In a mouse model, human adipose-derived mesenchymal stem cells from older donors failed to ameliorate the neurodegeneration associated with experimental autoimmune encephalomyelitis, and mice treated with cells from older donors had increased central nervous system inflammation, demyelination, and splenocyte proliferation. The age-related therapeutic differences corroborate recent findings that biologic aging occurs in stem cells.
Keywords: Mesenchymal stem cells, Experimental models, Cell transplantation, Adult stem cells, Aging, Multiple sclerosis, EAE, Experimental autoimmune encephalomyelitis
Abstract
There is a significant clinical need for effective therapies for primary progressive multiple sclerosis, which presents later in life (i.e., older than 50 years) and has symptoms that increase in severity without remission. With autologous mesenchymal stem cell therapy now in the early phases of clinical trials for all forms of multiple sclerosis (MS), it is necessary to determine whether autologous stem cells from older donors have therapeutic effectiveness. In this study, the therapeutic efficacy of human adipose-derived mesenchymal stem cells (ASCs) from older donors was directly compared with that of cells from younger donors for disease prevention. Mice were induced with chronic experimental autoimmune encephalomyelitis (EAE) using the myelin oligodendrocyte glycoprotein35–55 peptide and treated before disease onset with ASCs derived from younger (<35 years) or older (>60 years) donors. ASCs from older donors failed to ameliorate the neurodegeneration associated with EAE, and mice treated with older donor cells had increased central nervous system inflammation, demyelination, and splenocyte proliferation in vitro compared with the mice receiving cells from younger donors. Therefore, the results of this study demonstrated that donor age significantly affects the ability of human ASCs to provide neuroprotection, immunomodulation, and/or remyelination in EAE mice. The age-related therapeutic differences corroborate recent findings that biologic aging occurs in stem cells, and the differences are supported by evidence in this study that older ASCs, compared with younger donor cells, secrete less hepatocyte growth factor and other bioactive molecules when stimulated in vitro. These results highlight the need for evaluation of autologous ASCs derived from older patients when used as therapy for MS.
Introduction
Multiple sclerosis (MS) is a neurodegenerative disease that is characterized by inflammation and scar-like lesions throughout the central nervous system (CNS) due to repetitive attacks by immune cells with decreased self-tolerance for myelin [1–3]. There is no cure for MS [4], and no treatment ameliorates the severe forms of MS, such as primary progressive MS (PPMS) [5]. Thus, there is an unmet clinical need to develop therapeutics to treat these patients, who often present with debilitating symptoms without clinical remission [1, 5]. Based on animal studies, transplantation of mesenchymal stem cells (MSCs), also called multipotent stromal cells, holds promise as a therapy for all forms of MS [6–9]. MSCs home to areas of damage [10, 11], release trophic factors [7, 12], and exert neuroprotective [13–15] and immunomodulatory effects to inhibit T-cell proliferation [11, 15, 16]. MS-related clinical trials have all confirmed the safety of autologous MSC therapy [17–19].
It is unclear whether MSCs derived from older MS patients, specifically PPMS patients, have the same therapeutic potential as those derived from younger patients [1, 5]. Aging is known to have a negative impact on the regenerative capacity of most tissues [4, 20, 21], and human MSCs are susceptible to biologic aging [21–24], including changes in differentiation potential, proliferation ability, and gene expression [4, 25–27]. These age-related differences may affect the ability of older donor cells to migrate extensively, provide trophic support, persist long-term, and promote endogenous repair mechanisms [4]. However, there are no studies in animal models of neurological diseases that have compared the therapeutic potential of MSCs derived from young and old donors.
To address this problem, this study used the experimental autoimmune encephalomyelitis (EAE) mouse model of MS and compared older (>60 years) and younger (<35 years) human adipose-derived mesenchymal stem cell (ASC) treatment effects. Specifically, EAE-associated symptoms, motor function, spinal cord pathology, and T-cell proliferation were assessed. A recent study using the EAE model demonstrated that hepatocyte growth factor (HGF) is one of the main therapeutic factors released by MSCs after transplantation [7]. Therefore, the current study also compared the RNA levels of HGF and several bioactive molecules of cultured young and old donor ASCs to determine whether any age-dependent therapeutic differences are related to the inability of older donor cells to produce known therapeutic factors.
Materials and Methods
Experimental Animals
Female C57BL/6 mice (6–8 weeks) were obtained from Charles River Laboratories (Willimantic, CT, http://www.criver.com); these mice were randomly assigned to one of four mouse groups. The established mouse colony was maintained under standard housing conditions in the Tulane University School of Medicine Vivarium. All animal procedures were approved by the Institutional Animal Care and Use Committee at Tulane University and conformed to the requirements of the Animal Welfare Act. Animals were housed at four or five mice per cage with free access to food pellets and water. In total, there were four mouse groups studied: 21 Hanks' balanced saline solution (HBSS)-treated (i.e., sham-control) EAE-induced mice (EAE), 23 EAE-induced mice treated with human ASCs (hASCs) derived from younger donors (Young), 14 EAE-induced mice treated with hASCs derived from older donors (Old), and 17 HBSS-treated naïve (i.e., no EAE induction) mice were used in this study. For clarification purposes, the “Young” treatment group consisted of 6–8-week-old mice that received ASCs derived from young (<35 years) donors; the “Old” treatment group was a cohort of 6–8-week-old mice that received cells from old (>60 years) donors. The animals were maintained on a 12-hour/12-hour light/dark cycle, and video recording was consistently conducted at the same time of day.
Induction of EAE Using the Myelin Oligodendrocyte Glycoprotein35-55 Peptide
A myelin oligodendrocyte glycoprotein (MOG)35–55 peptide was administered to the mice to induce chronic EAE, which models PPMS more than other MS forms [28]. MOG35–55 peptide (AnaSpec, Fremont, CA, https://www.anaspec.com) was diluted (2 mg/ml) in 1× phosphate buffered saline (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) and added to equal amounts of Complete Freund's adjuvant (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com) with 8 mg/ml Mycobacterium tuberculosis H35RA (catalog no. 231131; BD Biosciences). These mixtures were thoroughly emulsified for 45 minutes using two emulsifying syringes and a micro-emulsifying needle (Cole Parmer, Vernon Hills, IL, http://www.coleparmer.com). For the induction of chronic EAE, the experimental animals were anesthetized by 4% isoflurane in oxygen and then injected at either side of the base of the tail with 100 μl of the MOG35–55 peptide emulsion (200 μl total per mouse) via a subcutaneous route. While under anesthesia, the mice were also injected with 200 ng of pertussis toxin (2 ng/μl; List Biologicals Laboratories, Campbell, CA, http://www.listlabs.com) through intraperitoneal (i.p.) administration. Each animal also received i.p. administration of 100 μl of HBSS, young hASCs (1 × 106 cells), or old hASCs (1 × 106 cells). This EAE induction day was designated as 0 days postimmunization (0 DPI). After 48 hours, the mice received an additional 100 μl of 200 ng of pertussis toxin (2 ng/μl) through the i.p. route. All solutions were injected with a 1-ml syringe with a 27-gauge 3/8-inch needle.
Collection, Culture, and Injection of Human ASCs
The hASCs were obtained from six female patients who were classified as younger (n = 3; <35 years old) or older (n = 3; >60 years) donors. All cells were isolated from processed lipoaspirates, characterized, and cultured as previously described [27]. The young donor ASCs had a mean ± SD age of 26.3 ± 3.8 years, and the old donor ASCs had a mean ± SD age of 63 ± 1.4 years. In addition to donor age, the race and selected demographics were obtained and analyzed; there were no other significant demographic differences, including body mass index. All cells were isolated after review and approval by the institutional review board of Tulane University School of Medicine, Pennington Biomedical Research Center, or Brigham and Women's Hospital/Harvard Medical School with informed patient consent.
Passage two (P2) hASCs were recovered from cryopreservation in α-minimum essential medium (α-MEM; Invitrogen) with 20% fetal bovine serum (FBS; Atlanta Biologicals, Lawrenceville, GA, http://www.atlantabio.com), 1% l-glutamine (Invitrogen), and 1% penicillin-streptomycin (pen-strep; Invitrogen). All cells were grown separately for each individual donor, and the medium was changed on the second day followed by every 2–3 days thereafter until the cells reached 70% confluence. The cells were washed thoroughly with 1× phosphate-buffered saline (PBS; Invitrogen), incubated at 37°C with trypsin for 3 minutes (Invitrogen), neutralized with an equal volume of complete media, and counted using a Countess Automated Cell Counter (Invitrogen). For expansion purposes, the cells were then replated at 250 cells per cm2. The media were changed every 2–3 days, and the cells were again lifted with trypsin once they reached 70% confluence. The viabilities of the lifted cells were consistently greater than 90% (data not shown), and all donor cell populations were grown and lifted on the same days. Additionally, these cells were analyzed using flow cytometry, and no differences were found in the size of the cells using side and forward light-scatter measurements [27]. For harvesting, the lifted and neutralized cells were centrifuged at 420g for 7 minutes at room temperature, and the cell pellet was resuspended with HBSS (Fisher Scientific, Pittsburgh, PA, http://www.thermofisher.com) containing calcium and magnesium but no phenol red. At this point the younger donor cells were pooled together and the older donor cells were pooled together for injection purposes. The two cell populations (i.e., younger and older cells) were kept off ice for 30 minutes and then returned to ice for no longer than 1 hour prior to injection. Before injection, the cell suspension was mixed thoroughly by inversion and warmed to room temperature. All treated animals received injections of 100 μl of the appropriate cell suspension (1 × 106 total younger or older ASCs) or HBSS into the left side of the peritoneal cavity at 0 DPI, as described above.
Clinical Scoring of EAE Mice
Beginning at 0 DPI, clinical scoring was performed daily to monitor the severity of the EAE-associated symptoms and averaged from three independent observers, who were blinded to the treatment groups. These observations were performed three times each day between 8:00 a.m. and 5:00 p.m. (i.e., when the mice were awake). Tail and hind limb paralysis were scored using the following scoring system: 0, complete absence; 1, tail atony (i.e., loss of tail tone); 2, hind limb weakness with no apparent paralysis; 3, partial hind limb paralysis affecting only one leg; 4, complete paralysis with no leg movement; and 5, death. Any death was recorded as a score of 5 for the day of death and any subsequent day for the remainder of the study.
Video Tracking for Motor Function Assessment
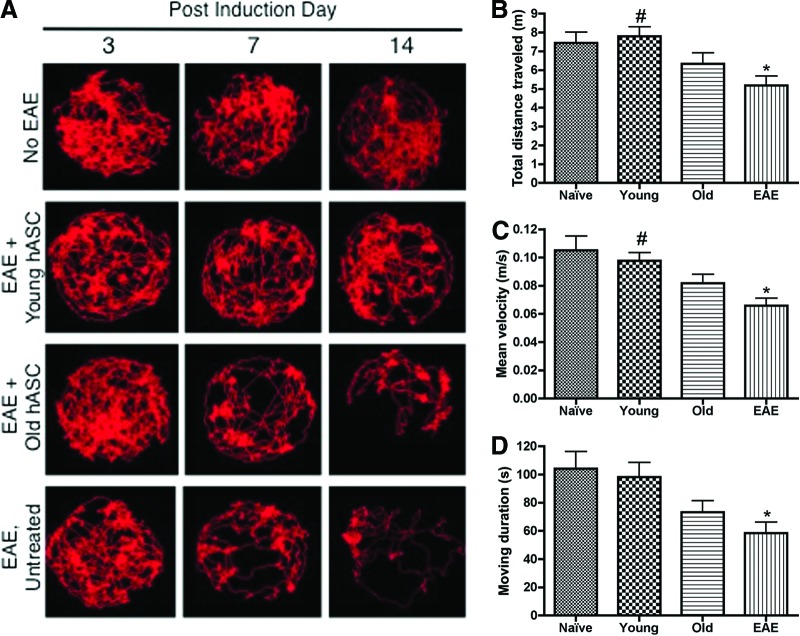
Video recording of each mouse was conducted at 3, 7, and 14 DPI using a LifeCam Cinema webcam with True 720p HD video and Debut Video Capture Software (Microsoft, Redmond, WA, http://www.microsoft.com) as described previously [29]. Briefly, all video files were uploaded for analysis using the EthoVision XT7 software (Noldus IT, Wageningen, The Netherlands, http://www.noldus.com) for quantification of various spatial endpoints, such as mean velocity, total distance traveled, and moving duration [30, 31]. EthoVision XT7 generated JPEG images of each mouse's tracks after the 5-minute recording period, and these tracks were compared per mouse group to visualize differences in activity within the arena. A blinded analyst assessed the track densities both visually and using Fiji/ImageJ software (National Institutes of Health, Bethesda, MD) as previously described [29, 32].
Tissue Processing and Histological Staining
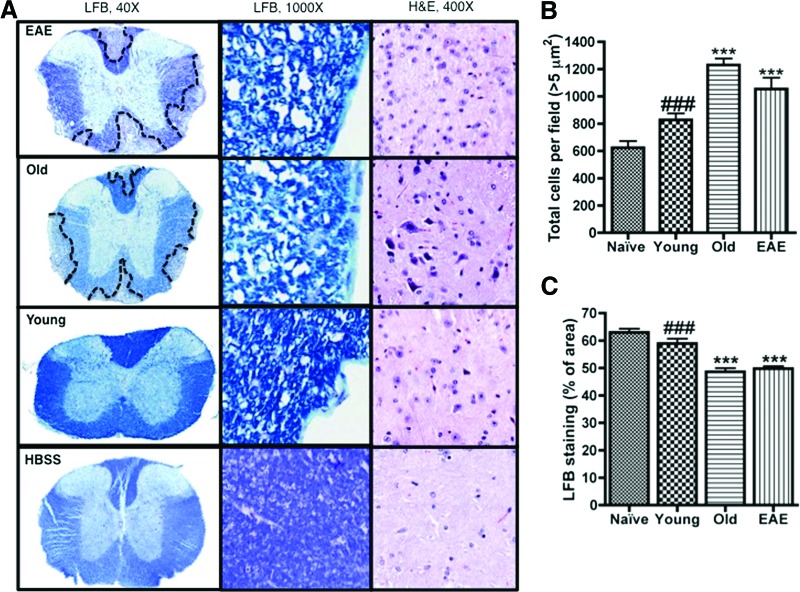
Animals were killed by exposure to CO2, and spinal cords were removed and stored at room temperature in 10% neutral buffered formalin. Paraffin-embedded sections were cut at 5 μm, mounted on glass slides, and subsequently stained with Luxol fast blue (LFB) and hematoxylin and eosin (H&E) for identification of intact myelin and infiltrating cells, respectively. Slides were scanned using an Aperio ScanScope CS instrument (Aperio Technologies, Inc., Vista, CA, http://www.aperio.com), and the LFB images were analyzed with Aperio software (Aperio Technologies) to quantify the intensity of the blue staining per section as a percentage of blue pixels per total pixels. For each mouse group, LFB stains of four mice (three sections per mouse) were analyzed. The H&E images were analyzed with Fiji/ImageJ software to quantify the number of total cells (i.e., cells ≥5 μm2) per field at ×400 magnification [32]. For each treatment group, H&E stains of three mice (two sections per mouse) were analyzed. The total number of cells per field for each representative H&E image corresponds to the average number of cells per field for the respective mouse group. The investigators who performed the tissue investigations were blinded to the mouse groups until the end of the experiment after graphs were generated and tissue sections were compared.
Protein Isolation
The spinal cords and spleens were collected and stored at 4°C in Allprotect tissue reagent solution (Qiagen, Hilden, Germany, http://www.qiagen.com). For protein isolation, each sample was homogenized in 0.2% Nonidet P40 in PBS using a plastic motorized pestle; approximately 10 ml of fluid was used per gram of sample. All samples were exposed to 10 rounds of sonication, with each round consisting of 5 seconds of sonication and 20 seconds on ice without sonication. The solutions were centrifuged twice for 5 minutes at 12,000g, and the supernatant was removed and saved in aliquots at −20°C for future enzyme-linked immunosorbent assay (ELISA) analyses. Protein concentration was determined using the BCA assay (Pierce, Rockford, IL, http://www.piercenet.com), and all protein lysates were centrifuged using Ultrafree-MC centrifugal filter units with microporous membranes (Millipore, Billerica, MA, http://www.millipore.com) before testing.
Cytokine/Chemokine ELISA
At 15 DPI, the sera of five mice per treatment group were pooled together and stored at −80°C prior to testing. A total of 25 μl of neat serum sample (n = 5 mice per sample) was loaded in duplicate onto four 96-well ELISA plates (Invitrogen) at room temperature. These precoated plates were used to detect interleukin (IL)-12, IL-17, interferon-γ (IFN-γ), or tumor necrosis factor-α (TNF-α). Specific polyclonal antibodies were added after washing followed by the addition of a substrate solution; all steps were performed according to the manufacturer's instructions. For each plate, the absorbance values were measured at 450 nm on a fluorescent microplate reader (FLUOstar Optima; BMG Labtech, Ortenberg, Germany, http://www.bmglabtech.com), and the values of cytokine levels were calculated based on the standard curve.
T-Cell Proliferation Assay
Immediately after euthanasia, four spleens per mouse group were briefly stored on ice in α-MEM (<1 hour), homogenized gently using the blunt end of a sterile 10-ml plastic syringe, filtered through a 70-μm cell strainer (BD Biosciences), and washed twice with 1× PBS. The filtrate was centrifuged at 1,200 rpm for 10 minutes, resuspended in 2 ml ammonium-chloride-potassium (ACK) red blood cell lysis buffer (Invitrogen) for 3 minutes, centrifuged again at 1,200 rpm for 7 minutes, and then resuspended in RPMI 1640 (Sigma-Aldrich) with 2 mM l-glut, 2 mM pen-strep, 10% FBS, and 50 μM β-mercaptoethanol. The splenocytes were counted using a Countess Automated Cell Counter (Invitrogen). For each 96-well plate, splenocytes of four mice per treatment group were plated in triplicate at 2 × 105 cells per well in 200 μl of complete RPMI 1640. For the PBS condition, the splenocytes were grown for 48 hours in complete RPMI 1640 with the addition of 10 μl of PBS. For the MOG condition, a 96-well plate was incubated with MOG35–55 peptide at 30 μg/ml and then blotted dry, leaving the plate coated with the peptide; the splenocytes were then added to the precoated plate and grown for 48 hours in complete RPMI 1640. For the stimulated condition, the splenocytes were grown for 48 hours in complete RPMI 1640 with the addition of 1 μg/ml anti-CD3 monoclonal antibody (mAb) and 2 μg per well of anti-CD28 mAb (BioLegend, San Diego, CA, http://www.biolegend.com).
A 5-bromo-2′-deoxyuridine (BrdU) cell proliferation assay (Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com) was used to determine the cell proliferation activity of each well. Briefly, 20 μl per well of 10× BrdU solution was added after the cells had grown for 48 hours. After 18 hours of incubation with BrdU, the plates were centrifuged at 300g for 10 minutes; the media were removed; and a fixation/denaturing solution, an anti-BrdU antibody, and a horseradish peroxidase-conjugated secondary antibody were added according to the manufacturer's instructions. To quantify the amount of BrdU that was incorporated by the proliferating cells, a TMB (tetramethylbenzidine) substrate was incubated for 30 minutes, and samples were measured at 450 nm on a fluorescent microplate reader (FLUOstar Optima) following the addition of the provided STOP solution.
ASC Stimulation, RNA Isolation, and Reverse Transcription-Polymerase Chain Reaction
P2 hASCs derived from younger or older donors were grown to 70% confluence in complete α-MEM, lifted as previously described with trypsin, and then seeded into six-well plates at a density of 2 × 105 ASCs per well. After 24 hours, the media were removed and replaced with either fresh, complete α-MEM or macrophage-conditioned medium that had been taken directly from confluent primary RAW 264.7 (catalog no. TIB-71; American Type Culture Collection, Manassas, VA, http://www.atcc.org) mouse macrophage cells grown in Dulbecco's modified Eagle's medium (American Type Culture Collection) with 10% FBS (Atlanta Biologicals) and 1% pen-strep (Invitrogen). Thus, there were four cell populations in all (e.g., Young, Young-Stimulated, Old, and Old-Stimulated). For RNA isolation, the cell pellets were obtained and homogenized using a QIAshredder (Qiagen) according to the manufacturer's instructions. The isolation was completed on ice using the RNeasy Qiagen protocol without modifications to the cell RNA isolation protocol. DNase 1× Amplification Grade (Invitrogen) was added to 1 μg of RNA for 15 minutes at room temperature, and 1 μl of EDTA (25 μM) was added at 65°C for 10 minutes to stop this reaction. DNase-treated RNA was then converted to complementary DNA (cDNA) using the iScript protocol and reagents (Bio-Rad, Hercules, CA, http://www.bio-rad.com). The cDNA were then tested for the presence of various cytokines/chemokines and growth factors (e.g., HGF, IL-1, IL-2, IL-8, IL-10, IL-12) and glyceraldehyde-3-phosphate dehydrogenase using real-time reverse transcription-polymerase chain reaction (PCR). All primer sequences were reported in a previous study [12]. All RNA and cDNA aliquots were quantified for RNA or DNA content using a NanoDrop Spectrophotometer 2000 (Thermo Scientific, Waltham, MA, http://www.thermoscientific.com).
Statistical Analysis
For comparisons of clinical scores, two-way analysis of variance (ANOVA) was performed using GraphPad Prism 4.0b for Macintosh at 10–14 and 26–30 DPI, and all values were reported as mean ± SEM. For tests with a significant (p < .05) time × group interaction, pairwise comparisons of least square means were made to further investigate the interaction. For the behavioral, neurophenotyping, and histological studies, statistical analysis of three or more groups was performed using one-way ANOVA followed by pairwise comparisons of the mouse groups using Bonferroni post hoc testing. Significance for the overall group effect and individual pairwise comparisons was defined as p < .05. A two-tailed Student's t test was performed to evaluate differences in RNA levels of various factors for the old and young hASCs. Disease incidence ratios (i.e., number of animals with EAE symptoms per number of animals injected with MOG) were compared using a contingency table and a χ2 test.
Results
Assessment of EAE Symptoms Using Observational and Automated Phenotyping Methods
All mice were weighed on DPI 0 before EAE induction, and there were no significant weight differences between the four mouse groups (p = .71). The EAE-induced mice were monitored daily for scoring of tail and hind limb paralysis, revealing a significant group × time interaction (p < .0001), group effect (p < .0001), and time effect (p < .0001) over the course of the study (Fig. 1A). Bonferroni post hoc testing was performed at 10–14 and 26–30 DPI for comparisons between the three mouse groups. None of the naïve mice presented with tail or hind limb paralysis over the duration of the study. The Young treatment group, which received hASCs from younger donors, had significantly improved scores compared with the sham-control EAE mice at 10–14 DPI (p < .01 to .001). However, at 26–30 DPI, the Young mice had significantly lower clinical scores, and thus less paralysis, than both the sham-control EAE and Old groups (p < .001). ASCs derived from older donors had no effect on the clinical presentation of the EAE-induced mice, as there were no significant differences in clinical score between the sham-control EAE and Old mouse groups at any time point in this study (Fig. 1A). To evaluate the distribution of clinical scores per treatment group, the number of animals with each clinical score at 14 DPI was recorded. As shown in Figure 1B, the Young mice (n = 23) had a mode of 0, with 10 mice showing no evidence of disease. In contrast, the Old (n = 14) and EAE (n = 21) groups had modes of 1 and 3, respectively (Fig. 1B). All EAE-induced mouse groups, regardless of treatment, demonstrated the characteristic chronic disease progression of EAE with presentation of symptoms between 7 and 10 DPI, peaking of symptoms by 15 DPI, and maintenance of disease severity after 15 DPI. However, the younger hASCs were able to alleviate EAE-associated symptoms, whereas the cells from older donors failed to improve the severity of the disease.
Figure 1.
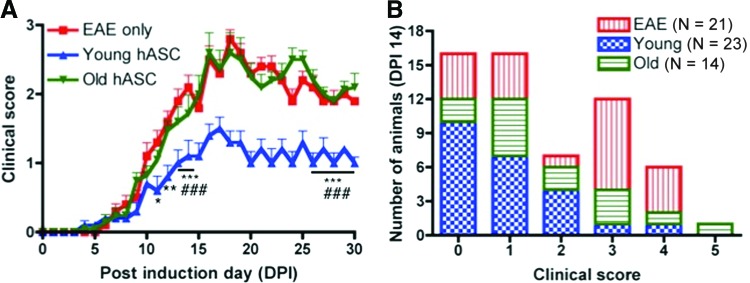
Young, but not old, donor adipose-derived mesenchymal stem cells improve EAE clinical presentation. (A): The clinical scores for the EAE (n = 21), Young (n = 23), and Old (n = 14) mouse groups were recorded daily for the duration of the study. The Young group had significantly lower scores and less paralysis compared with the EAE group for all time points tested (p < .01 to .001) and the Old group for 26–30 DPI (p < .001). All non-EAE control mice (Hanks' balanced saline solution, naïve) had scores of 0 for every time point and are not included in the group comparisons. All values represent means + SEM. Significance was defined as follows: *, p < .05; **, p < .01; ***, p < .001 versus EAE mice; ###, p < .001 versus Old mice. (B): The number of mice with each clinical score (0–5) is shown for each treatment group at 14 DPI. The majority of young mice had clinical scores of 0 (i.e., no disease), whereas the majority of Old and EAE groups presented with EAE-associated symptoms corresponding to scores of 1 and 3, respectively. Abbreviations: DPI, days postimmunization; EAE, experimental autoimmune encephalomyelitis; hASC, human adipose-derived mesenchymal stem cell.
Neither the younger nor the older hASCs significantly delayed the onset of the EAE symptoms, which started at 8.89 ± 0.85, 9.67 ± 0.84, and 10.47 ± 0.89 DPI for the EAE, Old, and Young mouse groups, respectively (Table 1). The disease incidences for the EAE, Old, and Young mouse groups were 19 of 21 (90.5%), 12 of 14 (85.7%), and 17 of 23 (73.9%), respectively, and there were no significant difference between the cell treatment groups and the sham-controls (Table 1). There was a significant group effect on the maximum clinical score achieved (F [2, 56] = 4.10; p < .022), and post hoc testing demonstrated that the Young group had less severe maximum scores than the EAE mouse group (p < .05; Table 1).
Table 1.
Comparisons of EAE-associated symptoms and pathological findings across treatment groups
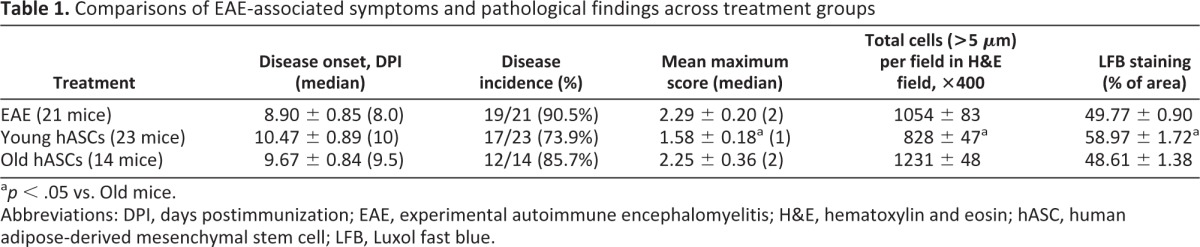
ap < .05 vs. Old mice.
Abbreviations: DPI, days postimmunization; EAE, experimental autoimmune encephalomyelitis; H&E, hematoxylin and eosin; hASC, human adipose-derived mesenchymal stem cell; LFB, Luxol fast blue.
As shown by the representative track visualizations (Fig. 2A), the EAE and Old mice showed a marked decrease in activity in the arena at 7 and 14 DPI. In contrast, the naïve and Young mice showed increased activity and utilization of the arena space compared with the EAE and Old mice. Analysis of automated and observational behaviors further demonstrated significant differences between the mouse groups, with a significant group effect on total distance traveled (F[3, 24] = 4.79; p < .009), average velocity (F(3, 24)] = 4.96; p < .026), and moving duration (F[3, 24] = 5.43; p < .021) during the 5-minute recording period on DPI 7 (Fig. 2B–2D). Compared with the naïve mice, the EAE mice had a significant decrease in total distance traveled, moving velocity, and time spent moving (p < .05), whereas the Young and Old groups did not differ from the naïve mice. The younger, but not older, hASC-treated mice showed improved distance traveled and velocity compared with EAE mice (p < .05; Fig. 2C).
Figure 2.
Young adipose-derived mesenchymal stem cell-treated mice have increased activity and motor function. (A): Track visualizations were generated using EthoVision XT7 for the naïve HBSS (n = 6), Young (n = 6), Old (n = 6), and EAE (n = 6) mouse groups at 3, 7, and 14 days postimmunization (DPI). For any given day, a mouse was recorded once for 5 minutes, and representative images are shown. The tracks for the EAE and Old mice showed a marked decrease in track density, especially in the inner zone of the arena, seen as early as 7 DPI. The Young and naïve HBSS mice demonstrated increased activity per recording period compared with the EAE and Old mouse groups. (B): The total distance traveled in 5 minutes was compared across all mouse groups at 7 DPI. (C): The average velocity over 5 minutes was compared across all mouse groups at 7 DPI. (D): The time spent in motion over 5 minutes was compared for all mouse groups at DPI 7. Comparisons between mouse groups were made using one-way analysis of variance and Bonferroni's post hoc testing. *, p < .05 versus naïve HBSS mice; #, p < .05 versus EAE mice. Abbreviations: EAE, experimental autoimmune encephalomyelitis; hASC, human adipose-derived mesenchymal stem cell; HBSS, Hanks' balanced saline solution.
Histological Analysis of the Spinal Cord
As a prototype for inflammatory neurodegenerative diseases, the EAE mouse has increased inflammation throughout the CNS with lesions that resemble the pathology in the human patient [17, 28, 33]. To determine the effects of hASC treatment on myelin levels in the EAE mice, spinal cords were obtained at sacrifice and analyzed for intact myelin after LFB staining. The LFB images (Fig. 3A) showed areas of demyelination that contained low intensity LFB staining throughout the spinal cord sections for both the EAE and Old mouse groups. The Young mice had significantly more intact myelin than both Old mice and EAE mice (Fig. 3C, p < .001). However, the older hASC treatment had no significant effect on myelin levels compared with the EAE mice. Compared with the naïve mice, the spinal cords from the Young mice had similar myelin levels and the Old and EAE spinal cords had significantly less intact myelin (Fig. 3C, p < .001). H&E staining was performed to evaluate the levels of infiltrating cells in the spinal cords of all treatment groups. The younger hASC treatment significantly decreased the number of infiltrating cells present in the spinal cords compared with the EAE control and the older hASC-treated mice (Fig. 3B, p < .001).
Figure 3.
Cell therapy effects on myelin and infiltrating cell levels in the spinal cord of EAE-induced mice. (A): Spinal cords were obtained at sacrifice from four mice per mouse group (naïve HBSS, Young, Old, and EAE). Each spinal cord was sectioned, mounted, and stained with LFB or H&E for comparison of intact myelin levels and infiltrating cells, respectively. Images were acquired at magnifications of ×40 and ×1,000 for the LFB-stained sections and ×400 for the H&E sections. (B): The H&E-stained sections (n = 3 mice; 2 sections per mouse) were quantified for the number of total cells per field at ×400 magnification using ImageJ/Fiji software. (C): The LFB-stained sections (n = 4 mice; three sections per mouse) were quantified for the intensity of the blue staining using Aperio software. Comparisons between mouse groups were made using one-way analysis of variance and Bonferroni's post hoc testing. ***, p < .001 versus naïve mice; ###, p < .001 versus Old mice. Abbreviations: EAE, experimental autoimmune encephalomyelitis; H&E, hematoxylin and eosin; HBSS, Hanks' balanced saline solution; LFB, Luxol fast blue.
Effects of Cell Therapy on Splenocyte Proliferation
Splenocytes isolated from the four mouse groups at sacrifice were grown in the presence of 1× PBS, MOG35–55, or anti-CD3 mAb and then tested for cell proliferation using a BrdU assay (Fig. 4). Comparing the cells grown with PBS only, the cells isolated from the naïve and Young mouse groups proliferated less than the cells of the Old group (p < .01). The cells isolated from the naïve and Young mice proliferated less than the EAE mouse cells when grown in the presence of MOG35–55 peptide (p < .05), even though there was no difference in proliferation between the Old and EAE cells after exposure to MOG35–55. All cells had increased proliferation when exposed to antibodies targeting CD3 and CD28; however, even in this stimulatory environment, the cells from the naïve mice had less proliferative capacities than the Old mouse splenocytes (p < .05).
Figure 4.
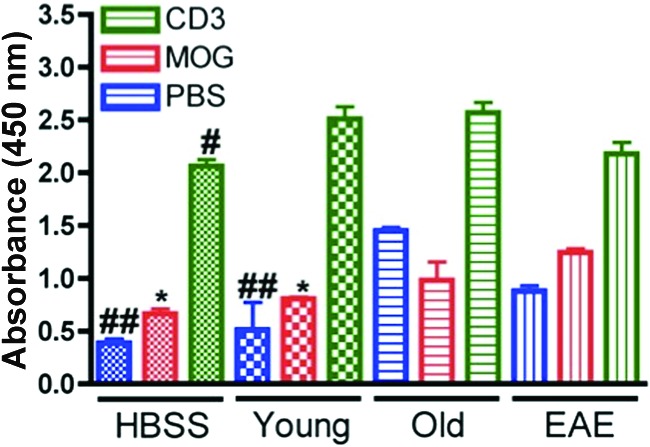
5-Bromo-2′-deoxyuridine (BrdU) cell proliferation assay with mouse splenocytes. Mouse spleens were isolated from the naïve HBSS (n = 4), Young (n = 4), Old (n = 4), and EAE (n = 4) mice at sacrifice, and splenocytes were cultured in the presence of PBS (blue), MOG35–55 (30 mg/ml; red), or CD3 and CD28 antibodies (green). A BrdU cell proliferation assay was used to compare the proliferation abilities of the four splenocyte populations in the three growth conditions. The splenocytes of the Old group had greater proliferation at baseline (i.e., with only PBS added) compared with the naïve and Young mice (p < .01); similarly, the Old group had increased proliferation compared with naïve HBSS mice after stimulation with CD3 and CD28 antibodies (p < .05). The EAE mice had greater proliferation after MOG35–55 exposure when compared with the naïve HBSS and Young groups (p < .05). Comparisons between mouse groups were made using one-way analysis of variance and Bonferroni's post hoc testing. *, p < .05 versus EAE mice; #, p < .05; ##, p < .01 versus Old mice. Abbreviations: EAE, experimental autoimmune encephalomyelitis; HBSS, Hanks' balanced saline solution; MOG, myelin oligodendrocyte glycoprotein35–55; PBS, phosphate-buffered saline.
Analysis of Proinflammatory Cytokines in the Serum
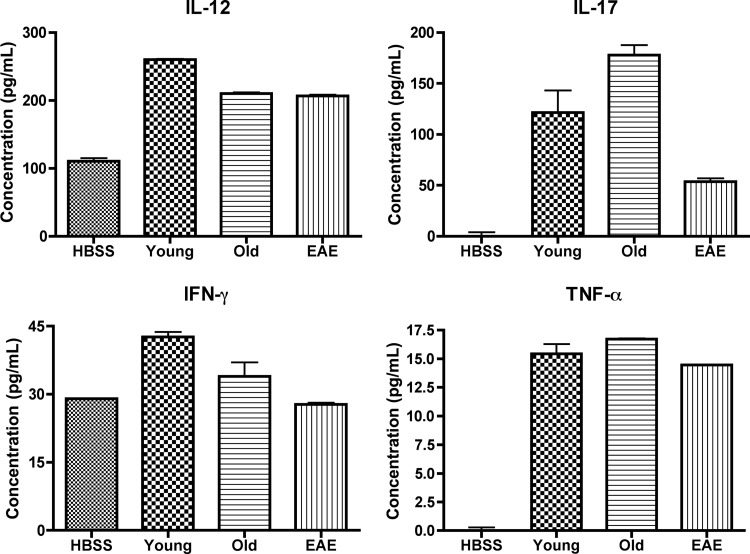
The sera of five mice per mouse group were obtained at 15 DPI, pooled together, and analyzed for levels of different proinflammatory cytokines using ELISAs (Fig. 5). The EAE-induced mice, regardless of treatment, had greater levels of IL-12, IL-17, IFN-γ, and TNF-α in their sera than the naïve mice. Serum levels of TNF-α were consistent across the Young, Old, and EAE groups. The serum levels of IFN-γ were increased in the Old group and further increased in the Young group, compared with the EAE controls. In contrast, IL-17 levels were increased in the Young group and further increased in the Old group compared with the EAE mice. Also, the Young mice had greater serum levels of IL-12 than any other group, including the Old group.
Figure 5.
Mouse serum protein levels of proinflammatory cytokines. Sera were pooled from five mice per mouse group at 15 days postimmunization and tested on four enzyme-linked immunosorbent assay plates to detect levels of IL-12, IL-17, IFN-γ, and TNF-α. The protein concentrations for all four cytokines were elevated for the EAE-induced mice compared with the HBSS controls. The Old mice had increased IL-17 levels compared with all groups, and the Young mice had increased IL-12 and IFN-γ compared with all groups. Abbreviations: EAE, experimental autoimmune encephalomyelitis; HBSS, Hanks' balanced saline solution; IFN, interferon; IL, interleukin; TNF, tumor necrosis factor.
Analysis of Cytokines and Growth Factors in hASCs Derived From Young and Old Donors
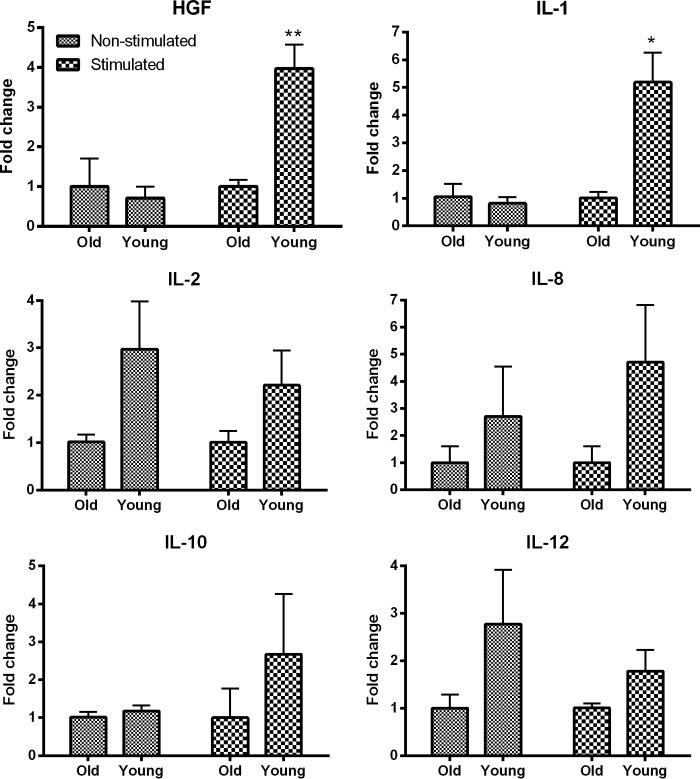
The RNA levels for different cytokine or growth factors in hASCs derived from younger and older donors were determined using real-time PCR (Fig. 6). Without stimulation, there were no significant differences between the younger and older hASCs for any factor. The younger and older cells were then exposed to macrophage-conditioned media, and the younger hASCs expressed significantly more HGF and IL-1α RNA, with slightly increased RNA levels of IL-2, IL-8, IL-10, and IL-12. Because of recent findings that HGF is a therapeutic factor released by MSCs in the EAE MS model [7], we investigated the role of HGF, specifically in regard to any pathways that may be dysregulated with age in MSCs. Analysis of younger and older hASCs using Ingenuity Pathway Analysis (IPA) and the Ingenuity Knowledge Base demonstrated an altered HGF-related signaling pathway (data not shown); specifically, extracellular signal-regulated kinase 1/2 (ERK1/2) and mitogen-activated protein kinase (MAPK) are markedly downregulated in older human ASCs, as previously reported by our laboratory using IPA, mRNA, and protein analyses [27]. The differentiation potential, proliferation ability, and morphology of the young and old ASCs were reported in the same study from our laboratory [27].
Figure 6.
Young human adipose-derived mesenchymal stem cell (hASCs) have increased RNA levels of various cytokine and growth factors compared with old hASCs. hASCs derived from young or old donors were grown with and without stimulation by macrophage-conditioned media. The hASCs derived from young donors had increased levels of RNA for HGF and IL-1 after stimulation and slight increases for IL-2, IL-8, IL-10, and IL-12 when left nonstimulated. Comparisons between mouse groups were made using one-way analysis of variance and Bonferroni's post hoc testing. *, p < .05; **, p < .01 versus Old mice. Abbreviations: HGF, hepatocyte growth factor; IL, interleukin.
Discussion
Older donor MSCs of various species have decreased differentiation potential and proliferative capability [12, 20, 22, 26] and altered gene expression [12, 22, 34] compared with younger donor MSCs. Furthermore, demonstrated differences in signaling pathways [27, 35], methylation patterns [36], oxidative stress levels [35, 37], microRNA expression [20, 27], telomere length [23, 24], and growth factor production [12, 34] suggest that MSCs derived from older donors may have decreased therapeutic effectiveness after transplantation. To test this possibility, our study is the first to compare the age-related therapeutic effects of human ASCs derived from older and younger donors using the EAE mouse model of MS.
To ensure that this study was clinically relevant to the population in question (i.e., late-onset PPMS patients), EAE was induced using the MOG35–55 peptide in order to simulate a chronic disease state. The improved clinical scores and motor function of the EAE-induced mice receiving younger donor ASCs indicate that younger, but not older, ASCs are capable of halting EAE disease progression before the development of hind limb paralysis. Although the stem cells from older donors do not seem to exacerbate EAE in the mouse, our results indicate that older donor MSCs have lost the ability to alleviate EAE-associated symptoms. The striking differences in clinical presentation between younger and older hASC-treated EAE mice emphasize the need to determine the therapeutic effectiveness of autologous cell transplantation in older patients, especially those with demyelinating or inflammatory diseases like MS.
To understand why the older ASCs had no therapeutic effect in the EAE model, we evaluated the effect of donor age on the MSCs' ability to improve EAE CNS pathology, decrease T-cell proliferation, and produce relevant therapeutic factors. Spinal cords of all mouse groups were evaluated for differences in intact myelin levels using LFB staining. The increased blue intensity associated with the younger ASC-treated mouse group and the extensive demyelination present in the older ASC-treated mice indicated that the younger donor cells were capable of providing neuroprotection through inhibition of demyelination or promotion of remyelination. Supporting this result, a recent study demonstrated that ASCs can stimulate endogenous progenitors, such as oligodendrocyte progenitors, to differentiate into their adult myelin-producing counterparts [4]. We also found that donor age has an effect on the ability of the MSCs to decrease inflammatory infiltration in the spinal cord. Collectively, our results suggest that older ASCs are less therapeutic for EAE mice because of their inability to decrease demyelination and inflammation in the spinal cord.
The increased inflammation in the spinal cords of the older ASC-treated mouse cohort led us to evaluate the immunomodulatory effects of young and old human MSCs. The T-cell proliferation in vitro assay is commonly used to determine whether the T cells residing in an animal's spleen are primed for proliferation because of their previous exposure to the MOG35–55 peptide in vivo. Thus, any treatment that inhibits T-cell proliferation in vivo should also result in decreased in vitro T-cell proliferation even when cultured in the presence of MOG35–55. In contrast, untreated animals should have increased T-cell proliferation after being cultured with MOG35–55 because of their previous exposure to the peptide in vivo. All T cells, regardless of mouse or treatment group, should proliferate in the presence of the CD3 and CD28 antibodies because of the direct stimulatory effect. In the current study, the T-cell proliferation assay results indicate that older MSCs may actually stimulate, and not inhibit, the proliferation of the T cells. In contrast, the results suggest that younger ASCs are capable of inhibiting the proliferation of T cells compared with the EAE cells in the presence of MOG35–55. A decrease in T-cell proliferation would result in a decreased number of T cells available to attack the CNS in the mice, which directly supports the histological results that the CNS damage and inflammation are less severe in the young ASC-treated mice than in the old ASC-treated mice.
Although the histopathological findings in the spinal cord and the T-cell proliferation assay demonstrated therapeutic effects in the affected mouse group receiving young hASCs, the serum proinflammatory cytokine levels at the peak of disease (DPI 15) were elevated compared with the naïve mice and not improved compared with the sham mice or mice receiving older hASCs. These ELISA results indicate that young hASCs are not capable of alleviating the systemic inflammation associated with EAE; these findings may explain why several mice receiving young cells became partially or completely paralyzed. Injected ASCs must be distributed in high enough numbers to perform systemic and local anti-inflammatory actions; thus, it is likely that a higher dose of ASCs should be initially used or an additional injection of ASCs should be provided (e.g., second injection at DPI 10) to increase the number of ASCs available to provide therapeutic benefit in the EAE-affected mice.
A recent article has demonstrated that HGF is a main therapeutic factor released by bone marrow-derived MSCs (BMSCs) when transplanted in EAE mice [7]. Bai et al. provided compelling evidence that both BMSCs and BMSC-conditioned media lose their therapeutic effectiveness when combined with anti-HGF antibodies [7]. Recent studies have also shown that HGF gene expression decreases with donor age in BMSCs and ASCs [12, 20, 34]. Collectively, these independent studies indicate that ASCs from older donors may have decreased levels of the main therapeutic agent necessary to provide CNS protection in EAE mice or MS patients. To test this hypothesis, the RNA levels of HGF and various cytokines were compared after culturing the old and young ASCs with and without stimulation.
Our data indicate that the younger cells have increased gene expression of HGF after stimulation compared with the older stem cells. HGF is a cytokine that promotes angiogenesis and cell survival [7], and it is also involved in the activation of ERK1/2 and MAPK pathways [38]. Directly supporting this finding, a previous study in our laboratory demonstrated that older donors have decreased expression of ERK1/2 and MAPK/p38 [27]. Therefore, the age-associated decline of HGF expression and disruption of signaling pathways in human ASCs may directly relate to the decreased therapeutic potential of ASCs from older donors. To further test this hypothesis, ongoing experiments in our laboratory have been designed: MSCs derived from older donors will be genetically modified to overexpress HGF or supplemented with recombinant HGF upon transplantation to achieve improved therapeutic efficacy, and HGF antibodies will be used in conjunction with in vivo experiments to test whether there is a loss of activity of the young cells. However, any protocols designed to increase HGF levels must also take into consideration the route of administration because of HGF's potent mitogenic effect on hepatocytes [7].
The age-related decrease of cytokines, specifically IL-1α, has been documented in several studies using human BMSCs and ASCs [20, 27]. Because MS involves chronic activation of microglia and macrophages [1, 3], it is appropriate to assume that the younger ASCs in this study provide better clinical benefit because of their ability to release of trophic factors after their activation in inflammatory disease states. Such effects are probably attributable to the age-related increases in nuclear factor-κB [20, 27] and DNA damage [35] and decreases in cell cycle regulators (e.g., p53, p21) [34], growth factors (e.g., HGF, vascular endothelial growth factor) [20, 34], and genomic stability (i.e., telomere length) [21]. A previous study from our laboratory highlighted significant age-related differences in the expression of microRNAs in human BMSCs and ASCs [27]. Our previous study demonstrated using histochemical staining of differentiation assays that ASCs have significantly less mineralization and lipid production in older donors than do those in younger donors (p < .05), demonstrating that the differentiation capabilities of ASCs are potentially linked to biological age of the donor [27]. Although it is unlikely that the therapeutic effects of the injected ASCs in the EAE mouse model are due to their in vivo differentiation, it is likely that other properties (e.g., migration ability and/or overall viability) could be similarly affected by biologic aging.
The stem cells of older donors have increased apoptosis [22], altered methylation patterns [36], altered secretomes (e.g., decreased IL-1α) [27, 37], and excessive activation of Wnt/b-catenin signaling [35]. A recent study compared young (1–3 months) and aged (18–24 months) mouse ASCs in normoxic (20%) and hypoxic (1%) conditions, and it was found that aged ASCs in hypoxic conditions have decreased proliferation potential and telomere lengths, increased frequency of apoptosis, and changed angiogenic properties [39]. Therefore, it is also possible that older human stem cells are also more sensitive to toxic environments, such as in vivo hypoxia after transplantation, and may have impaired therapeutic potential because of decreased proliferation or increased apoptosis.
This proof-of-concept study demonstrates that ASCs derived from older donors have a decreased therapeutic effectiveness compared with young donor cells when injected into an MS mouse model. It is likely that biologic aging of the human stem cells led to the differences in clinical and/or histopathological outcomes, and we predict that older donor MSCs would also lack therapeutic effectiveness in the human MS patient. Although a similar study has yet to be performed in a human population, several clinical trials have implemented autologous human MSC treatment for progressive MS patients. Thus far, all MS clinical trials that have included patients of young and old ages have demonstrated markedly mixed clinical outcomes, with some patients improving, others remaining unchanged, and several progressing in severity [19, 40–42]. Larger sample sizes are necessary for these MS clinical trials, but the inconsistent results and lack of benefit for most patients receiving autologous stem cell treatment may be related to the biologic age of the transplanted cells. Unfortunately, no MS clinical trial findings to date have been reported separately for older participants (>50 years). Thus, MS clinical studies should focus on demonstrating the therapeutic potential, or lack thereof, of human MSCs in patients of various ages; such a study would be instrumental in determining the therapeutic effectiveness of older cells in MS patients for the ultimate purpose of stem cell therapy optimization.
Conclusion
ASC transplantation is a novel therapy for MS patients who respond poorly to standard treatment regimens. However, as MSC clinical trials for MS patients become increasingly common, it becomes crucial to understand the biologic changes and therapeutic effects of older donor stem cells, which will be administered to older patients receiving autologous cell therapy. Numerous studies have demonstrated the age-related molecular, genetic, and in vitro changes of MSCs, but the current study is the first to demonstrate that MSCs derived from older donors are less effective than their younger counterparts upon transplantation in the EAE mouse model of MS. Collectively, these data support the use of autologous MSC transplantation for young MS patients only and highlight the need for more therapeutic testing of autologous transplantation in older MS patients. Potential allogeneic cell therapy or modified autologous protocols for late-onset MS patients may be necessary if older MSCs injected into humans, as in EAE mice, fail to provide neuroprotection, ameliorate symptoms of neurodegeneration, or release known therapeutic agents upon stimulation.
Acknowledgments
We thank Siddharth Gaikwad, Jeremy Green, Evan Kyzar, and the Neurophenotyping Core at Tulane University School of Medicine for their instruction, advice, and suggestions regarding the video recording studies. We also thank the veterinary and vivarial staff of the Tulane University Health Sciences Center Animal Facility for the daily care of the mice. We also thank Alan Tucker for performing flow cytometry for characterization of the MSC populations and for his assistance with the cell proliferation assay, and Dina Gaupp and Claire Llamas in the Tulane Histology Core for performance of the histological staining for this study. This work was supported by Tulane University.
Author Contributions
B.A.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; J.A.S. and X.Z.: conception and design, collection and/or assembly of data, data analysis and interpretation; S.Z., A.C.B., and A.C.P.: collection and/or assembly of data, data analysis and interpretation; K.M.P.I.: collection and/or assembly of data; A.V.K. and J.M.G.: conception and design, data analysis and interpretation; B.A.B.: conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.M.G. has compensated employment and intellectual property rights as co-owner and co-founder of LaCell LLC.
References
- 1.Goldenberg MM. Multiple sclerosis review. P T. 2012;37:175–184. [PMC free article] [PubMed] [Google Scholar]
- 2.Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lassmann H, van Horssen J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011;585:3715–3723. doi: 10.1016/j.febslet.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Jadasz JJ, Aigner L, Rivera FJ, et al. The remyelination Philosopher's Stone: Stem and progenitor cell therapies for multiple sclerosis. Cell Tissue Res. 2012;349:331–347. doi: 10.1007/s00441-012-1331-x. [DOI] [PubMed] [Google Scholar]
- 5.Lassmann H, van Horssen J, Mahad D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat Rev Neurol. 2012;8:647–656. doi: 10.1038/nrneurol.2012.168. [DOI] [PubMed] [Google Scholar]
- 6.Bai L, Lennon DP, Eaton V, et al. Human bone marrow-derived mesenchymal stem cells induce Th2-polarized immune response and promote endogenous repair in animal models of multiple sclerosis. Glia. 2009;57:1192–1203. doi: 10.1002/glia.20841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bai L, Lennon DP, Caplan AI, et al. Hepatocyte growth factor mediates mesenchymal stem cell–induced recovery in multiple sclerosis models. Nat Neurosci. 2012;15:862–870. doi: 10.1038/nn.3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constantin G, Marconi S, Rossi B, et al. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27:2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 9.Freedman MS, Bar-Or A, Atkins HL, et al. The therapeutic potential of mesenchymal stem cell transplantation as a treatment for multiple sclerosis: Consensus report of the International MSCT Study Group. Mult Scler. 2010;16:503–510. doi: 10.1177/1352458509359727. [DOI] [PubMed] [Google Scholar]
- 10.Gerdoni E, Gallo B, Casazza S, et al. Mesenchymal stem cells effectively modulate pathogenic immune response in experimental autoimmune encephalomyelitis. Ann Neurol. 2007;61:219–227. doi: 10.1002/ana.21076. [DOI] [PubMed] [Google Scholar]
- 11.Zappia E, Casazza S, Pedemonte E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106:1755–1761. doi: 10.1182/blood-2005-04-1496. [DOI] [PubMed] [Google Scholar]
- 12.Kilroy GE, Foster SJ, Wu X, et al. Cytokine profile of human adipose-derived stem cells: Expression of angiogenic, hematopoietic, and pro-inflammatory factors. J Cell Physiol. 2007;212:702–709. doi: 10.1002/jcp.21068. [DOI] [PubMed] [Google Scholar]
- 13.Uccelli A, Benvenuto F, Laroni A, et al. Neuroprotective features of mesenchymal stem cells. Best Pract Res Clin Haematol. 2011;24:59–64. doi: 10.1016/j.beha.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 14.Uccelli A, Zappia E, Benvenuto F, et al. Stem cells in inflammatory demyelinating disorders: A dual role for immunosuppression and neuroprotection. Expert Opin Biol Ther. 2006;6:17–22. doi: 10.1517/14712598.6.1.17. [DOI] [PubMed] [Google Scholar]
- 15.Kassis I, Grigoriadis N, Gowda-Kurkalli B, et al. Neuroprotection and immunomodulation with mesenchymal stem cells in chronic experimental autoimmune encephalomyelitis. Arch Neurol. 2008;65:753–761. doi: 10.1001/archneur.65.6.753. [DOI] [PubMed] [Google Scholar]
- 16.Bunnell BA, Betancourt AM, Sullivan DE. New concepts on the immune modulation mediated by mesenchymal stem cells. Stem Cell Res Ther. 2010;1:34. doi: 10.1186/scrt34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Uccelli A, Laroni A, Freedman MS. Mesenchymal stem cells as treatment for MS: Progress to date. Mult Scler. 2013;19:515–519. doi: 10.1177/1352458512464686. [DOI] [PubMed] [Google Scholar]
- 18.Karussis D, Karageorgiou C, Vaknin-Dembinsky A, et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch Neurol. 2010;67:1187–1194. doi: 10.1001/archneurol.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Connick P, Kolappan M, Crawley C, et al. Autologous mesenchymal stem cells for the treatment of secondary progressive multiple sclerosis: An open-label phase 2a proof-of-concept study. Lancet Neurol. 2012;11:150–156. doi: 10.1016/S1474-4422(11)70305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alt EU, Senst C, Murthy SN, et al. Aging alters tissue resident mesenchymal stem cell properties. Stem Cell Res. 2012;8:215–225. doi: 10.1016/j.scr.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Wu W, Niklason L, Steinbacher DM. The effect of age on human adipose derived stem cells. Plast Reconstr Surg. 2012 doi: 10.1097/PRS.0b013e3182729cfc. [DOI] [PubMed] [Google Scholar]
- 22.Stolzing A, Jones E, McGonagle D, et al. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech Ageing Dev. 2008;129:163–173. doi: 10.1016/j.mad.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Bonab MM, Alimoghaddam K, Talebian F, et al. Aging of mesenchymal stem cell in vitro. BMC Cell Biol. 2006;7:14. doi: 10.1186/1471-2121-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner W, Ho AD, Zenke M. Different facets of aging in human mesenchymal stem cells. Tissue Eng Part B Rev. 2010;16:445–453. doi: 10.1089/ten.TEB.2009.0825. [DOI] [PubMed] [Google Scholar]
- 25.Kretlow JD, Jin YQ, Liu W, et al. Donor age and cell passage affects differentiation potential of murine bone marrow-derived stem cells. BMC Cell Biol. 2008;9:60. doi: 10.1186/1471-2121-9-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou S, Greenberger JS, Epperly MW, et al. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pandey AC, Semon JA, Kaushal D, et al. MicroRNA profiling reveals age-dependent differential expression of nuclear factor κB and mitogen-activated protein kinase in adipose and bone marrow-derived human mesenchymal stem cells. Stem Cell Res Ther. 2011;2:49. doi: 10.1186/scrt90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sonobe Y, Jin S, Wang J, et al. Chronological changes of CD4(+) and CD8(+) T cell subsets in the experimental autoimmune encephalomyelitis, a mouse model of multiple sclerosis. Tohoku J Exp Med. 2007;213:329–339. doi: 10.1620/tjem.213.329. [DOI] [PubMed] [Google Scholar]
- 29.Scruggs BA, Bowles AC, Zhang X, et al. High-throughput screening of stem cell therapy for globoid cell leukodystrophy using automated neurophenotyping of twitcher mice. Behav Brain Res. 2013;236:35–47. doi: 10.1016/j.bbr.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noldus LP, Spink AJ, Tegelenbosch RA. EthoVision: A versatile video tracking system for automation of behavioral experiments. Behav Res Methods Instrum Comput. 2001;33:398–414. doi: 10.3758/bf03195394. [DOI] [PubMed] [Google Scholar]
- 31.Spink AJ, Tegelenbosch RA, Buma MO, et al. The EthoVision video tracking system: A tool for behavioral phenotyping of transgenic mice. Physiol Behav. 2001;73:731–744. doi: 10.1016/s0031-9384(01)00530-3. [DOI] [PubMed] [Google Scholar]
- 32.Schindelin J, Arganda-Carreras I, Frise E, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernard CC, Carnegie PR. Experimental autoimmune encephalomyelitis in mice: Immunologic response to mouse spinal cord and myelin basic proteins. J Immunol. 1975;114:1537–1540. [PubMed] [Google Scholar]
- 34.Wilson A, Shehadeh LA, Yu H, et al. Age-related molecular genetic changes of murine bone marrow mesenchymal stem cells. BMC Genomics. 2010;11:229. doi: 10.1186/1471-2164-11-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang DY, Pan Y, Zhang C, et al. Wnt/β-catenin signaling induces the aging of mesenchymal stem cells through promoting the ROS production. Mol Cell Biochem. 2013;374:13–20. doi: 10.1007/s11010-012-1498-1. [DOI] [PubMed] [Google Scholar]
- 36.Bork S, Pfister S, Witt H, et al. DNA methylation pattern changes upon long-term culture and aging of human mesenchymal stromal cells. Aging Cell. 2010;9:54–63. doi: 10.1111/j.1474-9726.2009.00535.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gala K, Burdzińska A, Idziak M, et al. Characterization of bone-marrow-derived rat mesenchymal stem cells depending on donor age. Cell Biol Int. 2011;35:1055–1062. doi: 10.1042/CBI20100586. [DOI] [PubMed] [Google Scholar]
- 38.Awasthi V, King RJ. PKC, p42/p44 MAPK, and p38 MAPK are required for HGF-induced proliferation of H441 cells. Am J Physiol Lung Cell Mol Physiol. 2000;279:L942–L949. doi: 10.1152/ajplung.2000.279.5.L942. [DOI] [PubMed] [Google Scholar]
- 39.Efimenko A, Starostina E, Kalinina N, et al. Angiogenic properties of aged adipose derived mesenchymal stem cells after hypoxic conditioning. J Transl Med. 2011;9:10. doi: 10.1186/1479-5876-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mohyeddin Bonab M, Yazdanbakhsh S, Lotfi J, et al. Does mesenchymal stem cell therapy help multiple sclerosis patients? Report of a pilot study. Iran J Immunol. 2007;4:50–57. doi: 10.22034/iji.2007.17180. [DOI] [PubMed] [Google Scholar]
- 41.Lee T. Stem cell therapy independent of stemness. World J Stem Cells. 2012;4:120–124. doi: 10.4252/wjsc.v4.i12.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tyndall A. Successes and failures of stem cell transplantation in autoimmune diseases. Hematology Am Soc Hematol Educ Program. 2011;2011:280–284. doi: 10.1182/asheducation-2011.1.280. [DOI] [PubMed] [Google Scholar]