Abstract
Isolation of Circulating tumor cells (CTCs) from peripheral blood or cancer cells from bone marrow has significant applications in cancer diagnosis, therapy monitoring and drug development. CTCs are cancer cells shed from primary tumors; they circulate in the bloodstream, leading to metastasis. The extraordinary rarity of CTCs in the bloodstream makes their isolation a significant technological challenge. Herein, we report the development of a platform combining multivalent DNA aptamer nanospheres with microfluidic devices for efficient isolation of cancer cells from blood. Gold nanoparticles (AuNPs) were used as an efficient platform for assembling a number of aptamers for high-efficiency cell capture. Up to 95 aptamers were attached onto each AuNP, resulting in enhanced molecular recognition capability. An increase of 39-fold in binding affinity was confirmed by flow cytometry for AuNP-aptamer conjugates (AuNP-aptamer) when compared with aptamer alone. With a laminar flow flat channel microfluidic device, the capture efficiency of human acute leukemia cells from a cell mixture in buffer increased from 49% using aptamer alone to 92% using AuNP-aptamer. We also employed AuNP-aptamer in a microfluidic device with herringbone mixing microstructures for isolation of leukemia cells in whole blood. The cell capture efficiency was also significantly increased with the AuNP-aptamer over aptamer alone, especially at high flow rates. Our results show that the platform combining DNA nanostructures with microfluidics has a great potential for sensitive isolation of CTCs, and is promising for cancer diagnosis and prognosis.
Keywords: gold nanoparticles, circulating tumor cells, multivalency, microfluidics, aptamer, cell isolation
The isolation of rare cells in peripheral blood such as circulating tumor cells (CTCs) is highly important but challenging.1–3 CTCs are cancer cells shed from either primary tumors or metastatic sites and are highly related to the initiation of metastasis and the spread of cancer to distant organs. Thus CTCs hold the key for understanding metastasis, diagnosing cancer and monitoring treatment response.4–6 However, the extraordinary rarity of CTCs makes their isolation and characterization technically challenging. Traditionally, methods based on flow cytometry have been used in clinics, but with a considerable number of false negatives and low detection sensitivity.7, 8 The only FDA-approved CTC enumeration method is CellSearch Assay, which uses antibody-coated magnetic beads for CTC isolation. However, it suffers from low CTC-capture efficiency.9, 10 Recently, microfluidic devices with monovalent capture ligands, including antibodies11–15 and nucleic acid aptamers,16–18 have been extensively used for immunocapture of rare tumor cells. However, most efforts for increasing the sensitivity of cell capture are based on engineering complicated structures inside the microfluidic devices, such as microposts, sinusoidal channels, and silicon nanopillars, etc., for enhancing ligand-cell interactions.19–23 These structures make the device fabrication time-consuming and induce significant nonspecific cell capture, causing low specificity.
Herein, we have investigated use of nanotechnology-based high affinity binding to enhance cell capture in microfluidic devices. Multivalent binding, the simultaneous interaction of multiple ligands on one entity with the complementary receptors on another, has been widely used for achieving high-affinity molecular recognition in biological processes.24–28 The multivalency-enhanced binding between the ligands and targets in those biological systems has been extensively investigated.29–31 To achieve multivalent binding, scaffolds from numerous nanoscale structures, such as dendrimers,32, 33 nanorods,34 nanoparticles,35 polymers36 and proteins, have been used by researchers for assembling multiple ligands. And dendrimer-mediated multivalent binding have been used for enhanced surface capture of cells.33 Recently, nucleic acid aptamers have been selected for targeting numerous cancers,37, 38 and nanomaterial-aptamer conjugates have been extensively used for enhanced molecular recognition, but none of them have been used for enhancing capturing cancer cells.39–43 Here, we hypothesize the nanoparticle-aptamer conjugates could greatly improve the efficiency of capturing cancer cells. We chose gold nanoparticles (AuNPs) as the multivalent ligand scaffolds to assemble multiple DNA aptamers (DNA nanospheres) owing to their easy synthesis and conjugation with DNA.44, 45 Our flow cytometric analysis demonstrated the multivalent binding between aptamers and cells through AuNP-conjugation. Then we developed a flat channel microfluidic device which is able to capture cancer cells from buffer or lysed blood with high efficiency and high throughput using the AuNP-aptamer conjugates (AuNP-aptamer). The enhanced binding affinity afforded by the AuNP-aptamer modified surface significantly increased the capture efficiency of target cancer cells. And the AuNP-aptamer maintained high capture efficiency with increased flow rate, which considerably improves the sample throughput of the microfluidic device.
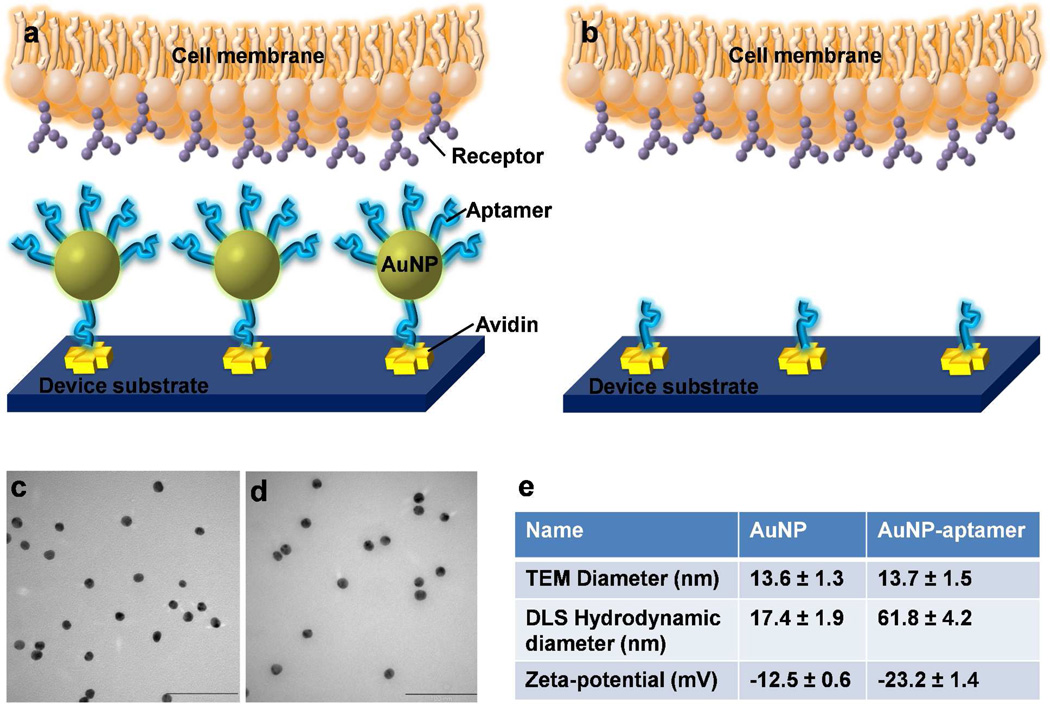
The scheme of the AuNP-aptamer mediated cell capture is shown in Figure 1. The microfluidic device surface is first coated with avidin by physical adsorption.16, 18 Then, biotinylated aptamer-conjugated AuNPs are immobilized onto the channel through biotin-avidin interaction. When a sample containing target cancer cells passes through the channel, cells are captured via the specific interaction between the aptamers and the target cell receptors. Since each AuNP is conjugated with ~95 aptamers, we hypothesize that the AuNP-aptamer binds to cell surface markers in a cooperative manner, leading to multivalent effect and resulting in enhanced cell capture efficiency. Besides the multivalent binding, the AuNP-aptamer modified surface increases the surface roughness23 and allows enhanced local topographic interactions between the AuNP-aptamers and nanoscale receptors on the cell surface,21, 46, 47 contributing to the increased cell capture.
Figure 1.
a–b) Illustration of enhanced cell capture using AuNP-aptamer modified surface. With AuNP conjugation (a), multiple aptamers on the AuNP surfaces bind with multiple receptors on the cell membrane, leading to cooperative, multivalent interactions; Without AuNP (b), aptamer alone binds with receptors via monovalent interaction, with much less interactions. c–d) Transmission electron microscopy (TEM) image of AuNPs (c), and AuNPs conjugated with aptamers (d), scale bar = 100 nm. e) Comparison between AuNP and AuNP-aptamer in terms of particle diameters from TEM images, hydrodynamic diameters from dynamic light scattering (DLS) measurements, and zeta-potential measurements.
RESULTS AND DISCUSSION
Synthesis and Characterization of AuNP-aptamer Conjugates
AuNPs were prepared following the methods detailed in the experimental section. Figure 1c shows the transmission electron microscopy (TEM) image of the AuNPs, with an average diameter of 13.6 nm. The as-prepared AuNPs were then functionalized with thiol-modified DNA aptamers, and the TEM image is shown in Figure 1d, with average size of 13.7 nm. A 24-unit polyethylene glycol (PEG) spacer between AuNP surface and aptamers was added to minimize the steric effects of the particle surface on aptamers and to increase the loading of DNA on AuNPs.48 Figure 1c& d show that the properties of AuNPs remained unchanged after conjugation with aptamers, without any aggregation. Dynamic light scattering (DLS) measurements showed that the hydrodynamic diameter of AuNPs was 17.4 nm. After conjugation with aptamers, the hydrodynamic diameter increased to 61.8 nm, demonstrating the successful conjugation of aptamers onto AuNPs (Figure S1 in the Supporting Info.). Zeta-potential measurements indicated that the AuNPs had a zeta potential of −12.5 mV. After modification with aptamers, the zeta-potential became −23.2 mV, which is attributed to the negative charges carried by DNA aptamers. The comparison of properties between AuNPs and AuNP-aptamers is made in Figure 1e.
Flow Cytometric Analysis Demonstrating High Affinity Binding
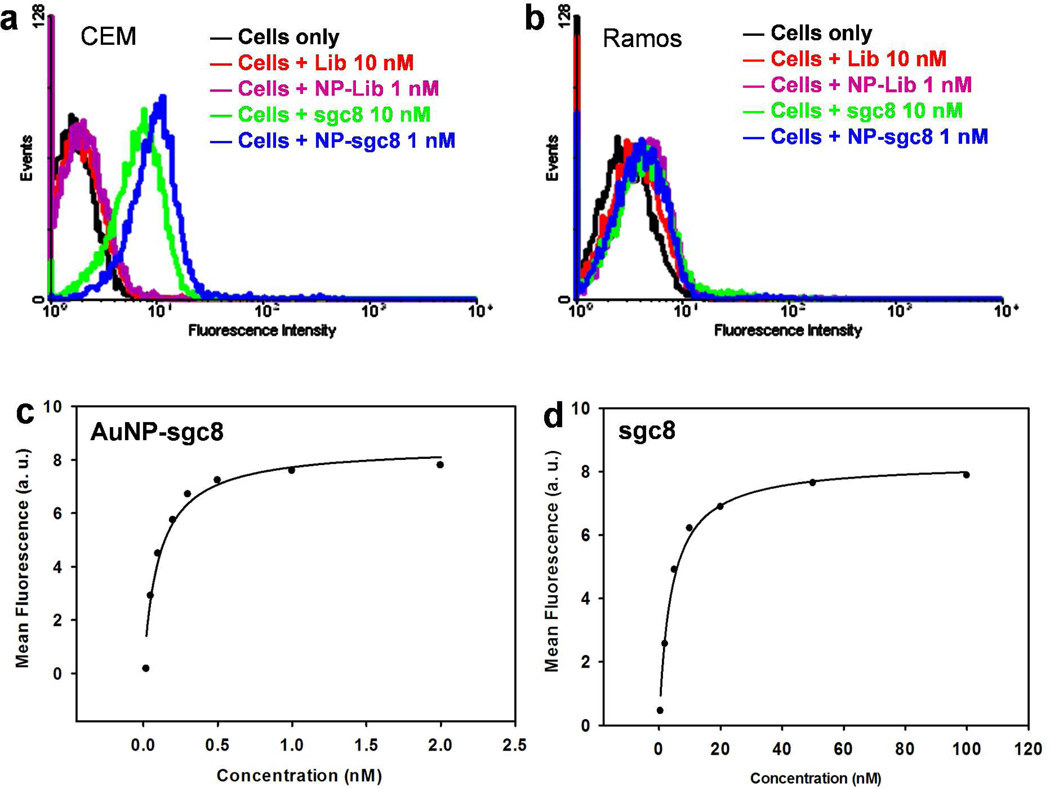
To investigate the AuNP-aptamer mediated multivalent binding, we directly measured the binding behaviors of AuNP-sgc8 aptamer conjugates (AuNP-sgc8) and free sgc8 aptamer (sgc8) using flow cytometry. Sgc8 is an aptamer that has specific binding with CEM cells (human acute lymphoblastic leukemia), with a nanomolar (nM) dissociation constant (Kd).37 Ramos cells (human Burkitt’s lymphoma) that do not bind with sgc8 aptamer were used as control cells here. Figure 2a shows a noticeable increase in fluorescence signal for both AuNP-sgc8 and free sgc8 aptamer compared to the random DNA library (Lib) and AuNP-Lib, proving that both have strong binding with their target cells. Besides, AuNP-sgc8 produces a higher fluorescence signal than free sgc8, even with 10 times lower concentration. As shown in Figure 2b, neither free sgc8 nor AuNP-sgc8 shows a signal increase when incubated with control Ramos cells, demonstrating the specificity of both free aptamers and AuNP-aptamers. Furthermore, the binding affinity of sgc8 and AuNP-sgc8 to CEM cells was measured quantitatively by studying their binding with varying concentrations of sgc8 and AuNP-sgc8 aptamers. As demonstrated in Figure 2c& d, AuNP-sgc8 shows a 39-times higher binding affinity (Kd = 0.10 ± 0.02 nM) than that of free sgc8 (Kd = 3.9 ± 0.5 nM). The lower dissociation constant of AuNP-sgc8 suggests a multivalent-mediated enhancement in binding affinity when multiple aptamers on the AuNP surface bind to multiple receptors on the cell membrane.
Figure 2.
Flow cytometry shows the strong and specific binding of AuNP-sgc8 aptamer conjugates (AuNP-sgc8) with target CEM cells. a) CEM cells selectively bind with free sgc8 and AuNP-sgc8 aptamers; negligible signal change was observed for cells incubated with random DNA library (Lib) or AuNP-Lib conjugates (NP-lib) compared with cells only. b) Control Ramos cells did not bind with either AuNP-sgc8 or sgc8 alone (with no signal shift for either case), demonstrating the specificity of free sgc8 and AuNP-sgc8 aptamers to CEM cells. c–d) Flow cytometry analysis determines the binding affinity of AuNP-sgc8 (c) and sgc8 alone (d) to CEM cells.
Enhanced Cancer Cell Capture in a Flat Channel Microdevice
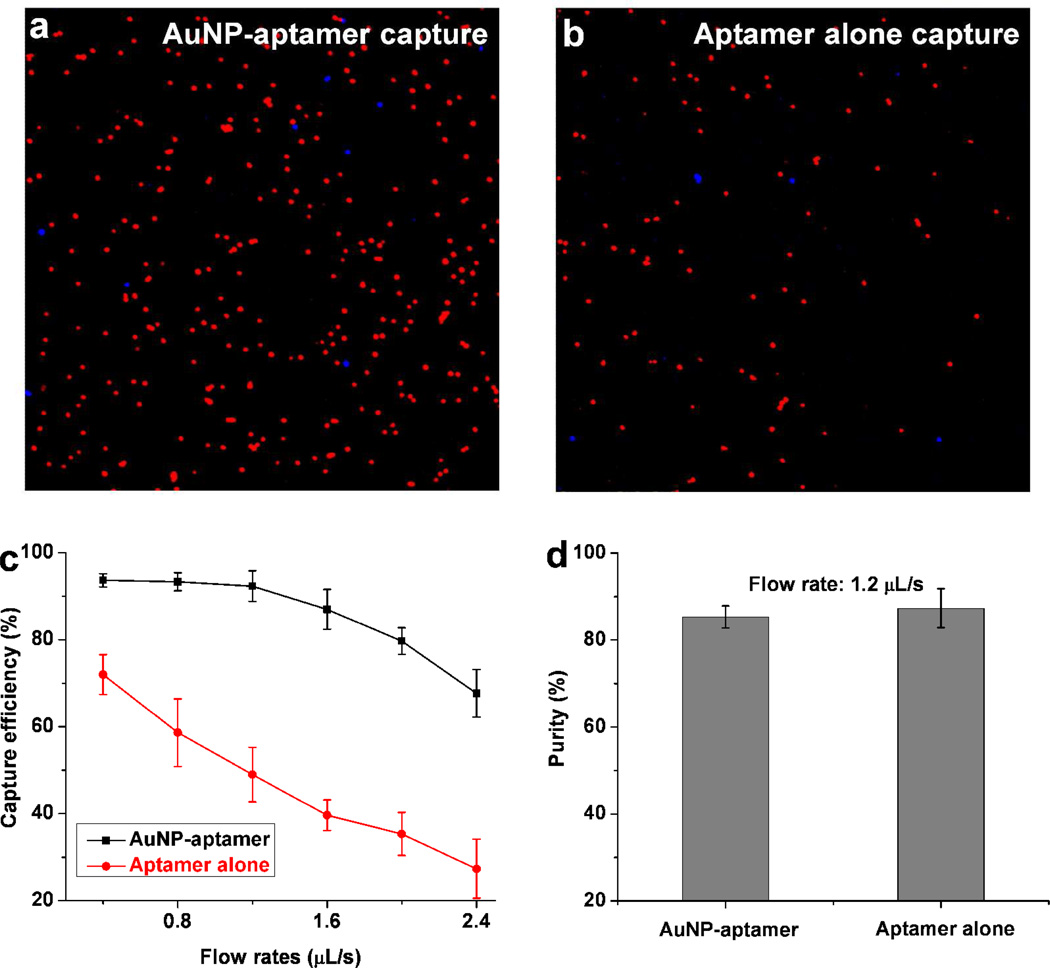
To study the cancer cell capture using AuNP-aptamer, we first developed a microfluidic laminar flow device with flat channels (S2b), which allowed us to directly compare the capture performance between AuNP-aptamer and aptamer alone. After coating surfaces with AuNP-sgc8 aptamer, a cell mixture containing 105 target CEM cells and 106 control Ramos cells (1:10 ratio) in 1 mL of phosphate buffered saline (PBS) was introduced into the channel. Note that the cell solution was continuously pumped into the device without any interruption. CEM and Ramos cells were pre-stained with Vybrant DiI (red) and DiD (blue), respectively. Figure 3a shows a representative image of cells captured using AuNP-aptamer, a high percentage of target CEM cells (red) was captured, while most control Ramos cells (blue) were washed away. In another set of experiments with the same conditions, sgc8 alone was used instead of AuNP-sgc8. Figure 3b shows a typical image of cells captured after washing using aptamer alone (without the nanoparticle conjugation). The results in Figure 3a& b clearly indicate that much more target CEM cells were captured using AuNP-aptamer than with aptamer alone, demonstrating that enhanced cell capture was achieved by the AuNP conjugation. The capture efficiency using AuNP-aptamer and aptamer alone was also studied at different flow rate conditions (with different shear stresses). We found that AuNP-aptamer exhibited more enhancement in the capture efficiency at higher flow rates, as shown in Figure 3c. At a flow rate of 1.2 µL/s, AuNP aptamer maintained a capture efficiency of (92 ± 4)%, while aptamer alone yielded a capture efficiency of only (49 ± 6)%. The capture efficiency was defined as the ratio of the number of the target cells captured to the number of the target cells initially seeded. The AuNP-aptamer enables significant increase in capture efficiency for the target cells. We also studied the purity of the captured cells and found that the capture purity is not affected by the AuNP conjugation. The purity was defined as the ratio of the number of the target cells captured to the number of total cells captured. As shown in Figure 3d, similar purity was obtained for AuNP-aptamer and aptamer alone when the same flow rate was used; this suggests that AuNP-aptamer does not introduce more nonspecific binding relative to aptamer alone, which is consistent with flow cytometry results on Figure 2b. However, the AuNP-aptamer allows us to use higher flow rates to maintain the capture efficiency, higher purity can thus be obtained because nonspecifically adsorbed cells are more easily washed away with a stronger shear force at a higher flow rate.18 8 Figure S3 demonstrates that purity of captured cells was improved by the increasing flow rates with proportionally increased shear stresses.
Figure 3.
a–b) Representative image of the target CEM cells (red) and control Ramos cells (blue) captured in the flat channel device using (a) AuNP-sgc8 aptamer conjugates; (b) sgc8 aptamer alone. Cell suspensions were continuously pumped into device without interruption. c) Comparison of CEM cell capture efficiency in PBS between AuNP-aptamer and aptamer alone when they were coated in a flat channel device, at flow rates from 0.4 µL/s to 2.4 µL/s. d) Comparison of the capture purity of target CEM cells between AuNP-aptamer and aptamer alone at the same flow rate; no significant difference was observed. Error bars represent standard deviations (n=3).
In addition to the DNA nanosphere-mediated multivalent binding, the enhanced cell capture also accrues from the nanosphere-modified surface. The increases in the surface roughness and total surface area compared with plain surface, allowed enhanced local topographic interactions between the aptamer-coated nanoparticle and nanoscale components on the cell surface.46 Moreover, the nanoparticle surfaces packed the aptamers in a highly dense manner, accommodating more aptamers to be immobilized than a plain surface, which is an additional advantages of using AuNP-aptamer. The increased ligand density also contributes to the enhanced interaction between cells and aptamers. Furthermore, the enhanced binding strength afforded by the multivalency effect lowers the detachment ratio of immobilized cells, thus increasing the capture efficiency compared to aptamer alone. To evaluate the versatility of our system, we also applied the system for capturing Ramos cells using AuNP-TD05 aptamer conjugates. TD05 is an aptamer with specific binding to Ramos cells.49 A capture efficiency of 90% was obtained with AuNP-TD05, while TD05 aptamer alone yielded only 41% capture, showing significant enhancement in capture efficiency as a result of using DNA nanosphere.
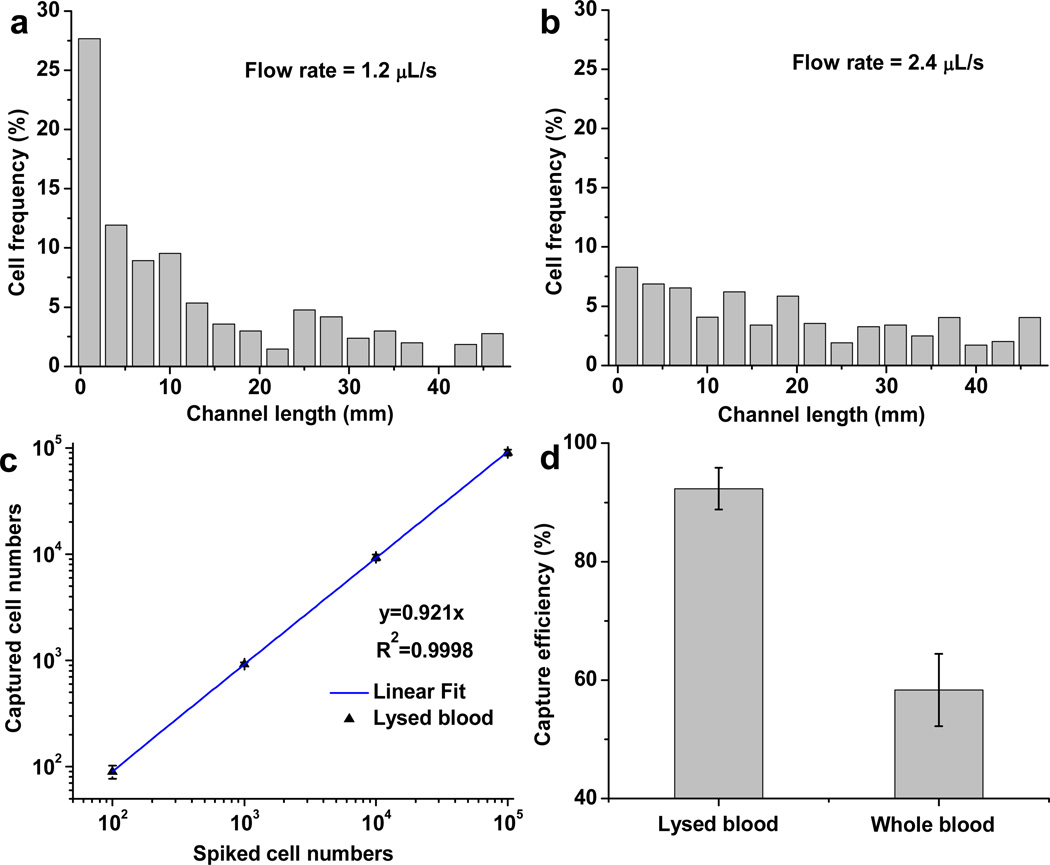
The reduced capture efficiency at higher flow rates (shown in Figure 3c) is due to increased flow-induced shear stress and the decreased interaction time between cells and aptamers on surfaces. We further characterized the distribution of captured cells at different locations of the 50 mm long microchannel with different flow rates. As shown in Figure 4a, at flow rate of 1.2 µL/s (with a shear stress of 0.4 dyn/cm2), 65% of the cells were captured in the first 25% of the channel coated with AuNP-aptamer. With an increased flow rate of 2.4 µL/s (Figure 4b), the cells captured were distributed along the channel because cells needed longer flow distance (travel length) to have an opportunity to interact with aptamers coated on the surfaces, and the attached cells experienced proportionally increased shear stresses. The cell-surface interaction is due to the ligand-receptor binding as well as gravitational force. With the AuNP-conjugation, the PEG spacer extends the aptamer strands into the 3D space of flow, increasing the accessibility and frequency of interactions between aptamers and cells to permit more efficient cell capture under higher flow rates.
Figure 4.
a–b) Spatial distribution of surface-captured CEM cells along the 50 mm-long microchannel in the flat channel device at different flow rates of (a) 1.2 µL/s and (b) 2.4 µL/s; c) Capture efficiency for 100,000, 10,000, 1000 and 100 CEM cells spiked in 1 mL of lysed blood, with flow rate of 1.2 µL/s; d) CEM cell capture efficiency from lysed blood or whole blood at the same flow rate (1.2 µL/s); 1000 CEM cells were spiked in 1 mL lysed blood or whole blood. Error bars represent the standard deviations of triplicate experiments.
To explore the clinical utility of the system, we assessed the isolation of CEM cells from lysed blood (blood with red blood cells lysed) at concentrations ranging from 105 to 100 cells/mL. As shown in Figure 4c, as few as 100 cells were efficiently isolated from 1 mL of lysed blood within 14 min. However, when we tried to capture cancer cells from unprocessed whole blood directly, the capture was significantly lower (even at a low flow rate), as shown in Figure 4d. The relatively low capture was primarily due to the reduced interaction chances between target cells and AuNP-aptamer, which is caused by abundant red blood cell blockage.
Efficient Isolation of Cancer Cells from Whole Blood using DNA Nanospheres in Micromixer Devices
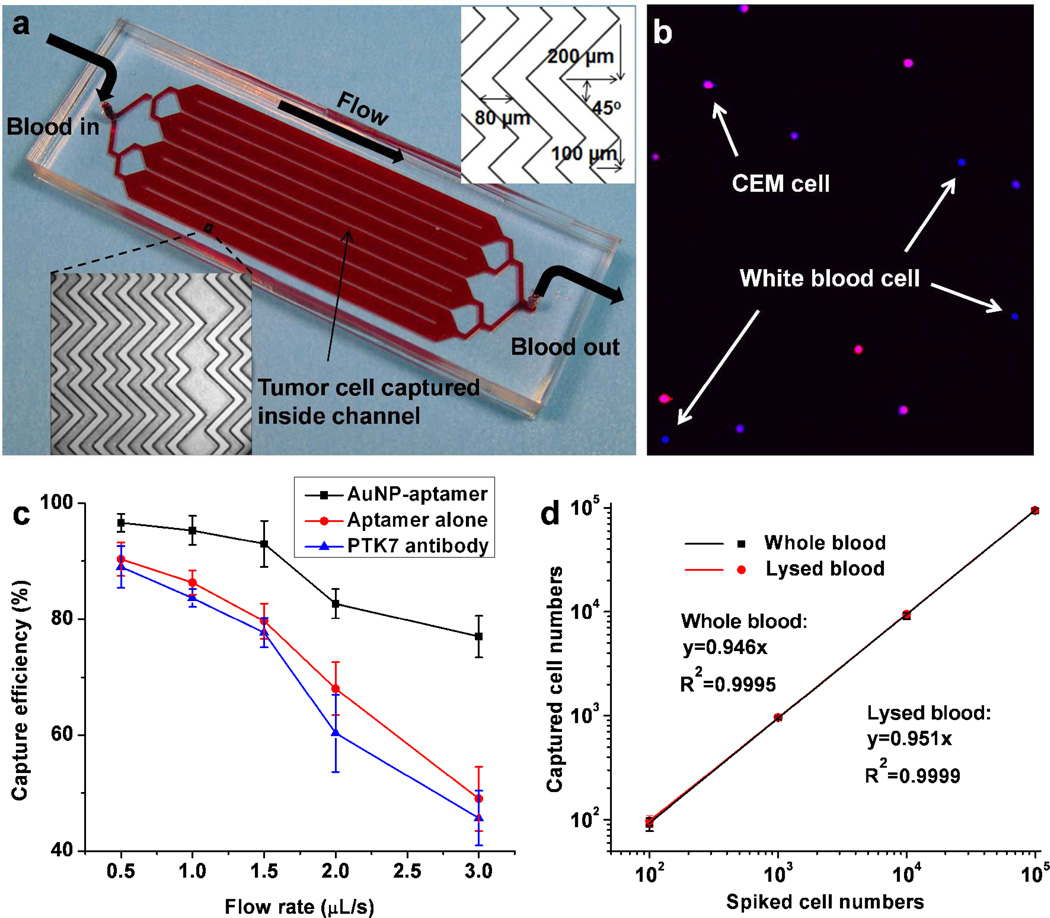
Although the laminar flow flat channel device achieved high efficiency when capturing cells in PBS or lysed blood, it showed a low capture efficiency (<60%) when capturing cells from whole blood. To enable the efficient capture of CTCs from whole blood, we integrated the AuNP-aptamer system into a herringbone groove-based micromixer device (Figure 5a). The staggered herringbone mixer generates microvortex and chaotic mixing inside the microchannel, which significantly enhances the cell-surface interactions, leading to higher capture efficiency.12, 22, 50 We first evaluated the isolation of 104 CEM cells (pre-stained by DiI, red) spiked in 1 mL of whole blood at a flow rate of 1 µL/s. After cell capture and rinsing, 4,6-diamidino-2-phenylindole (DAPI) was introduced into the device to test the purity of the target cells. DAPI stained all the cancer cells and leukocytes with blue color and verified that captured cells retain intact nuclei. As shown in Figure 5b, cells positive to both DAPI and DiI were target CEM cells (blue merged with red), while cells positive to DAPI only were white blood cells (blue only). A purity of (70 ± 6)% was obtained when capturing CEM cells from whole blood, with a capture efficiency of (95 ± 3)%. This capture purity from whole blood is much higher than those reported in literature (~50% & 14%).11, 12 Further, we tested the capture efficiency over a wide range of flow rates from 0.5 µ/s to 3 µL/s. Control experiments using identical device and conditions with aptamer alone (no AuNP-conjugation) were then conducted. Much higher capture efficiencies were obtained using AuNP-aptamer than aptamer alone, especially at high flow rates (Figure 5c). The combined effect of high affinity binding from AuNP-aptamer with the passive mixing provided by the herringbone structures enabled high capture efficiency from whole blood (93%) at high flow rate (1.5 µL/s). To compare the AuNP-aptamer based cell capture with traditional antibody based cell capture, protein tyrosine kinase 7 (PTK7) antibody was used for capturing CEM cells with identical device and conditions. For the binding between CEM cells and sgc8 aptamer, our previous study identified PTK7 as the marker for CEM cells.51 As shown in Figure 5c, the capture efficiency of CEM cells using anti-PTK7 is comparable with aptamer alone, but significantly less than AuNP-aptamer. To test the limit of detection for the AuNP-aptamer based cell capture system, cell spike numbers from 105 to 100 were explored, and >90% capture efficiency were obtained for all cases at the flow rate of 1.5 µL/s. Regardless of whether the red blood cells are intact or lysed, high capture efficiency is always obtained by the integration of AuNP-aptamer with a herringbone mixer (Figure 5d). In addition, with the flow rate of 1.5 µL/s (5.4 mL/h), 1 mL of blood sample can be processed in 11 minutes, which gives sufficient throughput for clinical applications. The system gives more benefit at higher flow rates, maintains a target cell capture efficiency of >75% for all flow rates up to 3 µL/s. With this flow rate, only 42 min is needed for processing 7.5 mL blood, the amount of blood needed to detect clinical relevant CTC number. Compared with reported work, this AuNP-aptamer modified mixer device enables >90% capture at a flow rate 5.4 mL/h, 2 to 4fold higher than reported aptamer-alone-based micropillar device (2.16 mL/h)18 and antibody-coated herringbone device (1.2 mL/h).12 The results show that the AuNP-aptamer-modified herringbone device has a great potential for clinical CTC isolation and enumeration.
Figure 5.
a) Device layout and dimensions of a microfluidic device containing herringbone mixers. b) Representative image of captured CEM cells (DiI+, DAPI+) from whole blood; the DAPI+ cells (blue only) are nonspecifically captured white blood cells. c) CEM cell capture efficiency in whole blood at various flow rates using AuNP-aptamer, aptamer alone and anti-PTK7 antibody, respectively. d) Calibration plot of cancer cell capture from whole blood and lysed blood with different cell concentrations at 1.5 µL/s, solid lines represent linear fitting. Error bars represent standard deviations (n=3).
CONCLUSIONS
In summary, we demonstrated the use of gold nanoparticles as an efficient high affinity vehicle for molecular assembly of aptamers for target cancer cell capture in microfluidic devices. Up to 95 aptamers were attached onto each AuNP, resulting in enhanced aptamer molecular recognition capability. Flow cytometry results demonstrated the high affinity binding effect using AuNP-aptamer conjugates. The capture efficiency for target cancer cells was significantly increased using the AuNP-aptamer conjugates because of the cooperative, multiple ligand-receptor interactions, as well as the increased surface roughness and ligand density. With the AuNP-aptamer surface immobilization, a flat channel microfluidic device was able to capture 100 cancer cells from 1 mL of lysed blood with ~90% capture efficiency within 14 min (4.3 mL blood/h). Using the integration of the AuNP-aptamer with a herringbone mixer design, efficient capture of rare cancer cells from whole blood was achieved, with a throughput of processing 1 mL of blood in 11 min. The high efficiency, throughput and purity make the system suitable for clinical isolation of CTCs from patient blood.
The use of leukemia cell-targeting aptamers allows the platform to be suitable for minimal residual disease (MRD) detection. MRD is the small amount of leukemia cells remaining in patient blood during or after treatment when the patient is at remission, which is the major cause for cancer relapse.52, 53 Our system capable of efficient isolation of rare cells is suitable for sensitive detection of MRD, which will be promising for monitoring treatment response and predicting cancer relapse. However, aptamers are currently not as widely used as antibodies, and limited numbers of aptamers have been developed for targeting CTCs in patient bloods. Our future efforts will include incorporating AuNPs with CTC-marker-binding aptamers [e.g., anti-EpCAM aptamer (epithelial cell adhesion molecule),54 anti-PSMA aptamer (prostate specific membrane antigen)]55 for capturing CTCs from cancer patients, as well as exploring release and culture of captured CTCs.
Spherical DNA nanostructures have been well developed and widely used for cancer cell detection; however, to our knowledge, this is the first use of aptamer nanospheres for enhancing cancer cell capture. Our results show that the combination of nanotechnology with a microfluidic device56 has a great potential for sensitive isolation of cancer cells from patient blood, and is promising for cancer diagnosis and monitoring treatment response.
METHODS
Synthesis and Characterization of Gold Nanoparticle-Aptamer Conjugates
Hydrogen tetrachloroaurate (III) (HAuCl4), trisodium citrate dihydrate, tris-(2-carboxyethyl) phosphine hydrochloride (TCEP), tris-(hydroxymethyl) aminomethane (Tris), and sodium acetate were obtained from Sigma-Aldrich (St. Louis, MO). Acetate buffer (500 mM, pH 5.2) was prepared using a mixture of sodium acetate and acetic acid. Tris acetate buffer (500 mM, pH 8.2) was prepared using Tris and acetic acid.
AuNPs were prepared using the protocols reported previously.57 Briefly, 100 mL of 1 mM HAuCl4 solution was heated till reflux. Then, 10 mL of 38.8 mM sodium citrate was added and reflux was continued for another 20 min. The diameter of such prepared AuNPs was ~13 nm, measured by transmission electron microscopy (TEM). The concentration of the AuNPs was ~13 nM, determined by UV-Vis measurement at 520 nm using a Cary Bio-300 UV spectrometer (Varian) (Figure S4).
DNA aptamers were synthesized in-house. Thiol modified-sgc8 aptamer sequence was: 5’-thiol-PEG-ATC TAA CTG CTG CGC CGC CGG GAA AAT ACT GTA CGG TTA GA-biotin-3’. The sequences of all aptamers used are listed in Table S1. For flow cytometric analysis, a fluorescein isothiocyanate (FITC) modifier was used to replace the biotin linker. All DNA aptamers were purified using a ProStar HPLC (Varian, Walnut Creek, CA) with a C18 column (Econosil, 5U, 250 × 4.6 mm) from Alltech Associates (Deerfield, IL), with triethylammonium acetate-acetonitrile as eluent. DNA concentration was determined by UV-Vis measurement at 260 nm.
Thiol-modified aptamers were conjugated on AuNPs using the reported protocols.48,57,58 Aptamers (9 µL, 1 mM) were added with acetate buffer (1 µL, 500 mM) and TCEP (1.5 µL, 10 mM) and incubated for 1 h at room temperature to activate the thiol group. Then the TCEP-treated aptamer was added to 3 mL of as-prepared AuNPs and incubated for 16 h. Finally, Tris acetate buffer (30 µL, 500 mM) and NaCl (300 µL, 1M) were added, and the mixture was incubated for 24 h. Unconjugated aptamers was then removed by centrifugation at 14,000 rpm for 15 min.
The aptamer concentration in the supernatant was measured, and the final conjugated aptamer concentration in the AuNPs was determined by subtracting the supernatant concentration from the previous aptamer concentration. The final AuNP concentration was 12.7 nM with an aptamer concentration was 1.2 µM, giving an average of approximately 95 aptamers on each AuNP. Dynamic light scattering (DLS) measurement was performed to evaluate the hydrodynamic diameter of the AuNPs before and after conjugation with aptamers using Zetasizer Nano ZS, (Malvern, Worcestershire, United Kingdom) (Figure S1). Zeta-potential measurements were performed using the same instrument. Fluorescence spectroscopy (Figure S5) also demonstrated the successful conjugation of aptamer on the AuNP. The fluorescence signal of each AuNP-aptamer conjugate is much higher than that of individual aptamer.
Device Design and Fabrication
A single flat channel device was initially used for proof-of-concept studies, and then eight flat channels were parallelized to form a high throughput device. As shown in Figure S2a, the single flat channel device was designed with a length of 50 mm, width of 2 mm, depth of 100 µm, and with single inlet and outlet. Three independent devices can be incorporated within one microscope slide size (3 in. × 1 in.). To increase the throughput, eight channels were connected through parallelization, and uniform flow was maintained in the eight channels. The size of the high throughput device is also 3 in. × 1 in., as shown in Figure S2b. Both of the two devices were made of polydimethylsiloxane (PDMS), and bonded to a 3 in. × 1 in. glass slide.
PDMS devices were fabricated according to the procedures reported by Whitesides’ group.59 The layout of the device was designed in AutoCAD and then sent to CAD/Art Services, Inc. (Bandon, OR) to produce a high resolution transparency photomask. Silicon wafers (Silicon Inc., Boise, ID) were first spin-coated with SU-8 2035 photoresist (MicroChem, Newton, MA) using a spin coater (Laurell Tech., North Wales, PA). Then the pattern on the photomask was transferred to the silicon substrate via UV exposure. After development, a silicon master patterned with the complementary structures was obtained. PDMS devices were fabricated by casting a liquid PDMS precursor against the master using Sylgard 184 reagents (Dow Corning, Midland, MI) according to the instructions of the manufacturer. To prevent the cured PDMS from sticking to the silicon master, TFOCS (Tridecafluoro-1,1,2,2-tetrahydrooctyl-1-trichlorosilane) (Sigma-Aldrich) was vacuum vaporized to the surface of the master. The channel depth, which was controlled by the spin speed of the SU-8, was measured using a Dektak 150 profilometer. The PDMS substrate was then sealed with a glass microscope slide, and inlet and outlet wells were created at the channel ends by punching holes in the PDMS sheet.
The design of herringbone mixer-device was inspired by several works in the literature,12, 50 and the dimensions were chosen for optimal cell capture, as shown in Figure 5a. The mixer device was fabricated as described above, but using a two-layer SU-8 fabrication technique, with two coating and exposure steps and a single developing step.60 The silicon mold consists of a first layer as the main channel and the second layer as the herringbone ridges, which become grooves after transfer to the PDMS substrate.
Cell Lines and Buffers
T-cell human acute lymphoblastic leukemia cells (CCRF-CEM cells, CCL-119) and B-cell human Burkitt’s lymphoma cells (Ramos cells, CRL-1596) were purchased from American Type Culture Collection (ATCC). CEM and Ramos cells were cultured in RPMI medium 1640 (ATCC) supplemented with 10% fetal bovine serum (FBS; heat-inactivated; GIBCO) and 100 units/mL penicillin-streptomycin (Cellgro, Manassas, VA). Both cultures were incubated at 37 °C under 5% CO2 atmosphere. Dulbecco’s phosphate buffered saline with calcium and magnesium (PBS) (Fisher Scientific, Hampton, NH) was used to wash cells. A solution of 50 mg/mL (5%) bovine serum albumin (BSA) (Fisher) and 0.1% Tween-20 (Fisher) in PBS was used for rinsing the unbound molecules on the surface, and resuspending cells for the cell capture. BSA and Tween-20 in PBS can fully passivate the surfaces to reduce nonspecific adsorption of cells in the channel.
Flow Cytometric Analysis
Flow cytometry was used to evaluate the targeting capabilities of AuNP-aptamer conjugatess toward specific cells. Fluorescence measurements were made with a FACScan cytometer (BD Immunocytometry Systems, San Jose, CA). Briefly, 200,000 cells were incubated with FITC-labeled free aptamer or AuNP-aptamer conjugates in 200 µL of PBS (containing 0.1% BSA) for 30 min on ice. After incubation, the cells were washed three times by centrifugation with 200 µ;L PBS, and 10, 000 counts were measured in the flow cytometer to determine the fluorescence. Varying concentrations of free sgc8 and AuNP-sgc8 aptamers were used to determine their binding affinities. The FITC-labeled random DNA library was used as a negative control to determine nonspecific binding. All of the experiments for the binding assay were repeated three times. The mean fluorescence intensity of target cells labeled by aptamers was used to calculate the specific binding by subtracting the mean fluorescence intensity of nonspecific binding from random DNA library.37 The equilibrium dissociation constants (Kd) of the aptamer-cell interaction were obtained by fitting the dependence of fluorescence intensity of specific binding on the concentration of the aptamers to the equation Y = BmaxX/(Kd + X) using SigmaPlot (Jandel, San Rafael, CA), where Y is the fluorescence intensity and X is the concentration of aptamers.
Cell Capture Assay in Microfluidic Devices
Immediately before cell capture experiments, cells were washed with PBS and resuspended at 106 cells/mL. By following the manufacturer’s instructions, CEM and Ramos cells were stained with Vybrant DiI (red) and DiD (blue) cell-labeling solutions (Invitrogen, Carlsbad, CA), respectively, then washed with PBS, and resuspended at 107 cells/mL in the PBS containing BSA and Tween-20. Labeled cells were stored on ice and further diluted to the desired concentrations before cell capture.
The single donor human whole blood was obtained from Innovative Research (Novi, MI), with anticoagulant of ethylenediaminetetraacetic acid (EDTA). Lysed blood was obtained by treating whole blood with red blood cell lysing buffer (Sigma-Aldrich) (containing NH4Cl) according to manufacturer’s instructions. Different concentrations of CEM cells were then spiked in whole blood or lysed blood.
To start cell capture experiments, one device volume (~100 µL) of 1 mg/mL avidin (Invitrogen) in PBS was first introduced into the device, followed by incubation for 15 min and then three rinses with PBS. Then, 100 µL of sgc8 aptamer or AuNP-sgc8 aptamer was introduced into the device and incubated for 15 min, followed by three rinses with the PBS containing BSA and Tween-20. Finally, 1 mL of cell mixture or blood sample spiked with cancer cells was continuously pumped into the device at a flow rate of 1.2 µL/s (or other flow rates specified in the text). For cell capture using antibody, anti-PTK7 biotin (Miltenyi Biotec, Auburn, CA) was used instead of sgc8 or AuNP-sgc8 aptamer. Afterwards, the device was washed three times with PBS to remove nonspecifically captured cells, followed by acquiring fluorescent images to determine the cell numbers. To test the purity of captured cells from lysed blood or whole blood, DAPI (Invitrogen) was introduced into the device to label the nonspecifically captured white blood cells. By following the manufacturer’s instructions, 300 nM DAPI was incubated with cells for 10 min, followed by rinsing with PBS.
The cell suspensions or blood samples were introduced into the device by pumping.18 A Micro4 syringe pump (World Precision Instruments, Sarasota, FL) with a 1 mL syringe was connected to the inlet of the device via polymer tubing and a female luer-to-barb adapter (IDEX Health & Science, Oak Harbor, WA). To avoid cell settling, a tiny magnetic stirring bar was placed inside the 1 mL syringe, with a stir plate beneath the syringe. The magnetic stirring bar kept cells in suspension while cell mixture or blood was being pumped through the device. The device was placed on the stage of an Olympus IX71 fluorescence microscope (Olympus America, Melville, NY) for detecting captured cell. To determine cell numbers, sets of images corresponding to the red fluorescent cells, blue fluorescent cells, and transmission images were acquired at different positions in each channel. Images were then imported into ImageJ (NIH), and cell counts were obtained using the Analyze Particles function after setting an appropriate threshold. Cell counts were further confirmed by comparing fluorescent images with transmission images; only those with appropriate cell morphology in the transmission images were counted.
Supplementary Material
Acknowledgment
This work was supported in part by National Cancer Institute of NIH (K25CA149080) and the University of Florida. We acknowledge K. Williams for manuscript review. We thank C. Cassano and X. Zheng for useful discussions.
Footnotes
Conflict of Interest: The authors declare no competing financial interest.
Supporting Information Available: DLS measurements of AuNPs and AuNP-aptamers, picture of flat channel microfluidic devices, purity of CEM cells as a function of flow rate, adsorption spectrum of AuNPs, fluorescence spectrum of fluorescein-labeled free aptamers and AuNP-aptamers and table of aptamer sequences. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES AND NOTES
- 1.Kling J. Beyond counting tumor cells. Nat Biotech. 2012;30:578–580. doi: 10.1038/nbt.2295. [DOI] [PubMed] [Google Scholar]
- 2.Yu M, Stott S, Toner M, Maheswaran S, Haber DA. Circulating tumor cells: approaches to isolation and characterization. The Journal of Cell Biology. 2011;192:373–382. doi: 10.1083/jcb.201010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galanzha EI, Shashkov EV, Kelly T, Kim J-W, Yang L, Zharov VP. In vivo magnetic enrichment and multiplex photoacoustic detection of circulating tumour cells. Nat Nano. 2009;4:855–860. doi: 10.1038/nnano.2009.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pantel K, Brakenhoff RH, Brandt B. Detection, clinical relevance and specific biological properties of disseminating tumour cells. Nat. Rev. Cancer. 2008;8:329–340. doi: 10.1038/nrc2375. [DOI] [PubMed] [Google Scholar]
- 5.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ, Terstappen LWMM, et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 6.Danila DC, Fleisher M, Scher HI. Circulating Tumor Cells as Biomarkers in Prostate Cancer. Clin. Cancer. Res. 2011;17:3903–3912. doi: 10.1158/1078-0432.CCR-10-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He W, Wang H, Hartmann LC, Cheng J-X, Low PS. In vivo quantitation of rare circulating tumor cells by multiphoton intravital flow cytometry. Proceedings of the National Academy of Sciences. 2007;104:11760–11765. doi: 10.1073/pnas.0703875104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Issadore D, Chung J, Shao H, Liong M, Ghazani AA, Castro CM, Weissleder R, Lee H. Ultrasensitive Clinical Enumeration of Rare Cells ex Vivo Using a Micro-Hall Detector. Sci. Transl. Med. 2012;4:141ra92. doi: 10.1126/scitranslmed.3003747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riethdorf S, Fritsche H, Müller V, Rau T, Schindlbeck C, Rack B, Janni W, Coith C, Beck K, Jänicke F, et al. Detection of Circulating Tumor Cells in Peripheral Blood of Patients with Metastatic Breast Cancer: A Validation Study of the Cell Search System. Clin. Cancer. Res. 2007;13:920–928. doi: 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 10.Balic M, Williams A, Lin H, Datar R, Cote RJ. Circulating Tumor Cells: From Bench to Bedside. Annu. Rev. Med. 2013;64:31–44. doi: 10.1146/annurev-med-050311-163404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagrath S, Sequist LV, Maheswaran S, Bell DW, Irimia D, Ulkus L, Smith MR, Kwak EL, Digumarthy S, Muzikansky A, et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature. 2007;450:1235-U10. doi: 10.1038/nature06385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stott SL, Hsu CH, Tsukrov DI, Yu M, Miyamoto DT, Waltman BA, Rothenberg SM, Shah AM, Smas ME, Korir GK, et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl. Acad. Sci. U. S. A. 2010;107:18392–18397. doi: 10.1073/pnas.1012539107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozkumur E, Shah AM, Ciciliano JC, Emmink BL, Miyamoto DT, Brachtel E, Yu M, Chen P-i, Morgan B, Trautwein J, et al. Inertial Focusing for Tumor Antigen– Dependent and –Independent Sorting of Rare Circulating Tumor Cells. Sci. Transl. Med. 2013;5:179ra47. doi: 10.1126/scitranslmed.3005616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schiro PG, Zhao M, Kuo JS, Koehler KM, Sabath DE, Chiu DT. Sensitive and High-Throughput Isolation of Rare Cells from Peripheral Blood with Ensemble-Decision Aliquot Ranking. Angew. Chem. Int. Ed. 2012;51:4618–4622. doi: 10.1002/anie.201108695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng X, Cheung LS-L, Schroeder JA, Jiang L, Zohar Y. A high-performance microsystem for isolating circulating tumor cells. Lab Chip. 2011;11:3269–3276. doi: 10.1039/c1lc20331b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Phillips JA, Xu Y, Xia Z, Fan ZH, Tan W. Enrichment of Cancer Cells Using Aptamers Immobilized on a Microfluidic Channel. Anal. Chem. 2009;81:1033–1039. doi: 10.1021/ac802092j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Y, Phillips JA, Yan J, Li Q, Fan ZH, Tan W. Aptamer-Based Microfluidic Device for Enrichment, Sorting, and Detection of Multiple Cancer Cells. Anal. Chem. 2009;81:7436–7442. doi: 10.1021/ac9012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sheng W, Chen T, Kamath R, Xiong X, Tan W, Fan ZH. Aptamer-Enabled Efficient Isolation of Cancer Cells from Whole Blood Using a Microfluidic Device. Anal. Chem. 2012;84:4199–4206. doi: 10.1021/ac3005633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gleghorn JP, Pratt ED, Denning D, Liu H, Bander NH, Tagawa ST, Nanus DM, Giannakakou PA, Kirby BJ. Capture of circulating tumor cells from whole blood of prostate cancer patients using geometrically enhanced differential immunocapture (GEDI) and a prostate-specific antibody. Lab Chip. 2010;10:27–29. doi: 10.1039/b917959c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams AA, Okagbare PI, Feng J, Hupert ML, Patterson D, Göttert J, McCarley RL, Nikitopoulos D, Murphy MC, Soper SA. Highly Efficient Circulating Tumor Cell Isolation from Whole Blood and Label-Free Enumeration Using Polymer-Based Microfluidics with an Integrated Conductivity Sensor. J. Am. Chem. Soc. 2008;130:8633–8641. doi: 10.1021/ja8015022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Wang H, Jiao J, Chen K-J, Owens GE, Kamei K-i, Sun J, Sherman DJ, Behrenbruch CP, Wu H, et al. Three-Dimensional Nanostructured Substrates toward Efficient Capture of Circulating Tumor Cells. Angew. Chem. Int. Ed. 2009;48:8970–8973. doi: 10.1002/anie.200901668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang S, Liu K, Liu J, Yu ZTF, Xu X, Zhao L, Lee T, Lee EK, Reiss J, Lee Y-K, et al. Highly Efficient Capture of Circulating Tumor Cells by Using Nanostructured Silicon Substrates with Integrated Chaotic Micromixers. Angew. Chem. Int. Ed. 2011;50:3084–3088. doi: 10.1002/anie.201005853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han W, Allio BA, Foster DG, King MR. Nanoparticle Coatings for Enhanced Capture of Flowing Cells in Microtubes. ACS Nano. 2009;4:174–180. doi: 10.1021/nn900442c. [DOI] [PubMed] [Google Scholar]
- 24.Mammen M, Choi S-K, Whitesides GM. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998;37:2754–2794. doi: 10.1002/(SICI)1521-3773(19981102)37:20<2754::AID-ANIE2754>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 25.Kitov PI, Sadowska JM, Mulvey G, Armstrong GD, Ling H, Pannu NS, Read RJ, Bundle DR. Shiga-like toxins are neutralized by tailored multivalent carbohydrate ligands. Nature. 2000;403:669–672. doi: 10.1038/35001095. [DOI] [PubMed] [Google Scholar]
- 26.delaFuente JM, Barrientos AG, Rojas TC, Rojo J, Canñda J, Fernández A, Penadés S. Gold Glyconanoparticles as Water-Soluble Polyvalent Models To Study Carbohydrate Interactions. Angew. Chem. 2001;113:2317–2321. doi: 10.1002/1521-3773(20010618)40:12<2257::AID-ANIE2257>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 27.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multivalent attachment of small molecules. Nat. Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 28.Fasting C, Schalley CA, Weber M, Seitz O, Hecht S, Koksch B, Dernedde J, Graf C, Knapp E-W, Haag R. Multivalency as a Chemical Organization and Action Principle. Angew. Chem. Int. Ed. 2012;51:10472–10498. doi: 10.1002/anie.201201114. [DOI] [PubMed] [Google Scholar]
- 29.Massich MD, Giljohann DA, Schmucker AL, Patel PC, Mirkin CA. Cellular Response of Polyvalent Oligonucleotide–Gold Nanoparticle Conjugates. ACS Nano. 2010;4:5641–5646. doi: 10.1021/nn102228s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim Y, Cao Z, Tan W. Molecular assembly for high-performance bivalent nucleic acid inhibitor. Proceedings of the National Academy of Sciences. 2008;105:5664–5669. doi: 10.1073/pnas.0711803105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao W, Cui CH, Bose S, Guo D, Shen C, Wong WP, Halvorsen K, Farokhzad OC, Teo GS, Phillips JA, et al. Bioinspired multivalent DNA network for capture and release of cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:19626–19631. doi: 10.1073/pnas.1211234109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S, Leroueil PR, Majoros IJ, Orr BG, Baker JR, Jr, Banaszak Holl MM. The Binding Avidity of a Nanoparticle-Based Multivalent Targeted Drug Delivery Platform. Chemistry & Biology. 2007;14:107–115. doi: 10.1016/j.chembiol.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 33.Myung JH, Gajjar KA, Saric J, Eddington DT, Hong S. Dendrimer-Mediated Multivalent Binding for the Enhanced Capture of Tumor Cells. Angew. Chem. Int. Ed. 2011;50:11769–11772. doi: 10.1002/anie.201105508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang Y-F, Chang H-T, Tan W. Cancer Cell Targeting Using Multiple Aptamers Conjugated on Nanorods. Anal. Chem. 2008;80:567–572. doi: 10.1021/ac702322j. [DOI] [PubMed] [Google Scholar]
- 35.Pavlov V, Xiao Y, Shlyahovsky B, Willner I. Aptamer-Functionalized Au Nanoparticles for the Amplified Optical Detection of Thrombin. J. Am. Chem. Soc. 2004;126:11768–11769. doi: 10.1021/ja046970u. [DOI] [PubMed] [Google Scholar]
- 36.Farokhzad OC, Jon S, Khademhosseini A, Tran T-NT, LaVan DA, Langer R. Nanoparticle-Aptamer Bioconjugates. Cancer Res. 2004;64:7668–7672. doi: 10.1158/0008-5472.CAN-04-2550. [DOI] [PubMed] [Google Scholar]
- 37.Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proceedings of the National Academy of Sciences. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat Rev Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang H, Yang R, Yang L, Tan W. Nucleic Acid Conjugated Nanomaterials for Enhanced Molecular Recognition. ACS Nano. 2009;3:2451–2460. doi: 10.1021/nn9006303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zheng D, Seferos DS, Giljohann DA, Patel PC, Mirkin CA. Aptamer Nano-flares for Molecular Detection in Living Cells. Nano Lett. 2009;9:3258–3261. doi: 10.1021/nl901517b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen T, Shukoor MI, Wang R, Zhao Z, Yuan Q, Bamrungsap S, Xiong X, Tan W. Smart Multifunctional Nanostructure for Targeted Cancer Chemotherapy and Magnetic Resonance Imaging. ACS Nano. 2011;5:7866–7873. doi: 10.1021/nn202073m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bamrungsap S, Chen T, Shukoor MI, Chen Z, Sefah K, Chen Y, Tan W. Pattern Recognition of Cancer Cells Using Aptamer-Conjugated Magnetic Nanoparticles. ACS Nano. 2012;6:3974–3981. doi: 10.1021/nn3002328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang Y-F, Liu H, Xiong X, Chen Y, Tan W. Nanoparticle-Mediated IgE–Receptor Aggregation and Signaling in RBL Mast Cells. J. Am. Chem. Soc. 2009;131:17328–17334. doi: 10.1021/ja907125t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mirkin CA, Letsinger RL, Mucic RC, Storhoff JJ. A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature. 1996;382:607–609. doi: 10.1038/382607a0. [DOI] [PubMed] [Google Scholar]
- 45.Cutler JI, Auyeung E, Mirkin CA. Spherical nucleic acids. J. Am. Chem. Soc. 2012;134:1376–1391. doi: 10.1021/ja209351u. [DOI] [PubMed] [Google Scholar]
- 46.Chen W, Weng S, Zhang F, Allen S, Li X, Bao L, Lam RH, Macoska JA, Merajver SD, Fu J. Nanoroughened Surfaces for Efficient Capture of Circulating Tumor Cells without Using Capture Antibodies. ACS Nano. 2013;7:566–575. doi: 10.1021/nn304719q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee S-K, Kim G-S, Wu Y, Kim D-J, Lu Y, Kwak M, Han L, Hyung J-H, Seol J-K, Sander C, et al. Nanowire Substrate-Based Laser Scanning Cytometry for Quantitation of Circulating Tumor Cells. Nano Lett. 2012;12:2697–2704. doi: 10.1021/nl2041707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hurst SJ, Lytton-Jean AKR, Mirkin CA. Maximizing DNA Loading on a Range of Gold Nanoparticle Sizes. Anal. Chem. 2006;78:8313–8318. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tang Z, Shangguan D, Wang K, Shi H, Sefah K, Mallikratchy P, Chen HW, Li Y, Tan W. Selection of Aptamers for Molecular Recognition and Characterization of Cancer Cells. Anal. Chem. 2007;79:4900–4907. doi: 10.1021/ac070189y. [DOI] [PubMed] [Google Scholar]
- 50.Stroock AD, Dertinger SKW, Ajdari A, Mezić I, Stone HA, Whitesides GM. Chaotic Mixer for Microchannels. Science. 2002;295:647–651. doi: 10.1126/science.1066238. [DOI] [PubMed] [Google Scholar]
- 51.Shangguan D, Cao Z, Meng L, Mallikaratchy P, Sefah K, Wang H, Li Y, Tan W. Cell-Specific Aptamer Probes for Membrane Protein Elucidation in Cancer Cells. J. Proteome Res. 2008;7:2133–2139. doi: 10.1021/pr700894d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cavé H, vander Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E, et al. Clinical Significance of Minimal Residual Disease in Childhood Acute Lymphoblastic Leukemia. N. Engl. J. Med. 1998;339:591–598. doi: 10.1056/NEJM199808273390904. [DOI] [PubMed] [Google Scholar]
- 53.Bottcher S, Ritgen M, Fischer K, Stilgenbauer S, Busch RM, Fingerle-Rowson G, Fink AM, Buhler A, Zenz T, Wenger MK, et al. Minimal Residual Disease Quantification Is an Independent Predictor of Progression-Free and Overall Survival in Chronic Lymphocytic Leukemia: A Multivariate Analysis From the Randomized GCLLSG CLL8 Trial. J. Clin. Oncol. 2012;30:980–988. doi: 10.1200/JCO.2011.36.9348. [DOI] [PubMed] [Google Scholar]
- 54.Song Y, Zhu Z, An Y, Zhang W, Zhang H, Liu D, Yu C, Duan W, Yang C. Selection of DNA Aptamers against Epithelial Cell Adhesion Molecule for Cancer Cell Imaging and Circulating Tumor Cell Capture. Anal. Chem. 2013;85:4141–4149. doi: 10.1021/ac400366b. [DOI] [PubMed] [Google Scholar]
- 55.Lupold SE, Hicke BJ, Lin Y, Coffey DS. Identification and Characterization Nuclease-stabilized RNA Molecules That Bind Human Prostate Cancer Cells via the Prostate-specific Membrane Antigen. Cancer Res. 2002;62:4029–4033. [PubMed] [Google Scholar]
- 56.Valencia PM, Farokhzad OC, Karnik R, Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat. Nanotechnol. 2012;7:623–629. doi: 10.1038/nnano.2012.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu J, Lu Y. Preparation of aptamer-linked gold nanoparticle purple aggregates for colorimetric sensing of analytes. Nat. Protocols. 2006;1:246–252. doi: 10.1038/nprot.2006.38. [DOI] [PubMed] [Google Scholar]
- 58.Zhang X, Servos MR, Liu J. Instantaneous and Quantitative Functionalization of Gold Nanoparticles with Thiolated DNA Using a pH-Assisted and Surfactant-Free Route. J. Am. Chem. Soc. 2012;134:7266–7269. doi: 10.1021/ja3014055. [DOI] [PubMed] [Google Scholar]
- 59.Qin D, Xia Y, Whitesides GM. Soft lithography for micro- and nanoscale patterning. Nat. Protocols. 2010;5:491–502. doi: 10.1038/nprot.2009.234. [DOI] [PubMed] [Google Scholar]
- 60.Mata A, Fleischman AJ, Roy S. Fabrication of multi-layer SU-8 microstructures. Journal of Micromechanics and Microengineering. 2006;16:276. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.