This study used a proteomics approach to characterize and quantify multipotent adult progenitor cell (MAPC) secretome components in vitro in the presence of inflammatory triggers or a tolerogenic CD74 ligand. Findings provide a background for experiments assessing MAPC response to molecular and cellular triggers and events, for eventual programming of MAPCs to be used in a wide range of clinical applications.
Keywords: Adult hematopoietic stem cells, Immunotherapy, Mesenchymal stem cells, Tolerance, Graft versus host disease, Proteomics, RTL1000
Abstract
Multipotent adult progenitor cells (MAPCs) are adult adherent stromal stem cells currently being assessed in acute graft versus host disease clinical trials with demonstrated immunomodulatory capabilities and the potential to ameliorate detrimental autoimmune and inflammation-related processes. Our previous studies documented that MAPCs secrete factors that play a role in regulating T-cell activity. Here we expand our studies using a proteomics approach to characterize and quantify MAPC secretome components secreted over 72 hours in vitro under steady-state conditions and in the presence of the inflammatory triggers interferon-γ and lipopolysaccharide, or a tolerogenic CD74 ligand, RTL1000. MAPCs differentially responded to each of the tested stimuli, secreting molecules that regulate the biological activity of the extracellular matrix (ECM), including proteins that make up the ECM itself, proteins that regulate its construction/deconstruction, and proteins that serve to attach and detach growth factors from ECM components for redistribution upon appropriate stimulation. MAPCs secreted a wide array of proteases, some detectable in their zymogen forms. MAPCs also secreted protease inhibitors that would regulate protease activity. MAPCs secreted chemokines and cytokines that could provide molecular guidance cues to various cell types, including neutrophils, macrophages, and T cells. In addition, MAPCs secreted factors involved in maintenance of a homeostatic environment, regulating such diverse programs as innate immunity, angiogenesis/angiostasis, targeted delivery of growth factors, and the matrix-metalloprotease cascade.
Introduction
There is accelerating interest in developing adult-derived mesenchymal stromal and other stem cells as therapeutic agents for the treatment of graft versus host disease (GVHD), cardiac indications, central nervous system (CNS) injury, and CNS disease pathologies [1–5]. Adult stromal cells have been isolated from multiple tissues and characterized [6]. Adult-derived adherent stromal cells demonstrate a number of favorable characteristics, including genetic stability, extensive expansion capacity, and low immunogenicity profiles [7]. These properties could prove invaluable in an allogeneic hematopoietic stem cell transplantation setting in which the patient receives bone marrow or blood stem cells from a closely but non-genotypically identical human leukocyte antigen (HLA)-matched donor [4, 8, 9]. Functional improvement after administration of adult-derived cells has been demonstrated in multiple preclinical models of injury or disease, but the mechanisms by which these outcomes are accomplished remain poorly understood [9, 10]. An understanding of the specific mechanisms by which beneficial effects are obtained after treatment with adult stem cells will be important for refining both dosage and route of delivery of these cells as cellular therapeutics continue to expand into clinical development.
Multipotent adult progenitor cells (MAPCs) (MultiStem; Athersys, Inc., Cleveland, OH, http://www.athersys.com) were originally described as a subpopulation of adherent stem cells that could be isolated from adult bone marrow and other adult tissues [11]. The bone marrow compartment contains populations of adult stromal cells that exist in a discernible hierarchy [12]. More primitive cells such as MAPCs or marrow-isolated adult multilineage inducible cells [13] and less primitive stem cells such as mesenchymal stem cells (MSCs) all copurify, and clonal populations are obtained by manipulating culture conditions [14].
Both MAPCs and MSCs have been shown to have strong immunomodulatory properties, and we have recently shown that rodent and human cell populations from bone marrow that were expanded at large scale via conditions specified for MAPC isolation are safe and can positively modulate the immune system [15, 16]. Based on these properties MAPCs have been “fast-tracked” into clinical trials. However, a detailed molecular understanding of the basic biology of these cells at rest and under conditions of variable stimuli remains absent and elusive. The studies described in this article were conducted to identify immunomodulatory factors secreted by MAPCs and to determine the effect of potential licensing or triggering agents on secretion of these factors. This information will be used to guide the design of future in vitro interaction studies between human MAPCs and peripheral blood mononuclear cells and in vivo experiments using a rodent model of GVHD evaluating the requirement, hierarchical importance, and redundancy of the various factors identified.
A straightforward proteomics approach involving liquid chromatography-coupled tandem mass spectrometry (LC-MS/MS) detection and bioinformatics was used to take an inventory and categorize the array of factors secreted by MAPCs. We sought to obtain a global overview of the immunomodulatory potential of MAPCs by characterizing the manufacturing capacity and secreted protein profiles of these cells over a 72-hour period under standard culture conditions in the absence of any exogenous stimuli, and then monitored changes in the secreted molecular constituents following treatment of MAPCs with inflammatory stimuli interferon (IFN)-γ and lipopolysaccharide (LPS), or a tolerogenic stimuli, RTL1000, a CD74 ligand [17–20]. A recent review by Hynes and Naba [21] provides an inventory of the molecular constituents and functions of the extracellular matrix (ECM), and the results presented here document that many of the known ECM regulatory components are directly manufactured by MAPCs, which secrete molecules that control angiogenesis, the matrix metalloproteinase (MMP) cascade, and effectors of both innate and adaptive immunity. In addition to classic secreted molecules, the MAPC secretome contains (a) premanufactured molecules that are stored in secretory granules and rapidly released from cells in response to external stimuli, and (b) cell-surface molecules proteolytically shed by ectodomain shedding. The results presented in this report reveal an array of factors known to be involved in regulating and integrating aspects of inflammation and angiogenesis, including the trafficking of leukocytes to sites of inflammation, and are discussed in the context of the potential utility of these cells in prevention and treatment of inflammatory disorders.
Materials and Methods
Chemicals and Reagents
IFN-γ was purchased from R&D Systems (Minneapolis, MN, http://www.rndsystems.com; catalog no. 285IF100), and LPS was from Sigma-Aldrich (St. Louis, MO, http://www.sigmaaldrich.com; catalog no. L4391).
RTL1000
We have previously described recombinant T cell receptor ligands (RTLs) derived from the β-1 and α-1 domains of major histocompatibility (MHC) class II molecules bearing covalently tethered antigens [22]. These molecules effectively tolerize Ag-specific CD4+ T cells [23] and modulate the inflammatory potential of CD11b+ monocytes [20]. Cloning, expression, and purification of the RTL protein constructs from inclusion bodies produced in Escherichia coli have been previously described [24]. Characterization and batch-to-batch quality control validation for RTLs [25] have also been described extensively in previous publications [22, 26]. The RTL1000 used in this study was recently successfully used in a phase I clinical trial for treatment of multiple sclerosis [27]. It is extremely stable as assessed by biochemical and biophysical integrity and biological activity (inhibiting clinical signs of experimental autoimmune encephalomyelitis in DR2-Tg mice) over a 42-month follow-up period.
Human MAPC Cultures and MAPC-Conditioned Media
MAPCs meet the formal criteria for designation of cells as MSCs, a prototype for adherent stem and progenitor cells, defined in a position statement of the International Society for Cellular Therapy [28]. This designation requires that the in vitro expanded cells be plastic-adherent and express cell-surface molecules, including CD73, CD90, and CD105, in the absence of hematopoietic markers, including CD14, CD34, CD45, and HLA-DR. MAPCs can be distinguished from MSCs on the basis of cellular phenotype, size, transcriptional profile, and expansion capacity. Compared with standard MSC culture conditions, MAPCs are isolated using hypoxic conditions in media supplemented with growth factors epidermal growth factor and platelet-derived growth factor and kept at subconfluent culture density [6]. MAPCs can be expanded under defined low-serum conditions for more than 100 population doublings without telomere shortening and remain karyotypically normal [11, 29]. Clinical-grade MAPCs (MultiStem) were manufactured by Athersys. Human MAPC cultures were derived from bone marrow aspirates donated by human volunteers (lots 061269, BMC135, and BMC167), and bone marrow was processed as previously described [30]. In brief, human MAPCs were isolated from bone marrow aspirate, obtained with consent from a healthy donor, and cultured in fibronectin-coated plastic tissue culture flasks. Cell cultures were maintained under low oxygen tension in a humidified 5% CO2 atmosphere. Cells were cultured to subconfluence in MultiStem culture medium consisting of low-glucose Dulbecco's modified Eagle's medium (Life Technologies/Invitrogen, Grand Island, NY, http://www.lifetech.com) supplemented with fetal bovine serum (Atlas Biologicals, Fort Collins, CO, http://www.atlasbio.com), insulin, transferrin, and selenium liquid medium supplement (Sigma-Aldrich), MCDB (Sigma-Aldrich), platelet-derived growth factor (R&D Systems), epidermal growth factor (R&D Systems), dexamethasone (Sigma-Aldrich), penicillin/streptomycin (Life Technologies), 2-phospho-l-ascorbic acid (Sigma-Aldrich), and linoleic acid-albumin (Sigma-Aldrich). Cells were passaged every 3–4 days and harvested using trypsin/EDTA (Life Technologies). Flow cytometric analysis of surface-expressed antigens confirmed the homogeneity of the MAPCs used in this study and confirmed that they met previously described release criteria [6], including staining positive (>90%) for CD49c and CD90 and negative (<5%) for MHC class II and CD45 (antibodies used were from BD Biosciences, San Jose, CA, http://www.bdbiosciences.com). Cells were cryopreserved in Plasma-Lyte A (Baxter, Deerfield, IL, http://www.baxter.com) with dimethyl sulfoxide and human serum albumin. Immediately prior to their use MAPCs were thawed, washed twice with xeno-free medium containing 1% human serum, and then cultured for a minimum of three passages. MAPCs were then treated with various stimuli, including IFN-γ (2 ng/ml), LPS (10 μg/ml), and RTL1000 (5 μg/ml). Conditioned medium (CM) was collected at 24 hours (15% confluent), 48 hours (30% confluent), and 72 hours (60% confluent) in 50-ml conical tubes (BD Biosciences). The conditioned medium samples were spun down at 400g for 5 minutes at 4°C to remove cells and debris, and the supernatants were transferred to new 50-ml conical tubes. MAPC-conditioned medium (MAPC-CM) samples were concentrated 50-fold with an Amicon Microcon Ultracel YM-3 3000 molecular weight cutoff centrifugal filter (Millipore, Billerica, MA, http://www.millipore.com), snap-frozen on dry ice, and stored at −80°C until analysis.
Processing of Media and MAPC-CM Samples and Immunodepletion of Major Serum Components From MAPC-CM for Secretome Analysis
Samples were thawed and assayed for protein content using a bicinchoninic acid (BCA) assay and bovine serum albumin standard (Pierce, Rockford, IL, http://www.piercenet.com) [31]. MAPC-CM samples were buffer exchanged into Agilent buffer A (Agilent Technologies, Santa Clara, CA, http://www.agilent.com; proprietary medium formulation) and concentrated, and the total amount of protein present in the samples was determined (BCA) (Pierce). Recognizing that the presence of even 1% serum limits the depth of coverage and identification of secreted cell products, MAPC-CM samples were immunodepleted using a serum immunodepletion column (Agilent multiple affinity removal system [MARS-14]) of the 14 most common serum proteins that make up 98% of the total protein in serum prior to characterization by LC-MS/MS. The immunoaffinity column antibodies and buffer components are designed to interact with the major serum components in a denaturing but nonreducing buffer A, resulting in the removal of the major serum components without removing secretome components that potentially bind to these major serum proteins (Virginia Curtiss, Ph.D., Agilent Technologies, personal communication).
Trypsinization of Secretome Samples
Serum-depleted medium and MAPC-CM samples were concentrated and assayed for protein content by BCA assay. Ten micrograms total protein per sample was treated with trypsin at 37°C overnight at a ratio of enzyme/substrate of 1:25, and the reaction was stopped by the addition of formic acid as previously described [32].
Mass Spectrometry Analysis of MAPC Secretome Peptides
Forty microliters (10 μg of total medium or MAPC-CM protein digested with trypsin) of each sample was separated by reverse phase liquid chromatography while collecting data-dependent MS/MS spectra on the eluted peptides. Peptides were separated using an Agilent 1100 series capillary LC system and an LTQ Velos linear ion trap mass spectrometer (ThermoFisher, San Jose, CA, http://www.thermofisher.com). Electrospray ionization was performed with an ion max source fitted with a 34 gauge metal needle and 2.4 kV potential. Samples were applied at 20 μl/minute to a trap cartridge (Michrom BioResources, Inc., Auburn, CA, http://www.michrom.com) and then switched onto a 0.5 × 250 mm Zorbax SB-C18 column with 5 μm particles (Agilent Technologies) using a mobile phase containing 0.1% formic acid, 7%–30% acetonitrile gradient over 220 minutes, and 10 μl/minute flow rate. Data-dependent collection of MS/MS spectra used the dynamic exclusion feature of the instrument control software (repeat count equal to 1, exclusion list size of 10, exclusion duration of 30 seconds, and exclusion mass width of −1 to +4) to obtain MS/MS spectra of the five most abundant parent ions (minimum signal threshold of 1 × 104) following each survey scan from m/z 350 to 2,000. The tune file was configured with no averaging of microscans, a maximum inject time of 200 ms, and automatic gain control targets of 3 × 104 in mass spectrometry mode and 1 × 104 in multiple-stage mass spectrometry mode.
Data Analysis
An in-house script was used to create DTA-format files from the RAW files using extract_msn.exe (version 5.0, ThermoFisher) with a molecular weight range of 550–4,000, an absolute threshold of 500, group scan setting of 1, and a minimum of 25 ions. A human species subset of the Sprot (version 2011.06) protein database (20,235 proteins) was prepared with concatenated reversed entries (and common contaminants) using scripts available at the Proteomic Analysis Workbench (http://www.ProteomicAnalysisWorkbench.com) and searched with SEQUEST (version 28, revision 12; ThermoFisher). Parent ion and fragment ion tolerances of 2.5 and 1.0 Da were used with calculated average and monoisotopic masses, respectively. Cysteine had a static modification mass of +57 Da, and trypsin specificity was used. An in-house suite of programs [33] was used to provide a Peptide Prophet-like discriminant function [34] scoring to identify “correct” peptides and discard “incorrect” peptides using sequence-reversed matches to estimate the false discovery rate (FDR). Of the 798,803 acquired MS2 scans from the 24 samples, there were 132,333 that passed score thresholds with 6,649 matches to reversed sequences, giving an estimated overall peptide FDR of 5.3%. Protein identification lists were prepared using standard parsimony principles. Proteins were required to have two or more fully tryptic peptides with distinct sequences. Different charge states of the same peptide sequence were not considered as unique peptides. The number of identified proteins per sample ranged from 59 to 287 (nonredundant counts, excluding contaminants), and the protein FDR per sample ranged from 1.2% to 6.4%. An in-house algorithm was used to compare identified peptide sets between proteins having peptides in common and group together highly homologous identifications into families. Shared and unique peptide status was recomputed across all proteins after family grouping. Shared peptide counts were fractionally split on the basis of relative total unique counts of the proteins that had those peptides in common. These corrected total spectral counts per protein were used in the quantitative comparisons described below. There are a number of factors that influence the number of spectral counts for a particular protein. For example, a protein would be under-represented or even undetected if, by virtue of its structure or association with other elements, it was not efficiently cleaved by trypsin. Similarly, certain peptides, such as ones shorter than 6 or longer than 20 residues, produce MS/MS spectra that are difficult to match to peptide sequences, and therefore, these peptides would be under-represented. One parameter that strongly affects the count of peptides in the analysis is the size of the parent protein. At equimolar concentrations, a large protein will generate more peptides than a small one of similar composition and structure. To normalize for this effect, we divided the spectral count for a given protein by the molecular mass of that protein so the abundance more closely resembled molar concentrations rather than weight concentrations. We recognize that the relationships may not be strictly linear and accordingly use the term “apparent abundance” when comparing the signals from two proteins. The list of all proteins identified, their normalized spectral counts, and annotations are found in supplemental online Table 1.
Bioinformatics
Swiss protein identifiers (more human-informative but less stable identifiers) were mapped to their Swiss-Prot accession numbers for subsequent analysis when needed. The potential for MAPCs to manufacture the secretome components identified by LC-MS/MS was confirmed by microarray analysis. Samples were run in duplicate on Human WG6 V2 gene chips (Illumina platform; Illumina Inc., San Diego, CA, http://www.illumina.com), and the data were analyzed using Illumina Bead Studio software, Excel (Microsoft, Redmond, WA, http://www.microsoft.com), and Invitrogen Linnea Pathway software (Life Technologies) (data not shown). Conversion of Illumina identifiers (Illumina_ID) to Unipro IDs and removal of redundancies was performed by Da-Wei Huang, M.D., using DAVID Bioinformatics resource 6.7 software (http://david.abcc.ncifcrf.gov) (Da-Wei Huang, M.D., National Institute of Allergy and Infectious Diseases, personal communication) [35, 36]. The secretome data set containing up- or downregulated proteins (≥2-fold), including proteins identified only in IFN-γ-, LPS-, or RTL1000-treated MAPC samples, was analyzed by Ingenuity Pathway Analysis (IPA) version 9.0 (Ingenuity Systems, Mountain View, CA, http://www.ingenuity.com). Statistically significant input protein identifications and IPA were mapped with a continuously updated and curated database of published literature regarding a range of functions, cellular locations, canonical pathways, and disease inter-relationships. The association between proteins in the data set and canonical pathways in the Ingenuity Pathways Knowledge Base was measured as a ratio of the number of molecules from the data that map to a pathway divided by the total number of molecules that map to the canonical pathway. A right-tailed Fisher's exact test was used to calculate the p value of the probability that the association between each protein in the data set and the canonical pathway was random [37].
Results
MAPC Secretome Analysis
Human MAPCs were cultured for a minimum of three passages under defined conditions in the absence of any exogenous stimuli, and medium alone and MAPC-CM samples were collected at 24, 48, and 72 hours. A protein identified by LC-MS/MS analysis was deemed a confident match if at least two unique peptides were detected for that protein, and spectral counts obtained at all time points for each sample were averaged for downstream comparison between treatment groups. One of our long-term goals is to program coherent innate and adaptive immune responses in vivo. Toward this effort we determined the effect of potential licensing agents on the MAPC secretome. MAPC-CM was monitored over 72 hours of treatment with the three licensing agents IFN-γ (2 ng/ml), LPS (10 μg/ml), and RTL1000 (5 μg/ml) [17–20]. Proteins were considered to be MAPC secretome candidates if more than 2.5 unique peptides were identified in any of the treatment group samples, disallowing any proteins with more than 2.5 unique peptides identified in medium-alone samples. The single exception to this cutoff criterion was thrombospondin-1. Although it was detected in medium (3.8 spectral counts), it was included in our analysis because it showed a 7.5-fold or greater increase in spectral counts in MAPC-CM. A wide range of functionally distinct classes of molecules was identified by high-performance liquid chromatography-MS/MS (Table 1), and the complete data set of 97 proteins identified is presented in supplemental online Table 1. Our data set includes 66 classically secreted proteins, 3 glycophosphatidylinositol-linked proteins, 4 single-pass type I membrane proteins, and 24 proteins that are classically identified as endoplasmic reticulum or cytoplasmic residents but that have been identified from previous studies as being secreted via various nonclassic routes or by ectodomain shedding mechanisms.
Table 1.
MAPC protein secretome: Functionally distinct classes of molecules identified by HPLC-MS/MS
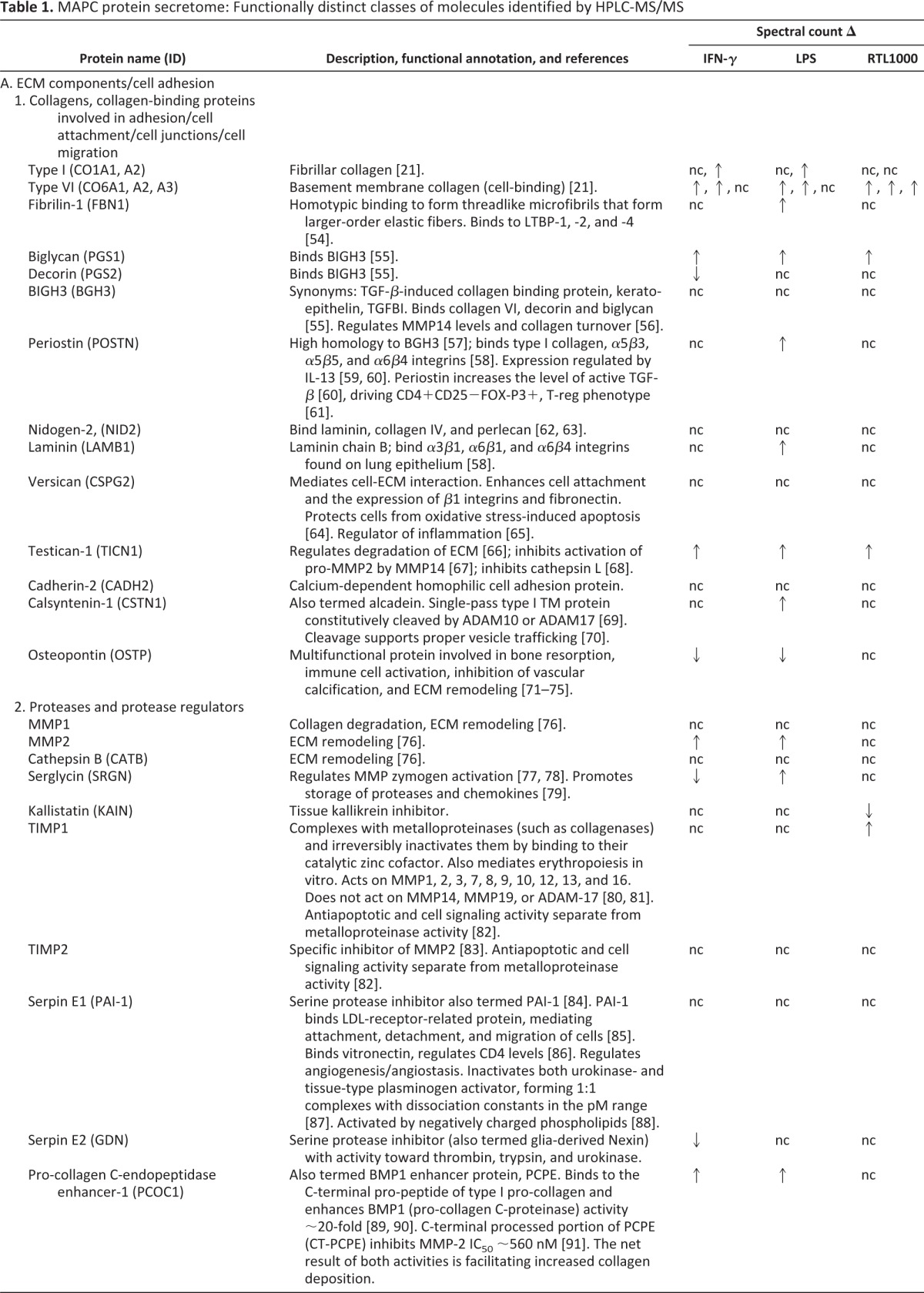
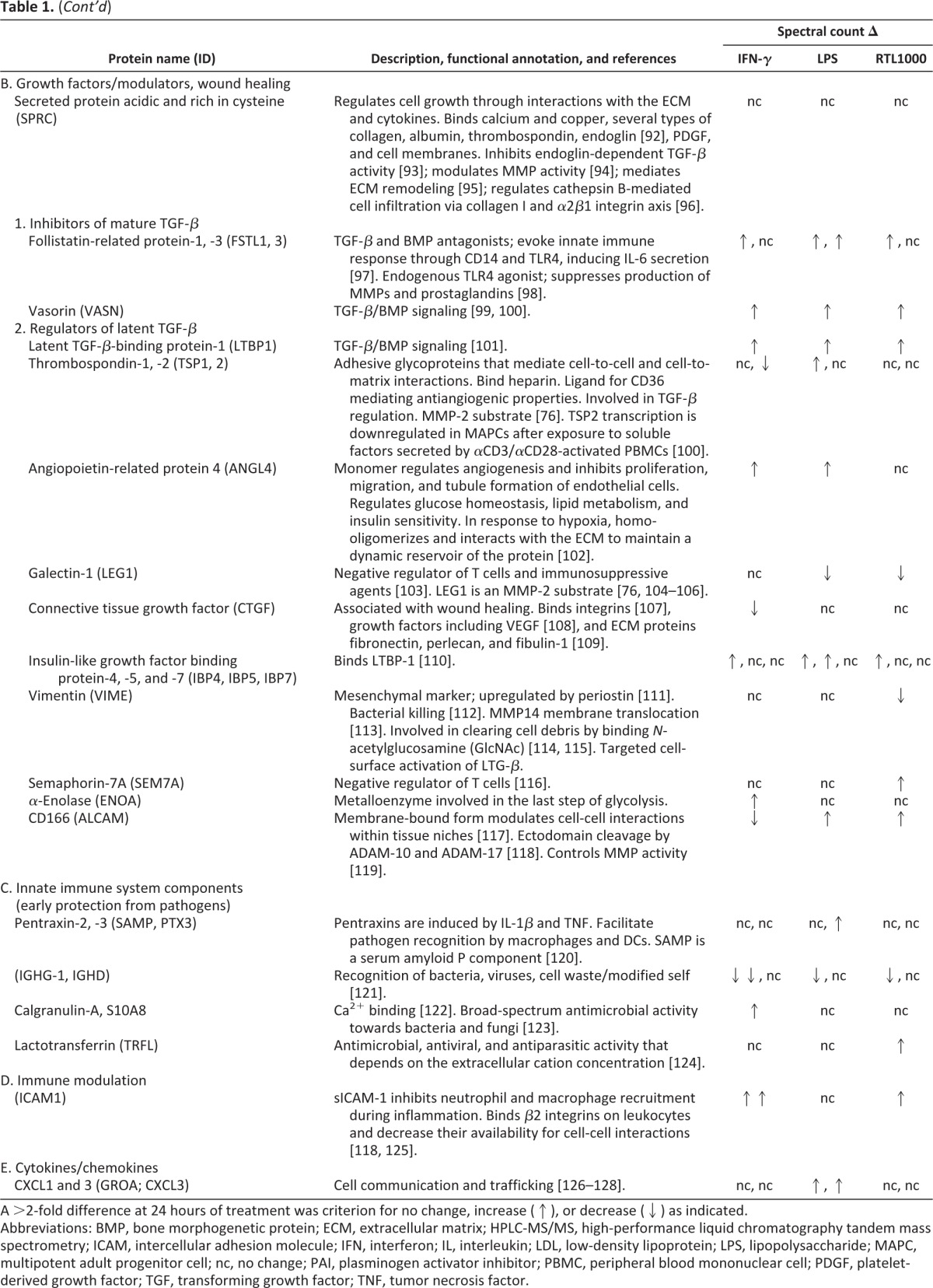
A >2-fold difference at 24 hours of treatment was criterion for no change, increase (↑), or decrease (↓) as indicated.
Abbreviations: BMP, bone morphogenetic protein; ECM, extracellular matrix; HPLC-MS/MS, high-performance liquid chromatography tandem mass spectrometry; ICAM, intercellular adhesion molecule; IFN, interferon; IL, interleukin; LDL, low-density lipoprotein; LPS, lipopolysaccharide; MAPC, multipotent adult progenitor cell; nc, no change; PAI, plasminogen activator inhibitor; PBMC, peripheral blood mononuclear cell; PDGF, platelet-derived growth factor; TGF, transforming growth factor; TNF, tumor necrosis factor.
MAPCs exposed to these agents showed a wide range of responses as reflected in the secretome (Fig. 1). Correlation curves plotting the averaged spectral counts of technical replicates at 24 hours (n = 2) suggested that all three treatments perturbed the MAPC secretome (Fig. 1C). When a twofold ratio change cutoff was applied to the 97 proteins in the MAPC secretome candidate list, IFN-γ, LPS, or RTL1000 treatment resulted in approximately 72% (n = 69) of the MAPC secretome candidates being of differential abundance. Of the 97 proteins detectable in the MAPC secretome at 24 hours, 24 were still detectable following IFN-γ treatment, 26 following LPS treatment, and 24 following RTL1000 treatment (Fig. 1A). Combined, 16 of the 97 proteins (17%) were detectable under all conditions. At 24 hours, IFN-γ treatment (2 ng/ml) [18] was associated with a decrease in abundance of 10 proteins and an increased abundance of 16 proteins. LPS (10 μg/ml) [17] was associated with a decrease in abundance of 11 proteins and an increased abundance of 41 proteins. RTL1000, a CD74 ligand (5 μg/ml) [20] was associated with a decrease in abundance of 10 proteins and an increased abundance of 26. The results indicate that MAPCs are able to recognize these stimuli and alter their protein secretion programs.
Figure 1.
IFN-γ, LPS, and RTL1000 induced upregulation and downregulation of MAPC secretome proteins at 24 hours. (A): Venn diagram showing the overlap, relationship, and spectral count changes of 71 MAPC secretome candidates. (B): Treatment signatures as defined by unique Spc Δ. (C): Highest abundance secretome proteins and Spc Δ following treatment. The blue line indicates baseline abundance (MAPCs, no treatment). Larger font indicates uniquely up- or downregulated secretome proteins. Inset: Correlation curves plotting the averaged spectral counts of technical replicates at 24 hours (n = 2). (D): Principal component analysis of the 97 detected proteins. Abbreviations: IFN, interferon; LPS, lipopolysaccharide; MAPC, multipotent adult progenitor cell; Spc Δ, spectral count changes; VEGFA, vascular endothelial growth factor A.
We used a combination of pathways analysis (Ingenuity IPA) and brute-force data mining coupled with structure-function homology modeling (Sybyl-X; Tripos, Inc., St. Louis, MO, http://www.tripos.com) to map interactions of the MAPC secretome proteins identified (Fig. 2). Forty-six of these proteins (47%) physically interacted with each other, clustering into two major groups (28 of 46, 61%; and 14 of 46, 30%). The proteins that directly interacted with each other are indicated by black lines in Figure 2, and the proteins that interacted as substrate/catalyst pairs are highlighted with substrates indicated by the arrowheads. Inclusion of indirect interactions (represented by dotted lines) expands the interactome to 58 of 97 (60%) of the MAPC secretome proteins. This array of interactions documents the complex interconnectedness and interdependence of the secretome. A three-dimensional workspace consisting of the ECM overlaid with the known interactions of MAPC secretome proteins identified is presented in Figure 3.
Figure 2.
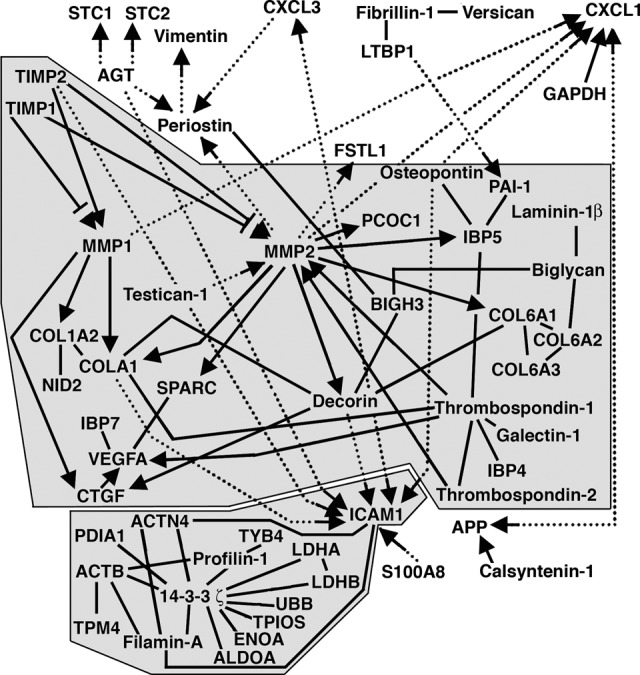
Known interactions between proteins identified in multipotent adult progenitor cell secretome. Proteins identified that directly interact with each other are indicated by solid black lines. Substrate/catalyst pairs are highlighted with substrates indicated by arrowheads. Indirect interactions are represented by dotted lines. Abbreviations: GAPDH, glyceraldehyde-3-phosphate dehydrogenase; LTBP, latent transforming growth factor binding protein; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; SPARC, secreted protein acidic and rich in cysteine; VEGFA, vascular endothelial growth factor A.
Figure 3.
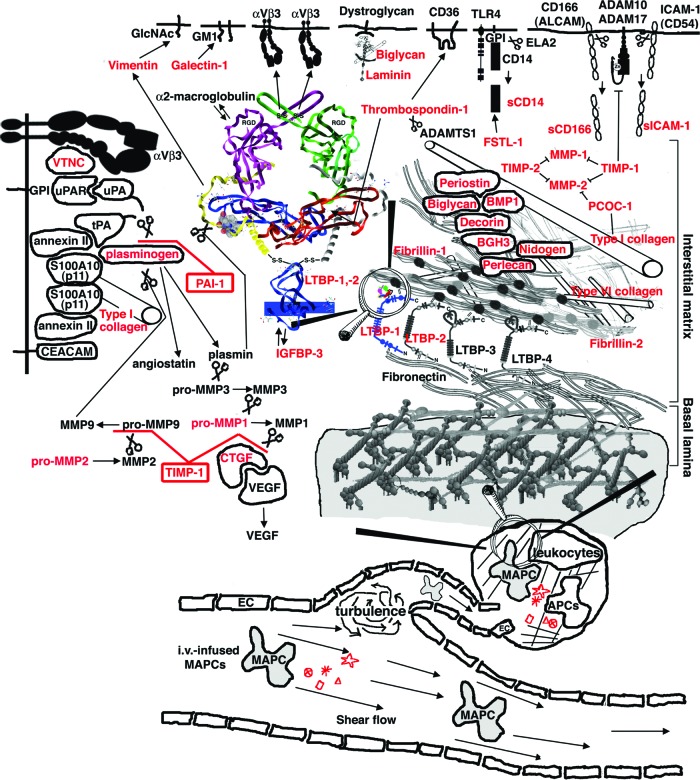
A schematic overview of our hypothesis. Intravenously delivered MAPCs and secretome components identified (red) are hypothesized to regulate broad aspects of growth factor delivery, the metalloproteinase cascade, and angiogenesis. Abbreviations: LTBP, latent transforming growth factor binding protein; MAPC, multipotent adult progenitor cell; MMP, matrix metalloproteinase; PAI, plasminogen activator inhibitor; uPA, urokinase plasminogen activator; uPAR, urokinase plasminogen activator receptor; VEGF, vascular endothelial growth factor.
Discussion
Our data document that MAPCs secrete molecules that regulate the biological activity of the ECM as well as secreting chemokines, cytokines, and an array of molecules participating in and regulating diverse programs, including angiogenesis/angiostasis, targeted delivery of growth factors, and the matrix metalloprotease cascade (Table 1). Molecules that regulate the biological activity of the ECM include proteins that make up the ECM itself, such as type I and VI collagens, and proteins that regulate its construction/deconstruction. These include proteases such as MMP1, MMP2, and cathepsin B. Also detected were protease inhibitors and regulators of protease activation, including serglycin, kallistatin, TIMP1 and TIMP2, plasminogen activator inhibitor-1 (PAI-1), glia-derived nexin, PCOC1, and histidine-rich glycoprotein. A wide array of collagen-binding proteins are secreted by MAPCs, including biglycan, decorin, BIGH3, periostin, versican, and osteopontin. The MAPC secretome contains proteins that modulate the activity of growth factors and serve to attach and de-attach growth factors from ECM components for redistribution upon appropriate stimulation. These regulatory proteins include secreted protein acidic and rich in cysteine, latent transforming growth factor binding proteins, follistatin-related proteins, vasorin, and thrombospondins. Additionally, MAPCs secrete innate immune system components that provide early protection from pathogens, such as pentraxins and lactotransferrin, and immunomodulatory proteins, including soluble intercellular adhesion molecule 1 (sICAM1), semaphorins, and galectins. It is important to note that many of the proteins identified in the MAPC secretome are multifunctional proteins that serve overlapping and alternative functions that depend upon variable signals from the local microenvironment.
The high abundance of protease inhibitor PAI-1 is consistent with identification of tryptic fragments from the intact activation peptide of plasminogen, suggesting that the plasminogen identified in the samples is (at least partially) in the zymogen (inactive) state (supplemental online Fig. 1). In addition, consistent with the high abundance of TIMP-1, a high-affinity regulator of MMP2 activity, the MMP2 identified in the MAPC secretome is also (at least partially) in its zymogen form. This is inferred from the identification of tryptic peptide fragments that traverse the propeptide-mature protein boundary, with propeptide tryptic peptide fragments representing 78% coverage of the propeptide domain versus 37% coverage of the mature MMP2 protein (supplemental online Fig. 2). The data are consistent with the concept that MAPCs secrete an array of factors that maintain the ECM in a state of repose, ready to respond appropriately to exogenous stimuli that would be expected to be generated as a result of local physical injury of tissue or infiltration by various cell types.
IFN-γ is a cytokine secreted by natural killer cells, monocytes/macrophages, and Th1 CD4+ cells, and it would be expected to be present in a local environment as a result of infiltrating leukocytes. LPS could be present in a local environment as a consequence of a bacterial infection. Cellular trafficking into inflammatory compartments results in proteolytic ectodomain-shedding of membrane proteins, including fragments of MHC class I and MHC class II molecules. We hypothesize that these fragments regulate local inflammatory processes. In our recently published studies, we documented that RTL1000, a recombinant molecule consisting of the β-1 and α-1 domains of MHC class II, binds and downregulates CD74, inhibiting leukocyte trafficking and inflammation, at least in part via decreased macrophage inhibitory factor signaling [20]. Consequently, RTL1000 was tested for the first time in experiments presented in this study to determine whether MAPCs could respond to this mimic of a highly localized tolerogenic mechanism. Our data documented that MAPCs were able to recognize these three stimuli with concomitant differential alteration of their protein secretion programs.
One of the major challenges in fully characterizing a secretome is that many of the molecules secreted and expected to be active in situ in cell-to-cell signaling interactions function at subnanogram to picogram levels, and these are difficult to detect using LC-MS/MS against a background of thousands of highly abundant serum proteins (in the mg/ml range) [38]. We used an immunodepletion strategy to remove the top 14 background serum components [39]. Having detected galectin-1, CXCL1, and CXCL3 in the MAPC secretome (Table 1), the level of detection we reached with our approach is estimated to be proteins present at concentrations of approximately 200 pg/ml [40]. In future studies we will incorporate selective enrichment technology that will allow detection of more of the lower abundance proteins expected to be present in the MAPC secretome [41]. A second caveat when analyzing this data set is that a protein was deemed a secretome candidate if more than 2.5 unique peptides were identified in any of the biological replicates under any of the conditions used, and thus this study was not designed to address the variation between biological replicates. The data accurately reflect the differential effects of IFN-γ, LPS, and RTL1000 on MAPCs cultured from a single individual donor.
Bioinformatics and pathways analysis were used to evaluate in detail how a representative subset of molecules present in the MAPC secretome could potentially function as a complex network of interacting molecules that regulate the ECM when MAPCs differentially respond to IFN-γ, LPS, and RTL1000. From a wound-healing perspective, the differential responses observed indicate that MAPCs could respond rapidly and efficiently to various types of insults and alterations that occur as a function of time that are induced in part by infiltrating inflammatory and immune cells. Molecules identified in the MAPC secretome have the ability to modulate the behavior of infiltrating cells, and pathways analysis suggests that the molecular components are integrated on a number of levels, with MAPCs secreting a complex mixture of collagens and collagen-interacting molecules. We hypothesize that this network of interacting molecules secreted by MAPCs serves to guide a simultaneously coordinated plethora of events. The events potentially include recruitment of cells (via CXCL1 and CXCL3, for example) and managing the behavior of infiltrating cells by regulating their effector functions (secretion of galectin-1 negatively regulating T-cell effector function, for example) appropriately for a wound-healing environment [42]. In addition, our data document that the MAPC secretome contains molecules that prevent opportunistic pathogens from spreading and enhancing tissue damage. MAPCs secrete molecules that regulate access of tissues by macrophages for clearing of debris while simultaneously coordinating with top-level memory/effector CD4+ and CD8+ cells, using their cytokine and chemokine arsenal to communicate and program the systemic response to specific stimuli.
Cellular therapeutics are rapidly entering the clinical arena. Establishing dose-response, defining optimal routes of delivery, and establishing potency are essential to the optimization of clinical application. All the steps first await a thorough understanding of the primary mechanisms by which MAPCs provide clinical benefit.
In our previously published studies it was documented that rodent and human MAPCs are safe and can modulate the immune system via the secretion of soluble factors [15, 16]. Good manufacturing practice-manufactured human MAPCs are now in phase II clinical trials for inflammatory bowel disease, acute myocardial infarction, and ischemic stroke. Two phase I clinical trials (safety studies) have been completed using MAPCs for prevention of GVHD and for treatment of acute myocardial infarction [43, 44]. The beneficial effects of MAPCs in vivo may be the product of multiple mechanisms, including a proangiogenic effect through trophic support [45–47] and the modulation of the immune response [15, 48]. Immunoregulatory properties of MAPCs may be important for GVHD prevention and treatment. Human and rat MAPCs inhibit allogeneic T-cell proliferation mediated by soluble factors that include indoleamine 2,3-dioxygenase [16]. At present, it is still unclear how many different molecular pathways are impacted. This study was designed to begin to catalog the array of primary targets.
The route of delivery also remains unclear and may be critical for optimal application of stromal cell therapies. Our focus has been on exploiting the immunomodulatory potential of MAPCs to protect patients from GVHD following allogeneic hematopoietic stem cell transplantation [16, 49]. Most studies have treated patients with systemic intravenous administration in which stromal cells first passage to the lung where they are initially retained [48, 50]. In one study, lung entrapment appeared to be required for treatment of peritoneal adhesion [51]. In other models, for example, chemical injury to the corneal epithelial surface, either local or systemic delivery of MSCs ameliorates damage [52]. These reports reflect the ongoing uncertainty regarding how clinical benefit is achieved. Finally, recent studies evaluating encapsulated stromal cell product support the concept that stromal cell homing to specific organs is not absolutely required to control inflammation [53]. These observations support the concept that MAPC immune regulation is a consequence of soluble factors that act systemically.
Conclusion
Our studies used a proteomics approach to characterize and quantify the changes induced in the MAPC secretome over 72 hours in vitro under steady-state conditions and in the presence of the inflammatory triggers IFN-γ and LPS, or a tolerogenic CD74 ligand, RTL1000. MAPCs differentially responded to each of the tested stimuli, and these studies provide a foundation for understanding and controlling the function of MAPCs. As such, the data presented here provide a strong background for hypothesis-driven experiments assessing MAPC response to molecular and cellular triggers and events for eventual programming of MAPCs to be used in a wide range of clinical applications.
Supplementary Material
Acknowledgments
We thank Dr. Virginia Curtiss at Agilent Technologies (Santa Clara, CA) as a resource for developing and refining our immunodepletion strategy.
Author Contributions
G.G.B.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; W.v.H.: conception and design, data interpretation, manuscript writing; L.F.N.: conception and design, manuscript writing; A. Reddy and L.L.D.: collection of data, data analysis and interpretation, manuscript writing; P.A.W.: assembly of data, data analysis, manuscript writing; A. Raber: collection of data, manuscript writing; A.B. and J.P.: data analysis and interpretation, manuscript writing; R.J.D.: data interpretation, manuscript writing; R.T.M.: conception and design, financial support, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
G.G.B. is the inventor of the RTL technology, has uncompensated intellectual property rights with Artielle ImmunoTherapeutics, Inc., and is a compensated consultant with Virogenomics, Inc. G.G.B. and Oregon Health and Science University (OHSU) have a significant financial interest in Artielle ImmunoTherapeutics, Inc., a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by the OHSU Conflict of Interest in Research Committees. W.v.H. is a compensated Athersys employee, has compensated intellectual property rights, and is a compensated stock holder. A. Raber is a compensated Athersys employee and has compensated stock options from Athersys. A.B. is a compensated Athersys employee. J.P. is a compensated Athersys employee, is head of research and development at Athersys, and has compensated stock options in Athersys. R.J.D. is a compensated Athersys employee, is an uncompensated patent holder with Athersys, and has compensated stock options in Athersys. Athersys has licensed OHSU's rights in intellectual property coinvented by R.T.M., which may apply to the use of MAPCs in this treatment area. This potential individual and institutional conflict of interest has been reviewed and managed by OHSU. W.v.H. and R.J.D. are coinventors of the MultiStem technology.
References
- 1.Biernaskie J, Sparling JS, Liu J, et al. Skin-derived precursors generate myelinating Schwann cells that promote remyelination and functional recovery after contusion spinal cord injury. J Neurosci. 2007;27:9545–9559. doi: 10.1523/JNEUROSCI.1930-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bambakidis NC, Butler J, Horn EM, et al. Stem cell biology and its therapeutic applications in the setting of spinal cord injury. Neurosurg Focus. 2008;24:E20. doi: 10.3171/FOC/2008/24/3-4/E19. [DOI] [PubMed] [Google Scholar]
- 3.Barnabé-Heider F, Frisen J. Stem cells for spinal cord repair. Cell Stem Cell. 2008;3:16–24. doi: 10.1016/j.stem.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 5.Kebriaei P, Robinson S. Treatment of graft-versus-host-disease with mesenchymal stromal cells. Cytotherapy. 2011;13:262–268. doi: 10.3109/14653249.2010.549688. [DOI] [PubMed] [Google Scholar]
- 6.Reading JL, Yang JH, Sabbah S, et al. Clinical-grade multipotent adult progenitor cells durably control pathogenic T cell responses in human models of transplantation and autoimmunity. J Immunol. 2013;190:4542–4552. doi: 10.4049/jimmunol.1202710. [DOI] [PubMed] [Google Scholar]
- 7.Gnecchi M, Zhang Z, Ni A, et al. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kebriaei P, Isola L, Bahceci E, et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol Blood Marrow Transplant. 2009;15:804–811. doi: 10.1016/j.bbmt.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Auletta JJ, Deans RJ, Bartholomew AM. Emerging roles for multipotent, bone marrow-derived stromal cells in host defense. Blood. 2012;119:1801–1809. doi: 10.1182/blood-2011-10-384354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caplan AI. All MSCs are pericytes? Cell Stem Cell. 2008;3:229–230. doi: 10.1016/j.stem.2008.08.008. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 12.Anjos-Afonso F, Bonnet D. Nonhematopoietic/endothelial SSEA-1+ cells define the most primitive progenitors in the adult murine bone marrow mesenchymal compartment. Blood. 2007;109:1298–1306. doi: 10.1182/blood-2006-06-030551. [DOI] [PubMed] [Google Scholar]
- 13.D'Ippolito G, Diabira S, Howard GA, et al. Marrow-isolated adult multilineage inducible (MIAMI) cells, a unique population of postnatal young and old human cells with extensive expansion and differentiation potential. J Cell Sci. 2004;117:2971–2981. doi: 10.1242/jcs.01103. [DOI] [PubMed] [Google Scholar]
- 14.Reyes M, Lund T, Lenvik T, et al. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001;98:2615–2625. doi: 10.1182/blood.v98.9.2615. [DOI] [PubMed] [Google Scholar]
- 15.Kovacsovics-Bankowski M, Mauch K, Raber A, et al. Pre-clinical safety testing supporting clinical use of allogeneic multipotent adult progenitor cells. Cytotherapy. 2008;10:730–742. doi: 10.1080/14653240802320245. [DOI] [PubMed] [Google Scholar]
- 16.Kovacsovics-Bankowski M, Streeter PR, Mauch KA, et al. Clinical scale expanded adult pluripotent stem cells prevent graft-versus-host disease. Cell Immunol. 2009;255:55–60. doi: 10.1016/j.cellimm.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Pevsner-Fischer M, Morad V, Cohen-Sfady M, et al. Toll-like receptors and their ligands control mesenchymal stem cell functions. Blood. 2007;109:1422–1432. doi: 10.1182/blood-2006-06-028704. [DOI] [PubMed] [Google Scholar]
- 18.Krampera M, Cosmi L, Angeli R, et al. Role for interferon-gamma in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells. 2006;24:386–398. doi: 10.1634/stemcells.2005-0008. [DOI] [PubMed] [Google Scholar]
- 19.Barrilleaux BL, Fischer-Valuck BW, Gilliam JK, et al. Activation of CD74 inhibits migration of human mesenchymal stem cells. In Vitro Cell Dev Biol Anim. 2010;46:566–572. doi: 10.1007/s11626-010-9279-1. [DOI] [PubMed] [Google Scholar]
- 20.Vandenbark AA, Meza-Romero R, Benedek G, et al. A novel regulatory pathway for autoimmune disease: Binding of partial MHC class II constructs to monocytes reduces CD74 expression and induces both specific and bystander T-cell tolerance. J Autoimmun. 2013;40:96–110. doi: 10.1016/j.jaut.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes RO, Naba A. Overview of the matrisome: An inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Burrows GG, Chou YK, Wang C, et al. Rudimentary TCR signaling triggers default IL-10 secretion by human Th1 cells. J Immunol. 2001;167:4386–4395. doi: 10.4049/jimmunol.167.8.4386. [DOI] [PubMed] [Google Scholar]
- 23.Huan J, Kaler LJ, Mooney JL, et al. MHC class II derived recombinant T cell receptor ligands protect DBA/1LacJ mice from collagen-induced arthritis. J Immunol. 2008;180:1249–1257. doi: 10.4049/jimmunol.180.2.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang JW, Mechling DE, Bachinger HP, et al. Design, engineering, and production of human recombinant t cell receptor ligands derived from human leukocyte antigen DR2. J Biol Chem. 2001;276:24170–24176. doi: 10.1074/jbc.M101808200. [DOI] [PubMed] [Google Scholar]
- 25.Burrows GG, Vandenbark AA, inventors. Oregon Health Sciences University, assignee. Recombinant MHC molecules useful for manipulation of antigen-specific T-cells. US patent 6/270/772. 2001 Aug 7;
- 26.Huan J, Meza-Romero R, Mooney JL, et al. Single-chain recombinant HLA-DQ2.5/peptide molecules block alpha2-gliadin-specific pathogenic CD4+ T-cell proliferation and attenuate production of inflammatory cytokines: A potential therapy for celiac disease. Mucosal Immunol. 2011;4:112–120. doi: 10.1038/mi.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yadav V, Bourdette DN, Bowen JD, et al. Recombinant T-cell receptor ligand (RTL) for treatment of multiple sclerosis: A double-blind, placebo-controlled, phase 1, dose-escalation study. Autoimmune Dis. 2012;2012:954739. doi: 10.1155/2012/954739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells: The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz RE, Reyes M, Koodie L, et al. Multipotent adult progenitor cells from bone marrow differentiate into functional hepatocyte-like cells. J Clin Invest. 2002;109:1291–1302. doi: 10.1172/JCI15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boozer S, Lehman N, Lakshmipathy U, et al. Global characterization and genomic stability of human MultiStem, a multipotent adult progenitor cell. J Stem Cells. 2009;4:17–28. [PubMed] [Google Scholar]
- 31.Bassnett S, Wilmarth PA, David LL. The membrane proteome of the mouse lens fiber cell. Mol Vis. 2009;15:2448–2463. [PMC free article] [PubMed] [Google Scholar]
- 32.Wilmarth PA, Tanner S, Dasari S, et al. Age-related changes in human crystallins determined from comparative analysis of post-translational modifications in young and aged lens: Does deamidation contribute to crystallin insolubility? J Proteome Res. 2006;5:2554–2566. doi: 10.1021/pr050473a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilmarth PA, Riviere MA, David LL. Techniques for accurate protein identification in shotgun proteomic studies of human, mouse, bovine, and chicken lenses. J Ocul Biol Dis Infor. 2009;2:223–234. doi: 10.1007/s12177-009-9042-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Keller A, Nesvizhskii AI, Kolker E, et al. Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 35.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 36.Dennis G, Jr., Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003;4:P3. [PubMed] [Google Scholar]
- 37.Zhan X, Desiderio DM. Signaling pathway networks mined from human pituitary adenoma proteomics data. BMC Med Genomics. 2010;3:13. doi: 10.1186/1755-8794-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Finoulst I, Vink P, Rovers E, et al. Identification of low abundant secreted proteins and peptides from primary culture supernatants of human T-cells. J Proteomics. 2011;75:23–33. doi: 10.1016/j.jprot.2011.03.034. [DOI] [PubMed] [Google Scholar]
- 39.Smith MP, Wood SL, Zougman A, et al. A systematic analysis of the effects of increasing degrees of serum immunodepletion in terms of depth of coverage and other key aspects in top-down and bottom-up proteomic analyses. Proteomics. 2011;11:2222–2235. doi: 10.1002/pmic.201100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Horie H, Kadoya T, Hikawa N, et al. Oxidized galectin-1 stimulates macrophages to promote axonal regeneration in peripheral nerves after axotomy. J Neurosci. 2004;24:1873–1880. doi: 10.1523/JNEUROSCI.4483-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eichelbaum K, Winter M, Berriel Diaz M, et al. Selective enrichment of newly synthesized proteins for quantitative secretome analysis. Nat Biotechnol. 2012;30:984–990. doi: 10.1038/nbt.2356. [DOI] [PubMed] [Google Scholar]
- 42.Wolf D, Wolf AM. Mesenchymal stem cells as cellular immunosuppressants. Lancet. 2008;371:1553–1554. doi: 10.1016/S0140-6736(08)60666-2. [DOI] [PubMed] [Google Scholar]
- 43.Maziarz RT, Devos T, Bachier C, et al. Prophylaxis of acute GVHD using Multistem® stromal cell Therapy: Preliminary results after administration of single or multiple doses in a Phase 1 trial. Biol Blood Marrow Transplant. 2012;18(suppl 1):S264–S265. [Google Scholar]
- 44.Penn MS, Ellis S, Gandhi S, et al. Adventitial delivery of an allogeneic bone marrow-derived adherent stem cell in acute myocardial infarction: Phase I clinical study. Circ Res. 2012;110:304–311. doi: 10.1161/CIRCRESAHA.111.253427. [DOI] [PubMed] [Google Scholar]
- 45.Aranguren XL, Luttun A, Clavel C, et al. In vitro and in vivo arterial differentiation of human multipotent adult progenitor cells. Blood. 2007;109:2634–2642. doi: 10.1182/blood-2006-06-030411. [DOI] [PubMed] [Google Scholar]
- 46.Aranguren XL, Pelacho B, Penuelas I, et al. MAPC transplantation confers a more durable benefit than AC133+ cell transplantation in severe hind limb ischemia. Cell Transplant. 2011;20:259–269. doi: 10.3727/096368910X516592. [DOI] [PubMed] [Google Scholar]
- 47.Lehman N, Cutrone R, Raber A, et al. Development of a surrogate angiogenic potency assay for clinical-grade stem cell production. Cytotherapy. 2012;14:994–1004. doi: 10.3109/14653249.2012.688945. [DOI] [PubMed] [Google Scholar]
- 48.Walker PA, Bedi SS, Shah SK, et al. Intravenous multipotent adult progenitor cell therapy after traumatic brain injury: Modulation of the resident microglia population. J Neuroinflammation. 2012;9:228. doi: 10.1186/1742-2094-9-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hill GR, Ferrara JL. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood. 2000;95:2754–2759. [PubMed] [Google Scholar]
- 50.Eggenhofer E, Benseler V, Kroemer A, et al. Mesenchymal stem cells are short-lived and do not migrate beyond the lungs after intravenous infusion. Front Immunol. 2012;3:297. doi: 10.3389/fimmu.2012.00297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang N, Shao Y, Mei Y, et al. Novel mechanism for mesenchymal stem cells in attenuating peritoneal adhesion: Accumulating in the lung and secreting tumor necrosis factor α-stimulating gene-6. Stem Cell Res Ther. 2012;3:51. doi: 10.1186/scrt142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Roddy GW, Oh JY, Lee RH, et al. Action at a distance: Systemically administered adult stem/progenitor cells (MSCs) reduce inflammatory damage to the cornea without engraftment and primarily by secretion of TNF-alpha stimulated gene/protein 6. Stem Cells. 2011;29:1572–1579. doi: 10.1002/stem.708. [DOI] [PubMed] [Google Scholar]
- 53.Zanotti L, Sarukhan A, Dander E, et al. Encapsulated mesenchymal stem cells for in vivo immunomodulation. Leukemia. 2013;27:500–503. doi: 10.1038/leu.2012.202. [DOI] [PubMed] [Google Scholar]
- 54.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 55.Reinboth B, Thomas J, Hanssen E, et al. Beta ig-h3 interacts directly with biglycan and decorin, promotes collagen VI aggregation, and participates in ternary complexing with these macromolecules. J Biol Chem. 2006;281:7816–7824. doi: 10.1074/jbc.M511316200. [DOI] [PubMed] [Google Scholar]
- 56.Nacu N, Luzina IG, Highsmith K, et al. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J Immunol. 2008;180:5036–5044. doi: 10.4049/jimmunol.180.7.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rios HF, Ma D, Xie Y, et al. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 2008;79:1480–1490. doi: 10.1902/jop.2008.070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sheppard D. Epithelial integrins. Bioessays. 1996;18:655–660. doi: 10.1002/bies.950180809. [DOI] [PubMed] [Google Scholar]
- 59.Woodruff PG, Boushey HA, Dolganov GM, et al. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci USA. 2007;104:15858–15863. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gordon ED, Sidhu SS, Wang ZE, et al. A protective role for periostin and TGF-β in IgE-mediated allergy and airway hyperresponsiveness. Clin Exp Allergy. 2012;42:144–155. doi: 10.1111/j.1365-2222.2011.03840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sidhu SS, Yuan S, Innes AL, et al. Roles of epithelial cell-derived periostin in TGF-beta activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA. 2010;107:14170–14175. doi: 10.1073/pnas.1009426107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bechtel M, Keller MV, Bloch W, et al. Different domains in nidogen-1 and nidogen-2 drive basement membrane formation in skin organotypic cocultures. FASEB J. 2012;26:3637–3648. doi: 10.1096/fj.11-194597. [DOI] [PubMed] [Google Scholar]
- 63.Ho MS, Bose K, Mokkapati S, et al. Nidogens: Extracellular matrix linker molecules. Microsc Res Tech. 2008;71:387–395. doi: 10.1002/jemt.20567. [DOI] [PubMed] [Google Scholar]
- 64.Wu Y, Wu J, Lee DY, et al. Versican protects cells from oxidative stress-induced apoptosis. Matrix Biol. 2005;24:3–13. doi: 10.1016/j.matbio.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Miao L, Wang L. Inflammation amplification by versican: The first mediator. Int J Mol Sci. 2012;13:6873–6882. doi: 10.3390/ijms13066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakada M, Miyamori H, Yamashita J, et al. Testican 2 abrogates inhibition of membrane-type matrix metalloproteinases by other testican family proteins. Cancer Res. 2003;63:3364–3369. [PubMed] [Google Scholar]
- 67.Nakada M, Yamada A, Takino T, et al. Suppression of membrane-type 1 matrix metalloproteinase (MMP)-mediated MMP-2 activation and tumor invasion by testican 3 and its splicing variant gene product, N-Tes. Cancer Res. 2001;61:8896–8902. [PubMed] [Google Scholar]
- 68.Bocock JP, Edgell CJ, Marr HS, et al. Human proteoglycan testican-1 inhibits the lysosomal cysteine protease cathepsin L. Eur J Biochem. 2003;270:4008–4015. doi: 10.1046/j.1432-1033.2003.03789.x. [DOI] [PubMed] [Google Scholar]
- 69.Hata S, Fujishige S, Araki Y, et al. Alcadein cleavages by amyloid beta-precursor protein (APP) alpha- and gamma-secretases generate small peptides, p3-Alcs, indicating Alzheimer disease-related gamma-secretase dysfunction. J Biol Chem. 2009;284:36024–36033. doi: 10.1074/jbc.M109.057497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maruta C, Saito Y, Hata S, et al. Constitutive cleavage of the single-pass transmembrane protein alcadeinα prevents aberrant peripheral retention of Kinesin-1. PLoS One. 2012;7:e43058. doi: 10.1371/journal.pone.0043058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kazanecki CC, Uzwiak DJ, Denhardt DT. Control of osteopontin signaling and function by post-translational phosphorylation and protein folding. J Cell Biochem. 2007;102:912–924. doi: 10.1002/jcb.21558. [DOI] [PubMed] [Google Scholar]
- 72.Uede T, Katagiri Y, Iizuka J, et al. Osteopontin, a coordinator of host defense system: A cytokine or an extracellular adhesive protein? Microbiol Immunol. 1997;41:641–648. doi: 10.1111/j.1348-0421.1997.tb01906.x. [DOI] [PubMed] [Google Scholar]
- 73.Singh M, Ananthula S, Milhorn DM, et al. Osteopontin: A novel inflammatory mediator of cardiovascular disease. Front Biosci. 2007;12:214–221. doi: 10.2741/2059. [DOI] [PubMed] [Google Scholar]
- 74.Wang KX, Denhardt DT. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 75.Scatena M, Liaw L, Giachelli CM. Osteopontin: A multifunctional molecule regulating chronic inflammation and vascular disease. Arterioscler Thromb Vasc Biol. 2007;27:2302–2309. doi: 10.1161/ATVBAHA.107.144824. [DOI] [PubMed] [Google Scholar]
- 76.Prudova A, auf dem Keller U, Butler GS, et al. Multiplex N-terminome analysis of MMP-2 and MMP-9 substrate degradomes by iTRAQ-TAILS quantitative proteomics. Mol Cell Proteomics. 2010;9:894–911. doi: 10.1074/mcp.M000050-MCP201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Winberg JO, Kolset SO, Berg E, et al. Macrophages secrete matrix metalloproteinase 9 covalently linked to the core protein of chondroitin sulphate proteoglycans. J Mol Biol. 2000;304:669–680. doi: 10.1006/jmbi.2000.4235. [DOI] [PubMed] [Google Scholar]
- 78.Winberg JO, Berg E, Kolset SO, et al. Calcium-induced activation and truncation of promatrix metalloproteinase-9 linked to the core protein of chondroitin sulfate proteoglycans. Eur J Biochem. 2003;270:3996–4007. doi: 10.1046/j.1432-1033.2003.03788.x. [DOI] [PubMed] [Google Scholar]
- 79.Kolset SO, Pejler G. Serglycin: A structural and functional chameleon with wide impact on immune cells. J Immunol. 187:4927–4933. doi: 10.4049/jimmunol.1100806. [DOI] [PubMed] [Google Scholar]
- 80.Grossman M, Tworowski D, Dym O, et al. The intrinsic protein flexibility of endogenous protease inhibitor TIMP-1 controls its binding interface and affects its function. Biochemistry. 2010;49:6184–6192. doi: 10.1021/bi902141x. [DOI] [PubMed] [Google Scholar]
- 81.Lee MH, Rapti M, Knauper V, et al. Threonine 98, the pivotal residue of tissue inhibitor of metalloproteinases (TIMP)-1 in metalloproteinase recognition. J Biol Chem. 2004;279:17562–17569. doi: 10.1074/jbc.M312589200. [DOI] [PubMed] [Google Scholar]
- 82.Chirco R, Liu XW, Jung KK, et al. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 83.Itoh Y, Ito A, Iwata K, et al. Plasma membrane-bound tissue inhibitor of metalloproteinases (TIMP)-2 specifically inhibits matrix metalloproteinase 2 (gelatinase A) activated on the cell surface. J Biol Chem. 1998;273:24360–24367. doi: 10.1074/jbc.273.38.24360. [DOI] [PubMed] [Google Scholar]
- 84.Cuzner ML, Gveric D, Strand C, et al. The expression of tissue-type plasminogen activator, matrix metalloproteases and endogenous inhibitors in the central nervous system in multiple sclerosis: Comparison of stages in lesion evolution. J Neuropathol Exp Neurol. 1996;55:1194–1204. doi: 10.1097/00005072-199612000-00002. [DOI] [PubMed] [Google Scholar]
- 85.Degryse B, Neels JG, Czekay RP, et al. The low density lipoprotein receptor-related protein is a motogenic receptor for plasminogen activator inhibitor-1. J Biol Chem. 2004;279:22595–22604. doi: 10.1074/jbc.M313004200. [DOI] [PubMed] [Google Scholar]
- 86.Bristow CL, Babayeva MA, LaBrunda M, et al. α1Proteinase inhibitor regulates CD4+ lymphocyte levels and is rate limiting in HIV-1 disease. PLoS One. 2012;7:e31383. doi: 10.1371/journal.pone.0031383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Mourik JA, Lawrence DA, Loskutoff DJ. Purification of an inhibitor of plasminogen activator (antiactivator) synthesized by endothelial cells. J Biol Chem. 1984;259:14914–14921. [PubMed] [Google Scholar]
- 88.Lambers JW, Cammenga M, Konig BW, et al. Activation of human endothelial cell-type plasminogen activator inhibitor (PAI-1) by negatively charged phospholipids. J Biol Chem. 1987;262:17492–17496. [PubMed] [Google Scholar]
- 89.Vadon-Le Goff S, Kronenberg D, Bourhis JM, et al. Procollagen C-proteinase enhancer stimulates procollagen processing by binding to the C-propeptide region only. J Biol Chem. 2011;286:38932–38938. doi: 10.1074/jbc.M111.274944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hopkins DR, Keles S, Greenspan DS. The bone morphogenetic protein 1/Tolloid-like metalloproteinases. Matrix Biol. 2007;26:508–523. doi: 10.1016/j.matbio.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Waldburger JM, Masternak K, Muhlethaler-Mottet A, et al. Lessons from the bare lymphocyte syndrome: Molecular mechanisms regulating MHC class II expression. Immunol Rev. 2000;178:148–165. doi: 10.1034/j.1600-065x.2000.17813.x. [DOI] [PubMed] [Google Scholar]
- 92.Castonguay R, Werner ED, Matthews RG, et al. Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J Biol Chem. 2011;286:30034–30046. doi: 10.1074/jbc.M111.260133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rivera LB, Brekken RA. SPARC promotes pericyte recruitment via inhibition of endoglin-dependent TGF-β1 activity. J Cell Biol. 2011;193:1305–1319. doi: 10.1083/jcb.201011143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Patterson J, Hubbell JA. SPARC-derived protease substrates to enhance the plasmin sensitivity of molecularly engineered PEG hydrogels. Biomaterials. 2011;32:1301–1310. doi: 10.1016/j.biomaterials.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 95.McCurdy SM, Dai Q, Zhang J, et al. SPARC mediates early extracellular matrix remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol. 2011;301:H497–H505. doi: 10.1152/ajpheart.01070.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Girotti MR, Fernandez M, Lopez JA, et al. SPARC promotes cathepsin B-mediated melanoma invasiveness through a collagen I/α2β1 integrin axis. J Invest Dermatol. 2011;131:2438–2447. doi: 10.1038/jid.2011.239. [DOI] [PubMed] [Google Scholar]
- 97.Murakami K, Tanaka M, Usui T, et al. Follistatin-related protein/follistatin-like 1 evokes an innate immune response via CD14 and toll-like receptor 4. FEBS Lett. 2012;586:319–324. doi: 10.1016/j.febslet.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 98.Hiraiwa N, Yabuta T, Yoritomi K, et al. Transactivation of the fucosyltransferase VII gene by human T-cell leukemia virus type 1 Tax through a variant cAMP-responsive element. Blood. 2003;101:3615–3621. doi: 10.1182/blood-2002-07-2301. [DOI] [PubMed] [Google Scholar]
- 99.Malapeira J, Esselens C, Bech-Serra JJ, et al. ADAM17 (TACE) regulates TGFbeta signaling through the cleavage of vasorin. Oncogene. 2011;30:1912–1922. doi: 10.1038/onc.2010.565. [DOI] [PubMed] [Google Scholar]
- 100.Caccia D, Zanetti Domingues L, Micciche F, et al. Secretome compartment is a valuable source of biomarkers for cancer-relevant pathways. J Proteome Res. 2011;10:4196–4207. doi: 10.1021/pr200344n. [DOI] [PubMed] [Google Scholar]
- 101.Sun T, Adra S, Smallwood R, et al. Exploring hypotheses of the actions of TGF-beta1 in epidermal wound healing using a 3D computational multiscale model of the human epidermis. PLoS One. 2009;4:e8515. doi: 10.1371/journal.pone.0008515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Grootaert C, Van de Wiele T, Verstraete W, et al. Angiopoietin-like protein 4: Health effects, modulating agents and structure-function relationships. Expert Rev Proteomics. 2012;9:181–199. doi: 10.1586/epr.12.12. [DOI] [PubMed] [Google Scholar]
- 103.Sioud M, Mobergslien A, Boudabous A, et al. Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins. Int J Oncol. 2011;38:385–390. doi: 10.3892/ijo.2010.869. [DOI] [PubMed] [Google Scholar]
- 104.Wang J, Lu ZH, Gabius HJ, et al. Cross-linking of GM1 ganglioside by galectin-1 mediates regulatory T cell activity involving TRPC5 channel activation: Possible role in suppressing experimental autoimmune encephalomyelitis. J Immunol. 2009;182:4036–4045. doi: 10.4049/jimmunol.0802981. [DOI] [PubMed] [Google Scholar]
- 105.Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8:825–834. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 106.Patnaik SK, Potvin B, Carlsson S, et al. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology. 2006;16:305–317. doi: 10.1093/glycob/cwj063. [DOI] [PubMed] [Google Scholar]
- 107.Babic AM, Chen CC, Lau LF. Fisp12/mouse connective tissue growth factor mediates endothelial cell adhesion and migration through integrin αvβ3, promotes endothelial cell survival, and induces angiogenesis in vivo. Mol Cell Biol. 1999;19:2958–2966. doi: 10.1128/mcb.19.4.2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hashimoto G, Inoki I, Fujii Y, et al. Matrix metalloproteinases cleave connective tissue growth factor and reactivate angiogenic activity of vascular endothelial growth factor 165. J Biol Chem. 2002;277:36288–36295. doi: 10.1074/jbc.M201674200. [DOI] [PubMed] [Google Scholar]
- 109.Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945–963. doi: 10.1038/nrd3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gui Y, Murphy LJ. Interaction of insulin-like growth factor binding protein-3 with latent transforming growth factor-beta binding protein-1. Mol Cell Biochem. 2003;250:189–195. doi: 10.1023/a:1024990409102. [DOI] [PubMed] [Google Scholar]
- 111.Hong L, Sun H, Lv X, et al. Expression of periostin in the serum of NSCLC and its function on proliferation and migration of human lung adenocarcinoma cell line (A549) in vitro. Mol Biol Rep. 2010;37:2285–2293. doi: 10.1007/s11033-009-9721-1. [DOI] [PubMed] [Google Scholar]
- 112.Mor-Vaknin N, Punturieri A, Sitwala K, et al. Vimentin is secreted by activated macrophages. Nat Cell Biol. 2003;5:59–63. doi: 10.1038/ncb898. [DOI] [PubMed] [Google Scholar]
- 113.Kwak HI, Kang H, Dave JM, et al. Calpain-mediated vimentin cleavage occurs upstream of MT1-MMP membrane translocation to facilitate endothelial sprout initiation. Angiogenesis. 2012;15:287–303. doi: 10.1007/s10456-012-9262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Komura K, Ise H, Akaike T. Dynamic behaviors of vimentin induced by interaction with GlcNAc molecules. Glycobiology. 2012;22:1741–1759. doi: 10.1093/glycob/cws118. [DOI] [PubMed] [Google Scholar]
- 115.Ise H, Kobayashi S, Goto M, et al. Vimentin and desmin possess GlcNAc-binding lectin-like properties on cell surfaces. Glycobiology. 2010;20:843–864. doi: 10.1093/glycob/cwq039. [DOI] [PubMed] [Google Scholar]
- 116.Czopik AK, Bynoe MS, Palm N, et al. Semaphorin 7A is a negative regulator of T cell responses. Immunity. 2006;24:591–600. doi: 10.1016/j.immuni.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 117.Crosnier C, Stamataki D, Lewis J. Organizing cell renewal in the intestine: Stem cells, signals and combinatorial control. Nat Rev Genet. 2006;7:349–359. doi: 10.1038/nrg1840. [DOI] [PubMed] [Google Scholar]
- 118.van Kilsdonk JW, van Kempen LC, van Muijen GN, et al. Soluble adhesion molecules in human cancers: Sources and fates. Eur J Cell Biol. 89:415–427. doi: 10.1016/j.ejcb.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 119.Lunter PC, van Kilsdonk JW, van Beek H, et al. Activated leukocyte cell adhesion molecule (ALCAM/CD166/MEMD), a novel actor in invasive growth, controls matrix metalloproteinase activity. Cancer Res. 2005;65:8801–8808. doi: 10.1158/0008-5472.CAN-05-0378. [DOI] [PubMed] [Google Scholar]
- 120.Manfredi AA, Rovere-Querini P, Bottazzi B, et al. Pentraxins, humoral innate immunity and tissue injury. Curr Opin Immunol. 2008;20:538–544. doi: 10.1016/j.coi.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 121.Li X, Ni R, Chen J, et al. The presence of IGHG1 in human pancreatic carcinomas is associated with immune evasion mechanisms. Pancreas. 2011;40:753–761. doi: 10.1097/MPA.0b013e318213d51b. [DOI] [PubMed] [Google Scholar]
- 122.McCormick MM, Rahimi F, Bobryshev YV, et al. S100A8 and S100A9 in human arterial wall. Implications for atherogenesis. J Biol Chem. 2005;280:41521–41529. doi: 10.1074/jbc.M509442200. [DOI] [PubMed] [Google Scholar]
- 123.Smith SM, Thomas CE, Birk DE. Pericellular proteins of the developing mouse tendon: A proteomic analysis. Connect Tissue Res. 2012;53:2–13. doi: 10.3109/03008207.2011.602766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Adlerova L, Bartoskova A, Faldyna M. Lactoferrin: A review. Veterinarni Medicina. 2008;53:457–468. [Google Scholar]
- 125.Wang HW, Babic AM, Mitchell HA, et al. Elevated soluble ICAM-1 levels induce immune deficiency and increase adiposity in mice. FASEB J. 2005;19:1018–1020. doi: 10.1096/fj.04-3094fje. [DOI] [PubMed] [Google Scholar]
- 126.Weber C, Kraemer S, Drechsler M, et al. Structural determinants of MIF functions in CXCR2-mediated inflammatory and atherogenic leukocyte recruitment. Proc Natl Acad Sci USA. 2008;105:16278–16283. doi: 10.1073/pnas.0804017105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kohler A, De Filippo K, Hasenberg M, et al. G-CSF-mediated thrombopoietin release triggers neutrophil motility and mobilization from bone marrow via induction of Cxcr2 ligands. Blood. 2011;117:4349–4357. doi: 10.1182/blood-2010-09-308387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hyun YM, Sumagin R, Sarangi PP, et al. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J Exp Med. 2012;209:1349–1362. doi: 10.1084/jem.20111426. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.