This study found that targeting the α7 nicotinic acetylcholine receptors (α7nAChRs) with a specific TC-7020 agonist led to a robust accumulation of endogenous fibroblast growth factor receptor 1 (FGFR1) in the cell nucleus and neuronal differentiation of neural progenitor cells in brain cortex, hippocampus, and substantia nigra. The reactivation of integrative nuclear FGFR1 signaling and neurogenesis in adult brain by the α7nAChR agonist offers a new strategy to treat brain injuries, neurodegenerative diseases, and neurodevelopmental diseases.
Keywords: Adult stem cells, Cell biology, Neural differentiation, Neural stem cell, Nucleus, Signal transduction
Abstract
Reactivation of endogenous neurogenesis in the adult brain or spinal cord holds the key for treatment of central nervous system injuries and neurodegenerative disorders, which are major health care issues for the world's aging population. We have previously shown that activation of developmental integrative nuclear fibroblast growth factor receptor 1 (FGFR1) signaling (INFS), via gene transfection, reactivates neurogenesis in the adult brain by promoting neuronal differentiation of brain neural stem/progenitor cells (NS/PCs). In the present study, we report that targeting the α7 nicotinic acetylcholine receptors (α7nAChRs) with a specific TC-7020 agonist led to a robust accumulation of endogenous FGFR1 in the cell nucleus. Nuclear FGFR1 accumulation was accompanied by an inhibition of proliferation of NS/PCs in the subventricular zone (SVZ) and by the generation of new neurons. Neuronal differentiation was observed in different regions of the adult mouse brain, including (a) βIII-Tubulin-expressing cortical neurons, (b) calretinin-expressing hippocampal neurons, and (c) cells in substantia nigra expressing the predopaminergic Nurr1+ phenotype. Furthermore, we showed that in vitro stimulation of neural stem/progenitor cells with α7nAChR agonist directly activated INFS and neuronal-like differentiation. TC-7020 stimulation of the βIII-Tubulin gene was accompanied by increased binding of FGFR1, CREB binding protein, and RNA polymerase II to a Nur77 targeted promoter region. TC-7020 augmented Nur77-dependent activation of nerve growth factor inducible-B protein responsive element, indicating that α7nAChR upregulation of βIII-Tubulin involves neurogenic FGFR1-Nur signaling. The reactivation of INFS and neurogenesis in adult brain by the α7nAChR agonist may offer a new strategy to treat brain injuries, neurodegenerative diseases, and neurodevelopmental diseases.
Introduction
Neurogenesis, the process of generating new neurons, underlies the global development of the central nervous system (CNS) but becomes severely restricted in the mature brain. Developmental neurogenesis is driven by groups of germinal neural stem/progenitor cells (NS/PCs) in ventricular walls that produce neurons and glia that populate the entire CNS. This process of differentiation and migration stops shortly after birth. In the mature brain, generation of new neurons occurs only in two germinal niches: the subgranular zone (SGZ) of the hippocampal dentate gyrus and the subventricular zone (SVZ) of the lateral ventricles [1, 2]. In other regions, neurogenesis is limited, and few new neurons are found in cortical and subcortical circuits of the mature brain [3].
Brain development engages multigene programs that drive stem cell self-renewal, mitotic expansion, and differentiation. Universal, integrative network modules responsible for the stem cell self-renewal (Oct3/4, Nanog, and Sox) and mitotic expansion (Cyclin E and CDK2) have been partially identified [4]. However, their manipulation to promote mitogenic expansion of NS/PCs in the adult brain has had only a small effect on the production of new neurons, because of inefficient NS/PC differentiation and survival [5]. We identified an analogous universal regulatory module, integrative nuclear fibroblast growth factor receptor 1 (FGFR1) signaling (INFS), which promotes postmitotic neuronal development and offers an efficient means for increasing neurogenesis [6–8]. Central to this mechanism is the release of newly synthesized FGFR1 molecules from the endoplasmic reticulum into the cytosol, followed by nuclear translocation along with the 23-kDa form of fibroblast growth factor-2 (FGF-2), which contains a nuclear localization signal (NLS) [9–14]. INFS is initiated by diverse neurogenic stimuli including retinoic acid, nerve growth factor, bone morphogenetic protein 7, and cyclic AMP. Nuclear FGFR1“feeds forward” this activation directly to the common transcriptional coactivator and essential gating factor, CREB binding protein (CBP), enabling gene activation and neuronal differentiation ([6, 15–17]; reviewed in [4, 8]).
Recent studies have shown that INFS is activated during in vivo neurogenesis and supports neuronal differentiation [6, 16, 18]. In proliferating SVZ NS/PCs, FGFR1 is concentrated in the cytoplasmic membrane and mediates mitogenic effects of extracellular FGFs. As cells exit the cell cycle, FGFR1 accumulates and remains in the nucleus during cell differentiation and migration from the SVZ to target brain regions, after which the nuclear targeting of FGFR1 is decreased or not found in mature neurons. In the developing brain, nuclear accumulation of FGFR1 can be observed in the immature βIII-Tubulin-expressing mouse cortical neurons at postnatal day 5 [18]. By postnatal day 15, the expression of β III-Tubulin is turned off and FGFR1 localization changes from nuclear to cytoplasmic. A similar pattern of FGFR1 is observed in developing dopamine neurons in the substantia nigra pars compacta (SNc) [6, 19]. Interference with FGFR1 signaling in developing dopamine neurons using dominant-negative FGFR1 mutant substantiates the role of FGFR1 in neuronal development in a transgenic mouse model [20, 21].
Transfection of 23-kDa FGF-2, which targets endogenous nuclear FGFR1, or an engineered constitutive nuclear FGFR1(SP−/NLS), in which the signal peptide (SP) is replaced with an NLS from FGF-2, effectively activates neuronal genes and promotes neuron-like differentiation in cultured human neural progenitor cells (hNPCs) and mouse embryonic stem cells (mESCs) [5, 15, 16, 22, 23]. The same transfection into the SVZ in vivo diminished proliferation of NS/PCs and stimulated massive neuronal differentiation and migration into the adult brain cortex and subcortical regions, which normally show no neurogenesis [14, 16]. These studies provide a proof of concept for the reinstatement of endogenous neurogenesis in adult brain via reactivation of the INFS developmental module.
An alternative, noninvasive approach to controlling brain neurogenesis would use pharmacologically active small molecules capable of activating INFS in brain NS/PCs after systemic administration [18]. Nicotinic acetylcholine receptor (nAChR) agonists represent one such class of potential agents. nAChRs are pentameric, ligand gated ion channels expressed in both undifferentiated and differentiating cells [24]. The nAChRs influence cell development and appear to affect several processes during brain neurogenesis [24]. Homopentameric α7nAChRs are highly abundant during embryonic and postnatal brain development [25, 26] and can be detected in nearly all regions of the mature brain [27, 28]. The early appearance and prolonged expression pattern of the α7nAChRs suggest that these receptors influence cell development, migration, and apoptosis. Indeed, stimulation of α7nAChRs promotes proliferation in neuroendocrine cells [29]; neurite outgrowth in rat olfactory culture [30]; neuronal differentiation of cultured hippocampal neuronal progenitors [31]; and hippocampal neuron maturation, integration, and survival in vivo [32]. α7nAChRs are expressed also on embryonic and brain stem cells and stimulate cell differentiation while inhibiting or activating proliferation [33–35].
In our earlier report we showed that nicotine stimulates INFS in adrenal medullary cells [10]. Recently TC-7020, a novel agonist, has been developed, which is highly selective for the α7 nAChR subtype, based on both binding affinity (Ki ∼2 nm) and function [36]. TC-7020 exhibits only a marginal affinity toward other nAChR subtypes (Ki >1,000 nM), including the major brain α4β2n subtype, the muscle and ganglion-type nAChRs, and more than 60 non-nicotinic receptor targets tested [36]. We used TC-7020 to determine whether a specific stimulation of α7nAChRs can initiate the INFS in vivo and reactivate neuronal development in the adult brain. The mechanisms of α7nAChR action were investigated further using cultured neuronal stem-like cells.
Materials and Methods
Plasmids
Plasmids expressing the following FGFR1 constructs have been described previously [22, 23]: FGFR1(TK−)-deleted tyrosine kinase domain, FGFR1(SP−/NLS)-signal peptide replaced with the NLS from the SV40 large T antigen, and FGFR1(SP−/NLS)(TK−). Plasmid nerve growth factor inducible-B protein responsive element 3 (NBRE3)-Luc containing three NGF-binding response elements in minimal POMC gene promoter (−34/+63) and Nur77-expressing pCMX vector were gifts from Dr. Jacques Drouin (Institut de Recherches Cliniques de Montréal) [37, 38]. The reference reporter plasmid, pGL4.70 (hRluc) promoterless, was from Promega, Madison, WI, http://www.promega.com). The pCAGGS-Nurr1–3xFlag was obtained from Dr. A. Ratzka and was described by Baron et al. [19].
Cell Cultures
hNPCs and human neuroblastoma (NB) cell line SK-N-BE(2) were cultured as previously described [15, 17].
Animals
Male mice (C57BL/10J/C3H/HeJ), approximately 4 months of age, were kept in a normal 12-hour light/12-hour dark cycle with free access to food and water [20]. All experiments involving mice were carried out in accordance with institutional animal care and use committee (IACUC) guidelines and approved by the local IACUC. 5-Bromo-2′-deoxyuridine (BrdU) injections (intraperitoneal [i.p.] injection) were carried out four times a day beginning 1 hour before the start of the dark cycle and then every 3 hours until all four injections had been given. The injections continued for 4 days. One day after the last BrdU injection, the mice received a single daily injection (1 hour into the dark cycle) of either saline or 1 mg/kg (i.p.) TC-7020 (provided by Targacept Inc., Winston-Salem, NC, http://www.targacept.com) [36, 39]. TC-7020/saline injections continued for 11 days, after which mice were perfused, and 40-μm coronal cryostat sections spanning the entire analyzed brain structures were prepared [14, 16].
Immunocytochemistry and Stereology
Immunocytochemistry and stereology were performed as previously described [20]. Briefly, tissue or cells were fixed in paraformaldehyde, permeabilized with 1% Triton X-100, and incubated with the primary antibody. The primary antibodies used were as follows: polyclonal FGFR1 (1:100; SC-121; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), monoclonal FGFR1 (1:200; antibody [Ab] 823; Abcam, Cambridge, MA, http://www.abcam.com), βIII-Tubulin (1:200; ab18207; Abcam), BrdU (1:200; MCA2060; AbD Serotec, Raleigh, NC, http://www.ab-direct.com), Nurr1 (1:100; SC-991; Santa Cruz Biotechnology), calretinin (1:1,000; SC-50453; Santa Cruz Biotechnology), tyrosine hydroxylase (TH) (1:1,000; SC-7847; Santa Cruz Biotechnology), Olig2 (1:1,000; AB15620; Millipore, Billerica, MA, http://www.millipore.com), and pan-neuronal maker (1:75; MAB2300; Millipore). Subsequently, tissue or cells were incubated with secondary antibodies containing the following fluorescent tags: goat anti-mouse Alexa 568 (1:1,500) or Alexa 488 (1:2,000), goat anti-rabbit Alexa 568 or Alexa 488 (1:2,000), or goat anti-rat Alexa 568 (1:2,000). The fluorescence was imaged using an Axioimager fluorescence microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com). The specificity of immunostaining was verified as previously described [6, 9, 11, 19, 40, 41]. Staining was not observed when the primary antibody was omitted or replaced with preimmune serum. Similar FGFR1 nuclear-cytoplasmic localization was observed by using three antibodies targeting different FGFR1 epitopes and by detection of transfected FGFR1-enhanced green fluorescent protein (EGFP) and FGFR1-Flag using native fluorescence and αFlag [17, 19]. The presence of and changes in the levels of nuclear FGFR1 immunoreactivity were confirmed by Western blot analysis of FGFR1 in subcellular fractions. For stereology, tissue was imaged under a BX-51 microscope (Olympus, Tokyo, Japan, http://www.olympus-global.com), and stereological cell counts were performed using the protocol described by Klejbor et al. [20] and consisting of (a) outlining the nuclei under low magnification, (b) random sampling at a magnification of ×20 using the same antero-posterior sequence (five sections from each brain), and (c) determining neuronal density within the tested fields of known surface areas. The raw data from the individual tested fields were recorded and weighed, and the mean cell density was calculated for each brain region. The density of cells in vehicle and TC-7020 mice were compared using analysis of variance (Kruskal-Wallis test).
Western Blotting
Cells were fractionated as described in [9, 10, 17, 42], and equal amounts of proteins from cytoplasmic or nuclear fractions were resolved on SDS-7.5% polyacrylamide gel and transferred to polyvinylidene difluoride (Millipore). Blots were probed with primary antibodies, and immune complexes were revealed by chemiluminescence using SuperSignal Femto Maximum Sensitivity Substrate (Pierce, Rockford, IL, http://www.piercenet.com) and a Fuji chemiluminescence imager (Fuji, Valhalla, NY, http://www.fujifilm.com). Antibodies used were FGFR1 (1:1,000; SC-121; Santa Cruz Biotechnology), GAPDH (1:1,000; SC137179; Santa Cruz Biotechnology), Matrin (1:10,000; A300-591A; Bethyl Laboratories), tyrosine hydroxylase (1:10,000; SC-14007; Santa Cruz Biotechnology), doublecortin (1:10,000; Santa Cruz Biotechnology), and βIII-Tubulin (1:25,000; AB18207; Abcam).
mRNA Level Determination Using Quantitative Polymerase Chain Reaction
Total RNA was isolated from 35-mm plates of NB cultures using Trizol. cDNA synthesis was carried out using 1 μg of RNA and the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, http://www.bio-rad.com). One-tenth of the synthesized cDNA was used as a template for real-time polymerase chain reaction (PCR). Twenty-five microliters of real-time PCRs were performed on the BioRad MyiQ Cycler with iQ SYBR Green Supermix (Bio-Rad). Real-time (RT) quantitative polymerase chain reaction (qPCR) using the following amplification cycles: one round of cycle 1 (initial denaturation for 8 minutes at 95°C), followed by 35 rounds of cycle 2 (denaturation for 15 seconds at 95°C and annealing for 1 minute at 60°C). Melt curve data collection was enabled by decreasing the set point temperature after cycle 2 by 0.5°C. The specificity of amplicons was confirmed by generating the melt curve profile of all amplified products. Gene expression was quantified as described [43].
Chromatin Immunoprecipitation Assays
NB cells were cross-linked with 1% formaldehyde (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com). Chromatin immunoprecipitation (ChIP) was performed and the results were calculated as previously described [17, 19]. Genomic DNA (200 μg) was precipitated with the indicated Ab or control IgG and subjected to duplicate qPCRs [17, 19]. The antibodies used were FGFR1 (AB10464; Abcam), CBP (SC-369; Santa Cruz Biotechnology), Nurr1/Nur77 (SC-990; Santa Cruz Biotechnology), and RNA polymerase (Pol) II (AB5095; Abcam).
The primer sequences for the human βIII-Tubulin gene NBRE-containing region were as follows: forward, 5′-TCTTCTCAGTGGGTTCAGGGC (−578 nucleotides); reverse, 5′GGTTCCGCCCTTGCGAC (−388 nucleotides). The FGFR1 nonbinding region of the Mesp2 gene was used as an internal control, to which no binding was detected (not shown).The results were calculated as immunoprecipitated (IP) DNA, where ΔΔCt = (CtIP Ab − CtIP IgG) − (Ctinput DNA − CtIP IgG) [17, 19].
Dual Luciferase Assays
Transfections of plasmids to NB cells and transcription assays were performed with the dual luciferase reporter system (Promega) as described in [17, 19]. Luminescence measurement was performed on a BioTek Plate Reader (BioTek, Winooski, VT, http://www.biotek.com). The data were calculated as the ratio of firefly to Renilla luciferase activity or normalized by protein concentration and transfection rate evaluated by cotransfection of EGFP. Experiments were repeated two to four times, and each was performed in quadruplicate.
Results
TC-7020 Reverses Postnatal Inactivation of INFS, Inhibits Cell Proliferation, and Increases Neuronal Differentiation in the Adult Mouse Brain SVZ Region
Nuclear accumulation of FGFR1 and coexpression of neuronal marker βIII-Tubulin can be observed in developing brain cortex at postnatal day 5 but is not found by postnatal day 15 [18]. To determine whether both INFS and neuronal development may be reactivated in the mature brain by an α7nAChR agonist, mice were injected with TC-7020 (1 mg/kg) or vehicle (0.9% NaCl). Previous work has shown that long-term dosage of the α7nAChR agonist results in the upregulation of α7nAChRs. In order to mimic a long-term dosage regime we decided to inject the mice for 11 days of TC-7020. After 11 daily injections, we analyzed the expression and nuclear localization of FGFR1, as an index of the INFS activity. In vehicle-injected mice weak FGFR1 immunoreactivity (IR) was observed throughout regions adjacent to the SVZ and in the brain cortex (Figs. 1A, 2B). Typical for adult brains [18], cells displayed a cytoplasmic, nonnuclear FGFR1-IR. Only few cells in the vicinity of SVZ/rostral migratory stream (RMS) showed nuclear FGFR1-IR. In contrast, mice treated with TC-7020 showed a ubiquitous presence of cells displaying intense nuclear FGFR1-IR. These cells extended from the SVZ/RMS to a more distal lateral periventricular region (Fig. 1A) and to the brain cortex (Fig. 2B).
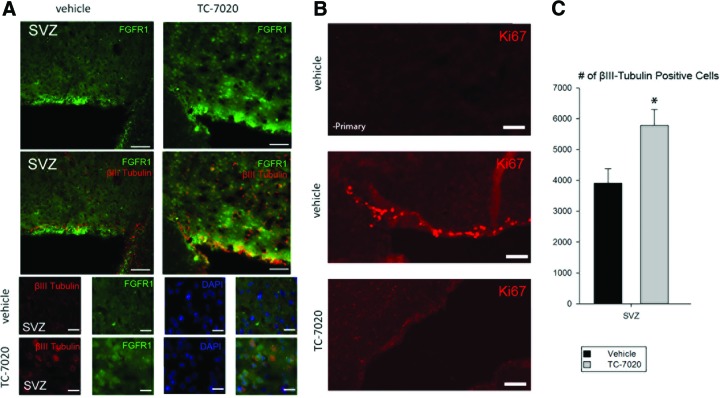
Figure 1.
Treatment with TC-7020 reactivates integrative nuclear FGFR1 signaling and reinstates βIII-Tubulin-expressing young neurons in adult mouse brain. Mice were injected with TC-7020 or vehicle (saline) for 11 days. Brains were coimmunostained with anti-FGFR1 and anti-βIII-Tubulin antibody (Ab), and nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bars = 50 μm (low magnification) and 25 μm (high magnification). (A): Lateral periventricular region including the SVZ and rostral migratory stream. Bottom: Individual and merged FGFR1, βIII-Tubulin, and DAPI stains to illustrate extranuclear FGFR1 in vehicle-injected controls and nuclear localization of FGFR1 in TC-7020-injected mice. Magnification, ×20 (top), ×40 (bottom). (B): Ki67 staining of the SVZ. Top: Vehicle, without primary Ab; middle: vehicle, Ki67 Ab; bottom: TC-7020, Ki67 Ab. (C): Stereological counts of βIII-Tubulin-expressing cells per 1 mm2 in SVZ from five control and four TC-7020-treated mice (p < .05, one-way analysis of variance). Abbreviations: FGFR1, fibroblast growth factor receptor 1; SVZ, subventricular zone.
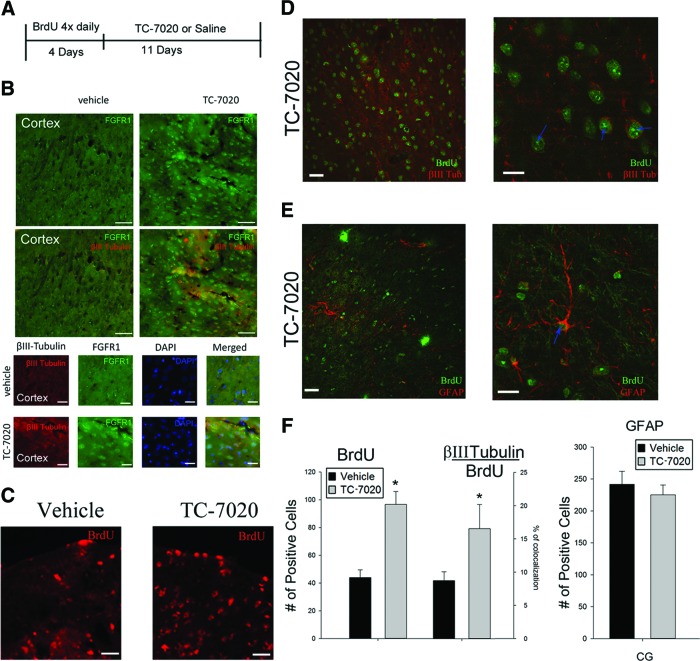
Figure 2.
TC-7020 promotes the generation of new neurons in the brain cortex. (A): Experiment time line: BrdU was injected for 4 days followed by 11 days of TC-7020 or vehicle injections. (B): Treatment with TC-7020 reactivated integrative nuclear FGFR1 signaling and reinstated βIII-Tubulin-expressing young neurons in cortex. Shown is the cingulate gyrus of the brain cortex. Magnification, ×20 (top), ×40 (bottom). In (A) and (B), the top panels show single FGFR1 and double FGFR1+βIII-Tubulin staining; bottom panels show individual and merged FGFR1, βIII-Tubulin, and 4′,6-diamidino-2-phenylindole stains to illustrate extranuclear FGFR1 in vehicle-injected controls and nuclear localization of FGFR1 in TC-7020-injected mice. (C): BrdU (red) immunostaining of brain cortex cingulate gyrus shows an increase in BrdU-retaining cells in TC-7020-injected mice. (D): BrdU (green) and βIII-Tubulin (red) coimmunostaining shows colocalization in the cingulate cortex of TC-7020-treated mice. (E): Lack of colocalization between BrdU and GFAP. One BrdU+/GFAP+ cell was found that displayed an astrocytic morphology and may represent an apoptotic cell. (F): Quantification of BrdU-positive cells and their fraction that expressed βIII-Tubulin (p < .01). Also shown is the quantification of GFAP-positive cells. Scale bars = 50 μm (low magnification) and 25 μm (high magnification). Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; CG, cingulate gyrus; FGFR1, fibroblast growth factor receptor 1; GFAP, glial fibrillary acidic protein.
In vivo transfection experiments have established that nuclear accumulation of FGFR1 promotes an exit from cell cycle and induces neuronal differentiation [6, 15, 17, 44]. Therefore, the effects of α7nAChR agonist on proliferation of the SVZ cells were evaluated by staining for Ki67, known to be expressed transiently in proliferating cells [45]. In vehicle-treated mice, all sections examined showed ubiquitous Ki67 cells in the superficial SVZ layers (Fig. 1B). In contrast, mice treated with TC-7020 displayed only single or no Ki67-positive cells. Thus, TC-7020 treatment inhibits proliferation of the SVZ cells.
To assess neuronal differentiation we stained brain sections for βIII-Tubulin, typically expressed in maturing neurons. In saline-treated mice, we observed fewer βIII-Tubulin expressing cells within the SVZ-periventricular region and in the brain cortex. Stereological quantification verified a 50% increase in the number of βIII-Tubulin-positive cells in the SVZ area (Fig. 1C). Coimmunostaining for βIII-Tubulin showed ubiquitous cells that colocalized cytoplasmic βIII-Tubulin-IR with nuclear FGFR1-IR. These cells were observed exclusively in TC-7020-treated mice. The colocalization of cytoplasmic βIII-Tubulin-IR and nuclear FGFR1-IR was further confirmed by confocal microscopy (supplemental online Fig. 1; supplemental online Videos 1, 2). Thus, administration of α7nAchR agonist TC-7020 induced an appearance of new neurons expressing nuclear FGFR1.
TC-7020 Promotes Neuronal Differentiation in the Brain Cortex and Substantia Nigra
A BrdU pulse-chase experiment was carried out to determine whether cells that stopped proliferation in response to TC-7020 had survived and differentiated (Fig. 2A). Mice were injected with BrdU for 4 days, followed by 11 days of TC-7020 or saline treatment after which animals were perfused and brains were analyzed by immunohistochemistry. We observed more BrdU-labeled cells within the cingulate cortex inTC-7020-treated mice than in control. An example of cortical anti-BrdU stain is shown in Figure 2C. Quantification of BrdU-IR cell densities showed a significant >2-fold increase in brain cortex cingulate gyrus (Fig. 2F), as well as the two hippocampal regions examined, the dentate gyrus and the CA1 area (Fig. 3A, 3B), after TC-7020 treatment.
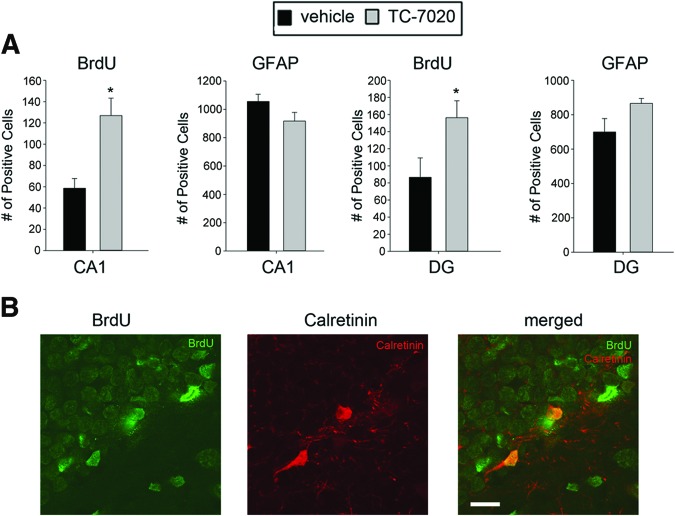
Figure 3.
TC-7020 promotes generation of neurons in the hippocampus. Experimental time line is shown in Figure 2A. (A): Quantification of hippocampal BrdU-positive cells shows increases in BrdU-retaining cells in the dentate gyrus and CA1 region after 7 days of TC-7020 treatment (p < .01). (B): BrdU (green) and calretinin (red) immunostaining in the hippocampus shows calretinin+/BrdU+ cells in TC-7020-treated animals. Approximately half of the BrdU+ cells expressed calretinin. Magnification, ×60. Scale bar = 25 μm. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; DG, dentate gyrus; GFAP, glial fibrillary acidic protein.
To identify the lineage of the BrdU-labeled cells, we performed double immunostaining with BrdU and glial fibrillary acidic protein (GFAP) or Olig2 or neuronal marker antibodies. In all mice examined, we only encountered a single double-labeled BrdU+/GFAP+ cell with an astrocyte-like morphology (Fig. 2E). However, this could also be an apoptotic cell, since BrdU can incorporate into apoptotic cells. Furthermore, counting GFAP+ cells showed no significant difference between saline- and TC-7020-injected mice (Figs. 2F, 3A, 3B). Therefore, the BrdU pulse-labeled cells did not differentiate to astrocytes. In addition, we have carried a broad coimmunostaining for BrdU and the Olig2 protein in the brains of TC-7020-treated mice. Olig2 is expressed by both developing and mature oligodendrocytes. We observed only rare colocalizations of Olig2 and BrdU in brain cortex and the SVZ area, which was not amenable to quantitative evaluation (supplemental online Fig. 2).
Double immunostaining for BrdU and βIII-Tubulin revealed the expression of the neuronal marker in approximately 8% of the BrdU+ cortical cells in control mice. The fraction of βIII-Tubulin+/BrdU+ cells increased more than twofold in TC-7020-injected mice. Given the twofold increase in total BrdU+ cells (Fig. 2E), TC-7020 produced an overall fourfold increase in the newly generated BrdU pulse-labeled βIII-Tubulin-positive neurons. Therefore, TC-7020 promotes neuronal but not astroglial differentiation in the brain cortex.
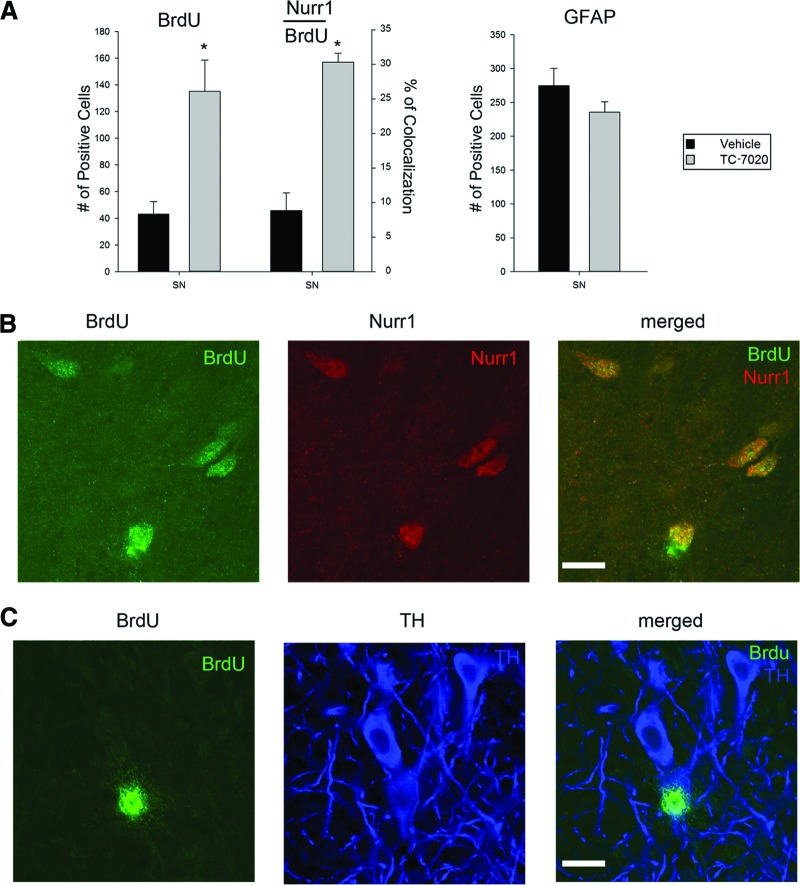
In the hippocampus, double immunostaining showed an expression of calretinin, a marker of early/mature hippocampal neurons, colocalizing in several BrdU+ cells in TC-7020-treated mice (Fig. 3B). The SNc also showed a threefold increase in the density of BrdU-labeled cells in TC-7020-treated mice compared with saline-treated controls (Fig. 4A). As in the other regions, there was no measurable overlap between BrdU and GFAP staining and no significant differences in the numbers of GFAP+ cells after TC-7020 treatment (Fig. 4A). Because of the prevalence of dopaminergic neurons in the SNc region, we performed double immunostaining for BrdU and Nurr1, a transcriptional factor that is expressed in developing dopamine neurons and that promotes their maturation [19, 46]. In control brains approximately 7% of the BrdU-labeled cells expressed Nurr1, which increased to more than 30% in TC-7020-treated mice (Fig. 4A; examples of Nurr1+/BrdU+ cells are shown on Fig. 4B). These data, taken together with the overall 3-fold increase in BrdU+ cells induced by TC-7020, show nearly a 12-fold increase in the newly generated immature SNc neurons after TC-7020 treatment. BrdU/TH double immunostaining was performed to determine whether the BrdU pulse-labeled cells acquired a mature dopamine phenotype. Few TH+/BrdU+ cells were found in TC-7020-treated mice (Fig. 4C), indicating that a longer time may be needed for the full differentiation of Nurr1+/BrdU+ neurons. No TH+/BrdU+ cells were detected in saline-treated control mice (not shown).
Figure 4.
TC-7020 promotes generation of Nurr1-expressing neurons in substantia nigra pars compacta (SNc). Mice were treated as outlined in Figure 2A. (A): Quantification of BrdU+ cells (p < .01) and their fraction that coexpresses Nurr1 (p < .05). In the absence of BrdU and GFAP colocalization, all GFAP-positive cells were also quantified. (B): Double immunostaining of the SNc region with anti-BrdU (green) and anti-Nurr1 (red) antibody (Ab). Confocal images show BrdU+ cells coexpress orphan Nurr1 receptor marking the developing dopamine neurons. Magnification, ×60. (C): Occasional coimmunostaining of BrdU+ (green) cells with anti-TH Ab (blue). Scale bars = 25 μm. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; GFAP, glial fibrillary acidic protein; SN, substantia nigra; TH, tyrosine hydroxylase.
TC-7020 Promotes Nuclear Accumulation of FGFR1 and Neuronal-Like Differentiation in Cultured Progenitor Cells
α7nAChRs are expressed ubiquitously throughout the brain and are present on both the developing cells and mature neurons. Hence, our in vivo experiments could not distinguish whether the activation of INFS and accompanying neurogenesis could be due to a direct α7nAChR activation of the brain progenitor cells or whether this process is mediated through α7nAChR-activated neuronal networks that influence brain stem and progenitor cells. To determine whether α7 acetylcholine receptors (α7AChRs) can directly affect the INFS, we carried out in vitro experiments in which cultured stem-like cells were treated with TC-7020. Our first model was hNPCs, in which nuclear FGFR1 accumulation can be induced by cAMP or brain-derived neurotrophic factor, leading to neuronal differentiation [15]. In the present study, incubation of hNPCs with TC-7020 induced a robust nuclear accumulation of FGFR1 (Fig. 5A). In cultures treated with 1 μM TC-7020 essentially all cells displayed a marked extension of neurites stained with Pan-Neuronal Marker Ab (Millipore) (Fig. 5B), which recognizes nuclear NeuN and cytoplasmic neuronal cytoskeleton proteins. Measurement of the neurite length showed a statistically significant increase in neurite length induced by α7nAChR agonist. Furthermore, none of the hNPCs showed oligodendrocyte- or astrocyte-like morphology.
Figure 5.
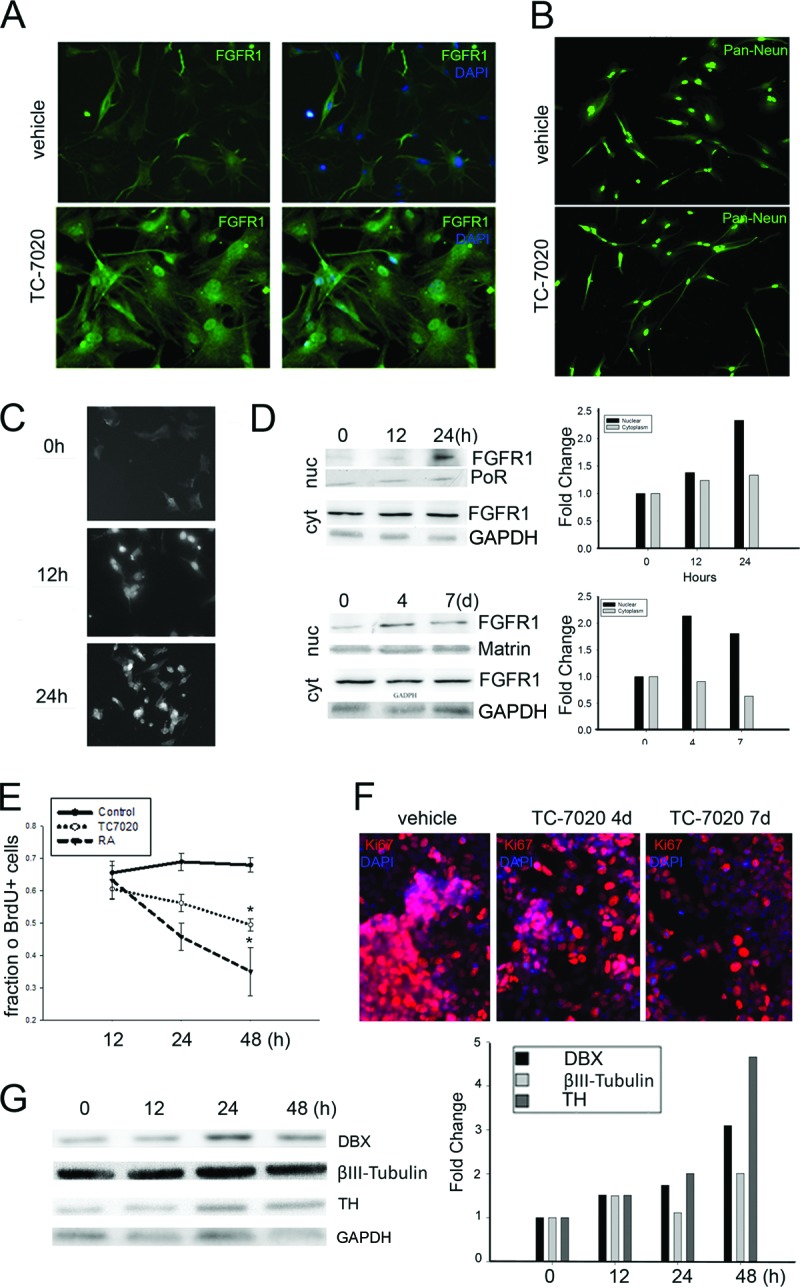
The effects of TC-7020 on cultured human neural progenitor cells (hNPCs) or human neuroblastoma (NB) cells. (A): hNPCs were incubated without or with 1 μM TC-7020 for 48 hours, stained with DAPI, and immunostained with αFGFR1. Images illustrate nuclear accumulation of FGFR1 in TC-7020-treated cultures. Magnification, ×20. (B): hNPCs were incubated in control medium or with TC-7020 for 48 hours. Morphological changes were observed after immunostaining with Pan-Neuronal Marker antibody (Ab), which labels nuclear NeuN and cytoplasmic cytoskeletal proteins. The average length of neurites extending from cell soma measured on multiple dishes was 79 ± 7.8 pixels in control cells; length increased to 136 ± 7.8 pixels after TC-7020 treatment (p < .05). Magnification, ×10. (C–G): NB cells were treated with 1 μM TC-7020 for the indicated periods of time. (C): Monoclonal FGFR1 McAb6 was used for immunocytochemistry. Magnification, ×20. (D): Cells were treated with 1 μM TC-7020 for 12 or 24 hours (short-term experiment) or 4 or 7 days (long-term experiment) or maintained in control medium. Nuclear and cytoplasmic proteins (40 μg of protein per lane) were immunoblotted with polyclonal αFGFR1 Ab. Approximately 80 kDa of FGFR1 was detected in the cytoplasmic and nuclear fractions. The TC-7020 induced changes are evident relative to protein content in individual lanes stained with Ponceau S Red or Matrin Ab (nucleus) or GAPDH Ab (cytoplasm) and were measured by densitometry (bar graph). The same results were obtained in three independent experiments (not shown). (E, F): Treatment of NB cells with TC-7020 inhibited NB proliferation. (E): Cells were incubated with 10 μM BrdU for 2 hours before the end of treatment and immunostained for αBrdU. The TC-7020-induced decrease in the number BrdU+ cells is compared with the inhibition of cell proliferation by 1 μM RA. (F): Cells were stained for Ki67 Ab (red), and nuclei were counterstained with DAPI (blue). A time-dependent decrease in the number of Ki67+ cells was observed in TC-7020-treated cells. The experiments were repeated three times with the same results (not shown). Magnification, ×20. (G): Treatment of NB cells with TC-7020 increased expression of neuronal differentiation-associated proteins. Total cell lysates were electrophoresed and immunoblotted with Ab against DBX, βIII-Tubulin, TH, or the housekeeping GAPDH (loading control). Bands were densitometrically scanned, and the contents of neuronal proteins were expressed relative to GAPDH. TC-7020 induced time-dependent increases in the expression of DBX, TH, and βIII-Tubulin. Abbreviations: BrdU, 5-bromo-2′-deoxyuridine; cyt, cytoplasmic fraction; DAPI, 4′,6-diamidino-2-phenylindole; DBX, doublecortin; FGFR1, fibroblast growth factor receptor 1; nuc, nuclear fraction; PoR, Ponceau Red; RA, all-trans retinoic acid; TH, tyrosine hydroxylase.
Our second model, human NB cells, has been used to study neuronal differentiation [47–50] typically induced by retinoic acid (RA). Also, the fast-growing NB cultures provide ample material for biochemical analyses. NB cultures treated with TC-7020 (1 μM) for 12 or 24 hours showed a marked increase of nuclear FGFR1-IR (Fig. 5C), similar to that in hNPCs. Western blots confirmed this increase (Fig. 5D) and showed that nuclear FGFR1 remained elevated during 4- or 7-day TC-7020 treatment; this elevation was accompanied by a depletion of cytoplasmic FGFR1 (Fig. 5D).
To assess the effect of TC-7020 on cell proliferation, NB cultures were incubated with BrdU for 2 hours before harvesting. Although no difference in the number of BrdU-labeled control and TC-7020-treated cells was observed after 12 or 24 hours, by 48 hours a statistically significant reduction was seen in TC-7020-treated cultures (Fig. 5E). The effects of TC-7020 were delayed compared with RA inhibition of NB proliferation. The number of mitotically active cells was also evaluated by Ki67 immunostaining. As observed in vivo, TC-7020 treatment reduced the number of Ki67-expressing cells (Fig. 5F). To ascertain whether TC-7020-treated cells undergo differentiation we analyzed the expression of neuronal proteins. Western blots showed increases in the neuroblast marker doublecortin, immature neuronal βIII-Tubulin, and catecholaminergic neuronal TH in cells treated with TC-7020 for 12 hours and further upregulation during 48 hours of treatment (Fig. 5G). Thus, direct treatment of undifferentiated cells withTC-7020 stimulates INFS accompanied by a reduction in cell proliferation and increased expression of neuronal proteins.
TC-7020 Promotes Binding of FGFR1 and Activation of the βIII-Tubulin Gene
RT qPCR analysis of NB cells treated with TC-7020 for 48 hours revealed a threefold increase in βIII-Tubulin mRNA and a 1.5-fold increase of doublecortin mRNA (Fig. 6A). mRNA increases were not affected by transfection of control β-galactosidase-expressing plasmid but were abolished by dominant-negative FGFR1(TK−) with deleted tyrosine kinase domain (Fig. 6B). In contrast, transfection of full length constitutive nuclear FGFR1(SP−/NLS) [15, 22, 51] increased mRNA levels of doublecortin and βIII-Tubulin without TC-7020 treatment. Thus, gene activation by TC-7020 requires FGFR1 signaling, and nuclear FGFR1 is sufficient to activate doublecortin and βIII-Tubulin neuronal genes.
Figure 6.
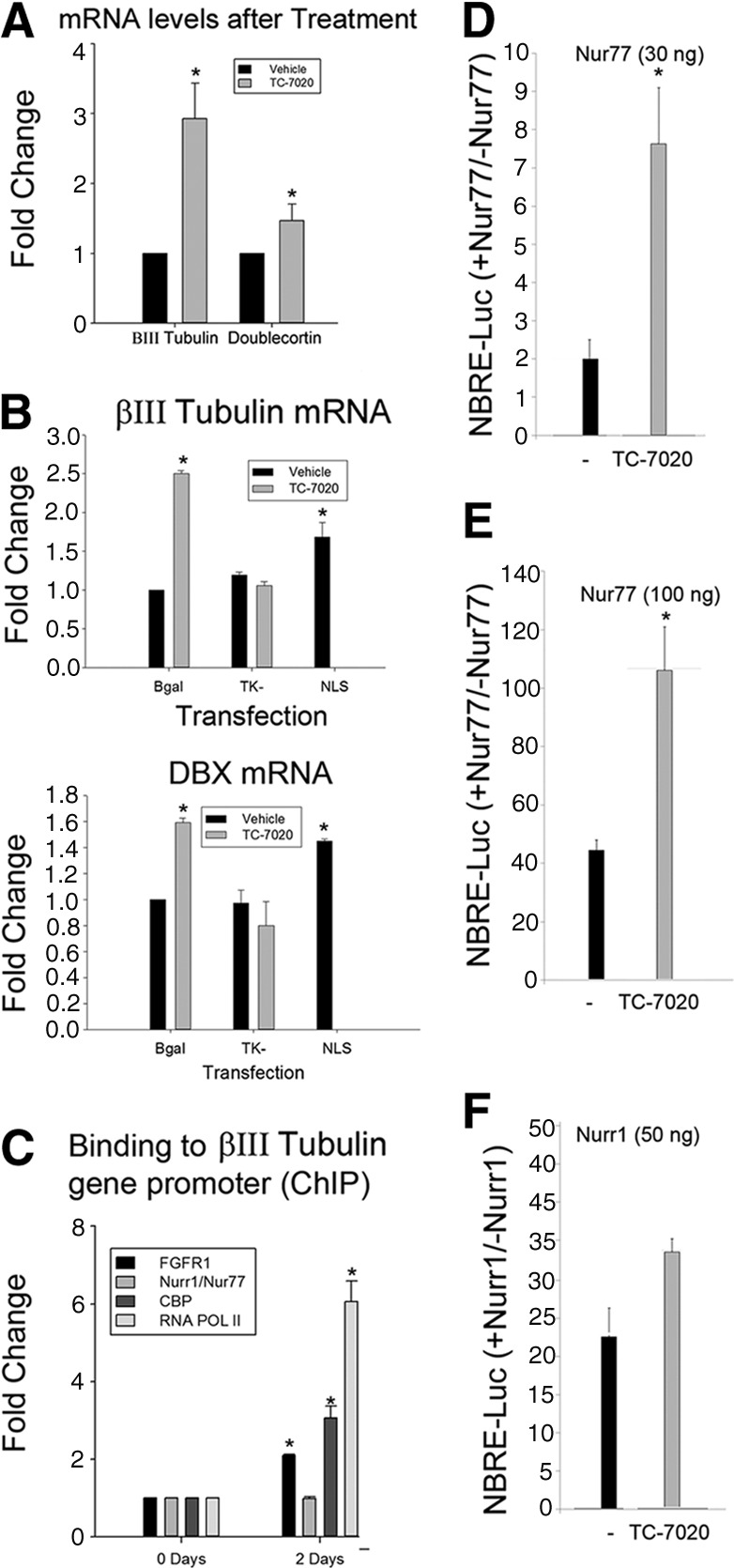
TC-7020 activation of the βIII-Tubulin gene is accompanied by FGFR1 binding to the βIII-Tubulin gene promoter in human neuroblastoma (NB) cells. (A): Real-time quantitative polymerase chain reaction analysis revealed a threefold increase in βIII-Tubulin mRNA and a 1.5-fold increase in DBX mRNA after 48 hours of treatment with 1 μM TC-7020. (B): Cultures were transfected with dominant-negative FGFR1(TK−), constitutive active nuclear FGFR1(SP−/nuclear localization signal [NLS]), or control β-galactosidase. Twenty-four hours later, some of the transfected cultures were treated with 1 μM TC-7020 for an additional 48 hours. TC-7020 upregulation of βIII-Tubulin and DBX mRNA was blocked by dominant-negative FGFR1(TK−). Transfection of constitutive active nuclear FGFR1(SP−/NLS) upregulated βIII-Tubulin and DBX mRNA. (C): Chromatin immunoprecipitation assay of the NBRE-containing βIII-Tubulin promoter region with antibodies against FGFR1, CBP, Nurr1/Nur77, RNA Pol II, or control IgG. Bars indicate specific binding above control IgG. (D, E): TC-7020 augments Nur77-dependent transcription of the NBRE-luciferase in NB cells. The NBRE-luciferase reporter containing three NBREs fused to the TATA box was cotransfected with the indicated amounts of Nur77 or Nurr1 or with control β-galactosidase expressing vector and with Renilla reference plasmid. Luciferase activity was measured in reference to Renilla activity. Results are shown as the ratio of NBRE-Luciferase activity in the presence and absence of Nur77. TC-7020 had no significant effect on promoter activity in the absence of Nur77 or Nurr1 (not shown). *, p > .001, TC-7020 versus control condition. Abbreviations: CBP, CREB binding protein; ChIP, chromatin immunoprecipitation; DBX, doublecortin; FGFR1, fibroblast growth factor receptor 1; NBRE, nerve growth factor inducible-B protein response element.
In mESCs, FGFR1 complexes with nuclear receptors Nur77, Nurr1, and RXR/RAR and is involved in the activation of diverse neurogenic and neuronal genes [17]. We used ChIP-qPCR to analyze the binding of FGFR1 and its partners, including the gene activation marker CBP, to an NBRE-containing proximal region of the βIII-Tubulin promoter. Constitutive binding of Nur77 to this promoter region was unaffected by TC-7020. In contrast, 48-hour treatment of NB cells with TC-7020 increased binding of FGFR1 as well CBP and RNA Pol II, indicative of increased transcription (Fig. 6C).
To determine whether TC-7020 can influence the transcriptional activity of Nur77, we used a luciferase gene reporter containing an isolated NBRE. TC-7020 augmented severalfold the Nur77-dependent activation of the NBRE (Fig. 6D 6F), an effect that was similar to Nur77 activation by transfected constitutive nuclear FGFR1(SP−/NLS) [17]. Also, TC-7020 activation of Nur77 appeared stronger than the activation of Nurr1 (Fig. 6E). Thus, TC-7020 upregulation of βIII-Tubulin may involve the neurogenic FGFR1-Nur77 signaling.
Discussion
The principal finding of the present study is that specifically targeting α7nAChRs reactivates the developmental INFS module along with the postmitotic neuronal development in adult brain SVZ and hippocampus. The generation of new neurons was also observed in the brain cortex and SNc, where little neurogenesis occurs in the mature brain.
Evidence that the α7nAChR activates INFS and promotes neuronal differentiation derives from the experimental observations of an upregulation of nuclear FGFR1 and the mitotic arrest of SVZ cells in vivo and NB cells in vitro, accompanied by the expression of neuronal and lack of glial marker proteins. α7AChR treatment, similar to transfection of 23-kDa FGF2 or FGFR1(SP−/NLS) [14, 44], halts cellular proliferation in a region of the SVZ as indicated by depletion of Ki67 immunoreactive cells. The survival of nonproliferating cells was documented in BrdU prelabeling experiments. Following 11 days of α7nAChR agonist administration we observed an increase in BrdU-positive cells in neurogenic SVZ and dentate gyrus regions, as well as the brain cortex and midbrain SNc. Since Ki67 immunostaining showed a lack of local cell generation in the cortex or midbrain SNc, the increased appearance of BrdU-prelabeled cells in these non-neurogenic regions may reflect the migration of new cells that were generated elsewhere, that is, in the SVZ and SGZ. It is important to note that some quiescent progenitor cells might exist outside the SVZ or SGZ and give rise to astrocytes, oligodendrocytes, and possibly neurons [52, 53].
The stimulation of neuronal differentiation by TC-7020 is indicated by the robust increase in double-labeled βIII-Tubulin+/BrdU+ cells in cortex or Nurr1+/BrdU+ cells in the SNc. BrdU labeling has the potential disadvantage that at high doses it can lead to toxicity affecting cell proliferation and postmitotic development. However, in our earlier investigation we established that the dosages of BrdU used in our experiments affected neither cell proliferation nor cell differentiation [16].
Previous studies showed that the activation of INFS via transfection of nuclear FGFR1(SP−/NLS) or its 23-kDa FGF-2 ligand causes SVZ cells to exit the cell cycle and turn off expression of stem cell-associated nestin, followed by an induction of neuroblast-specific doublecortin and neuronal βIII-Tubulin but not GFAP [14, 16]. In the present study, the absence of GFAP-expressing cells among the population of BrdU prelabeled cells demonstrated that TC-7020-induced cells in SVZ NS/PCs, hippocampus, cortex, or SNc progress toward the neuronal phenotype and not toward the astrocytic lineage. Also, the present experiments using Olig2 marker do not support significant oligodendrocytic differentiation of BrdU-prelabeled newly generated cells. These findings are consistent with the previously established INFS stimulation of neuronal development and the lack of astrocytic development stimulation in mature brain [16]. However, only 30% of BrdU-positive cells expressed immature neuronal protein βIII-Tubulin, indicating the neuronal-specific fate. Our results are in agreement with studies in which the phenotype of newly generated BrdU+ brain cells was analyzed (reviewed in [3, 54]). When brain-derived neurotrophic factor was used to stimulate neurogenesis in the adult brain, 2 weeks after BrdU labeling a significant portion (20%–30%) of BrdU+ colocalized with early neuronal βIII-Tubulin, whereas only a small percentage colocalized with GFAP and the percentage of BrdU+ oligodendrocytes was negligible [55]. Also, in the RMS/olfactory neuronogenic pathway, less than 50% of BrdU-labeled cells expressed neuronal or glial markers [56]. In the substantia nigra (SN) approximately 20% of BrdU+ cells were identified as differentiated [53]. Similar observations were made during hormone-induced neurogenesis in sexually dimorphic brain regions [57]. Consistent with these and other studies [3, 54], our results showed that a substantial number of BrdU+ cells expressed neither neuronal nor glial maker proteins. Cells that do not express neuronal or glial markers were proposed to represent neural stem cells and immature neuroblasts or glioblasts [56]. Lugert et al. [58] followed the progeny of newborn mouse brain cells at different time points after BrdU administration. After 5 days' chase most BrdU+ cells had become immature (doublecortin [DBX]+) neuroblasts (50%–60%), and after 30 days' chase the number of neuroblasts decreased and the number of mature NeuN neurons increased. In our earlier studies we analyzed gradual neurogenesis induced by an activation of INFS using in vivo transfection of SVZ cells [16]. The induction of neurogenesis coincided with reduction of nestin- and GFAP-expressing cells. Two and 3 weeks after BrdU pulse labeling we established the presence of DBX-positive neuroblasts in periventricular brain regions, including the striatum, septum, and brain cortex; the number of neuroblasts was markedly increased in mice transfected with nuclear FGFR1 or its ligand. These changes were accompanied by the appearance of fewer, more mature βIII-Tubulin+, TH+, and Neurofilament L+ cells, demonstrating differentiation along the neuronal lineage pathway. In vitro activation of the INFS mechanism resulted in a gradual morphological neuronal development and expression of neuroblast DBX and mature neuronal TH protein [16]. Taken together these results show a variable extent of neuronal differentiation of BrdU-labeled brain cells. Cells that do not colocalize BrdU with βIII-Tubulin, GFAP, or Olig2 may be slated for later differentiation, as shown previously [58]. Pencea et al. [55] found that in brain regions in which 20%–30% of BrdU+ cells expressed βIII-Tubulin, a small percentage of newly generated cells express mitogen-activated protein-2, a marker of mature neurons, suggesting that the majority of the newly generated neurons have not sufficiently matured [55]. In the present study, we report similar findings in the SN, where 30% of BrdU+ cells colocalized with predopaminergic Nurr1, but only single cells displayed a TH+ mature phenotype. Alternatively, the immature cells may be slated for apoptosis [59] or remain undifferentiated and support local neuronal function as proposed by Ahmed et al. [57]. These possibilities will be addressed in future studies on the long-term effects of α7nAChR agonists or INFS targeting gene therapy.
The rate of neurogenesis can be increased by mitogenic factors that stimulate cell proliferation, including extracellular FGFs (reviewed in [5]). In addition, accelerated neuronal differentiation of the progenitor cells and survival of their neuronal progeny may act to hasten generation of new neurons [16]. Indeed, the present results demonstrate that although the proliferation of the SVZ cells is reduced by TC-7020, the increase in neuronal differentiation creates a net increase in neurogenesis. These new observations are in agreement with our earlier finding that transfections of the nuclear form of FGFR1 or FGF-2 depleted the proliferating nestin-positive NS/PCs, whereas the expression of doublecortin-positive cells was markedly increased [16]. Such a mechanism is consistent with the evidence that under control conditions, the differentiation rate of the NS/PCs is low because of the combination of infrequent conversions into neuroblasts, along with the natural developmental cell death of a portion of the neuroblasts [60]. Hence, we conclude that the generation of neurons may be effectively regulated at the postmitotic stage. Increases in neurogenesis could potentially be mediated by improving the rate of cell survival. Although this could contribute to the neurogenic effects of TC-7020, in several neuronal cell models, including the NB and hNPCs used here, TC-7020 and INFS [8, 61] can stimulate the appearance of differentiating neuronal cells. Given that they both can activate expression of genes associated with differentiation, we propose that TC-7020 stimulates neuronal differentiation in vivo.
In the intact mammalian brain, active neurogenesis is restricted to the SGZ of the hippocampus and the SVZ-olfactory bulb axis [62]. Recent evidence, however, also demonstrates that neurogenesis in the cortex and striatum may be induced under some pathologic conditions and is associated with the occurrence of doublecortin-expressing neuroblasts [63, 64]. Thus, the TC-7020 induction of new neurons that extends to the brain cortex, hippocampus, or SNc observed in the present study re-emphasizes the possibility of latent neurogenesis in these brain regions. Activation of cortical neurogenesis by TC-7020 raises hope for new treatments of cortical injuries, stroke, and neurodegeneration in Alzheimer's and Huntington's diseases. Similarly, TC-7020 activation of hippocampal neurogenesis could be applicable to treatments of dementias resulting from the loss of hippocampal neurons.
An important advance made by the present study is the demonstration that the α7AChR agonist can stimulate neurogenesis in adult SNc. TC-7020 increased more than 10-fold the production of SNc cells, which appear to enter the dopamine (DA) differentiation pathway as indicated by their expression of Nurr1. Nurr1 is a transcription factor, along with nuclear FGFR1, which drives the development of DA neurons [19, 20, 46] and directly activates the th gene [19]. The exact origin of new TC-7020-induced SNc neurons was not directly determined by the current study. Although the SNc neurons could arise from cells in the adjacent midbrain aqueduct, studies have suggested that substantia nigra also contains quiescent progenitors, which infrequently enter the mitotic cycle [52, 53]. Such cells could also potentially generate new neurons in the adult SNc.
Direct α7nAChR Activation of Proliferating Progenitors or Action Through Neuronal Networks?
The α7nAChRs are expressed in several brain regions and are found on both mature neurons and immature developing cells. By promoting calcium influx, α7nAChRs can modulate the release of diverse neurotransmitters, including GABA, which has been shown to play an important role in regulation of progenitor cells in the hippocampus [65–69]. A similar system of GABAergic innervation may play a role in SVZ progenitor populations. There is evidence that tonic GABA produced by neuroblasts acts as negative feedback for proliferation [70, 71]; however, it is unclear whether GABA plays a role in differentiation of the SVZ and SGZ progenitors. The direct neurogenic action of α7nAChR agonists on NS/PCs is indicated by our cell culture experiments. The effects of systemic TC-7020 are reproduced by direct treatment of cultured hNPCs and NB cells, both of which can differentiate to neurons [72–74]. When treated with TC-7020 these cells expressed nuclear FGFR1, indicating that TC-7020 can activate INFS by direct action on the NS/PC-like cells. Nuclear accumulation of FGFR1 was accompanied by an acquisition of neuronal morphology, with neurofilament-expressing neurites in hNPCs and an exit from the cell cycle and upregulation neuronal proteins in NB.
Mechanism of Neurogenic Action of α7nAChR: The Role of INFS
α7nAChRs are calcium-permeable ion channels, and their activation raises intracellular calcium levels and activates various calcium-dependent pathways, including protein kinase A, protein kinase C, inositol triphosphate kinase, and MEK-extracellular signal-regulated kinase (ERK) pathways [75]. Pathways that use PKA, PKC, and ERK have been shown to promote neuronal differentiation [31, 76–78]. The INFS has been shown to serve as a common mechanism activated by these and other neurogenic signals, which propagates neuronal differentiation and associated gene activation via a feed-forward-end-gate mechanism [3, 8]. The further pieces of evidence that INFS mediates TC-7020 activation of neurogenesis are severalfold: (a) administration of TC-7020 resulted in a marked increase in the nuclear FGFR1 content, which in the brain occurred in developing βIII-Tubulin expressing neurons and in vitro in differentiating hNPCs and NB cells; (b) neurogenic effects of TC-7020 observed in the present study are reproduced by transfection of a nuclear form of FGFR1(SP−/NLS) or its ligand (FGF-2) into the SVZ in vivo [18, 44] and in hNPCs, mESCs, and neuroblastoma lines in vitro [6, 15, 17]; (c) effects of TC-7020 are diminished by transfection of dominant-negative FGFR1(TK−); (d) TC-7020 increased FGFR1 binding to an NBRE-like promoter region of the active βIII-Tubulin gene and activated Nur77-dependent gene transcription severalfold. The nuclear receptor complexes of FGFR1/Nur77 and FGFR1/Nurr1 were recently shown to activate diverse neurogenic and neuronal genes in embryonic stem cells and brain dopamine neurons [17, 19]. Thus, activation of nuclear Nur receptors may constitute one molecular mechanism for TC-7020-induced neurogenesis.
Conclusion
Stem cell therapies may represent the first true disease-modifying treatments for diverse neurological diseases and injuries such as Alzheimer's disease, Parkinson's disease, multiple sclerosis, Huntington's disease, amyotrophic lateral sclerosis, spinal cord injury, and stroke. However, many hurdles remain, including safety and a variety of host responses that could hinder efficacy. Stimulation of endogenous neurogenesis in the adult brain by the α7nAChR agonist TC-7020 presents an intriguing opportunity to treat neuronal losses associated with many pathological conditions. Reactivation of neurogenesis in the adult brain by targeting the INFS with α7AChR agonist may represent an important step toward these therapeutic goals.
Supplementary Material
Acknowledgments
The authors gratefully acknowledge the generous gift of NBRE3-Luc and Nur77-expressing pCMX plasmids from Dr. Jacques Drouin (Institut de Recherches Cliniques de Montréal), and Nurr1-expressing plasmid from Drs. Andreas Ratzka and Peter Claus (Hannover Medical School, Hannover, Germany). We thank Christopher Terranova and Dr. John Aletta for critical reviewing the manuscript. This work was supported by NYSTEM contracts C026415 and C026714 (to M.K.S.). TC-7020 was provided as a gift by Targacept Inc.
Author Contributions
N.S.S.: design, collection and assembly of data, manuscript writing; I.K.: collection and assembly of data; B.B.: design, assembly of data; Y.-W.L.: collection and assembly of data; J.M.: collection and data analysis; E.K.S.: design, collection and assembly of data; D.P.: collection of data; M.B.: data analysis and interpretation, manuscript writing; M.K.S.: design, data analysis, manuscript writing.
Disclosure of Potential Conflicts of Interest
M.B. has compensated employment and stock options from Targacept and uncompensated intellectual property rights.
References
- 1.Gates MA, Thomas LB, Howard EM, et al. Cell and molecular analysis of the developing and adult mouse subventricular zone of the cerebral hemispheres. J Comp Neurol. 1995;361:249–266. doi: 10.1002/cne.903610205. [DOI] [PubMed] [Google Scholar]
- 2.Kuhn HG, Winkler J, Kempermann G, et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J Neurosci. 1997;17:5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sohur US, Emsley JG, Mitchell BD, et al. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos Trans R Soc Lond B Biol Sci. 2006;361:1477–1497. doi: 10.1098/rstb.2006.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stachowiak MK, Stachowiak EK, Aletta JM, et al. A common integrative nuclear signaling module for stem cell development. In: Stachowiak MK, Tzanakakis ES, editors. Stem Cells: From Mechanisms to Technologies. Hackensack, NJ: World Scientific Publishing; 2011. pp. 87–132. [Google Scholar]
- 5.Guillemot F. Cellular and molecular control of neurogenesis in the mammalian telencephalon. Curr Opin Cell Biol. 2005;17:639–647. doi: 10.1016/j.ceb.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Fang X, Stachowiak EK, Dunham-Ems SM, et al. Control of CREB-binding protein signaling by nuclear fibroblast growth factor receptor-1: A novel mechanism of gene regulation. J Biol Chem. 2005;280:28451–28462. doi: 10.1074/jbc.M504400200. [DOI] [PubMed] [Google Scholar]
- 7.Stachowiak MK, Fang X, Myers JM, et al. Integrative nuclear FGFR1 signaling (INFS) as a part of a universal “feed-forward-and-gate” signaling module that controls cell growth and differentiation. J Cell Biochem. 2003;90:662–691. doi: 10.1002/jcb.10606. [DOI] [PubMed] [Google Scholar]
- 8.Stachowiak MK, Maher PA, Stachowiak EK. Integrative nuclear signaling in cell development: A role for FGF receptor-1. DNA Cell Biol. 2007;26:811–826. doi: 10.1089/dna.2007.0664. [DOI] [PubMed] [Google Scholar]
- 9.Kim EL, Esparza FM, Stachowiak MK. The roles of CRE, TRE, and TRE-adjacent S1 nuclease sensitive element in the regulation of tyrosine hydroxylase gene promoter activity by angiotensin II. J Neurochem. 1996;67:26–36. doi: 10.1046/j.1471-4159.1996.67010026.x. [DOI] [PubMed] [Google Scholar]
- 10.Stachowiak MK, Maher PA, Joy A, et al. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol Biol Cell. 1996;7:1299–1317. doi: 10.1091/mbc.7.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunham-Ems SM, Lee YW, Stachowiak EK, et al. Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control. Mol Biol Cell. 2009;20:2401–2412. doi: 10.1091/mbc.E08-06-0600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers JM, Martins GG, Ostrowski J, et al. Nuclear trafficking of FGFR1: A role for the transmembrane domain. J Cell Biochem. 2003;88:1273–1291. doi: 10.1002/jcb.10476. [DOI] [PubMed] [Google Scholar]
- 13.Reilly JF, Maher PA. Importin beta-mediated nuclear import of fibroblast growth factor receptor: Role in cell proliferation. J Cell Biol. 2001;152:1307–1312. doi: 10.1083/jcb.152.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bharali DJ, Klejbor I, Stachowiak EK, et al. Organically modified silica nanoparticles: A nonviral vector for in vivo gene delivery and expression in the brain. Proc Natl Acad Sci USA. 2005;102:11539–11544. doi: 10.1073/pnas.0504926102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stachowiak EK, Fang X, Myers J, et al. cAMP-induced differentiation of human neuronal progenitor cells is mediated by nuclear fibroblast growth factor receptor-1 (FGFR1) J Neurochem. 2003;84:1296–1312. doi: 10.1046/j.1471-4159.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 16.Stachowiak EK, Roy I, Lee Y-W, et al. Targeting novel integrative nuclear FGFR1 signaling by nanoparticle-mediated gene transfer stimulates neurogenesis in adult brain. Integr Biol (Camb) 2009;1:394–403. doi: 10.1039/b902617g. [DOI] [PubMed] [Google Scholar]
- 17.Lee YW, Terranova C, Birkaya B, et al. A novel nuclear FGF Receptor-1 partnership with retinoid and Nur receptors during developmental gene programming of embryonic stem cells. J Cell Biochem. 2012;113:2920–2936. doi: 10.1002/jcb.24170. [DOI] [PubMed] [Google Scholar]
- 18.Stachowiak EK, Roy I, Stachowiak MK. Triggering neuronogenesis by endogenous brain stem cells with DNA nanoplexes. In: Stachowiak MK, Tzanakakis ES, editors. Stem Cells: From Mechanisms to Technologies. Hackensack, NJ: World Scientific Publishing; 2011. pp. 333–359. [Google Scholar]
- 19.Baron O, Förthmann B, Lee YW, et al. Cooperation of nuclear FGFR1 and Nurr1 offers a new interactive mechanism in postmitotic development of mesencephalic dopaminergic neurons. J Biol Chem. 2012;287:19827–19840. doi: 10.1074/jbc.M112.347831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klejbor I, Myers JM, Hausknecht K, et al. Fibroblast growth factor receptor signaling affects development and function of dopamine neurons: Inhibition results in a schizophrenia-like syndrome in transgenic mice. J Neurochem. 2006;97:1243–1258. doi: 10.1111/j.1471-4159.2006.03754.x. [DOI] [PubMed] [Google Scholar]
- 21.Klejbor I, Kucinski A, Wersinger SR, et al. Serotonergic hyperinnervation and effective serotonin blockade in an FGF receptor developmental model of psychosis. Schizophr Res. 2009;113:308–321. doi: 10.1016/j.schres.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng H, Moffett J, Myers J, et al. Novel nuclear signaling pathway mediates activation of fibroblast growth factor-2 gene by type 1 and type 2 angiotensin II receptors. Mol Biol Cell. 2001;12:449–462. doi: 10.1091/mbc.12.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peng H, Myers J, Fang X, et al. Integrative nuclear FGFR1 signaling (INFS) pathway mediates activation of the tyrosine hydroxylase gene by angiotensin II, depolarization and protein kinase C. J Neurochem. 2002;81:506–524. doi: 10.1046/j.1471-4159.2002.00833.x. [DOI] [PubMed] [Google Scholar]
- 24.Resende RR, Adhikari A. Cholinergic receptor pathways involved in apoptosis, cell proliferation and neuronal differentiation. Cell Commun Signal. 2009;7:20. doi: 10.1186/1478-811X-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Broide RS, O'Connor LT, Smith MA, et al. Developmental expression of alpha 7 neuronal nicotinic receptor messenger RNA in rat sensory cortex and thalamus. Neuroscience. 1995;67:83–94. doi: 10.1016/0306-4522(94)00623-d. [DOI] [PubMed] [Google Scholar]
- 26.Falk L, Nordberg A, Seiger A, et al. Higher expression of alpha7 nicotinic acetylcholine receptors in human fetal compared to adult brain. Brain Res Dev Brain Res. 2003;142:151–160. doi: 10.1016/s0165-3806(03)00063-4. [DOI] [PubMed] [Google Scholar]
- 27.Quik M, Polonskaya Y, Gillespie A, et al. Localization of nicotinic receptor subunit mRNAs in monkey brain by in situ hybridization. J Comp Neurol. 2000;425:58–69. doi: 10.1002/1096-9861(20000911)425:1<58::aid-cne6>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 28.Adams CE, Broide RS, Chen Y, et al. Development of the alpha7 nicotinic cholinergic receptor in rat hippocampal formation. Brain Res Dev Brain Res. 2002;139:175–187. doi: 10.1016/s0165-3806(02)00547-3. [DOI] [PubMed] [Google Scholar]
- 29.Quik M, Chan J, Patrick J. alpha-Bungarotoxin blocks the nicotinic receptor mediated increase in cell number in a neuroendocrine cell line. Brain Res. 1994;655:161–167. doi: 10.1016/0006-8993(94)91610-1. [DOI] [PubMed] [Google Scholar]
- 30.Coronas V, Durand M, Chabot JG, et al. Acetylcholine induces neuritic outgrowth in rat primary olfactory bulb cultures. Neuroscience. 2000;98:213–219. doi: 10.1016/s0306-4522(00)00143-3. [DOI] [PubMed] [Google Scholar]
- 31.Cui W, Cui GZ, Li W, et al. Bis(12)-hupyridone, a novel multifunctional dimer, promotes neuronal differentiation more potently than its monomeric natural analog huperzine A possibly through alpha7 nAChR. Brain Res. 2011;1401:10–17. doi: 10.1016/j.brainres.2011.05.042. [DOI] [PubMed] [Google Scholar]
- 32.Campbell NR, Fernandes CC, Halff AW, et al. Endogenous signaling through alpha7-containing nicotinic receptors promotes maturation and integration of adult-born neurons in the hippocampus. J Neurosci. 2010;30:8734–8744. doi: 10.1523/JNEUROSCI.0931-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paraoanu LE, Steinert G, Koehler A, et al. Expression and possible functions of the cholinergic system in a murine embryonic stem cell line. Life Sci. 2007;80:2375–2379. doi: 10.1016/j.lfs.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Landgraf D, Barth M, Layer PG, et al. Acetylcholine as a possible signaling molecule in embryonic stem cells: Studies on survival, proliferation and death. Chem Biol Interact. 2010;187:115–119. doi: 10.1016/j.cbi.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Lin W, Hirata N, Sekino Y, et al. Role of α7-nicotinic acetylcholine receptor in normal and cancer stem cells. Curr Drug Targets. 2012;13:656–665. doi: 10.2174/1389450111209050656. [DOI] [PubMed] [Google Scholar]
- 36.Marrero MB, Lucas R, Salet C, et al. An alpha7 nicotinic acetylcholine receptor-selective agonist reduces weight gain and metabolic changes in a mouse model of diabetes. J Pharmacol Exp Ther. 2010;332:173–180. doi: 10.1124/jpet.109.154633. [DOI] [PubMed] [Google Scholar]
- 37.Maira M, Martens C, Batsche E, et al. Dimer-specific potentiation of NGFI-B (Nur77) transcriptional activity by the protein kinase A pathway and AF-1-dependent coactivator recruitment. Mol Cell Biol. 2003;23:763–776. doi: 10.1128/MCB.23.3.763-776.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maira M, Martens C, Philips A, et al. Heterodimerization between members of the Nur subfamily of orphan nuclear receptors as a novel mechanism for gene activation. Mol Cell Biol. 1999;19:7549–7557. doi: 10.1128/mcb.19.11.7549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ke L, Eisenhour CM, Bencherif M, et al. Effects of chronic nicotine treatment on expression of diverse nicotinic acetylcholine receptor subtypes. I. Dose- and time-dependent effects of nicotine treatment. J Pharmacol Exp Ther. 1998;286:825–840. [PubMed] [Google Scholar]
- 40.Stachowiak EK, Maher PA, Tucholski J, et al. Nuclear accumulation of fibroblast growth factor receptors in human glial cells: Association with cell proliferation. Oncogene. 1997;14:2201–2211. doi: 10.1038/sj.onc.1201057. [DOI] [PubMed] [Google Scholar]
- 41.Dunham-Ems SM, Pudavar HE, Myers JM, et al. Factors controlling fibroblast growth factor receptor-1's cytoplasmic trafficking and its regulation as revealed by FRAP analysis. Mol Biol Cell. 2006;17:2223–2235. doi: 10.1091/mbc.E05-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Somanathan S, Stachowiak EK, Siegel AJ, et al. Nuclear matrix bound fibroblast growth factor receptor is associated with splicing factor rich and transcriptionally active nuclear speckles. J Cell Biochem. 2003;90:856–869. doi: 10.1002/jcb.10672. [DOI] [PubMed] [Google Scholar]
- 43.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stachowiak EK, Roy I, Lee YW, et al. Targeting novel integrative nuclear FGFR1 signaling by nanoparticle-mediated gene transfer stimulates neurogenesis in the adult brain. Integr Biol (Camb) 2009;1:394–403. doi: 10.1039/b902617g. [DOI] [PubMed] [Google Scholar]
- 45.Canavese G, Azzoni C, Pizzi S, et al. p27: A potential main inhibitor of cell proliferation in digestive endocrine tumors but not a marker of benign behavior. Hum Pathol. 2001;32:1094–1101. doi: 10.1053/hupa.2001.28234. [DOI] [PubMed] [Google Scholar]
- 46.Jankovic J, Chen S, Le WD. The role of Nurr1 in the development of dopaminergic neurons and Parkinson's disease. Progr Neurobiol. 2005;77:128–138. doi: 10.1016/j.pneurobio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 47.Abemayor E, Sidell N. Human neuroblastoma cell lines as models for the in vitro study of neoplastic and neuronal cell differentiation. Environ Health Perspect. 1989;80:3–15. doi: 10.1289/ehp.89803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ghigo D, Priotto C, Migliorino D, et al. Retinoic acid-induced differentiation in a human neuroblastoma cell line is associated with an increase in nitric oxide synthesis. J Cell Physiol. 1998;174:99–106. doi: 10.1002/(SICI)1097-4652(199801)174:1<99::AID-JCP11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 49.Lee JH, Kim KT. Induction of cyclin-dependent kinase 5 and its activator p35 through the extracellular-signal-regulated kinase and protein kinase A pathways during retinoic-acid mediated neuronal differentiation in human neuroblastoma SK-N-BE(2)C cells. J Neurochem. 2004;91:634–647. doi: 10.1111/j.1471-4159.2004.02770.x. [DOI] [PubMed] [Google Scholar]
- 50.Silvagno F, Guarnieri V, Capizzi A, et al. Synergistic effect of retinoic acid and dehydroepiandrosterone on differentiation of human neuroblastoma cells. FEBS Lett. 2002;532:153–158. doi: 10.1016/s0014-5793(02)03667-0. [DOI] [PubMed] [Google Scholar]
- 51.Hu Y, Fang X, Dunham SM, et al. 90-kDa ribosomal S6 kinase is a direct target for the nuclear fibroblast growth factor receptor 1 (FGFR1): Role in FGFR1 signaling. J Biol Chem. 2004;279:29325–29335. doi: 10.1074/jbc.M311144200. [DOI] [PubMed] [Google Scholar]
- 52.Leung CT, Coulombe PA, Reed RR. Contribution of olfactory neural stem cells to tissue maintenance and regeneration. Nat Neurosci. 2007;10:720–726. doi: 10.1038/nn1882. [DOI] [PubMed] [Google Scholar]
- 53.Lie DC, Dziewczapolski G, Willhoite AR, et al. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riddle DR, Lichtenwalner RJ. Neurogenesis in the adult and aging brain. In: Riddle DR, editor. Brain Aging: Models, Methods, and Mechanisms. Frontiers in Neuroscience. Boca Raton, FL: CRC Press; 2007. [Google Scholar]
- 55.Pencea V, Bingaman KD, Wiegand SJ, et al. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fukushima N, Yokouchi K, Kawagishi K, et al. Differential neurogenesis and gliogenesis by local and migrating neural stem cells in the olfactory bulb. Neurosci Res. 2002;44:467–473. doi: 10.1016/s0168-0102(02)00173-6. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed EI, Zehr JL, Schulz KM, et al. Pubertal hormones modulate the addition of new cells to sexually dimorphic brain regions. Nat Neurosci. 2008;11:995–997. doi: 10.1038/nn.2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lugert S, Basak O, Knuckles P, et al. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell. 2010;6:445–456. doi: 10.1016/j.stem.2010.03.017. [DOI] [PubMed] [Google Scholar]
- 59.Cui L, Qu H, Xiao T, et al. Stromal cell-derived factor-1 and its receptor CXCR4 in adult neurogenesis after cerebral ischemia. Restor Neurol Neurosci. 2013;31:239–251. doi: 10.3233/RNN-120271. [DOI] [PubMed] [Google Scholar]
- 60.Biebl M, Cooper CM, Winkler J, et al. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- 61.Chmurzynska A, Stachowiak M, Pruszynska-Oszmalek E. Maternal protein and folic acid intake during gestation does not program leptin transcription or serum concentration in rat progeny. Genes Nutr. 2012;7:217–222. doi: 10.1007/s12263-011-0239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- 63.Arvidsson A, Collin T, Kirik D, et al. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 64.Magavi SS, Leavitt BR, Macklis JD. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 65.Li G, Bien-Ly N, Andrews-Zwilling Y, et al. GABAergic interneuron dysfunction impairs hippocampal neurogenesis in adult apolipoprotein E4 knockin mice. Cell Stem Cell. 2009;5:634–645. doi: 10.1016/j.stem.2009.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tozuka Y, Fukuda S, Namba T, et al. GABAergic excitation promotes neuronal differentiation in adult hippocampal progenitor cells. Neuron. 2005;47:803–815. doi: 10.1016/j.neuron.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Alkondon M, Pereira EF, Eisenberg HM, et al. Choline and selective antagonists identify two subtypes of nicotinic acetylcholine receptors that modulate GABA release from CA1 interneurons in rat hippocampal slices. J Neurosci. 1999;19:2693–2705. doi: 10.1523/JNEUROSCI.19-07-02693.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alkondon M, Albuquerque EX. Nicotinic acetylcholine receptor alpha7 and alpha4beta2 subtypes differentially control GABAergic input to CA1 neurons in rat hippocampus. J Neurophysiol. 2001;86:3043–3055. doi: 10.1152/jn.2001.86.6.3043. [DOI] [PubMed] [Google Scholar]
- 69.Kanno T, Yaguchi T, Yamamoto S, et al. 8-[2-(2-pentyl-cyclopropylmethyl)-cyclopropyl]-octanoic acid stimulates GABA release from interneurons projecting to CA1 pyramidal neurons in the rat hippocampus via pre-synaptic alpha7 acetylcholine receptors. J Neurochem. 2005;95:695–702. doi: 10.1111/j.1471-4159.2005.03398.x. [DOI] [PubMed] [Google Scholar]
- 70.Liu X, Wang Q, Haydar TF, et al. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat Neurosci. 2005;8:1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Platel JC, Dave KA, Bordey A. Control of neuroblast production and migration by converging GABA and glutamate signals in the postnatal forebrain. J Physiol. 2008;586:3739–3743. doi: 10.1113/jphysiol.2008.155325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Piper DR, Mujtaba T, Keyoung H, et al. Identification and characterization of neuronal precursors and their progeny from human fetal tissue. J Neurosci Res. 2001;66:356–368. doi: 10.1002/jnr.1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Piper DR, Mujtaba T, Rao MS, et al. Immunocytochemical and physiological characterization of a population of cultured human neural precursors. J Neurophysiol. 2000;84:534–548. doi: 10.1152/jn.2000.84.1.534. [DOI] [PubMed] [Google Scholar]
- 74.Jeong H, Kim MS, Kim SW, et al. Regulation of tyrosine hydroxylase gene expression by retinoic acid receptor. J Neurochem. 2006;98:386–394. doi: 10.1111/j.1471-4159.2006.03866.x. [DOI] [PubMed] [Google Scholar]
- 75.Ren K, Puig V, Papke RL, et al. Multiple calcium channels and kinases mediate alpha7 nicotinic receptor neuroprotection in PC12 cells. J Neurochem. 2005;94:926–933. doi: 10.1111/j.1471-4159.2005.03223.x. [DOI] [PubMed] [Google Scholar]
- 76.Mullenbrock S, Shah J, Cooper GM. Global expression analysis identified a preferentially NGF-induced transcriptional program regulated by sustained MEK/ERK and AP-1 activation during PC12 differentiation. J Biol Chem. 2011;286:45131–45145. doi: 10.1074/jbc.M111.274076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kudo TA, Kanetaka H, Mizuno K, et al. Dorsomorphin stimulates neurite outgrowth in PC12 cells via activation of a protein kinase A-dependent MEK-ERK1/2 signaling pathway. Genes Cells. 2011;16:1121–1132. doi: 10.1111/j.1365-2443.2011.01556.x. [DOI] [PubMed] [Google Scholar]
- 78.von Kriegsheim A, Baiocchi D, Birtwistle M, et al. Cell fate decisions are specified by the dynamic ERK interactome. Nat Cell Biol. 2009;11:1458–1464. doi: 10.1038/ncb1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.