Murine stromal vascular fraction (SVF) cells were compared with culture-expanded adipose-derived stem cells (ASCs) for the treatment of experimental autoimmune encephalitis (EAE) in mice. It was found that SVF cells effectively inhibited EAE disease progression more than culture-expanded ASCs did, suggesting that they might represent a valuable tool for stem cell-based therapy in chronic inflammatory disease of the central nervous system.
Keywords: Adipose, Adult stem cells, Tissue-specific stem cells, Stem cells, Neuroimmune
Abstract
Administration of adipose-derived stromal/stem cells (ASCs) represents a promising therapeutic approach for autoimmune diseases since they have been shown to have immunomodulatory properties. The uncultured, nonexpanded counterpart of ASCs, the stromal vascular fraction (SVF), is composed of a heterogeneous mixture of cells. Although administration of ex vivo culture-expanded ASCs has been used to study immunomodulatory mechanisms in multiple models of autoimmune diseases, less is known about SVF-based therapy. The ability of murine SVF cells to treat myelin oligodendrocyte glycoprotein35–55-induced experimental autoimmune encephalitis (EAE) was compared with that of culture-expanded ASCs in C57Bl/6J mice. A total of 1 × 106 SVF cells or ASCs were administered intraperitoneally concomitantly with the induction of disease. The data indicate that intraperitoneal administration of ASCs significantly ameliorated the severity of disease course. They also demonstrate, for the first time, that the SVF effectively inhibited disease severity and was statistically more effective than ASCs. Both cell therapies also demonstrated a reduction in tissue damage, a decrease in inflammatory infiltrates, and a reduction in sera levels of interferon-γ and interleukin-12. Based on these data, SVF cells effectively inhibited EAE disease progression more than culture-expanded ASCs.
Introduction
Multiple sclerosis (MS) is an autoimmune disease characterized by inflammatory demyelinating lesions, extensive mononuclear cell infiltration into the central nervous system (CNS), and loss of motor function. Despite improved understanding of the mechanisms by which MS is manifested, current treatment options for this disease (such as interferon-β-1a, interferon-1β, glatiramer acetate, fingolimod, mitoxantrone, and natalizumab) have limited efficacy in providing symptomatic relief and complete remission, especially in patients affected with the chronic form of MS [1, 2]. The application of adult stem cells as a potential treatment in MS patients has been of recent interest, especially for patients who do not respond to the pharmacologic immunosuppression regimens with steroids or corticotropin.
Adipose tissue makes up one of the largest organs in the body and serves as an important endocrine organ regulating many facets of homeostasis [3–5]. Made up of mature adipocytes and other nonadipocyte cells, adipose tissue can be manually disrupted and/or treated with collagenase to isolate the stromal vascular fraction (SVF). Although not a fully defined cell population, the SVF includes vascular smooth muscle cells, fibroblasts, mast cells, macrophages, lymphocytes, endothelial cells, preadipocytes, and adipose-derived stromal/stem cells (ASCs) [5–10]. There is an increasing interest in the biology and therapeutic potential of SVF because of the direct and rapid isolation procedure in a xenobiotic-free environment [9, 11–13].
The SVF can also be cultured in plasticware to select and expand ASCs. ASCs are a fibroblast-like adherent cell population; these cells maintain their characteristics for several passages and can be induced to differentiate into several cell types in vitro, including adipocytes, chondrocytes, osteoblasts, cardiomyocytes, smooth muscle cells, hepatic cells, endothelial cells, and neuronal-like cells [14–22]. Comparable to the better studied bone marrow stromal cells (BMSCs), ASCs have similar self-renewal abilities, common surface epitopes, growth kinetics, and cytokine expression profiles [4, 14, 23–25]. Whereas the harvesting of BMSCs has been associated with morbidity, pain, and low yield, ASCs can be obtained at a high yield (the yield of stem cells per tissue volume is 100–500-fold higher in adipose tissue than bone marrow) with minimal discomfort under local anesthesia [11, 26–28]. In addition, recent data indicate that ASCs are potently immunomodulatory, making them an appealing candidate for regenerative medicine and tissue engineering [4, 29].
Both ASCs and SVF cells have been used clinically to treat acute and chronic diseases afflicting a range of tissues and organs, including soft tissue defects, breast reconstruction, and autoimmune disease [3, 11]. Anti-inflammatory and regenerative effects of nonexpanded SVF cells have yielded promising results in canine osteoarthritis and equine tendon ligament injuries [30]. With the encouraging outcomes in clinical work, it is essential to examine the effects and mechanisms of SVF and ASC treatment in a murine model. The myelin oligodendrocyte glycoprotein (MOG)35–55-induced experimental autoimmune encephalomyelitis (EAE) in C57Bl/6J mice correlates to chronic, progressive MS and is an appropriate model to investigate the effects of treatment with ASCs or SVF [31, 32]. Although it has been reported that mesenchymal lineage stem cells from the bone marrow have a therapeutic impact in the EAE model, to date, only a single study has investigated murine ASCs, and SVF has not been assessed [33–38]. The goal of this study was to directly compare SVF cells with ASCs as interventions for disease progression in the EAE model.
Materials and Methods
Cell Isolation and Culture
Isolation of Stromal Vascular Fraction
Subcutaneous white adipose tissue was collected from male enhanced green fluorescence protein (eGFP) transgenic mice (C57Bl/6-Tg(UBC-GFP)30Scha/J strain; Jackson Laboratory, Bar Harbor, ME, http://www.jax.org), ranging in age from 2 to 6 months. Adipose tissue was washed in Hanks' balanced saline solution (HBSS; Life Technologies, Grand Island, NY, http://www.lifetech.com), minced with a scalpel, and treated with 0.1% (wt/vol) collagenase type I (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) in HBSS for 2 hours, while shaking at 160 rpm at 37°C. The collagenase was neutralized with prewarmed complete culture medium (CCM) that consisted of Dulbecco's Modified Eagle Medium:Nutrient Mixture F-12 (Life Technologies), 20% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA, http://www.atlantabio.com), 100 units/ml penicillin (Life Technologies), 100 μg/ml streptomycin (Life Technologies), and 2 mM l-glutamine (Life Technologies). Digested tissue was filtered through a 70-μm-pore cell strainer (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com) to remove debris. Cells were spun at 400g for 10 minutes, and cell numbers and viability were counted with trypan blue.
Isolation and Expansion of ASCs
The stromal vascular fraction was plated in CCM in a 75-cm2 flask (Corning Enterprises, Corning, NY, http://www.corning.com) and incubated at 37°C with 5% humidified CO2. After 24 hours, nonadherent cells were removed by washing with phosphate-buffered saline (PBS) and fresh CCM. When cells (passage 0) reached 70%–80% confluence, cells were subcultured by lifting with 0.25% trypsin/1 mM EDTA (Gibco, Grand Island, NY, http://www.invitrogen.com) and plated (passage 1) at 100 cells per cm2 in CCM on a 150-cm2 tissue culture dish (Nunc, Rochester, NY, http://www.nuncbrand.com). Medium was replaced every 3–4 days, and cultures were split when cells reached 70% confluence. For all experiments, subconfluent cells (≤70% confluent) between passage 2 and passage 6 were used.
Colony-Forming Unit Assay
ASCs were plated at 100 cells on a 10-cm2 plate in CCM and incubated for 14 days at 37°C with 5% humidified CO2. Plates were then rinsed three times with PBS, and 3 ml of a 3% crystal violet solution (Sigma-Aldrich) was added for 30 minutes at room temperature. The plates were washed three times with PBS and once with tap water. Only the colonies that were 2 mm2 or more in diameter were counted. Each experiment was performed in triplicate.
Differentiation
Adipogenic Differentiation
ASCs were cultured in six-well plates in CCM until 90% confluent. Medium was then replaced with fresh medium containing adipogenic supplements, consisting of 1 μM dexamethasone (Sigma-Aldrich), 5 μg/ml insulin, 0.5 mM isobuytlmethylxanthine (Sigma-Aldrich), and 50 μM indomethacin (Sigma-Aldrich) and changed every third day. After 3 weeks, cells were fixed in 10% formalin for 1 hour at 4°C and stained for 15 minutes at room temperature with Oil Red O (Sigma-Aldrich), and images were acquired on a Nikon Eclipse TE200 (Nikon, Melville, NY, http://www.nikon.com) with a Nikon DXM1200F digital camera using Nikon ACT-1 software, version 2.7. Images were acquired at a magnification of ×10.
Osteogenic Differentiation
ASCs were cultured in six-well plates in CCM until 90% confluent, and then the medium was replaced with medium containing osteogenic supplements, which consisted of 50 μM ascorbate 2-phosphate (Sigma-Aldrich), 20 mM β-glycerol phosphate (Sigma-Aldrich), 50 ng/ml l-thyroxine sodium pentahydrate, and 1 nM dexamethasone. After 3 weeks, cells were fixed in 10% formalin for 1 hour at 4°C and stained for 10 minutes with 40 mM alizarin red (pH 4.1) to visualize calcium deposition in the extracellular matrix. Images were acquired on a Nikon Eclipse TE200 with a Nikon DXM1200F digital camera using Nikon ACT-1 software, version 2.7. Images were acquired at a magnification of ×10.
Flow Cytometry
ASCs were harvested with 0.25% trypsin/1 mM EDTA (Gibco) for 4 minutes at 37°C. A total of 3 × 105 cells were concentrated by centrifugation at 500g for 5 minutes, suspended in 100 μl of PBS containing 1% (wt/vol) bovine serum albumin, and incubated at room temperature for 30 minutes with a panel of monoclonal antibodies specific for CD106, CD29, Sca-1, CD31, CD11b, and CD45. All monoclonal antibodies were purchased from BD Pharmingen/BD Biosciences (San Jose, CA, http://www.bdbiosciences.com). The samples were then analyzed by FACScan (FACSCalibur; BD Biosciences) with CellQuest software. The SVF was characterized using the same method with the monoclonal antibodies to the following: CD14, CD16, CD18, CD25, CD36, CD44, CD146, CD117, Mac-1, F4/80, and Foxp3 (all purchased from eBioscience Inc., San Diego, CA, http://www.ebioscience.com) and CD3, CD4, CD8 CD11b, CD19, CD31, CD34, and CD45 (purchased from BD Pharmingen). A minimum of 10,000 events were analyzed and compared with isotype controls.
EAE Induction and Treatment Protocols
All animal experiments were approved by Tulane University School of Medicine's Institutional Animal Care and Use Committee and were conducted in accordance with the U.S. Public Health Service Policy on Human Care and Use of Laboratory Animals. Female C57Bl/6 mice, 6–8 weeks old, were purchased from Charles River Laboratories (Wilmington, MA, http://www.criver.com). Chronic EAE was induced in these animals by subcutaneous immunization with 200 μl of 200 ng MOG35–55 (AnaSpec, San Diego, CA, https://www.anaspec.com) mixed 1:1 in Complete Freund's adjuvant with 8 mg/ml Mycobacterium tuberculosis H37RA (BD DIFCO, Franklin Lakes, NJ, http://www.bd.com), and 100 μl was injected subcutaneously at each side of the base of the tail. Mice also received 100 μl of 200 ng of pertussis toxin (List Biological Laboratories, Campbell, CA, http://www.listlabs.com) by intraperitoneal (i.p.) injection concomitantly and again 2 days later. To evaluate the clinical and pathological efficacies of ASCs and SVF in chronic EAE, cells were administered concomitantly with disease induction. Concurrently with disease induction, 100 μl of 1 × 106 cells suspended in HBSS was injected with a 27-gauge needle into the left side of the peritoneal cavity during EAE induction (day 0). Sham-treated, EAE-induced mice received equal volumes of HBSS without cells.
Clinical Scoring and Statistical Analysis
Naïve mice, EAE sham-treated mice, EAE mice treated with ASCs, and EAE mice treated with SVF each had 12 mice per group and were monitored daily for clinical signs of disease by three independent, blinded investigators. Clinical scores were based on a scale of 0–5, with a score of 0 indicating no disease; 1, limp tail (loss of tail tone); 2, hind limb weakness; 3, partial hind limb paralysis; 4, complete hind limb paralysis; and 5, moribund or dead. No animals were excluded from analysis. Clinical scores are presented as mean ± SEM for each group, with dead animals given a score of 5 on the day of death and for the remainder of the experiment. Statistical analysis was determined by two-way ANOVA on days 10–14 and again on days 26–30 using GraphPad Prism version 4.0b (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). Significance was assumed if the p value was ≤.01. The disease onset, disease incidence, and mean maximum scores were recorded for each mouse and expressed as mean ± SD. The cumulative disease score was calculated by summing the daily clinical score for each mouse during the course of observation.
Tissue Processing and Histological Analysis
After 30 days after disease induction, 5 of the 12 animals per group were euthanized by exposure to CO2 and perfused with sterile PBS. The lumbar and sacral regions of the spinal cords were removed, postfixed in 10% formalin (Thermo Fisher Scientific, Waltham, MA, http://www.thermofisher.com), and then embedded in paraffin. Sections were cut at 6-μm thickness on a microtome and stained for hematoxylin and eosin (H&E; Thermo Fisher Scientific) to reveal perivascular inflammatory infiltrates and Luxol Fast Blue/Cresyl Violet (LFB; IHC World, Ellicott City, MD, http://www.ihcworld.com) and Toluidine Blue (Thermo Fisher Scientific) for myelin detection. Histological analysis was performed on a Nikon Eclipse E800 microscope, acquired with Slidebook version 5.0 software (Olympus, Center Valley, PA, http://www.olympus-global.com), and analyzed using Fiji/ImageJ software. Quantification for each stain was performed on nine random sections per animal and five animals per group. All images were analyzed by investigators blinded to the status of the animal. The demyleination score was measured by the ratio of area of intact myelein against the same values for the naïve group, which was set to 1. An index of cellular debris was determined by the percentage of positive pixels divided by the percentage of positive pixels of the naïve group, which was set to 1. The percentage of inflammatory infiltrates was measured by the number of total cells (i.e., cells 5 μm2) per field at a magnification of ×400.
Cytokine Detection
At 30 days after disease induction, blood was collected from all mice during intracardial perfusions. Cytokines were analyzed by ELISA Immunoassay (Life Technologies), according to the manufacturer's instructions. Briefly, serum was added in 96-well precoated plates and incubated at room temperature. After washing, a specific polyclonal antibody followed by substrate solution was added, and the color development was measured at 450 nm on a fluorescent microplate reader (FLUOstar optima; BMG Labtech Inc., Durham, NC, http://www.bmglabtech.com). The concentration of cytokines was calculated using the standard curve. Statistical analysis using a two-tailed Student's t test was performed to evaluated differences between groups. Significance was assumed if the p value was ≤.05.
Results
Characterization of the Fat Stromal Vascular Fraction
Subcutaneous adipose tissue obtained from 2–6-month old male eGFP transgenic mice was processed by collagenase digestion and differential centrifugation. Approximately 0.55 ± 0.21 g of adipose tissue was harvested per mouse, averaging 1.82 × 106 ± 9.9 × 104 mononuclear SVF cells per gram of adipose tissue, with a viability between 89% and 94% when measured with trypan blue (data not shown). After lysing red blood cells, the cell populations in the SVF were analyzed for the presence of lineage-specific markers via flow cytometry. The most abundant populations detected were committed adipocyte progenitors (26.26%) and ASCs (21.38%; Table 1). Additionally, 24.31% expressed leukocyte markers, 7.98% of cells expressed hematopoietic stem cell-like markers, and 4.20% expressed T-cell markers. Cells below detectable levels (<2%) included the following: endothelial cells, vascular smooth muscle cells, B cells, mast cells, granulocytes, monocytes, macrophages, natural killer cells, and T-regulatory cells.
Table 1.
Characterization of stromal vascular fraction from C57BL/6 mice
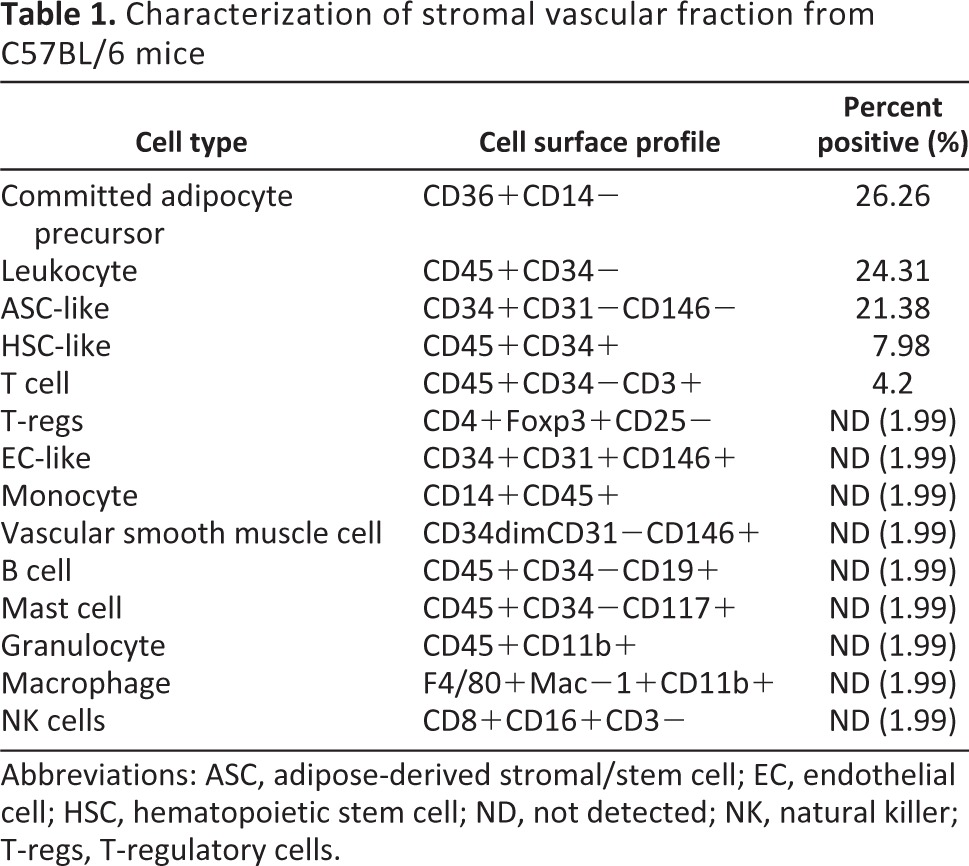
Abbreviations: ASC, adipose-derived stromal/stem cell; EC, endothelial cell; HSC, hematopoietic stem cell; ND, not detected; NK, natural killer; T-regs, T-regulatory cells.
Characterization of Cultured Adipose-Derived Stem Cells
In passage 1 after the initial plating of the SVF cells, ASCs appeared as a monolayer of large, flat cells and had a mean doubling time of 55.2 ± 4.8 hours. At passage 2, cells acquired a spindle shape, appeared fibroblast-like, and had a mean doubling time of 81.6 ± 4.8 hours. From passage 2 on, the morphological appearance and length of doubling time was maintained during subsequent passages.
To demonstrate the multipotent differentiation potential of ASCs, passage 2 cells were induced to differentiate into adipogenic and osteogenic lineages. When ASCs were cultured in adipogenic medium for 3 weeks, more than 50% of the cells became lipid-retaining cells that stained positive with Oil Red O (Fig. 1A, middle). In osteogenic medium, more than 30% of the cells were induced into an osteogenic lineage confirmed by alizarin red staining (Fig. 1A, right). The ASCs successfully generated colony-forming units by generating an average of 20 ± 4.6 colonies per plate when plated at 100 cells on a 10-cm2 plate (Fig. 1B). ASCs were phenotypically defined as >90% positive for CD29 and Sca-1, 60%–80% positive for CD106, and negative (< 2% positive) for CD31, CD11b, and CD45 via flow cytometry (Fig. 1C).
Figure 1.
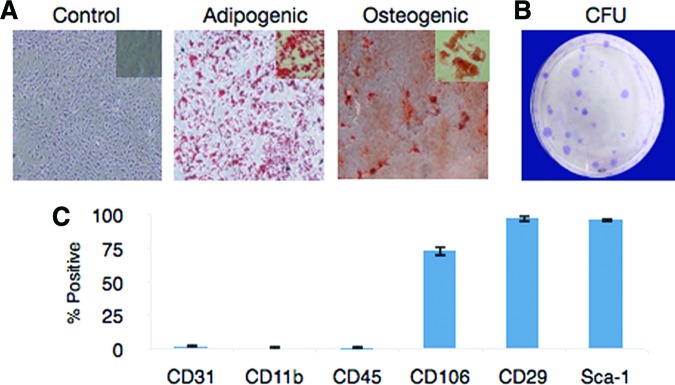
Characterization of mouse adipose-derived stem cells from C57Bl/6 stromal vascular fraction. Adipose-derived stromal/stem cells (ASCs) were grown in complete culture medium (CCM) until they were 70% confluent and then switched to differentiation medium. After 21 days, cells were fixed and stained with alizarin red for osteogenesis and Oil Red O for adipogenesis. Representative images for each group are shown (A). Magnification is ×10 for all panels. (B): CFUs were seeded at a density of 100 cells per 10-cm2 dish and incubated in CCM for 14 days. Cells were fixed and stained with crystal violet. A representative image is shown. (C): ASCs were stained with antibodies against the indicated antigens and analyzed by flow cytometry. Each experiment was performed in triplicate. Abbreviation: CFU, colony-forming units.
Intraperitoneal Injection of ASCs or SVF Ameliorates MOG35–55-Induced EAE
To evaluate whether the SVF cells or ASCs could affect disease progression in an autoimmune disease such as EAE, a preventative protocol was investigated in which cells were administered i.p. at the time of disease induction. Both SVF treatment and ASC treatment halted disease progression when compared with controls treated with vehicle alone, resulting in a statistically significant reduction of cumulative disease scores (Fig. 2; Table 2). On average, control mice developed the first clinical signs at 9 days postinduction (DPI), peaked around day 14 with hind limb paralysis, and then presented a stable disease course, typical of this model (Fig. 2; Table 2). Mice treated with ASCs had a slight but statistically insignificant delayed onset of disease compared with controls, with the average first clinical signs appearing at 9.3 DPI. EAE mice treated with SVF cells had a drastic delay in disease onset (14 DPI). It is important to note that only 3 of the 12 experimental animals treated with the SVF demonstrated clinical signs, which first presented at 10 DPI. Although the mean maximum score of the ASC-treated group was not significantly different compared with SVF-treated mice (1.9 ± 0.7 vs. 1.7 ± 0.9, respectively), the disease incidence was vastly altered. Only one of the SVF-treated mice reached a clinical score of 3, which presented only for 3 days, and then reduced to a clinical score of 2 for the remainder of the disease course (Fig. 2B). These data suggest that the i.p. injection of ASCs or SVF cells results in delayed and reduced disease severity. Moreover, it also indicates the SVF cells have a very potent neuroprotective effect in chronic EAE.
Figure 2.
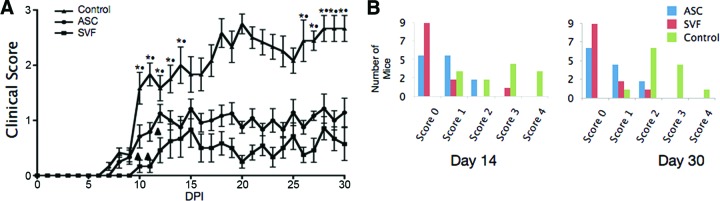
Clinical evaluation of ASC or SVF treatment in myelin oligodendrocyte glycoprotein35–55-induced experimental autoimmune encephalitis. (A): Improved clinical scores were seen in both ASC-treated (●) and SVF-treated (■) mice compared with controls (▴). Values are means from three independent reviewers. Bars indicate ±SEM. *, p ≤ .01, comparing controls and ASC-treated mice; ●, p ≤ .01 between controls and SVF-treated mice; ▴, p ≤ .05 between SVF-treated and ASC-treated mice. (B): Left: The clinical scores at day 14 DPI show the distribution during peak disease, demonstrating that the majority of SVF-treated mice displayed no symptoms and ASC-treated mice showed decreased symptoms. Right: The clinical scores at the end of the course show that the treated groups stably maintained their reduced state of disease. Abbreviations: ASC, adipose-derived stromal/stem cell; DPI, days postinjury; SVF, stromal vascular fraction.
Table 2.
Clinical features of EAE mice treated with ASCs or SVF
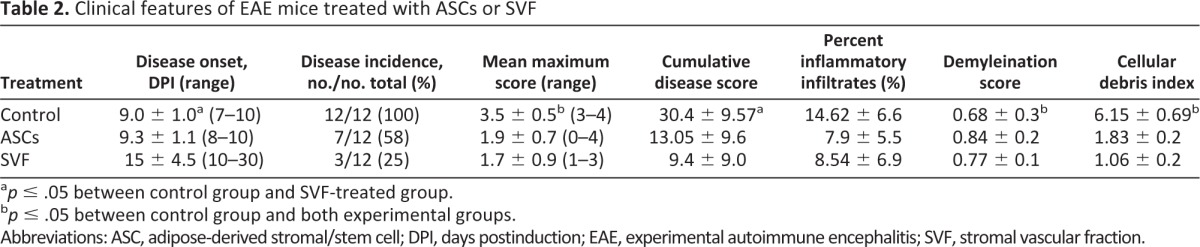
ap ≤ .05 between control group and SVF-treated group.
bp ≤ .05 between control group and both experimental groups.
Abbreviations: ASC, adipose-derived stromal/stem cell; DPI, days postinduction; EAE, experimental autoimmune encephalitis; SVF, stromal vascular fraction.
Pathological Features Are Diminished With SVF or ASC Treatment
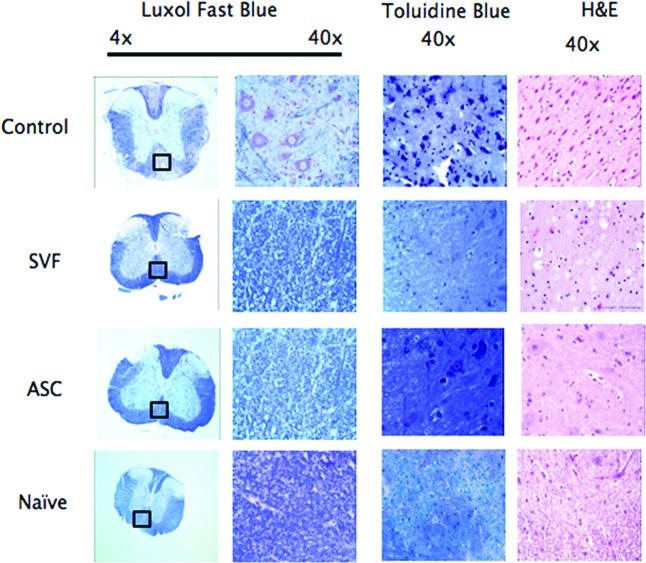
To determine whether the reduced disease severity correlated with pathological features, spinal cords from naïve, HBSS-treated, ASC-treated, and SVF-treated EAE mice were stained with Luxol Fast Blue to analyze regions of demyelination. Quantification was performed on nine sections per animal and five animals per group. All images were analyzed by investigators blinded to the status of the animal. Indexes were normalized to the average value obtained in naïve mice (set to 1).
The extent of demyelinated regions was reduced in both the SVF-treated (score of 0.77) and ASC-treated mice (score of 0.84) compared with control EAE-treated mice (score of 0.68) (Fig. 3; Table 2). Similarly, both treatment groups demonstrated reduced myelin breakdown products and debris compared with controls (an index of 1.8 in ASC-treated mice and 1.0 in SVF-treated mice, compared with 6.1 in controls), consistent with a neuroprotective effect of both cell therapies in EAE (Fig. 3; Table 2). The influx of immune infiltrates was measured by H&E staining of spinal cords. The percentage of infiltrating cells was significantly decreased in animals treated with either ASCs (7.9%) or SVF (8.5%) compared with controls (14.6%), indicating that both cell treatments are also capable of reducing inflammatory cell infiltrates into the CNS during EAE induction (Fig. 3; Table 2).
Figure 3.
Treatment with SVF or ASCs reduces cellular infiltration and tissue damage in experimental autoimmune encephalitis (EAE). Spinal cords from Hanks' balanced saline solution (HBSS)-treated, ASC-treated, SVF-treated, and naïve mice were obtained after euthanasia at 30 days postinjury and processed for histological staining using Luxol Fast Blue (LFB), Toluidine Blue (TB), and H&E. Quantification was performed on nine random sections per animal and five animals per group. LFB staining identified multiple areas of demyelination in HBSS-treated EAE mice but only scattered foci in both treated groups. Similarly, sections labeled with TB showed increased myelin debris and greater numbers of demyelinated axons in the control mice compared with naïve or treated mice. Comparisons of the H&E images show a decrease in the number of infiltrating immune cells in the spinal cord after administration of both ASCs and SVF. Abbreviations: ASC, adipose-derived stromal/stem cell; H&E, hematoxylin and eosin; SVF, stromal vascular fraction.
SVF and ASCs Both Suppressed Interferon-γ and Interleukin-12 in the Sera of EAE Mice
Tumor necrosis factor-α (TNFα), interleukin-12 (IL-12), and interferon-γ (IFN-γ) are cytokines that have been shown to be responsible for Th1 cell stimulation and that play a central role in the pathology of MS and EAE [39, 40]. To determine whether the protective effect of ASC or SVF treatment was related to these factors, levels in the sera were assayed by enzyme-linked immunosorbent assay. Although the IFN-γ sera levels of ASC-treated (51.33 pg/ml) and SVF-treated (54.46 pg/ml) mice were both lower than in the control (77.71 pg/ml), the two experimental groups were not statistically significant between each other. Neither ASC nor SVF treatment affected TNFα levels. The IL-12 sera levels were significantly decreased in ASC-treated mice (79.26 pg/ml) and further decreased in SVF-treated mice (35.57 pg/ml) compared with control mice (235.18 pg/ml) (Fig. 4). Collectively, these data suggest that ASC and SVF treatment can decrease serum levels of Th1-type inflammatory cytokines, such as IL-12 and IFN-γ.
Figure 4.
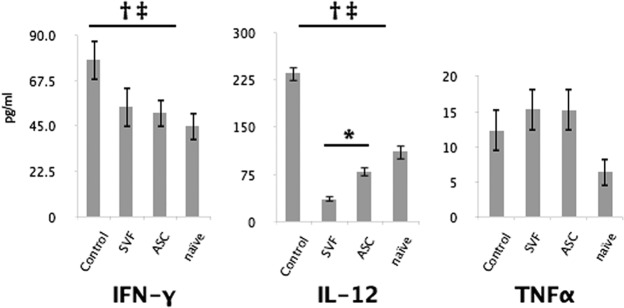
Serum levels of inflammatory cytokines. At 30 days after disease induction, blood was collected from all mice during intracardial perfusion and analyzed by ELISA Immunoassay. IL-12 levels were decreased in ASC-treated mice and further decreased in SVF-treated mice compared with control mice (middle). Both treatment groups had a similar decrease in levels of IFN-γ (left). No difference was seen in TNFα (right). Bars indicate ±SD. †, p ≤ .05 between controls and SVF-treated mice; ‡, p ≤ .05 between controls and ASC-treated mice; *, p ≤ .05 between treatment groups. Abbreviations: ASC, adipose-derived stromal/stem cell; IFN-γ, interferon-γ; IL-12, interleukin-12; SVF, stromal vascular fraction; TNFα, tumor necrosis factor-α.
Discussion
Multipotent marrow stromal cells (MSCs) are a promising therapy for the treatment of autoimmune diseases of the CNS because of their immunomodulatory and neuroprotective effects. BMSCs, the most studied type of MSCs, have demonstrated the ability to ameliorate both chronic and relapsing-remitting EAE [36, 41, 42]. In both experimental conditions, BMSCs have shown to be effective by immune suppression exerted on autoreactive B and T cells and inducing a Th2-polarized immune response [33, 35, 36, 42, 43]. However, the source and availability of MSCs is becoming a crucial issue for their clinical application. Although BMSCs have demonstrated promising results, the invasive nature of bone marrow biopsies may limit their practicality for wider clinical applications. Since adipose tissue contains a large number of ASCs, is easy to obtain in large quantities, and is easily accessible, it has become an appealing cell source for regenerative medicine and tissue engineering. ASCs have been shown to hold many of the same properties of BMSCs, such as the ability to differentiate, inhibit T-cell activation and proliferation, and produce anti-inflammatory molecules, and have been shown to aid in tissue repair through the secretion of cytokines [16]. The uncultured counterpart of ASCs, the SVF, is a particularly promising candidate for regenerative medicine because the cells can be isolated within hours of obtaining the lipoaspirate and no culture expansion of the cells is required, which would reduce any potential risks associated with growing cells in vitro and remove the need for complex laboratories. In addition to the clinical risks of ex vivo expansion, the variables used in cell culture, such as percentage and source of serum used, type of basal medium, medium supplements, culture surface substrate, cell seeding density, passage number, and confluence of culture, are undoubtedly giving rise to contrasting and confusing results in research. They also contain a heterogeneous population of cells, each type of which may independently contribute or work synergistically together, providing a beneficial effect.
Although the use of ASCs and SVF cells has been investigated in alleviating symptoms in autoimmune diseases such as graft-versus-host-disease, rheumatoid arthritis, Crohn's disease, and stroke, few studies have investigated ASCs or SVF cells in the treatment of MS or its mouse model EAE [18, 30]. One group showed that murine ASCs had a significant beneficial effect on chronic EAE by acting simultaneously in the lymphoid organs as well as the inflamed CNS and causing a dramatic change in antigen-specific T-cells [37]. Another group, Riordan et al., demonstrated improved function in MS patients treated with SVF [30]. However, the mechanism and effects of SVF therapy are not well understood, and a comparison of uncultured versus ex vivo-expanded cells in the treatment of EAE has yet to be evaluated. Furthermore, quantitative and statistically significant data are lacking in animal models used to determine mechanism and effects.
This study is among the first to show that ASCs have a significant beneficial effect in preventing chronic EAE not only when ex vivo-expanded but also without expansion. Although in both cases, the amelioration of clinical scores was accompanied by a strong reduction of spinal cord inflammation, demyelination, and axonal damage, the SVF cells provided a significant reduction in incidence of disease compared with their ex vivo-expanded counterpart.
Characterizing the molecular mechanisms mediating ASC- and SVF-based therapy is of critical importance to clinical applications and represents an important contribution to the understanding of ASC- and SVF-based therapy. IFN-γ is a cytokine associated with a number of autoinflammatory and autoimmune diseases because of its role in Th1 cell stimulation, differentiation, and function via STAT1 and STAT4 pathways [39]. These autoreactive T cells play a central role in the direct regulation of T-cell activation and survival during autoimmune inflammation in the pathology of MS and EAE [39, 40]. In this study, IFN-γ was reduced comparably between treatment groups. These results point to an effector role of both ASCs and SVF cells, which would occur during the early inflammatory phase of disease, supporting the possibility that ASCs and SVF could both affect the generation of encephalitogenic effector T cells. Although BMSCs have been shown to reduce levels of IFN-γ by direct contact, it is unclear whether ASCs and SVF cells use the same mechanism [44]. Although the mechanisms mediating such effects are still only partially understood, it is likely that they involve both direct cell-to-cell contact and paracrine signaling via soluble factors.
In addition to IFN-γ, IL-12 is responsible for Th1 cell stimulation, differentiation, and function and plays a central role in the pathology of MS [39]. In this study, IL-12 was reduced in both treatment groups, but levels were further reduced in the SVF treatment group compared with the ASC treatment group. Although this is the first study to show that both ASC treatment and SVF treatment have reduced levels of IL-12, BMSCs have been shown to reduce levels of IL-12 in a chronic EAE model [44, 45]. Interestingly, murine BMSCs were shown to exert opposing effects on Th1 cells depending on the time of disease onset and the level of effector T-cell activation, suppressing all T cells when administered early in T-cell activation and able to decrease IFN-γ and increase IL-17 only when T cells became activated [46].
These results indicate that IL-12 may play an important mechanistic role during the increased potency of SVF-based therapy. It is possible the SVF cells, beyond their ex vivo-expanded counterpart, have the ability to further reduce the level of effector T-cell activation, keeping the disease progression in a more naïve state by reducing IL-12 and, therefore, Th1 stimulation and differentiation. It remains unclear whether this is a result of the ASCs being uncultured and retaining more of their in vivo properties, whether it is a result of administering a heterogeneous population, or whether it is a product resulting from the interaction of ASCs with one of the other cell types present.
Although BMSCs have been shown to reduce TNFα levels by direct contact in vitro, this study showed that neither ASCs or SVF cells affected TNFα levels in an EAE model [44]. This may be due to BMSCs using a different mechanism or the cells being administered at different time points during T-cell activation and differentiation. This also reiterates the speculation that the timing of stem cell interaction with T cells may drastically change immunomodulatory results and, therefore, disease progression.
Conclusion
Further work needs to address whether it is the uncultured ASCs in the SVF fraction that have such a potent effect or whether the effect is a synergistic one with one or more of the other cell populations found in the SVF fraction. Future studies also need to further elucidate the mechanism, whether the injected therapeutic cells are causing a shift in the immune response in the lymphatic system before other pathogenic events via IL-17 or other notable cytokines, whether the injected therapeutic cells are influencing the endothelium to prevent breakdown of the blood-brain barrier and subsequent infiltration of immune cells into the CNS, or whether the injected therapeutic cells are directly affecting the myelin breakdown. In conclusion, these findings show that when compared with ex vivo-expanded ASCs, SVF cells are easier and safer to access, further delay and reduce EAE disease course and pathology, and further reduce Th1-type cytokines. These results show that SVF cells have relevant therapeutic potential in an animal model of chronic MS and might represent a valuable tool for stem cell-based therapy in chronic inflammatory disease of the CNS.
Acknowledgments
We thank Alan Tucker, Dina Gaupp, and Claire Llamas for their valuable technical assistance. This work was supported by funds from Tulane University (to B.A.B.).
Author Contributions
J.A.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; X.Z. and A.C.P.: collection and/or assembly of data, data analysis and interpretation; S.M.A., C.M., S.Z., B.A.S., A.L.S., S.A.S., M.M.B.: collection and/or assembly of data; J.M.G.: conception and design, data analysis and interpretation; B.A.B.: conception and design, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support.
Disclosure of Potential Conflicts of Interest
J.M.G. has compensated employment and is co-owner and co-founder of LaCell LLC.
References
- 1.Vlahiotis A, Sedjo R, Cox ER, et al. Gender differences in self-reported symptom awareness and perceived ability to manage therapy with disease-modifying medication among commercially insured multiple sclerosis patients. J Manag Care Pharm. 2010;16:206–216. doi: 10.18553/jmcp.2010.16.3.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.The MS disease-modifying medications. [Accessed April 11, 2012]. Available at http://www.nationalmssociety.org.
- 3.Tran TT, Kahn CR. Transplantation of adipose tissue and stem cells: Role in metabolism and disease. Nat Rev Endocrinol. 2010;6:195–213. doi: 10.1038/nrendo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 5.Peinado JR, Pardo M, de la Rosa O, et al. Proteomic characterization of adipose tissue constituents, a necessary step for understanding adipose tissue complexity. Proteomics. 2012;12:607–620. doi: 10.1002/pmic.201100355. [DOI] [PubMed] [Google Scholar]
- 6.Hausman GJ. Techniques for studying adipocytes. Stain Technol. 1981;56:149–154. doi: 10.3109/10520298109067302. [DOI] [PubMed] [Google Scholar]
- 7.Pettersson P, Cigolini M, Sjöström L, et al. Cells in human adipose tissue developing into adipocytes. Acta Med Scand. 1984;215:447–451. doi: 10.1111/j.0954-6820.1984.tb17677.x. [DOI] [PubMed] [Google Scholar]
- 8.Caspar-Bauguil S, Cousin B, Galinier A, et al. Adipose tissues as an ancestral immune organ: Site-specific change in obesity. FEBS Lett. 2005;579:3487–3492. doi: 10.1016/j.febslet.2005.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Casteilla L, Planat-Bénard V, Cousin B, et al. Plasticity of adipose tissue: A promising therapeutic avenue in the treatment of cardiovascular and blood diseases? Arch Mal Coeur Vaiss. 2005;98:922–926. [PubMed] [Google Scholar]
- 10.Weisberg SP, McCann D, Desai M, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gimble JM, Guilak F, Bunnell BA. Clinical and preclinical translation of cell-based therapies using adipose tissue-derived cells. Stem Cell Res Ther. 2010;1:19. doi: 10.1186/scrt19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schäffler A, Büchler C. Concise review: Adipose tissue-derived stromal cells: Basic and clinical implications for novel cell-based therapies. Stem Cells. 2007;25:818–827. doi: 10.1634/stemcells.2006-0589. [DOI] [PubMed] [Google Scholar]
- 13.Danoviz ME, Bassaneze V, Nakamuta JS, et al. Adipose tissue-derived stem cells from humans and mice differ in proliferative capacity and genome stability in long-term cultures. Stem Cells Dev. 2011;20:661–670. doi: 10.1089/scd.2010.0231. [DOI] [PubMed] [Google Scholar]
- 14.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 15.Halvorsen YC, Wilkison WO, Gimble JM. Adipose-derived stromal cells: Their utility and potential in bone formation. Int J Obes Relat Metab Disord. 2000;24(suppl 4):S41–S44. doi: 10.1038/sj.ijo.0801503. [DOI] [PubMed] [Google Scholar]
- 16.Planat-Benard V, Silvestre JS, Cousin B, et al. Plasticity of human adipose lineage cells toward endothelial cells: Physiological and therapeutic perspectives. Circulation. 2004;109:656–663. doi: 10.1161/01.CIR.0000114522.38265.61. [DOI] [PubMed] [Google Scholar]
- 17.Rangappa S, Fen C, Lee EH, et al. Transformation of adult mesenchymal stem cells isolated from the fatty tissue into cardiomyocytes. Ann Thorac Surg. 2003;75:775–779. doi: 10.1016/s0003-4975(02)04568-x. [DOI] [PubMed] [Google Scholar]
- 18.Zuk PA, Zhu M, Mizuno H, et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 19.Majumdar MK, Banks V, Peluso DP, et al. Isolation, characterization, and chondrogenic potential of human bone marrow-derived multipotential stromal cells. J Cell Physiol. 2000;185:98–106. doi: 10.1002/1097-4652(200010)185:1<98::AID-JCP9>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 20.Konno M, Hamazaki TS, Fukuda S, et al. Efficiently differentiating vascular endothelial cells from adipose tissue-derived mesenchymal stem cells in serum-free culture. Biochem Biophys Res Commun. 2010;400:461–465. doi: 10.1016/j.bbrc.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 21.Xu Y, Malladi P, Wagner DR, et al. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Curr Opin Mol Ther. 2005;7:300–305. [PubMed] [Google Scholar]
- 22.Zuk PA, Zhu M, Ashjian P, et al. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi Y, Sekiya I, Yagishita K, et al. Comparison of human stem cells derived from various mesenchymal tissues: Superiority of synovium as a cell source. Arthritis Rheum. 2005;52:2521–2529. doi: 10.1002/art.21212. [DOI] [PubMed] [Google Scholar]
- 24.De Ugarte DA, Morizono K, Elbarbary A, et al. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs. 2003;174:101–109. doi: 10.1159/000071150. [DOI] [PubMed] [Google Scholar]
- 25.Izadpanah R, Trygg C, Patel B, et al. Biologic properties of mesenchymal stem cells derived from bone marrow and adipose tissue. J Cell Biochem. 2006;99:1285–1297. doi: 10.1002/jcb.20904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bredeson C, Leger C, Couban S, et al. An evaluation of the donor experience in the canadian multicenter randomized trial of bone marrow versus peripheral blood allografting. Biol Blood Marrow Transplant. 2004;10:405–414. doi: 10.1016/j.bbmt.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 27.D'Andrea F, De Francesco F, Ferraro GA, et al. Large-scale production of human adipose tissue from stem cells: A new tool for regenerative medicine and tissue banking. Tissue Eng Part C Methods. 2008;14:233–242. doi: 10.1089/ten.tec.2008.0108. [DOI] [PubMed] [Google Scholar]
- 28.Casteilla L, Dani C. Adipose tissue-derived cells: From physiology to regenerative medicine. Diabetes Metab. 2006;32:393–401. doi: 10.1016/s1262-3636(07)70297-5. [DOI] [PubMed] [Google Scholar]
- 29.Lombardo E, DelaRosa O, Mancheño-Corvo P, et al. Toll-like receptor-mediated signaling in human adipose-derived stem cells: Implications for immunogenicity and immunosuppressive potential. Tissue Eng Part A. 2009;15:1579–1589. doi: 10.1089/ten.tea.2008.0340. [DOI] [PubMed] [Google Scholar]
- 30.Riordan NH, Ichim TE, Min WP, et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J Transl Med. 2009;7:29. doi: 10.1186/1479-5876-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tafreshi AP, Mostafavi H, Zeynali B. Induction of experimental allergic encephalomyelitis in C57/BL6 Mice: An animal model for multiple sclerosis. Iran J Allergy Asthma Immunol. 2005;4:113–117. [PubMed] [Google Scholar]
- 32.Racke MK. Experimental autoimmune encephalomyelitis (EAE) Curr Protoc Neurosci. 2001 doi: 10.1002/0471142301.ns0907s14. Chapter 9:Unit 9.7. [DOI] [PubMed] [Google Scholar]
- 33.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barhum Y, Gai-Castro S, Bahat-Stromza M, et al. Intracerebroventricular transplantation of human mesenchymal stem cells induced to secrete neurotrophic factors attenuates clinical symptoms in a mouse model of multiple sclerosis. J Mol Neurosci. 2010;41:129–137. doi: 10.1007/s12031-009-9302-8. [DOI] [PubMed] [Google Scholar]
- 35.Rafei M, Campeau PM, Aguilar-Mahecha A, et al. Mesenchymal stromal cells ameliorate experimental autoimmune encephalomyelitis by inhibiting CD4 Th17 T cells in a CC chemokine ligand 2-dependent manner. J Immunol. 2009;182:5994–6002. doi: 10.4049/jimmunol.0803962. [DOI] [PubMed] [Google Scholar]
- 36.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 37.Gordon D, Pavlovska G, Glover CP, et al. Human mesenchymal stem cells abrogate experimental allergic encephalomyelitis after intraperitoneal injection, and with sparse CNS infiltration. Neurosci Lett. 2008;448:71–73. doi: 10.1016/j.neulet.2008.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 39.Chen SJ, Wang YL, Fan HC, et al. Current status of the immunomodulation and immunomediated therapeutic strategies for multiple sclerosis. Clin Dev Immunol. 2012;2012:970789. doi: 10.1155/2012/970789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reynolds JM, Martinez GJ, Chung Y, et al. Toll-like receptor 4 signaling in T cells promotes autoimmune inflammation. Proc Natl Acad Sci USA. 2012;109:13064–13069. doi: 10.1073/pnas.1120585109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassis I, Grigoriadis N, Gowda-Kurkalli B, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65:753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- 42.Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 43.Lanz TV, Opitz CA, Ho PP, et al. Mouse mesenchymal stem cells suppress antigen-specific TH cell immunity independent of indoleamine 2,3-dioxygenase 1 (IDO1) Stem Cells Dev. 2010;19:657–668. doi: 10.1089/scd.2009.0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Han KH, Ro H, Hong JH, et al. Immunosuppressive mechanisms of embryonic stem cells and mesenchymal stem cells in alloimmune response. Transpl Immunol. 2011;25:7–15. doi: 10.1016/j.trim.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 45.Wehner R, Taubert C, Mende T, et al. Engineered extracellular matrix components do not alter the immunomodulatory properties of mesenchymal stromal cells in vitro. J Tissue Eng Regen Med. 2012 doi: 10.1002/term.1500. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 46.Carrión F, Nova E, Luz P, et al. Opposing effect of mesenchymal stem cells on Th1 and Th17 cell polarization according to the state of CD4+ T cell activation. Immunol Lett. 2011;135:10–16. doi: 10.1016/j.imlet.2010.09.006. [DOI] [PubMed] [Google Scholar]