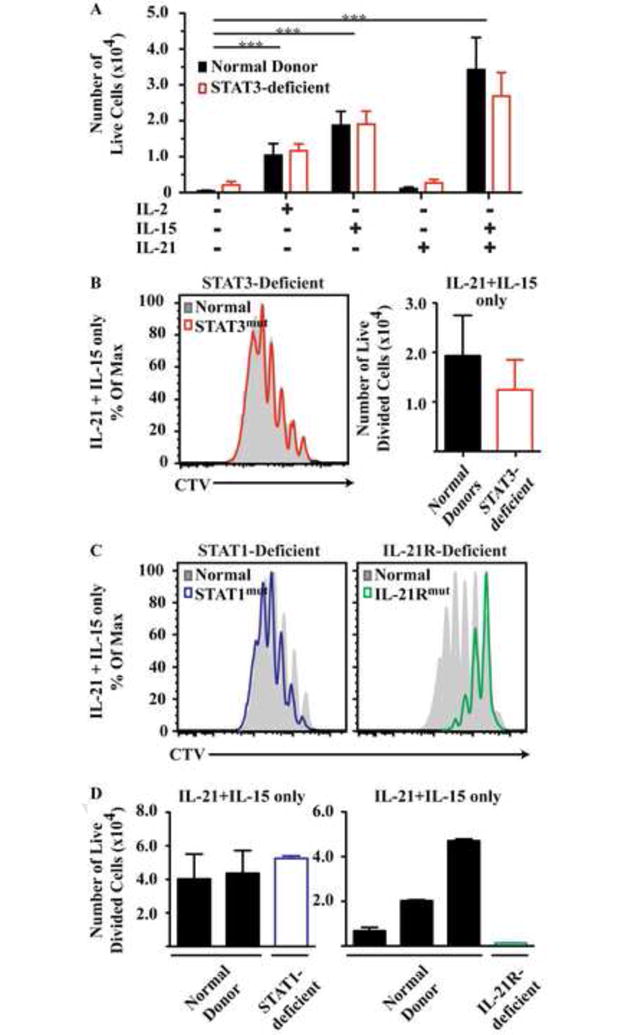
Naïve CD8+ T cells were cultures with cytokines only and (A) the numbers of live cells were determined (mean ± SEM, n=5). (B, C) Histograms show representative CTV profiles of cells stimulated with IL-21/IL-15. Graph in (B) shows the number of divided cells in IL-21/IL-15 cultures (mean ± SEM; n=5). (D) Each bar represents an individual patient or normal donor for experiments using cells from STAT1- and IL-21R-deficient patients. * P<0.05; **, P<0.001; *** P<0.001.
STAT3 is required for IL-21 induced expression of granzyme B
The capacity of CD8+ T cells to produce the cytotoxic molecules granzyme B and perforin is important for their effector function40. IL-2, IL-15 and IL-21 can all induce expression of these molecules7-9, 16, 41-43. Therefore, we assessed the impact of STAT1, STAT3 and IL21R mutations on the ability of these cytokines to induce granzyme B. Co-culture with IL-21 plus IL-15 induced higher granzyme B expression in normal naïve CD8+ T cells than did IL-2, IL-15 or IL-21 alone (Fig 3A). However, both IL21RMUT and STAT3MUT CD8+ T cells cultured with IL-15 and IL-21 failed to upregulate granzyme B (p<0.001) to the same level as control cells (Fig 3A-C). In contrast, STAT1MUT CD8+ T cells showed normal upregulation of granzyme B after stimulation with IL-15/IL-21 (Fig 3B, 3C). Thus, acquisition of the lytic machinery by naïve CD8+ T cells stimulated with IL-21 in combination with IL-15 was dependent on STAT3 signaling downstream of a functional IL21R.
Figure 3. Cytokine-induced expression of granzyme B is impaired in IL-21R-deficient or STAT3-deficient naïve CD8+ T cells.
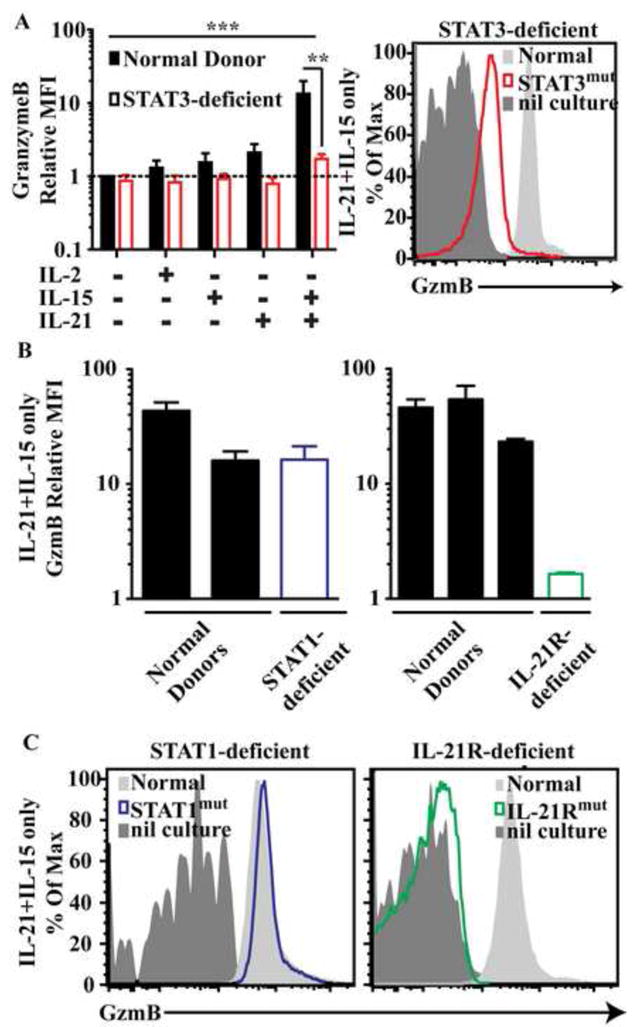
Naïve CD8+ T cells were cultured with cyokines only and (A and B) graphs depict fold increase (mean ± SEM; n=5) in MFI of granzyme B over normal cells cultured with media alone (A). Each bar graph in (B) represents mean and range of duplicate cultures from an individual patient or normal donor for experiments using cells from STAT1- and IL-21R deficient patients. (A) and (C) are representative plots of cells from normal donors or the indicated patients stimulated with IL-21/IL-15 and normal donor cells from unstimulated cultures. ** P<0.001; *** P<0.001.
TCR/costimulation rescues defective IL-21 responses in STAT3MUT CD8+ T cells
During an immune response CD8+ T cells also receive signals via the TCR and costimulatory molecules. Therefore, we examined naïve CD8+ T cell responses following culture with cytokines and anti-CD3/anti-CD28/anti-CD2 stimulus (provided by TAE beads). Addition of IL-15 or IL-21/IL-15 to cells from healthy donors resulted in the recovery of a significantly more CD8+ T cells than stimulation with TAE beads alone, or TAE beads plus IL-2 (Fig 4A). Proliferation analysis revealed that treatment of naïve CD8+ T cells with TAE beads plus IL-21 increased the total percentage of divided cells and the average number of divisions the cells had undergone compared to those cultured with TAE beads alone (Fig 4B,C). Interestingly, we detected no significant defect in the ability of TAE-activated naive STAT3MUT (Fig 4A-C) or STAT1MUT (Fig 4D) CD8+ T cells to respond to IL-21 or IL-21/IL-15 co-stimulation. However, mutations in IL-21R decreased recovery of viable cells and progression through division in cultures containing IL-21 (Fig 4E). IL-7, another γc cytokine, also enhanced proliferation of normal naïve CD8+ T cells that had been stimulated with TAE beads (not shown). Although previous studies have found that IL-7 can activate STAT344, 45, the ability of IL-7 to promote naïve CD8+ T cell proliferation was unaffected by mutations in STAT3 (not shown).
Figure 4. STAT3-deficient naïve CD8+ T cells proliferate normally in response to TCR engagement and activating cytokines.
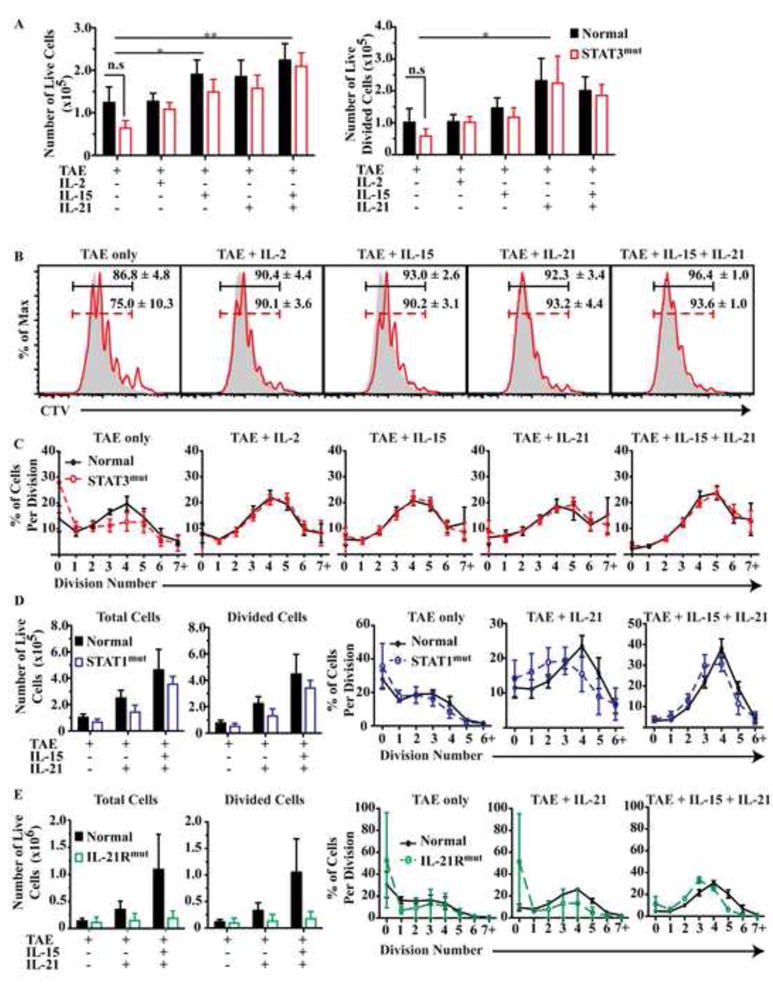
Naive CD8+ T cells from healthy donors (n=6-8 [A-C], 3 [D], 5 [E]), STAT3-deficient (A-C; n=6-8), STAT1-deficient (D; n=3); or IL-21R-deficient (E; n=2) patients, were cultured with TAE beads alone or together with cytokines. Total numbers of live cells that had entered division (A, D and E) from each culture were determined (mean ± SEM). (B) Histograms show representative CTV. Numbers give % of divided cells (mean ± SEM). (C-E) Percentage of CD8+ T cells in each division was determined. * P<0.05; ** P<0.001; *** P<0.001
In cultures receiving cytokines alone, STAT3 mutations impaired the ability of IL-21 to upregulate granzyme B (Fig 3). We therefore determined whether signals provided by TCR/costimulation modulated this impairment. Addition of IL-2, IL-15, IL-21 or IL-21/IL-15 to TAE-stimulated cultures strongly (i.e. >20-fold) up-regulated granzyme B expression in normal naïve CD8+ T cells (Fig 5A,D). In contrast to these cytokines, the effect of IL-7 on granzyme B induction was modest (ie <10% granzyme B+ cells; ref16 and not shown).Mutations in STAT3 (Figure 5A,D) or STAT1 (Figure 5B,D) did not impair the ability of TAE-stimulated T cells to upregulate granzyme B in response to any of the cytokines tested. However, IL-21RMUT naïve CD8+ T cells were unable to upregulate granzyme B following IL-21 stimulation and this was partially recovered by IL-15 (Fig 5C,D). These results demonstrate that stimulation through TCR/costimulation alters the activation of the naïve CD8+ T cells such that STAT3 mutations no longer prevent IL-21-induced expression of the cytotoxic mediator granzyme B.
Figure 5. IL-21 induced granzyme B production is intact in TCR-stimulated STAT3-deficient CD8+ T cells.
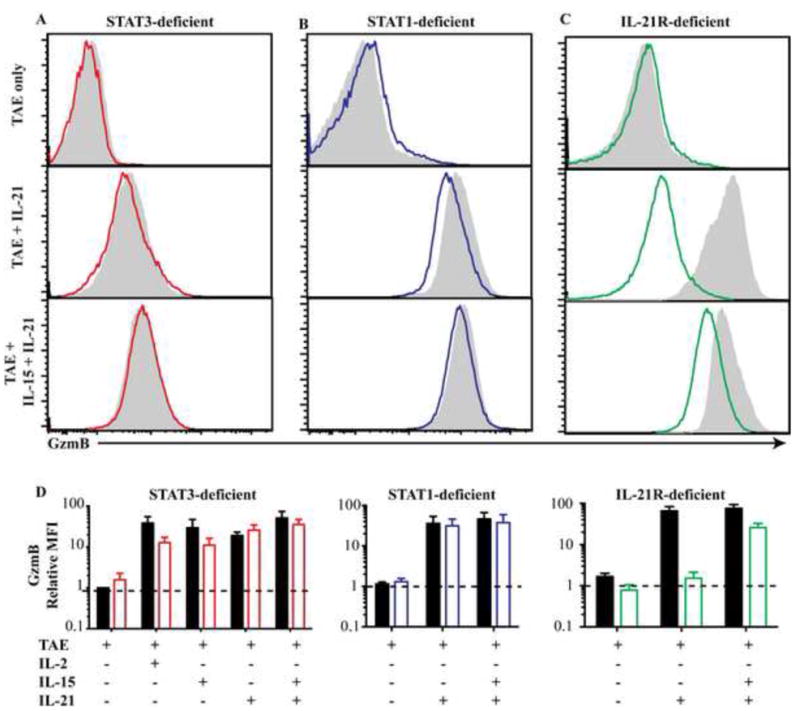
Naive CD8+ T cells from (A) STAT3-deficient (n=6-8), (B) STAT1-deficient (n=3), (C) IL-21R-deficient (n=2) patients or healthy donors (n=6-8), were cultured with TAE beads alone or together with cytokines. Representative histograms of granzyme B expression (filled – normal, coloured lines – patients) are depicted in (A-C). Graphs in (D) show fold-increase (mean ± SEM) in MFI of granzyme B expression by cytokine-stimulated normal and patient cells over those cultured with TAE beads alone.
Mutations in STAT3 and IL21R alter the frequencies of memory CD8+ T cells
Since IL-21 signals through IL-21R to activate STAT1 and STAT3 and regulates effector function we speculated that impaired IL-21R, STAT1 or STAT3 function may also affect CD8+ T cell differentiation in vivo. Therefore, we examined PB CD8+ T cell populations and phenotypes in patients with mutations in these molecules. We found that CD8+ T cells represented 21.0 ± 1.1% of PB lymphocytes in healthy donors. This did not differ for STAT3MUT (23.5 ± 1.5%), STAT1MUT (31.9 ± 3.8%) or IL-21RMUT patients (17.4 ± 2.6%; Fig 6A).
Figure 6. Mutations in STAT3 but not STAT1 impair generation of effector/memory CD8+ T cells in vivo.
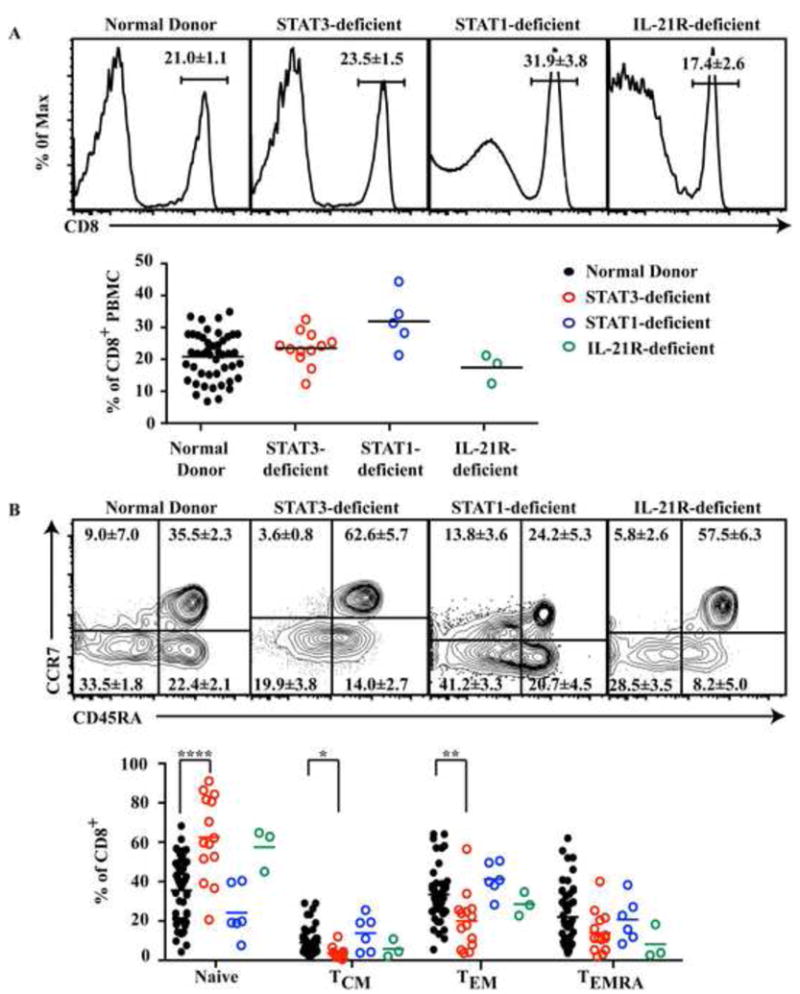
PB from healthy donors (n=46 or 51), STAT3-deficient (n=13), STAT1-deficient (n=5 or 6) or IL-21R-deficient (n=3) patients were assessed for the percentage of (A) total CD8+ T cells or (B) naïve, TCM, TEM and TEMRA cells. Each symbol corresponds to an individual donor or patient and lines represent means. Histograms and dot plots are from one representative donor or patient. ** P<0.001; *** P<0.001
In humans the CD8+ T cell population can be divided into subsets based on differential expression of CD45RA and CCR746. The CD8+ T cell compartment of normal donors thus comprises naïve (35.5 ± 2.3%), TCM (8.9 ± 7.0%), TEM (33.5 ± 1.8%) and TEMRA (22.1 ± 2.1%) cells (Fig 6B). The distribution of these subsets in STAT1-deficient patients did not differ from normal controls (Fig 6B). However, the frequency of naïve CD8+ T cells in STAT3-deficient patients was significantly increased (62.6 ± 5.7%, p< 0.001) compared to normal controls. This was associated with substantial decreases in TEM, TEMRA and TCM cells in STAT3-deficient patients (19.9%± 3.8%; 14.0 ± 2.7% and 3.6 ± 0.8%, respectively; Fig 6B). Furthermore, analysis of CD8+ T cell subsets from the three IL-21R-deficient patients suggested that memory in these patients may also be dysregulated, with increased naïve (57.5 ± 6.3%) and reduced TEMRA (8.2 ± 5.0%) (Fig 6B). It was recently reported that populations of memory and effector CD8+ T cells reach adult levels by ∼5-10 years of age47, 48. This is consistent with our finding that the proportions of memory and effector CD8+ T cells in the cohort of STAT1-deficient patients, the average age of which was 13 years (Table E1), were normal (Fig 6B). Thus, it is unlikely that the decreased frequencies of non-naïve CD8+ T cells in STAT3 and IL-21R-deficient individuals reflects the inclusion of some younger patients in these cohorts. These results suggest that STAT3, but not STAT1, plays an important role in generating and/or maintaining memory CD8+ T cells. Analysis of further IL-21R-deficient patients will be required to determine whether or not the required STAT3 activation is occurring downstream of IL-21 signaling or whether other cytokines, such as IL-1049, are also involved.
To further understand the decrease in memory CD8+ T cell in STAT3-deficient patients we analyzed expression of genes that control their differentiation and survival (Fig E1). BCL2 was significantly higher in STAT3MUT naïve compared to normal naïve CD8+ T cells (Fig E1). This suggests Bcl-2 may play a role in the survival, and thus increased frequency, of naïve CD8+ T cells in STAT3-deficient patients. However, we observed no other ifferences in expression of pro- or anti-apoptotic molecules between patients and controls (Fig E1). Similarly, although transcription factors responsible for CD8+ T cell function and differentiation were differentially expressed across CD8+ T cell populations, we observed no significant differences between normal and STAT3MUT CD8+ T cells (Fig E1).
STAT3-deficient TEM and TEMRA CD8+ T cells have a phenotype suggestive of sustained activation
CD8+ T cell subsets were further assessed for expression of a range of molecules that change during differentiation from naïve to effector cells50, 51. STAT3MUT cells showed dysregulated expression of many of these molecules (Fig 7). For example, 2B4 was highest on normal CD8+ TEM and TEMRA cell, but was increased 2-4 fold on STAT3MUT TEM cells (p<0.001). In contrast, 2B4 was expressed at normal levels on STAT1MUT CD8+ T cells (Fig 7, E2A, E3A). CD57 expression is associated with poorly proliferative terminally differentiated T cells51, 52. Consistent with this, the greatest frequency of CD57+ cells was found in the TEMRA population. However, proportions of CD57+ cells in TEM and TEMRA populations of STAT3MUT CD8+ T cells were significantly increased relative to normal and STAT1MUT cells (Fig 7). CD127 (IL-7Rα) is highly expressed on naïve and TCM cells, and downregulated on TEM and TEMRA cells. However, in STAT3MUT patients all memory populations displayed significantly decreased levels of CD127 compared to healthy donors (Fig 7). Not all activation molecules displayed altered expression however as CD95 was not altered on CD8+ T cells from either the STAT1MUT or STAT3MUT patients (Fig 7, E2A, E3A).
Figure 7. CD8+ T cells from STAT3-deficient display a more activated phenotype.
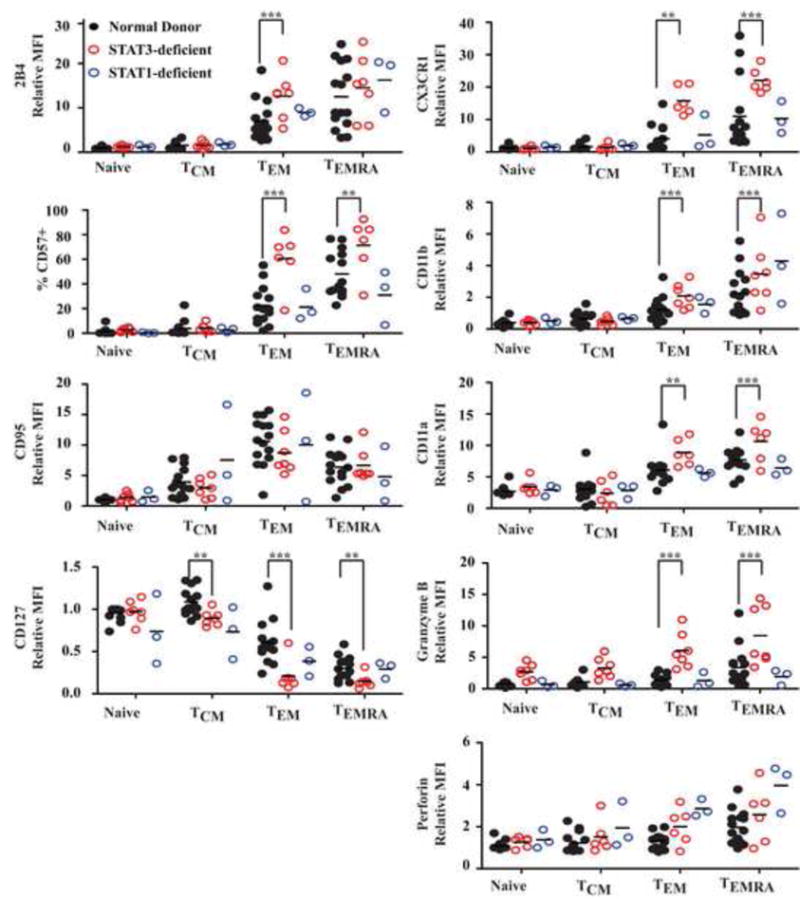
Subsets of naïve, TCM, TEM and TEMRA CD8+ T cells in PB of healthy donors (n=15), STAT3-deficient (n=7), STAT1-deficient (n=3) and IL21R-deficient (n=2) patients were assessed for expression of 2B4, CD57, CD95, CD127, CX3CR1, CD11a, CD11b, granzyme B or perforin. Graphs represent fold-change in MFI of the molecules relative to naïve cells, or the % of positive cells. **, P<0.001; ***, P<0.001
Chemokine receptors and adhesion molecules are important for regulating migration to secondary lymphoid organs and inflamed tissues. CX3CR1, CD11a and CD11b are highest on TEMRA and TEM cells from normal donors. However, their expression on STAT3MUT, but not STAT1MUT, TEM and TEMRA cells was significantly (2-3-fold) higher than on normal cells (Fig 7, E2B, E3B). Interestingly, STAT3MUT TEM and TEMRA CD8+ T cells had significantly higher expression of granzyme B but not perforin. The altered expression of these molecules by STAT3MUT CD8+ T cells suggests they have undergone aberrant differentiation in vivo and the residual effector memory populations exhibit a more senescent/exhausted phenotype.
DISCUSSION
To establish protective anti-viral and anti-tumor immunity, naïve CD8+ T cells must proliferate and acquire effector function, processes believed to be regulated by IL-21. Indeed, IL-21 has been found to increase in vitro proliferation and survival of mouse and human CD8+ T cells6-12, 38, as well as induce effector molecules - IFN-γ, granzyme B, perforin - and enhance their overall cytotoxicity7-10, 16, 42, 43. Furthermore, in vivo delivery of IL-21 to cancer patients upregulated granzyme B and perforin in CD8+ T cells17. Consistent with previous studies in multiple cell types6, 15, 17, 26, IL-21 induced phosphorylation of STAT1, STAT3 and STAT5 in human CD8+ T cells. Yet, the contribution of individual STATs and cytokines to the development and effector function of human CD8+ T cells has not previously been defined. To clarify the requirements for these molecules in human CD8+ T cell function, we analyzed CD8+ T cell differentiation in vivo, and IL-21 signaling in vitro, in individuals with loss-of function mutations in IL21R, STAT1 or STAT3.
The ability of IL-21 to promote proliferation of naïve CD8+ T cells was unaffected by mutations in STAT1 or STAT3. In contrast, impaired STAT3 signaling abolished upregulation of granzyme B in response to IL-15/IL-21 where no exogenous TCR stimulus was provided. This paralleled our observations from cells unable to signal through the IL-21R, thereby suggesting that intact signaling through the IL-21R/STAT3 axis is required for GZMB transcription and subsequent cytotoxicity in human CD8+ T cells stimulated with IL-15/IL-21. These findings are physiologically relevant because they infer that the increase in expression in granzyme B observed in CD8+ T cells of cancer patients who were administered IL-21 in the absence of specific T-cell activation17 was STAT3-dependent. This is supported by work showing that IL-21-induced phosphoSTAT3 binds upstream of Gzmb in murine CD4+ T cells53. The inability of STAT3-deficient CD8+ T cells to upregulate granzyme B in response to IL-15/IL-21 would be further compounded by impaired production of IL-21 by STAT3-deficient CD4+ T cells54. Surprisingly, we observed that when naïve STAT3MUT CD8+ T cells were provided with extrinsic TCR stimulus and IL-21 they were capable of upregulating granzyme B to similar levels as controls. IL-21-induced granzyme B upregulation was also intact in STAT1-deficient naïve CD8+ T cells suggesting that TCR/costimulation did not result in a switch from a STAT3 to a STAT1 pathway downstream of IL-21R for granzyme B regulation. Rather TCR/costimulatory signaling is likely to alter the sensitivity of cells to STAT3 such that residual STAT3 activity in STAT3MUT CD8+ T cells is sufficient to induce a cytotoxic response.
Studies in mice have suggested that IL-21 is important for controlling some viral infections, such as vaccina18 and chronic LCMV19-21. AD-HIES patients generally do not exhibit heightened susceptibility to primary viral infection, but some (<20%) do have an impaired ability to control reactivation of herpes viruses (HSV, EBV, VZV)29, 30. On the other hand, the few IL-21R-deficient individuals documented to date appear capable of mounting protective responses to these viruses, yet experience ongoing infection with norovirus and rhinovirus37. Both of these PIDs though are associated with increased susceptibility to infection with bacterial and fungal pathogens (Staphylococcus aureus, Candida albicans, Pneumocystis, cryptosporidia)28, 29, 37, 55, 56. Our findings actually provide an explanation for the relatively mild susceptibility of STAT3MUT and IL-21RMUT individuals to primary viral infection, despite predictions from mouse models18-21, 49: first, delivery of strong TCR and costimulatory signals during viral infection would facilitate normal induction of granzyme B in IL-21-stimulated STAT3MUT cells. Second, granzyme B induction by IL-2 and IL-15 is intact in STAT3MUT and IL-21RMUT CD8+ T cells. Thus, during most viral infections, combined signals by TCR/costimulation, IL-2, IL-15 and/or IL-21 in naïve CD8+ T cells would be sufficient to generate a protective cytotoxic thereby circumventing the dependency on signaling through IL-21R or STAT3.
An interesting feature of AD-HIES is predisposition to B-lymphoma29, 31, 32, 57. It is possible that during an anti-tumor response immune activation is not as strong as during viral infection; thus the relative contribution of IL-21R/STAT3 signaling in regulating granzyme B may be greater. Consequently, STAT3 mutations may contribute to impaired CD8+ T cell immune surveillance against B-cell malignancies. This is reminiscent of the susceptibility of perforin-deficient individuals to hematological neoplasms, including B-lymphoma58.
The reported role of IL-21 in memory cell development12, 18, 49 prompted us to investigate the phenotype of CD8+ T cells from STAT1- and STAT3-deficient patients. Recent studies reported reduced frequencies of TCM cells in AD-HIES patients30 thereby implicating STAT3 in the development of „central memory’ CD8+ T cells30, 49. We found significant decreases not only in TCM but also in TEM cells in STAT3-deficient, but not STAT1-deficient, individuals. These changes were not due to alterations in the total percentages of CD8+ T cells or age-related variations in the number of naïve versus memory cells (Fig E4A-D). TEM and TEMRA populations from STAT3-deficient patients also displayed a phenotype of exaggerated differentiation often associated with increased/sustained exposure to antigen50, 51, 59. Assessment of IL-21R-deficient patients suggested IL-21 may contribute to establishing some of the memory populations in STAT3-deficient individuals. However, to determine whether IL-21 is the STAT3-activating cytokine required for maintaining CD8+ T cell memory analysis of additional IL-21R-deficient patients will be required.
Several explanations can be proposed for the memory CD8+ T cell deficiency in AD-HIES. First, STAT3 mutations may affect CD8+ T cell homeostasis or differentiation. However, proliferation of STAT3MUT naïve CD8+ T cells in response to IL-7 or IL-21/IL-15, which regulate homeostasis of CD8+ T cells2, 9, 38, 42, 60-64, was normal. Thus, there is no evidence that the homeostatic proliferation and survival of naïve T cells induced by these cytokines requires STAT3, implying that the memory cell deficit is unlikely to be caused by impaired proliferation. Second, impaired STAT3 function may alter CD8+ memory T cell numbers through effects on differentiation. CD8+ T cell differentiation is regulated by multiple transcription factors that control opposing fates: Eomes and Bcl-6 favor “central memory” CD8+ T cell development, whereas T-bet and Blimp-1 promote differentiation to “effector” CD8+ T cells13, 65-69. The observations that Socs3, Tbx21, Bcl6 and Prdm1 are direct targets of STAT353, together with reduced expression of these genes in Stat3-/- murine CD8+ T cells post-infection with LCMV49, and of SOCS3 and BCL6 in ex vivo isolated STAT3-deficient human naïve CD8+ T cells30, suggest that STAT3 regulates CD8+ T cell differentiation by controlling expression of transcription factors. Consistent with this we observed decreased SOCS3 expression in IL-21-stimulated STAT3-deficient naïve CD8+ T cells in vitro. Thus, as proposed previously49, decreased SOCS3 expression may contribute to aberrant differentiation resulting in the enhanced activated phenotype we observed in TEM and TEMRA cells in STAT3MUT patients. However, we saw no significant differences in the levels of other transcription factors between normal and STAT3-deficient CD8+ T cells either directly ex vivo (Fig E1) or following IL-21 stimulation (Fig E5). This suggests that the IL-21/STAT3 axis proposed to drive Bcl-6 expression and CD8+ TCM cell generation may be an oversimplification. Instead, STAT3 appears to have broader effects, being required for the generation or maintenance of not only TCM cells, but also TEM and TEMRA populations.
Lastly, reduced memory CD8+ T cell frequencies in STAT3MUT individuals may reflect reduced signaling through IL-7R (CD127). IL-7 is another homeostatic cytokine important for memory T cell maintenance39, 61, 63, 64. Interestingly, CD127 was significantly decreased on all memory populations from STAT3-deficient patients compared to normal donors consistent with a more activated phenotype. Thus, reduced expression of CD127 on STAT3-deficient CD8+ T cells may limit their responsiveness to IL-7 signals, thereby compromising the pro-survival effects of IL-7 and compounding any decrease in memory cell numbers caused by reduced memory cell differentiation.
These findings reveal STAT3, but not STAT1, as an important downstream component of IL-21 signaling that mediates induction of effector function in CD8+ T cells. However, a high level of redundancy for the induction of cytotoxic function seems to exist suggesting that in most circumstances a functional level of killing would still be generated. This is consistent with mild susceptibility to viral infections in either AD-HIES or IL-21R-deficiency. In contrast, STAT3 signals, possibly initiated by IL-21, are critical for regulating the pool of all memory CD8+ T cell subsets in humans. Collectively, these insights significantly add to our understanding of the function of IL-21 and STAT3 in human CD8+ T cell development and behavior, and disease pathogenesis in individuals with mutations in STAT3 and IL21R.
Supplementary Material
Key Messages.
loss-of function mutations in STAT3, causing autosomal dominant hyper-IgE syndrome, and IL21R, compromise differentiation of human CD8+ T cells to memory and effector cells
mutations in STAT3, but not STAT1, abrogate the ability of human CD8+ T cells to differentiate into granzyme B-expressing effector cells in response to IL-21
Acknowledgments
We thank the patients and their families for their involvement in this work.
This work was funded by project and program grants from the National Health and Medical Research Council (NHMRC) of Australia (to EKD, SGT, CSM, DAF, MCC), Cancer Council NSW (to SGT, UP) and Rockefeller University Center for 541 Clinical and Translational science (5UL1RR024143, to JLC). CSM is a recipient of a Career Development Fellowship and SGT a Principal Research Fellowship from the NHMRC of Australia.
Abbreviations
- AD-HIES
autosomal dominant hyper-IgE syndrome
- AR
autosomal recessive
- IL-21R
IL-21 receptor
- BCL-2
B cell lymphoma 2
- EOMES
Eomesodermin
- LCMV
Lymphocytic choriomeningitis virus
- PID
primary immune deficiency
- TCR
T cell receptor
- TCM
central memory T cells
- TEM
effector memory T cells
- TEMRA
effector memory T cells expressing CD45RA
- TAE
T cell activation and expansion beads
- EBV
Epstein Barr Virus
- CMV
cytomegalovirus
- VZV
varicella zoster virus
- CTV
Cell Trace Violet
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest
SGT and EKD contributed equally to this study
References cited
- 1.Becker TC. Interleukin 15 Is Required for Proliferative Renewal of Virus-specific Memory CD8 T Cells. J Exp Med. 2002;195:1541–8. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lodolce JP, Burkett PR, Koka RM, Boone DL, Ma A. Regulation of lymphoid homeostasis by interleukin-15. Cytokine Growth Factor Rev. 2002;13:429–39. doi: 10.1016/s1359-6101(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 3.Surh CD, Sprent J. Homeostasis of Naive and Memory T Cells. Immunity. 2008;29:848–62. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Pipkin ME, Sacks JA, Cruz-Guilloty F, Lichtenheld MG, Bevan MJ, Rao A. Interleukin-2 and Inflammation Induce Distinct Transcriptional Programs that Promote the Differentiation of Effector Cytolytic T Cells. Immunity. 2010;32:79–90. doi: 10.1016/j.immuni.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kalia V, Sarkar S, Subramaniam S, Haining WN, Smith KA, Ahmed R. Prolonged Interleukin-2Rα Expression on Virus-Specific CD8+ T Cells Favors Terminal-Effector Differentiation In Vivo. Immunity. 2010;32:91–103. doi: 10.1016/j.immuni.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Zeng R, Spolski R, Casas E, Zhu W, Levy DE, Leonard WJ. The molecular basis of IL-21-mediated proliferation. Blood. 2007;109:4135–42. doi: 10.1182/blood-2006-10-054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey KA, Mescher MF. IL-21 Promotes Differentiation of Naive CD8 T Cells to a Unique Effector Phenotype. J Immunol. 2007;178:7640–8. doi: 10.4049/jimmunol.178.12.7640. [DOI] [PubMed] [Google Scholar]
- 8.Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, et al. IL-21 Limits NK Cell Responses and Promotes Antigen-Specific T Cell Activation: A Mediator of the Transition from Innate to Adaptive Immunity. Immunity. 2002;16:559–69. doi: 10.1016/s1074-7613(02)00295-9. [DOI] [PubMed] [Google Scholar]
- 9.Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, et al. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J Exp Med. 2005;201:139–48. doi: 10.1084/jem.20041057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barker BR, Parvani JG, Meyer D, Hey AS, Skak K, Letvin NL. IL-21 Induces Apoptosis of Antigen-Specific CD8+ T Lymphocytes. J Immunol. 2007;179:3596–603. doi: 10.4049/jimmunol.179.6.3596. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Bleakley M, Yee C. IL-21 Influences the Frequency, Phenotype, and Affinity of the Antigen-Specific CD8 T Cell Response. J Immunol. 2005;175:2261–9. doi: 10.4049/jimmunol.175.4.2261. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen H, Weng Np. IL-21 preferentially enhances IL-15-mediated homeostatic proliferation of human CD28+ CD8 memory T cells throughout the adult age span. J Leukoc Biol. 2010;87:43–9. doi: 10.1189/jlb.0209086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ichii H, Sakamoto A, Hatano M, Okada S, Toyama H, Taki S, et al. Role for Bcl-6 in the generation and maintenance of memory CD8+ T cells. Nat Immunol. 2002;3:558–63. doi: 10.1038/ni802. [DOI] [PubMed] [Google Scholar]
- 14.Ostiguy V, Allard EL, Marquis M, Leignadier J, Labrecque N. IL-21 promotes T lymphocyte survival by activating the phosphatidylinositol-3 kinase signaling cascade. J Leukoc Biol. 2007;82:645–56. doi: 10.1189/jlb.0806494. [DOI] [PubMed] [Google Scholar]
- 15.Strengell M, Sareneva T, Foster D, Julkunen I, Matikainen S. IL-21 Up-Regulates the Expression of Genes Associated with Innate Immunity and Th1 Response. J Immunol. 2002;169:3600–5. doi: 10.4049/jimmunol.169.7.3600. [DOI] [PubMed] [Google Scholar]
- 16.Parmigiani A, Pallin MF, Schmidtmayerova H, Lichtenheld MG, Pahwa S. Interleukin-21 and cellular activation concurrently induce potent cytotoxic function and promote antiviral activity in human CD8 T cells. Hum Immunol. 2011;72:115–23. doi: 10.1016/j.humimm.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frederiksen KS, Lundsgaard D, Freeman JA, Hughes SD, Holm TL, Skrumsager BK, et al. IL-21 induces in vivo immune activation of NK cells and CD8(+) T cells in patients with metastatic melanoma and renal cell carcinoma. Cancer Immunol Immunother. 2008;57:1439–49. doi: 10.1007/s00262-008-0479-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Novy P, Huang X, Leonard WJ, Yang Y. Intrinsic IL-21 Signaling Is Critical for CD8 T Cell Survival and Memory Formation in Response to Vaccinia Viral Infection. J Immunol. 2011;186:2729–38. doi: 10.4049/jimmunol.1003009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsaesser H, Sauer K, Brooks DG. IL-21 Is Required to Control Chronic Viral Infection. Science. 2009;324:1569–72. doi: 10.1126/science.1174182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frohlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, et al. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science. 2009;324:1576–80. doi: 10.1126/science.1172815. [DOI] [PubMed] [Google Scholar]
- 21.Yi JS, Du M, Zajac AJ. A vital role for interleukin-21 in the control of a chronic viral infection. Science. 2009;324:1572–6. doi: 10.1126/science.1175194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ma HL, Whitters MJ, Konz RF, Senices M, Young DA, Grusby MJ, et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-gamma. J Immunol. 2003;171:608–15. doi: 10.4049/jimmunol.171.2.608. [DOI] [PubMed] [Google Scholar]
- 23.Di Carlo E, Comes A, Orengo AM, Rosso O, Meazza R, Musiani P, et al. IL-21 induces tumor rejection by specific CTL and IFN-gamma-dependent CXC chemokines in syngeneic mice. J Immunol. 2004;172:1540–7. doi: 10.4049/jimmunol.172.3.1540. [DOI] [PubMed] [Google Scholar]
- 24.Moroz A, Eppolito C, Li Q, Tao J, Clegg CH, Shrikant PA. IL-21 enhances and sustains CD8+ T cell responses to achieve durable tumor immunity: comparative evaluation of IL-2, IL-15, and IL-21. J Immunol. 2004;173:900–9. doi: 10.4049/jimmunol.173.2.900. [DOI] [PubMed] [Google Scholar]
- 25.Markley JC, Sadelain M. IL-7 and IL-21 are superior to IL-2 and IL-15 in promoting human T cell-mediated rejection of systemic lymphoma in immunodeficient mice. Blood. 2010;115:3508–19. doi: 10.1182/blood-2009-09-241398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Asao H, Okuyama C, Kumaki S, Ishii N, Tsuchiya S, Foster D, et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J Immunol. 2001;167:1–5. doi: 10.4049/jimmunol.167.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Habib T, Senadheera S, Weinberg K, Kaushansky K. The common gamma chain (gamma c) is a required signaling component of the IL-21 receptor and supports IL-21-induced cell proliferation via JAK3. Biochemistry. 2002;41:8725–31. doi: 10.1021/bi0202023. [DOI] [PubMed] [Google Scholar]
- 28.Grimbacher B, Holland SM, Puck JM. Hyper-IgE syndromes. Immunol Rev. 2005;203:244–50. doi: 10.1111/j.0105-2896.2005.00228.x. [DOI] [PubMed] [Google Scholar]
- 29.Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore) 2012;91:e1–19. doi: 10.1097/MD.0b013e31825f95b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Siegel A, Heimall J, Freeman A, Hsu A, Brittain E, Brenchley J, et al. A Critical Role for STAT3 Transcription Factor Signaling in the Development and Maintenance of Human T Cell Memory. Immunity. 2011;35:806–18. doi: 10.1016/j.immuni.2011.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumánovics A, Perkins S, Gilbert H, Cessna M, Augustine N, Hill H. Diffuse Large B Cell Lymphoma in Hyper-IgE Syndrome Due To STAT3 Mutation. J Clin Immunol. 2010;30:886–93. doi: 10.1007/s10875-010-9452-z. [DOI] [PubMed] [Google Scholar]
- 32.Jiao H, Toth B, Erdos M, Fransson I, Rakoczi E, Balogh I, et al. Novel and recurrent STAT3 mutations in hyper-IgE syndrome patients from different ethnic groups. Mol Immunol. 2008;46:202–6. doi: 10.1016/j.molimm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 33.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–19. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 34.Minegishi Y, Saito M, Tsuchiya S, Tsuge I, Takada H, Hara T, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–62. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 35.Boisson-Dupuis S, Kong XF, Okada S, Cypowyj S, Puel A, Abel L, et al. Inborn errors of human STAT1: allelic heterogeneity governs the diversity of immunological and infectious phenotypes. Curr Opin Immunol. 2012;24:364–78. doi: 10.1016/j.coi.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casanova JL, Holland Steven M, Notarangelo Luigi D. Inborn Errors of Human JAKs and STATs. Immunity. 2012;36:515–28. doi: 10.1016/j.immuni.2012.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kotlarz D, Zietara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013;210:433–43. doi: 10.1084/jem.20111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Alves NL, Arosa FA, van Lier RAW. IL-21 Sustains CD28 Expression on IL-15-Activated Human Naive CD8+ T Cells. J Immunol. 2005;175:755–62. doi: 10.4049/jimmunol.175.2.755. [DOI] [PubMed] [Google Scholar]
- 39.Melchionda F, Fry TJ, Milliron MJ, McKirdy MA, Tagaya Y, Mackall CL. Adjuvant IL-7 or IL-15 overcomes immunodominance and improves survival of the CD8+ memory cell pool. J Clin Inves. 2005;115:1177–87. doi: 10.1172/JCI23134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Trapani JA, Smyth MJ. Functional significance of the perforin/granzyme cell death pathway. Nat Rev Immunol. 2002;2:735–47. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 41.Tamand DL, Redelman D, Alves BN, Vollger L, Bethley C, Hudig D. Induction of granzyme B and T cell cytotoxic capacity by IL-2 or IL-15 without antigens: Multiclonal responses that are extremely lytic if triggered and short-lived after cytokine withdrawal. Cytokine. 2006;36:148–59. doi: 10.1016/j.cyto.2006.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White L, Krishnan S, Strbo N, Liu H, Kolber MA, Lichtenheld MG, et al. Differential effects of IL-21 and IL-15 on perforin expression, lysosomal degranulation, and proliferation in CD8 T cells of patients with human immunodeficiency virus-1 (HIV) Blood. 2007;109:3873–80. doi: 10.1182/blood-2006-09-045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Strengell M, Matikainen S, Sirén J, Lehtonen A, Foster D, Julkunen I, et al. IL-21 in Synergy with IL-15 or IL-18 Enhances IFN-γ Production in Human NK and T Cells. J Immunol. 2003;170:5464–9. doi: 10.4049/jimmunol.170.11.5464. [DOI] [PubMed] [Google Scholar]
- 44.Lin JX, Migone TS, Tsang M, Friedmann M, Weatherbee JA, Zhou L, et al. The role of shared receptor motifs and common Stat proteins in the generation of cytokine pleiotropy and redundancy by IL-2, IL-4, IL-7, IL-13, and IL-15. Immunity. 1995;2:331–9. doi: 10.1016/1074-7613(95)90141-8. [DOI] [PubMed] [Google Scholar]
- 45.Yu CR, Young HA, Ortaldo JR. Characterization of cytokine differential induction of STAT complexes in primary human T and NK cells. J Leukoc Biol. 1998;64:245–58. doi: 10.1002/jlb.64.2.245. [DOI] [PubMed] [Google Scholar]
- 46.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999 doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 47.van Gent R, van Tilburg CM, Nibbelke EE, Otto SA, Gaiser JF, Janssens-Korpela PL, et al. Refined characterization and reference values of the pediatric T- and B-cell compartments. Clin Immunol. 2009;133:95–107. doi: 10.1016/j.clim.2009.05.020. [DOI] [PubMed] [Google Scholar]
- 48.Schatorje EJ, Gemen EF, Driessen GJ, Leuvenink J, van Hout RW, de Vries E. Paediatric reference values for the peripheral T cell compartment. Scand J Immunol. 2012;75:436–44. doi: 10.1111/j.1365-3083.2012.02671.x. [DOI] [PubMed] [Google Scholar]
- 49.Cui W, Liu Y, Weinstein Jason S, Craft J, Kaech Susan M. An Interleukin-21- Interleukin-10-STAT3 Pathway Is Critical for Functional Maturation of Memory CD8+ T Cells. Immunity. 2011;35:792–805. doi: 10.1016/j.immuni.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Appay V, van Lier RAW, Sallusto F, Roederer M. Phenotype and function of human T lymphocyte subsets: Consensus and issues. Cytometry A. 2008;73A:975–83. doi: 10.1002/cyto.a.20643. [DOI] [PubMed] [Google Scholar]
- 51.Focosi D, Bestagno M, Burrone O, Petrini M. CD57+ T lymphocytes and functional immune deficiency. J Leukoc Biol. 2010;87:107–16. doi: 10.1189/jlb.0809566. [DOI] [PubMed] [Google Scholar]
- 52.Brenchley JM, Karandikar NJ, Betts MR, Ambrozak DR, Hill BJ, Crotty LE, et al. Expression of CD57 defines replicative senescence and antigen-induced apoptotic death of CD8+ T cells. Blood. 2003;101:2711–20. doi: 10.1182/blood-2002-07-2103. [DOI] [PubMed] [Google Scholar]
- 53.Kwon H, Thierry-Mieg D, Thierry-Mieg J, Kim HP, Oh J, Tunyaplin C, et al. Analysis of Interleukin-21-Induced Prdm1 Gene Regulation Reveals Functional Cooperation of STAT3 and IRF4 Transcription Factors. Immunity. 2009;31:941–52. doi: 10.1016/j.immuni.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ma CS, Avery DT, Chan A, Batten M, Bustamante J, Boisson-Dupuis S, et al. Functional STAT3 deficiency compromises the generation of human T follicular helper cells. Blood. 2012;119:3997–4008. doi: 10.1182/blood-2011-11-392985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minegishi Y. Hyper-IgE syndrome. Curr Opin Immunol. 2009;21:487–92. doi: 10.1016/j.coi.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 56.Kotlarz D, Zietara N, Uzel G, Weidemann T, Braun CJ, Diestelhorst J, et al. Loss-of-function mutations in the IL-21 receptor gene cause a primary immunodeficiency syndrome. J Exp Med. 2013 doi: 10.1084/jem.20111229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Heimall J, Freeman A, Holland S. Pathogenesis of Hyper IgE Syndrome. Clinic Rev Allerg Immunol. 2010;38:32–8. doi: 10.1007/s12016-009-8134-1. [DOI] [PubMed] [Google Scholar]
- 58.Brennan AJ, Chia J, Trapani JA, Voskoboinik I. Perforin deficiency and susceptibility to cancer. Cell Death Differ. 2010;17:607–15. doi: 10.1038/cdd.2009.212. [DOI] [PubMed] [Google Scholar]
- 59.Randall KL, Chan SS, Ma CS, Fung I, Mei Y, Yabas M, et al. DOCK8 deficiency impairs CD8 T cell survival and function in humans and mice. J Exp Med. 2011;208:2305–20. doi: 10.1084/jem.20110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Berard M, Brandt K, Paus SB, Tough DF. IL-15 Promotes the Survival of Naive and Memory Phenotype CD8+ T Cells. J Immunol. 2003;170:5018–26. doi: 10.4049/jimmunol.170.10.5018. [DOI] [PubMed] [Google Scholar]
- 61.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–32. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 62.Zhang X, Sun S, Hwang I, Tough DF, Sprent J. Potent and Selective Stimulation of Memory Phenotype CD8+ T Cells In Vivo by IL-15. Immunity. 1998;8:591–9. doi: 10.1016/s1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 63.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191–8. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 64.Maraskovsky E, Teepe M, Morrissey P, Braddy S, Miller R, Lynch D, et al. Impaired survival and proliferation in IL-7 receptor-deficient peripheral T cells. J Immunol. 1996;157:5315–23. [PubMed] [Google Scholar]
- 65.Banerjee A, Gordon SM, Intlekofer AM, Paley MA, Mooney EC, Lindsten T, et al. Cutting Edge: The Transcription Factor Eomesodermin Enables CD8+ T Cells To Compete for the Memory Cell Niche. J Immunol. 2010;185:4988–92. doi: 10.4049/jimmunol.1002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ichii H, Sakamoto A, Arima M, Hatano M, Kuroda Y, Tokuhisa T. Bcl6 is essential for the generation of long-term memory CD4+ T cells. Int Immuno. 2007;19:427–33. doi: 10.1093/intimm/dxm007. [DOI] [PubMed] [Google Scholar]
- 67.Ichii H, Sakamoto A, Kuroda Y, Tokuhisa T. Bcl6 Acts as an Amplifier for the Generation and Proliferative Capacity of Central Memory CD8 + T Cells. J Immunol. 2004;173:883–91. doi: 10.4049/jimmunol.173.2.883. [DOI] [PubMed] [Google Scholar]
- 68.Intlekofer AM, Takemoto N, Wherry EJ, Longworth SA, Northrup JT, Palanivel VR, et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat Immunol. 2005;6:1236–44. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 69.Rutishauser RL, Martins GA, Kalachikov S, Chandele A, Parish IA, Meffre E, et al. Transcriptional Repressor Blimp-1 Promotes CD8+ T Cell Terminal Differentiation and Represses the Acquisition of Central Memory T Cell Properties. Immunity. 2009;31:296–308. doi: 10.1016/j.immuni.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


