This study developed a limbal epithelium-like structure that could be maintained for at least 5 months in vitro.
Keywords: Adult stem cells, Cell culture, Colony formation, Differentiation, Experimental models, Long-term repopulation, Stem cell culture
Abstract
Corneal epithelial stem cells are located in the limbus, the junction between the cornea and the conjunctiva. A limbal epithelium model in vitro would be useful for the study of epithelial stem cells, as well as improving the quality of cultivated epithelial sheets for the treatment of limbal stem cell deficiency. In this study, we succeeded in constructing a limbal epithelium-like structure that could be maintained for at least 5 months in vitro. We modified conventional medium by replacing epidermal growth factor with keratinocyte growth factor (KGF) and adding Y-27632, a rho kinase inhibitor. Using this medium, epithelial cells freshly isolated from human limbus were cocultured with human mesenchymal stem cell-derived feeder cells. Cells formed a stratified layer without air exposure, and both basal and suprabasal layers maintained their unique morphologies for up to 5 months. Basal layers expressed the progenitor marker p63 uniformly and K15 heterogeneously. Expressions of PAX6, K3, and K12 indicated that cell sheets underwent normal differentiation in the corneal epithelium lineage. Although medium was changed daily after day 7, cell debris was observed every day, suggesting that cell sheets underwent turnover. Furthermore, secondary colonies were observed from cells dissociated from 1-month and 3-month cultured sheets. In conclusion, human limbal epithelial cell sheet cultures with KGF and Y-27632 maintained stratification, high expression of both stem/progenitor markers and differentiation markers, and colony-forming cells long-term. This protocol may be useful as an in vitro limbal epithelial model for basic studies.
Introduction
Stem cells of corneal epithelium are located in the limbus, the junction between the cornea and the conjunctiva [1]. The basal layer of limbal epithelium express the stem/progenitor markers cytokeratin 15 (K15) [2], p63 [3], and ABCG2 [4, 5], whereas the suprabasal layer of the limbal epithelium and the whole layer of the corneal epithelium express the cornea-specific differentiation markers K3 and K12 [6, 7]. The limbus contains melanocytes and melanin, whereas the cornea does not, suggesting that the limbus is protected from ultraviolet light [8]. Stem cells are thought to have high proliferation potential (self-renewal ability), proven by the higher colony forming efficiency (CFE) and higher replicative ability of limbal epithelial cells compared with corneal epithelial cells [9, 10]. Damage to the limbus causes limbal stem cell deficiency (LSCD), which leads to chronic corneal epithelial loss and conjunctival invasion, indicating that the limbus is a critical region to maintain corneal epithelium [11].
Since it is impossible to study human epithelial stem cells in vivo, an in vitro model would be useful for the study of these cells. Engineering the limbal epithelial phenotype in vitro would also be useful as a source of stem cells for the treatment of LSCD patients [12–23] and to reduce the need of experimental animal models for ophthalmic drug tests [24]. Differentiation of epithelial cells is mediated by the Rho/ROCK pathway and inhibited by the chemical inhibitor of ROCK, Y-27632 [25]. This may be the reason why Y-27632 increases the CFE of human keratinocytes [25, 26]. Y-27632 also increases the CFE of other cell types, such as human embryonic stem cells (ESCs) [27], primate corneal endothelial cells [28], and rabbit limbal epithelial cells [29]. In human ESCs, Y-27632 inhibits apoptosis caused by the loss of cadherin-dependent cell-cell contact [27, 30, 31]. In rabbit limbal epithelial cells, Y-27632 promotes the rapid adherence of inoculated cells to the culture plate and scavenges accumulated reactive oxygen species (ROS) [29]. In addition to the improvement of CFE, long-term administration of ROCK inhibitor greatly increases the proliferation capacity of human keratinocytes without impairment of the differentiation capacity [32]. These results suggest that ROCK inhibitor may be effective for expanding limbal epithelial stem cells in vitro.
Limbal basal cells express FGFRIIIb, the receptor for fibroblast growth factor 7/keratinocyte growth factor (KGF) [33, 34]. KGF stimulates the proliferation of epithelial cells [35–37]. Although epithelial cells themselves do not produce KGF, they secrete interleukin-1, which stimulates the production of KGF in mesenchymal cells [38]. Limbal fibroblasts secrete higher levels of KGF and less hepatocyte growth factor (HGF) than central corneal fibroblasts [39], and KGF stimulates the expansion of explant cultured rabbit limbal epithelium and increases the expression of p63 compared with HGF [40]. In contrast to epidermal growth factor (EGF), KGF does not inhibit the induction of differentiation markers K1 in epidermal keratinocytes [36] and K3 in limbal epithelial cells [37] in serum-free, feeder-free cultures. These facts suggest that KGF stimulates the proliferation of limbal epithelial cells without impairing differentiation, which may be beneficial to culture limbal epithelial cell sheets that include both undifferentiated and differentiated cell layers as observed in vivo.
In this study, we attempted to use primary cultured human limbal epithelial cell sheets as an in vitro limbal epithelium model. Several methods of culturing epithelial cell sheets are known [9, 12, 13, 15, 16, 22, 23, 41–43]. In clinical studies at our institution, epithelial cells freshly isolated from human limbus are cultured in cell culture inserts, with human feeder layer cells separated in the bottom of a paired well [43]. To improve the quality of our sheets, we supplemented the medium with KGF and Y-27632 instead of EGF, which resulted in higher expression of both stem/progenitor markers and differentiation markers, as well as a high content of colony-forming cells in the epithelial cell sheets. These sheets maintained the morphology, stratification, marker expressions, and CFE for up to 5 months in vitro, suggesting the maintenance of stem/progenitor cells by this protocol.
Materials and Methods
Preparation of Feeder Layer Cells
Human mesenchymal stem cells (HMSCs) (SanBio Inc., Mountain View, CA, http://www.san-bio.com) and NIH/3T3 cells were used as feeder cells. HMSCs were used as feeders for primary cultured epithelial cell sheets because of local regulations requiring minimal use of xenogenic factors for clinical use [43]. NIH/3T3 cells were used as a feeder layer for the colony formation assay, immunostaining of colonies, subculture of epithelial cells, and culture of experimental epithelial cell sheets. HMSCs were fed with α-minimal essential medium (Life Technologies, Carlsbad, CA, http://www.invitrogen.com) supplemented with fetal bovine serum (FBS; 10%) and the antibiotics streptomycin (100 μg/ml; Meiji Seika Pharma, Tokyo, Japan, http://www.meiji-seika-pharma.co.jp/english) and penicillin G (100 U/ml; Meiji), twice a week. Semiconfluent cells were dissociated by enzyme treatment (37°C for 10 minutes; TrypLE Express; Life Technologies) and subcultured at a density of 3 × 105 cells per 75-cm2 flask. For use as feeder cells, HMSCs were seeded in six-well plates (1 × 105 cells per well) and cultured until cells reached confluence. Confluent cells were treated with mitomycin C (MMC; final concentration, 4 μg/ml; Kyowa Hakko Kirin, Tokyo, Japan, http://www.kyowa-kirin.co.jp/english) at 37°C for 2 hours. NIH/3T3 cells (3 × 105 cells per 75-cm2 flask) were subcultured every 3 days by enzyme dissociation (TrypLE Express). NIH/3T3 cells were fed with Dulbecco's modified Eagle's medium (DMEM; Life Technologies) including FBS (10%) and antibiotics. Confluent NIH/3T3 cells were treated with MMC (4 μg/ml, 37°C, 2 hours), and dissociated cells were cryopreserved until use. One day before use, NIH/3T3 feeder cells were seeded to the culture dishes at a density of 2.5 × 104 cells per cm2.
Primary Culture of Human Limbal Epithelial Cell Sheets
Human limbal epithelial cells were obtained from U.S. eye bank eyes after the central cornea was used for transplantation. Iris, ciliary body, Descemet's membrane with corneal endothelium, conjunctiva, and excess sclera were surgically removed from the corneosclera. Limbal epithelium was isolated by the treatment with Dispase II (Roche, Basel, Switzerland, http://www.roche.com) in a low-cell-binding centrifuge tube (STEMFUL; Sumitomo Bakelite Co., Ltd., Tokyo, Japan, http://www.sumibe.co.jp/english/index.html) at a concentration of 4.0 U/ml in DMEM/F12, followed by the dissociation by pipetting. Epithelial cells (0.5–2 × 105 cells per insert) were inoculated on cell culture inserts (3450; Corning Enterprises, Corning, NY, http://www.corning.com) and cocultured with HMSC feeder in the bottom of six-well plates, as described previously [43]. When indicated, epithelial cells were directly seeded on NIH/3T3 feeder layers in the cell culture insert and cocultured with another NIH/3T3 feeder layer in the bottom of a paired well as described previously [44]. Supplemental hormonal epithelial medium (SHEM) containing human recombinant EGF (10 ng/ml; PeproTech, Rocky Hill, NJ, http://www.peprotech.com) or KGF (10 ng/ml; 100-19; PeproTech), as well as DMEM/F12 (96%), FBS (4%), insulin (10 μg/ml; Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com), tri-iodo-thyronine (2 nM; Sigma-Aldrich), hydrocortisone (500 ng/ml; Nichi-iko Pharmaceutical, Toyama, Japan, http://www.nichiiko.co.jp/english/index.html), isoproterenol hydrochloride (250 ng/ml; Kowa Company, Aichi, Japan, http://www.kowa.co.jp/eng), and antibiotics, was used as culture medium. ROCK inhibitor Y-27632 (final concentration, 10 μM; Nakalai Tesque, Kyoto, Japan, http://www.nacalai.co.jp/global) was supplemented when indicated. In some cases, cell culture inserts were coated with fibrin (Kaketsuken, Kumamoto, Japan, http://www.kaketsuken.or.jp/en/) as described previously [45], and aprotinin (final concentration, 150 KIU/ml; Wako Pure Chemical Industries, Osaka, Japan, http://www.wako-chem.co.jp/english/) was added to the medium to prevent the degradation of fibrin gels. Cells were fed at day 3, day 5, and every day after day 7 for up to 5 months. Feeder cells were not changed throughout the culture, and epithelial cells were not air lifted.
Colony Forming Assay of Primary Human Limbal Epithelial Cells
Freshly isolated epithelial cells were passed through a 40-μm mesh (Cell Strainer; BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) to remove cell aggregations, then seeded in an NIH/3T3 feeder-prepared 100-mm dish at a density of 1 × 103 cells per dish. Cells were fed with SHEM supplemented with EGF, EGF and Y-27632 (E+Y), KGF, or KGF and Y-27632 (K+Y). After 10–14 days of culture, cells were fixed with 10% formalin and stained with rhodamine B. Since the CFEs of primary cells varied greatly between experiments, we normalized CFE data by setting the CFE of EGF culture as 1.
Colony Forming Assay of Cultivated Human Limbal Epithelial Cell Sheets
Epithelial cell sheets cultured for 1 month and 3 months were dissociated by enzyme treatment (TrypLE Express with Y-27632) at 37°C for 30 minutes. Y-27632 (10 μM) was added to prevent any effects due to cell dissociation. Cells were seeded on NIH/3T3 feeder-prepared 100-mm dishes at a density of 1 × 103 cells per dish. To compare the CFEs of sheets cultured using different media, a colony formation assay was performed using the same medium (SHEM E+Y) in all groups, since EGF stimulated more growth than KGF (supplemental online Fig. 1B, 1C), resulting in larger colonies, which are easy to visualize compared with smaller colonies.
Serial Cultivation of Human Limbal Epithelial Cells
Epithelial cells freshly isolated from limbus were seeded on NIH/3T3 feeder layers in 75-cm2 flasks. Cells were fed every 3–4 days with SHEM supplemented with EGF, E+Y, KGF, or K+Y. When cells reached semiconfluence, cells were dissociated by enzyme (TrypLE Express) and subcultured to new feeder-prepared flasks at a density of 1 × 105 cells per 75-cm2 flask. The number of cell population doublings (PDs) in each passage was calculated as PDs = log2 (Number of cells collected/Number of cells seeded).
Reconstruction of Epithelial Cell Sheets From Subcultured Epithelial Cells
An epithelial cell sheet cultured with SHEM K+Y for 5 months was dissociated by enzyme (TrypLE Express), and 1 × 105 cells were subcultured on NIH/3T3 feeders in 75-cm2 flasks using SHEM K+Y. After four passages, cells (1 × 105 cells per insert) were seeded on the NIH/3T3 duplex feeders as described in the primary culture methods. Cells were fed every day for 2 weeks to allow stratification.
Immunostaining
For whole mount staining of sheets, cell sheets were cut using a sharp blade (biopsy punch; Kai Medical Inc., Seki, Japan, http://www.kaimedical.com/en2). For immunohistochemistry, 10-μm-thick frozen sections were prepared. For immunostaining of colonies, freshly isolated cells (5 × 102 cells per well) were cultured on NIH/3T3 feeder-prepared six-well plates for 10 days. All samples were fixed with ice-cold 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 minutes. After treatment with blocking solution consisting of normal donkey serum (10%) and Triton X (0.1%) in PBS for 1 hour at room temperature (RT), antibodies specific for cytokeratin 15 (K15, mouse IgG2a, clone LHK15, MS-1068; Thermo Fisher Scientific, Waltham, MA, https://www.thermoscientific.com), K12 (rabbit IgG, sc-25722; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), K3 (mouse IgG1, clone AE5, sc-80000; Santa Cruz Biotechnology), PAX6 (rabbit IgG, PRB-278P; Covance, Princeton, NJ, http://www.covance.com), p63 (mouse IgG2a, clone 4A4, sc-8431; Santa Cruz Biotechnology), and CDH1 (rabbit IgG, sc-7870; Santa Cruz Biotechnology) were diluted in blocking buffer at 1:100 and applied to samples for 1–2 hours at RT. After washing three times with PBS, anti-mouse IgG antibody (Alexa Fluor 488-conjugated, 1:100; Life Technologies), anti-rabbit IgG antibody (Alexa Fluor 555-conjugated, 1:100), and 4′,6-diamidino-2-phenylindole (1 μg/ml; Dojindo Laboratories, Kumamoto, Japan, http://www.dojindo.com) were applied for 1 hour at RT. Samples were mounted with coverslips using mounting medium (PermaFluor; Thermo Fisher Scientific) and observed using a fluorescence microscope.
Statistical Analysis
One-way analysis of variance followed by Scheffe's F test was used to compare four groups, and Student's t test was used to compare two groups, at a significance level of p < .05.
Results
The Effects of KGF and the ROCK Inhibitor Y-27632 on Cultured Human Limbal Epithelial Cells
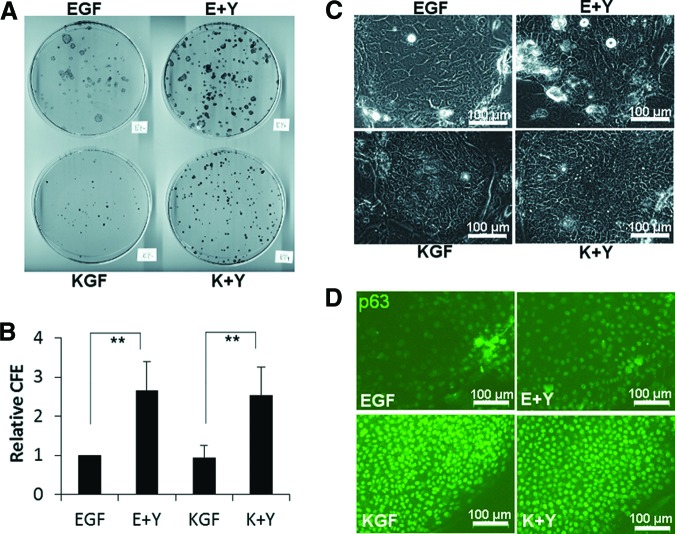
Colony formation assays were performed to examine the effects of Y-27632, KGF, and their combination on primary human limbal epithelial cells in the presence of 3T3 feeder cells (Fig. 1A, 1B). Since CFE varied among donor cell source (supplemental online Fig. 1), CFE was normalized as CFE of EGF = 1 (relative CFE; Fig. 1B). Y-27632 significantly increased the relative CFE in both EGF groups (EGF culture and E+Y culture) and KGF groups (KGF culture and K+Y culture). The relative CFE of E+Y culture was 2.7 ± 0.7-fold (mean ± SD; n = 7) as large as that of EGF culture, as recently reported [29]. Similarly, the relative CFE in K+Y culture was 2.8 ± 1.0-fold as large as that in KGF culture. Although relative CFE did not differ between EGF and KGF, the morphology of colonies was different between these groups. Colonies in KGF consisted of densely packed small cells compared with EGF (Fig. 1C). Colony size was smaller in KGF (Fig. 1A), reflecting the slow cell growth compared with EGF (supplemental online Fig. 1B, 1C). Immunostaining showed that expression of the epithelial stem/progenitor marker p63 was higher in KGF than EGF (Fig. 1D). Both EGF culture and KGF culture without Y-27632 ceased growth at passage 4 in the serial cultivation assay (supplemental online Fig. 1D; 19.6 ± 1.04 PDs in EGF and 16.0 ± 1.6 PDs in KGF; n = 3), whereas E+Y culture and K+Y culture continued to grow over passage 5 (32.0 ± 1.2 PDs and 29.5 ± 1.4 PDs, respectively).
Figure 1.
The effects of EGF, KGF, and Y-27632 on the colony formation of human limbal epithelial cells. (A): Rhodamine B-stained 100-mm dish. (B): Relative CFE; n = 7. **, p < .01. CFE was normalized as CFE of EGF = 1. (C): Phase contrast micrograph of colonies at day 7. (D): Immunostaining of colonies at day 10 using anti-p63 antibody (green). Scale bars = 100 μm (C, D). Abbreviations: CFE, colony forming efficiency; E+Y, epidermal growth factor and Y-27632; EGF, epidermal growth factor; K+Y, keratinocyte growth factor and Y-27632; KGF, keratinocyte growth factor.
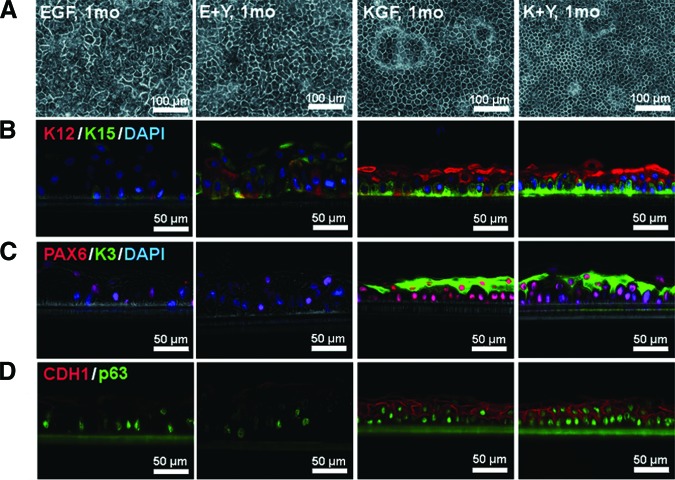
The Effects of KGF and ROCK Inhibitor Y-27632 on the Morphology of Cultivated Epithelial Cell Sheets
Next we confirmed the effects of combining KGF and Y-27632 on the culture of epithelial cell sheets. Limbal epithelial cells were primary cultured with human feeder cells that were separated from epithelial cells by cell culture inserts [43], as was required for clinical application. As observed in colonies on 3T3 feeders, the morphology of basal cells was different between EGF (EGF sheets and E+Y sheets) and KGF groups (KGF sheets and K+Y sheets). Cell sheets in KGF were dense, and the border between cells was easy to observe using a phase contrast microscope (Fig. 2A). Immunohistochemistry showed higher expressions of epithelial stem/progenitor markers (K15, p63), differentiation-related markers (K3, K12), transcriptional factor PAX6, and epithelial cadherin (CDH1) in KGF compared with EGF (Fig. 2B–2D). K15 was heterogeneously expressed in the basal layers of KGF groups, whereas it was random in E+Y sheets and rare in EGF sheets (Fig. 2B, green). K12 was expressed in suprabasal cells and some basal cells in KGF sheets (Fig. 2B, red), whereas K3 was observed only in suprabasal cells (Fig. 2C, green). CDH1 was prominent in the cell borders of KGF sheets (Fig. 2D; supplemental online Fig. 2). The effect of KGF was also confirmed in traditional epithelial sheets cultured in contact with 3T3 feeder cells, which also showed enhanced expression of p63, K3, and K12 (supplemental online Fig. 3).
Figure 2.
The effects of EGF, KGF, and Y-27632 on the morphology of human limbal epithelial cell sheets. (A): Phase contrast micrograph of cells cultured for 1 month in the indicated medium. (B–D): Cryosections stained with antibodies specific for K15 (green, [B]), K12 (red, [B]), K3 (green, [C]), PAX6 (red, [C]), p63 (green, [D]), and CDH1 (red, [D]). Phase contrast images were merged with immunofluorescence images. Where indicated, cell nuclei were stained with DAPI. Culture conditions were the same as for the top panel. Scale bars = 100 μm (A) and 50 μm (B–D). Abbreviations: DAPI, 4′,6-diamidino-2-phenylindole; E+Y, epidermal growth factor and Y-27632; EGF, epidermal growth factor; K+Y, keratinocyte growth factor and Y-27632; KGF, keratinocyte growth factor; mo, month.
The Effects of KGF and the ROCK Inhibitor Y-27632 on the Colony Forming Ability of Cultivated Epithelial Cell Sheets
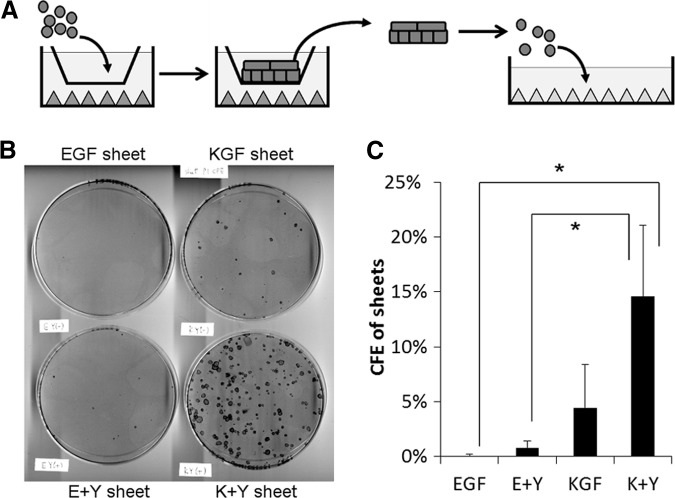
To confirm the proliferation potential of cell sheets, cells dissociated from cell sheets were seeded on feeder-prepared dishes at clonal density (Fig. 3). Cells prepared from K+Y sheets showed the highest CFE (14.5 ± 6.5%; n = 3). The CFE of KGF sheets was 4.5 ± 3.9%. E+Y sheets showed significantly lower CFE (0.8 ± 0.6%) compared with K+Y sheets. EGF sheets showed lowest CFE (0.0 ± 0.1%).
Figure 3.
The effects of EGF, KGF, and Y-27632 on the colony forming ability of human limbal epithelial cell sheets. (A): Scheme of colony formation assay. Cell sheets cultured with EGF, E+Y, KGF, or K+Y medium were dissociated by enzyme treatment, followed by subculture on the indicator dishes at clonal density. All dishes were fed with same medium. (B): Rhodamine B-stained indicator dishes. (C): CFE of sheets; n = 3. *, p < .05. Abbreviations: CFE, colony forming efficiency; E+Y, epidermal growth factor and Y-27632; EGF, epidermal growth factor; K+Y, keratinocyte growth factor and Y-27632; KGF, keratinocyte growth factor.
Long-Term Maintenance of Cultivated Epithelial Cell Sheets by Using KGF and ROCK Inhibitor Y-27632
We cultured cell sheets in EGF medium or K+Y medium for up to 3 months in eight cases (Fig. 4) and for 5 months in one case (supplemental online Fig. 4). Cells in EGF sheets showed large, elongated shapes at 3 months and were lost by 5 months, whereas all K+Y sheets maintained cell morphology up to 5 months. Although medium was changed daily after day 7, desquamation was observed every day, suggesting the turnover of superficial cells in these cell sheets. Expressions of stem/progenitor markers (K15, p63), differentiation markers (K12 and K3), PAX6, and CDH1 were maintained in long-term in K+Y sheets (Fig. 4B; supplemental online Fig. 4B) as shown in a 1-month-cultured sheet (Fig. 2). The CFE of 3-month-cultured K+Y sheets was 23.3 ± 7.3% (n = 5), similar to that of 1-month-cultured K+Y sheets (19.0 ± 12.6%; n = 7), which slightly decreased at 5 months (8.6%). However, cells dissociated from the 5-month-cultured K+Y sheet were able to be subcultured for at least seven passages (28.0 PDs; supplemental online Fig. 4C) and were able to reconstruct stratified epithelial cell sheets after passage 4 (25.4 PDs at passage 4; supplemental online Fig. 4D).
Figure 4.
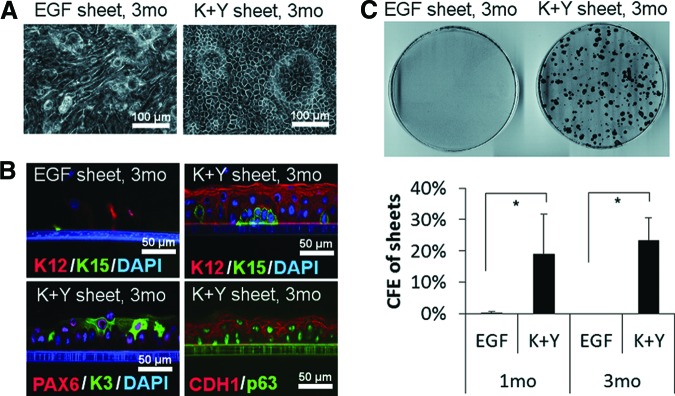
Morphology and colony forming ability of human limbal epithelial cell sheets cultured for 3 months. (A): Phase contrast micrograph of cell sheets cultured with EGF medium or K+Y medium for 3 months. (B): Immunohistochemistry of 3-month cultured EGF sheets and K+Y sheets using the specific antibodies indicated. Phase contrast images were merged with immunofluorescence images. (C): Colony formation assays of cells dissociated from 3-month cultured EGF sheets and K+Y sheets. Top: Rhodamine B-stained indicator dishes. Bottom: CFE of 1-month cultured sheets and 3-month cultured sheets. Scale bars = 100 μm (A) and 50 μm (B). Abbreviations: CFE, colony forming efficiency; EGF, epidermal growth factor; K+Y, keratinocyte growth factor and Y-27632; mo, months.
Discussion
In order for limbal epithelial cell sheets in vitro to be equivalent to limbal epithelium in vivo, the cell sheets should contain stem/progenitor cells to maintain differentiated layers. In our culture system, cell morphology was maintained for the long term; 3 months in eight cases (Fig. 4) and 5 months in one case (supplemental online Fig. 4). Cell debris was observed throughout the culture, although the culture medium was changed daily after day 7, suggesting that the cell sheets underwent turnover. Histology and immunohistochemistry showed that tissue organization of cell sheets was similar to that of limbal epithelium in vivo, consisting of small basal cells and large differentiated suprabasal cells (Figs. 2, 4). Tissue histology did not change from 1 month to 5 months, and cell polarity and morphology were stable. The existence of K15-high cell clusters (Fig. 2) and colony-forming cells (Fig. 3) in cell sheets suggests the presence of stem/progenitor cells. This idea is supported by the serial cultivation assay and sheet reconstruction assay of 5-month-cultured K+Y sheets (supplemental online Fig. 4). Expressions of PAX6 and corneal epithelium-specific differentiation markers K3 and K12 indicate that cell sheets maintained their linage as corneal epithelium and did not transform to conjunctival epithelium or epidermis. From these results, we conclude that our limbal epithelial cell sheets had stem/progenitor cells generating differentiated cells in corneal epithelium linage, showing the similarity with limbal epithelium in vivo.
We primary cultured human limbal epithelial cell sheets with KGF- and Y-27632-supplemented medium for up to 5 months. Although long-term supplementation of Y-27632 has shown a deleterious effect on epidermal keratinocytes [26], another group reported a beneficial effect [32]. In the former report, human keratinocytes treated with Y-27632 from day 0 to day 14 showed an increase in CFE in primary culture but a decrease in CFE after passage [26]. In the latter report, continuous administration of Y-27632 throughout culture enabled human keratinocytes to be subcultured for up to 150 passages without impairing the differentiation capacity [32]. In this study, we found that cell sheets cultured with Y-27632 tended to increase CFE (Fig. 3C) and serial cultivation with Y-27632 increased cell proliferation (supplemental online Fig. 1D). The latter report and our results support the fact that long-term administration of Y-27632 is beneficial to maintain stem cells.
How does Y-27632 work in the maintenance of stem cells in culture? Y-27632 increased CFE up to threefold in both EGF and KGF medium (Fig. 1), indicating that the effect of Y-27632 is independent of KGF. Y-27632 is known to protect dissociated human ESCs from apoptosis [27], which is induced by the loss of cadherin-dependent cell-cell contact [30, 31]. Y-27632 also inhibits the differentiation of dissociated keratinocytes suspended as single cells [25]. In addition, Y-27632 promotes the rapid adherence of rabbit limbal epithelial cells [29]. These studies suggest that Y-27632 protects human epithelial cells from the influence of dissociation. The effect of Y-27632 does not seem to be limited to the period immediately after cell preparation but continues for several days. In human keratinocytes, Y-27632 treatment after cell inoculation increased CFE; Y-27632 treatment for the first 6 days out of 14 days seemed to slightly increase CFE compared with Y-27632 treatment for first 3 days of 14 days [26]. This is consistent with our preliminary study where Y-27632 treatment for the first 4 days out of 10 days showed lower CFE compared with Y-27632 at 10 days (data not shown). Recently, Zhou et al. showed that Y-27632 increases glutathione level in rabbit limbal epithelial cells and decreases intracellular ROS during clonal expansion [29]. Y-27632 may contribute to the long-term maintenance of epithelial cell sheets by increasing seeding efficiency at the beginning of the culture and protecting cells from ROS stress.
KGF is not expressed in epithelial cells, but it is expressed in fibroblasts [33] and is increased in fibroblasts by the stimulation from epithelial cells [39, 46]. The absence of KGF production in epithelial cells may be related to the requirement of fibroblasts for serial cultivation of epithelial cells in serum-containing medium [41]. Basal layers of the limbal epithelium are known to express the KGF receptor, and KGF increases p63 expression in rabbit limbal epithelial cell sheets [40]. These facts suggest that KGF is required for the maintenance of undifferentiated epithelial cells, including stem cells. As expected, KGF increased stem/progenitor cell marker expression (Fig. 2). Although K15 and K12 expressions were slightly increased by Y-27632 in EGF groups, both KGF and K+Y sheets showed similar strong expression patterns (Fig. 2B), indicating that KGF, and not Y-27632, mainly contributed to the increased expression of these markers. Despite p63-positive cells being observed in EGF groups (Fig. 2D), the CFE of EGF group sheets was low (Fig. 3), suggesting that p63 alone may not be indicative of the stemness of the cell. KGF does not completely suppress differentiation, since differentiated cells were observed in KGF cultured limbal epithelial cell sheets (Figs. 2, 4). Similarly, KGF does not inhibit the induction of differentiation markers in epidermal keratinocytes [36] or corneal epithelial cells [37]. Overexpression of KGF in adult murine corneal stroma increases proliferation of corneal epithelial cells, but K12 expression was observed in corneal epithelium [47]. These results indicate that KGF is not a strong repressor of epithelial cell differentiation; in other words, KGF does not perturb the normal differentiation of epithelial cells. Epithelial stem cells may require KGF to maintain undifferentiated states but can respond to signals that induce differentiation or migration.
Cultivated epithelial cell sheets are also useful for treating LSCD patients, because they supply stem cells along with differentiated cells that form barriers of the ocular surface [10, 14–23]. Maintenance of progenitor cell markers, differentiation markers, and colony-forming cells for 3 months suggests that the quality of the K+Y sheet is stable for the long term in vitro, which may help scheduling of transplantation in clinical cases. In addition, we found that our limbal epithelial model was robust and easy to separate from the culture inserts, whereas cell sheets cultured with EGF was more fragile and adhered to inserts (supplemental online Fig. 5). Strong expression of E-cadherin/CDH1 in the cell-cell border may explain this robustness. For these reasons, our limbal epithelial model may be a better option for transplantation in LSCD patients. Although we used human feeder cells which were separated from the epithelial cells, traditional sheet culture used murine 3T3 feeder cells, which were in direct contact with the epithelial cells. We confirmed that epithelial cell sheets directly in contact with 3T3 feeder cells also increased the expressions of p63, K12, and K3 by KGF and Y-27632 (supplemental online Fig. 3), suggesting the usefulness of KGF and Y-27632 for other sheet culture methods.
Conclusion
We cultured primary human limbal epithelial cell sheets with feeder cells in transitional serum and hormone containing medium, with modifications using KGF and Y-27632 instead of EGF. Our cell sheets showed limbal epithelium-like features, stratification, expression of stem/progenitor cell markers and corneal epithelium-linage differentiation markers, and the existence of colony-forming cells. These features were maintained long-term in vitro, suggesting the homeostasis of cell sheets. We conclude that our cell sheets are useful as a limbal epithelial model in vitro for the basic study of stem/progenitor cells and for clinical application to treat LSCD patients.
Supplementary Material
Acknowledgments
We thank Dr. Emi Inagaki and Tomomi Sekiguchi for technical assistance, and Dr. Shin Hatou for his useful suggestions. We also thank all members of room 3N605, room 6N9, and room 9S2 in the Department of Ophthalmology, Keio University School of Medicine, for general assistance. This work was supported by a grant from the Ministry of Health, Labor and Welfare, Japan (to S.S. and K.T.).
Author Contributions
H.M.: conception and design, collection and assembly of data, data analysis and interpretation, manuscript writing; S. Yokoo and S. Yamagami: conception and design; S. Yoshida and T.K.: data analysis and interpretation; K.T.: administrative support; S.S.: conception and design, data analysis and interpretation, manuscript writing.
Disclosure of Potential Conflicts of Interest
K.T. and S.S. have compensated research funding.
References
- 1.Ordonez P, Di Girolamo N. Limbal epithelial stem cells: Role of the niche microenvironment. Stem Cells. 2012;30:100–107. doi: 10.1002/stem.794. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida S, Shimmura S, Kawakita T, et al. Cytokeratin 15 can be used to identify the limbal phenotype in normal and diseased ocular surfaces. Invest Ophthalmol Vis Sci. 2006;47:4780–4786. doi: 10.1167/iovs.06-0574. [DOI] [PubMed] [Google Scholar]
- 3.Pellegrini G, Dellambra E, Golisano O, et al. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–3161. doi: 10.1073/pnas.061032098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen Z, de Paiva CS, Luo L, et al. Characterization of putative stem cell phenotype in human limbal epithelia. Stem Cells. 2004;22:355–366. doi: 10.1634/stemcells.22-3-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe K, Nishida K, Yamato M, et al. Human limbal epithelium contains side population cells expressing the ATP-binding cassette transporter ABCG2. FEBS Lett. 2004;565:6–10. doi: 10.1016/j.febslet.2004.03.064. [DOI] [PubMed] [Google Scholar]
- 6.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodrigues M, Ben-Zvi A, Krachmer J, et al. Suprabasal expression of a 64-kilodalton keratin (no. 3) in developing human corneal epithelium. Differentiation. 1987;34:60–67. doi: 10.1111/j.1432-0436.1987.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 8.Higa K, Shimmura S, Miyashita H, et al. Melanocytes in the corneal limbus interact with K19-positive basal epithelial cells. Exp Eye Res. 2005;81:218–223. doi: 10.1016/j.exer.2005.01.023. [DOI] [PubMed] [Google Scholar]
- 9.Lindberg K, Brown ME, Chaves HV, et al. In vitro propagation of human ocular surface epithelial cells for transplantation. Invest Ophthalmol Vis Sci. 1993;34:2672–2679. [PubMed] [Google Scholar]
- 10.Pellegrini G, Golisano O, Paterna P, et al. Location and clonal analysis of stem cells and their differentiated progeny in the human ocular surface. J Cell Biol. 1999;145:769–782. doi: 10.1083/jcb.145.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tseng SC. Concept and application of limbal stem cells. Eye (Lond) 1989;3:141–157. doi: 10.1038/eye.1989.22. [DOI] [PubMed] [Google Scholar]
- 12.Pellegrini G, Traverso CE, Franzi AT, et al. Long-term restoration of damaged corneal surfaces with autologous cultivated corneal epithelium. Lancet. 1997;349:990–993. doi: 10.1016/S0140-6736(96)11188-0. [DOI] [PubMed] [Google Scholar]
- 13.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. [DOI] [PubMed] [Google Scholar]
- 14.Schwab IR, Reyes M, Isseroff RR. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea. 2000;19:421–426. doi: 10.1097/00003226-200007000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Koizumi N, Inatomi T, Suzuki T, et al. Cultivated corneal epithelial transplantation for ocular surface reconstruction in acute phase of Stevens-Johnson syndrome. Arch Ophthalmol. 2001;119:298–300. [PubMed] [Google Scholar]
- 16.Rama P, Bonini S, Lambiase A, et al. Autologous fibrin-cultured limbal stem cells permanently restore the corneal surface of patients with total limbal stem cell deficiency. Transplantation. 2001;72:1478–1485. doi: 10.1097/00007890-200111150-00002. [DOI] [PubMed] [Google Scholar]
- 17.Shimazaki J, Aiba M, Goto E, et al. Transplantation of human limbal epithelium cultivated on amniotic membrane for the treatment of severe ocular surface disorders. Ophthalmology. 2002;109:1285–1290. doi: 10.1016/s0161-6420(02)01089-8. [DOI] [PubMed] [Google Scholar]
- 18.Grueterich M, Espana EM, Touhami A, et al. Phenotypic study of a case with successful transplantation of ex vivo expanded human limbal epithelium for unilateral total limbal stem cell deficiency. Ophthalmology. 2002;109:1547–1552. doi: 10.1016/s0161-6420(02)01105-3. [DOI] [PubMed] [Google Scholar]
- 19.Sangwan VS, Vemuganti GK, Iftekhar G, et al. Use of autologous cultured limbal and conjunctival epithelium in a patient with severe bilateral ocular surface disease induced by acid injury: A case report of unique application. Cornea. 2003;22:478–481. doi: 10.1097/00003226-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 20.Nishida K, Yamato M, Hayashida Y, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 21.Ang LP, Nakamura T, Inatomi T, et al. Autologous serum-derived cultivated oral epithelial transplants for severe ocular surface disease. Arch Ophthalmol. 2006;124:1543–1551. doi: 10.1001/archopht.124.11.1543. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura T, Inatomi T, Sotozono C, et al. Transplantation of autologous serum-derived cultivated corneal epithelial equivalents for the treatment of severe ocular surface disease. Ophthalmology. 2006;113:1765–1772. doi: 10.1016/j.ophtha.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;363:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 24.Reichl S, Kölln C, Hahne M, et al. In vitro cell culture models to study the corneal drug absorption. Expert Opin Drug Metab Toxicol. 2011;7:559–578. doi: 10.1517/17425255.2011.562195. [DOI] [PubMed] [Google Scholar]
- 25.McMullan R, Lax S, Robertson VH, et al. Keratinocyte differentiation is regulated by the Rho and ROCK signaling pathway. Curr Biol. 2003;13:2185–2189. doi: 10.1016/j.cub.2003.11.050. [DOI] [PubMed] [Google Scholar]
- 26.Terunuma A, Limgala RP, Park CJ, et al. Efficient procurement of epithelial stem cells from human tissue specimens using a Rho-associated protein kinase inhibitor Y-27632. Tissue Eng Part A. 2010;16:1363–1368. doi: 10.1089/ten.tea.2009.0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe K, Ueno M, Kamiya D, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- 28.Okumura N, Ueno M, Koizumi N, et al. Enhancement on primate corneal endothelial cell survival in vitro by a ROCK inhibitor. Invest Ophthalmol Vis Sci. 2009;50:3680–3687. doi: 10.1167/iovs.08-2634. [DOI] [PubMed] [Google Scholar]
- 29.Zhou Q, Duan H, Wang Y, et al. ROCK inhibitor Y-27632 increases the cloning efficiency of limbal stem/progenitor cells by improving their adherence and ROS-scavenging capacity. Tissue Eng Part C Methods. 2013;19:531–537. doi: 10.1089/ten.tec.2012.0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen G, Hou Z, Gulbranson DR, et al. Actin-myosin contractility is responsible for the reduced viability of dissociated human embryonic stem cells. Cell Stem Cell. 2010;7:240–248. doi: 10.1016/j.stem.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohgushi M, Matsumura M, Eiraku M, et al. Molecular pathway and cell state responsible for dissociation-induced apoptosis in human pluripotent stem cells. Cell Stem Cell. 2010;7:225–239. doi: 10.1016/j.stem.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 32.Chapman S, Liu X, Meyers C, et al. Human keratinocytes are efficiently immortalized by a Rho kinase inhibitor. J Clin Invest. 2010;120:2619–2626. doi: 10.1172/JCI42297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li DQ, Tseng SC. Three patterns of cytokine expression potentially involved in epithelial-fibroblast interactions of human ocular surface. J Cell Physiol. 1995;163:61–79. doi: 10.1002/jcp.1041630108. [DOI] [PubMed] [Google Scholar]
- 34.Schlötzer-Schrehardt U, Kruse FE. Identification and characterization of limbal stem cells. Exp Eye Res. 2005;81:247–264. doi: 10.1016/j.exer.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 35.Rubin JS, Osada H, Finch PW, et al. Purification and characterization of a newly identified growth factor specific for epithelial cells. Proc Natl Acad Sci USA. 1989;86:802–806. doi: 10.1073/pnas.86.3.802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchese C, Rubin J, Ron D, et al. Human keratinocyte growth factor activity on proliferation and differentiation of human keratinocytes: Differentiation response distinguishes KGF from EGF family. J Cell Physiol. 1990;144:326–332. doi: 10.1002/jcp.1041440219. [DOI] [PubMed] [Google Scholar]
- 37.Wilson SE, He YG, Weng J, et al. Effect of epidermal growth factor, hepatocyte growth factor, and keratinocyte growth factor, on proliferation, motility and differentiation of human corneal epithelial cells. Exp Eye Res. 1994;59:665–678. doi: 10.1006/exer.1994.1152. [DOI] [PubMed] [Google Scholar]
- 38.Maas-Szabowski N, Shimotoyodome A, Fusenig NE. Keratinocyte growth regulation in fibroblast cocultures via a double paracrine mechanism. J Cell Sci. 1999;112:1843–1853. doi: 10.1242/jcs.112.12.1843. [DOI] [PubMed] [Google Scholar]
- 39.Li DQ, Tseng SC. Differential regulation of keratinocyte growth factor and hepatocyte growth factor/scatter factor by different cytokines in human corneal and limbal fibroblasts. J Cell Physiol. 1997;172:361–372. doi: 10.1002/(SICI)1097-4652(199709)172:3<361::AID-JCP10>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 40.Cheng CC, Wang DY, Kao MH, et al. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway. J Cell Sci. 2009;122:4473–4480. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- 41.Rheinwald JG, Green H. Serial cultivation of strains of human epidermal keratinocytes: The formation of keratinizing colonies from single cells. Cell. 1975;6:331–343. doi: 10.1016/s0092-8674(75)80001-8. [DOI] [PubMed] [Google Scholar]
- 42.Yokoo S, Yamagami S, Usui T, et al. Human corneal epithelial equivalents for ocular surface reconstruction in a complete serum-free culture system without unknown factors. Invest Ophthalmol Vis Sci. 2008;49:2438–2443. doi: 10.1167/iovs.06-1448. [DOI] [PubMed] [Google Scholar]
- 43.Omoto M, Miyashita H, Shimmura S, et al. The use of human mesenchymal stem cell-derived feeder cells for the cultivation of transplantable epithelial sheets. Invest Ophthalmol Vis Sci. 2009;50:2109–2115. doi: 10.1167/iovs.08-2262. [DOI] [PubMed] [Google Scholar]
- 44.Miyashita H, Shimmura S, Higa K, et al. A novel NIH/3T3 duplex feeder system to engineer corneal epithelial sheets with enhanced cytokeratin 15-positive progenitor populations. Tissue Engineering Part A. 2008;14:1275–1282. doi: 10.1089/ten.tea.2007.0212. [DOI] [PubMed] [Google Scholar]
- 45.Higa K, Shimmura S, Kato N, et al. Proliferation and differentiation of transplantable rabbit epithelial sheets engineered with or without an amniotic membrane carrier. Invest Ophthalmol Vis Sci. 2007;48:597–604. doi: 10.1167/iovs.06-0664. [DOI] [PubMed] [Google Scholar]
- 46.Smola H, Thiekotter G, Fusenig NE. Mutual induction of growth factor gene expression by epidermal-dermal cell interaction. J Cell Biol. 1993;122:417–429. doi: 10.1083/jcb.122.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hayashi M, Hayashi Y, Liu CY, et al. Overexpression of FGF7 enhances cell proliferation but fails to cause pathology in corneal epithelium of Kerapr-rtTA/FGF7 bitransgenic mice. Mol Vis. 2005;11:201–207. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.