Abstract
Rationale
Mitochondrial dysfunction has been implicated in several cardiovascular diseases; however, the roles of mitochondrial oxidative stress and DNA damage in hypertensive cardiomyopathy are not well understood.
Objective
We evaluated the contribution of mitochondrial reactive oxygen species (ROS) to cardiac hypertrophy and failure by using genetic mouse models overexpressing catalase targeted to mitochondria and to peroxisomes.
Methods and Results
Angiotensin II increases mitochondrial ROS in cardiomyocytes, concomitant with increased mitochondrial protein carbonyls, mitochondrial DNA deletions, increased autophagy and signaling for mitochondrial biogenesis in hearts of Angiotensin II treated mice. The causal role of mitochondrial ROS in Angiotensin II-induced cardiomyopathy is shown by the observation that mice that overexpress catalase targeted to mitochondria, but not mice that overexpress wild-type peroxisomal catalase, are resistant to cardiac hypertrophy, fibrosis and mitochondrial damage induced by Angiotensin II, as well as heart failure induced by overexpression of Gαq. Furthermore, primary damage to mitochondrial DNA, induced by zidovudine administration or homozygous mutation of mitochondrial polymerase gamma, is also shown to contribute directly to the development of cardiac hypertrophy, fibrosis and failure.
Conclusions
These data indicate the critical role of mitochondrial ROS in cardiac hypertrophy and failure and support the potential use of mitochondrial-targeted antioxidants for prevention and treatment of hypertensive cardiomyopathy.
Keywords: mitochondria, reactive oxygen species, angiotensin, cardiomyopathy, heart failure
Hypertension is a prevalent human disease that imposes a major risk for development of wide spectrum of cardiac and vascular diseases, including atherosclerosis, cardiomyopathy, stroke and kidney diseases. Angiotensin II (Ang) is a key component of the Renin-Angiotensin System that plays a pivotal role in hypertension and causes left ventricular hypertrophy1. Left ventricular hypertrophy increases the risk of chamber dilatation, heart failure, stroke and sudden cardiac death2. Angiotensin II binds to angiotensin receptor-1, a Gq coupled receptor, then stimulates NADPH oxidase to produce reactive oxygen species (ROS)3. NADPH-generated ROS has been shown to stimulate mitochondrial ROS production and induce mitochondrial dysfunction in endothelial and vascular smooth muscle cells4–5. While the role of ROS-mediated mitochondrial damage in hypertensive cardiomyopathy has not been fully investigated, suggestive evidence comes from observations that defects in genes encoding mitochondrial enzymes are associated with some forms of idiopathic hypertrophic and dilated cardiomyopathies6 and that mitochondrial DNA (mtDNA) deletions have been found in experimental models of heart failure7. However, the causal link between mtDNA deletions and heart failure remains to be established.
We have previously shown that mice overexpressing catalase targeted to mitochondria (mCAT) have 17–21% extension of lifespan, which was not seen in mice overexpressing wild type catalase in peroxisomes (pCAT), which is cytoplasmic and concentrated in peroxisomes8. We have recently shown that mCAT mice have reduced age-dependent left ventricular hypertrophy and diastolic dysfunction, concomitant with attenuation of age-dependent increases in cardiac mtDNA deletions and oxidative damage9. We therefore hypothesized that mCAT might also be beneficial in the setting of Angiotensin II induced cardiac hypertrophy and a genetic model of heart failure. The purposes of this study were to: 1) investigate the effect of Ang on cardiac mitochondrial ROS, function and turnover; 2) determine the effect of reduction of mitochondrial vs. cytosolic ROS on cardiac hypertrophy, fibrosis and failure, and 3) investigate the effect of acquired mitochondrial DNA mutation on cardiac hypertrophy and failure. Our results show that Ang induces mitochondrial damage and increased turnover through increased mt-ROS. The reduction of mitochondrial oxidative damage by mCAT confers protection against cardiac hypertrophy, fibrosis and failure, while protection was not conferred by overexpression of pCAT. Furthermore, acquired increases in mtDNA damage were shown to contribute directly to cardiac hypertrophy and failure.
Methods
For detailed methods please see the online Data Supplement.
Neonatal ventricular cardiomyocyte culture, flow cytometry and confocal microscopy
Neonatal mouse ventricular myocytes were stimulated with Ang (1µM) for 2 h, then loaded with MitoSOX (5µM), tetramethylrhodamine ethyl ester (TMRE, 10mM) or Dihydroethidium (DHE, 5µM) and Dihydrochlorofluorescein (DCFDA, 5µM), then measured by flow cytometry and/or confocal microscopy.
Animal models, Angiotensin II and AZT delivery, echocardiography and blood pressure measurement
The genetic mouse models used in this study are summarized in Online Table I. The floxed stop sequence preceding mCAT in the inducible BAC model (Online Figure I) was excised by tamoxifen-induced Rosa26TAM- or MHCTAM -promoted Cre. Overexpression of human catalase targeted to mitochondria was shown to be specific to mitochondria (Online Figure II–III). Four to seven male mice were included in each experimental group. Ang (1.1 mg/kg/d) was delivered for 4 weeks with Alzet 1004 pumps. AZT (240 mg/kg/d) was administered for 8 weeks in drinking water. Blood pressure response to Ang in WT, pCAT, mCAT and MHC-i-mCAT mice was compared by continuous telemetry using intravascular catheter PA-C10 (DSI, MN). Echocardiography was performed at baseline and at the end of experiments using Siemens Acuson CV-70 equipped with 13MHz probe9.
Biochemical and Molecular Analysis
Cardiac mitochondrial protein carbonyl was measured using OxiSelect protein carbonyl ELISA kit (Cell Biolabs, San Diego, CA). Mitochondrial DNA deletion frequency and gene expression were analyzed with quantitative polymerase chain reactions.
Electron microscopy was performed on a JEOL JEM 1200EXII transmission electron microscope at 15,000X. Quantitative analysis of damage mitochondria and autophagosomes was quantified blindly from 10 images from different fields (15,000× magnification). Damaged mitochondria were defined by loss of electron density in more than 20% of the area of a mitochondrion. Autophagosomes or autolysosomes were identified by the characteristic structure of a double or multi-lamellar smooth membrane completely surrounding compressed mitochondria or membrane bound electron-dense material10
Mitochondrial respiratory function was measured in situ using saponin-permeabilized cardiac fibers isolated from the left ventricle11.
Statistical Analysis
All data were presented as means ± SEM. Comparisons between two groups were performed using Student t-tests. One-way or two-way ANOVA was used to compare differences among multiple groups, followed by Tukey post-hoc test for significance. P<0.05 was considered significant.
Results
Ang induces mitochondrial ROS and mitochondrial damage
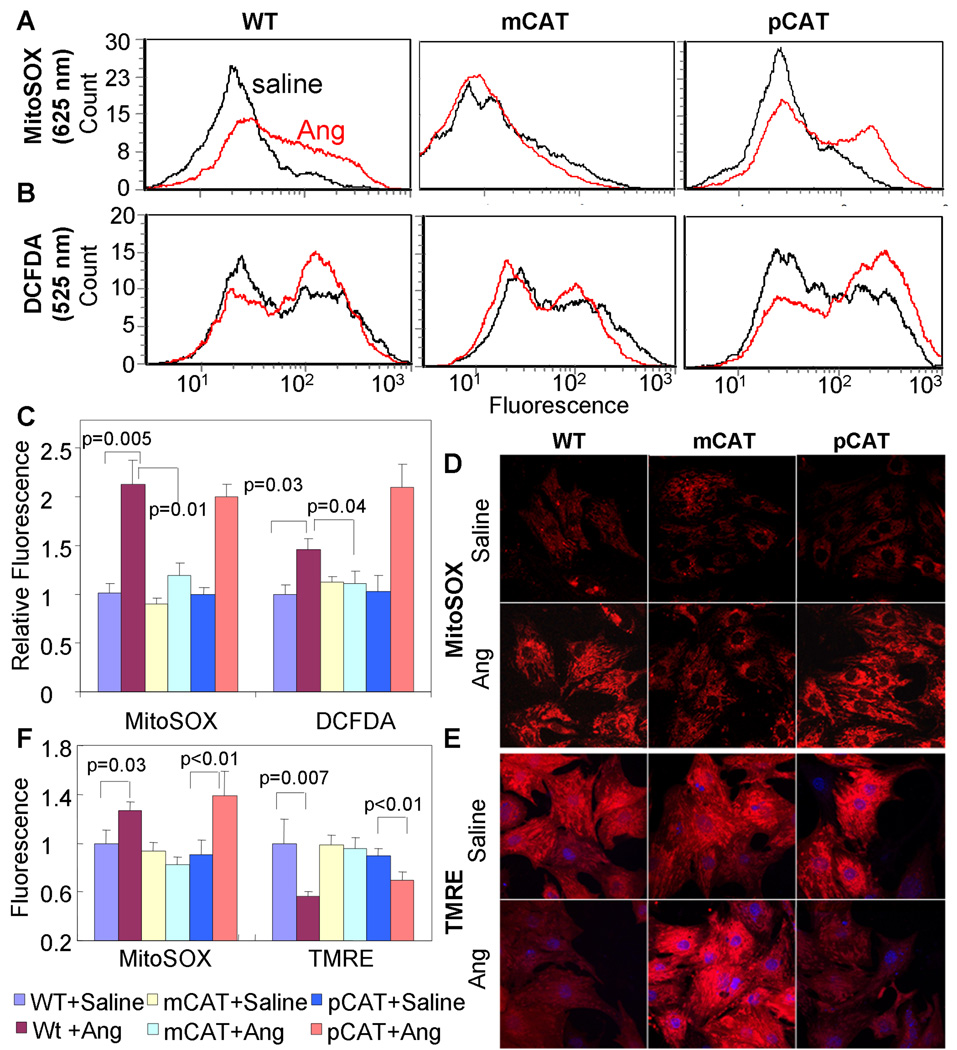
Neonatal cardiomyocytes exposed to Angiotensin II (1µM) show a greater than 2-fold increase in mitochondrial superoxide (measured with MitoSOX fluorescence by flow cytometry) and approximately a 50% increase in total ROS (DCFDA fluorescence); however, the presence of catalase targeted to mitochondria (mCAT) reduces this to levels indistinguishable from that of untreated wild type (WT) cardiomyocytes, while overexpression of wild type peroxisomal catalase (pCAT) does not show any beneficial effect (Fig. 1A–C). This mCAT protective effect is abolished by 3-amino 1,2,4-triazole, a catalase inhibitor, suggesting that this effect is mediated by the enzymatic function of catalase (Online Figure IV). Confocal microscopy confirmed that Ang induced a significant increase in MitoSOX fluorescence, which was prevented in mCAT but not in pCAT neonatal cardiomyocytes (Fig. 1 D,F). Ang treatment also induced a significant decline in TMRE fluorescence (Fig.1E–F), indicating a decline in mitochondrial membrane potential. This was also present in pCAT but not in mCAT cardiomyocytes, indicating its dependence on mitochondrial ROS (Fig. 1E–F). The overexpression of pCAT was competent in attenuating other sources of ROS, as shown by the substantial attenuation of H2O2-induced increases in DCFDA (total ROS) and dihydroethidium (cytosolic superoxide) (Online Figure V A,B). Exogenous H2O2 also increased mitochondrial superoxide (MitoSOX), but this was attenuated to the same extent by pCAT and mCAT (Online Figure V C). These results suggest that mitochondria are the primary sites of ROS generated in response to Ang in cardiomyocytes, and that mt-ROS is most effectively scavenged by mitochondrial-localized antioxidant.
Fig. 1.
Angiotensin-induced mitochondrial oxidative stress and reduction of mitochondrial membrane potential in neonatal mouse cardiomyocytes. Ang (1µM) induced a significant increase in mitochondrial ROS (MitoSOX, A) and total cellular ROS (DCFDA,B) measured by flow cytometry in WT cardiomyocytes (left column A–B). Ang-induced mitochondrial (A) and total ROS (B) is substantially inhibited in mCAT (middle column) but not pCAT (right column) cardiomyocytes, summarized in panel C (mean ± SEM of histograms of triplicate samples). Confocal microscopy shows similar results obtained with MitoSOX (D) and demonstrates that mitochondrial membrane potential, indicated by TMRE fluorescence (E), is reduced in WT cardiomyocytes treated with Ang. Both mitochondrial ROS (MitoSOX) and mitochondrial membrane potential (TMRE) are protected in mCAT but not pCAT cardiomyocytes (middle and right panels of D and E, respectively). Confocal images are quantitated in panel F (see supplemental methods).
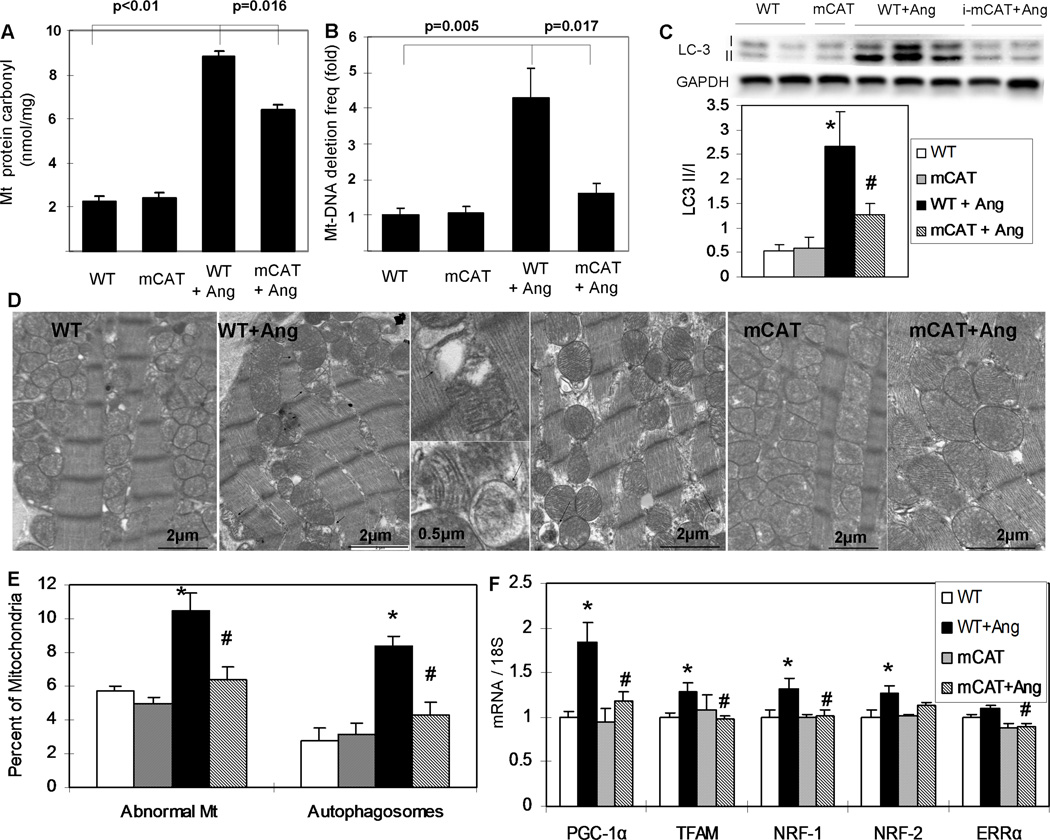
To investigate the effect of Ang in vivo, we administered a pressor dose (1.1mg/kg/d) for 4 weeks. WT, mCAT and pCAT mice displayed similar magnitudes of blood pressure increases after Ang, in which both systolic and diastolic BP increased by ~25 mmHg (Online Figure VI). As an indicator of oxidative damage, the protein carbonyl content of the cardiac mitochondria increased by greater than 4-fold in WT mice after Ang, with partial protection in mCAT mice (Fig. 2A). We calculated mtDNA copy number by the relative ratio of DNA from the mitochondrial gene ND1 to the nuclear gene cytochrome P4501A1 and found that Ang induced a decline of mtDNA copy number in WT mice by 38% (p=0.005), but only a modest 19% decline in mCAT mice (p=0.16). To estimate the frequency of mtDNA deletions we applied the random mutation capture assay12, as the abundance of mtDNA deletions has been shown to have an inverse correlation with mitochondrial function in heart and skeletal muscle12. We found that Ang significantly increased the mtDNA deletion frequency by 4.3-fold in WT mice, and this increase was substantially protected in mCAT mice (Fig. 2B).
Fig. 2.
Ang (1.1mg/kg/d) for 4 weeks induced cardiac mitochondrial damage, autophagy and biogenesis through mitochondrial ROS. (A) Protein carbonyl content was quantified using a DNPH-based enzyme immunoassay of cardiac mitochondrial protein extracts. Ang significantly increased cardiac mt-protein carbonylation, which was significantly protected in mCAT mice, n=9–10 (B) Mitochondrial DNA deletion frequencies quantified by the random mutation assay showed a greater than 4-fold increase after Ang, and this was substantially attenuated in mCAT mouse hearts, n=8–10 (C) Western blots showed that Ang significantly increased the LC-3 II/I ratio, which was significantly attenuated by MHC-i-mCAT. (D) Transmission electron microscopy showed damaged mitochondria (arrowheads) and autophagosomes/autolysosome (arrows) (magnification as indicated). (E) Quantitative analysis of electron micrographs showed that Ang significantly increased the proportion of damaged WT mitochondria (see Methods for definition), as well as the number of autophagosomes/autolysosomes that appear to contain damaged mitochondria. Both of these findings were significantly attenuated in Ang-treated mCAT mouse hearts. (F) Expression of genes implicated in mitochondrial biogenesis was significantly increased in WT hearts after Ang, but was substantially attenuated in Ang-treated mCAT mouse hearts. *p<0.05 for WT vs. WT+Ang, #p<0.05 for WT+Ang vs. mCAT+Ang, n=6–12.
Ang induces mitochondrial autophagy and biogenesis in mouse hearts through mitochondrial ROS
Using transmission electron microscopy, we found that Ang induced mitochondrial damage (loss of electron density, Fig. 2D, arrowheads) and the quantitative analysis showed a significant increase in the numbers of damaged mitochondria (Fig. 2E). Increased mitochondrial damage appeared to be associated with increased number of autophagosomes/autolysosomes with the characteristic double-membrane structure seen by electron microscopy (Fig. 2D arrows and 2E). All of the above changes were significantly attenuated in mCAT littermates. Western blots of LC-3 displayed a significant increase in LC-3 II/I ratio, a corellate of autophagy, after 4 weeks of Ang (Fig.2C). This was attenuated in mCAT mice (Fig. 2C). ROS-induced mitochondrial damage and turnover is expected to result in increased signaling for mitochondrial biogenesis, a principal regulator of which is peroxisome proliferator-activated receptor gamma coactivator-1 alpha (PGC-1α). PGC-1α is transcriptionally upregulated by ROS13. As shown in Fig. 2F, 4-weeks of Ang significantly increased expression of PGC-1α by 84%, with a concomitant increase in expression of PCG1α target genes. In contrast, Ang-treated mCAT mice showed little activation of PCG1α or its target genes, suggesting that the preservation of mitochondrial function by mCAT prevents activation of mitochondrial biogenesis signaling.
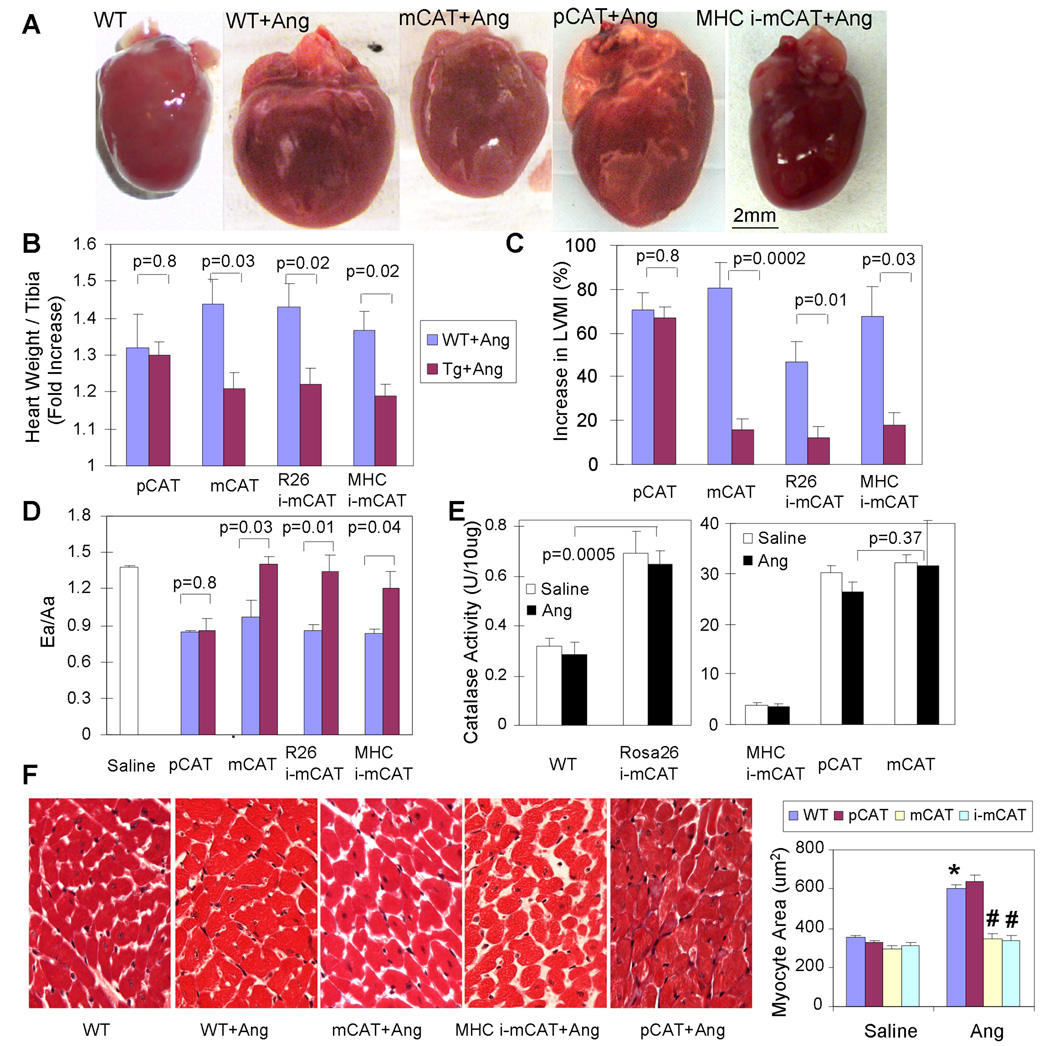
Overexpression of mCAT, but not pCAT, blocks Ang-induced cardiac hypertrophy and Gαq overexpression-induced heart failure
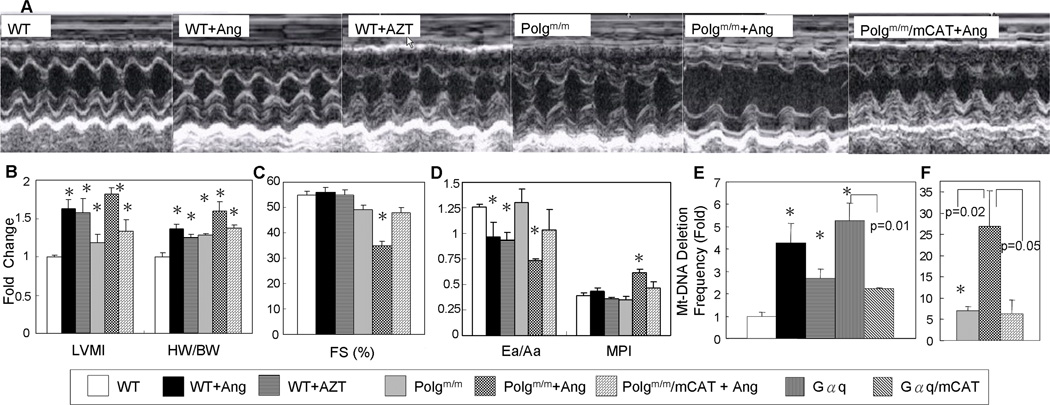
To demonstrate that mitochondria are the primary target of oxidative damage, we compared the protective effects of overexpression of pCAT14–15 vs. mCAT in response to 4 weeks of Ang treatment. As shown in Fig. 3A, Ang-induced cardiac hypertrophy is protected in mCAT but not pCAT mice. Compared to saline-treated littermate controls, Ang significantly increased heart weight of WT and littermate pCAT mice (Fig. 3B, see Online Table II for full data); echocardiographic left ventricular mass index (LVMI) significantly increased in these mice (Fig. 3C, see Online Table III for detail echocardiographic data) and diastolic function measured by Ea/Aa decreased to 0.84–0.86 (Fig. 3D, Online Table III). (Ea/Aa by tissue Doppler imaging is the ratio of myocardial velocities during early and late ventricular diastolic filling; early filling normally predominates, but can be reduced by fibrosis or slow calcium reuptake, in which case atrial-dependent late filling predominates). In all cases, pCAT mice showed no differences from their WT littermates. In contrast, in both C57Bl6 (Fig. 3) and C3H/C57Bl6 hybrid mice (Online Table III) the constitutively expressed mCAT model showed appreciable protection from Ang-induced hypertrophy (size, heart weight and LVMI) and the decline of diastolic function, compared to their respective WT littermates, This was closely recapitulated in two models of tamoxifen induced mCAT expression, ubiquitously expressed Rosa-26 i-mCAT and cardiac-specific MHC i-mCAT. To emphasize that it is the location and not the level of catalase overexpression that is crucial for this effect, we measured total catalase activity from cardiac protein extracts and showed that the total cellular catalase activity for R26 i-mCAT, MHC i-mCAT, pCAT and mCAT were 2.2, 12.2, 95.4 and 101.8 fold above the WT level, respectively (Fig. 3E). In other words, placing an activity of catalase in mitochondria equal to that found in WT peroxisomes (R26-i-mCAT) was highly protective, whereas increasing the WT peroxisomal catalase activity by 95-fold was not. It was noteworthy that mCAT and WT mouse hearts did not show any significant difference in other antioxidant pathway, such as glutathione peroxidase (GPx), glutathione reductase (GRx) or glucose-6 phosphate dehydrogenase (data not shown), although mCAT mouse hearts were shown to have significantly higher GSH and significantly lower GSSG/GSH compared with WT mouse hearts (Online Figure VII), consistent with reduced utilization of GPx and GRx, both of which depend on GSH for reduction of mitochondrial H2O2.
Fig. 3.
Ang-induced cardiac hypertrophy was protected in mice overexpressing mCAT but not pCAT. As seen by heart dimension (A), heart weight normalized to tibia length† (B) and LVMI†(C), Ang resulted in significant cardiac hypertrophy in WT littermates of each genotype (blue bars). (D) Ea/Aa is normally > 1, as seen in WT treated with saline, but Ang results in diastolic dysfunction in WT (blue bars), shown by Ea/Aa <1. In Panels A–D all Ang-induced changes are significantly attenuated in mice with either constitutive or inducible overexpression of mCAT, but not by overexpression of pCAT (red bars). (E) Total cellular catalase activity (per mg cardiac protein by Amplex Red assay) showed activities for R26-i-mCAT, MHC-i-mCAT, pCAT and mCAT that were 2.2, 12.2, 95.4 and 101.8 fold greater than WT, respectively. There was no significant difference in catalase activity after Ang treatment (black bars). (F) Cardiomyocytes cross-sectional area significantly increased after Ang, which was attenuated in mCAT but not pCAT. †Normalized to saline-treated WT littermates of each group (See Online Tables II–III for full data). *p<0.01 compared with saline treated groups. # p<0.01 compared with Ang- treated WT.
Cardiac histopathology demonstrated cardiomyocyte hypertrophy after Ang, which was significantly ameliorated by mCAT but not pCAT (Fig. 3F). To confirm the protective effect of mCAT, we demonstrated that the upregulation of atrial natriuretic peptide (ANP), a fetal gene reactivated during hypertrophy was substantially decreased in mCAT hearts (Online Figure VIII).
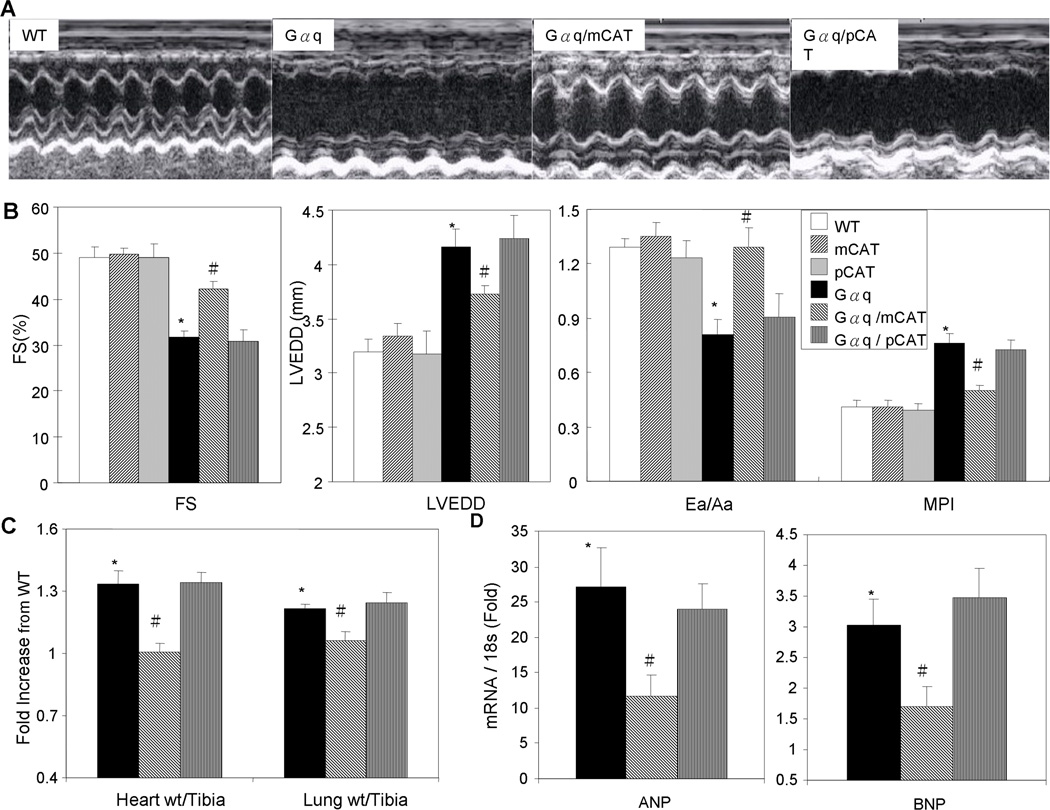
To extend our observations to a model of heart failure, we used the genetic mouse model overexpressing Gαq. Gαq is coupled to several catecholamine and angiotensin II receptors and its cardiac overexpression has been shown to cause heart failure in mice by 14–16 weeks of age16. We confirmed that Gαq mice had substantial impairment of systolic function at 16 weeks age, as shown by decline in FS, with enlargement of the LV chamber (increase in LV end-diastolic dimension), impaired diastolic function (decreased Ea/Aa) and worsening of myocardial performance index (MPI, which rises as a greater fraction of systole is isovolemic, see Methods) (Fig. 4B). While the compound heterozygous Gαq/pCAT mice did not show any protection against any of these parameters of heart failure, the Gαq/mCAT mice demonstrated a significant preservation of systolic and diastolic function as well as attenuation of cardiac chamber enlargement (Fig. 4B). Increases in heart weight and lung weight further supported the presence of congestive heart failure in Gαq mice (Fig. 4C, Online Table II), and these were also significantly protected in mCAT but not pCAT mice. Upregulation of cardiac ANP and BNP genes in failing Gαq hearts was also significantly attenuated in Gαq/mCAT, but not Gαq/pCAT hearts (Fig. 4D).
Fig. 4.
Gαq overexpression caused heart failure was protected in mCAT but not pCAT mice. (A) Echocardiography showed that 16-week-old Gαq heterozygous mice had poor contractility and enlargement of the LV chamber. The heart failure phenotypes in Gαq mice were substantially attenuated by overexpression of mCAT but not pCAT. (B) Quantitative analysis of echocardiography demonstrated that compared to WT mice, Gαq mice had significantly lower fractional shortening (FS), larger LV end-diastolic dimension (LVEDD), lower Ea/Aa by tissue Doppler and worsening of the MPI. All of these changes were significantly attenuated in compound heterozygous Gαq/mCAT mice but not Gαq/pCAT mice. (C) Relative to WT littermates, both normalized heart weight and lung weight were significantly increased in Gαq and Gαq/pCAT mice, but were substantially protected in Gαq/mCAT mice. (D) Relative to WT littermates, expression of ANP and BNP genes (normalized to 18S) was significantly upregulated in Gαq and Gαq/pCAT mice, but this increase was significantly less in Gαq/mCAT mice. *p<0.05 for WT vs. Gαq, #p<0.05 for Gαq vs. Gαq/mCAT, n= 5–10/group.
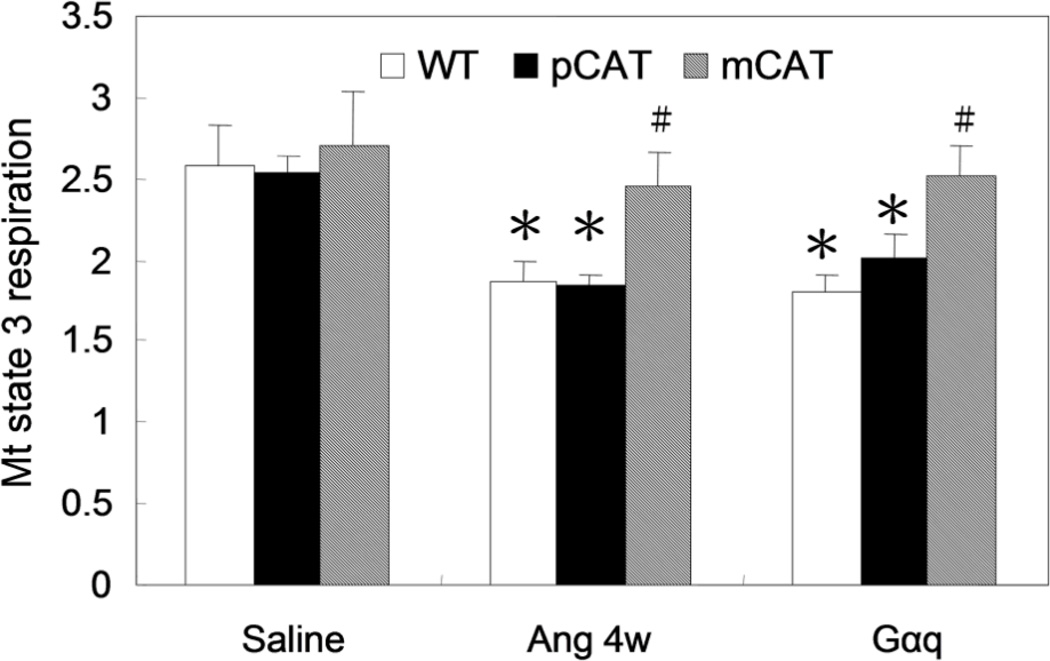
Direct evidence of the protection of mitochondrial function conferred by mCAT to Ang-treated or Gαq-overexpressed hearts is shown by analysis of in situ state 3 (maximal stimulated) mitochondrial respiration, determined by measuring the O2 consumption of saponin permeabilized cardiac muscle in the presence of excess substrate (pyruvate and malate) and ADP (Fig. 5). Whereas Ang resulted in a 28% decline in respiratory capacity in WT and pCAT hearts, mCAT hearts had substantially better preservation of state 3 mitochondrial respiration. A similar result was obtained with MHC-i-mCAT mice (Online Figure IX). Gαq overexpression induced a 30% reduction in mitochondrial respiratory capacity and this was also preserved in mCAT but not pCAT mice (Fig. 5).
Fig. 5.
Mitochondrial state 3 respiration was measured using saponin-permeabilized cardiac muscle fibers from the LV apex, in the presence of excess pyruvate/ malate, stimulated by ADP, and normalized to tissue weight (nmol O2/min/mg). The state 3 respiratory capacity in WT mice significantly declined by 28% after 4 weeks of Ang II, and this was protected in mCAT but not pCAT mice. Gαq overexpression caused a 30% reduction of maximal respiratory capacity and this was protected in Gαq/mCAT but not Gαq/pCAT mice. *p<0.05 compared with WT, #p<0.05 for mCAT+Ang vs. WT+Ang and Gαq/mCAT vs. Gαq, n=4–6/group.
Mitochondrial DNA damage contributes to the development of cardiac hypertrophy and failure
The antiretroviral drug Zidovudine (AZT), a nucleoside analog, interferes with mtDNA replicative fidelity and can induce mitochondrial damage in the heart17. We demonstrated that 8 weeks of AZT treatment of WT mice induced LV hypertrophy to a degree comparable to that of Ang treatment, as shown by increased LVMI and heart weight (Fig. 6A–B). To test the hypothesis that mtDNA mutations could accelerate heart failure, we examined mice with homozygous mutation of mitochondrial polymerase gamma (Polgm/m) which have defective exonuclease proofreading capacity18. These mice have been shown to have accelerated aging phenotypes, cardiac hypertrophy and mitochondrial dysfunction in parallel with the accumulation of mtDNA mutations and deletions18–19. At only16 wks of age, the increase in LVMI and heart weight in Polgm/m mice is significant (Fig. 6A–B, Online Table II), but no systolic or diastolic dysfunction is evident (Fig. 6C–D). However, when Polgm/m mice are treated with Ang, the cardiac failure phenotype is greatly augmented. While Ang treatment caused a decline in diastolic function (Ea/Aa) and preserved systolic function in WT mice, the same treatment in Polgm/m mice induced heart failure with worsening of MPI, significant impairment of both systolic and diastolic function and dramatic cardiac hypertrophy (Fig. 6B–D, Online Table II). Ang-induced heart failure in Polgm/m mice is ameliorated by mCAT (Fig. 6B–D, Online Table II). In Ang- and AZT- induced cardiac hypertrophy, the mtDNA deletion frequency significantly increased by 4.3 and 2.7 fold, respectively, while in Gαq mice and Ang-treated Polgm/m mice with heart failure, mtDNA deletion frequencies dramatically increased by 5.2 and 26.9 fold, respectively (Fig. 6F). mCAT overexpression in Gαq- and Ang-treated Polgm/m mice, which attenuated heart failure, as noted above, also significantly decreased these mtDNA deletion frequencies (Fig. 6E). All of this evidence suggests that mtDNA damage contributes directly to cardiac hypertrophy and failure. Interestingly, the significant upregulation of mitochondrial biogenesis signaling that was observed in cardiac hypertrophy induced by Ang, AZT or Polgm/m was blunted in mice with heart failure (Gαq- and Ang-treated Polgm/m) (Online Figure X).
Fig. 6.
Mitochondrial DNA damage can induce cardiac hypertrophy and failure. (A) Echocardiography demonstrated that both Ang and AZT (zidovudine) treatment of WT mice induced LV hypertrophy with preserved systolic function. In contrast, Ang treatment of Polgm/m mice caused significant impairment of LV contractility and severe hypertrophy, which is ameliorated by mCAT. (B) Relative to untreated WT mice, both echocardiographic LVMI and normalized heart weight increased significantly after Ang or AZT. These parameters of cardiac hypertrophy increased even more dramatically when Polgm/m mice were treated with Ang. WT mice treated with Ang or AZT had preserved systolic function measured by FS (C) and a slight decline in diastolic function (Ea/Aa) with no change in MPI (D). Polgm/m mice showed normal systolic and diastolic function, but Polgm/m mice treated with Ang developed heart failure with worsening of myocardial performance and impairment of both systolic and diastolic function, all of which were attenuated by mCAT (C–D). (E) Mutation assay showed that Ang, AZT and Gαq resulted in significantly increased mtDNA deletion frequencies: 4.3, 2.7 and 5.2-fold above WT, respectively. Expression of mCAT in Gαq mice, which attenuated heart failure (Figures 4–5), also significantly decreased the mtDNA deletion frequency. Polgm/m mice have a 7-fold increase in mtDNA deletion frequency, which rises to 26.9-fold after Ang treatment and this is alleviated by mCAT. *p<0.05 compared with WT. n=6–10 for echocardiography, n=8–12 for deletion assay.
Masson·s trichrome staining demonstrated that Ang treatment and Gαq overexpression were accompanied by significant ventricular fibrosis, which was substantially attenuated in mCAT but not pCAT mouse hearts (Online Figure XI A). Similarly, both Ang and Gαq overexpression increased pro-collagen type 1α2 transcript levels by ~2-fold, and this was significantly protected in mCAT but not pCAT hearts (Online Figure XI B). Consistent with the finding that homozygous mutation of Polg contributes to the transition from hypertrophy to failure in response to Ang, we found that Polgm/m further aggravated cardiac fibrosis induced by Ang (Online Figure XI).
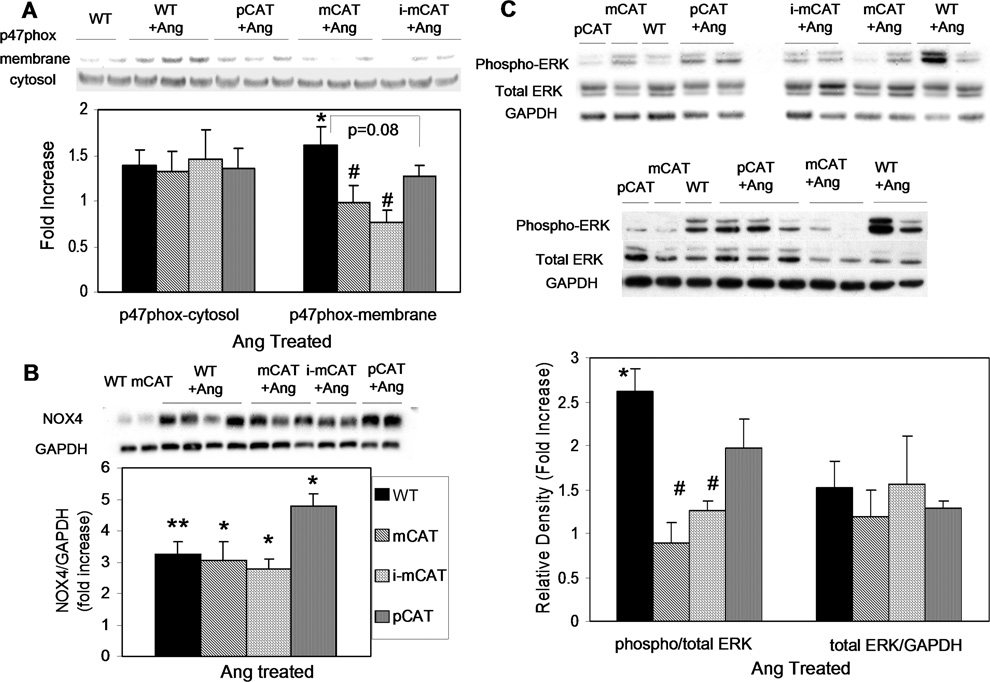
Ang induced ROS mediated signaling
NADPH oxidase (NOX) has been shown to play a crucial role in Ang signaling, including NOX220, activation of which depends on p47phox complex translocation to the plasma membrane. Western blots demonstrated that Ang significantly increased p47-phox membrane translocation in WT hearts, and this was attenuated in mCAT (p<0.05) and pCAT hearts (p=0.08) (Fig. 7A). The NOX4 isoform of NADPH oxidase has recently been shown to be associated with mitochondria in cardiomyocytes and upregulated by Ang21. Ang-induced upregulation of NOX4 was, however, not significantly altered by mCAT or pCAT (Fig. 7B). Ang also increased phosphorylation of ERK1/2, a member of the MAPK family known to be a key player in cardiac hypertrophy, and this phosphorylation was significantly lower in mCAT but not pCAT hearts (Fig. 7C), suggesting that the MAP kinase is activated through mt-ROS sensitive mechanisms (Online Figure XII).
Fig. 7.
(A) Ang increased p47phox membrane translocation, which was attenuated by mCAT (p<0.05) and pCAT (p=0.08). (B) Ang significantly increased NOX4 expression (p<0.01), which was not altered by mCAT or pCAT. (C) Ang-induced phosphorylation of ERK1/2 is significantly reduced in mCAT but not pCAT mice. *p<0.05 for Ang-treated vs. saline treated; #p<0.05 compared with WT; n=5–7.
Discussion
This study demonstrates that mt-ROS plays a critical role in the development of Ang-induced cardiac hypertrophy. The overexpression of mCAT ameliorated LV hypertrophy, fibrosis and diastolic dysfunction induced by 4 weeks of Ang. This beneficial effect was seen even when catalase was expressed in mitochondria at a very modest level (activity similar to that of WT peroxisomal catalase), but it was not observed in mice that greatly overexpressed WT catalase (pCAT). This fact emphasizes that it is the mitochondrial localization of catalase and not the expression level that is crucial for cardioprotection. Furthermore, mCAT protection appears to be autonomous to the heart, evidenced by similar protection in mice with cardiomyocyte-specific (MHC-i-mCAT) vs. ubiquitous mCAT expression (R26-i-mCAT, Fig 3.B–D). The absence of mCAT dosage effect in this study is consistent with our previous observation of lifespan extension, in which two different lines of mCAT with ~100× catalase and ~10× catalase overexpression demonstrated similar benefit on lifespan extension8. This suggests that a low dose of mitochondrial catalase is adequate to break the vicious cycle of mitochondrial ROS induced ROS (see below).
Mitochondria are major site of generation of ROS as a byproduct of oxidative phosphorylation. In fact, by their proximity to ROS, mitochondrial proteins, lipids and DNA are believed to be primary targets of oxidative damage during stress22, creating a mitochondrial free radical “vicious cycle” of injury. Angiotensin II binds to ATR1, a Gαq coupled-receptor, then activates NADPH oxidase through a PKC-dependent manner, and the ROS from NADPH oxidase might increase mitochondrial ROS production, as previously shown in endothelial cells4. The current study is the first to provide direct evidence that amplification of ROS within mitochondria is a key mediator of Ang- and Gαq- induced cardiac hypertrophy and failure. The mechanisms of ROS amplification might include ROS induced ROS release as well as a ROS-mtDNA damage vicious cycle (Online Figure XII). For example, ROS production (from NOX2 and/ or NOX4) can increase electron leakage from the electron transports chain, further stimulating ROS production. This mechanism is also consistent with observations that primary damage to mtDNA is sufficient to elevate ROS, cause cardiac hypertrophy and accentuate Ang effect to induce heart failure. Thus, breaking the ROS vicious cycle within mitochondria by mCAT is effective to attenuate both cardiac hypertrophy and failure.
Although the NADPH oxidase NOX2 isoform has been shown to mediate Ang-induced cardiac hypertrophy20, Ago, et al. have recently demonstrated that NOX4 is collocalized to mitochondria and is upregulated in response to Ang21. They also showed that overexpression of NOX4 significantly increased mitochondrial ROS, decreased mitochondrial membrane potential and mitochondrial function, and induced the mitochondrial apoptotic pathway21. Nox4 is thought to be constitutively active and does not require activation by p47phox or p67phox. While Ang-induced upregulation of NOX4 is not affected by mCAT or pCAT, the membrane translocation of p47phox (activation of NOX2) was attenuated by mCAT (p<0.05) and pCAT (p=0.08). This effect suggests positive-feedback between mitochondrial and cytosolic ROS and NOX2 activation (Online Figure XII). The relative roles of NOX2 and NOX4 in cardiomyocytes, however, remain to be elucidated. Based on the observations reported here, we propose that cardiac ROS produced by NADPH oxidase (NOX2 and/or NOX4) is amplified within mitochondria; mitochondrial hydrogen peroxide is freely diffusible and can subsequently activate redox-sensitive nuclear and cytoplasmic signaling, including ERK1/2 (Figs. 7C, Online Figure XII). ROS are also known to activate several other MAPK and NF-κB pathways23, 24, which interact with Ca-mediated signaling25. The present study provides evidence that Ang-induced cardiac ROS is primarily mitochondrial, causes a decline of mitochondrial membrane potential and increases cardiac mitochondrial protein oxidative damage and mtDNA deletions. The deleterious effects of Ang on mitochondria are associated with an increase in autophagosomes and increased signaling of mitochondrial biogenesis in attempt to replenish the damaged mitochondria and restore energy production (Fig. 2). The fact that mCAT, but not pCAT reduces mt-ROS and substantially ameliorates all of the above Ang-induced changes suggests that these events are mediated by mt-ROS.
To recapitulate the effect of chronic exposure to Ang on heart, we used transgenic mice overexpressing the Gαq protein. Gαq is a subunit of the G-protein that is coupled to cathecholamine and angiotensin II receptors. Cardiac specific overexpression of Gαq has been shown to cause heart failure in mice by 16 weeks of age, despite the absence of increased blood pressure16. Overexpression of mCAT, but not pCAT was able to ameliorate heart failure and pathological cardiac remodeling in the Gαq mouse model and attenuated the increased mtDNA deletions and depressed mitochondrial respiratory capacity observed in Gαq mice. These findings reinforce the hypothesis that mt-ROS mediated mitochondrial damage plays a critical role in development of cardiac hypertrophy and failure.
MtDNA mutations and deletions have been observed in several forms of cardiomyopathy in humans6. Whether this DNA damage is the cause or consequence of the cardiomyopathy remains unclear. We found that AZT treatment substantially increased mtDNA deletions, concomitant with the development of cardiac hypertrophy (Fig. 6). It has been previously shown that AZT-induced cardiomyopathy in mice was also ameliorated by mCAT26. Mice with homozygous mutation of exonuclease domain of mitochondrial polymerase gamma (Polgm/m) have impaired DNA proofreading capacity. These mice accumulate mtDNA deletions even at young age (Fig. 6F) and display cardiac hypertrophy with preserved systolic function (Fig. 6B and F). Furthermore, while Ang did not affect systolic function in WT mice, the same treatment in Polgm/m mice induced a greater than 25-fold increase in mtDNA deletions, and resulted in severe cardiac hypertrophy, significant systolic heart failure, chamber dilation and fibrosis. These findings suggest that mtDNA deletions induce cardiac hypertrophy, but when augmented by Ang-induced mt-ROS, this results in systolic heart failure. Again, this is consistent with the vicious cycle model of mt-ROS induced mitochondrial damage and its interruption by mCAT (Online Figure XII).
An additional link between mt-ROS and cardiac failure is likely to be mitochondrial energetic failure, such as the decline in mitochondrial respiration shown in Figure 5. Consistent with previous reports27, we found that PGC-1α, the main regulator of mitochondrial biogenesis, is upregulated during compensated hypertrophy and that this signal was blunted in heart failure (Online Figure X). In contrast, it has been shown that many signaling pathways that induced mitochondrial biogenesis, such as AMPK, calcineurin and MAPK signaling, are often upregulated rather than downregulated in heart failure28. The molecular mechanisms underlying the loss of PGC-1α in transition to heart failure merit further investigation, and this report suggests that it will be important to further pursue the interactions of mt-ROS, mtDNA deletions and energetic failure as one of the mechanisms leading to heart failure.
Clinical trials applying antioxidants to attenuate the progression of cardiovascular diseases have shown equivocal benefits, at best29. These studies have, however, generally employed cellular antioxidants, whereas there are now several promising mitochondrially-targeted small molecule antioxidants, including mitochondrial-targeted ubiquinone (MitoQ), the catalase mimetic complex Mn(III)-salen chloride (Euk8) and SS peptide antioxidants30–32. Our study therefore provides a strong rationale to investigate the potential application of such mitochondrial-targeted antioxidant drugs in the treatment or prevention of hypertensive cardiomyopathy and heart failure.
Supplementary Material
NOVELTY AND SIGNIFICANCE.
What Is Known?
Oxidative stress and mitochondrial dysfunction have been implicated in several cardiovascular diseases
However, the roles of mitochondrial oxidative stress and DNA damage in hypertensive cardiomyopathy are not well understood
What New Information Does This Article Contribute?
Transgenic mice that express catalase in the mitochondria, but not mice that overexpress catalase in peroxisomes are protected from Angiotensin-II treatment or Gαq overexpression induced cardiac hypertrophy and failure.
This observation directly demonstrates that mitochondrial reactive oxygen species (ROS) play a critical role in the development of cardiac hypertrophy and failure.
This study supports the potential use of mitochondrial-targeted antioxidants for prevention and treatment of hypertensive cardiomyopathy and heart failure.
While both oxidative stress and mitochondrial dysfunction have been implicated in several cardiovascular diseases, the roles of mitochondrial oxidative stress and DNA damage in hypertensive cardiomyopathy and heart failure are not well understood. This was directly addressed by using genetic mouse models expressing catalase targeted to mitochondria, and comparing these to mice expressing catalase in peroxisomes (the natural location of catalase). The causal role of mitochondrial ROS was shown by the observation that only mice that express catalase targeted to mitochondria are resistant to cardiac hypertrophy, fibrosis, and mitochondrial damage induced by Angiotensin II, as well as heart failure induced by overexpression of Gαq. Mechanistically, mitochondrial catalase blocked angiotensin-II induced accumulation of mitochondrial protein carbonyls, mitochondrial DNA deletions, increased autophagy, signaling for mitochondrial biogenesis and activation of the ROS-sensitive MAP kinase ERK1/2 in the hearts. These observations indicate a critical role of mitochondrial ROS in the mechanisms that contribute to cardiac hypertrophy and failure and support the potential use of mitochondrial-targeted antioxidants for prevention and treatment of hypertensive cardiomyopathy and heart failure.
Acknowledgments
Funding Sources: NIH AG013280, AG001751, HL101186, AG028455 and AG035844.
Non-Standard Abbreviations List
- Ang
Angiotensin II
- ATR1
Angiotensin II receptor-1
- AZT
Zidovudine
- BP
Blood pressure
- FS
Fractional Shortening
- i-mCAT
inducible catalase targeted to mitochondria
- LC-3
Microtubule associated protein Light Chain 3
- LVH
Left ventricular hypertrophy
- MAPK
Mitogen-activated protein kinase
- Mt
mitochondrial
- MHC
Myosin heavy chain
- MPI
Myocardial performance index
- ND1
NADH Dehydrogenase subunit 1
- NOX
NADPH Oxidase
- pCAT
Catalase targeted to peroxisomes
- PGC-1
Peroxisome proliferator-activated receptor gamma coactivator-1
- Polg
Polymerase gamma
- ROS
Reactive oxygen species
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: No conflicts to disclose
Contributor Information
Dao-Fu Dai, Department of Pathology, University of Washington, Seattle.
Simon C. Johnson, Department of Pathology, University of Washington, Seattle.
Jason J. Villarin, Department of Radiology, University of Washington, Seattle.
Michael T. Chin, Department of Cardiovascular Medicine, University of Washington, Seattle.
Madeline Nieves-Cintrón, Department of Physiology and Biophysics, University of Washington, Seattle.
Tony Chen, Department of Pathology, University of Washington, Seattle.
David J. Marcinek, Department of Radiology, University of Washington, Seattle.
Gerald W. Dorn, II, Department of Internal Medicine, Washington University School of Medicine, St. Louis, MO.
Y. James Kang, Departments of Medicine, and Pharmacology and Toxicology, University of Louisville School of Medicine, Louisville, KY.
Tomas A. Prolla, Department of Genetics & Medical Genetics, University of Wisconsin-Madison.
Luis F. Santana, Department of Physiology and Biophysics, University of Washington, Seattle.
Peter S. Rabinovitch, Department of Pathology, University of Washington, Seattle.
References
- 1.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–556. doi: 10.1016/s0092-8674(01)00241-0. [DOI] [PubMed] [Google Scholar]
- 2.Gardin JM, Lauer MS. Left ventricular hypertrophy: the next treatable, silent killer? Jama. 2004;292:2396–2398. doi: 10.1001/jama.292.19.2396. [DOI] [PubMed] [Google Scholar]
- 3.Mollnau H, Wendt M, Szocs K, Lassegue B, Schulz E, Oelze M, Li H, Bodenschatz M, August M, Kleschyov AL, Tsilimingas N, Walter U, Forstermann U, Meinertz T, Griendling K, Munzel T. Effects of angiotensin II infusion on the expression and function of NAD(P)H oxidase and components of nitric oxide/cGMP signaling. Circ Res. 2002;90:E58–E65. doi: 10.1161/01.res.0000012569.55432.02. [DOI] [PubMed] [Google Scholar]
- 4.Doughan AK, Harrison DG, Dikalov SI. Molecular mechanisms of angiotensin II-mediated mitochondrial dysfunction: linking mitochondrial oxidative damage and vascular endothelial dysfunction. Circ Res. 2008;102:488–496. doi: 10.1161/CIRCRESAHA.107.162800. [DOI] [PubMed] [Google Scholar]
- 5.Kimura S, Zhang GX, Nishiyama A, Shokoji T, Yao L, Fan YY, Rahman M, Abe Y. Mitochondria-derived reactive oxygen species and vascular MAP kinases: comparison of angiotensin II and diazoxide. Hypertension. 2005;45:438–444. doi: 10.1161/01.HYP.0000157169.27818.ae. [DOI] [PubMed] [Google Scholar]
- 6.DiMauro S, Schon EA. Mitochondrial respiratory-chain diseases. N Engl J Med. 2003;348:2656–2668. doi: 10.1056/NEJMra022567. [DOI] [PubMed] [Google Scholar]
- 7.Marin-Garcia J, Goldenthal MJ, Moe GW. Abnormal cardiac and skeletal muscle mitochondrial function in pacing-induced cardiac failure. Cardiovasc Res. 2001;52:103–110. doi: 10.1016/s0008-6363(01)00368-6. [DOI] [PubMed] [Google Scholar]
- 8.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 9.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mizushima N, Yoshimori T, Levine B. Methods in mammalian autophagy research. Cell. 2010;140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuznetsov AV, Veksler V, Gellerich FN, Saks V, Margreiter R, Kunz WS. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 12.Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- 13.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell. 2006;127:397–408. doi: 10.1016/j.cell.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 14.Kang YJ, Chen Y, Epstein PN. Suppression of doxorubicin cardiotoxicity by overexpression of catalase in the heart of transgenic mice. J Biol Chem. 1996;271:12610–12616. doi: 10.1074/jbc.271.21.12610. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Z, Kang YJ. Cellular and subcellular localization of catalase in the heart of transgenic mice. J Histochem Cytochem. 2000;48:585–594. doi: 10.1177/002215540004800502. [DOI] [PubMed] [Google Scholar]
- 16.D'Angelo DD, Sakata Y, Lorenz JN, Boivin GP, Walsh RA, Liggett SB, Dorn GW., 2nd Transgenic Galphaq overexpression induces cardiac contractile failure in mice. Proc Natl Acad Sci U S A. 1997;94:8121–8126. doi: 10.1073/pnas.94.15.8121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamperth L, Dalakas MC, Dagani F, Anderson J, Ferrari R. Abnormal skeletal and cardiac muscle mitochondria induced by zidovudine (AZT) in human muscle in vitro and in an animal model. Lab Invest. 1991;65:742–751. [PubMed] [Google Scholar]
- 18.Kujoth GC, Hiona A, Pugh TD, Someya S, Panzer K, Wohlgemuth SE, Hofer T, Seo AY, Sullivan R, Jobling WA, Morrow JD, Van Remmen H, Sedivy JM, Yamasoba T, Tanokura M, Weindruch R, Leeuwenburgh C, Prolla TA. Mitochondrial DNA mutations, oxidative stress, and apoptosis in mammalian aging. Science. 2005;309:481–484. doi: 10.1126/science.1112125. [DOI] [PubMed] [Google Scholar]
- 19.Trifunovic A, Wredenberg A, Falkenberg M, Spelbrink JN, Rovio AT, Bruder CE, Bohlooly YM, Gidlof S, Oldfors A, Wibom R, Tornell J, Jacobs HT, Larsson NG. Premature ageing in mice expressing defective mitochondrial DNA polymerase. Nature. 2004;429:417–423. doi: 10.1038/nature02517. [DOI] [PubMed] [Google Scholar]
- 20.Bendall JK, Cave AC, Heymes C, Gall N, Shah AM. Pivotal role of a gp91(phox)-containing NADPH oxidase in angiotensin II-induced cardiac hypertrophy in mice. Circulation. 2002;105:293–296. doi: 10.1161/hc0302.103712. [DOI] [PubMed] [Google Scholar]
- 21.Ago T, Kuroda J, Pain J, Fu C, Li H, Sadoshima J. Upregulation of Nox4 by hypertrophic stimuli promotes apoptosis and mitochondrial dysfunction in cardiac myocytes. Circ Res. 2010;106:1253–1264. doi: 10.1161/CIRCRESAHA.109.213116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 23.Clerk A, Fuller SJ, Michael A, Sugden PH. Stimulation of "stress-regulated" mitogen-activated protein kinases (stress-activated protein kinases/c-Jun N-terminal kinases and p38-mitogen-activated protein kinases) in perfused rat hearts by oxidative and other stresses. J Biol Chem. 1998;273:7228–7234. doi: 10.1074/jbc.273.13.7228. [DOI] [PubMed] [Google Scholar]
- 24.Frantz S, Kelly RA, Bourcier T. Role of TLR-2 in the activation of nuclear factor kappaB by oxidative stress in cardiac myocytes. J Biol Chem. 2001;276:5197–5203. doi: 10.1074/jbc.M009160200. [DOI] [PubMed] [Google Scholar]
- 25.Liu Q, Wilkins BJ, Lee YJ, Ichijo H, Molkentin JD. Direct interaction and reciprocal regulation between ASK1 and calcineurin-NFAT control cardiomyocyte death and growth. Mol Cell Biol. 2006;26:3785–3797. doi: 10.1128/MCB.26.10.3785-3797.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kohler JJ, Cucoranu I, Fields E, Green E, He S, Hoying A, Russ R, Abuin A, Johnson D, Hosseini SH, Raper CM, Lewis W. Transgenic mitochondrial superoxide dismutase and mitochondrially targeted catalase prevent antiretroviral-induced oxidative stress and cardiomyopathy. Lab Invest. 2009;89:782–790. doi: 10.1038/labinvest.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ventura-Clapier R, Garnier A, Veksler V. Transcriptional control of mitochondrial biogenesis: the central role of PGC-1alpha. Cardiovasc Res. 2008;79:208–217. doi: 10.1093/cvr/cvn098. [DOI] [PubMed] [Google Scholar]
- 28.Tian R, Musi N, D'Agostino J, Hirshman MF, Goodyear LJ. Increased adenosine monophosphate-activated protein kinase activity in rat hearts with pressure-overload hypertrophy. Circulation. 2001;104:1664–1669. doi: 10.1161/hc4001.097183. [DOI] [PubMed] [Google Scholar]
- 29.Steinhubl SR. Why have antioxidants failed in clinical trials? Am J Cardiol. 2008;101:14D–19D. doi: 10.1016/j.amjcard.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 30.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP, Sammut IA. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. Faseb J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 31.van Empel VP, Bertrand AT, van Oort RJ, van der Nagel R, Engelen M, van Rijen HV, Doevendans PA, Crijns HJ, Ackerman SL, Sluiter W, De Windt LJ. EUK-8, a superoxide dismutase and catalase mimetic, reduces cardiac oxidative stress and ameliorates pressure overload-induced heart failure in the harlequin mouse mutant. J Am Coll Cardiol. 2006;48:824–832. doi: 10.1016/j.jacc.2006.02.075. [DOI] [PubMed] [Google Scholar]
- 32.Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.